Abstract
Copper misbalance has been linked to fat accumulation in animals and experimental systems; however, information about copper homeostasis in human obesity is limited. In this study, the copper status of obese individuals was evaluated by measuring their levels of copper and cuproproteins in serum, adipose and hepatic tissues. The analysis of serum trace elements showed significant positive and element-specific correlation between copper and BMI after controlling for gender, age, and ethnicity. Serum copper also positively correlated with leptin, insulin, and the leptin/BMI ratio. When compared to lean controls, obese patients had elevated circulating cuproproteins, such as semucarbazide-sensitive amine oxidase (SSAO) and ceruloplasmin, and higher SSAO activity and copper levels in visceral fat. Although hepatic steatosis reduces copper levels in the liver, obese patients with no or mild steatosis have higher copper content in the liver compared to lean controls. In conclusion, obese patients evaluated in this study had altered copper status. Strong positive correlations of copper levels with BMI and leptin suggest that copper and/or cuproproteins may be functionally linked to fat accumulation.
Introduction
Copper (Cu) is a trace element that is vital to human health. Tight regulation of Cu levels in cells and tissues is necessary for normal physiologic processes. Cu is a co-factor of enzymes involved in mitochondrial function, inflammatory response, and anti-oxidative functions. Cu has also been reported to play a role in fat metabolism. Dietary Cu deficiency combined with high fructose induces non-alcoholic fatty liver disease (NAFLD) in rats.1 Imbalance of Cu along with changes in the activity and abundance of Cu-dependent enzymes has been linked to diabetes and obesity (refs). Mice lacking the Cu transporter ATP7B in hepatocytes accumulate copper in the liver and develop steatosis and obesity.2 Downregulation of the Cu-dependent enzyme, semicarbazide-sensitive amine oxidase (SSAO), in mice leads to mild obesity.3 At the cellular level, the loss of Cu-dependent SSAO activity results in unbalanced utilization of metabolic fuels (glucose and fatty acids), accumulation of fat, and adipocyte hypertrophy.4
The emerging role of Cu in lipid metabolism may provide a new target for obesity research. However, information concerning Cu homeostasis in obese patients is limited. Elevation in serum Cu has been reported in obesity.5-7 In serum, the majority of Cu is bound to cuproproteins,8 and cuproproteins such as ceruloplasmin (Cp)9 and SSAO are higher in obese patients.10 Cp is a Cu-dependent ferroxidase secreted predominantly by the liver. Cp is involved in iron metabolism and serves as a marker of inflammation in clinical settings.11 SSAO is an amine oxidase, which converts primary amines to the corresponding aldehydes with the release of ammonia and hydrogen peroxide.12 SSAO is predominantly expressed in adipose tissue, smooth muscle, and endothelial cells. The soluble SSAO is derived from the membrane-bound SSAO and released into circulation predominantly from the adipose tissue.13
The increase of serum Cu in obese patients may reflect an increased secretion of cuproprotein(s) from tissues. Higher expression/secretion of cuproproteins would require higher supply of Cu to tissues. Currently, information about Cu levels in metabolic tissues such as adipose tissue or liver in obesity is lacking, and the relationship between total serum Cu and levels of cuproproteins like SSAO and Cp is also unclear. To address these gaps in our knowledge, we conducted a cross-sectional study, in which we analyzed the levels of Cu in circulation and two major metabolic tissues (adipose and liver) in obese individuals.
Materials and methods
Study design and participants
A cross-sectional study was conducted from October 2013 to March 2015 at the Johns Hopkins Medical Institutions.14 Obese patients (BMI > 40 kg m−2 or >35 kg m−2 with one or more obesity-associated comorbidities) undergoing bariatric surgery (Roux-en Y gastric bypass (RYGB) or vertical sleeve gastrectomy (VSG)) were recruited from the Johns Hopkins Center for Bariatric Surgery. Patients with previous weight loss surgery were excluded. Lean controls (BMI < 26 kg m−2) with no history of diabetes or cardiovascular disease were recruited from the Johns Hopkins Hospital. A total of 62 lean and 55 obese patients were included in this study. Participants were verbally briefed about the study and signed written informed consent. All human studies were approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Human tissue specimens
Visceral adipose tissue from obese patients was obtained from the Greater Omentum, and immediately placed into a sterile container containing All Protect Tissue Reagent (Qiagen) for stabilization until frozen on dry ice and stored at −80 °C. De-identified visceral fat tissue from healthy adults with a BMI < 26 was obtained from the Bayview Medical Center pathology laboratory. Fresh tissue was collected, frozen and stored at −80 °C. The body mass index (BMI) was calculated as weight/height2 (kg m−2). Obese liver tissues were obtained from the left lateral lobe of the liver and immediately placed into a sterile container containing the RNA stabilization reagent (Qiagen, Hilden, Germany) until frozen on dry ice and stored at −80 °C for future analysis. Normal human liver tissues were obtained through the Liver Tissue Cell Distribution System (Minneapolis, MN), funded by the NIH contract #HHSN276201200017C. All blood samples were collected in the morning following an overnight fast. Blood samples were centrifuged at 3200g for 7 min and the serum was aliquoted and stored at −80 °C for subsequent assays.
Inductively coupled plasma mass spectroscopy (ICP-MS).
For trace metal measurements, 200 μL of each serum sample was transferred into an acid-rinsed 15 mL conical tube and diluted 21× with 1% HNO3 (trace metal grade, Fisher-Scientific). ICP-MS analysis was performed using an Agilent 7700× equipped with an ASX 500 autosampler as previously described15 with the following variation. Elemental recovery was evaluated by measuring the NIST reference material (water, SRM 1643e, and serum, SRM 1598a) and was found to be >90% for all determined elements.
Analysis of Cu levels in tissues by atomic absorption spectroscopy.
70% nitric acid was added to adipose or liver tissue in an Eppendorf tube in an equal ratio (volume : weight). The tissues in nitric acid were incubated at 55 °C for 30 min. The samples were then diluted 40 times with ultrapure water, and the debris was removed by centrifugation at 14 000 × g for 1 min. Cu concentration in the supernatant was measured using atomic absorption spectroscopy (AA-6650G, Shimadzu, Columbia, MO). The samples with the same weight were homogenized using homogenizing buffer (50 mM HEPES, 1/1000 Igepal, 150 mM NaCl, 0.25 M sucrose, 0.5 μM AEBSF and 1 : 100 protease inhibitor solution). The samples received 30 strokes with tight-fitting pistons. Samples were then centrifuged at 1000 × g for 15 min, and the supernatant was collected as tissue homogenates. The protein concentration was determined by the BCA assay. The Cu/protein ratio was measured in triplicate and averaged for each sample.
