Abstract
Background
Live kinase B1 (LKB1) is a tumor suppressor that is mutated in Peutz-Jeghers syndrome (PJS) and a variety of cancers. Lkb1 encodes serine-threonine kinase (STK) 11 that activates AMP-activated protein kinase (AMPK) and its 13 superfamily members, regulating multiple biological processes, such as cell polarity, cell cycle arrest, embryo development, apoptosis, and bioenergetics metabolism. Increasing evidence has highlighted that deficiency of LKB1 in cancer cells induces extensive metabolic alterations that promote tumorigenesis and development. LKB1 also participates in the maintenance of phenotypes and functions of normal cells through metabolic regulation.
Scope of review
Given the important role of LKB1 in metabolic regulation, we provide an overview of the association of metabolic alterations in glycolysis, aerobic oxidation, the pentose phosphate pathway (PPP), gluconeogenesis, glutamine, lipid, and serine induced by aberrant LKB1 signals in tumor progression, non-neoplastic diseases, and functions of immune cells.
Major conclusions
In this review, we summarize layers of evidence demonstrating that disordered metabolisms in glucose, glutamine, lipid, and serine caused by LKB1 deficiency promote carcinogenesis and non-neoplastic diseases. The metabolic reprogramming resulting from the loss of LKB1 confers cancer cells with growth or survival advantages. Nevertheless, it also causes a metabolic frangibility for LKB1-deficient cancer cells. The metabolic regulation of LKB1 also plays a vital role in maintaining cellular phenotype in the progression of non-neoplastic diseases. In addition, lipid metabolic regulation of LKB1 plays an important role in controlling the function, activity, proliferation, and differentiation of several types of immune cells. We conclude that in-depth knowledge of metabolic pathways regulated by LKB1 is conducive to identifying therapeutic targets and developing drug combinations to treat cancers and metabolic diseases and achieve immunoregulation.
Keywords: Metabolism, LKB1, Tumorigenesis, Lipid, Immune cell, Glucose
1. Introduction
Liver kinase B1 (LKB1), also known as serine/threonine kinase (STK11), was originally detected to be mutated in Peutz-Jeghers syndrome and more recently identified as a pivotal tumor suppressor [1,2]. Increasing evidence suggests that inactivated somatic mutations of LKB1 are involved in the pathogenesis of several types of cancers, including gastrointestinal cancer [3], non-small cell lung cancer (NSCLC) [[4], [5], [6]], pancreatic cancer [7], cervical cancer [8], and melanoma [9,10]. LKB1 not only suppresses malignant cell transformation and the progression of cancers [[11], [12], [13]], but also plays a vital role in regulating the dynamics of hematopoietic stem cells [[14], [15], [16]], the development of nerve and muscle [17,18], disease progression of polycystic kidneys and fibrosis [19,20], and immune cell functions [[21], [22], [23]]. Metabolic regulation of LKB1 exhibits an essential role in this context.
The functions of LKB1 mainly depend on the phosphorylation and activation of 13 members of the AMP-activated protein kinase (AMPK) superfamily, including AMPK1/2, microtubule affinity-regulating kinase (MARK)1/2/3/4, brain selective kinase (BRSK)1/2, salt-inducible kinase (SIK)1/2/3, NUAK family sucrose non-fermenting (SNF)1-like kinase (NUAK)1/2, and SNF-related kinase (SNRK) [24,25]. LKB1 regulates a wide range of cellular and biological functions. Among the downstream targets of LKB1, the energy sensor AMPK mediates the prominent metabolic regulation via modulating metabolic enzyme activities and inducing transcriptional adaptive responses [26,27]. However, increasing evidence indicates that LKB1 also regulates metabolism in an AMPK-independent manner. Given the need for LKB1 for the metabolic regulation of multiple biological functions, this review summarizes metabolic pathways regulated by LKB1 and elucidates the processes modulated by dysfunctional LKB1 in disease progression and cell function.
2. LKB1 modulates glucose metabolism
2.1. Glycolysis
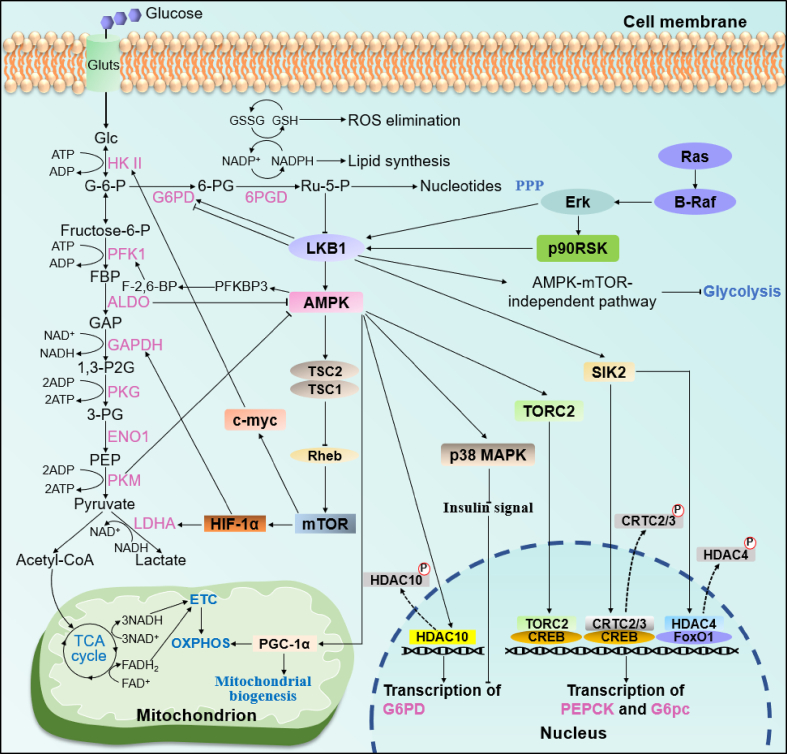
Cancer cells primarily depend on glycolysis to produce ATP and metabolic intermediates to meet the requirements of proliferation and development, followed by increased lactate production and glucose consumption, which is denoted as the “Warburg effect” [28]. As a key tumor suppressor and metabolic regulator, LKB1 participates in the regulation of metabolic reprogramming, which induces excess glycolysis. LKB1 inhibits HPV-induced glycolysis by suppressing hexokinase-II (HK-II), the first rate-limiting enzyme of the glycolytic pathway (Figure 1), inhibiting HPV-stimulated tumor progression [8]. One study found that re-expressing LKB1 in A549 cells, a human non-small cell lung cancer cell line naturally lacking LKB1, reduced the extracellular acidification rate (ECAR), an indicator of glycolysis, by ∼20% relative to control cells without LKB1 [29]. This metabolic phenotype transformation was induced by an increased expression of hypoxia-inducible factor 1α (HIF-1α), which is ascribed to the deficiency of LKB1 [29]. AMPK is the foremost downstream target of LKB1 for mediating metabolic regulation. Thus, it is plausible that glycolysis would increase under AMPK deficiency conditions. Indeed, inactivation of AMPKα promotes a metabolic shift from oxidative phosphorylation to aerobic glycolysis in Myc-induced lymphomagenesis in an HIF-1α-dependent manner [30]. However, in A549 cells with ectopic LKB1 expression, although targeting active AMPK mutants to mitochondria results in a decreased ECAR, ablations of AMPKβ subunits were also found to significantly decrease ECAR and lactic acid concentrations [31]. These results suggest that the activation of AMPK not only inhibits excessive “Warburg effect,” but also is required for basal glycolysis metabolism. Consistent with these findings, AMPK phosphorylates 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKBP3) to induce a switch from oxidative respiration to glycolysis by regulating the concentration of fructose 2,6-bisphosphate (F2,6BP), an allosteric activator of glycolysis rate-limiting enzyme phosphofructokinase 1 (PFK1) [32,33]. Absence of AMPK results in the suppression of F2,6BP signaling, reducing glucose flux to glycolysis. As an energy sensor, LKB1-AMPK signaling is also under the control of fructose-1,6-bisphosphate (FBP) and aldolase (ALDO) [34]. As a critical intermediate, FBP reflects the level of glycolysis and modulates ALDO to sense glucose availability [34]. Decreased FBP levels or ALDO knockdown activates AMPK to provide sufficient energy for cells by promoting catabolism, such as fatty acid oxidation (FAO) [34].
Figure 1.
LKB1 in glucose metabolic reprogramming. LKB1 controls glucose metabolism by inducing the expression of several genes that encode the enzymes of glycolysis, aerobic oxidation, the pentose phosphate pathway, and gluconeogenesis. Abbreviations: hexokinase II (HK II), phosphofructokinase 1 (PFK1), aldolase (ALDO), glyceraldehyde 3-phosphatedehydrogenase (GAPDH), phosphoglycerate kinase (PGK), α-enolase (ENO1), pyruvate kinase (PK), glucose (Glc), glucose-6-phosphate (G-6-P), fructose-6-phosphate (F-6-P), fructose-1,6-bisphosphate (FBP), glyceraldehyde-3-phosphate (GAP), 1,3-diphosphoglycerate (1,3-P2G), 3-phosphoglycerate (3-PG), phosphoenolpyruvate (PEP), 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKBP3), fructose 2,6-bisphosphate (F-2,6-BP).
