Abstract
Atrial high rate episodes (AHREs) are defined as asymptomatic atrial tachyarrhythmias detected by cardiac implantable electronic devices with atrial sensing, providing automated continuous monitoring and tracings storage, occurring in subjects with no previous clinical atrial fibrillation (AF) and with no AF detected at conventional electrocardiogram recordings. AHREs are associated with an increased thrombo-embolic risk, which is not negligible, although lower than that of clinical AF. The thrombo-embolic risk increases with increasing burden of AHREs, and moreover, AHREs burden shows a dynamic pattern, with tendency to progression along with time, with potential transition to clinical AF. The clinical management of AHREs, in particular with regard to prophylactic treatment with oral anticoagulants (OACs), remains uncertain and heterogeneous. At present, in patients with confirmed AHREs, as a result of device tracing analysis, an integrated, individual and clinically-guided assessment should be applied, taking into account the patients’ risk of stroke (to be reassessed regularly) and the AHREs burden. The use of OACs, preferentially non-vitamin K antagonists OACs, may be justified in selected patients, such as those with longer AHREs durations (in the range of several hours or ≥24 h), with no doubts on AF diagnosis after device tracing analysis and with an estimated high/very high individual risk of stroke, accounting for the anticipated net clinical benefit, and informed patient’s preferences. Two randomized clinical trials on this topic are currently ongoing and are likely to better define the role of anticoagulant therapy in patients with AHREs.
Keywords: Anticoagulation, Atrial fibrillation, Atrial high rate episodes, Stroke, Pacemaker, Continuous monitoring, Thrombo-embolic risk, Subclinical atrial fibrillation
Clinical atrial fibrillation: definition and symptoms assessment
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its prevalence is expected to increase in the next years, also in relationship with progressive population ageing.1,2
It is important to precise the definition of clinical AF. According to Guidelines3–5 any arrhythmia that has the electrocardiogram (ECG) characteristics of AF and lasts sufficiently long for a 12-lead ECG to be recorded, or at least 30 s on a rhythm strip, should be considered as clinical AF. The presence of arrhythmia-related symptoms is not required for the definition of clinical AF, which therefore can be symptomatic or asymptomatic.
The true epidemiological burden of AF is largely unknown since an important proportion of patients may present asymptomatic AF or may present with non-specific symptoms.6,7 Despite continuous improvements in prevention, diagnosis and treatment of AF, morbidity and mortality associated with AF are still high, especially in the elderly population.8–10 Nowadays, physicians should acknowledge that a considerable proportion of AF episodes are completely asymptomatic. These episodes may or may not be detected at the time of occurrence of a clinical event (even at the time of stroke or systemic thromboembolism, etc.) or by chance during occasional checks, with still some resistance to institution of an appropriate prophylaxis with oral anticoagulants (OACs) in patients at risk.8,11,12
Asymptomatic atrial fibrillation: epidemiological and clinical implications
The proportion of AF that is asymptomatic is not well defined, but reported rates vary from 10% to 40%, depending on patient populations and settings, especially in terms of patient age, prevalence of risk factors for AF, methods for AF detection, and length of patient follow-up duration.6,7,12–14 Many studies indicate that asymptomatic AF is more common among male subjects and when AF is non-paroxysmal.11,13 In a clinical perspective, a key question is if asymptomatic AF carries a lower risk of thrombo-embolic events as compared to symptomatic AF. According to a series of studies, the implications in terms of outcomes, and specifically in terms of risk of stroke, are even worse for asymptomatic AF than for symptomatic AF.6,13,15 Therefore, the presence/absence of symptoms should not interfere with prescription of OACs in patients considered at risk according to the CHA2DS2VASc score.4,5,16,17
Conversely, assessment of presence/absence of symptoms and evolution of symptoms along with time, usually through grading according to the European Heart Rhythm Association (EHRA) score, is important for defining the most appropriate treatment strategy in terms of rate/rhythm control.4,18,19 Despite some heterogeneity, the results of a meta-analysis of six studies (two randomized clinical trials and four observational studies) show no differences in stroke/thromboembolism between asymptomatic and symptomatic AF and therefore the former (more frequently associated with male sex) should not be addressed differently in terms of stroke prevention and other cardiovascular prevention therapies.20
Detection of patients with unknown, untreated asymptomatic AF is the basis for initiatives of AF screening, targeted to appropriate antithrombotic prophylaxis in patients with untreated AF at risk of stroke.9,10,21
Atrial high rate episodes/subclinical AF: definitions and characterization
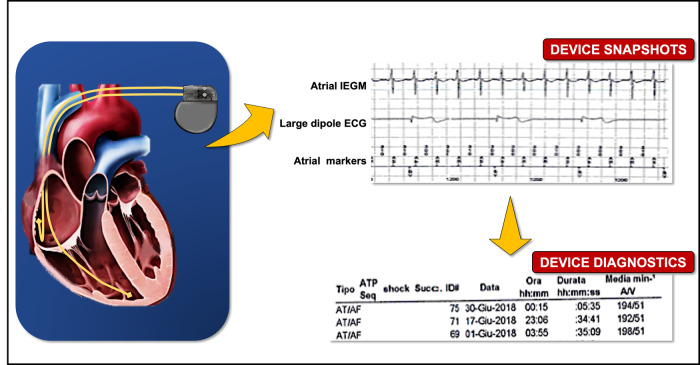
Cardiac implantable electronic devices (CIEDs) have undergone considerable technological progress in the last decades with possibility to treat bradycardia through implanted pacemakers (PM), ventricular tachyarrhythmias through implantable cardioverter-defibrillators (ICDs) and heart failure in appropriately selected patients through devices (PMs or ICDs) for cardiac resynchronization (CRT). CIEDs are now capable to analyse, record and possibly treat different types of arrhythmias through one or more intracavitary catheters.11,15,21,22 Furthermore, the atrial lead allows us to continuously monitor the atrial activity and record any arrhythmic episode characterized by a high atrial rate, named atrial high rate episodes (AHREs) (Figure 1).
Figure 1.
Atrial high rate episode (AHRE) detected by a dual-chamber pacemaker and the corresponding intracavitary electrogram (EGM).
Therefore, a key characteristics of AHREs episodes is that they are recorded exclusively through the continuous monitoring of CIEDs and include various atrial arrhythmias such as AF, atrial flutter and atrial tachycardias, often with transition from regular to irregular rhythm in the same patient, with recordings that can be stored in devices’ memory, as intracavitary electrograms (EGMs) (Figure 1).