Immunoblotting
Serum was diluted 5 times with PBS, and 5 μL of diluted serum was used for protein separation and Western blotting. Before the electrophoretic separation of proteins, each sample was combined with an equal volume of 2× Laemmli sample buffer containing 5% β-mercaptoethanol. Proteins were then resolved on 8% Laemmli SDS-PAGE and transferred to a PVDF membrane at 90 V for 90 min in Towbin buffer. The PVDF membrane was incubated in blocking buffer (3% BSA in PBS) for 30 min before overnight incubation in primary antibodies in PBS at 4 °C. Primary antibodies used in immunoblotting were: the rat monoclonal anti-SSAO 7-106 antibody (AK 982/02), and the rabbit polyclonal antibody to transferrin (Abcam, ab66952). All primary antibodies were diluted in PBS at a dilution of 1 in 1000. Secondary antibodies were: the goat polyclonal anti-rabbit IgG HRP-conjugate (Santa Cruz, SC-2004) and the goat polyclonal anti-rat IgG HRP-conjugate (Santa Cruz, SC-2006). The PVDF membrane was incubated in secondary antibodies diluted in PBST (0.1% TWEEN20 in PBS) at a dilution of 1 : 5000 for 1 h at room temperature.
SSAO activity
Tissue homogenates were prepared on ice by homogenizing pieces of frozen visceral fat from human subjects in 200 μL of homogenizing buffer (50 mM HEPES, 1/1000 Igepal, 150 mM NaCl, 0.25 M sucrose, 0.5 μM AEBSF and 1 : 100 protease inhibitor solution). The samples received 30 strokes with tight-fitting pistons. Samples were then centrifuged at 1000 × g for 15 min to remove the debris, and the supernatant was collected. The protein concentration of the lysate was determined by the BCA assay.
The SSAO activity was determined fluorometrically at 37 °C by measuring hydrogen peroxide production using the Amplex Red Monoamine Oxidase Assay Kit (Molecular Probes, The Netherlands) according to manufacturer’s instructions. Briefly, tissue homogenates of 10 μg protein were pre-incubated for 30 min at 37 °C with 100 μM monoamine oxidase B inhibitor pargyline. The reaction was started by the addition of 100 μL of the reaction buffer (400 μM Amplex Red, 2 U mL−1 horseradish peroxidase, 2 mM benzylamine, 50 mM sodium phosphate, pH 7.4) and the fluorescence (excitation: 530 nm, emission: 590 nm) was monitored every two minutes for 60 min at 37 °C in a microplate reader. In parallel, 1 mM semicarbazide (SSAO inhibitor) was added to the same reaction mixture as a negative (background) control. Resorufin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) was used as a standard.
RNA isolation, reverse transcription, and real-time PCR
Human visceral adipose tissue samples (~100 mg) were homogenized using RLT buffer (Qiagen), incubated at RT for 5 min, and centrifuged at 12 000g × 10 min at 4 °C. The homogenate was carefully transferred into QIAshredder avoiding the fat layer. RNA was isolated using the QIAshredder and RNeasy Mini Kit (Qiagen). The first-strand cDNA was synthesized from 1 μg of RNA using the high capacity RNA-to-cDNA Kit (Applied Biosystems). The qPCR reaction was performed using the Power SYBR Green Master Mix on the QuantStudio6 Real-Time PCR system (Applied Biosystems) using human specific primers (Table S2, ESI†). Relative quantities were determined by the CT method. Transcript levels were normalized to Rpl19 as an internal control.
Statistical analysis
Statistical analysis was performed using Prism software version 6.0 (GraphPad Software). Two-tailed Student’s t-test was used when appropriate. All data in the figures and the text are shown as the mean ± SEM. The p-value of less than 0.05 was considered significant. Correlations between the Cu levels and continuous variables were analyzed using Pearson’s correlation and linear regression. Linear regression analysis was performed for each independent variable with BMI. If variables reached statistical significance in univariate analysis, they were included into multivariate analysis with BMI as a dependent variable. All statistical analyses in Table S1 (ESI†) were performed using STATA statistical software 11.0 (StataCorp LP, College Station, TX); data in Tables 1 and 2 were generated using R Project for Statistical Computing software version 1.66.
Table 1.
Association between trace metals and BMI
| BMI |
||||||
|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
|||||
| Obese |
Combined |
Combined |
||||
| r2 | p | r2 | p | β | p | |
| Gender | −0.016 | 0.69 | 0.022 | 0.06 | 0.849 | 0.77 |
| Age | 0.035 | 0.09 | 0.006 | 0.20 | 0.043 | 0.66 |
| Ethnicity | −0.014 | 0.63 | −0.006 | 0.60 | −1.425 | 0.57 |
| Mn | −0.017 | 0.77 | −0.009 | 0.99 | −0.330 | 0.64 |
| Zn | −0.002 | 0.34 | 0.041 | 0.02 | 0.004 | 0.61 |
| Fe | −0.018 | 0.80 | 0.041 | 0.02 | 0.001 | 0.15 |
| Cu | 0.0700 | 0.03 | 0.150 | <0.0001 | 0.017 | 0.001 |
Table 2.
Association between Cu levels in the serum and metabolic parameters
| Cu |
||||
|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
|||
| β | P | β | p | |
| Age | 3.84 | 0.08 | 3.41 | 0.09 |
| Gender | −258.14 | <0.0001 | 243.89 | <0.0001 |
| Ethnicity | 34.66 | 0.54 | 37.54 | 0.47 |
| AST | 2.793 | 0.52 | ||
| ALT | 3.349 | 0.14 | ||
| Total cholesterol | 0.49 | 0.59 | ||
| Triglycerides | 0.47 | 0.14 | ||
| LDL | 0.95 | 0.34 | ||
| HDL | −2.713 | 0.08 | ||
| Fasting glucose | 0.30 | 0.87 | ||
| Insulin | 12. 528 | 0.04 | 12.14 | 0.04 |
| Hemoglobin A1C | 13.244 | 0.04 | ||
| Adiponectin | −0.005 | 0.11 | ||
| Leptin | 0.003 | <0.001 | 0.002 | 0.01 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; β, unstandardized coefficient.
