Abstract
Atrial fibrillation is a highly prevalent cardiac arrhythmia. It is associated with numerous co mobilities. Approximately 30% of diabetic patients have atrial fibrillation and 15% of atrial fibrillation regulation patients have diabetes mellitus. Diabetes increases the likelihood of the development of atrial fibrillation and contributes to the high risk of thromboembolism seen in patients with both diabetes and atrial fibrillation. Chronic kidney disease is often a consequence of diabetes and presents an additional challenge to the management of patients with both atrial fibrillation and diabetes. All non-vitamin K oral anticoagulants are partially eliminated via the kidney and must be carefully prescribed according to strict dosing schedules to avoid anticoagulation overdose. However, NOACs have the advantage of being associated with less progressive impairment of renal function compared with vitamin K antagonist therapy in both diabetics and non-diabetics. Otherwise, diabetic patients benefit from NOAC therapy as opposed to vitamin K antagonists to a similar extent as patients without diabetes. This review deals with anticoagulation treatment in patients with fibrillation and diabetes mellitus, often complicated by progressive renal impairment.
Keywords: Atrial fibrillation, Diabetes mellitus, Renal failure, Oral anticoagulants, Direct oral anticoagulants, Vitamin K antagonists
Introduction
The prevalence of atrial fibrillation continues to increase because of better case recognition and improved survival of patients already with atrial fibrillation or with comorbidities which are likely to lead to atrial fibrillation. Among these comorbidities are diabetes and chronic kidney disease (CKD), both of which promote the development of atrial fibrillation, worsen outcomes associated with atrial fibrillation, and present challenges with the treatment of the arrhythmia with anticoagulant therapy. This review focuses on the prognosis and management of atrial fibrillation and its complications in patients with diabetes, impaired renal function, or both conditions.
Diabetes
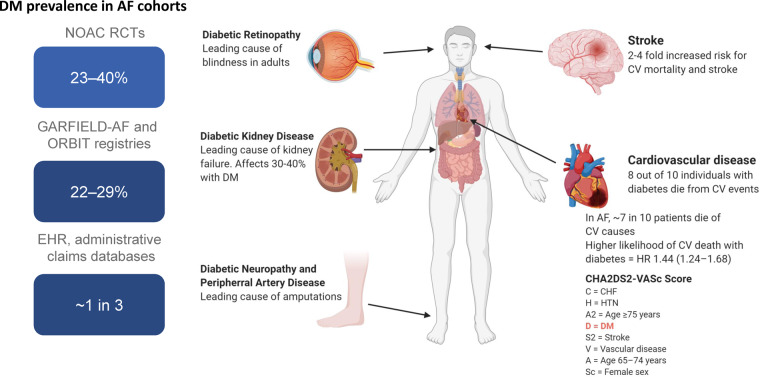
The global burden of diabetes is currently estimated to affect 463 million individuals or 1 in 11, according to the International Diabetes Federation, and projections estimate a 48% increase in the prevalence to 700 million people by 2045.1 The vast majority (80–90%) have type 2 diabetes. Diabetes predisposes to the development of atrial fibrillation, thus in the Framingham study, the odds for atrial fibrillation was increased 40% for men and 60% for women.2 In people with atrial fibrillation not receiving anticoagulation, diabetes is a risk factor for ischaemic stroke3–5 (Figure 1).
Figure 1.
Comorbid diabetes is highly prevalent and increases the risk of stroke and cardiovascular death in patients with atrial fibrillation. DM, diabetes mellitus; HR, hazard ratio; GARFIELD, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; NOAC, non-vitamin K oral anticoagulant; ORBIT, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; RCT, randomized controlled trial.
In addition to the increased risk for atrial fibrillation, diabetes is associated with a two- to four-fold increased risk for atherosclerotic cardiovascular disease (CVD) compared to the background population, and 30–40% with diabetes are affected by chronic kidney disease characterized by increased albuminuria and/or decreased glomerular filtration rate (GFR; or diabetic kidney disease, DKD) (Figure 1). The presence of kidney disease in diabetes increases the risk of CVD and the combination is associated with excess mortality. Increasing albuminuria or decreasing GFR increases the risk of CVD and mortality6 and the risk for end-stage kidney disease (ESKD). Furthermore, albuminuria and GFR levels form the basis on which chronic kidney disease is staged according to the Kidney Disease Improving Global Outcome guidelines.7 Early-onset DKD may shorten life expectancy by ∼15 years,8 and excess mortality in diabetes is primarily due to mortality in DKD9; with a six-fold increased risk for mortality with albuminuria, and 15-fold increased risk with albuminuria and reduced GFR.9
As already stated, the prevalence of atrial fibrillation is increased in diabetes. It has been estimated that 15% of subjects with diabetes have atrial fibrillation10 and conversely that 30% of atrial fibrillation patients have diabetes, among a population from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) registry, a prospective, nationwide, outpatient registry of people with incident and prevalent AF including 9749 subjects with atrial fibrillation.11 In a large electronic health record database with administrative claims data, one in three with atrial fibrillation had diabetes.12 Similarly, analysis of a large database including 293 124 individuals found diabetes to be a strong independent risk factor with 100% increased odds for atrial fibrillation.13 Even the metabolic syndrome, with a cluster of multiple cardiovascular (CV) risk factors including hypertension, obesity, dyslipidaemia, and dysglycaemia, often preceding manifest diabetes, is associated with excess risk for atrial fibrillation, and even more when combined with chronic kidney disease.14