At present, there is no consensus on which is the most appropriate definition of AHRE, both in terms of duration and atrial frequency of the episode. The definition adopted by the majority of the studies in literature and by the guidelines of the European Society of Cardiology (ESC) sets the time limit of 5–6 min and an atrial rate ≥175 b.p.m.5
These cut-offs aim at minimizing the possible inclusion of artefacts, since some episodes of high atrial frequency may not represent an atrial arrhythmia of clinical interest, but could simply be noise signals recorded by the atrial lead.22,23
The term ‘subclinical AF’ and AHRE have been used in literature interchangeably, but a precise definition is needed. According to consensus document and guidelines,5,22 the following definitions should be used:
AHRE refers to events characterized by an atrial rate above a programmed cut-off (>175 b.p.m., with a duration of at least 5 min) detected by CIEDs with atrial sensing (atrial lead/atrial sensing features) that allows automated continuous monitoring of atrial rhythm and tracings storage. The tracings of CIED-recorded AHREs need to be checked for confirmation of its arrhythmic nature in order to exclude artefacts/double counting or repetitive non-reentrant ventriculo-atrial (VA) synchrony resulting in false positives detections.
Subclinical AF refers to AHRE confirmed to have the characteristic of an atrial tachyarrhythmia (AF, atrial flutter, atrial tachycardia) or AF episodes detected by insertable cardiac monitors or wearable monitors and confirmed by analysis of intracardiac electrograms or ECG-recorded rhythm.
Despite some variable use occurred in literature, a key component of AHRE/subclinical AF is the occurrence in subjects with no previous detection of clinical AF (i.e. without surface ECG tracings showing AF) and without symptoms attributable to AF.
AHRE/subclinical AF: dynamic characteristics, incidence, and relationship with clinical AF
The concept of AHRE has raised some concern in the cardiology community since in this term are included different forms of atrial tachyarrhythmias, with a wide range of atrial cycles (from regular atrial tachycardias to fast AF) and different patterns of organization of the atrial rhythm, with frequent transitions from one pattern to another, as demonstrated by studies performed in patients with dual-chamber PMs with dedicated algorithms capable to detect phases with regular, slower atrial rhythm susceptible to termination by pacing24–26 (Figure 1). Moreover, as emphasized by guidelines,5 in case of AHRE detected by a CIED, it is needed to validate the arrhythmia through device diagnostics, by analysis of the EGMs stored in device memory, in order to rule-out a series of events leading to oversensing and that do not correspond to a true atrial tachyarrhythmias, such as repetitive non-reentrant VA synchrony, PM-mediated tachycardia, far-field R wave oversensing, myotonic potentials, lead failure, electromagnetic interference, or other non-cardiac signals.15,23
The term ‘AF burden’ has been introduced in the most recent years to describe the temporal pattern of AF and atrial tachyarrhythmias, in terms of presence and duration of arrhythmic episodes corresponding to AHRE/subclinical AF, as detected by continuous monitoring with an implanted device. ‘AF burden’ is now specifically defined as the overall time spent in AF during a specified period of time (usually 24 h).5,15,23
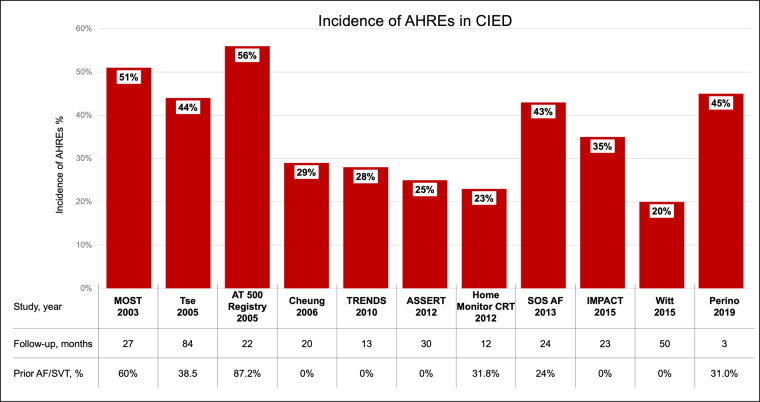
In patients with a CIED, the incidence of AHRE, after an average follow-up of 1 year, is usually estimated to be around 20%.22 However, it is difficult to report precise estimates of the true incidence and prevalence of AHREs because of the heterogeneity of the studies in literature in terms of definition of AHREs, specific design (retrospective or prospective), duration of follow-up, sample size, CIEDs recognition algorithms, etc.27 In a wider perspective, taking into account all the data reported in literature, the incidence of AHRE >5 min can be considered to vary between 10% and 68%.28,29 As a matter of fact, as shown in Figure 2, a considerable variability between the cohorts of patients analysed has to be considered, since some studies included groups of unselected patients with common indications for PM/ICD implantation, while others included only subgroups of patients at high risk of thrombo-embolic events and some studies included also patients with previous history of AF.30–40 Bearing in mind these findings, and the subsequent reports from the literature, there is evidence that subclinical AF episodes are common in patients implanted with CIEDs, both in terms of incidence and prevalence.15,22,28
Figure 2.
Incidence of CIED-detected AHREs on the basis of data from literature (refs30–40). AF, atrial fibrillation; AHRE, atrial high-rate episode; CIED, cardiac implanted electronic device; SVT, supraventricular tachycardia.