Results
Serum Cu levels positively correlate with BMI
To characterize the status of essential metals in obesity, we performed a cross-sectional study of obese patients (n = 55) and lean controls (n = 62). The baseline characteristics of the study subjects have been described16 and are summarized in Table S1 (ESI†). Briefly, the cohort included 117 patients. The 55 obese patients had BMI values in the range of 35.6–68.6 kg m−2, and the 62 lean controls had BMI values from 17.6 to 26 kg m−2. The obese group consisted of 22 men and 89 women in the age range of 22 to 66 years old; 16 obese patients had type 2 diabetes, 34 patients had hypertension, and 12 patients had hyperlipidemia. Fasting glucose, hemoglobin A1c (HbA1c), insulin, HOMA-IR, alanine aminotransferase (ALT), total cholesterol, triglycerides, and leptin were significantly higher in the obese group, whereas HDL-cholesterol and adiponectin were significantly lower in obese patients relative to lean controls (Table S1, ESI†).
The concentrations of Cu and other trace metals (Fe, Zn, and Mn) in serum were measured for each study participant using ICP-MS. Cu levels were significantly higher in obese patients, in both males and females, relative to lean controls (Fig. 1A; female: 1149.8 ± 29.6 μg L−1 vs. 992.3 ± 49.9 μg L−1; male: 955.4 ± 41.1 μg L−1 vs. 758.3 ± 35.3 μg L−1). Women in both the lean and the obese group had higher serum Cu compared to men (Fig. 1A; control: 992.3 ± 49.9 μg L−1 vs. 758.3 ± 35.3 μg L−1; obese: 1149.8 ± 29.6 μg L−1 vs. 955.4 ± 41.1 μg L−1). BMI positively correlated with Cu (p < 0.0001), Zn (p = 0.02), and Fe (p = 0.02) (Table 1 and Fig. 1B). Following multiple regression analysis, which controlled for age, gender, ethnicity, and other metals, Cu was the only essential metal that retained its significance as a predictor of BMI (p = 0.001; Table 1). Within the obese group, only Cu levels significantly and positively correlated with BMI (Fig. 1C; p = 0.03). These results demonstrate that Cu is tightly and specifically linked to fat accumulation.
Fig. 1.
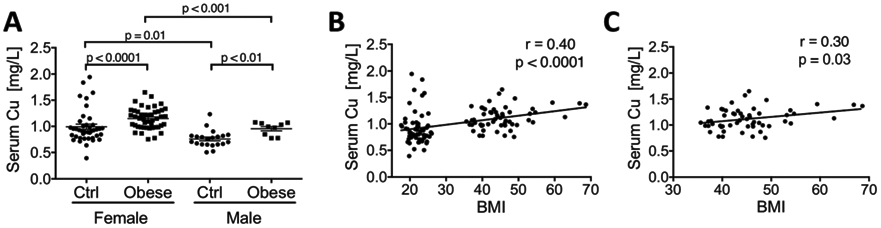
Cu levels in serum are higher in obese patients and positively correlate with BMI. (A) Serum Cu levels in female and male obese patients and lean controls (Ctrl). (B) Serum Cu concentration plotted against BMI values for both obese and lean patients. (C) Serum Cu levels are plotted against BMI values only for obese individuals. (Pearson’s r and p values were calculated by using a linear regression analysis. Student’s t-test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns p > 0.05. The data are presented as mean ± SEM.)
Leptin and insulin levels significantly correlate with the serum Cu levels
To better understand the relationship between Cu and various metabolic parameters in obesity, we examined the associations between serum Cu and alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, triglycerides, HDL, LDL, fasting glucose, insulin, HbA1c, adiponectin, and leptin. Univariate analysis of the data combined from the lean and obese groups revealed a positive association between Cu and insulin (p = 0.04; Fig. 2A), Cu and leptin (p < 0.0001; Fig. 2B), and Cu and HbA1c (p = 0.04; Table 2). Age and ethnicity did not significantly influence serum Cu; however, as mentioned above, gender was a predictor of serum Cu loads (p < 0.0001; Table 2). We performed multiple regression analysis of metabolic parameters while controlling for age, gender, and ethnicity, and found that leptin (p = 0.001) and insulin (p = 0.04) retained significant association with serum Cu levels, whereas HbA1c did not.
Fig. 2.
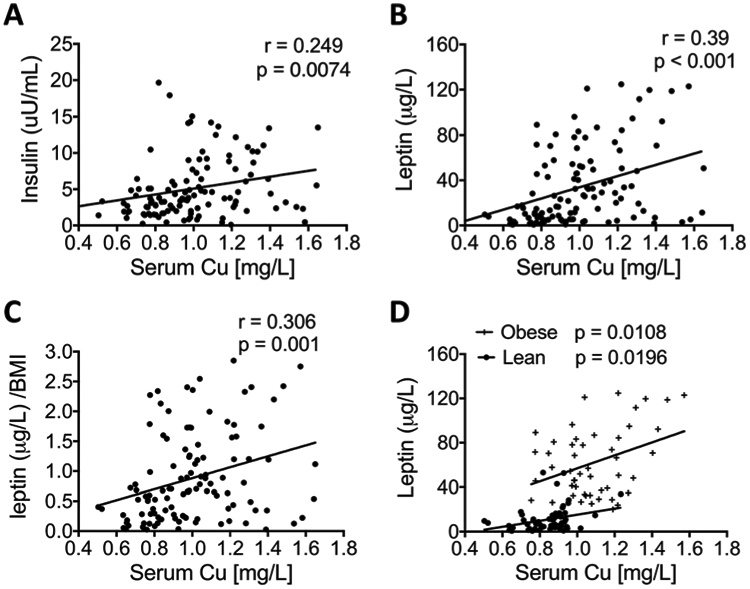
Correlations between serum Cu content and serum metabolic parameters. The serum concentrations of (A) leptin, (B) insulin, and (C) leptin/BMI levels in a group combining all obese and lean subjects are plotted against their Cu levels. (D) Leptin levels in either obese patients or lean controls (Ctrl) are plotted against their Cu levels. (Pearson’s r and p values were calculated by using a linear regression analysis.)
Considering that both insulin and leptin are significantly correlated with BMI, to remove the confounder factor of BMI, we analyzed the relation between serum Cu levels and insulin/BMI, or leptin/BMI. After normalizing by BMI, insulin lost its significant association with serum Cu, but leptin retained its significance as a predictor. Serum Cu levels were positively correlated with leptin/BMI (p = 0.001; Fig. 2C). We then examined their relation in the lean group and obese group separately. After removing the 5 statistical outliers, serum Cu levels were significantly associated with leptin in both lean and obese groups (Fig. 2D; obese group p = 0.0108; lean group p = 0.0196). Taken together, these results suggest that serum Cu positively correlates with leptin and insulin, and specifically and significantly with leptin.
