Abstract
Background
In patients with non-small cell lung cancer (NSCLC), both chronic obstructive pulmonary disease (COPD) and systemic inflammatory biomarkers, such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), have significant association with prognosis. NLR and PLR also predict mortality in patients with COPD alone. A combination of the two parameters may be helpful in a more individualized approach for predicting prognosis in NSCLC.
Methods
Medical records of patients with stage IIIB and IV NSCLC from January 2012 to January 2018 in seven university hospitals were reviewed. Patients were categorized into four subgroups based on pulmonary function test results and cutoffs for NLR or PLR.
Results
A total of 277 patients were evaluated and categorized into non-COPD and COPD groups; 194 patients were in the non-COPD group and 83 patients were in the COPD group. The non-COPD group showed significantly longer overall survival (OS) compared with the COPD group (P = 0.019). Median survival was significantly different between high/low PLR groups (P < 0.001), between high/low NLR groups (P = 0.001), and between high/low c-reactive protein (CRP) groups (P < 0.001). PLR, NLR and CRP showed significant correlations with each other. PLR showed a significant negative linear correlation with FVC (absolute) (r = −0.149, P = 0.015), FVC (%) (r = −0.192, P = 0.002), DLCO (absolute) (r = −0.271, P < 0.001), DLCO (%) (r = −0.139, P = 0.032), and NLR (r = 0.718, P < 0.001). In the multivariate analysis, the high PLR, COPD sub-group showed significantly higher risk for mortality (HR 2.066 (1.175–3.633), P = 0.012) compared with the low-PLR non-COPD group. However, COPD-NLR subtype was not an independent predictor for OS.
Conclusion
A combination of COPD status and PLR may be a cost-effective and readily available prognostic marker in patients with advanced NSCLC.
Keywords: chronic obstructive pulmonary disease, non-small cell lung cancer, survival, platelet, lymphocyte, inflammation, biomarker
Introduction
Lung cancer is one of major causes of cancer-related deaths worldwide.1,2 Lung cancer and chronic obstructive pulmonary disease (COPD) are interrelated in many ways. With a prevalence of 40–70% in patients with COPD, studies have reported that patients with COPD have a high risk of lung cancer development.3–8 Gao et al showed that COPD and emphysema are poor prognostic factors in patients with lung cancer.9 In addition, negative effects of COPD on the postoperative outcomes of patients with lung cancer have been reported.10,11 In our previous articles, COPD defined by spirometry was shown to be associated with shorter overall survival in NSCLC, even in the never-smoker subgroup.12,13
Systemic inflammation is shown to be an important risk factor that affects prognosis in cancer.14,15 A series of studies on the association between systemic inflammatory response and prognosis in non-small cell lung cancer (NSCLC) reported several biomarkers correlated with prognosis. Among them, an elevated platelet to lymphocyte ratio (PLR) is associated with shorter overall survival (OS) and poor progression-free survival (PFS) in NSCLC.16,17 An elevated PLR was also associated with poor prognosis in patients with surgically treated NSCLC,18 and also was identified as a risk factor for brain metastasis of lung adenocarcinoma.19
While COPD is prevalent and an important comorbidity with prognostic value in lung cancer,10,20,21 systemic inflammatory biomarkers also show predictive value in COPD. In a study by Kumar et al, increased PLR was significantly associated with increased mortality in patients experiencing acute exacerbation (AE) of COPD.22 In the study by Yao et al, neutrophil-lymphocyte ratio (NLR) and PLR were both significantly increased in non-survivor patients with COPD AE.23
Indeed, in patients diagnosed with COPD and lung cancer, clinical presentations may differ depending on levels of inflammatory biomarkers that affect both diseases. Thus, a more individualized clinical approach is necessary for this subgroup. A combination of airway obstruction and inflammatory markers, such as PLR and NLR, may provide accurate prediction of prognosis in lung cancer. However, no studies have been published yet on this topic.
Since COPD and systemic inflammation were shown to be associated with prognosis in NSCLC, phenotyping using these two factors may be helpful in treatment of NSCLC. In this multicenter retrospective cohort study, the relationship between inflammatory biomarkers and lung functions in lung cancer was assessed, and the combination of both parameters as a predictive tool was evaluated.
Methods
Patient Selection
From a cohort of patients with NSCLC, a total of 277 patients with advanced (stage IIIB-IV) NSCLC were selected for the present study. They were diagnosed with NSCLC between January 2012 and January 2018 and enrolled from seven university hospitals: Yeouido St. Mary’s Hospital, Seoul St. Mary’s Hospital, Bucheon St. Mary’s Hospital, Incheon St. Mary’s Hospital, Eunpyeong St. Mary’s Hospital, St. Vincent Hospital, and Uijeongbu St. Mary’s Hospital.24 Inclusion criteria for the study selection were patients with 1) complete pretreatment blood count (CBC) differential data at the time of initial pathologic diagnosis and 2) all clinical data available from the electronic medical record. Exclusion criteria were patients 1) with small cell lung cancer, 2) with significant infection requiring antibiotic treatment at the time of enrollment, 3) with an underlying hematologic disease,24 and 4) patients who were treated with immune checkpoint inhibitors, because COPD was reported to be associated with improved treatment outcomes in NSCLC in previous studies.25,26
Clinical and Laboratory Data
For all enrolled patients, data including sex, age, histology, tumor stage by tumor–node–metastasis stage (AJCC 2009), Eastern Cooperative Oncology Group performance status (ECOG PS), and smoking status were collected.
Chemotherapy and Adverse Reactions
Systemic conventional chemotherapies given to patients included cisplatin/carboplatin combination regimens.24 Targeted therapies included erlotinib, gefitinib, and afatinib for positive EGFR mutations and crizotinib for positive ALK translocations.