The prevalent combination of diabetes and atrial fibrillation is also reflected in the randomized controlled clinical trials (RE-LY, ROCKET-AF, ARISTOTLE, ENGAGE AF-TIMI 48) investigating non-vitamin K oral anticoagulants (NOACs) compared with warfarin, in subjects with non-valvular atrial fibrillation, where 23–40% of subjects included had diabetes.15 Diabetes has been shown to increase the risk of incident AF,7 as it promotes maladaptive and pro-fibrillatory structural (mediated by oxidative stress, advanced glycosylation end products, and connective tissue changes), electromechanical, and autonomic nervous system changes.16
Not only is diabetes associated with increased occurrence of atrial fibrillation but also based on follow-up of 11 140 type 2 diabetes subjects, the combination of diabetes and atrial fibrillation is associated with increased risk for death (61% increased risk) and major cerebrovascular deaths (68% increased risk).17 This increased event rate may be explained by the underlying pathophysiological mechanisms where diabetes with hyperglycaemia and AF contribute to the risk of stroke and thromboembolism via partly shared pathophysiological pathways. Thus, both promote oxidative stress and inflammation which leads to endothelial damage, increasing susceptibility to thrombus formation. Also, diabetes and AF are independently associated with platelet and fibrinogen activation contributing to changes in the blood constituents which also increases the likelihood of thrombus formation. Adding the changes in left atrial dimensions and abnormal flow and stasis further increases the propensity for thromboembolism.16
This was supported and extended from the ORBIT-AF register showing that patients with diabetes (30%) were younger, more likely to have hypertension, chronic kidney disease, heart failure, coronary heart disease, and stroke. Compared to patients without diabetes, patients with diabetes also had a lower Atrial Fibrillation Effects on Quality of Life score of 80 (interquartile range, IQR: 62.5–92.6) vs. 82.4 (IQR: 67.6–93.5; P = 0.025) driven by the daily activities’ domain score, whereas the symptoms and treatment concern domain scores were similar.
The overall use of anticoagulants (warfarin or dabigatran) was significantly greater among people with diabetes. The use of warfarin only was higher among those with diabetes compared to those without diabetes (74.3% vs. 70.4%; P < 0.001) but the use of dabigatran was similar in the two groups. Aspirin was more commonly used among people with diabetes (46.5% vs. 43.4%; P = 0.006). Diabetes was associated with higher mortality risk, including overall [hazard ratio (HR): 1.63; 95% confidence interval (CI): 1.04–2.56, for age < 70 years vs. HR: 1.25; 95% CI: 1.09–1.44, for age ≥ 70 years] and CV mortality (HR: 2.20; 95% CI: 1.22–3.98, for age < 70 years vs. 1.24; 95% CI: 1.02–1.51 for age ≥ 70 years). Diabetes conferred a higher risk of non-CV death, sudden cardiac death, hospitalization, CV hospitalization, and non-CV and non-bleeding-related hospitalization, but no increase in risks of thromboembolic events, bleeding-related hospitalization, new-onset heart failure (HF), and AF progression.11
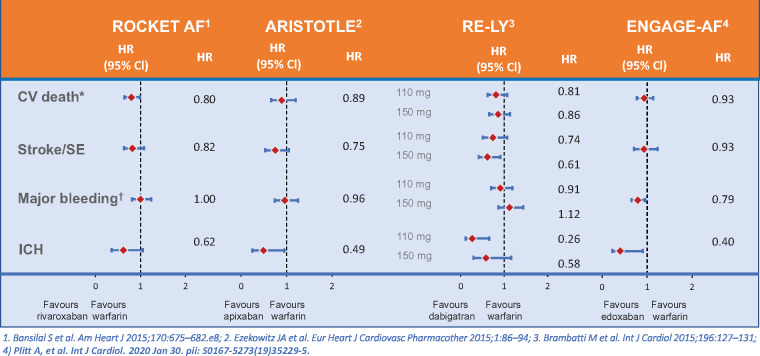
In the ROCKET-AF study comparing rivaroxaban and warfarin in nonvalvular atrial fibrillation in 14 264 subjects with a prevalence of diabetes of 40%, a higher likelihood of CV death was observed with diabetes with an HR of 1.44 (1.24–1.68).18 This study furthermore demonstrated that the NOAC rivaroxaban gave similar protection against the primary endpoint of stroke and systemic embolism as did warfarin, without increasing overall major bleeding risk (Figure 2). In addition, rivaroxaban provided a significant benefit on the secondary outcome of CV death with an HR of 0.80 (0.64–0.99) and a significant interaction with diabetes (P = 0.037).
Figure 2.
Outcomes of diabetic patients in Phase 3 non-vitamin K oral anticoagulants versus warfarin(19,70–72). CV = cardiovascular; ICH = intra cranial hemorrhage SE = systemic embolus.
In the ARISTOTLE trial, there were similar benefits on reducing stroke, decreasing mortality, and causing less intracranial bleeding with apixaban compared to warfarin in people with and without diabetes. People with diabetes receiving apixaban had lower rates of stroke and systemic embolism (HR of 0.75, 95% CI 0.53–1.05), all-cause mortality (HR 0.83, 95% CI 0.67–1.02), CV mortality (HR 0.89, 95% CI 0.66–1.20), intracranial haemorrhage (HR 0.49, 95% CI 0.25–0.95), and a similar rate of myocardial infarction (HR 1.02, 95% CI 0.62–1.67) compared with warfarin19 (Figure 2). In a real-world data study of a cohort of n = 77 752 with atherosclerosis and atrial fibrillation, the risk for major adverse CV events (CV death or hospitalization with a primary billing code for myocardial infarction or ischaemic stroke) was also increased with diabetes (n = 37 114) with an HR of 1.11 (1.05–1.18).20
The four Phase 3 studies comparing NOACs with warfarin were meta-analysed and the analysis demonstrated a reduction in stroke/systemic embolism in diabetic [risk ratios (RR) 0.80, 95% CI 0.68–0.93; P = 0.004] and nondiabetic patients (RR 0.83, 0.73–0.93; P = 0.001; P for interaction 0.72). In general, there was no interaction between diabetes and NOAC effect in AF. There was a reduction in vascular mortality in diabetes with NOACs compared to warfarin, which was not seen in non-diabetic subjects, but the P-value for interaction was not significant21 (Figure 2).
