Abstract
Image registration is a process that underlies many new techniques in radiation oncology – from multimodal imaging and contour propagation in treatment planning to dose accumulation throughout treatment. Deformable image registration (DIR) is a subset of image registration subject to high levels of complexity in process and validation. A need for local guidance to assist in high‐quality utilisation and best practice was identified within the Australian community, leading to collaborative activity and workshops. This report communicates the current limitations and best practice advice from early adopters to help guide those implementing DIR in the clinic at this early stage. They are based on the state of image registration applications in radiotherapy in Australia and New Zealand (ANZ), and consensus discussions made at the ‘Deforming to Best Practice’ workshops in 2018. The current status of clinical application use cases is presented, including multimodal imaging, automatic segmentation, adaptive radiotherapy, retreatment, dose accumulation and response assessment, along with uptake, accuracy and limitations. Key areas of concern and preliminary suggestions for commissioning, quality assurance, education and training, and the use of automation are also reported. Many questions remain, and the radiotherapy community will benefit from continued research in this area. However, DIR is available to clinics and this report is intended to aid departments using or about to use DIR tools now.
Deformable image registration is a powerful tool in radiotherapy; however, many questions remain around best practice. Key considerations and recommendations are given based on workshop consensus and expert opinion.
![]()
Introduction
Image registration, whether rigid image registration (RIR) or deformable image registration (DIR), is a core process used in the radiotherapy treatment chain. Given two images, IA and IB, image registration is the process by which the spatial transformation from IA is performed, such that its similarity with IB is maximised. This transformation can then be used to transform any data residing in the frame of reference of IA to the frame of reference of IB. Image registration can be computed within a general framework presented in Figure 1. RIR is the registration of images using only rigid translations and rotations between frames of reference, while DIR can provide a non‐linear registration of each point in the images. The theory of similarity metrics, optimisation, regularisation and transformation has been well reported 1 , 2 and is outside the scope of this report. Image registration, as illustrated in Figure 2, is used for multimodality treatment planning, image segmentation, image‐guided treatments, treatment response assessment, replanning and plan adaptation. DIR is becoming widely used, with commercially available modules within radiotherapy treatment planning systems and specific medical image toolkits. DIR has the potential to provide improved ability to more accurately map data between multiple image sets, providing efficiency gains, enhanced use of multimodality imaging and improved quantification of radiotherapy treatments. DIR, however, must be implemented and used with caution, as it is an ill‐defined process, the accuracy of which is highly subject to variation in algorithm and user input. Most DIR algorithms do not explicitly model biomechanical properties, and it is entirely possible for results to be physically implausible. For a comprehensive summary of the state of DIR in radiotherapy, see Rigaud et al. 3
Figure 1.
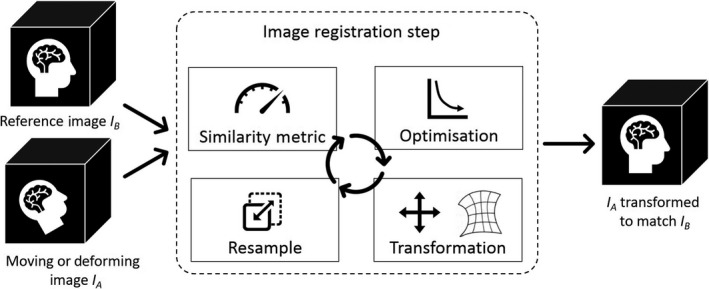
The typical image registration process, where a moving or deforming image is transformed to match a reference image. The same process is used for rigid and deformable registration; however, different similarity metrics, optimisation and transformation algorithms are used.
Figure 2.