Secreted Cu-containing enzymes are elevated in the serum of obese patients
In circulation, more than 95% of total Cu is bound to Cu-containing proteins.8 Cp is the major cuproprotein in serum, and the levels of Cp are elevated in obese patients.9,17,18 SSAO is another abundant Cu-dependent enzyme; the activity of SSAO in serum was shown to significantly and positively correlate with BMI.10 Consequently, we hypothesized that the elevation of serum Cu observed in our studies is caused, at least in part, by an increased abundance of these important cuproproteins. To test whether changes in the circulating levels of Cp or SSAO contribute to elevated serum Cu, we compared the abundance of serum CP and SSAO and the corresponding total copper levels in lean controls and obese female patients (Table 3). Serum samples with a comparable protein concentration from each group were selected, and the same volumes of serum were then loaded and analyzed by western blotting (Table 3). This analysis revealed that both Cp and SSAO were significantly increased in obese patients compared to lean controls, whereas the levels of transferrin (iron carrier) were not elevated, and even decreased (Fig. 3). These results are in agreement with the previous reports that show elevation of Cp and SSAO in the serum of obese patients.17,19
Table 3.
Characteristics of samples used in Fig. 3
| Loading order | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Cu level (mg L−1) | 0.83 | 0.82 | 0.86 | 0.86 | 1.45 | 1.51 | 1.42 | 1.38 |
| Pro. conc. (μg μL−1) | 61.7 | 50.1 | 75.1 | 61.8 | 67.5 | 50.4 | 51.6 | 56.3 |
| Age | 24 | 23 | 32 | 30 | 66 | 26 | 49 | 47 |
| BMI | 20.4 | 19.9 | 20.9 | 23.3 | 49 | 67 | 40 | 37 |
| Tissue source | Female lean | Female lean | Female lean | Female lean | Female obese | Female obese | Female obese | Female obese |
Fig. 3.
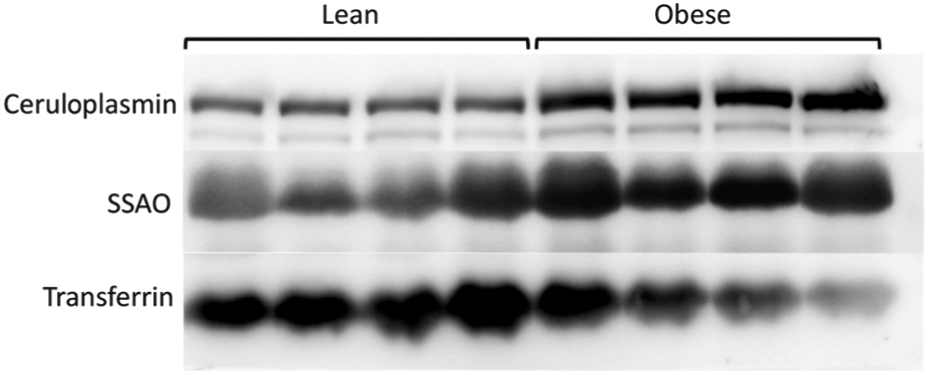
Serum ceruloplasmin and SSAO are elevated in obese subjects. A representative Western blot of equal volumes of serum from obese patients (n = 4) and lean controls (n = 4). The copper values and other characteristics of these samples are summarized in Table 3. The Cu-containing enzymes ceruloplasmin and SSAO are elevated in obese individuals, whereas iron-carrying transferrin (Tf) is not elevated.
SSAO activity and Cu levels in visceral fat positively correlate with BMI
Adipose tissue is one of the major sources of soluble SSAO found in circulation,13 especially under conditions of biological stress.20 Therefore higher levels of serum SSAO may reflect an elevation of SSAO in adipose tissue. To test whether this is the case, we measured the SSAO activity in the visceral fat from 25 obese patients (3 outliers) and 6 lean controls. Demographic and clinical characteristics of these patients were similar to the overall obese cohort (Table 1), and the average BMI was 44.38 ± 1.35 kg m−2. The average BMI of the 6 lean subjects was 24.48 ± 0.33 kg m−2. We found that the activity of adipose SSAO was significantly increased in obese patients relative to lean controls (Fig. 4A; 19.5 ± 1.5 vs. 10.4 ± 1.7 ng per min per mg protein). Further linear regression analysis showed that the SSAO activity in adipose tissue positively correlated with the BMI values (Fig. 4B; p = 0.0001).
Fig. 4.
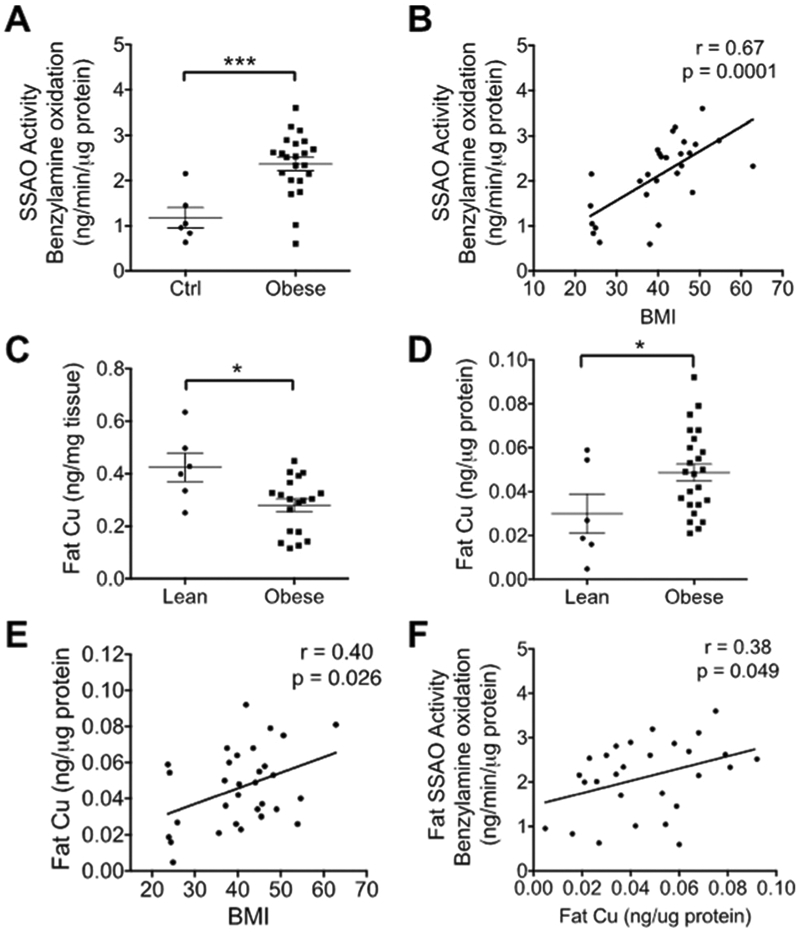
SSAO activity and Cu levels in visceral fat. (A) The activity of SSAO in the visceral fat of obese patients is significantly higher than in lean controls. (B) The activity of SSAO is plotted against BMI values. (C) Cu levels measured per tissue weight are lower in obese subjects. (D) Cu levels normalized per protein content of visceral fat are higher in obese patients compared to lean controls. (E) Cu levels normalized to protein are plotted against BMI. (F) SSAO activity is plotted against the visceral fat Cu normalized to protein. (B, E, F, Pearson’s r and p values were calculated by using a linear regression analysis. Student’s t-test, ****p < 0.0001, ***p < 0.001, **p<0.01, *p<0.05, ns p>0.05. The data are presented as mean ± SEM.)