Observational data from registries have found similar results. A study using MarketScan claims data demonstrated in a cohort of 77 752 with coronary artery disease or at least three risk factors, and NVAF, of whom 48% had diabetes, that AF patients receiving an oral anticoagulant (OAC) compared to patients without OAC, were less likely to experience major adverse cardiovascular evnts (MACE) (8.9% vs 11.6%, P < 0.0001) including ischaemic stroke (5.4% vs. 6.7%, P < 0.0001).22 In another study using MarketScan claims data for a cohort of only type 2 diabetes with non-valvular AF (NVAF), 10 700 users of the NOAC rivaroxaban and 13 946 users of warfarin were identified. The study found rivaroxaban was associated with a 25% (95% CI 4–41) reduced risk of MACE compared to warfarin. Major bleeding risk did not significantly differ between cohorts (HR 0.95).23
When applying the CHA2DS2-VASc score to assess risk for stroke in atrial fibrillation the D (diabetes) is defined as fasting blood glucose >7 mmol/L, or treatment with oral hypoglycaemic drugs, and/or insulin.24 It has now been included in the ESC guideline that the excess risk for stroke associated with diabetes, is very similar in type 1 and type 2 diabetes except perhaps for a slightly increased risk in type 2 diabetes compared to type 1 in patients <65 years of age.25 The risk for stroke increases with longer diabetes duration, as well as with more diabetes comorbidities such as retinopathy.24,26,27 In diabetes guidelines, diabetes is also diagnosed by elevated haemoglobin A1c >6.5% or 48 mmol/L, which does not require fasting and this could be an alternative.
The impact of diabetes therapy on the risk for stroke and AF has been debated. Although the SGLT2 inhibitor empagliflozin was associated with a reduction in major adverse CV events in the CV outcome study EMPA-REG, a non-significant trend towards an increase in stroke was seen,28 but this has not been seen in other SGLT2 inhibitor studies. With the GLP1 receptor agonists, a reduction in fatal and non-fatal stroke has been seen in the CV outcome studies HR 0.84 (95% CI 0.76–0.93).29 This was not evaluated in the context of atrial fibrillation. It has been suggested that metformin and pioglitazone may reduce the risk for AF.30 Recently, it was suggested that the SGLT2 inhibitor dapagliflozin-reduced occurrence of AF in type 2 diabetes in the CV outcome study DECLARE-TIMI 58. Dapagliflozin reduced the risk of AF/AFL events by 19% [264 vs. 325 events; 7.8 vs. 9.6 events per 1000 patient-years; HR 0.81 (95% CI 0.68–0.95); P = 0.009]. The reduction in AF/AFL events was consistent regardless of the presence or absence of a history of AF/AFL or ASCVD at baseline. Dapagliflozin also reduced the total number (first and recurrent) of AF/AFL events incidence rate ratio, 0.77 (95% CI 0.64–0.92); P = 0.005).31 This finding was exploratory but very interesting as the SGLT2 inhibitors are now increasingly recommended for patients with type 2 diabetes,32 but the finding should be confirmed in additional studies. This has not been seen with other glucose-lowering medications, and intensive glycaemic control does not affect the risk for AF.24
A prevalent complication in diabetes is the diabetic foot, or major adverse limb events (MALE) in clinical studies. In diabetes management, this is the most expensive complication related to diabetes. Interestingly, NOACs were found to reduce the risk of MALE in atrial fibrillation patients with diabetes when compared with warfarin. This was seen in a retrospective claims’ analysis in patients with atrial fibrillation and comorbid diabetes. The study identified 10 700 people treated with rivaroxaban and 13 946 patients treated with warfarin. At baseline, 11% of people had peripheral artery disease, 5.1% had coronary artery disease, and 5.1% had a prior MALE. Compared to warfarin, rivaroxaban was associated with a 63% (95% CI 35–79) reduced risk of MALE. For major limb amputations, the reduced risk with rivaroxaban compared with warfarin was 80% (95% CI 31–94).23 Similar results were found based on an analysis from the Taiwan National Health Insurance Research Database, where a total of 20 967 and 5812 consecutive AF patients with diabetes taking NOACs and warfarin, respectively were identified. Here, NOAC treatment was associated with a lower risk of major adverse CV events [adjusted HR: 0.88 (95% CI 0.78–0.99), P = 0.028], major adverse limb events [HR: 0.72 (95% CI 0.57–0.92); P = 0.0083], and major bleeding [HR: 0.67 (95% CI 0.59–0.76); P < 0.0001] compared to warfarin.33
Given the limitation of non-randomized observations, this needed to be confirmed in a pre-specified subgroup of people with peripheral artery disease and diabetes in the Phase III COMPASS study which compared low-dose rivaroxaban + aspirin to aspirin alone in 7470 participants with peripheral artery disease.34 In the COMPASS study, rivaroxaban 2.5 mg twice daily with aspirin 100 mg once daily compared to aspirin alone, reduced MALE including major amputation with an HR of 0.54 (95% CI 0.35–0.82; P = 0.0037).34 The effect was smaller in people with comorbid diabetes, but there was no significant interaction with diabetes.35
Thus, diabetes is prevalent, associated with increased risk for atrial fibrillation, and also other adverse CV and kidney outcomes. It may be argued that diabetes in atrial fibrillation should be considered more than ‘1’ point in the CHA2DS2-VASc risk score.