The study patients were assessed for treatment-related adverse reactions either at the outpatient clinic or during admission on a regular basis. Treatment was either delayed or changed to another regimen when patients experienced grade III or IV adverse reactions.
EGFR Testing
Excluding other uncommon epidermal growth factor receptor (EGFR) profiles, EGFR mutations were defined as an exon 21 point mutation or an exon 19 deletion. EGFR genotyping was performed by peptide nucleic acid (PNA)-mediated PCR clamping methods using the PNAClampTM EGFR Mutation Detection Kit (PANAGENE, Inc., Daejeon, Korea).24,27
OS and PFS
For response evaluation, computed tomography scan of target organs was performed to the patients after every two treatment cycles. To evaluate treatment response, Response Evaluation Criteria in Solid Tumors version 1.1 was used,28 and both treating physicians and independent radiologists evaluated the responses. OS was defined as time duration from the date of lung cancer diagnosis to death. PFS was defined as time duration from lung cancer diagnosis to radiologically confirmed disease progression after first-line treatment. If patients died or lost contact during the follow-up period, they were considered censored.24
Spirometry and Definition of COPD
Spirometry tests were performed by qualified technicians following the standardized protocol for pulmonary function tests recommended by the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force.29 The Morris reference equation was applied to calculate normal predictive values for spirometric data.30 According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, post-bronchodilator fixed criteria [forced expiratory volume in 1 s divided by forced vital capacity (FEV1/FVC)<0.7] was applied for definition of COPD.31
PLR, NLR and CRP
PLR and NLR were calculated from pretreatment CBC measured at the time of lung cancer diagnosis. PLR was defined as platelet count divided by absolute lymphocyte count, and NLR was defined as absolute neutrophil count divided by lymphocyte count.
The cutoff value of 181.2 used to categorize high and low PLR groups was determined from a previous publication on the prognostic value of PLR.32 The optimal cutoff used to define high/low NLR groups is 3.5, as used in previous publications.33,34 Regarding c-reactive protein (CRP), the median value of 1.7 mg/dL was used as the cutoff to categorize high and low CRP groups.
Definition of COPD-PLR, COPD-NLR and COPD-CRP Subgroups
All patients with both pulmonary function test and CBC results at the time of diagnosis were categorized into four COPD-PLR groups. Patients with fixed airway obstruction (FEV1/FVC<0.7) and PLR higher than the cutoff value were categorized into the COPD, high PLR group. Those with fixed airway obstruction and PLR lower than the cutoff value were categorized into the COPD, low PLR group. Among patients without fixed airway obstruction, those with PLR higher than the cutoff value and PLR lower than the cutoff value were categorized into non-COPD, high PLR and non-COPD, low PLR groups, respectively.
Subgroup definition using cutoffs of NLR and fixed airway obstruction was performed in a similar manner. Among patients with fixed airway obstruction, patients with NLR higher than the predetermined cutoff and those with lower NLR were grouped into COPD, high NLR and COPD, low NLR groups, respectively. Patients without fixed airway obstruction were grouped into non-COPD, high NLR and non-COPD, low NLR groups according to NLR value. In addition, the study patients were categorized into 4 subgroups using median value of CRP and fixed airway obstruction.
Statistical Analysis
The Statistical Package for Social Sciences software version 20.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses. Data of continuous variables are shown as means or medians with ranges, and were compared using two-sided t-tests or the Mann–Whitney U-test depending on the distribution status. The Chi-squared test was used to compare categorical parameters.
The median OS and PFS are presented with 95% confidence intervals. Survival curves were constructed using Kaplan–Meier analysis. A Log rank test was performed to determine significant differences in survival outcomes between groups. Univariate analysis using the Cox regression model was performed to determine variables significantly associated with OS. Statistically significant variables were entered into multivariate analysis using the Cox proportional hazards regression model. A P value less than 0.05 was considered statistically significant by all analyses.
For comparison among three or more groups, alpha correction was performed for statistically significant P-value cutoffs. For normally distributed parameters, analysis of variance (ANOVA) was used to compare continuous variables between groups. For non-normally distributed parameters, the Kruskal-Wallis test was performed. For post hoc pairwise comparison, Bonferonni method was used. Spearman’s rho method was used to perform correlation analyses.
Ethics Statement
The present study was approved by the Ethics Committees of Seoul St. Mary’s Hospital, Incheon St. Mary’s Hospital, Yeouido St. Mary’s Hospital, Bucheon St. Mary’s Hospital, St. Paul’s Hospital, St. Vincent Hospital, and Uijeongbu St. Mary’s Hospital (XC17REDI0069U). The need for informed consent was waived by the Institutional Review Boards. Due to the retrospective nature of the data, the requirement for informed consent was waived. This study was conducted in accordance with the Declaration of Helsinki. All patient data in the present study were fully anonymized before evaluation.
Results
Patient Characteristics
A total of 277 patients were evaluated in the present study. Clinical characteristics of the enrolled patients are shown in Table 1. Among the study patients, 246 (88.8%) patients were diagnosed with non-squamous cancer, and the other 31 (11.2%) patients had squamous cancer. Mean age was 64.9 years, and 44.8% of patients were female. Median survival duration of the overall patients was 19.6 months. Among the patients with advanced NSCLC, 7.2% had stage IIIB, while 92.8% had stage IV. Mean body mass index (BMI) was 23.3 kg/m2. Among 268 tested patients, the EGFR mutation was positive in 92 patients (34.5%). For first-line treatment, 166 (59.9%) underwent conventional chemotherapy, 68 (24.5%) received targeted therapy, and 33 (11.9%) underwent supportive care only. Among study patients, 45.8% were ever smokers and 85.5% had good performance status (Eastern Cooperative Oncology Group score 0 or 1).