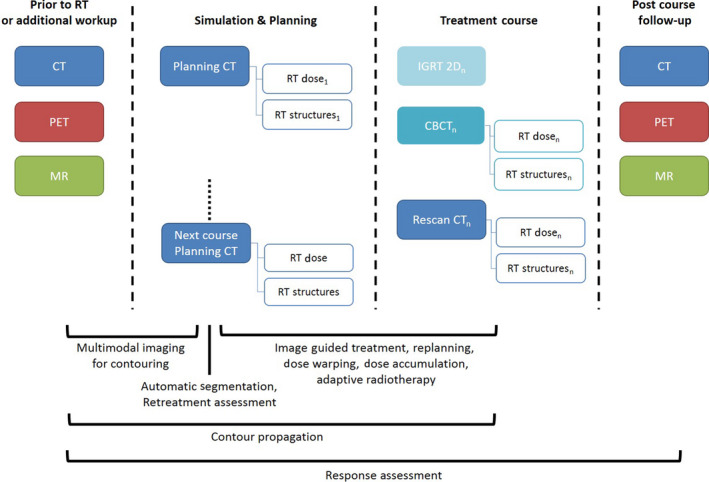
An example patient process map, indicating the imaging data that can be acquired at each phase of treatment, and below, the image registration‐related tasks (both deformable and rigid) are indicated across the time period and types of images they may occur.
A recent report from the American Association of Physicists in Medicine (AAPM) Task Group 132, ‘Use of image registration and fusion algorithms and techniques in radiotherapy’, 1 reviews the status of RIR and DIR in radiotherapy and makes recommendations for treatment planning and delivery. The report covers commissioning and quality assurance (QA) of image registration systems, clinical issues and sources of uncertainty. However, the report does not provide recommendations on the advanced applications of DIR such as image or dose deformation, and some recommendations are specific to the US workforce and practice.
A need for local guidance to assist in high‐quality utilisation and best practice was identified within the Australian community, leading to the collaborative activity and workshops outlined below. This report aims to communicate the key areas of concern based on the state and limitations of RIR and DIR applications in radiotherapy for Australia and New Zealand (ANZ) and provide advice for use based on consensus from discussions made at the ‘Deforming to Best Practice’ workshops and with local experts.
Methods
A collaborative group of radiation oncology medical physicists (ROMPs), radiation therapists (RTs) and radiation oncologists formed the ‘Society for Medical Image Registration and Fusion (or SMIRF)’ to look at safe, high‐quality implementation of DIR. SMIRF facilitated two workshops with local expert presenters. The aims were to provide education on RIR and DIR and their application in radiotherapy; provide a forum to discuss implementation and use of current clinical tools; and collect consensus opinion on the current state of the art.
Deforming to Best Practice workshops were convened on 15‐16 June 2018 in Sydney and 13 July 2018 in Melbourne. The workshops were attended by 125 and 55 registrants in Sydney and Melbourne, respectively, comprising medical physicists (43%), radiation therapists (47%), radiation oncologists (ROs, 2%), computer scientists and other professionals (8%). Prior to the workshops, registrants were asked to complete a survey on their clinic’s use of RIR and DIR, which was used to structure the workshop content and guide discussion. Throughout the workshops, an online polling tool (Mentimeter, Stockholm, Sweden) was used to collect responses from the audience in real time, for display and subsequent discussion. Informal raise‐of‐hand polling was also used for questions arising from discussion. Key considerations for DIR are drawn from the content experts presented and discussion which was transcribed during the workshop. Where no consensus was decided, further literature review by SMIRF content experts was undertaken to provide advice. Figure 3 describes the process to form the presented advice. The AGREE 4 and RIGHT 5 checklists for clinical guideline development were used where relevant in the drafting of these findings.
Figure 3.