In the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT), subclinical atrial tachyarrhythmias with at least 6 min duration were detected within 3 months in around 10% of patients implanted with a CIED with no previous history of clinical AF.35 During a follow-up period of 2.5 years, additional subclinical atrial tachyarrhythmias occurred in around 25% of patients and around 16% of those who had subclinical atrial tachyarrhythmias developed symptomatic AF.35
These findings highlight an interesting and clinically important aspect of AHREs. Indeed, AF burden and AHRE duration show a dynamic pattern, with tendency to progression along with time and transition from burden in the range of minutes-few hours to 12–23 h and even more than 23 h.41 In a study that enrolled 6580 patients, new AF with an AF burden of ≥5 min was detected in 2244 patients (34%) during a mean follow-up of 2.4 years and around half of these patients transitioned to a higher AF-burden threshold during follow-up. A higher duration of daily AF burden manifested at first detection and a CHADS2 score ≥2 were factors significantly associated with faster transition to a subsequent higher burden. Approximately one-fourth of the patients transitioned from a lower threshold to a daily AF burden of ≥23 h during follow-up.41 Diederichsen et al.42 studied 590 individuals undergoing continuous monitoring via loop recorder, aged ≥70 years with no history of clinical AF and with at least one of the following risk factors: arterial hypertension, diabetes, previous stroke, or heart failure. A total of 205 (35%) subjects developed AHREs lasting ≥6 min and 33 (16%) of these patients progressed to AHREs >24 h.42
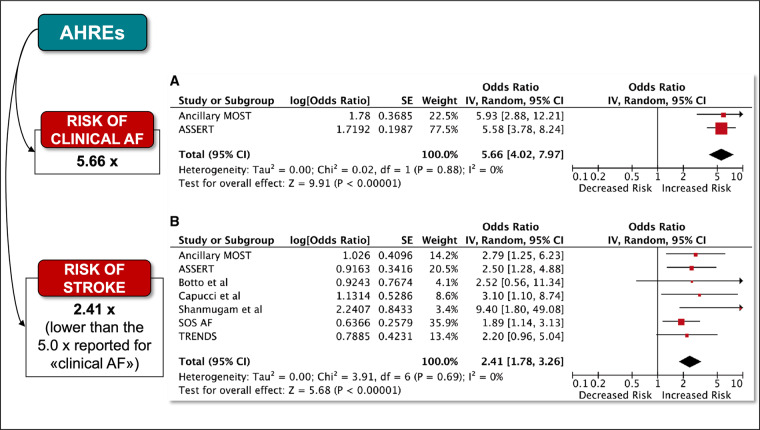
Therefore, in daily clinical practice, it is not uncommon to document the dynamic nature of AHREs and also the progression of these episodes to clinical AF. In the meta-analysis by Mahajan et al.27 taking into account the ancillary study of the MOde Selection Trial (MOST)30 and the ASSERT study,35 the risk of development of clinical AF in patients with AHRE was quantified as being 5.66-fold higher than patients without AHREs (Figure 3).
Figure 3.
(A) Association between subclinical and clinical AF. (B) Association of subclinical atrial fibrillation and stroke risk. From Mahajan et al.,27 with permission. AF, atrial fibrillation; AHRE, atrial high-rate episode.
Of note, progression of AF burden should be interpreted similarly to the progression of clinical AF12,43 being related to a progressively advancing atrial structural remodelling and worsening of underlying atrial cardiomyopathy.10,44
Clinical implications of AHRE/subclinical AF with regard to adverse outcomes and stroke
An assessment of the clinical implications of AHRE, after careful exclusion of oversensing, has to consider the duration of AHRE and the extent of AF burden. AHREs with a very short duration, ranging from three atrial premature complexes to 15–20 s, corresponding to non-sustained atrial tachyarrhythmias, are currently considered of no specific clinical significance since in the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes (RATE) during a follow-up of 23 months were found not significantly associated with episodes of longer duration, nor with an increased risk of adverse clinical events (including death or hospitalizations) nor with a significantly increased risk of stroke/systemic thromboembolism.45
While most of the interest on the prognostic significance of AHREs >5–6 min has been focused on the risk of stroke, the association with other outcomes should not be ignored. A study from the USA including 224 patients with no history of AF implanted with a dual-chamber PM found that AHREs were associated, even after adjustment for age, sex, and cardiovascular diseases, with a 2.8-fold increase in the risk of cardiovascular mortality.46 A more recent study from UK found that AHREs are associated with the risk of major adverse cardiovascular events (including acute heart failure, myocardial infarction, cardiovascular hospitalization, ventricular tachycardia/fibrillation,) during long-term follow-up and that the risk was higher in patients with AHRE ≥24 h, as compared to patients with AHREs ≥5 min.47 Similarly, in a sub-analysis of the ASSERT study, Wong et al.48 evaluated 415 patients with AHREs lasting between 6 min and 24 h and noted that over a mean follow-up of 2 years, 15.7% of patients transitioned to AHREs >24 h or clinical AF. Furthermore, the hospitalization rate for heart failure among patients with AHREs progression was higher than that of patients without progression (8.9% per year vs. 2.5% per year) and the progression of AHREs was independently associated with hospitalizations for heart failure even after multivariable adjustment [hazard ratio (HR): 4.58]. Similar results were obtained even when patients with prior history of heart failure were omitted from the analysis (HR: 7.06), or when AHREs progression was limited to >24 h alone, without clinical AF (HR: 3.68).48
Several studies investigated the risk of stroke associated with AHREs and in a general view it is clear that patients with AHREs have a high thrombo-embolic risk which, although possibly lower than that related to clinical AF, is certainly not negligible.
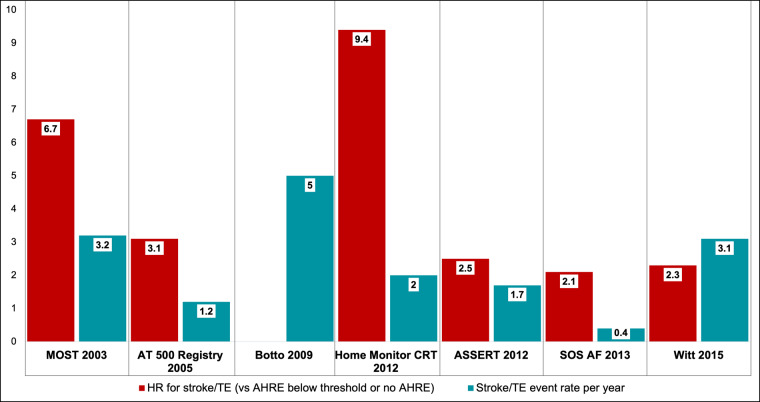
Figure 4 shows the HR for the risk of stroke in patients with AHRE when compared with patients with no AHRE or AHRE below the threshold and the observed actual rate of stroke/systemic embolism in studies from the literature.30,32,35–37,39,49 Glotzer et al.30 in a retrospective, ancillary study of the MOST based on 312 patients, found that patients with AHREs were at increased risk to develop clinical AF [HR 5.93, 95% confidence interval (CI) 2.88–12.2], to experience a non-fatal stroke or to die (HR 2.79, 95% CI 1.51–5.15) than patients without AHREs. Of note, 60% of patients included in the analysis had previous supraventricular arrhythmias. In 2012, the ASSERT study showed that the presence of AHREs was a predictor of stroke or systemic embolism even after adjusting the analysis for known stroke predictors (HR 2.5; 95% CI 1.28–4.89).35 In this prospective, multicentre, observational study, 2580 patients with no history of AF were enrolled. AHREs were defined as atrial rate ≥190/min, lasting ≥6 min and the average follow-up was 2.5 years.35 Numerous further evidences support the hypothesis that AHREs are related to a higher risk of thrombo-embolic events. In 2012, Shanmugam et al. (Home Monitor CRT study)36 reported that, in a population of 560 heart failure patients with CRT device, patients with AHREs >3.8 h (atrial rate > 180/min) were 9.4 times more likely to develop thrombo-embolic events than patients without AHREs (P = 0.006) (32% of patients included in the study had previous episodes of AF). Interesting results have been reported by Benezet-Mazuecos et al.50 who showed that AHREs (defined as atrial rate ≥ 225/min, duration ≥ 5 min) are an independent predictor of silent ischaemic stroke (HR 9.76, 95% CI 1.76–54.07), (31% of the patients included in the study had previous episodes of AF).