Table 1.
Baseline Clinical Characteristics of the Study Patients
| Overall Patients (n=277) (n,%) | Non-COPD (n=194) (n,%) | COPD (n=83) (n,%) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 124 (44.8) | 100 (51.5) | 24 (28.9) | 0.001 |
| Age (year) | 64.9±11.2 | 61.8±10.8 | 72.0±8.4 | <0.001 |
| Median survival time (months) | 19.6 [15.5–23.7] | 21.3 [16.7–25.8] | 10.7 [7.2–14.1] | 0.019 |
| Stage | 0.104 | |||
| IIIB | 20 (7.2) | 11 (5.7) | 9 (10.8) | |
| IV | 257 (92.8) | 183 (94.3) | 74 (89.2) | |
| BMI (kg/m2) | 23.3±3.5 | 23.7±3.5 | 22.6±3.3 | 0.027 |
| EGFR mutation | 92 (34.5)* 267 tested | 71 (38.0)* 187 tested | 21 (26.2)* 80 tested | 0.065 |
| First-line treatment | 0.064 | |||
| Conventional chemotherapy | 166 (59.9) | 113 (58.3) | 53 (63.8) | |
| Targeted therapy | 68 (24.5) | 53 (27.3) | 15 (18.1) | |
| Supportive care only | 33 (11.9) | 18 (9.3) | 15 (18.1) | |
| Smoking | <0.001 | |||
| Never smoker | 149 (53.8) | 119 (61.7) | 30 (36.1) | |
| Ever smoker | 127 (45.8) | 74 (38.3) | 53 (63.9) | |
| ECOG | 0.001 | |||
| 0–1 | 236 (85.5) | 174 (90.2) | 62 (74.7) | |
| 2–3 | 40 (14.5) | 19 (9.8) | 21 (25.3) | |
| Pathology | 0.043 | |||
| Non-squamous | 246 (88.8) | 177 (91.2) | 69 (83.1) | |
| Squamous | 31 (11.2) | 17 (8.8) | 14 (16.9) | |
| FEV1 (liter) | 2.0±1.5 | 2.0±0.7 | 1.9±2.5 | 0.845 |
| FEV1 (% predicted) | 78.8±22.4 | 82.7±22.3 | 69.9±20.0 | <0.001 |
| FVC (liter) | 2.7±2.0 | 2.5±0.9 | 3.0±3.3 | 0.195 |
| FVC (% predicted) | 77.7±21.8 | 76.9±21.3 | 79.5±23.1 | 0.381 |
| FEV1/FVC (% predicted) | 74.0±11.1 | 79.4±6.7 | 61.3±8.7 | <0.001 |
| DLCO (abs) | 12.4±4.6 | 13.1±4.7 | 10.8±3.9 | <0.001 |
| DLCO (%) | 70.2±20.0 | 71.7±19.7 | 66.8±20.3 | 0.079 |
| NLR | 4.07±3.49 | 4.05±3.72 | 4.13±2.92 | 0.841 |
| PLR | 189.6±86.1 | 189.3±87.2 | 190.2±84.0 | 0.940 |
| CRP | 3.74±0.42 | 3.55±0.49 | 4.13±0.81 | 0.259 |
| Hemoglobin (g/dl) | 13.0 [12.0–14.0] | 13.0 [12.0–14.0] | 13.0 [11.7–14.3] | 0.400 |
Note: *Only patients who are tested were considered.
Abbreviations: COPD, chronic obstructive pulmonary disease; CRP, c-reactive protein; BMI, body mass index; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio.
Study patients were categorized into non-COPD and COPD groups; 194 patients were in the non-COPD group and 83 patients were in the COPD group. The mean age of the COPD group was 72.0 years, and that of the non-COPD group was 61.8±10.8 years (P < 0.001). The proportion of females was significantly higher in the non-COPD group (51.5%) than in the COPD group (28.9%) (P =0.002). The percentage of ever-smokers was significantly higher in the COPD group than in the non-COPD group (63.9% vs 38.3%, respectively; P = 0.009).
The proportion of patients with Eastern Cooperative Oncology Group (ECOG) performance status scores 0 or 1 was not significantly different between the two groups (P = 0.515). The proportion of patients with squamous carcinoma was significantly higher in the COPD group than in the non-COPD group (16.9% vs 8.8%, respectively; P = 0.043).
Mean absolute FEV1 and predicted percentage of FEV1 were 1.9±2.5 L and 69.9±20.0% in the COPD group; the respective values in the non-COPD group were 2.0±0.7 L and 82.7±22.3% (P = 0.001).
Comparison Between High/Low PLR Groups, High/Low NLR Groups and High/Low CRP Groups
Supplementary table compares the clinical characteristics between the three sets of high and low inflammatory groups. The high PLR group had significantly lower BMI compared to the low PLR group (P = 0.024). Other parameters did not show significant difference. Among pulmonary function parameters, the high PLR group showed significantly lower FVC (%) (p=0.020). Both NLR and PLR were significantly higher in the high PLR group (P < 0.001 and P < 0.001, respectively).
The high NLR group had significantly lower BMI compared to the low NLR group (P = 0.001). Other clinical parameters showed no significant difference, while FEV1 (%) and FVC (%) were significantly lower in the higher NLR group (P = 0.031 and P = 0.005, respectively). NLR, PLR, and CRP were significantly higher in the high NLR group (P = 0.001, P < 0.001, and P = 0.001, respectively).
Among patients who had baseline CRP results, 80 patients were categorized into a high CRP group, and the other 80 patients were categorized into a low CRP group. The high CRP group had a significantly higher proportion of squamous cell carcinoma (p=0.010). The high CRP group showed significantly lower FEV1 (%), and FVC (%) (P < 0.001 and P < 0.001, respectively) when compared to the low CRP group. In addition, NLR level was significantly higher in the high CRP group than in the low CRP group (Supplementary Table).