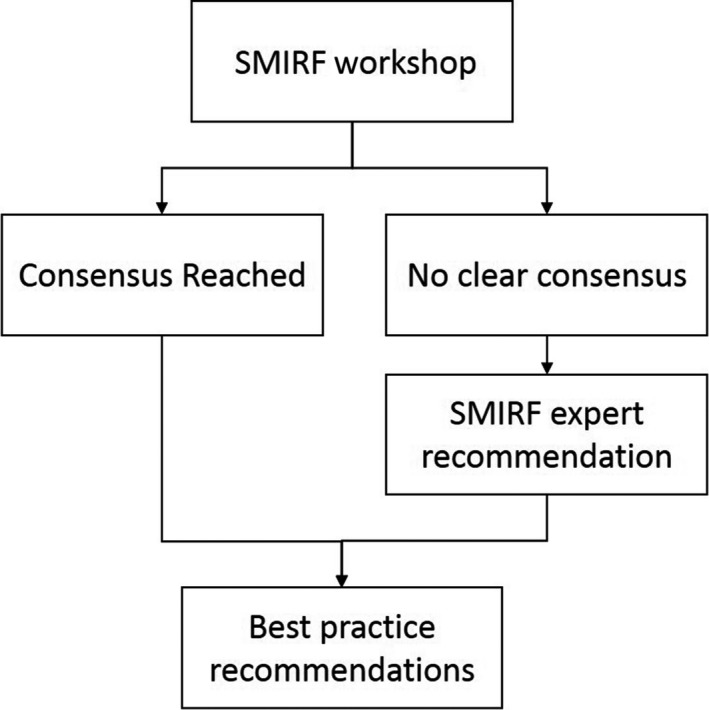
Development process of the advice in this report.
Results
Definitions of terms used in this report are listed in Table 1. Results are presented as general good image registration practice, the current status of image registration use and applications from workshop participants in Australasia, and then, consensus advice for specific clinical applications of DIR is suggested.
Table 1.
Definitions and acronyms used in this report, following the AAPM TG‐132 report. 1
| Term | Definition |
|---|---|
| Image registration (IR) | The process to generate a transform to convert one image to another image. Registration involves minimising the difference between moving and fixed images, using a similarity metric, to find a satisfactory solution. May also refer to the transform itself. |
| Rigid image registration (RIR) | A registration using a single 3D or 6D vector applied to the whole image. This may be manually performed by a user or an automatic process using an iterative optimisation process. |
| Deformable image registration (DIR) | A registration where the transform can vary across the image (i.e. a non‐rigid mapping of voxels). Transforms may be free form (spline‐based), flow‐based (e.g. demons), piecewise or finite‐element models. |
| Deformation vector field (DVF) | A transform describing the vector needed for each voxel to generate a warped image. Can be visualised as an overlaid grid, arrow vector field or colour map. |
| Warped image | The result of applying a DIR to the moving image. It is now a derived image and should be considered synthetic or a secondary source. |
| Fusion | The viewing of two images overlaid with a registration applied. |
| pCT | Radiotherapy simulation or planning CT. |
| rCT | A rescan CT or additional planning CT acquired during treatment. |
Key considerations for DIR
General best practice considerations for performing DIR are outlined in Table 2 (technical) and Table 3 (process‐based), but these are not intended to be a simple recipe for good registrations as it is impossible to provide standardised instructions that can apply to every algorithm and application. The initial manual RIR is a critical step for effective automated RIR and DIR. The best RIR may not be the best starting point for DIR, so it is important to understand how specific image registration software packages work. For instance, two packages may have the same algorithm, but one package may work on the actual image voxel values of the image while another package works based on the displayed window levels. Image registration can only work with the information the user provides, and so, it is critical to use appropriate bounding boxes and thresholds and work directly on the images of interest to obtain acceptable results. By setting bounding box subregions, a registration can focus directly on areas of interest without affecting or accounting for the whole image. A common key point to all registration tasks is to reduce ‘upstream’ issues/differences where possible and to minimise impact on ‘downstream’ tasks.
Table 2.
General technical considerations when performing DIR.
| Determining the bounding box or Region of Interest (ROI) for registration |
|
| Initial RIR is critical for effective DIR |
|
| Contrast within the ROI |
|
| Understand the limitations of RIR and DIR |
|
| Iterative deformation can improve accuracy |
|
Table 3.