Impact of chronic kidney disease in patients with diabetes and atrial fibrillation
It is well established that patients with NVAF and chronic kidney disease (CKD) are at a higher risk of ischaemic stroke,24 although the presence of CKD (or renal impairment) unlike the diagnosis of diabetes is not implemented in the currently recommended risk stratification.24 Indeed, the inclusion of renal dysfunction into the CHA2DS2-VASc score lacks substantial additive predictive value.36 This is likely because the predictive value of CKD is already captured by other score components within CHA2DS2-VASc that strongly associate with CKD, most importantly age, hypertension, and diabetes. Hence, hypertension and diabetes represent the most common global causes of CKD,37 while the prevalence of both CKD and diabetes associates with age. In diabetes and CKD, several overlapping pathophysiological factors related to alterations in the three components of Virchow’s triad (changes in blood constituents, blood flow, and vessel wall and/or atrial tissue) contribute to the prothrombotic state in coexisting AF with either CKD or diabetes.38,39
Interestingly, diabetic retinopathy did not emerge as an independent predictor for either stroke or severe bleeding,26 whereas regardless of AF, patients with CKD are per se at increased risk of bleeding due to several mechanisms that contribute to a prohaemorrhagic state.38,39 During anticoagulation, the increased bleeding risk may be further exacerbated by the significant effects of renal impairment on the pharmacokinetics and/or pharmacodynamics of anticoagulant drugs.40 Hence, abnormal renal function defined as chronic dialysis, renal transplantation, or serum creatinine ≥200 μmol/L (which would relate to a CrCl of about 25 or 30 mL/min in a female or male patient at the age of 70 years and body weight of 70 kg) is a parameter accounting for 1 point in the HAS-BLED score41 that is recommended as a tool for bleeding risk assessment in patients with AF.24
Against this background, the use of NOACs and particularly their safety in patients with CKD was followed with great interest from the beginning, because the four available NOACs for anticoagulation in AF are all to varying degrees eliminated by the kidney.40 In this regard, it was reassuring that in patients with renal impairment the safety and efficacy of NOAC compared with warfarin treatment was consistent with patients without CKD in the four landmark NOAC RCTs.42–45 However, patients with a creatinine clearance (CrCl) <25–30 mL/min as estimated with the Cockcroft–Gault equation were generally excluded from these pivotal Phase 3 RCTs. Consequently, adequate safety and efficacy data are lacking in patients with CKD stage 4 (CrCL of 15–29 mL/min) and CKD stage 5, i.e. ESKD (CrCL < 15 mL/min) including the relatively large group of patients with diabetes and advanced CKD.37 Nevertheless, NOAC use in AF patients with CKD and a CrCl greater than 30 mL/min is based on evidence from RCT and thus not restricted in this vulnerable patient group.24,40
The same considerations may apply to stroke risk assessment and selection of oral anticoagulants in these patients including patients with DKD and a CrCl >30 mL/min. Moreover, pharmacokinetic data indicated that the change in NOAC plasma levels for the three factor Xa inhibitors apixaban, edoxaban, and rivaroxaban in severe CKD (CrCl 15–30 mL/min) were similar to patients with moderate renal impairment and led to their approval in patients with severe CKD.46 Accordingly, reduced dosing regimens for these drugs are feasible options for anticoagulation in patients with severe non-ESKD.24,46
The use of OAC in patients with ESKD on maintenance dialysis is still a matter of debate.38,39,47 In Europe, NOACs are not approved and not recommended in patients with CrCl <15 mL/min.24,46 In this regard, it is important to emphasize that although the FDA does mention the usage of oral anticoagulant in patients on dialysis with recommended dosing for VKA, apixaban, and rivaroxaban, the FDA itself has not endorsed this indication for use in this population as recently noted.39
Data from observational studies suggest a possible reduction in the risk of bleeding in patients with ESKD who take an NOAC compared to VKA48,49 and possibly a similar risk of embolic and major bleeding events associated with the treatment with apixaban and rivaroxaban.50 Currently, there are at least three ongoing RCTs that are designed as safety (Phase 2) trials enrolling relatively low numbers (150–855) patients on dialysis including patients with diabetes. They will compare NOACs with VKAs or oral anticoagulation with no use anticoagulants (NCT03987711, NCT02933697, and NCT02886962). The challenge to conduct studies in this setting has been recently highlighted by the early termination of the RENAL-AF trial, investigating apixaban vs. warfarin in AF patients on haemodialysis, with inconclusive data on relative stroke and bleeding rates.51
Observational studies in AF patients and renal outcomes