Survival Comparison Between Subgroups According to Presence of COPD and Inflammatory Markers
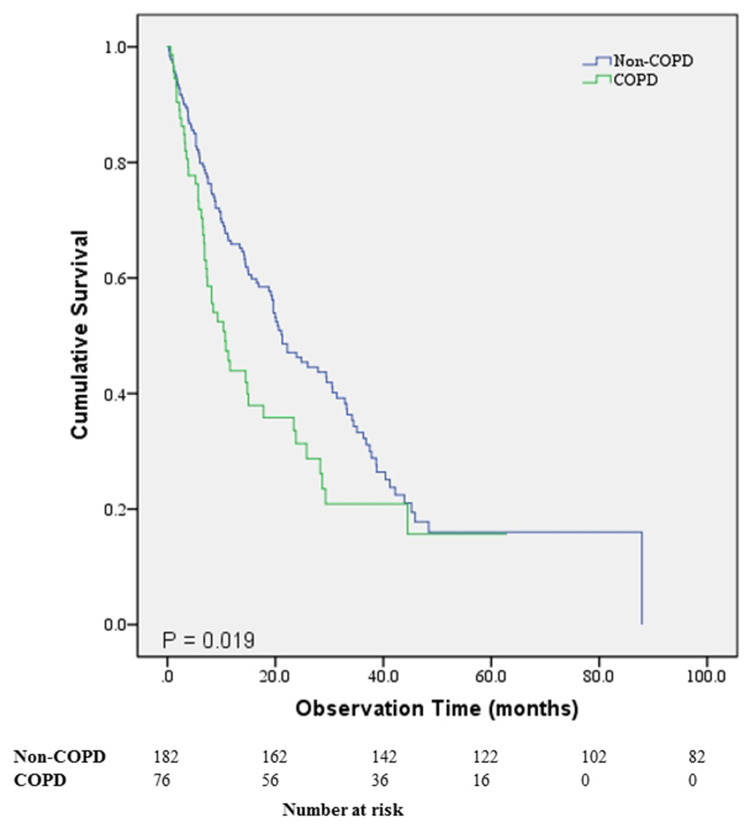
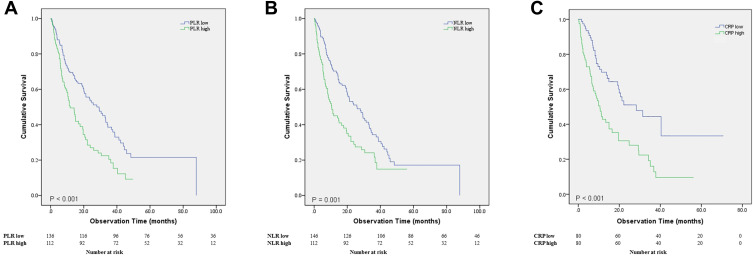
The non-COPD group had significantly longer OS compared to the COPD group (P = 0.019) (Figure 1). Median survival was 21.3 months (95% CI, 16.7–25.8 months) for the non-COPD group and 10.7 months (95% CI, 7.2–14.1 months) for the COPD group. PFS was not significantly different between the two groups. Overall survival was significantly different for the high and low PLR groups (P < 0.001) (Figure 2A). Median survival of the low PLR group and high PLR group was 29.3 months (95% CI, 20.0–38.6 months) and 11.8 months (95% CI, 8.1–15.5 months), respectively. Overall survival was significantly different between the high and low NLR groups (P = 0.001) (Figure 2B). Median survival of the low NLR group was 26.0 months (95% CI, 18.8–33.2 months) and 10.6 months for the high NLR group (95% CI, 5.8–15.4 months). The high CRP group showed significantly lower median survival than the low CRP group (P < 0.001). Median survival of the low CRP group was 28.3 months (95% CI, 15.0–41.6 months) and 10.6 months (95% CI, 8.0–13.2 months) for the high CRP group (Figure 2C).
Figure 1.
Survival comparison of the patients with non-small cell lung cancer according to the presence of chronic obstructive pulmonary disease.
Figure 2.
(A) Survival comparison between the high and low PLR groups (P< 0.001). (B) Survival comparison between the high and low NLR groups (P= 0.001). (C) Survival comparison between the high and low CRP groups (P< 0.001).
Abbreviation: PLR, platelet–lymphocyte ratio; NLR, neutrophil–lymphocyte ratio; CRP, c-reactive protein.
Relationship Between Lung Function and Inflammatory Markers
PLR showed a significant negative linear correlation with FVC (absolute) (r = −0.149, P = 0.015), FVC (%) (r = −0.192, P = 0.002), DLCO (absolute value) (r = −0.271, P < 0.001), and DLCO (%) (r = −0.139, P = 0.032). In addition, FVC (%) (r = −0.262, P < 0.001), FEV1 (absolute) (r = −0.203, P < 0.001), FEV1 (%) (r = −0.217, P < 0.001), DLCO (absolute value) (r = −0.235, P < 0.001), and DLCO (%) (r = −0.152, P = 0.016) showed significant correlations with NLR.
CRP showed a significant correlation with FVC (%) (r = −0.236, P = 0.003), FEV1 (absolute) (r = −0.156, P = 0.049), FEV1 (%) (r = −0.288, P < 0.001), and DLCO (%) (r = −0.205, P = 0.013). PLR, NLR and CRP all showed strong correlations with each other (Table 2).
Table 2.