Figure 4.
Risk of stroke in patients with AHREs when compared with patients with no AHREs or AHREs below the threshold and the observed actual rate of stroke/systemic embolism in studies from the literature (refs.30,32,35–37,39,49). AHRE, atrial high rate episode.
The TRENDS study (A Prospective Study of the Clinical Significance of Atrial Arrhythmias Detected by Implanted Device Diagnostics)34 prospectively evaluated 2486 patients with an implantable electronic device and CHADS2 ≥ 1. They found that the risk of annual thrombo-embolic events was approximately two-fold higher in patients with a high burden of AHREs (defined as ≥5.5 h) compared to patients with a low (<5.5 h) or zero burden (2.4% vs. 1.1% per year; HR 2.20, 95% CI 0.96–5.05).34
A large dataset of patients with CIED-detected AHREs was collated in the SOS (Stroke preventiOn Strategies) AF project a pooled analysis of data from three prospective studies (PANORAMA, Italian Clinical Services Project, and TRENDS), with a total of 10 016 patients.37 During a median follow-up of 24 months, 43% of patients with implanted devices experienced ≥1 day with ≥5 min of AHRE burden. In a Cox regression analysis adjusted for CHADS2 score and use of anticoagulants at baseline, AHRE burden was an independent predictor of stroke. In a dichotomized analysis that compared various potential cut-off thresholds for AHRE burden (5 min, 1 h, 6 h, 12 h, and 23 h) a 1-h threshold of AHRE burden was associated with an HR for ischaemic stroke of 2.11 (95% CI 1.22–3.64, P = 0.008).37 In any case, the absolute risk of ischaemic stroke in patients with AHREs was low (0.39% annual rate in the whole cohort). Also, in a Japanese study conducted by Kawakami et al.51 AHREs with atrial rate ≥175/min and duration ≥5 min were associated with stroke or systemic embolism especially in the subgroup of patients with high thrombo-embolic risk (CHADS2 score >2), (HR 3.73, 95% CI 1.06–13.1). In this study,51 around one-fourth of the patients had history of AF and, indeed, it has to be stressed that one of the main limitation of the majority of studies on AHREs is the inclusion of patients with a history of previous clinical AF (Figure 2), thus making it difficult to evaluate the effect of AHREs alone on thrombo-embolic risk.
Rather than the duration of the arrhythmic episode itself, it seems to be the quantity of such episodes in a given period of time, expressed precisely as arrhythmic burden, to be a factor associated with the stroke risk.5,15,23 In a study by Turakhia et al.,52 9850 patients with CIEDs remotely monitored in the Veterans Administration Health Care System between 2002 and 2012 were analysed, focusing on 187 patients with acute ischaemic stroke and continuous heart rhythm monitoring through an implantable cardiac device for 120 days before the stroke. The presence of AHREs >5.5 h was found associated with an increased risk of stroke. The results from a sub-analysis of the ASSERT study are slightly different instead.53 In fact, the thrombo-embolic risk of the 2455 patients analysed in this trial was actually increased only for episodes of AHREs >24 h (HR 3.24, 95% CI 1.51–6.95), while the increase in stroke risk was not significant for AHREs between 6 min and 24 h.53
The studies by Capucci et al.32 and Botto et al.49 offered additional information, also in relationship with clinical risk stratification. The first32 followed 725 patients with dual-chamber pace-makers for a median follow-up of 22 months, showing that the embolic risk was approximately three times higher in patients with AHREs >24 h (HR 3.1, 95% CI 1.1–10.5) while it was not for AHREs <24 h. The second,49 on the other hand, evaluated a cohort of 568 patients with dual-chamber PMs, divided into subgroups based on the CHADS2 score and AF burden. It emerged that patients with AHREs >5 min and CHADS2 ≥2, or AHREs >24 h and CHADS2 ≥1 had a much higher annualized risk of thrombo-embolic events than patients with AHREs <5 min and CHADS2 ≤2, or AHREs <24 h and CHADS2 ≤1, or AHREs >24 h and CHADS2 = 0 (5% vs. 0.8%; P = 0.035).
An overall assessment of the relationship between device-detected AF burden is available in the meta-analysis published by Mahajan et al.27 where after a systematic review of all the literature on AHRE available up to 2016, the annual stroke rate in patients with subclinical AF with a burden higher than the cut-off duration employed in the specific study (varying from 5 min to 24 h) was 1.89/100 person-year, with a 2.4-fold increased risk of stroke as compared to patients with subclinical AF below the cut-off duration (absolute risk was 0.93/100 person-year) (Figure 3).
In addition, the availability in CIEDs of continuous monitoring of the atrial rhythm, extended to periods of months/years, has allowed to obtain a detailed picture of the temporal relationships between occurrence of AHREs/subclinical AF episodes, and occurrence of ischaemic stroke/systemic thromboembolism.23 Many studies evaluating patients implanted with a CIED, with or without previous atrial tachyarrhythmias, highlighted that ischaemic stroke may occur without a strict temporal correlation with episodes of atrial tachyarrhythmias, in terms of presence of AHREs/subclinical AF at the time of stroke or in the days before.15,54,55
Clinical management of the risk of stroke associated with AHREs
The categorization, clinical characterization, and management of AHREs, especially with regard to prophylactic treatment with OACs are still matter of debate.