Intensive glycolysis induced by LKB1 deficiency not only serves as a primary energy supply for cancer cells, but also plays an important role in maintaining cellular morphology [17,35]. Schwann cell (SC)-specific LKB1 ablation does not result in any overt abnormalities in cell polarity in development process, but leads to the degeneration of myelinated and unmyelinated axons in adults after nerve development is complete due to metabolic deregulation induced by LKB1 deletion [17]. Further investigations revealed that LKB1-deficient SCs release increased amounts of lactate, which provides further support for deteriorating axons [17], suggesting that the increased glycolysis induced by LKB1 deletion manifests as a compensatory response to sustain distressed axons. Of note, the metabolic changes in LKB1-deficient SCs were found to be largely independent of AMPK and mammalian targets of rapamycin (mTOR) [17], which lowers the significance of the need for mediating the AMPK signal for LKB1 metabolic function, consistent with the contrasting actions of LKB1 and AMPK in glycolysis [31]. In summary, LKB1 deficiency facilitates glycolysis in an AMPK-independent or -dependent manner, which plays a pivotal role in energy supply and maintaining the survival and development of aberrant cells.
2.2. Aerobic oxidation
Although cancer cells require enhanced glycolysis to sustain their excessive proliferation, aerobic oxidation is also a principal approach for energy sources in these cells. Which pathway is selected to provide energy for cancer cells to a large extent depends on the metabolic microenvironment. The alternative reflects the metabolic plasticity of cancer cells. However, whether lack of LKB1 contributes to the enhanced tricarboxylic acid (TCA) cycle flux and oxidation respiratory reaction in cancer cells for their survival, proliferation, and migration remains to be elucidated. Interestingly, A549 cells were found to display an increased total abundance of metabolic intermediates derived from the TCA cycle, with no significant changes in the oxygen consumption rate (OCR) relative to A549 cells with re-expressed LKB1 [29]. These results are in accordance with the function of AMPK as an energy sensor tasked with activating the oxidative metabolism pathway. AMPK activation prevailingly depends on LKB1, in particular under conditions of energy and nutrition stress. Knockdown of pyruvate kinase (PK) isoforms in H1299 cells (a human non-small cell lung cancer cell line with intact LKB1) was reported to facilitate mitochondrial biogenesis and the electron transport chain (ETC), which are induced by AMPK activation [36]. However, silencing PKM in A549 cells exhibited a heterogenic response and failed to show similarities in terms of changes to mitochondrial biogenesis because LKB1 deficiency failed to activate AMPK, which led to insufficient sustaining in energy homeostasis and resulted in apoptosis [36]. Loss of LKB1 consistently facilitates the proliferation of cancer cells, but also lowers the metabolic plasticity in cancer cells, which leads to an increased sensitivity of these cells to nutrition deprivation [37]. In addition to silencing PKM, treating multiple types of cells with AMPK activators highlights the pivotal role of the LKB1-AMPK pathway in mitochondrial biogenesis and oxidative phosphorylation (OXPHOS). For example, Rev-erb-α deficiency in muscles results in reduced mitochondrial content and oxidative function [38], while the combination of curcumin treatment and endurance training increases COX-IV expression and mitochondrial DNA copy number as well as citrate synthase activity in skeletal muscle [39]. However, the overexpression of CAB39L suppresses gastric cancer development via a reinforced effect in OXPHOS and mitochondrial biogenesis [40]. These reactions are mediated by the LKB1-AMPK-PGC1α axis.
There is an inconsistency in terms of LKB1-AMPK activation in ATP-linked respiration. For example, ectopic expression of LKB1 in A549 cells did not increase OCR [29], whereas ATP-linked respiration was significantly increased by LKB1 expression although basal OCR did not increase [41]. In a 13C-labeled metabolic flux analysis, Parker et al. reported that NSCLC cells expressing functional LKB1 displayed higher levels of flux through OXPHOS than those in LKB1-deficient cells. Ectopic expression of LKB1 markedly enhanced the capability of cells to oxidize major mitochondrial substrates, such as fatty acids, pyruvate, and glutamine [41]. These results suggested that an increased oxidative TCA cycle is linked to an increase in mitochondrial ATP production.
In summary, although LKB1 deficiency induces an increase in the TCA cycle flux in cancer cells, the OXPHOS level does not display a corresponding change. LKB1-AMPK signaling positively regulates mitochondrial biogenesis and OXPHOS subunits expression, maintaining energy homeostasis. Thus, intervention therapies targeting the effect of LKB1 on aerobic oxidation could be potentially beneficial for treating diseases as follows: (i) in LKB1-deficient cancer cells, energy starvation or targeting mitochondrial metabolism is conducive to promote apoptosis in cancer cells due to insufficient maintenance of energy homeostasis, as tumors with Kras and Lkb1 mutations showed a strong response to phenformin (a mitochondrial inhibitor and analog of metformin) [42,43]; (ii) in intact LKB1 cancers, the activation of the LKB1-AMPK signal contributes to inhibiting the Warburg effect as well as suppressing tumor progression; and (iii) in normal cells with intact LKB1 and mitochondria damage or oxidative function deregulation, metformin (an AMPK activator) may be an effective therapeutic agent.
2.3. Pentose phosphate pathway (PPP)
The excessive proliferation of cancer cells requires not only a sufficient energy supply, but also precursors for biosynthesizing basic cellular components [28]. The increased aerobic glycolysis in cancer cells (Warburg effect) allows the diversion of multiple biological macromolecules into other metabolic pathways, such as pyruvate into the TCA cycle or glucose-6-phosphate (G-6-P) into oxidative PPP, and plays a pivotal role in energy and material supply of cancer cells [28,44]. PPP commonly produces ribulose-5-phosphate (Ru-5-P), a precursor for nucleotide synthesis, and nicotinamide adenine dinucleotide phosphate (NADPH), which is required for lipid biosynthesis and the elimination of reactive oxygen species (ROS) produced by the rapid proliferation of cancer cells [44]. Taking into account the metabolic coordination role of oxidative PPP in glycolysis, nucleoside biosynthesis, lipid synthesis, and oxidative homeostasis, cancer cells usually exhibit enhanced PPP, allowing them to grow and proliferate [45]. Indeed, the suppression of glucose-6-phosphate dehydrogenase (G6PD), the first enzyme in PPP, decreases NADPH and glutathione (GSH) levels and impairs ROS scavenging, which facilitates oxaliplatin-induced apoptosis in colorectal cancer [46]. Furthermore, the inhibition of 6-phosphogluconate dehydrogenase (6PGD), the third enzyme in PPP, contributes to decreased lipogenesis and RNA biosynthesis and increased ROS, resulting in the attenuation of cancer cell proliferation and tumor growth [47].
LKB1 participates in the regulation of PPP. Ru-5-P produced from 6-phosphogluconate (6-PG) upon the catalysis of 6PGD inhibits AMPK activation by impairing the active LKB1 complex, relieving the suppression of acetyl-CoA carboxylase 1 (ACC1) and intensifying lipogenesis [47]. In addition, activating the LKB1-AMPK signal promotes the phosphorylation of histone deacetylase 10 (HDAC10) at S393 and S540 in lung cancer cells, which leads to the translocation of HDAC10 from the nucleus into the cytoplasm, increasing histone acetylation levels and G6PD transcription [48]. However, LKB1 is involved in tumor suppression and likely to play an inhibitory role in PPP. Recent studies consistently demonstrated that the activation of AMPK inhibits G6PD expression at the transcriptional level [49,50]. Mechanistically, AMPK activation increases p38 mitogen-activated protein kinase (p38 MAPK) activity, inhibiting insulin signal transduction and suppressing G6PD expression [50]. In terms of what this indicates for the role of LKB1 in mediating the divergent regulation of G6PD, it has been reported that knockdown of LKB1 in H1299 cells or re-expression of LKB1 in A549 cells does not change the Ru-5-P level [47], suggesting that the alteration of LKB1 does not directly affect PPP. Thus, although LKB1 mediates the regulation of PPP, the transformation of PPP largely depends on upstream gene changes, such as the overexpression of 4-hydroxyphenylpyruvate dioxygenase (HPD) [48], or agent treatment, such as flavonoid-derivative GL-V9 treatment [49], which alters the redox balance. Therefore, the regulation of LKB1 to promote or inhibit G6PD may depend on the impact of PPP upstream signaling or the nutrition microenvironment, which requires further study.
2.4. Gluconeogenesis
LKB1 not only regulates glucose catabolism, but also affects anabolism via the gluconeogenesis pathway. Liver LKB1 conditional knockout (KO) mice displayed hyperglycemia that was induced by increased gluconeogenic gene expression in LKB1-deficient hepatic cells [51,52]. Mechanistically, cAMP-response element-binding protein (CREB) is responsible for regulating the transcription of catalyzing enzyme genes in gluconeogenesis, especially rate-limiting enzymes such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase catalytic subunit (G6PC). The activation of LKB1-AMPK signaling results in the dephosphorylation and transportation of transducer of regulated CREB activity 2 (TORC2) into the nucleus, where it promotes CREB-dependent transcription, increasing gluconeogenesis [53]. Of note, metformin is a first-line anti-diabetic drug that activates AMPK in a LKB1-dependent manner, seemingly inhibiting gluconeogenesis by activating the LKB1-AMPK signal. However, a metformin-induced decrease in glucose production was found to be enhanced in both AMPK- and LKB1-deficient hepatocytes compared to wild-type cells [52]. Thus, the inhibition of metformin in gluconeogenesis occurs in a transcription-independent manner, decreasing intracellular ATP content and inducing glucose production [52].
The dephosphorylation of CREB-regulated transcription coactivators 2/3 (CRTC2/3) and histone deacetylases (HDAC4) results in their accumulation in the nucleus, where they respectively cooperate with CREB and increase the acetylation of forkhead box transcription factor O1 (FoxO1), facilitating the transcription of gluconeogenic genes [54]. SIK2, a downstream target of LKB1, phosphorylates CRTC2 and HDACs, leading to their accumulation in the cytoplasm, inhibiting gluconeogenic gene expression [54,55].