Correlation Analysis Between Inflammatory Markers and Pulmonary Function Parameters
| PLR | NLR | CRP | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| FVC (absolute) | −0.149 | 0.015 | −0.105 | 0.080 | −0.069 | 0.388 |
| FVC (%predicted) | −0.192 | 0.002 | −0.262 | <0.001 | −0.236 | 0.003 |
| FEV1 (absolute) | 0.101 | 0.098 | −0.203 | <0.001 | −0.156 | 0.049 |
| FEV1 (% predicted) | −0.094 | 0.126 | −0.217 | <0.001 | −0.288 | <0.001 |
| FEV1/FVC | 0.101 | 0.098 | 0.045 | 0.460 | −0.048 | 0.545 |
| DLCO (absolute) | −0.271 | <0.001 | −0.235 | <0.001 | −0.112 | 0.179 |
| DLCO (% predicted) | −0.139 | 0.032 | −0.152 | 0.016 | −0.205 | 0.013 |
| PLR | – | – | 0.718 | <0.001 | 0.187 | 0.022 |
| NLR | 0.718 | <0.001 | – | – | 0.427 | <0.001 |
| CRP | 0.187 | 0.022 | 0.427 | <0.001 | – | – |
Note: P-value calculated using Spearman’s rho.
Abbreviations: CRP, c-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio.
OS and PFS Comparison Between COPD-PLR Groups, COPD-NLR Groups, and COPD CRP Groups
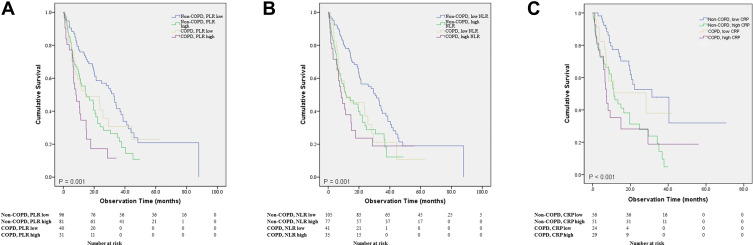
Kaplan-Meier graph was also used to compare overall survival between the subgroups. Compared to the low PLR non-COPD group, the high PLR non-COPD group had higher risk of mortality (HR = 9.240, P = 0.002), and the high PLR COPD group showed the highest risk of mortality (HR = 22.263, P < 0.001) (Figure 3A).
Figure 3.
(A) Survival comparison between the 4 COPD-PLR subgroups (P= 0.001). (B) Survival comparison between the 4 COPD-NLR subgroups (P= 0.001). (C) Survival comparison between the 4 COPD-CRP subgroups (P<0.001).
Compared to the low NLR non-COPD group, the high NLR non-COPD group, low NLR COPD group, and high NLR COPD group all showed significantly higher risk of mortality (HR = 12.570, P < 0.001; HR = 6.508, P = 0.011; and HR = 12.405, P < 0.001, respectively) (Figure 3B). OS was also compared between the COPD-CRP subgroups, showing significant difference between the groups (P<0.001). However, low CRP non-COPD group did not show significant difference with other subgroups (Figure 3C).
PFS was compared between COPD-PLR groups and was not significantly different (P = 0.078) (Supplementary Figure 1A). No significant difference in PFS was present between the COPD-NLR groups or between the COPD-CRP groups (Supplementary Figure 1B and 1C).
Comparison of Clinical Characteristics Between COPD-PLR Groups
Baseline clinical characteristics were compared between the four subgroups (Table 3). Sex, age, smoking status, and ECOG scores were significantly different between the four subgroups (P=0.002, P<0.001, P<0.001, and P=0.001, respectively). The COPD groups showed high proportions of males, ever smokers, and poor ECOG scores. Among pulmonary function parameters, significant differences were present for FEV1 (absolute), FEV1 (%), FEV/FVC, and DLCO (absolute) (P<0.001, P<0.001, P<0.001, and P<0.001, respectively). COPD groups with both low and high PLR showed significantly lower predicted FEV1 (%) compared to the non COPD, low PLR group.
Table 3.
Comparison of Clinical Characteristics Between COPD-PLR Subgroups
| Non-COPD, Low PLR (n=106) (n,%) | Non-COPD, High PLR (n=83) (n,%) | COPD, Low PLR (n=44) (n,%) | COPD, High PLR (n=34) (n,%) | p-value | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 60 (56.6) | 39 (47.0) | 13 (29.5) | 9 (26.5) | 0.002 |
| Age (year) | 60.7±10.5 | 63±11.3 | 72.3±8.6 | 70.6±8.1 | <0.001bcde |
| Mean survival time (months) (mean±SE) | 37.0±3.7 | 20.0±2.0 | 26.1±4.3 | 12.2±2.1 | <0.001 |
| Median survival time (95% CI) | 32.9 [27.0–38.8] | 15.0 [6.6–23.4] | 14.5 [0–34.1] | 8.2 [3.7–12.6] | <0.001 |
| Stage | 0.420 | ||||
| IIIB | 7 (6.6) | 4 (4.8) | 5 (11.4) | 4 (11.8) | |
| IV | 99 (93.4) | 79 (95.2) | 39 (88.6) | 30 (88.2) | |
| BMI (kg/m2) | 24.0±3.7 | 23.1±3.2 | 23.3±2.9 | 22.3±3.4 | 0.049 |
| EGFR mutation | (n=105) 41 (39.0) | (n=77) 30 (39.0) | (n=42) 9 (21.4) | (n=33) 11 (33.3) | 0.196 |
| First line treatment | |||||
| Conventional chemotherapy | 60 (56.6) | 50 (60.2) | 30 (68.1) | 22 (64.7) | 0.057 |
| Targeted therapy | 26 (24.5) | 27 (32.5) | 7 (15.9) | 7 (20.6) | |