Although CIED-detected AHREs are associated with a 2.0-fold to 2.5-fold increase in stroke risk compared with patients without these arrhythmias,28,34,35,37 the absolute risk of stroke among these patients is lower than the risk among patients with clinical AF.27 Moreover, CIED-detected AHREs may occur with temporal dissociation with stroke events, thus suggesting that they may represent a marker, rather than a risk factor for stroke.
The controversy on the use of anticoagulants in patients with AHREs/subclinical AF arises from the lack of robust scientific data, which in turn translates into a heterogeneous clinical management of patients and the potential risk of bleeding due to anticoagulant therapy.5 A recent survey has clearly underlined that there is an important heterogeneity in the perception of the thrombo-embolic risk associated with AHREs of different durations.56 The burden threshold of AHREs that physicians used as a cut-off to start an anticoagulant was extremely variable too. The decision to prescribe an anticoagulant depended on the overall clinical scenario and in particular on the presence of a previous stroke. This remarkable heterogeneity in the approach to the patient, from a diagnostic, therapeutic and screening point of view, is also highlighted by a previous survey conducted by Dobreanu et al.57 In particular, physicians showed a greater propensity to prescribe anticoagulant therapy in patients with multiple and longer AHREs and higher CHA2DS2-VASC score.57
Although the threshold at which it is appropriate to initiate oral anticoagulation in patients at risk is not defined, data from literature indicate that the risk of stroke is surely markedly increased when the duration of subclinical AF/AHRE is longer than 24 h, as shown by an analysis of ASSERT data.53 Two randomized trials, ARTESiA58 and NOAH-AFNET 6,59 are currently exploring the potential benefits of non-vitamin K antagonists in the specific setting of AHREs.58,59 In Table 1, the main characteristics of these two ongoing trials are shown.58,59
Table 1.
ARTESiA and NOAH trials: comparison of the two studies
| Feature | ARTESiA58 | NOAH59 |
|---|---|---|
| Planned number of patients to enrol | 4000 | 3400 |
| Double-blind, randomized, controlled trial | Yes | Yes |
| Inclusion if cardiac implanted electronic device-detected AHRE ≥6 min | Yes | Yes |
| Inclusion if atrial fibrillation detected by implanted loop recorder ≥6 min | Yes | No |
| Exclusion if single episode of AHRE >24 h | Yes | No |
| Exclusion if AF at baseline 12-lead ECG | Yes | Yes |
| CHA2DS2VASc score for inclusion | ≥4 | ≥2 |
| Active drug | Apixaban 5.0 mg or 2.5 mg twice daily | Edoxaban 60 mg or 30 mg daily |
| Comparator | Aspirin 80 mg daily | Usual care: Placebo or Aspirin 100 mg daily (if clinically indicated) |
| Primary endpoints | Stroke or systemic embolism, bleeding | Composite of stroke, systemic embolism, and cardiovascular death |
| Secondary endpoints | Ischaemic stroke, myocardial infarction, cardiovascular, and all-cause mortality, composites | Components of composite, all-cause death, and others |
| Censoring if single episode of AHRE >24 h | Yes | No |
| Study protocol publication | Lopes et al.58 | Kirchhof et al.59 |
AHRE, atrial high rate episode.
While waiting for the results of these randomized trials, decision-making on oral anticoagulation has to consider individualization of decision-making and monitoring on top of clinical risk stratification based on CHA2DS2VASc.16 Patients with subclinical AF/AHRE show a substantial dynamicity with transitions from lower to higher AF burden categories depending on the AF burden at first detection and CHADS2 score.41 The longer the amount of AF burden at first detection, the higher the probability of a faster transition to an AF burden >23–24 h, the threshold that in literature has been reported to be associated with an important increase in the risk of associated stroke.53
In the absence of direct evidence, which will be available after completion of the two ongoing RCTs, ARTESiA58 and NOAH-AFNET 6,59 some considerations can be done for addressing clinical decision-making in patients with AHRE:
There is need for individualized decision-making, taking into account risk stratification for stroke, using CHA2DS2VASc in combination with the amount of detected AF burden. Even if CHA2DS2VASc was previously validated in the setting of clinical AF, and not in the setting of AHRE, characterized by a less pronounced increase in the risk of stroke as compared to non-AF subjects,27 it may be used as a reference with the aim to identify, even in this context, ‘truly low-risk patients’; in combination with device-detected AF burden, that in many observational studies appears to modulate the observed event rate of stroke during follow-up, especially in non-anticoagulated patients.49,60,61 The combination of higher AHREs burden and higher CHA2DS2VASc score allowed to identify subgroups of patients with an event rate of stroke >1%/year, currently accepted as a valuable threshold for prescription of OAC and specifically of non-vitamin k antagonists OACs.61
At present, the wide variability in the perceptions of the role of OAC in AHREs56 translates into variable rates of OAC initiation after device-detected subclinical AF, even for episodes that last >24 h.40,56 In a large-scale observational study, it was found that the strongest association of OAC with reduction in stroke was observed for device-detected AF >24 h (HR 0.27 after propensity-score adjustment)40 and this finding is noteworthy, even despite all the limitations of uncontrolled studies. It has to be stressed that most of the uncertainty for management of AHRE with OAC is for AHRE <24 h53 and, as a matter of fact, patients with AHRE >24 h are not included in ARTESiA58 and will be only a subgroup of patients enrolled in NOAH-AFNET 659 (Table 1), so decision-making for AHREs >24 h will remain, even in the next future, largely dependent on clinical judgement, without a dedicated controlled study.
In all the patients with AHRE modifiable stroke risk factors should be identified and managed in each patient.5
Patients with subclinical AF/AHRE may develop atrial tachyarrhythmias lasting more than 24 h or clinical AF and therefore careful monitoring of these patients is needed, even considering remote monitoring to reduce the time to action62,63 especially with longer AHRE and higher risk profile. As soon as the clinical pictures appears to be modified (new symptoms, such as palpitations or even light-headedness, dyspnoea, or fatigue) device interrogation coupled with a 12-lead ECG (to detect ‘clinical’ AF) is recommended.