Of note, unlike AF patients on dialysis, to the best of our knowledge, there are no ongoing RCTs to explore the impact of OAC with VKA and NOACs in patients with non-dialysis advanced CKD particularly in patients with Stage 4 CKD (CrCl < 30 mL/min). We thus still face an unmet need of evidence from RCTs in this vulnerable patient cohort. Nevertheless, because there is a progressively increased risk of both ischaemic stroke and haemorrhage as renal function declines it is important to evaluate the effectiveness of anticoagulation in this setting, which is the aim of ongoing prospective registries with blinded outcome adjudication in Europe (XARENO, NCT02663076) and Eastern Asia (XARENAL, NCT03746301).Importantly, there is a substantial number of observational studies available in this condition that include by virtue of the relatively high prevalence also AF patients with CKD and diabetes. This is important since a progressive decline in renal function is often observed in diabetes particularly in patients with pre-existing CKD, which associates with a poor prognosis.52
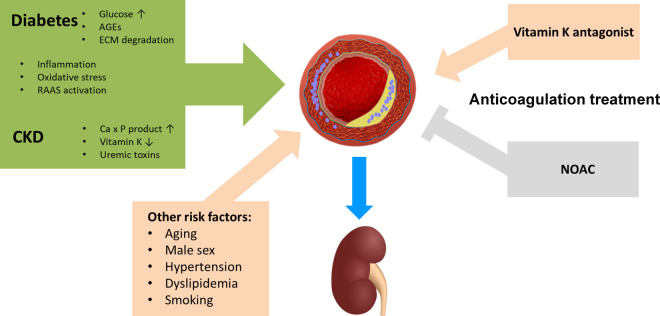
Increased vascular calcification processes for which diabetes and CKD share complementary pathophysiology53,54 has been identified as a possible mechanism contributing to the decline in renal function in patients with diabetes and CKD (Figure 3). The latter is frequently worsened in the presence of other risk factors for calcification, such as advanced age and hypertension,54 which are very common in AF patients. While in patients with CKD, a disturbance in calcium–phosphate balance, accumulation of uremic toxins, and severe vitamin K deficiency have been implicated in the pathogenesis of vascular calcification, several other signalling pathways in addition to elevated glucose levels play a role in diabetes although the mechanisms are still not fully understood53,54 (Figure 3). In anticoagulated AF patients and particularly in patients with comorbid CKD and diabetes, the use of VKA may aggravate vascular calcification55 including calcification in the vascular bed of the kidney, thereby contribute to worsening renal function in these patients.56,57 This is mechanistically based on inhibition of the vitamin K-dependent gamma-glutamyl carboxylation that applies not only to the clotting factors II, VII, IX, and X but also to the other vitamin K dependent gamma-carboxyglutamic acid (Gla) proteins including the matrix Gla protein (MGP).54,58 Matrix Gla protein, which is in the vascular wall mainly produced in vascular smooth muscle cells, represents the most potent endogenous protector against vascular calcification and its diminished function has been linked to vascular calcification during VKA treatment.58 In contrast, NOACs, such as the factor Xa inhibitor rivaroxaban, due to their different mode of action do not only lack this negative effect, but may even provide beneficial protective effects against vascular injury and renal functional decline by decreasing vascular inflammation, remodelling, and vascular calcifications through reduced protease-activated receptor (PAR) signalling via PAR-1 and PAR-259,60 (Figure 3).
Figure 3.
Factors for arterial calcification and kidney injury: differential impact of anticoagulation with Vitamin K antagonists versus non-vitamin K antagonists (NOACs). CKD = chronic kidney disease, ECM = extracellular matrix; RAAS = renin-angiotensin-aldosterone system.
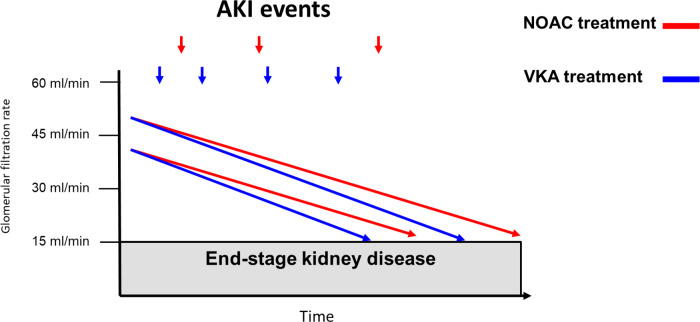
The possible differential impact of anticoagulation with either VKA vs. NOACs provided the biological basis to investigate the effects of these treatments for renal outcomes in addition to thromboembolic and bleeding events in AF patients with diabetes in retrospective real-world studies. Accordingly, a recent study using United States IBM MarketScan data included patients with NVAF and diabetes that newly initiated on rivaroxaban (N = 10 017) or warfarin (N = 11 665).61 Patients with Stage 5 CKD or undergoing haemodialysis at baseline were excluded and baseline covariates between cohorts were adjusted using inverse probability of treatment weighting based on propensity scores. In comparison to warfarin, rivaroxaban was associated with lower risks of acute kidney injury (AKI) events (HR 0.83, 95% CI 0.74–0.92) and development of Stage 5 CKD or need for haemodialysis (HR 0.82, 95% CI 0.70–0.96). The protective effect in favour of rivaroxaban was particularly pronounced in the subgroup of diabetic patients with pre-existing Stage 3–4 CKD for both AKI (HR 0.63, 95% CI 0.49–0.79) and the risk of stage 5 CKD or need for haemodialysis (HR 0.66, 95% CI 0.46–0.94).61 A similar retrospective study on data from a claims database in Germany evaluated confounder-adjusted risks for AKI and ESKD in AF patients with diabetes who received rivaroxaban (n = 6997) or the VKA phenprocoumon (n = 8545).62 The relative risks for AKI was decreased by 28% (HR 0.72, 95% CI 0.53–0.97) and for ESKD by 68% (HR 0.32, 95% CI 0.19–0.53) in AF patients with diabetes prescribed rivaroxaban vs. phenprocoumon.
Despite the methodological limitations of these observational retrospective studies including potential residual confounding due to the absence of randomization, it appears that rivaroxaban treatment associates with a lower risk of both AKI and progression to Stage 5 CKD or dialysis in diabetic patients with NVAF (Figure 4). Similar results towards a benefit in reducing the risk for AKI and/or progression to Stage 5 CKD were also obtained in other retrospective real-world studies in the overall population of AF patients that included also diabetic patients but were not restricted to patients with comorbid diabetes.63,64
Figure 4.