| Supportive care only | 11 (10.4) | 3 (3.6) | 7 (15.9) | 4 (11.8) | |
| Smoking | <0.001 | ||||
| Never smoker | 65 (61.3) | 53 (64.6) | 17 (38.6) | 9 (26.5) | |
| Ever smoker | 41 (38.7) | 29 (35.4) | 27 (61.4) | 25 (73.5) | |
| ECOG | 0.001 | ||||
| 0–1 | 93 (87.7) | 77 (93.9) | 34 (77.3) | 23 (67.6) | |
| 2–3 | 13 (12.3) | 5 (6.1) | 10 (22.7) | 11 (32.4) | |
| Pathology | 0.087 | ||||
| Non-squamous | 95 (89.6) | 77 (92.8) | 39 (88.6) | 26 (76.5) | |
| Squamous | 11 (10.4) | 6 (7.2) | 5 (11.4) | 8 (23.5) | |
| FEV1 (liter) | 2.1±0.7 | 1.9±0.7 | 2.3±3.3 | 1.6±0.6 | 0.104 |
| FEV1 (% predicted) | 85.3±22.3 | 80.2±22.1 | 69.8±15.9 | 70.1±21.7 | <0.001bc |
| FVC (liter) | 2.6±0.9 | 2.4±0.9 | 2.8±1.3 | 3.4±5.0 | 0.106 |
| FVC (% predicted) | 80.6±20.7 | 73.1±21.5 | 80.8±20.0 | 77.7±25.9 | 0.089 |
| FEV1/FVC (% predicted) | 78.7±6.4 | 80.0±6.7 | 60.9±9.3 | 63.1±6.5 | <0.001bcde |
| DLCO (abs) | 13.9±4.6 (n=95) | 12.2±4.7 (n=76) | 11.5±4.1 (n=38) | 10.4±3.4 (n=31) | 0.001c |
| DLCO (%) | 73.5±18.4 | 69.4±21.4 | 67.0±21.1 | 67.2±20.0 | 0.229 |
| NLR | 2.5±1.4 | 5.6±3.8 | 2.7±1.2 | 5.5±2.4 | <0.001acdf |
| PLR | 130.8±32.8 | 264.0±77.3 | 127.2±29.1 | 271.8±56.4 | <0.001acdf |
Notes: Post-hoc pairwise comparison: aNon-COPD, low PLR vs Non-COPD, high PLR, bNon-COPD, low PLR vs COPD, low PLR, cNon-COPD, low PLR vs COPD, high PLR, dNon-COPD, high PLR vs COPD, low PLR, eNon-COPD, high PLR vs COPD, high PLR, fCOPD, low PLR vs COPD, high PLR, P-value significance cutoff=0.0125.
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; SE, standard error.
Association of Clinical Parameters with Overall Survival
Baseline parameters including COPD-PLR subtype and COPD-NLR subtype were entered into a Cox regression hazard model for mortality (Table 4). In the univariate analysis, age, sex, EGFR mutation, ECOG, active anticancer treatment, hemoglobin, and COPD-PLR subtype were significant. In the multivariate analysis, male sex, wild type EGFR, not receiving active anticancer treatment, and low hemoglobin were significantly associated with shorter OS (HR 1.477 (1.016–2.148), P=0.041; HR 0.501 (0.338–0.740), P=0.001; HR 2.937 (1.497–5.762), P=0.002; and HR 0.846 (0.756–0.947), P=0.004, respectively). The high PLR, COPD group had significantly increased risk for mortality (HR 2.066 (1.175–3.633), P=0.012) compared with the low-PLR non-COPD group. In the model with the COPD-NLR subgroup entered in place of COPD-PLR, COPD-NLR was significant for mortality in univariate analysis but not in multivariate analysis.
Table 4.
Associations of Clinical Variables Affecting Overall Survival of the Patients
| Characteristics | Univariate | Multivariate (Model 1) | Multivariate (Model 2) | ||||
|---|---|---|---|---|---|---|---|
| P | P | HR | 95% CI | P | HR | 95% CI | |
| Age | 0.012 | 0.574 | 1.005 | 0.987–1.024 | 0.568 | 1.005 | 0.987–1.024 |
| Male/Female | 0.047 | 0.041 | 1.477 | 1.016–2.148 | 0.028 | 1.234 | 1.023–1.489 |
| Histology (non-squamous/squamous) | 0.173 | - | - | ||||
| EGFR mutation (mutant/wild-type) | 0.001 | 0.001 | 0.501 | 0.338–0.740 | 0.001 | 0.518 | 0.353–0.761 |
| ECOG (0–1/≥2) | 0.022 | 0.104 | 1.587 | 0.909–2.772 | 0.127 | 1.513 | 0.888–2.578 |
| First line treatment (active anticancer treatment/supportive care only) | <0.001 | 0.002 | 2.937 | 1.497–5.762 | <0.001 | 4.033 | 2.276–7.147 |
| Hemoglobin | 0.001 | 0.004 | 0.846 | 0.756–0.947 | 0.001 | 0.824 | 0.739–0.919 |
| BMI | 0.807 | - | - | ||||
| COPD-PLR subtype* | <0.001 | 0.048 | |||||
| Non-COPD, PLR low | 1 | ||||||
| Non-COPD, PLR high | 0.034 | 1.577 | 1.034–2.404 | ||||
| COPD, PLR low | 0.451 | 1.229 | 0.719–2.102 | ||||
| COPD, PLR high | 0.012 | 2.066 | 1.175–3.633 | ||||
| COPD-NLR subtype* | 0.001 | 0.096 | |||||
| Non-COPD, NLR low | 1 | ||||||
| Non-COPD, NLR high | 0.018 | 1.654 | 1.089–2.512 | ||||
| COPD, NLR low | 0.114 | 1.511 | 0.906–2.519 | ||||
| COPD, NLR high | 0.103 | 1.582 | 0.911–2.746 | ||||
Notes: *For comparison of HR, the reference group is Non-COPD, PLR low for the COPD, PLR subtypes, and Non-COPD, NLR low for the COPD-NLR subtypes.