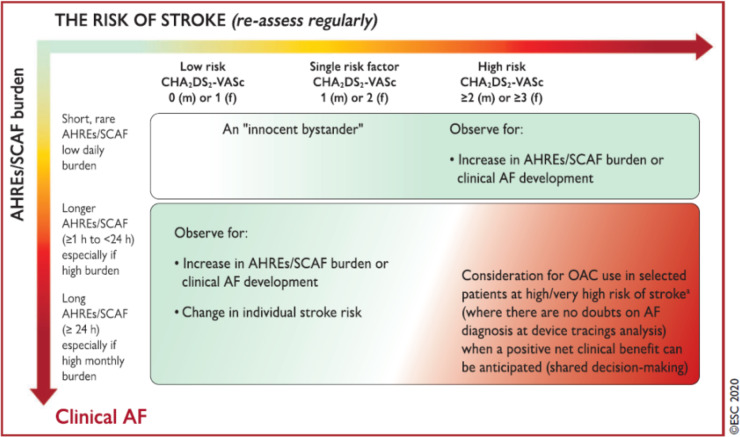
According with the 2020 ESC guidelines,5 individualized decision-making has to consider the risk of stroke (to be reassessed regularly) and also the AHRE/subclinical AF burden, thus resulting in an integrated assessment with variable weight of AHRE, from an ‘innocent bystander’ to an important and evolutive finding, associated with a substantial risk of stroke/thromboembolism. As a result of an integrated, individual and clinically-guided assessment, use of OAC, preferentially non-vitamin K antagonists OACs, may be justified in selected patients, such as patients with longer durations of AHRE/subclinical AF, in the range of several hours or ≥24 h, with no doubts on AF diagnosis after device tracing analysis and with an estimated high/very high individual risk of stroke, accounting for the anticipated net clinical benefit, and the informed patient’s preferences5 (Figure 5).
Figure 5.
Clinical management approach to CIED-detected AHREs according to the ESC Guidelines. From ref.,5 with permission. AF, atrial fibrillation; AHRE, atrial high rate episode; OAC, oral anticoagulant; SCAF, subclinical atrial fibrillation.
Conclusion
AHREs detection during CIEDs follow-up in patients with no history of clinical AF is frequent and it will be even more frequent in the future. AHREs are associated with an increased thrombo-embolic risk which, although it is lower than that of clinical AF, is not negligible. The thrombo-embolic risk increases with increasing duration of AHREs. Continuous monitoring of patients, preferably via remote monitoring, is of paramount importance to promptly intercept the evolution towards a high burden of AHRE or towards clinical AF. Unfortunately, the lack of high-quality scientific evidences on the topic is reflected in the heterogeneity of physicians’ clinical management of AHREs. The long-awaited results of the ARTESiA58 and NOAH-AFNET 659 studies are likely to better define the role of anticoagulant therapy in patients with AHREs <24 h and reduce the uncertainties that currently surround the clinical management of these patients. In the meantime, in the presence of AHRE/subclinical AF, with confirmation of diagnosis at device tracing analysis, individualized decision-making is needed, considering the risk of stroke (to be reassessed regularly) and also AHRE/subclinical AF burden. The use of oral anticoagulation, preferentially non-vitamin K antagonist OACs, may be justified in selected patients, such as patients with longer durations of AHRE/subclinical AF, in the range of several hours or ≥24 h, with no doubts on AF diagnosis after device tracing analysis and with an estimated high/very high individual risk of stroke, accounting for the anticipated net clinical benefit, and after appropriate patient information.
Funding
This paper was published as part of a supplement financially supported by Bayer AG and the scientific content has not been influenced in any way by the sponsor.
Conflict of interest: G.B. has received small speaker’s fees from Medtronic, Boston, Biotronik, Boehringer, and Bayer, outside of the submitted work. T.P.: consultant for Bayer/Jansen and BMS/Pfizer (no fees). G.Y.H.L.: consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi-Sankyo; speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo; no fees were directly received personally. The other authors reported no conflicts of interest.
References
- 1. Boriani G, Diemberger I, Martignani C, Biffi M, Branzi A. The epidemiological burden of atrial fibrillation: a challenge for clinicians and health care systems. Eur Heart J 2006;27:893–894. [DOI] [PubMed] [Google Scholar]
- 2. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: european perspective. Clin Epidemiol 2014;6:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J-Y, Ponikowski P, Rutten FH; Developed with the special contribution of the European Heart Rhythm Association (EHRA). Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020:doi:10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 6. Siontis KC, Gersh BJ, Killian JM, Noseworthy PA, McCabe P, Weston SA, Roger VL, Chamberlain AM. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: characteristics and prognostic implications. Heart Rhythm 2016;13:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994;74:236–241. [DOI] [PubMed] [Google Scholar]
- 8. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, Potpara T, Dan G-A, Kalarus Z, Tavazzi L, Maggioni AP, Lip GYH, Boriani G, Lip GYH, Tavazzi L, Maggioni AP, Dan G-A, Potpara T, Nabauer M, Marin F, Kalarus Z, Fauchier L, Goda A, Mairesse G, Shalganov T, Antoniades L, Taborsky M, Riahi S, Muda P, García Bolao I, Piot O, Nabauer M, Etsadashvili K, Simantirakis E, Haim M, Azhari A, Najafian J, Santini M, Mirrakhimov E, A Kulzida K, Erglis A, Poposka L, Burg M, Crijns H, Erküner Ö, Atar D, Lenarczyk R, Martins Oliveira M, Shah D, Dan G-A, Serdechnaya E, Potpara T, Diker E, Lip GYH; EORP-AF Long-Term General Registry Investigators. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: the EORP-AF general long-term registry. Europace 2019;21:1013–1022. [DOI] [PubMed] [Google Scholar]
- 9. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao T-F, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin L-Å, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu C-W, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP; AF-Screen Collaborators. Screening for atrial fibrillation: a report of the AF-screen international collaboration. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 10. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, Brandes A, Bustamante A, Casadei B, Crijns HJGM, Doehner W, Engström G, Fauchier L, Friberg L, Gladstone DJ, Glotzer TV, Goto S, Hankey GJ, Harbison JA, Hobbs FDR, Johnson LSB, Kamel H, Kirchhof P, Korompoki E, Krieger DW, Lip GYH, Løchen M-L, Mairesse GH, Montaner J, Neubeck L, Ntaios G, Piccini JP, Potpara TS, Quinn TJ, Reiffel JA, Ribeiro ALP, Rienstra M, Rosenqvist M, Themistoclakis S, Sinner MF, Svendsen JH, Van Gelder IC, Wachter R, Wijeratne T, Yan B. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN international collaboration. Circulation 2019;140:1834–1850. [DOI] [PubMed] [Google Scholar]