SIK1 and SIK3 were recently demonstrated to be key effectors in LKB1-mediated tumor suppression and are potentially more essential than AMPK [4,56]. Interestingly, SIK2 lacks the function of tumor suppression [4,56]. Adversely, SIK2 promotes tumorigenesis in prostate cancer [57]. Thus, SIK2-induced inhibition of gluconeogenesis may be an important mechanism for the function of SIK2 in tumorigenesis by suppressing tumor progression via increased gluconeogenesis [58]. However, further studies are needed in this regard.
3. LKB1 modulates glutamine metabolism
Glutamine is an important nutrient substance providing carbon and nitrogen for biosynthesis and energy metabolism, thus facilitating the maintenance of cell growth and proliferation. Although cancer cells depend on enhanced aerobic glycolysis for proliferation, many types of cancers show addiction to glutamine. Several oncogenes and tumor suppressors are associated with controlling glutamine metabolism [59]. Glutamine transforms α-ketoglutaric acid (α-KG) and flows into the TCA cycle, which is a major source of energy in addition to glucose metabolism. In LKB1-deficient NSCLC cells (A549 and A427), ectopic expression of LKB1 resulted in a reduced production of glutamine-derived glutamate. Moreover, LKB1 deficiency was previously found to induce enhanced TCA cycle flux along with increased glycolysis. However, a large proportion of glutamine-derived carbon is concerted into the TCA cycle relative to glucose-derived carbon in A549 cells compared to the re-expression of LKB1 [29], consistent with the suppression of the glutamine-dependent TCA cycle by metformin [60]. These findings suggest that LKB1 participates in the regulation of the glutamine flux.
Glutamine metabolism also plays a key role in maintaining oxidative homeostasis. Glutamate converted from glutamine under glutaminase catalysis is the precursor of glutathione (GSH), which promotes ROS detoxification [61]. A549 cells with LKB1 re-expression display increased apoptotic rates under ROS stress induced by treatment with H2O2 (400 μM) compared to control A549 cells. This is partly due to the upregulation of glutamine transformation in LKB1 deficiency [5]. LKB1 deficiency induces a dependence on glutamine, such that cancer cells lacking LKB1 may display an increased sensitivity to glutamine inhibition or deprivation. Indeed, most NSCLC cell lines with LKB1 deficiency display a high sensitivity for glutamine withdrawal [5]. However, LKB1 overexpression has been found to significantly decrease sensitivity of A549 cells to glutamine inhibitor (CB-839) [5]. Mechanistically, glutamine-cysteine ligase (GCL) catalyzes the formation of gamma-glutamylcysteine (γ-Glu-Gly) from glutamine and cysteine. LKB1 deficiency promotes nuclear factor E2-related factor 2 (NRF2)-dependent GCL expression, facilitating γ-Glu-Gly synthesis, which couples with glycine to form a larger GSH pool [5]. This is consistent with the finding that knockdown of NRF2 decreases the levels of 13C-labeled GSH in A549 cells with [U–13C5] glutamine medium [62].
The regulation of LKB1 in glutamine metabolism also plays a key role in the progression of polycystic kidney disease (PKD), which is characterized by the growth of fluid-filled cysts in the kidneys. It has been reported that LKB1 mutant kidneys display increased dependence on glutamine, which provides non-essential amino acid (NEAA) and GSH for cell growth [19]. Importantly, the loss of LKB1 alone does not induce PKD development. However, ablation of LKB1 in TSC1-deficient kidneys was found to accelerate the onset and progression of PKD [19].
It could be speculated that LKB1 is a non-dominated signal in many types of disease progression. LKB1 deficiency results in several metabolic changes that are compensated by other pathways to sustain cell growth and proliferation in its absence. Nevertheless, the loss of LKB1 leads to metabolic friability, and LKB1 deficiency may co-occur with other deficiencies, such as Kras activation in NSCLC cells [11], HPV infection in cervical cancer cells [8], B-RAF in melanoma [63], or TSC1 mutants in kidneys [19], which may accelerate disease progression.
4. LKB1 modulates lipid metabolism
4.1. Cancer cells with LKB1 deficiency display lipid metabolism disorder
Reprogramming lipid metabolism is one of the most important hallmarks of cancer cells [64]. Lipids not only constitute the basic membranal structure, but also act as signaling molecules. Cancer cells with lipid metabolism disorder generally display increased lipid uptake, de novo fatty acid (FA) synthesis, storage, and lipolysis, which satisfy the demands of cancer cells for their rapid proliferation and growth [65]. Illustratively, a subpopulation of CD44bright cells in oral carcinomas was found to express high levels of the FA receptor CD36, which is responsible for the uptake of exogenous lipids. The enhanced CD36 expression gives cancer cells the ability to initiate metastasis [66]. Fatty acid synthase (FASN), a key lipogenic enzyme in charge of the de novo biogenesis of FAs, confers growth and survival advantages in many types of human cancers [67]. Excessive lipids and cholesterol in cancer cells are stored in lipid droplets (LDs), and many types of tumors display high LDs, which are considered hallmarks of cancer aggression [65].
KL (Kras-sustained activation combined with Lkb1 loss) tumors were demonstrated to bear increased LDs relative to KP (Kras-sustained activation combined with Trp53 loss) tumors, suggesting that LKB1 deficiency results in lipid accumulation [37]. Furthermore, accumulated LDs are an important energy source for KL tumors in response to nutrient deprivation [37]. Autophagy mediates intracellular recycling, which supports mitochondrial activity [37]. Without autophagy, tumor cells heavily depend on fatty acid oxidation (FAO) for energy homeostasis, which depletes lipid storage and may result in cellular energy crisis [37].
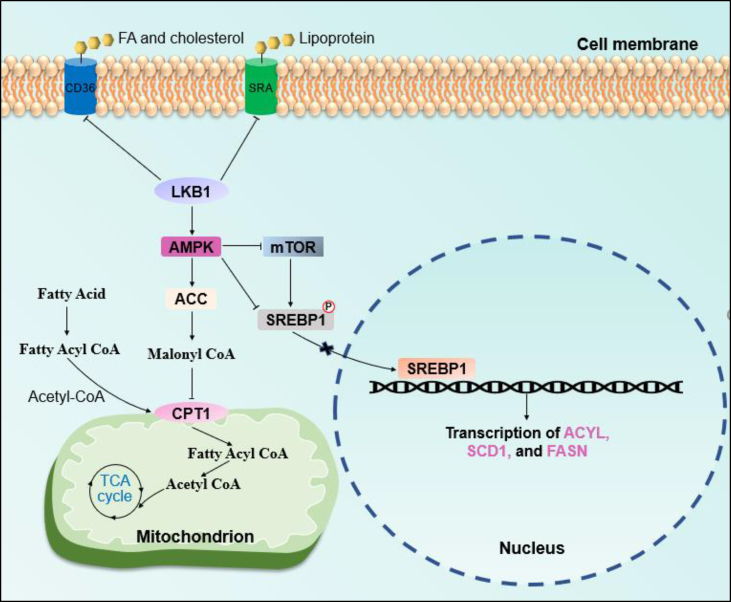
Two signals mediate the accumulation of lipids in LKB1-deficient cells (Figure 2). First, acetyl-coenzyme A carboxylase (ACC) catalyzes the carboxylation of acetyl-CoA to form malonyl-CoA, which is the basic substrate of FA synthesis under FASN catalysis [68]. Malonyl-CoA also inhibits FAO by blocking carnitine palmitoyltransferase 1 (CPT-1), which is responsible for transporting FA to the mitochondria [6]. ACC is a key downstream target of the LKB1-AMPK signal mediating the lipid metabolic regulation of LKB1. The activation of LKB1-AMPK phosphorylates ACC and inhibits its function [69]. It has been reported that ACC1 activity is necessary for the growth and survival of several types of cancers [6,70,71]. ND-646, an allosteric inhibitor of ACC enzymes, mimics the physiological regulation of ACC function via AMPK, markedly suppressing lung tumor growth via the inhibition of fatty acid synthesis and the depletion of cellular fatty acids [6]. Another liver-specific ACC inhibitor, ND-645, also inhibits hepatic de novo FA synthesis and suppresses the development of hepatocellular carcinoma (HCC) [70]. Since LKB1 deficiency removes the inhibition of ACC, targeting ACC in LKB1-proficient cancer cells may produce beneficial clinical outcomes. Consistent with this hypothesis, HeLa cells with LKB1 re-expression were found to display an increased sensitivity to ACC knockdown [72], and ND-646 was reported to have a stronger effect on the suppression of KP tumors than that in KL tumors [6]. If this hypothesis is warranted, it will benefit precision medicine and provide a basis for using ACC inhibitors in cancer therapy based on LKB1 status.
Figure 2.
LKB1 in lipid metabolic reprogramming. LKB1 regulates lipid metabolism in its uptake, de novo synthesis, and fatty acid β-oxidation.