The increased activity of SSAO in obesity suggested that Cu levels in the visceral fat could also be altered. Consequently, the total Cu content in visceral fat was quantified using the same samples (25 obese patients and 6 lean controls) that were previously used to measure the activity of SSAO. When normalized to wet weight of tissue, the amount of Cu per mg of adipose tissue was significantly lower in obese patients compared to lean controls (Fig. 4C; 0.28 ± 0.02 vs. 0.42 ± 0.05 ng per mg tissue). However, in “obese” samples, the contribution of fat to the total weight of tissue is much higher compared to control, and the adipocyte size is also increased. Therefore, the Cu levels expressed per wet weight of fat may not accurately reflect changes of cellular Cu levels. Consequently, we measured the total protein content in the same mass of visceral fat, and normalized Cu levels to total protein. The Cu levels per total protein in adipose tissue were significantly higher in obese patients (Fig. 4D; 0.05 ± 0.004 vs. 0.035 ± 0.009 ng per mg protein) and positively correlated with BMI (Fig. 4E; p = 0.04). Furthermore, the Cu levels in visceral fat positively correlated with the SSAO activity (Fig. 4F; p = 0.049). To further explore the source of elevated Cu in obese patients, we measured the mRNA levels for the copper uptake transporter CTR1. We also analyzed the mRNA levels for the Cu transporting ATPase ATP7A, which transports Cu into the secretory pathway and facilitates Cu delivery to SSAO. Real time quantitative PCR showed that both the CTR1 (Fig. 5A; p = 0.04) and ATP7A (Fig. 5B; p = 0.04) mRNA levels were higher in the obese group. CTR1’s mRNA levels were also positively correlated with BMI (Fig. 5C). Thus, the obese subjects upregulate the Cu handling machinery, in part to support increased SSAO activity in the fat tissue of obese patients.
Fig. 5.
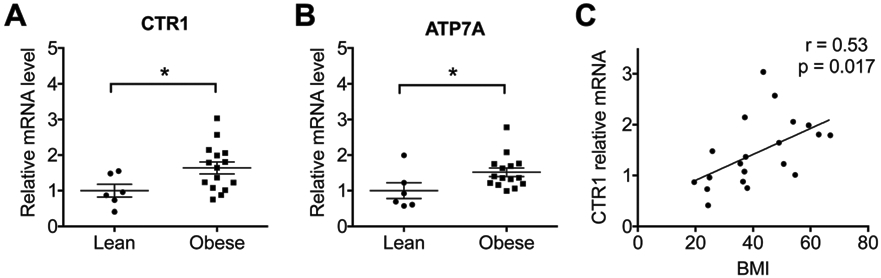
Relative mRNA levels of CTR1 and ATP7A in visceral fat. Relative mRNA levels of CTR1 (A) and ATP7A (B) in the visceral fat of obese patients are higher than in lean controls. (C) Relative CTR1 mRNA levels positively correlate with BMI. (C, Pearson’s r and p values were calculated by using a linear regression analysis. Student’s t-test, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns p > 0.05. The data are presented as mean ± SEM.)
Hepatic Cu is increased in obese patients and positively correlates with adipose Cu
Cp is a secreted Cu-dependent enzyme that is produced primarily by hepatocytes. The expression of Cp is increased in obese individuals,17 in agreement with our findings. However, little is known about hepatic Cu levels in the context of obesity. We obtained liver biopsies from 33 obese and 19 normal livers and measured Cu concentration in liver homogenates. The normal livers were from the “Liver Tissue Cell Distribution System”, and were the biopsy samples from 10 females and 9 males (age 19–64). The average BMI of the obese patients was 45.67 ± 1.42 kg m−2, and their demographic and clinical characteristics were similar to the overall obese cohort (Table 1). Obesity is often associated with liver abnormalities. Lower hepatic and blood Cu levels have been reported in patients with nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH).21,22 In our obese group, 4/33 (12%) patients had mild steatohepatitis with fibrosis; 7/33 (21%) had moderate steatosis, among them 2 had fibrosis; 16/33 patients had mild steatosis, among them 2 had fibrosis; and 6/33 patients had no steatosis and no fibrosis. Cu levels in the liver varied and were decreased in patients with most severe steatosis (Fig. 6A). However, obese patients with no or mild steatosis had hepatic Cu levels that were significantly higher compared to controls (Fig. 6B; 4.99 ± 0.39 vs. 2.56 ± 0.49 ng per mg tissue). The obese patients with moderate steatosis and steatohepatitis had comparable Cu levels to the control group (Fig. 6B). Interestingly, positive correlations were observed between the serum Cu and fat Cu or hepatic Cu (Fig. 6C and D), and Cu levels in the liver were significantly associated with Cu levels in visceral fat (Fig. 6E; p = 0.01). These correlations suggest that the elevation of Cu in adipose tissue and liver may be linked, implying a potential crosstalk between these two tissues via serum Cu proteins. The positive correlations may indicate that elevated serum Cu enzymes are products of enhanced Cu utilization by tissues for increased production/secretion of cuproenzymes.
Fig. 6.