- 11. Arnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, Deharo J-C, Svendsen HJ, Heidbüchel H, Isa R, Kalman JM, Lane DA, Louw R, Lip GYH, Maury P, Potpara T, Sacher F, Sanders P, Varma N, Fauchier L; ESC Scientific Document Group; EHRA Scientific Documents Committee. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS) Europace. 2019;euz046. [DOI] [PubMed] [Google Scholar]
- 12. Boriani G, Valzania C, Biffi M, Diemberger I, Ziacchi M, Martignani C. Asymptomatic lone atrial fibrillation—how can we detect the arrhythmia? Curr Pharm Des 2014;21:659–666. [DOI] [PubMed] [Google Scholar]
- 13. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L, Tavazzi L, Maggioni AP, Lip GYH. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–518.e2. [DOI] [PubMed] [Google Scholar]
- 14. Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 15. Boriani G, Pettorelli D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vascul Pharmacol 2016;83:26–35. [DOI] [PubMed] [Google Scholar]
- 16. Lip GYH, Banerjee A, Boriani G, Chiang C. e, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, Patel S, Moores L. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–1201. [DOI] [PubMed] [Google Scholar]
- 17. Proietti M, Lane DA, Boriani G, Lip GYH. Stroke prevention, evaluation of bleeding risk, and anticoagulant treatment management in atrial fibrillation contemporary international guidelines. Can J Cardiol 2019;35:619–633. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Imberti JF, Valenti AC, Malavasi VL, Vitolo M. Managing atrial fibrillation: the need for an individualized approach even in the emergency department. Intern Emerg Med 2020;15:9–12. [DOI] [PubMed] [Google Scholar]
- 19. Potpara TS, Lip GYH, Blomstrom-Lundqvist C, Boriani G, Van Gelder IC, Heidbuchel H, Hindricks G, John Camm A. The 4S-AF scheme (stroke risk; symptoms; severity of burden; substrate): a novel approach to in-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemost 2020:doi: 10.1055/s-0040-1716408. [DOI] [PubMed] [Google Scholar]
- 20. Xiong Q, Proietti M, Senoo K, Lip GY. Asymptomatic versus symptomatic atrial fibrillation: a systematic review of age/gender differences and cardiovascular outcomes. Int J Cardiol 2015;191:172–177. [DOI] [PubMed] [Google Scholar]
- 21. Mairesse GH, Moran P, Van Gelder IC, Elsner C, Rosenqvist M, Mant J, Banerjee A, Gorenek B, Brachmann J, Varma N, Glotz de Lima G, Kalman J, Claes N, Lobban T, Lane D, Lip GYH, Boriani G, Fauchier L, Jung W, Savelieva I, Freedman B, Chen SA, Isa R, Turakhia M, Sapp JL, Lip G, Gorenek B, Sticherling C, Fauchier L, Goette A, Jung W, Vos MA, Brignole M, Elsner C, Dan G-A, Marin F, Boriani G, Lane D, Blomstrom Lundqvist C, Savelieva I; ESC Scientific Document Group. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017;19:1589–1623. [DOI] [PubMed] [Google Scholar]
- 22. Gorenek B, Bax J, Boriani G, Chen S-A, Dagres N, Glotzer TV, Healey JS, Israel CW, Kudaiberdieva G, Levin L-Å, Lip GYH, Martin D, Okumura K, Svendsen JH, Tse H-F, Botto GL, Sticherling C, Linde C, Kutyifa V, Bernat R, Scherr D, Lau C-P, Iturralde P, Morin DP, Savelieva I, Lip G, Gorenek B, Sticherling C, Fauchier L, Goette A, Jung W, Vos MA, Brignole M, Elsner C, Dan G-A, Marin F, Boriani G, Lane D, Lundqvist CB, Savelieva I; ESC Scientific Document Group. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017;19:1556–1578. [DOI] [PubMed] [Google Scholar]
- 23. Boriani G, Diemberger I, Ziacchi M, Valzania C, Gardini B, Cimaglia P, Martignani C, Biffi M. AF burden is important—fact or fiction? Int J Clin Pract 2014;68:444–452. [DOI] [PubMed] [Google Scholar]
- 24. Boriani G, Tukkie R, Manolis AS, Mont L, Pürerfellner H, Santini M, Inama G, Serra P, de Sousa J, Botto GL, Mangoni L, Grammatico A, Padeletti L; on behalf of the MINERVA Investigators. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J 2014;35:2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Padeletti L, Pürerfellner H, Mont L, Tukkie R, Manolis AS, Ricci R, Inama G, Serra P, Scheffer MG, Martins V, Warman EN, Vimercati M, Grammatico A, Boriani G. New-generation atrial antitachycardia pacing (Reactive ATP) is associated with reduced risk of persistent or permanent atrial fibrillation in patients with bradycardia: results from the MINERVA randomized multicenter international trial. Heart Rhythm 2015;12:1717–1725. [DOI] [PubMed] [Google Scholar]
- 26. Boriani G, Padeletti L. Management of atrial fibrillation in bradyarrhythmias. Nat Rev Cardiol 2015;12:337–349. [DOI] [PubMed] [Google Scholar]
- 27. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, Khokhar KB, Thiyagarajah A, Middeldorp ME, Nalliah CJ, Hendriks JML, Kalman JM, Lau DH, Sanders P. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–1415. [DOI] [PubMed] [Google Scholar]
- 28. Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017;14:701–714. [DOI] [PubMed] [Google Scholar]
- 29. Boriani G, Vitolo M. Atrial fibrillation in patients with cardiac implantable electronic devices: new perspectives with important clinical implications. Kardiol Pol 2019;77:1119–1120. [DOI] [PubMed] [Google Scholar]
- 30. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 31. Tse HF, Lau CP. Prevalence and clinical implications of atrial fibrillation episodes detected by pacemaker in patients with sick sinus syndrome. Heart 2005;91:362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, Ricci R, Favale S, Zolezzi F, Di Belardino N, Molon G, Drago F, Villani GQ, Mazzini E, Vimercati M, Grammatico A. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 33. Cheung JW, Keating RJ, Stein KM, Markowitz SM, Iwai SEI, Shah BK, Lerman BB, Mittal S. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol 2006;17:1323–1328. [DOI] [PubMed] [Google Scholar]