Sterol regulatory element-binding protein 1 (SREBP1) is another master regulator of the FA metabolism located downstream of the LKB1-AMPK signal. SREBP1 is a membrane-bound basic helix-loop-helix leucine zipper (bHLH-Zip) transcription factor. It regulates the synthesis of FA, triglycerides, and cholesterol by controlling the transcription of ATP citrate lyase (ACYL), ACC, stearoyl-CoA desaturase 1 (SCD1), and FASN [73,74]. SREBP1 has two subtypes: SREBP1a is responsible for FA and cholesterol synthesis, whereas SREBP1c is responsible for FA synthesis [75]. The activation of AMPK directly phosphorylates SREBP1c at Ser372 in hepatocytes, inhibiting SREBP1c cleavage and nuclear translocation, preventing its transcriptional activity [76]. In addition, mTOR mediates the regulation of AMPK for SREBP1 [75,77]. Several recent studies demonstrated the significance of the regulation of lipid metabolism by SREBP1 during the progression of cancers. Sorafenib, a multi-kinase inhibitor, was found to induce apoptosis in liver cancer cells by blocking SCD1-mediated FA synthesis via the ATP-AMPK-mTOR-SREBP1 signaling pathway [77]. HCC-associated protein TD26 promotes HCC cell proliferation and tumor growth by disrupting the AMPK-mediated suppression of SREBP1 activity via its interaction with truncated SREBP1 [78]. However, few studies have directly demonstrated that excessive SREBP1 activity is responsible for facilitating tumor progression under LKB1 deficiency conditions. Despite this, we believe that targeting SREBP1 has the potential to result in a satisfactory effect on LKB1-deficient cancers.
4.2. LKB1 deficiency-induced lipid metabolic disorder accelerates disease progress in the liver and kidneys
The liver is one of the most important metabolic organs. Lipid accumulation in the liver is a characteristic of several hepatopathies. As such, activation of LKB1-AMPK signaling in the liver could represent an effective method of alleviating those hepatic diseases. Indeed, the activation of AMPK via multiple methods, including metformin, sirtuin-1 (SIRT1)-dependent resveratrol, γ-mangostin, chalcones, and honokiol, contributes to reducing lipid accumulation induced by high concentrations of glucose or a high-fat diet and is the primary therapeutic target for accelerated atherosclerosis and dyslipidemia in diabetes, age-related diseases, and non-alcoholic fatty liver disease (NAFLD) [[79], [80], [81], [82], [83]]. Based on the key role of LKB1-AMPK signaling in lipid metabolism in the liver, multiple induction factors may facilitate hepatopathy development by disrupting LKB1-AMPK signaling. For example, microRNA-122 (mir-122) and elastin-derived peptides (EDPs) induce hepatic lipogenesis, inflammation, and fibrosis by inhibiting the LKB1-AMPK pathway [84,85]. The significance of the LKB1-AMPK pathway in hepatopathy has been well documented, and many novel agents that ameliorate NAFLD via the regulation of lipid metabolism through the LKB1-AMPK pathway have recently been reported. Because lipid accumulation is also an important hallmark of cancer, we hypothesized that combining first-line anti-tumor drugs with agents that inhibit lipid accumulation may produce an enhanced therapeutic effect in LKB1-proficient cancers. Several potential agents that have been demonstrated to improve lipid metabolism are listed in Table 1.
Table 1.
Agents targeting lipid metabolism via the LKB1-AMPK signaling pathway.
| Agents | Effects | Pharmacological action |
|---|---|---|
| γ/α-mangostin [83] | Inhibiting lipid synthesis and enhancing FAO in HepG2 and Lo2 cells | An inhibitor of mutant IDH1 (IDH1-R132H); gamma-mangostin is a novel competitive 5-hydroxytryptamine 2A (5-HT2A) receptor antagonist |
| VOdipic-Cl [88] | Reducing lipid accumulation by inducing autophagy in vivo and in vitro | Induce autophagy to attenuate lipid accumulation via activating LKB1-AMPK |
| Honokiol [81] | Decreasing hepatic TG, lipogenic protein levels, and fat accumulation in mice fed high-fat diets | A bioactive, biphenolic phytochemical inhibiting Akt activation and enhancing ERK1/ERK2 phosphorylation |
| Chalcones (4HD, XAG, CAR, and FKB) | Attenuating lipid accumulation in HepG2 cells | Anti-inflammatory, anti-oxidative, anti-bacterial, anti-cancer, and anti-parasitic activities via inhibiting TNFα-induced NF-κB activation and activating BMP signaling |
| Gentiopicroside [89] | Reducing lipogenesis and promoting FAO in HepG2 cells | A naturally occurring iridoid glycoside that inhibits cytochrome P450 activity |
| Celastrol [90] | Ameliorating NAFLD by decreasing lipid synthesis and improving the anti-oxidative and anti-inflammatory status | A proteasome inhibitor |
| Bouchardatine analog (R17) [91] | Reversing high-fat diet (HFD)-induced hepatic triglyceride content, inflammation, injury, and fibrogenesis | A derivative of bouchardatine (an alkaloid from Bouchardatia neurococca) that activates AMPK by inhibiting ATP synthase activity |
| Phillyrin [92] | Preventing high-glucose-induced lipid accumulation in HepG2 cells | Isolated from Forsythia suspensa Vahl (Oleaceae), it has potentially inductive effects on rat cytochrome P450 (CYP) 1A2 and CYP2D1 activities |
| JD5037 [93] | Improving glycemic control and increasing FAO in HepG2 cells and HFD mice | A cannabinoid receptor type 1 (CB1R) antagonist |
| EGCG-rich GTE [94] | Decreasing body gain, preventing hepatic fat accumulation, reducing hypertriglyceridemia, and improving hyperglycemia and insulin resistance in HFD mice | Upregulates sirtuin 1 and AMPK and downregulates enzymes related to de novo lipogenesis |
| Anhydroicaritin [95] | Ameliorating obesity, insulin resistance, fatty accumulation in the liver, and hyperlipemia in diet-induced obese mice | A prenylflavonoid that regulates MAPK/ERK/JNK and JAK2/STAT3/AKT signals |
| GomisinJ [96] | Inhibiting lipid accumulation in oleic acid (OA)-induced HepG2 cells | Inhibits fetuin-A and activates AMPK |
| Cordycepin [97] | Reducing OA-induced lipid accumulation by activating AMPK in HepG2 cells | A nucleoside derivative isolated from Cordyceps and a potent inhibitor of IL-1β-induced chemokine production |
| CDCQ [98] | Suppressing high-glucose-induced lipid accumulation in HepG2 cells | A chlorogenic acid derivative that blocks the expression of SREBP-1 and FAS via activating LKB1/SIRT1 and AMPK |
Abbreviations: CDCQ: 3-caffeoyl, 4-dihydrocaffeoylquinic acid; EGCG-rich GTE: epigallocatechin gallate-rich green tea extract.
Chronic kidney disease (CKD) is a major chronic illness worldwide, of which tubulointerstitial fibrosis (TIF) is the most consistent pathological characteristic [86]. Several recent studies have shown that a defective energy metabolism, in particular when impaired by FAO, plays a key role in the pathogenesis of renal fibrosis [20,87]. Based on LKB1's regulatory role in lipid metabolism, LKB1 deficiency may participate in the development of CKD. Indeed, deletion of Lkb1 in renal tubular epithelial cells (TECs) was found to result in CKD and enhanced fibrosis [20]. LKB1 deficiency in TECs leads to increased lipid accumulation due to decreased FAO levels resulting from the decreased expression of rate-limiting enzymes in the β-oxidation pathway, such as Cpt1/2 and acyl-CoA oxidase (ACOX1) [20]. Although AMPK and peroxisome proliferator activated-receptor (PPAR)-α agonists attenuate LKB1 deficiency-induced fibrosis, the mechanism by which lipid accumulation causes epithelial differentiation remains unclear and requires further study. Table 1 provides a list of agents that target lipid metabolism via the LKB1-AMPK signaling pathway. These have been confirmed to inhibit lipid accumulation and may also have therapeutic potential for CKD.
4.3. LKB1 sustains immune cell functions via lipid metabolic regulation
Studies are increasingly focusing on LKB1's effect on immune cell function. Dendritic cells (DCs) play a crucial role in mediating protective immune responses against pathogens and tumors. DCs modulate peripheral tolerance by promoting the proliferation and differentiation of regulatory T cells (Tregs), which participate in immune tolerance and suppression [99]. It has been reported that LKB1 deficiency in DCs facilitates the proliferation of peripheral Tregs [21,100]. Mechanistically, LKB1 deficiency in DCs relieves suppression of the mTOR signal, which results in dysregulated metabolism leading to aberrant maturation of DCs from a quiescent state [21]. Dysregulated metabolism in LKB1-deficient DCs is characterized by higher OCR, ECAR, upregulated hallmarks of the cholesterol homeostasis pathway, and increased lipid accumulation relative to LKB1-proficient DCs [21]. Although researchers have not yet determined how aberrant lipid metabolism including the cholesterol pathway and accumulation of lipids in LKB1-deficient DCs contributes to the maturation of DCs, genes involved in cholesterol homeostasis have been found to significantly alter LKB1-deficient DCs according to gene-set enrichment analysis (GSEA) and proteome profiling results. These findings indicate a vital role of lipid metabolism in the activity of DCs controlled by LKB1. Researches have also emphasized the key role of mTOR in the mediation of LKB1-deficiency-induced DC maturation. The role of mTOR signal in lipid metabolism [101,102] suggests that aberrant alterations of lipid metabolism may partially mediate the function of mTOR in the induction of DC maturation under LKB1 deficiency.