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; HR, hazard ratio; CI, confidence interval; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio.
Discussion
The present study showed that a combination of fixed airway obstruction and PLR is an independent predictor of overall survival in advanced NSCLC. Among patients with NSCLC, NLR, PLR and CRP showed significant correlation with each other, and weak but significant correlations with lung function parameters, suggesting possible interrelation between systemic inflammation and pulmonary function in patients with advanced NSCLC.
A growing number of reports suggest that in both COPD and lung cancer, there are close associations between disease prognosis and systemic inflammatory status. In the previous study, NLR was positively correlated with CRP levels in patients with COPD, suggesting its value as an inflammatory marker in such patients.35 Increased levels of NLR and PLR were associated with mortality in patients with acute exacerbation of COPD.22,23 As with lung cancer, increased PLR was associated with poor survival in advanced NSCLC.32
We previously assumed that COPD may contribute to the systemic inflammatory response, and ultimately influence prognosis in patients with advanced lung cancer. From the study by Szentkereszty et al, the levels of IFNγ, TNFα, IL-10, and NLR were significantly different between a COPD and a non-COPD NSCLC group.36 However, there was no significant difference in the levels of NLR and PLR between COPD and non-COPD groups in our study. This may be due to the considerable proportion of patients with mild COPD in our study population, who did not show evident deterioration of lung function. The results indicate that the level of systemic inflammation is not sufficient to make a difference. However, our correlation analysis result suggests weak but significant correlations between inflammatory markers (NLR, PLR, CRP), and pulmonary function parameters. The interrelationship between COPD and systemic inflammatory response in NSCLC is complex, and a larger study population is necessary to obtain more concrete explanations regarding inflammatory status and pulmonary function in NSCLC.
Consistent with previous studies, our study showed that patients in groups with high PLR or NLR showed significantly worse survival compared to low PLR or NLR groups, respectively. Furthermore, there was a significant difference in survival between COPD and non-COPD groups. Among the four subgroups stratified by PLR cutoff and COPD status, patients with both fixed airway obstruction and high PLR had poor outcomes. This may be due to a combination or synergistic effect of two separate negative factors, increased systemic inflammation and fixed airway obstruction. Previous studies showed that patients experiencing more acute exacerbations of COPD show increased NLR and PLR compared to groups without exacerbations.23,37 It is possible that acute COPD exacerbation may have been more frequent in the high PLR, COPD group, ultimately influencing the prognosis of this group. Nevertheless, exacerbation frequency was not evaluated in this study. A future study evaluating the influence of COPD control on prognosis of advanced NSCLC would be useful.
In the present study, COPD-NLR was not an independent predictor for survival, but COPD-PLR showed a significant association in the multivariate analysis. We assume that COPD-PLR has an advantage over COPD-NLR, because PLR may be more sensitive to cancer activity than NLR, which would eventually have a significant impact on the prognosis of patients. Elevated PLR may be interpreted as simultaneous thrombocytosis and lymphocytopenia.32 Thrombocytosis in cancer is associated with proliferation, metastasis, evasion of immune detection, and chemoresistance.38,39 Furthermore, lymphocytopenia may result in decreased immune activity towards cancer cells, as lymphocytes are reported to play major anticancer roles.40,41 In the study on predictability for prognosis of patients undergoing curative surgery for NSCLC, PLR was superior to NLR, lymphocyte to monocyte to ratio, and CRP.42 However, this hypothesis needs further validation in the future study.
This study has several limitations. First, median survival was relatively long for advanced NSCLC. The enrollment criteria included the presence of pulmonary function data at the time of lung cancer diagnosis, so patients who were unable to perform the tests could not be evaluated, which may have led to selection bias. Patients with significant concurrent infections requiring antibiotics at the time of cancer diagnosis were also excluded. These factors may have affected survival data. Secondly, the proportion of never smokers was large and may have affected the proportion of EGFR mutations and use of EGFR TKI as first-line treatment, which may have influenced the median survival of overall study patients. Third, patients were staged according to AJCC 7th edition, as most of them were diagnosed with lung cancer before 2017. Lastly, COPD was defined based on the spirometry results, and it is possible that tumor burdens or central airway involvement by cancer may have affected the definition of COPD. Furthermore, the detailed description of COPD status, such as exacerbation history and exhaled nitric oxide are lacking in our study. In this study, we focused on the predictive value of the combination of values of two parameters, which can be easily attained from baseline studies at diagnosis of cancer, rather than the pathophysiological link. Future studies including strict definition of COPD are necessary.
Conclusions
A combination of COPD status and PLR may be a cost-effective and readily available prognostic marker in patients with advanced NSCLC. A combination of both parameters may give more perspective than a single prognostic marker. More studies are necessary to validate the predictability of this combination biomarker.
Funding Statement
No funding was received.