Differential impact of anticoagulation with VKA versus NOAC on renal outcomes in atrial fibrillation. AKI = acute kidney injury; NOAC = non-vitamin K oral anticoagulant, VKA = vitamin K oral anticoagulant.
Apart from the generally increased risk of bleeding in patients with AF with CKD, there are also concerns about the risk of anticoagulation nephropathy (ARN) in these patients.65,66 Anticoagulation nephropathy is characterized by profuse glomerular haemorrhage leading to renal tubules filled with red cells and red cell casts resulting in AKI.65,66 While ARN may develop in response to any anticoagulant, including VKA and NOACs, it has been particularly associated with overdosing of warfarin with INR levels >3.65,66 Older patients with diabetes and CKD are particularly prone to develop ARN that can trigger episodes of AKI that may occur more frequently in AF patients as previously thought.66 Recurrences of these AKI episodes can in turn accelerate the progression of CKD and are associated with an increased mortality rate.67 Thus, although the pathogenesis of AKI is multifactorial, ARN may contribute to AKI events particularly in AF patients with CKD and diabetes. In this regard, the observational evidence for a significant risk reduction for AKI events by rivaroxaban vs. VKA may at least in part be attributable to a decrease in the rate of ARN.
Summary/conclusion: pending
In the recent 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS), the importance of an integrated management of AF patients was emphasized to deliver optimized treatment.24 In this guidelines, a core element to guide and simplify management represents the previously suggested68 simple Atrial fibrillation Better Care (ABC) holistic pathway (‘A’ Anticoagulation/Avoid stroke; ‘B’ Better symptom management; ‘C’ Cardiovascular and Comorbidity optimization).24 In the ABC pathway, optimal management of comorbidities plays a pivotal role to improve outcome in patients with AF. As discussed in this review, diabetes per se and even more so when associated with CKD represents one of the most important comorbidities that requires optimal management by guideline-directed therapy.69 This is also acknowledged in the 2019 ESC Guidelines on diabetes, pre-diabetes, and CVDs developed in collaboration with the European Association for the Study of Diabetes, by recommending that anticoagulation in all AF patients with diabetes.69 This supports our notion that diabetes in atrial fibrillation should be considered more than ‘1’ score in the CHA2DS2-VASc score. In addition to the increased risk for stroke, diabetes is frequently associated with chronic kidney disease. It impacts choice of anticoagulation as reviewed: NOAC provide similar or better protection against stroke compared to VKA, but reduced risk for bleeding, and in addition rivaroxaban compared to VKA provided benefit on CV mortality, MALE, and progression of CKD.
Funding
This paper was published as part of a supplement financially supported by Bayer AG and the scientific content has not been influenced in any way by the sponsor.
Conflict of interest: R.K.: Bayer, Berlin-Chemie Menarini, Daiichi Sankyo, Ferrer, Merck, Sanofi, and Servier. A.J.C.: Institutional grants and personal fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/BMS. Personal fees from Abbott and Boston Scientific. P.R.: Consultancy and/or speaking fees (to Steno Diabetes Center Copenhagen) from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MSD, Mundipharma, Gilead, Vifor, Novo Nordisk, and Sanofi Aventis and research grants from AstraZeneca and Novo Nordisk.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 9th edn 2019, Brussels, Belgium. Available at: https://www.diabetesatlas.org
- 2. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840–844. [PubMed] [Google Scholar]
- 3. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. The Atrial Fibrillation Investigators. Arch Intern Med 1997;157:1237–1240. [PubMed] [Google Scholar]
- 4. Overvad TF, Skjoth F, Lip GY, Lane DA, Albertsen IE, Rasmussen LH, Larsen TB. Duration of diabetes mellitus and risk of thromboembolism and bleeding in atrial fibrillation: nationwide cohort study. Stroke 2015;46:2168–2174. [DOI] [PubMed] [Google Scholar]
- 5. Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, Singer DE. Effect of diabetes and glycemic control on ischemic stroke risk in AF patients: ATRIA study. J Am Coll Cardiol 2016;67:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney-Disease-Improving-Global-Outcomes(KDIGO)-CKD-Work-Group. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 8. Wen CP, Chang CH, Tsai MK, Lee JH, Lu PJ, Tsai SP, Wen C, Chen CH, Kao CW, Tsao CK, Wu X. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int 2017;92:388–396. [DOI] [PubMed] [Google Scholar]
- 9. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Hu D. The link between diabetes and atrial fibrillation: cause or correlation? J Cardiovasc Dis Res 2010;1:10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, Singer DE, Hylek EM, Go AS, Peterson ED, Piccini JP, Fonarow GC. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF registry. J Am Coll Cardiol 2017;70:1325–1335. [DOI] [PubMed] [Google Scholar]
- 12. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol 2005;105:315–318. [DOI] [PubMed] [Google Scholar]
- 14. Choe WS, Choi EK, Han KD, Lee EJ, Lee SR, Cha MJ, Oh S. Association of metabolic syndrome and chronic kidney disease with atrial fibrillation: a nationwide population-based study in Korea. Diabetes Res Clin Pract 2019;148:14–22. [DOI] [PubMed] [Google Scholar]
- 15. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 16. Plitt A, McGuire DK, Giugliano RP. Atrial fibrillation, type 2 diabetes, and non-vitamin K antagonist oral anticoagulants: a review. JAMA Cardiol 2017;2:442–448. [DOI] [PubMed] [Google Scholar]