- 34. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 35. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 36. Shanmugam N, Boerdlein A, Proff J, Ong P, Valencia O, Maier SKG, Bauer WR, Paul V, Sack S. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace 2012;14:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, Gasparini M, Lewalter T, Camm JA, Singer DE. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GYH, Ip J, Holcomb R, Akar JG, Halperin JL. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J 2015;36:1660–1668. [DOI] [PubMed] [Google Scholar]
- 39. Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm 2015;12:2368–2375. [DOI] [PubMed] [Google Scholar]
- 40. Perino AC, Fan J, Askari M, Heidenreich PA, Keung E, Raitt MH, Piccini JP, Ziegler PD, Turakhia MP. Practice variation in anticoagulation prescription and outcomes after device-detected atrial fibrillation. Circulation 2019;139:2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di S. Stefano L, Sepsi M, Landolina M, Lunati M, Lewalter T, Camm AJ. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018;15:376–383. [DOI] [PubMed] [Google Scholar]
- 42. Diederichsen SZ, Haugan KJ, Brandes A, Graff C, Krieger D, Kronborg C, Holst AG, Nielsen JB, Køber L, Højberg S, Svendsen JH. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk: from the LOOP study. Am Heart J 2020;219:117–127. [DOI] [PubMed] [Google Scholar]
- 43. Potpara TS, Stankovic GR, Beleslin BD, Polovina MM, Marinkovic JM, Ostojic MC, Lip GYH. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade Atrial Fibrillation study. Chest 2012;141:339–347. [DOI] [PubMed] [Google Scholar]
- 44. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim Y-H, Lip GYH, Ma C-S, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swiryn S, Orlov MV, Benditt DG, DiMarco JP, Lloyd-Jones DM, Karst E, Qu F, Slawsky MT, Turkel M, Waldo AL. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation 2016;134:1130–1140. [DOI] [PubMed] [Google Scholar]
- 46. Gonzalez M, Keating RJ, Markowitz SM, Liu CF, Thomas G, Ip JE, Lerman BB, Cheung JW. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm 2014;11:2214–2221. [DOI] [PubMed] [Google Scholar]
- 47. Pastori D, Miyazawa K, Li Y, Székely O, Shahid F, Farcomeni A, Lip GYH. Atrial high-rate episodes and risk of major adverse cardiovascular events in patients with cardiac implantable electronic devices. Clin Res Cardiol 2020;109:96–102. [DOI] [PubMed] [Google Scholar]
- 48. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Capucci A, Botto G, Grönefeld G, Israel CW, Connolly SJ, Healey JS. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol 2018;71:2603–2611. [DOI] [PubMed] [Google Scholar]
- 49. Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, Favale S, Molon G, Ricci R, Biffi M, Russo G, Vimercati M, Corbucci G, Boriani G. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–248. [DOI] [PubMed] [Google Scholar]
- 50. Benezet-Mazuecos J, Rubio JM, Cortes M, Iglesias JA, Calle S, de la Vieja JJ, Quinones MA, Sanchez-Borque P, de la Cruz E, Espejo A, Farre J. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace 2015;17:364–369. [DOI] [PubMed] [Google Scholar]
- 51. Kawakami H, Nagai T, Saito M, Inaba S, Seike F, Nishimura K, Inoue K, Okura T, Sumimoto T, Uemura S, Higaki J, Ikeda S. Clinical significance of atrial high-rate episodes for thromboembolic events in Japanese population. Heart Asia 2017;9:e010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol 2015;8:1040–1047. [DOI] [PubMed] [Google Scholar]
- 53. Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau C-P, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 54. Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, Ziegler PD. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8:1416–1423. [DOI] [PubMed] [Google Scholar]
- 55. Boriani G, Santini M, Lunati M, Gasparini M, Proclemer A, Landolina M, Padeletti L, Botto GL, Capucci A, Bianchi S, Biffi M, Ricci RP, Vimercati M, Grammatico A, Lip GYH. Improving thromboprophylaxis using atrial fibrillation diagnostic capabilities in implantable cardioverter-defibrillators: the multicentre Italian ANGELS of AF Project. Circ Cardiovasc Qual Outcomes 2012;5:182–188. [DOI] [PubMed] [Google Scholar]
- 56. Boriani G, Healey JS, Schnabel RB, Lopes RD, Calkins H, Camm JA, Freedman B. Oral anticoagulation for subclinical atrial tachyarrhythmias detected by implantable cardiac devices: an international survey of the AF-SCREEN Group. Int J Cardiol 2019;296:65–70. [DOI] [PubMed] [Google Scholar]
- 57. Dobreanu D, Svendsen JH, Lewalter T, Hernández-Madrid A, Lip GY, Blomström-Lundqvist C; conducted by the Scientific Initiatives Committee, European Heart Rhythm Association. Current practice for diagnosis and management of silent atrial fibrillation: results of the European Heart Rhythm Association survey. Europace 2013;15:1223–1225. [DOI] [PubMed] [Google Scholar]
- 58. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, Mairesse GH, Mabo P, Camm AJ, Healey JS. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 59. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener H-C, Goette A, Huening A, Lip GYH, Simantirakis E, Vardas P. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boriani G, Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Ricci R, Biffi M, De Santo T, Corbucci G, Lip GYH, for the Italian AT-500 Registry Investigators. Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke 2011;42:1768–1770. [DOI] [PubMed] [Google Scholar]
- 61. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA(2)DS(2)-VASc score. Circulation 2019;140:1639–1646. [DOI] [PubMed] [Google Scholar]
- 62. De Simone V, Zanotto G, Guarise P, Venturato A, Cassinadri E, Bassi M, Bozzolin M, Tondelli S, Giacopelli D, Morando G. Effects of remote monitoring of cardiac implantable electronic devices after stroke or transient ischemic attack. J Cardiovasc Med (Hagerstown) 2019;20:551–556. [DOI] [PubMed] [Google Scholar]
- 63. Boriani G, Da Costa A, Ricci RP, Quesada A, Favale S, Iacopino S, Romeo F, Risi A, Mangoni di S Stefano L, Navarro X, Biffi M, Santini M, Burri H; On Behalf Of The MORE-CARE Investigators. The MOnitoring resynchronization dEvices and CARdiac patiEnts (MORE-CARE) randomized controlled trial: phase 1 results on dynamics of early intervention with remote monitoring. J Med Internet Res 2013;15:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]