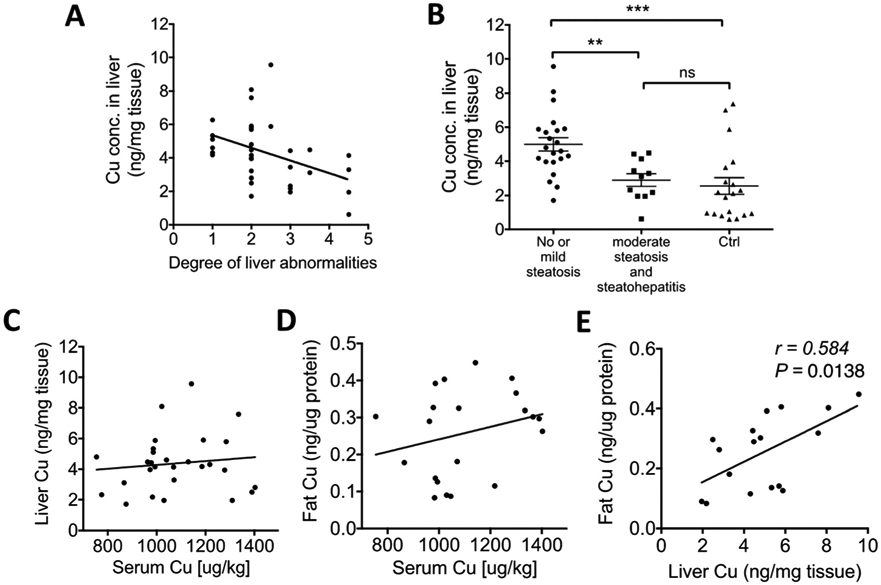
Hepatic Cu is higher in obese patients and positively correlates with Cu in adipose tissue. (A) Hepatic Cu levels grouped based on the degree of liver abnormalities: (1) no steatosis and no fibrosis, (2) mild steatosis and no fibrosis, (2.5) mild steatosis and mild fibrosis, (3) moderate steatosis and no fibrosis, (3.5) moderate steatosis and fibrosis, and (4.5) mild steatohepatitis with fibrosis. (B) Cu levels in the liver of lean controls and obese patients. (C) Hepatic Cu levels are plotted against serum Cu levels (p > 0.05). (D) Cu concentration in adipose tissue (fat) is plotted against serum Cu concentration (p > 0.05). (E) Adipose Cu levels (per protein) are plotted against liver Cu levels (per protein). (A and C–E, Pearson’s r and p values were calculated by using a linear regression analysis. Student’s t-test, **p < 0.01, *p < 0.05. The data are presented as mean ± SEM.)
In summary, obese patients have elevated Cu and cuproenzymes in the serum along with higher Cu in tissues mostly responsible for secreted cuproproteins (e.g. visceral fat and the normal liver).
Discussion
Cu serves as an essential cofactor for several enzymes critical for multiple cellular pathways, including mitochondrial respiration, melatonin production, wound healing, and neurotransmitter synthesis. The suggested daily dietary intake of Cu for an adult is 1.0 to 1.6 mg according to the third National Health and Nutrition Survey.23 Cu imbalance has been documented in pathologic conditions, such as inflammation,24 obesity, hypercholesterolemia, and fatty liver.21,25 These studies have demonstrated that the loss of tight regulation of Cu and Cu-dependent enzymes in tissues and circulation may contribute to impaired whole-body metabolism. The role of Cu in lipid metabolism has recently emerged. In white adipose tissue, Cu appears to regulate lipolysis26 and governs the absorption and utilization of major metabolic fuels through the activity of the Cu-containing enzyme SSAO.4 Here, to better understand the role of Cu metabolism in energy storage at the whole organism level, we characterized Cu and SSAO levels in obese patients. We found that the expansion of adipose tissue in obesity positively correlates with systemic Cu levels in serum (which are higher in obese patients in Table 4), in agreement with the previously published studies.7 We also show that Cu is elevated in visceral adipose tissue and liver with little steatosis in obese patients (Table 4), likely due to the concurrent increase in the cuproenzymes SSAO and Cp. This new information highlights Cu imbalance in obesity. Our results also suggest that the upregulation of biosynthesis of Cu-dependent enzymes in tissues could precede/underlie their increased levels in serum.
Table 4.
Cu levels in the lean and obese groups
| Cu levels | Lean | Obese |
|---|---|---|
| Serum | 913.1 ± 37.68 μg L−1 | 1118 ± 27.37 μg L−1 |
| Fat | 35 ± 9 ng mg−1 | 50 ± 4 ng mg−1 |
| Liver | 2.56 ± 0.49 ng mg−1 | 4.99 ± 0.39 ng mg−1 |
Our findings that serum Cu levels strongly correlate with BMI suggest a tight link between Cu homeostasis and adiposity. This correlation is observed even after controlling for age, gender, and ethnicity. Previously, Omar et al. reported higher serum Cu levels in 32 obese subjects compared to healthy controls.7 Both serum Cu and Zn levels were also found to be higher in obese children.27 In another study, Cu, Zn, Fe and Mn were measured in obese women with or without diabetes, and Cu was the only metal found to be significantly higher in obese women compared to healthy lean controls.28
Serum Cu levels were higher in females, in both obese and control groups. This observation is consistent with several previous publications demonstrating higher Cu levels in women than men.29 Studies of mice with a strict control on Cu intake showed a similar gender effect on Cu. These results suggest a gender-specific difference in Cu handling rather than dietary intake resulting in gender-specific differences in Cu levels.30 Previous studies found that chronic estrogen administration was associated with an increase in the serum Cu and ceruloplasmin levels.31,32 These effects of estrogen may explain the gender-specific differences in Cu levels.
Besides gender, serum Cu levels are also significantly correlated with the levels of leptin and insulin. Leptin is a hormone that is synthesized and secreted by adipose tissue and acts in the brain to regulate food intake and energy expenditure. Previous studies found that leptin and insulin were upregulated in obese subjects, and positively correlated with fat mass.33-35 Our findings are consistent with these earlier results and further show a new association between serum Cu and circulating levels of leptin (and insulin) in obesity. Leptin and insulin are thought to be “adiposity signals” for the long-term regulation of body weight and have a compensatory role in obesity.34,36 We suggest that the correlation between Cu and leptin/insulin, in addition to the Cu–BMI correlation, illustrates the link between Cu and adiposity. Interestingly, Olusi et al. reported a positive correlation between serum Cu levels and leptin in healthy individuals.37 We also showed that the correlation between Cu and leptin is independent of BMI (leptin/BMI). The tight correlation between Cu and leptin may also suggest a potential role of Cu in leptin secretion.