LKB1 also participates in programming the metabolism and function of Tregs. LKB1 coordinates the balance between immunity and tolerance by regulating the expression of immune regulatory molecules, including program cell death 1 (PD1) and the TNF receptor superfamily members GITR and OX40 [23]. Metabolite set enrichment analysis of Tregs revealed that LKB1 deficiency induced the downregulation of multiple metabolic programs and enhanced biosynthesis of unsaturated fatty acids as evidenced by a notable lipid accumulation in LKB1-deficient Tregs [23]. Despite this, the mechanism by which excessive lipid accumulation participates in the regulation of Tregs remains unclear [23]. Recent studies investigated the lipid metabolic regulation of LKB1 in Tregs’ functions and found that LKB1 deficiency increased cholesterol levels via the upregulation of CD36, which is responsible for the uptake of cholesterol from the extracellular environment and the downregulation of ATP-binding cassette transporter types A1 and G1 (Abca1/Abcg1) responsible for the efflux of cholesterol [103]. As a result, Tregs lose their suppressive activity and differentiate into inflammatory cells expressing Th1 and Th17 cytokines due to impairment of the mevalonate pathway resulting from the suppression of lipid metabolic gene expression [103]. Of note, LKB1-mediated regulation of Tregs was independent of AMPK [23,103,104], in contrast to the lipid metabolic regulation of LKB1-AMPK signaling in multiple cancer cells. This provides evidence for a novel therapeutic strategy for cancer treatment by targeting LKB1-AMPK signaling to inhibit lipid accumulation in cancers. Combined with agents targeting the mevalonate pathway, this treatment could help enhance anti-tumor immunity.
The enrichment of foam cells is a hallmark of atherosclerosis and known to promote lesion expansion. Thus, the transformation of macrophages into foam cells is a key process in the development of atherosclerosis [105]. Macrophages uptake lipoproteins during this process, and the uncontrolled accumulation of lipoproteins results in the transformation of macrophages into lipid-rich foam cells [106]. It was previously reported that the expression of LKB1 is decreased in macrophages during the development of atherosclerosis, promoting lipoprotein uptake and foam cell formation. Scavenger receptor A (SRA) is responsible for the uptake of modified lipoproteins. In this context, LKB1 functions as a negative regulator of the differentiation of macrophages and the formation of foam cells by directly phosphorylating SRA and facilitating its degradation, decreasing the accumulation of lipids in macrophages [22].
In summary, the signaling and metabolic pathways controlled by LKB1 play a vital role in the function, activity, proliferation, and differentiation of several immune cells. Thus, the positive and negative regulation of LKB1 could represent potential therapeutic strategies for many diseases, including anti-tumor immunity, autoimmune disease, and transplant rejection, among others. In addition, disorders of lipid metabolism affect the function of immune cells to a large extent as previously mentioned. Furthermore, several LKB1-independent pathways have been found to induce aberrant lipid metabolism in natural killer (NK) cells [107], CD4+ T cells [108], and tissue-resident memory T (TRM) cells [109], emphasizing the important role of lipid metabolism in immune cells. As such, targeting lipid metabolism may also be an effective method of regulating the function and activity of multiple immune cells.
5. Loss of LKB1 links to serine metabolism
The serine, glycine, and one-carbon (SGOC) metabolic network not only supports NADPH and one-carbon units for nucleotide synthesis, but also participates in the methionine cycle, generating S-adenosylmethionine (SAM), which is a universal methyl donor [110]. Kottakis et al. reported that LKB1 loss combined with KRAS mutations induced the activation of the SGOC network, increasing SAM generation and promoting DNA methylation at retrotransposon elements, which is associated with transcriptional silencing [7]. This study also found that LKB1 restricts serine metabolism by downregulating the expression of multiple serine pathway enzymes, including phosphoserine aminotransferase 1 (PSAT1), phosphoserine phosphatase (PSPH), serine hydroxymethyltransferase 1 (SHMT1), and SHMT2, in an AMPK-mTOR-dependent manner [7]. Consistently high levels of PSAT1, PSPH, and SHMT expression correlate with the clinical stage, proliferation, metastasis, and poor prognosis of multiple types of cancers [[111], [112], [113]]. Phosphoglycerate dehydrogenase (PHGDH) catalyzes the first and rate-limiting step of glucose-derived serine synthesis. Although PHGDH is not regulated by LKB1 based on current observations, its high expression significantly correlates with lymph node metastasis and poor prognosis in NSCLC [114]. Targeting PHGDH with small molecule inhibitor (NCT-502) in MDA-MB-468 (a breast cancer cell line with high PHGDH expression) xenografts reduces tumor volume [115]. These findings highlight serine metabolism's important role in tumorigenesis and tumor progression. Hence, targeting LKB1 signaling or the SGOC pathway with NCT-502, methotrexate, or pemetrexed that inhibits tetrahydrofolate synthesis is a potential therapeutic strategy for treating cancers, especially in LKB1-deficient tumors. However, the mechanism by which the LKB1-AMPK-mTOR signaling pathway regulates the expression of serine pathway enzymes remains unclear. As such, further study is required to establish a theoretical basis for targeting this pathway.
6. The double-edged sword behavior of LKB1
LKB1 plays multifaceted roles in metabolism to maintain functions and survival of multiple types of cells. The vital role of LKB1 in metabolism also determines that LKB1 may function oppositely under certain conditions. For example, inhibitory or stimulative effects of LKB1 on cancer cells may mainly depend on two critical factors: the types of cells and external stimuli.
LKB1 is critical for maintaining metabolic homeostasis in hematopoietic stem cells (HSC) [14,15]. LKB1 loss in bone marrow cells results in mitochondrial defects, maladjustment of lipid and nucleotide metabolism, and decreased cellular ATP levels [14]. LKB1-deficient adult mice displayed exhaustion of the HSC pool. LKB1 is also involved in the regulation of stem cell fates, such as the induced pluripotent stem (iPS) cell-derived mesenchymal stem cells, neural crest cells, and intestinal stem cells [[116], [117], [118]]. It is believed that LKB1 plays an essential role in stem cell homeostasis. However, in cancer stem cells (CSCs), LKB1 may help sustain the survival of cluster cells. In pancreatic ductal adenocarcinoma (PDAC), LKB1 co-expresses and co-locates with CD133 in malignant areas and sustains CD133+/CD44+ CSC populations [119]. In another report, researchers identified that LKB1 mediated CD44 expression and maintained the stemness properties of CSCs in PDAC [120]. In addition, LKB1 is necessary for circulating tumor cells (CTCs) to survive from anoikis during early phases of intravasation [121]. These findings suggest that LKB1 functions as a pro-oncogene in specific types of cells, such as cells with stemness, highlighting the cell-type dependence of LKB1 functions. Thus, the pro-oncogenic activity of LKB1 should be considered when targeting LKB1 for cancer therapy.
External stimuli are another key factor determining LKB1's role as a pro-oncogenic or cancerocidal facilitator. LKB1 promotes adaptive metabolic reprogramming in response to energy stress in NSCLC cells [41]. LKB1-deficient tumor cells are hypersensitive to energy stress-induced apoptosis via activating AMPK [122]. It was reported that AMPK promotes tumor cell survival via regulating NADPH homeostasis and activating mTORC2 during energetic stress [72,123]. Energy stress is a commonly restricted condition when LKB1-AMPK signals function as pro-oncogenic factors. Indeed, LKB1 confers metabolic flexibility to tumor cells [41]. As a vital metabolic regulator, LKB1 promotes cell survival during energy stress to avoid energy shortage-induced apoptosis. As a tumor suppressor, LKB1 inhibits proliferation of cancer cells. Altogether, we deem that LKB1 deficiency leads cancer cells to metabolic disorder, whereas intact LKB1 enhances the survival of cancer cells responding to energy stresses.
7. Conclusions and perspectives
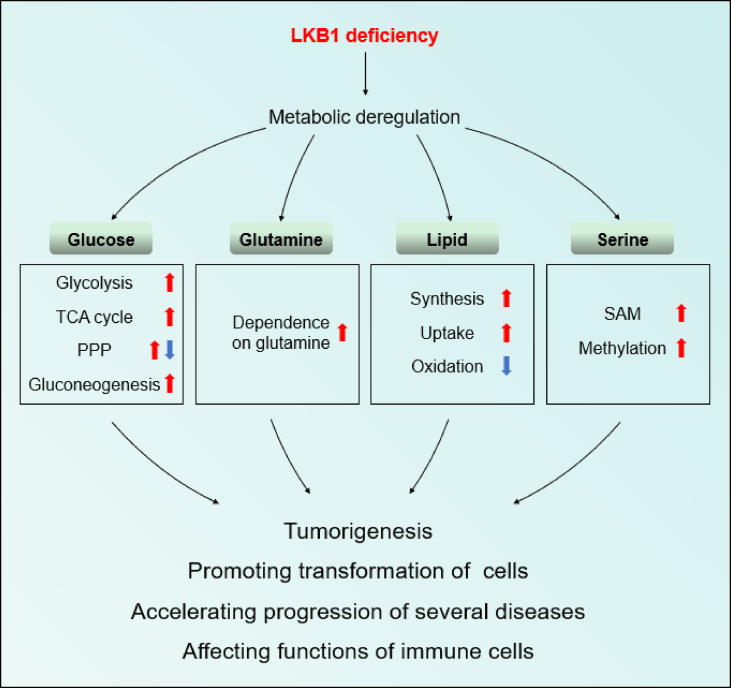
LKB1 plays important roles in multiple metabolic pathways, including glucose, glutamine, lipid, and serine metabolism. Disordered metabolisms caused by LKB1 deficiency affect the progression of multiple diseases and the functions of several types of cells (Figure 3). LKB1 is responsible for coordinating an extensive metabolic network. Thus, metabolic reprogramming induced by LKB1 loss confers cells with growth or survival advantages and acclimatizes cells to microenvironments, such as via the transformation of OXPHOS into glycolysis in hypoxic microenvironments [28], elevated glutamine utilization to eliminate ROS [5], and increased lipid storage to cope with energy crises [37]. Thus, the microenvironment impacts the kind of metabolic pathways that LKB1-deficient cells reprogram, which is a vital factor that should be used as a guide when developing approaches for targeted therapy.