- 17. Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, Woodward M, Cooper M, Harrap S, Hamet P, Poulter N, Lip GY, Patel A; ADVANCE Collaborative Group. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J 2009;30:1128–1135. [DOI] [PubMed] [Google Scholar]
- 18. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, Breithardt G, Singer DE, Hankey GJ, Hacke W, Becker RC, Berkowitz SD, Nessel CC, Mahaffey KW, Fox KA, Califf RM; Committee RAS, Investigators RASC. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ezekowitz JA, Lewis BS, Lopes RD, Wojdyla DM, McMurray JJ, Hanna M, Atar D, Cecilia Bahit M, Keltai M, Lopez-Sendon JL, Pais P, Ruzyllo W, Wallentin L, Granger CB, Alexander JH. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharmacother 2015;1:86–94. [DOI] [PubMed] [Google Scholar]
- 20. Miao B, Hernandez AV, Alberts MJ, Mangiafico N, Roman YM, Coleman CI. Incidence and predictors of major adverse cardiovascular events in patients with established atherosclerotic disease or multiple risk factors. J Am Heart Assoc 2020;9:e014402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patti G, Di Gioia G, Cavallari I, Nenna A. Safety and efficacy of nonvitamin K antagonist oral anticoagulants versus warfarin in diabetic patients with atrial fibrillation: A study-level meta-analysis of phase III randomized trials. Diabetes Metab Res Rev 2017;33:e2876. [DOI] [PubMed] [Google Scholar]
- 22. Miao B, Hernandez AV, Roman YM, Alberts MJ, Coleman CI, Baker WL. Four-year incidence of major adverse cardiovascular events in patients with atherosclerosis and atrial fibrillation. Clin Cardiol 2020;43:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker WL, Beyer-Westendorf J, Bunz TJ, Eriksson D, Meinecke AK, Sood NA, Coleman CI. Effectiveness and safety of rivaroxaban and warfarin for prevention of major adverse cardiovascular or limb events in patients with non-valvular atrial fibrillation and type 2 diabetes. Diabetes Obes Metabol 2019;21:2107–2114. [DOI] [PubMed] [Google Scholar]
- 24. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 25. Fangel MV, Nielsen PB, Larsen TB, Christensen B, Overvad TF, Lip GYH, Goldhaber SZ, Jensen MB. Type 1 versus type 2 diabetes and thromboembolic risk in patients with atrial fibrillation: a Danish nationwide cohort study. Int J Cardiol 2018;268:137–142. [DOI] [PubMed] [Google Scholar]
- 26. Lip GYH, Clementy N, Pierre B, Boyer M, Fauchier L. The impact of associated diabetic retinopathy on stroke and severe bleeding risk in diabetic patients with atrial fibrillation: the Loire valley atrial fibrillation project. Chest 2015;147:1103–1110. [DOI] [PubMed] [Google Scholar]
- 27. Arbelo E, Aktaa S, Bollmann A, D'Avila A, Drossart I, Dwight J, Hills MT, Hindricks G, Kusumoto FM, Lane DA, Lau DH, Lettino M, Lip GYH, Lobban T, Pak HN, Potpara T, Saenz LC, Van Gelder IC, Varosy P, Gale CP, Dagres N; ESC Scientific Document Group. Quality indicators for the care and outcomes of adults with atrial fibrillation. Europace 2020;doi: 10.1093/europace/euaa253. [DOI] [PubMed] [Google Scholar]
- 28. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; Investigators E-RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 29. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 30. Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Budaj A, Kiss RG, Padilla F, Gause-Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation 2020;141:1227–1234. [DOI] [PubMed] [Google Scholar]
- 32. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan YH, Lee HF, Li PR, Liu JR, Chao TF, Wu LS, Chang SH, Yeh YH, Kuo CT, See LC, Lip GYH. Effectiveness, safety, and major adverse limb events in atrial fibrillation patients with concomitant diabetes mellitus treated with non-vitamin K antagonist oral anticoagulants. Cardiovasc Diabetol 2020;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–229. [DOI] [PubMed] [Google Scholar]
- 35. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, Branch KRH, Probstfield J, Bosch J, Shestakovska O, Szarek M, Maggioni AP, Widimský P, Avezum A, Diaz R, Lewis BS, Berkowitz SD, Fox KAA, Ryden L, Yusuf S, Aboyans V, Alings M, Commerford P, Cook-Bruns N, Dagenais G, Dans A, Ertl G, Felix C, Guzik T, Hart R, Hori M, Kakkar A, Keltai K, Keltai M, Kim J, Lamy A, Lanas F, Liang Y, Liu L, Lonn E, Lopez-Jaramillo P, Metsarinne K, Moayyedi P, O’Donnell M, Parkhomenko A, Piegas L, Pogosova N, Sharma M, Stoerk S, Tonkin A, Torp-Pedersen C, Varigos J, Verhamme P, Vinereanu D, Yusoff K, Zhu J; COMPASS Committee. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS trial. Circulation 2020;141:1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224–232. [DOI] [PubMed] [Google Scholar]
- 37. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Potpara TS, Ferro CJ, Lip GYH. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nat Rev Nephrol 2018;14:337–351. [DOI] [PubMed] [Google Scholar]
- 39. Kumar S, Lim E, Covic A, Verhamme P, Gale CP, Camm AJ, Goldsmith D. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol 2019;74:2204–2215. [DOI] [PubMed] [Google Scholar]
- 40. Weir MR, Kreutz R. Influence of renal function on the pharmacokinetics, pharmacodynamics, efficacy, and safety of non-vitamin K antagonist oral anticoagulants. Mayo Clin Proc 2018;93:1503–1519. [DOI] [PubMed] [Google Scholar]
- 41. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 42. Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, Paolini JF, Hankey GJ, Mahaffey KW, Patel MR, Singer DE, Califf RM. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 2011;32:2387–2394. [DOI] [PubMed] [Google Scholar]
- 43. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012;33:2821–2830. [DOI] [PubMed] [Google Scholar]