White adipose tissue (WAT) is an important endocrine organ that secretes a large number of polypeptide hormones, including leptin, and other adipokines and cytokines. WAT is also primarily responsible for secreting SSAO.13 SSAO is expressed and localized on the plasma membrane of adipose tissue and smooth muscle of blood vessels.38 It is a Cu-requiring amine oxidase that regulates glucose and fatty acid uptake and storage,4 as well as recruitment of leukocytes during inflammation.39,40 The activity of circulating SSAO is increased in diabetes, fatty liver disease, and obesity.10,41 We found an upregulation of SSAO in circulation and visceral adipose tissue in the context of obesity. At the same time, fat Cu levels per gram of protein are positively correlated with SSAO activity, which may provide the Cu for SSAO synthesis. Altogether, we posit that elevation of SSAO in visceral fat increases the abundance of secreted SSAO, which in turn contributes to a rise in the amount of total serum Cu.
Cp is the major Cu-containing protein in serum, and is an acute-phase plasma protein primarily produced and secreted by the liver and activated immune cells (e.g., monocytes and macrophages). We have demonstrated that liver Cu levels are significantly higher in obesity, which may contribute to an increase in Cp production we observed in serum. Previous studies suggest that proinflammatory cytokines secreted by adipose tissue are associated with Cp synthesis in the liver.42 We found a positive correlation between liver Cu levels and fat Cu levels, which suggests potential tissue crosstalk.
Conclusions
In conclusion, we found that Cu levels are higher in circulation, as well as locally within the adipose tissue and liver of obese patients. Cu levels in serum and adipose tissue both positively correlate with the body mass index (BMI), as well as SSAO activity in adipose tissue. Serum SSAO and Cp are elevated in obese patients. We also found that total Cu levels positively correlate with leptin, insulin and leptin/BMI, suggesting a protective role in obesity. To our knowledge, this is the first study investigating Cu levels not only in the serum but also in two major metabolic tissues (adipose and liver). A limitation of our study is the relatively small sample size. Nevertheless, the study has yielded strong evidence in support of altered Cu status in obesity in both serum and tissues. Our data emphasize the need for further studies on the role of Cu and Cu-dependent enzymes in the development and treatment of obesity.
Supplementary Material
Significance to metallomics.
Copper homeostasis is essential for the normal metabolism of humans and animals. Several genetic disorders are caused by copper misbalance, and copper dysregulation co-occurs commonly with metabolic abnormalities. The described changes of copper homeostasis in human obesity and especially the correlation between copper status and body mass index highlight the need for better understanding of the metals’ role in normal metabolism and in disease.
Acknowledgements
This work was supported by grant S10RR025512 to MR, DK084171 to GWW, the Pediatric Endocrine Society (RMW), and the Endocrine Society (RMW).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mt00148d
Conflicts of interest
There are no conflicts to declare.
References
- 1.Song M, Schuschke DA and Zhou Z, et al. , Modest fructose beverage intake causes liver injury and fat accumulation in marginal copper deficient rats, Obesity, 2013, 21, 1669–1675, DOI: 10.1002/oby.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muchenditsi A, et al. , Targeted inactivation of copper transporter Atp7b in hepatocytes causes liver steatosis and obesity in mice, Am. J. Physiol.: Gastrointest. Liver Physiol, 2017, 313, G39–G49, DOI: 10.1152/ajpgi.00312.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bour S, et al. , Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 deficiency reduces leukocyte infiltration into adipose tissue and favors fat deposition, Am. J. Pathol, 2009, 174, 1075–1083, DOI: 10.2353/ajpath.2009.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Ralle M, Wolfgang JM, Dhawan N, Burkhead LJ, Rodriguez S, Kaplan HJ, Wong WG and Lutsenko S, Copper-dependent amine oxidase 3 governs selection of metabolic fuels in adipocytes, PLoS Biol., 2018, 16(9), e2006519, DOI: 10.1371/journal.pbio.2005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Zhang C and Bu J, Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S., Nutrients, 2017, 9(2), 104, DOI: 10.3390/nu9020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima SCVC, et al. , Assessment of copper and lipid profile in obese children and adolescents, Biol. Trace Elem. Res, 2006, 114, 1–19, DOI: 10.1385/BTER:114:1:19. [DOI] [PubMed] [Google Scholar]
- 7.Omar S, et al. , [Serum copper levels in obesity: a study of 32 cases], Tunis. Med, 2001, 79, 370–373. [PubMed] [Google Scholar]
- 8.Yamada T, Agui T, Suzuki Y, Sato M and Matsumoto K, Inhibition of the copper incorporation into ceruloplasmin leads to the deficiency in serum ceruloplasmin activity in Long-Evans cinnamon mutant rat, J. Biol. Chem, 1993, 268, 8965–8971. [PubMed] [Google Scholar]
- 9.Cignarelli M, et al. , Relationship of obesity and body fat distribution with ceruloplasmin serum levels, Int. J. Obes. Relat. Metab. Disord, 1996, 20(9), 809–813. [PubMed] [Google Scholar]
- 10.Mészáros Z, et al. , Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes mellitus: correlation with body mass index and serum triglyceride, Metab., Clin. Exp, 1999, 48(1), 113–117. [DOI] [PubMed] [Google Scholar]
- 11.Shukla N, Maher J, Masters J, Angelini GD and Jeremy JY, Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor?, Atherosclerosis, 2006, 187, 238–250. [DOI] [PubMed] [Google Scholar]
- 12.Lyles GA, Mammalian plasma and tissue-bound semicarba ide-sensitive amine oxidases: biochemical, pharmacological and toxicological aspects, Int. J. Biochem. Cell Biol, 1996, 28(3), 259–274. [DOI] [PubMed] [Google Scholar]
- 13.Abella A, et al. , Adipocytes release a soluble form of VAP-1/SSAO by a metalloprotease-dependent process and in a regulated manner, Diabetologia, 2004, 47(3), 429–438, DOI: 10.1007/s00125-004-1346-2. [DOI] [PubMed] [Google Scholar]