Figure 3.
Metabolic reprogramming induced by LKB1 deficiency promotes disease progress. LKB1 deficiency induces the metabolic reprogramming of glucose, glutamine, lipid, and serine, which promotes cellular transformation, tumorigenesis, and progression of diseases and affects functions of DCs, Tregs, and macrophages.
LKB1 deficiency in cancer cells with oncogenic activation, such as Kras mutation and HPV infection, has been proven to facilitate tumorigenesis, cancer aggression, and poor prognoses [7,8]. However, LKB1 loss alone does not induce tumorigenesis [19,20]. We believe that LKB1 deficiency represents the second echelon in the tumorigenesis process, although metabolic dysregulation caused by LKB1 deficiency plays a leading role in the promotion of tumor development. LKB1 deficiency induces metabolism disorders, which provide cancer cells with the energy and substances needed for their growth, proliferation, and survival. However, as observed in LKB1-deficient tumors, LKB1 loss also confers cancer cells frangibility to further metabolic stresses, providing a basis for the development of novel therapeutic strategies for the treatment of cancer.
Studies are increasingly focusing on investigating the effects of abnormal metabolism on immune cell function and cellular transformation. The alteration of lipid metabolism, especially in the cholesterol homeostasis pathway, plays a vital role in the function of DCs and Tregs [21,103]. The key roles of DCs and Tregs in anti-tumor immunity, autoimmune diseases, and immunological rejection indicate that targeting LKB1 signals or the cholesterol pathway may provide a curative option for these diseases. Lipid deposition induced by LKB1 deficiency promotes phenotypic transformations in macrophages and TECs [20,22], inhibits the uptake and de novo synthesis of lipids, and accelerates FAO via LKB1-dependent or -independent pathways, which are effective methods of attenuating the transformation. Our review on the metabolic regulation of LKB1 provides a basis for identifying therapeutic targets and developing drug combination programs for treating cancers, metabolic diseases, and immunoregulation in future studies.
Funding
This study in the authors’ laboratory was supported by grants from the National Natural Science Foundation of China (Nos. 81970648, 81772921, 81772924, and 82020108024) and International Cooperation Project of the Department of Science and Technology of Jilin Province (Nos. 20190701049GH and 20190701006GH).
Conflict of interest
None declared.
Contributor Information
Zhi-Xiang Xu, Email: zhixiangxu@jlu.edu.cn.
Honglan Zhou, Email: walkerzhouhl@163.com.
Yishu Wang, Email: wangys@jlu.edu.cn.
References
- 1.Song L., Guo J., Chang R., Peng X., Li J., Xu X. LKB1 obliterates Snail stability and inhibits pancreatic cancer metastasis in response to metformin treatment. Cancer Science. 2018;109(5):1382–1392. doi: 10.1111/cas.13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korsse S.E., Peppelenbosch M.P., van Veelen W. Targeting LKB1 signaling in cancer. Biochimica et Biophysica Acta. 2013;1835(2):194–210. doi: 10.1016/j.bbcan.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Ollila S., Domènech-Moreno E., Laajanen K., Wong I.P., Tripathi S., Pentinmikko N. Stromal Lkb1 deficiency leads to gastrointestinal tumorigenesis involving the IL-11-JAK/STAT3 pathway. Journal of Clinical Investigation. 2018;128(1):402–414. doi: 10.1172/JCI93597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollstein P.E., Eichner L.J., Brun S.N., Kamireddy A., Svensson R.U., Vera L.I. The AMPK-related kinases SIK1 and SIK3 mediate key tumor-suppressive effects of LKB1 in NSCLC. Cancer Discovery. 2019;9(11):1606–1627. doi: 10.1158/2159-8290.CD-18-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galan-Cobo A., Sitthideatphaiboon P., Qu X., Poteete A., Pisegna M.A., Tong P. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Research. 2019;79(13):3251–3267. doi: 10.1158/0008-5472.CAN-18-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson R.U., Parker S.J., Eichner L.J., Kolar M.J., Wallace M., Brun S.N. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22(10):1108–1119. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kottakis F., Nicolay B.N., Roumane A., Karnik R., Gu H., Nagle J.M. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016;539(7629):390–395. doi: 10.1038/nature20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Q., Chen J., Li Y., Werle K.D., Zhao R.X., Quan C.S. LKB1 inhibits HPV-associated cancer progression by targeting cellular metabolism. Oncogene. 2017;36(9):1245–1255. doi: 10.1038/onc.2016.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su K.H., Dai S., Tang Z., Xu M., Dai C. Heat shock factor 1 is a direct antagonist of AMP-activated protein kinase. Molecular Cell. 2019;76(4):546–561. doi: 10.1016/j.molcel.2019.08.021. e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olvedy M., Tisserand J.C., Luciani F., Boeckx B., Wouters J., Lopez S. Comparative oncogenomics identifies tyrosine kinase FES as a tumor suppressor in melanoma. Journal of Clinical Investigation. 2017;127(6):2310–2325. doi: 10.1172/JCI91291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji H., Ramsey M.R., Hayes D.N., Fan C., McNamara K., Kozlowski P. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S., Iwakawa R., Takahashi K., Kohno T., Nakanishi Y., Matsuno Y. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene. 2007;26(40):5911–5918. doi: 10.1038/sj.onc.1210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onozato R., Kosaka T., Achiwa H., Kuwano H., Takahashi T., Yatabe Y. LKB1 gene mutations in Japanese lung cancer patients. Cancer Science. 2007;98(11):1747–1751. doi: 10.1111/j.1349-7006.2007.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurumurthy S., Xie S.Z., Alagesan B., Kim J., Yusuf R.Z., Saez B. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 2010;468(7324):659–663. doi: 10.1038/nature09572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan B., Hu J., Jiang S., Liu Y., Sahin E., Zhuang L. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468(7324):701–704. doi: 10.1038/nature09595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakada D., Saunders T.L., Morrison S.J. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468(7324):653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beirowski B., Babetto E., Golden J.P., Chen Y.J., Yang K., Gross R.W. Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nature Neuroscience. 2014;17(10):1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan T., Xu Z., Liu J., Wu W., Wang Y. Lkb1 regulation of skeletal muscle development, metabolism and muscle progenitor cell homeostasis. Journal of Cellular Physiology. 2017;232(10):2653–2656. doi: 10.1002/jcp.25786. [DOI] [PubMed] [Google Scholar]
- 19.Flowers E.M., Sudderth J., Zacharias L., Mernaugh G., Zent R., DeBerardinis R.J. Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nature Communications. 2018;9(1):814. doi: 10.1038/s41467-018-03036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S.H., Malaga-Dieguez L., Chinga F., Kang H.M., Tao J., Reidy K. Deletion of Lkb1 in renal tubular epithelial cells leads to CKD by altering metabolism. Journal of the American Society of Nephrology. 2016;27(2):439–453. doi: 10.1681/ASN.2014121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Du X., Wei J., Long L., Tan H., Guy C. LKB1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity. Cell Research. 2019;29(5):391–405. doi: 10.1038/s41422-019-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z., Zhu H., Dai X., Wang C., Ding Y., Song P. Macrophage liver kinase B1 inhibits foam cell formation and atherosclerosis. Circulation Research. 2017;121(9):1047–1057. doi: 10.1161/CIRCRESAHA.117.311546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K., Blanco D.B., Neale G., Vogel P., Avila J., Clish C.B. Homeostatic control of metabolic and functional fitness of Treg cells by LKB1 signalling. Nature. 2017;548(7669):602–606. doi: 10.1038/nature23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lizcano J.M., Goransson O., Toth R., Deak M., Morrice N.A., Boudeau J. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. The EMBO Journal. 2004;23(4):833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaleel M., McBride A., Lizcano J.M., Deak M., Toth R., Morrice N.A. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Letters. 2005;579(6):1417–1423. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Cantó C., Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cellular and Molecular Life Sciences : CMLS. 2010;67(20):3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L., Yao Y., Zhao J., Cao J., Ma H. Dehydroepiandrosterone protects against hepatic glycolipid metabolic disorder and insulin resistance induced by high fat via activation of AMPK-PGC-1α-NRF-1 and IRS1-AKT-GLUT2 signaling pathways. International Journal of Obesity. 2020;(2005) doi: 10.1038/s41366-019-0508-8. [DOI] [PubMed] [Google Scholar]
- 28.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faubert B., Vincent E.E., Griss T., Samborska B., Izreig S., Svenssonc R.U. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. PNAS. 2014;111(7):2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faubert B., Boily G., Izreig S., Griss T., Samborska B., Dong Z. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metabolism. 2013;17(1):113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang S., Wang Y., Luo L., Shi F., Zou J., Lin H. AMP-activated protein kinase regulates cancer cell growth and metabolism via nuclear and mitochondria events. Journal of Cellular and Molecular Medicine. 2019;23(6):3951–3961. doi: 10.1111/jcmm.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doménech E., Maestre C., Esteban-Martínez L., Partida D., Pascual R., Fernández-Miranda G. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nature Cell Biology. 2015;17(10):1304–1316. doi: 10.1038/ncb3231. [DOI] [PubMed] [Google Scholar]