- 44. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014;129:961–970. [DOI] [PubMed] [Google Scholar]
- 45. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, Braunwald E. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation 2016;134:24–36. [DOI] [PubMed] [Google Scholar]
- 46. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H, Lip GYH, Weitz J, Fauchier L, Lane D, Boriani G, Goette A, Keegan R, MacFadyen R, Chiang C-E, Joung B, Shimizu W; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–1393. [DOI] [PubMed] [Google Scholar]
- 47. De Vriese AS, Caluwé R, Raggi P. The atrial fibrillation conundrum in dialysis patients. Am Heart J 2016;174:111–119. [DOI] [PubMed] [Google Scholar]
- 48. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, Tilea A, Stack AG, Balkrishnan R, Yao X, Noseworthy PA, Shah ND, Saran R, Nallamothu BK. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 2018;138:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coleman CI, Kreutz R, Sood N, Bunz TJ, Eriksson D, Meinecke AK, Baker WL. Effectiveness and safety of rivaroxaban versus warfarin in nonvalvular atrial fibrillation patients with severe kidney disease or undergoing hemodialysis. Am J Med 2019;132:1078–1083. [DOI] [PubMed] [Google Scholar]
- 50. Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol 2020;104:328–335. [DOI] [PubMed] [Google Scholar]
- 51. Belley-Cote EP, Eikelboom JW. Anticoagulation for stroke prevention in patients with atrial fibrillation and end-stage renal disease—first, do no harm. JAMA Network Open 2020;3:e202237. [DOI] [PubMed] [Google Scholar]
- 52. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012;380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol 2017;37:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vossen LM, Kroon AA, Schurgers LJ, de Leeuw PW. Pharmacological and nutritional modulation of vascular calcification. Nutrients 2019;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rennenberg RJ, van Varik BJ, Schurgers LJ, Hamulyak K, Ten Cate H, Leiner T, Vermeer C, de Leeuw PW, Kroon AA. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood 2010;115:5121–5123. [DOI] [PubMed] [Google Scholar]
- 56. Bohm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, Schumacher H, Brueckmann M, Schirmer SH, Kratz MT, Yusuf S, Diener HC, Hijazi Z, Wallentin L. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY Trial. J Am Coll Cardiol 2015;65:2481–2493. [DOI] [PubMed] [Google Scholar]
- 57. Posch F, Ay C, Stoger H, Kreutz R, Beyer-Westendorf J. Exposure to vitamin K antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res Pract Thromb Haemost 2019;3:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of vitamin K antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients 2015;7:9538–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rattazzi M, Faggin E, Bertacco E, Nardin C, Pagliani L, Plebani M, Cinetto F, Guidolin D, Puato M, Pauletto P. Warfarin, but not rivaroxaban, promotes the calcification of the aortic valve in ApoE-/- mice. Cardiovasc Therap 2018;36:e12438. [DOI] [PubMed] [Google Scholar]
- 60. Di Lullo L, Tripepi G, Ronco C, D'Arrigo G, Barbera V, Russo D, Di Iorio BR, Uguccioni M, Paoletti E, Ravera M, Fusaro M, Bellasi A. Cardiac valve calcification and use of anticoagulants: preliminary observation of a potentially modifiable risk factor. Int J Cardiol 2019;278:243–249. [DOI] [PubMed] [Google Scholar]
- 61. Hernandez AV, Bradley G, Khan M, Fratoni A, Gasparini A, Roman YM, Bunz TJ, Eriksson D, Meinecke A-K, Coleman CI. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes 2020;6:301–307. [DOI] [PubMed] [Google Scholar]
- 62. Bonnemeier H, Kreutz R, Kloss S, Enders M, Häckl D, Schmedt N. Comparative safety and effectiveness of non-vitamin-K oral anticoagulants vs phenprocoumon in patients with non-valvular atrial fibrillation and diabetes—results from the RELOADed study. Eur Stroke J 2019;4:167–168. [Google Scholar]
- 63. Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, Noseworthy PA. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2017;70:2621–2632. [DOI] [PubMed] [Google Scholar]
- 64. Coleman CI, Kreutz R, Sood N, Bunz TJ, Meinecke AK, Eriksson D, Baker WL. Rivaroxaban's impact on renal decline in patients with nonvalvular atrial fibrillation: a US MarketScan Claims Database Analysis. Clin Appl Thromb Hemost 2019;25:107602961986853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wheeler DS, Giugliano RP, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost 2016;14:461–467. [DOI] [PubMed] [Google Scholar]
- 66. Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol 2018;29:2787–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, Bhatt UY, Hebert LA. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011;80:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–628. [DOI] [PubMed] [Google Scholar]
- 69. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 70. Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, Becker RC, Breithardt G, Hacke W, Hankey GJ, Nessel CC, Singer DE, Berkowitz SD, Piccini JP, Mahaffey KW, Fox KA. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). Am Heart J 2015;170:675–682.e678. [DOI] [PubMed] [Google Scholar]
- 71. Brambatti M, Darius H, Oldgren J, Clemens A, Noack HH, Brueckmann M, Yusuf S, Wallentin L, Ezekowitz MD, Connolly SJ, Healey JS. Comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: results from the RE-LY trial. Int J Cardiol 2015;196:127–131. [DOI] [PubMed] [Google Scholar]
- 72. Plitt A, Ruff CT, Goudev A, Morais J, Ostojic MC, Grosso MA, Lanz HJ, Park JG, Antman EM, Braunwald E, Giugliano RP. Efficacy and safety of edoxaban in patients with diabetes mellitus in the ENGAGE AF-TIMI 48 trial. Int J Cardiol 2020;304:185–191. [DOI] [PubMed] [Google Scholar]