- 14.Wolf RM, et al. , C1q/TNF-Related Protein-9 (CTRP9) Levels Are Associated With Obesity and Decrease Following Weight Loss Surgery, J. Clin. Endocrinol. Metab, 2016, 101, 2211–2217, DOI: 10.1210/jc.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharjee A, et al. , Activity of Menkes Disease Protein ATP7A is Essential for Redox Balance in Mitochondria, J. Biol. Chem, 2016, 291(32), 16644–16658, DOI: 10.1074/jbc.M116.727248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf RM, et al. , C1q/TNF-related protein-9 (CTRP9) levels are associated with obesity and decrease following weight loss surgery, J. Clin. Endocrinol. Metab, 2016, 101(5), 2211–2217, DOI: 10.1210/jc.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim OY, Shin MJ, Moon J and Chung JH, Plasma ceruloplasmin as a biomarker for obesity: a proteomic approach, Clin. Biochem, 2011, 44(5–6), 351–356, DOI: 10.1016/j.clinbiochem.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Safavi SM, Ziaei R and Maracy MR, Association of serum ceruloplasmin level with obesity: some components of metabolic syndrome and high-sensitive C-reactive protein in Iran, J. Obes, 2012, 951093, DOI: 10.1155/2012/951093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meszaros Z, et al. , Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes mellitus: correlation with body mass index and serum triglyceride, Metab., Clin. Exp, 1999, 48(1), 113–117, DOI: 10.1016/S0026-0495(99)90019-7. [DOI] [PubMed] [Google Scholar]
- 20.Stolen CM, et al. , Origins of serum semicarbazide-sensitive amine oxidase, Circ. Res, 2004, 95, 50–57, DOI: 10.1161/01.RES.0000134630.68877.2F. [DOI] [PubMed] [Google Scholar]
- 21.Aigner E, et al. , A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease, Am. J. Gastroenterol, 2010, 105, 1978–1985, DOI: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 22.Nobili V, et al. , Levels of serum ceruloplasmin associate with pediatric nonalcoholic fatty liver disease, J. Pediatr. Gastroenterol. Nutr, 2013, 56, 370–375, DOI: 10.1097/MPG.0b013e31827aced4. [DOI] [PubMed] [Google Scholar]
- 23.Trumbo P, Yates AA, Schlicker S and Poos M, Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, J. Am. Diet. Assoc, 2001, 101, 294–301, DOI: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 24.Jomova K and Valko M, Advances in metal-induced oxidative stress and human disease, Toxicology, 2011, 283(2–3), 65–87. [DOI] [PubMed] [Google Scholar]
- 25.Lefevre M, Keen CL, Lonnerdal B, Hurley LS and Schneeman BO, Copper deficiency-induced hypercholesterolemia: effects on HDL subfractions and hepatic lipoprotein receptor activity in the rat, J. Nutr, 1986, 116(9), 1735–1746, DOI: 10.1093/jn/116.9.1735. [DOI] [PubMed] [Google Scholar]
- 26.Krishnamoorthy L, et al. , Copper regulates cyclic-AMP-dependent lipolysis, Nat. Chem. Biol, 2016, 12, 586–592, DOI: 10.1038/nchembio.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakinci C, a Paç FZ Küçükbay, M. Tayfun and A. Gül, Serum zinc, copper, and magnesium levels in obese children, Acta Paediatr. Jpn, 1997, 39, 339–341. [DOI] [PubMed] [Google Scholar]
- 28.Yerlikaya FH, Toker A and Aribaş A, Serum trace elements in obese women with or without diabetes, Indian J. Med. Res, 2013, 137(2), 339–345. [PMC free article] [PubMed] [Google Scholar]
- 29.Milne DB and Johnson PE, Assessment of copper status: effect of age and gender on reference ranges in healthy adults, Clin. Chem, 1993, 39, 883–887. [PubMed] [Google Scholar]
- 30.Quinn JF, et al. , Gender effects on plasma and brain copper, Int. J. Alzheimer’s Dis, 2011, 150916, DOI: 10.4061/2011/150916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta SW and Eikum R, Effect of estrogen on serum and tissue levels of copper and zinc, Adv. Exp. Med. Biol, 1989, 258, 155–162. [DOI] [PubMed] [Google Scholar]
- 32.Ganaraja B, Pavithran P and Ghosh S, Effect of estrogen on plasma ceruloplasmin level in rats exposed to acute stress, Indian J. Med. Sci, 2004, 58, 150–154. [PubMed] [Google Scholar]
- 33.Bahrami E, et al. , Insulin and leptin levels in overweight and normal-weight Iranian adolescents: the CASPIAN-III study, J. Res. Med. Sci, 2014, 19, 387–390. [PMC free article] [PubMed] [Google Scholar]
- 34.Baskin DG, Lattemann DF, Seeley RJ, Woods SC, Porte D Jr. and Schwartz MW, Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight, Brain Res., 1999, 848,114–123, DOI: 10.1016/S0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz MW, Peskind E, Raskind M, Boyko EJ and Porte D Jr., Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans, Nat. Med, 1996, 2, 589–593. [DOI] [PubMed] [Google Scholar]
- 36.Benoit SC, Clegg DJ, Seeley RJ and Woods SC, Insulin and leptin as adiposity signals, Recent Prog. Horm. Res, 2004, 59, 267–285, DOI: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 37.Olusi S, Al-Awadhi A, Abiaka C, Abraham M and George S, Serum copper levels and not zinc are positively associated with serum leptin concentrations in the healthy adult population, Biol. Trace Elem. Res, 2003, 91(2), 137–144, DOI: BTER-91-2-137[pii]\n 10.1385/BTER:91:2:137. [DOI] [PubMed] [Google Scholar]
- 38.Obata T, Diabetes and semicarbazide-sensitive amine oxidase (SSAO) activity: a review, Life Sci., 2006, 79, 417–422, DOI: 10.1016/j.lfs.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Enrique-Tarancón G, et al. , Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells, J. Biol. Chem, 1998, 273(14), 8025–8032, DOI: 10.1074/jbc.273.14.8025. [DOI] [PubMed] [Google Scholar]
- 40.Pannecoeck R, et al. , Vascular adhesion protein-1: role in human pathology and application as a biomarker, Crit. Rev. Clin. Lab. Sci, 2015, 52, 284–300, DOI: 10.3109/10408363.2015.1050714. [DOI] [PubMed] [Google Scholar]
- 41.Weiss HG, et al. , Plasma amine oxidase: a postulated cardiovascular risk factor in nondiabetic obese patients, Metab., Clin. Exp, 2003, 52(6), 688–692, DOI: 10.1016/S0026-0495(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 42.Engstrom G, et al. , Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke – a population-based study, Diabetes, 2003, 52(2), 442–447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