- 33.Bando H., Atsumi T., Nishio T., Niwa H., Mishima S., Shimizu C. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2005;11(16):5784–5792. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C.-S., Hawley S.A., Zong Y., Li M., Wang Z., Gray A. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548(7665):112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y.-A.A., Chen Y., Dao D.Q., Mayoral S.R., Wu L., Meijer D. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nature Communications. 2014;5:4991. doi: 10.1038/ncomms5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakasam G., Singh R.K., Iqbal M.A., Saini S.K., Tiku A.B., Bamezai R.N.K. Pyruvate kinase M knockdown-induced signaling via AMP-activated protein kinase promotes mitochondrial biogenesis, autophagy, and cancer cell survival. Journal of Biological Chemistry. 2017;292(37):15561–15576. doi: 10.1074/jbc.M117.791343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt V., Khayati K., Hu Z.S., Lee A., Kamran W., Su X. Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesis. Genes & Development. 2019;33(3–4):150–165. doi: 10.1101/gad.320481.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woldt E., Sebti Y., Solt L.A., Duhem C., Lancel S., Eeckhoute J. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19(8):1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray Hamidie R.D., Yamada T., Ishizawa R., Saito Y., Masuda K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism - Clinical and Experimental. 2015;64(10):1334–1347. doi: 10.1016/j.metabol.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Li W., Wong C.C., Zhang X., Kang W., Nakatsu G., Zhao Q. CAB39L elicited an anti-Warburg effect via a LKB1-AMPK-PGC1α axis to inhibit gastric tumorigenesis. Oncogene. 2018;37(50):6383–6398. doi: 10.1038/s41388-018-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker S.J., Svensson R.U., Divakaruni A.S., Lefebvre A.E., Murphy A.N., Shaw R.J. LKB1 promotes metabolic flexibility in response to energy stress. Metabolic Engineering. 2017;43(Pt B):208–217. doi: 10.1016/j.ymben.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shackelford D.B., Abt E., Gerken L., Vasquez D.S., Seki A., Leblanc M. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23(2):143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Momcilovic M., McMickle R., Abt E., Seki A., Simko S.A., Magyar C. Heightening energetic stress selectively targets LKB1-deficient non-small cell lung cancers. Cancer Research. 2015;75(22):4910–4922. doi: 10.1158/0008-5472.CAN-15-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends in Biochemical Sciences. 2014;39(8):347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biological Reviews of the Cambridge Philosophical Society. 2015;90(3):927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju H.Q., Lu Y.X., Wu Q.N., Liu J., Zeng Z.L., Mo H.Y. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene. 2017;36(45):6282–6292. doi: 10.1038/onc.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin R., Elf S., Shan C., Kang H.B., Ji Q., Zhou L. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nature Cell Biology. 2015;17(11):1484–1496. doi: 10.1038/ncb3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan C., Lu Z., Li Z., Sheng H., Fan J., Qi Q. 4-hydroxyphenylpyruvate dioxygenase promotes lung cancer growth via pentose phosphate pathway (PPP) flux mediated by LKB1-AMPK/HDAC10/G6PD axis. Cell Death & Disease. 2019;10(7):525. doi: 10.1038/s41419-019-1756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L., He Z., Yao J., Tan R., Zhu Y., Li Z. Regulation of AMPK-related glycolipid metabolism imbalances redox homeostasis and inhibits anchorage independent growth in human breast cancer cells. Redox Biology. 2018;17:180–191. doi: 10.1016/j.redox.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohan A.B., Talukdar I., Walsh C.M., Salati L.M. A role for AMPK in the inhibition of glucose-6-phosphate dehydrogenase by polyunsaturated fatty acids. Biochemical and Biophysical Research Communications. 2009;388(1):117–121. doi: 10.1016/j.bbrc.2009.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw R.J., Lamia K.A., Vasquez D., Koo S.-H., Bardeesy N., Depinho R.A. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science (New York, N.Y.) 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. Journal of Clinical Investigation. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo S.-H., Flechner L., Qi L., Zhang X., Screaton R.A., Jeffries S. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437(7062):1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 54.Sakamoto K., Bultot L., Goransson O. The salt-inducible kinases: emerging metabolic regulators. Trends in Endocrinology and Metabolism. 2018;29(12):827–840. doi: 10.1016/j.tem.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Patel K., Foretz M., Marion A., Campbell D.G., Gourlay R., Boudaba N. The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver. Nature Communications. 2014;5:4535. doi: 10.1038/ncomms5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray C.W., Brady J.J., Tsai M.K., Li C., Winters I.P., Tang R. An LKB1-SIK Axis suppresses lung tumor growth and controls differentiation. Cancer Discovery. 2019;9(11):1590–1605. doi: 10.1158/2159-8290.CD-18-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bon H., Wadhwa K., Schreiner A., Osborne M., Carroll T., Ramos-Montoya A. Salt-inducible kinase 2 regulates mitotic progression and transcription in prostate cancer. Molecular Cancer Research : MCR. 2015;13(4):620–635. doi: 10.1158/1541-7786.MCR-13-0182-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bian X.-L., Chen H.-Z., Yang P.-B., Li Y.-P., Zhang F.-N., Zhang J.-Y. Nur77 suppresses hepatocellular carcinoma via switching glucose metabolism toward gluconeogenesis through attenuating phosphoenolpyruvate carboxykinase sumoylation. Nature Communications. 2017;8:14420. doi: 10.1038/ncomms14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends in Biochemical Sciences. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griss T., Vincent E.E., Egnatchik R., Chen J., Ma E.H., Faubert B. Metformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesis. PLoS Biology. 2015;13(12) doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10(11) doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Zheng B., Jeong J.H., Asara J.M., Yuan Y.-Y., Granter S.R., Chin L. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Molecular Cell. 2009;33(2):237–247. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng C., Geng F., Cheng X., Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Communications (London, England) 2018;38(1):27. doi: 10.1186/s40880-018-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Q., Luo Q., Halim A., Song G. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer Letters. 2017;401:39–45. doi: 10.1016/j.canlet.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C.S.-O. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 67.Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Reviews. Cancer. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 68.Currie E., Schulze A., Zechner R., Walther T.C., Farese R.V. Cellular fatty acid metabolism and cancer. Cell Metabolism. 2013;18(2):153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinberg G.R., Kemp B.E. AMPK in health and disease. Physiological Reviews. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 70.Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metabolism. 2019;29(1) doi: 10.1016/j.cmet.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tcga R.N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeon S.-M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goldstein J.L., DeBose-Boyd R.A., Brown M.S. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 74.Galbraith L., Leung H.Y., Ahmad I. Lipid pathway deregulation in advanced prostate cancer. Pharmacological Research. 2018;131:177–184. doi: 10.1016/j.phrs.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 75.Shao W., Espenshade P.J. Expanding roles for SREBP in metabolism. Cell Metabolism. 2012;16(4):414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabolism. 2011;13(4):376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G., Kuang S., Cao R., Wang J., Peng Q., Sun C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids the ATP-AMPK-mTOR-SREBP1 signaling pathway. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2019;33(9):10089–10103. doi: 10.1096/fj.201802619RR. [DOI] [PubMed] [Google Scholar]
- 78.Wang C., Tong Y., Wen Y., Cai J., Guo H., Huang L. Hepatocellular carcinoma-associated protein TD26 interacts and enhances sterol regulatory element-binding protein 1 activity to promote tumor cell proliferation and growth. Hepatology (Baltimore, Md.) 2018;68(5):1833–1850. doi: 10.1002/hep.30030. [DOI] [PubMed] [Google Scholar]
- 79.Zang M., Zuccollo A., Hou X., Nagata D., Walsh K., Herscovitz H. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. Journal of Biological Chemistry. 2004;279(46):47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 80.Hou X., Xu S., Maitland-Toolan K.A., Sato K., Jiang B., Ido Y. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. Journal of Biological Chemistry. 2008;283(29):20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo M.S., Kim J.H., Kim H.J., Chang K.C., Park S.W. Honokiol activates the LKB1-AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicology and Applied Pharmacology. 2015;284(2):113–124. doi: 10.1016/j.taap.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 82.Zhang T., Yamamoto N., Ashida H. Chalcones suppress fatty acid-induced lipid accumulation through a LKB1/AMPK signaling pathway in HepG2 cells. Food & function. 2014;5(6):1134–1141. doi: 10.1039/c3fo60694e. [DOI] [PubMed] [Google Scholar]
- 83.Gu L., Cai N., Lyu Y., Yao L., Wang F., Xu H. γ-Mangostin ameliorates free fatty acid-induced lipid accumulation via the SIRT1/LKB1/AMPK pathway in HepG2 and L02 cells. Journal of Agricultural and Food Chemistry. 2019;67(50):13929–13938. doi: 10.1021/acs.jafc.9b05632. [DOI] [PubMed] [Google Scholar]
- 84.Long J.-K., Dai W., Zheng Y.-W., Zhao S.-P. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Molecular Medicine (Cambridge, Mass.) 2019;25(1):26. doi: 10.1186/s10020-019-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Romier B., Ivaldi C., Sartelet H., Heinz A., Schmelzer C.E.H., Garnotel R. Production of elastin-derived peptides contributes to the development of nonalcoholic steatohepatitis. Diabetes. 2018;67(8):1604–1615. doi: 10.2337/db17-0490. [DOI] [PubMed] [Google Scholar]