Abbreviations
COPD, chronic obstructive pulmonary disease; NSCLC, non-small cell lung cancer; PLR, platelet to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; AE, acute exacerbation; NLR, neutrophil to lymphocyte ratio; CBC, complete blood count; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PNA, peptide nucleic acid; ALK, anaplastic lymphoma kinase; FFPE, fixed paraffin-embedded; ATS/ERS, American Thoracic Society/European Respiratory Society; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.McErlean A, Ginsberg MS. Epidemiology of lung cancer. Semin Roentgenol. 2011;46(3):173–177. doi: 10.1053/j.ro.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 3.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60(7):570–575. doi: 10.1136/thx.2004.037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8(4):e34–e35. doi: 10.1097/JTO.0b013e31828950e3 [DOI] [PubMed] [Google Scholar]
- 6.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi: 10.1016/j.lungcan.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 7.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129(5):1305–1312. doi: 10.1378/chest.129.5.1305 [DOI] [PubMed] [Google Scholar]
- 8.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 9.Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269–279. doi: 10.1111/resp.12661 [DOI] [PubMed] [Google Scholar]
- 10.Bugge A, Lund MB, Brunborg C, Solberg S, Kongerud J. Survival after surgical resection for lung cancer in patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2016;101(6):2125–2131. doi: 10.1016/j.athoracsur.2015.12.057 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y, Kage H, Murakawa T, et al. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg. 2015;21(3):194–200. doi: 10.5761/atcs.oa.14-00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JU, Yeo CD, Rhee CK, et al. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis. 2019;14:929–938. doi: 10.2147/COPD.S190244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim JU, Yeo CD, Rhee CK, et al. Overall survival of driver mutation-negative non-small cell lung cancer patients with COPD under chemotherapy compared to non-COPD non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2139–2146. doi: 10.2147/COPD.S167372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 16.Gu X, Sun S, Gao XS, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3430 patients. Sci Rep. 2016;6:23893. doi: 10.1038/srep23893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Gao L, Zhang B, Zhang L, Wang C. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:22618. doi: 10.1038/srep22618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3720 patients. Int J Cancer. 2016;139(1):164–170. doi: 10.1002/ijc.30060 [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Bian C, Xia D, et al. Combining carcinoembryonic antigen and platelet to lymphocyte ratio to predict brain metastasis of resected lung adenocarcinoma patients. Biomed Res Int. 2017;2017:8076384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Lu Y, Lin L, Meng K, Fan J. Does chronic obstructive pulmonary disease relate to poor prognosis in patients with lung cancer?: a meta-analysis. Medicine (Baltimore). 2019;98(11):e14837. doi: 10.1097/MD.0000000000014837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Dou S, Dong W, et al. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis. 2018;13:3767–3776. doi: 10.2147/COPD.S168048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar P, Law S, Sriram KB. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(6):1509–1516. doi: 10.21037/jtd.2017.05.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao C, Liu X, Tang Z. Prognostic role of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–2290. doi: 10.2147/COPD.S141760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim JU, Yeo CD, Kang HS, et al. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PLoS One. 2018;13(7):e0200341. doi: 10.1371/journal.pone.0200341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin SH, Park HY, Im Y, et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer. 2019;145(9):2433–2439. doi: 10.1002/ijc.32235 [DOI] [PubMed] [Google Scholar]
- 26.Biton J, Ouakrim H, Dechartres A, et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am J Respir Crit Care Med. 2018;198(7):928–940. doi: 10.1164/rccm.201706-1110OC [DOI] [PubMed] [Google Scholar]
- 27.Yeo CD, Kim JW, Kim KH, et al. Detection and comparison of EGFR mutations in matched tumor tissues, cell blocks, pleural effusions, and sera from patients with NSCLC with malignant pleural effusion, by PNA clamping and direct sequencing. Lung Cancer. 2013;81(2):207–212. doi: 10.1016/j.lungcan.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 30.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103(1):57–67. [DOI] [PubMed] [Google Scholar]
- 31.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Res J. 2017;49:3. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 32.Lim JU, Yeo CD, Kang HS, et al. Elevated pretreatment platelet-to-lymphocyte ratio is associated with poor survival in stage IV non-small cell lung cancer with malignant pleural effusion. Sci Rep. 2019;9(1):4721. doi: 10.1038/s41598-019-41289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin G-N, Peng J-W, Liu -P-P, Liu D-Y, Xiao -J-J, Chen X-Q. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. 2017;13(5):e189–e194. doi: 10.1111/ajco.12273 [DOI] [PubMed] [Google Scholar]
- 34.Derman BA, Macklis JN, Azeem MS, et al. Relationships between longitudinal neutrophil to lymphocyte ratios, body weight changes, and overall survival in patients with non-small cell lung cancer. BMC Cancer. 2017;17(1):141. doi: 10.1186/s12885-017-3122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunay E, Sarinc Ulasli S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 2014;37(2):374–380. doi: 10.1007/s10753-013-9749-1 [DOI] [PubMed] [Google Scholar]
- 36.Szentkereszty M, Komlosi ZI, Szucs G, et al. Effect of COPD on inflammation, lymphoid functions and progression-free survival during first-line chemotherapy in advanced non-small cell lung cancer. Pathol Oncol Res. 2020;26(2):1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Gazzar AG, Kamel MH, Elbahnasy OKM, El-Naggar ME. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med. 2020;14(1):111–116. doi: 10.1080/17476348.2019.1675517 [DOI] [PubMed] [Google Scholar]
- 38.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979;53(4):604–618. doi: 10.1182/blood.V53.4.604.604 [DOI] [PubMed] [Google Scholar]
- 39.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee TK, Horner RD, Silverman JF, Chen YH, Jenny C, Scarantino CW. Morphometric and morphologic evaluations in stage III non-small cell lung cancers. Prognostic significance of quantitative assessment of infiltrating lymphoid cells. Cancer. 1989;63(2):309–316. doi: [DOI] [PubMed] [Google Scholar]
- 41.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Q, Diao P, Li CL, et al. Preoperative platelet-lymphocyte ratio is a superior prognostic biomarker to other systemic inflammatory response markers in non-small cell lung cancer. Medicine (Baltimore). 2020;99(4):e18607. doi: 10.1097/MD.0000000000018607 [DOI] [PMC free article] [PubMed] [Google Scholar]