- 86.Mount P.F., Power D.A. Balancing the energy equation for healthy kidneys. The Journal of Pathology. 2015;237(4):407–410. doi: 10.1002/path.4600. [DOI] [PubMed] [Google Scholar]
- 87.Kang H.M., Ahn S.H., Choi P., Ko Y.-A., Han S.H., Chinga F. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature Medicine. 2015;21(1):37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y., Liu F., Zhang F., Liu P., Xu T., Ding W. Vanadium(IV)-chlorodipicolinate alleviates hepatic lipid accumulation by inducing autophagy via the LKB1/AMPK signaling pathway in vitro and in vivo. Journal of Inorganic Biochemistry. 2018;183:66–76. doi: 10.1016/j.jinorgbio.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Li X., Zhang Y., Jin Q., Xia K.-L., Jiang M., Cui B.-W. Liver kinase B1/AMP-activated protein kinase-mediated regulation by gentiopicroside ameliorates P2X7 receptor-dependent alcoholic hepatosteatosis. British Journal of Pharmacology. 2018;175(9):1451–1470. doi: 10.1111/bph.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Geng C., Liu X., Li M., Gao M., Liu X. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Molecular Metabolism. 2017;6(1):138–147. doi: 10.1016/j.molmet.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao Y., Lu Y.-T., Li C., Song Q.-Q., Xu Y.-H., Xu Z. Bouchardatine analogue alleviates non-alcoholic hepatic fatty liver disease/non-alcoholic steatohepatitis in high-fat fed mice by inhibiting ATP synthase activity. British Journal of Pharmacology. 2019;176(16):2877–2893. doi: 10.1111/bph.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Do M.T., Kim H.G., Choi J.H., Khanal T., Park B.H., Tran T.P. Phillyrin attenuates high glucose-induced lipid accumulation in human HepG2 hepatocytes through the activation of LKB1/AMP-activated protein kinase-dependent signalling. Food Chemistry. 2013;136(2):415–425. doi: 10.1016/j.foodchem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Liu J., Godlewski G., Jourdan T., Liu Z., Cinar R., Xiong K. Cannabinoid-1 receptor antagonism improves glycemic control and increases energy expenditure through sirtuin-1/mechanistic target of rapamycin complex 2 and 5'Adenosine monophosphate-activated protein kinase signaling. Hepatology (Baltimore, Md.) 2019;69(4):1535–1548. doi: 10.1002/hep.30364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bae U.-J., Park J., Park I.W., Chae B.M., Oh M.-R., Jung S.-J. Epigallocatechin-3-Gallate-Rich green tea extract ameliorates fatty liver and weight gain in mice fed a high fat diet by activating the sirtuin 1 and AMP activating protein kinase pathway. American Journal of Chinese Medicine. 2018;46(3):617–632. doi: 10.1142/S0192415X18500325. [DOI] [PubMed] [Google Scholar]
- 95.Zheng Z.-G., Zhou Y.-P., Zhang X., Thu P.M., Xie Z.-S., Lu C. Anhydroicaritin improves diet-induced obesity and hyperlipidemia and alleviates insulin resistance by suppressing SREBPs activation. Biochemical Pharmacology. 2016;122:42–61. doi: 10.1016/j.bcp.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 96.Kim M., Lim S.J., Lee H.-J., Kim S.Y., Nho C.W. Gomisin J inhibits oleic acid-induced hepatic lipogenesis by activation of the AMPK-dependent pathway and inhibition of the hepatokine fetuin-A in HepG2 cells. Journal of Agricultural and Food Chemistry. 2015;63(44):9729–9739. doi: 10.1021/acs.jafc.5b04089. [DOI] [PubMed] [Google Scholar]
- 97.Wu C., Guo Y., Su Y., Zhang X., Luan H., Zhang X. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit. Journal of Cellular and Molecular Medicine. 2014;18(2):293–304. doi: 10.1111/jcmm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pil Hwang Y., Gyun Kim H., Choi J.H., Truong Do M., Tran T.P., Chun H.K. 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea attenuates high glucose-induced hepatic lipogenesis in human HepG2 cells through activation of the liver kinase B1 and silent information regulator T1/AMPK-dependent pathway. Molecular Nutrition & Food Research. 2013;57(3):471–482. doi: 10.1002/mnfr.201200529. [DOI] [PubMed] [Google Scholar]
- 99.Audiger C., Rahman M.J., Yun T.J., Tarbell K.V., Lesage S. The importance of dendritic cells in maintaining immune tolerance. Journal of immunology (Baltimore, Md. : 1950) 2017;198(6):2223–2231. doi: 10.4049/jimmunol.1601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pelgrom L.R., Patente T.A., Sergushichev A., Esaulova E., Otto F., Ozir-Fazalalikhan A. LKB1 expressed in dendritic cells governs the development and expansion of thymus-derived regulatory T cells. Cell Research. 2019;29(5):406–419. doi: 10.1038/s41422-019-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caron A., Richard D., Laplante M. The roles of mTOR complexes in lipid metabolism. Annual Review of Nutrition. 2015;35:321–348. doi: 10.1146/annurev-nutr-071714-034355. [DOI] [PubMed] [Google Scholar]
- 102.Guri Y., Colombi M., Dazert E., Hindupur S.K., Roszik J., Moes S. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32(6) doi: 10.1016/j.ccell.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Timilshina M., You Z., Lacher S.M., Acharya S., Jiang L., Kang Y. Activation of mevalonate pathway via LKB1 is essential for stability of T cells. Cell Reports. 2019;27(10) doi: 10.1016/j.celrep.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 104.He N., Fan W., Henriquez B., Yu R.T., Atkins A.R., Liddle C. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(47):12542–12547. doi: 10.1073/pnas.1715363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moore K.J., Sheedy F.J., Fisher E.A. Macrophages in atherosclerosis: a dynamic balance. Nature Reviews. Immunology. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickhout J.G., Basseri S., Austin R.C. Macrophage function and its impact on atherosclerotic lesion composition, progression, and stability: the good, the bad, and the ugly. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(8):1413–1415. doi: 10.1161/ATVBAHA.108.169144. [DOI] [PubMed] [Google Scholar]
- 107.Michelet X., Dyck L., Hogan A., Loftus R.M., Duquette D., Wei K. Metabolic reprogramming of natural killer cells in obesity limits anti-tumor responses. Nature Immunology. 2018;19(12):1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 108.Sawaf M., Fauny J.-D., Felten R., Sagez F., Gottenberg J.-E., Dumortier H. Defective BTLA functionality is rescued by restoring lipid metabolism in lupus CD4+ T cells. JCI Insight. 2018;3(13) doi: 10.1172/jci.insight.99711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan Y., Tian T., Park C.O., Lofftus S.Y., Mei S., Liu X. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543(7644):252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao X., Locasale J.W. Serine metabolism links tumor suppression to the epigenetic landscape. Cell Metabolism. 2016;24(6):777–779. doi: 10.1016/j.cmet.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Liao L., Yu H., Ge M., Zhan Q., Huang R., Ji X. Upregulation of phosphoserine phosphatase contributes to tumor progression and predicts poor prognosis in non-small cell lung cancer patients. Thoracic Cancer. 2019;10(5):1203–1212. doi: 10.1111/1759-7714.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao S., Ge A., Xu S., You Z., Ning S., Zhao Y. PSAT1 is regulated by ATF4 and enhances cell proliferation via the GSK3β/β-catenin/cyclin D1 signaling pathway in ER-negative breast cancer. Journal of Experimental & Clinical Cancer Research : Climate Research. 2017;36(1):179. doi: 10.1186/s13046-017-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pandey S., Garg P., Lee S., Choung H.-W., Choung Y.-H., Choung P.-H. Nucleotide biosynthesis arrest by silencing SHMT1 function via vitamin B6-coupled vector and effects on tumor growth inhibition. Biomaterials. 2014;35(34):9332–9342. doi: 10.1016/j.biomaterials.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 114.Zhu J., Ma J., Wang X., Ma T., Zhang S., Wang W. High expression of PHGDH predicts poor prognosis in non-small cell lung cancer. Translational Oncology. 2016;9(6):592–599. doi: 10.1016/j.tranon.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pacold M.E., Brimacombe K.R., Chan S.H., Rohde J.M., Lewis C.A., Swier L.J.Y.M. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nature Chemical Biology. 2016;12(6):452–458. doi: 10.1038/nchembio.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang P., Ma T., Guo D., Hu K., Shu Y., Xu H.H.K. Metformin induces osteoblastic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(2):437–446. doi: 10.1002/term.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Radu A.G., Torch S., Fauvelle F., Pernet-Gallay K., Lucas A., Blervaque R. LKB1 specifies neural crest cell fates through pyruvate-alanine cycling. Science Advances. 2019;5(7):eaau5106. doi: 10.1126/sciadv.aau5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Y., Yan Y., Tripathi S., Pentinmikko N., Amaral A., Päivinen P. LKB1 represses ATOH1 via PDK4 and energy metabolism and regulates intestinal stem cell fate. Gastroenterology. 2020;158(5) doi: 10.1053/j.gastro.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 119.Cheng X., Kim J.Y., Ghafoory S., Duvaci T., Rafiee R., Theobald J. Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Molecular Oncology. 2016;10(6):806–824. doi: 10.1016/j.molonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumazoe M., Takai M., Hiroi S., Takeuchi C., Kadomatsu M., Nojiri T. The FOXO3/PGC-1β signaling axis is essential for cancer stem cell properties of pancreatic ductal adenocarcinoma. Journal of Biological Chemistry. 2017;292(26):10813–10823. doi: 10.1074/jbc.M116.772111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trapp E.K., Majunke L., Zill B., Sommer H., Andergassen U., Koch J. LKB1 pro-oncogenic activity triggers cell survival in circulating tumor cells. Molecular Oncology. 2017;11(11):1508–1526. doi: 10.1002/1878-0261.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., DePinho R.A. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kazyken D., Magnuson B., Bodur C., Acosta-Jaquez H.A., Zhang D., Tong X. AMPK directly activates mTORC2 to promote cell survival during acute energetic stress. Science Signaling. 2019;12(585) doi: 10.1126/scisignal.aav3249. [DOI] [PMC free article] [PubMed] [Google Scholar]