General process and workflow considerations when performing DIR.
| Review registrations |
|
| Registration naming and storage conventions |
|
| Consider reproducibility of registrations |
|
| Acquire all images in similar position where possible |
|
General principles of good image registration practice, RIR or DIR, should be applied when registering and fusing images in the treatment planning process: define the purpose of registration and accuracy required, document which images are being registered, focus on the areas of importance and communicate/document uncertainty and compromises in the registration for the benefit of all downstream processes. Image registration request and report forms such as those given in TG‐132 Appendix B are recommended as a model for communicating the quality of all image registrations used for treatment planning.
Current status and considerations for clinical applications
A summary of advice for each clinical application is given in Table 4 (DIR between particular imaging modalities) and Table 5 (applications of DIR for deriving contours and dose). The following describes the current status of applying DIR to clinical applications by image modality.
Table 4.
Summary of key considerations for DIR between various image modalities used in radiotherapy.
| Image modalities | Key Considerations | Ref |
|---|---|---|
|
CT‐pCT registration |
No specific considerations extending Table 2. | |
| CBCT‐pCT registration |
Limitations of CBCT (FOV, HU accuracy, length limits) should be evaluated when estimating dose calculated on CBCT. Consider using tissue, air and bone overrides. |
8 |
| MR‐pCT registration |
MR‐pCT DIR should not be used routinely with the current tools available, unless multiple users have evaluated results on both technical and clinical grounds. |
11 |
| PET‐pCT registration |
Validate the consistent frame of reference between the PET and its attenuation correction CT before coupling other registrations. PET‐pCT DIR should only be performed using the intermediate registration between the attenuation correction CT and pCT. |
15 |
Table 5.
Summary of key considerations for clinical application use cases of DIR
| Clinical Application | Key considerations | Ref |
|---|---|---|
|
Contour propagation between pCT and rCT |
Any structure derived from another should not be propagated, but instead re‐created from the corrected propagated anatomical structures (e.g. margin expansions and Boolean products). | 1 |
|
Propagation of rigid/deformed isodose contours (e.g. for retreatments) are to be assessed for accuracy level achieved, as they cannot be corrected with subsequent editing. All deformably propagated structures should be reviewed and any errors corrected/assessed prior to further use |
31 | |
|
Atlas Segmentation |
Dice similarity coefficient should be used in combination with other metrics such as volume, location and surface measures. | 1, 33 |
| The clinical impact of automatically generated contours should be evaluated through determination of the dosimetric differences when using automatic versus manual segmentation for each department. | 36 | |
| Use pre‐ and post‐processing steps to save time (e.g. build atlases with smoothed and cleaned contours; atlas contours contain every third slice then interpolate as a final step). | 37 | |
| Adaptive Radiotherapy | Offline adaptation is feasible with current tools but resource‐intensive. Each department needs to assess their capacity to implement. | 31 |
| Online adaptation tools may be available, but workflows and expertise are not necessarily developed yet. More development is needed. | 66 | |
| Replanning | DIR can increase efficiency of replanning workflows for contouring. Automated workflows reduce manual steps and may reduce errors. The same careful review as manual replanning is required. | 31, 68 |
| Retreatment | The best estimate of previous dose depends on the scenario and available tools. Uncertainties of warping previous dose should be weighed against gains from providing a spatially correlated indication of past treatment. | |
| Dose Accumulation | Current tools and workflows for dose accumulation are not ready for routine clinical application, and the value gained from dose accumulation is not yet proven. Use should be evaluated as suitable by multiple users on both technical and clinical grounds. | 3, 31, 69 |
| Brachytherapy | Many challenges exist in brachytherapy DIR, and it should not be used in routine clinical application yet. Use should be evaluated as suitable by multiple users on both technical and clinical grounds. | 52 |
| Response Assessment | Large potential for quantitative response assessment and combination with functional or radiomic information. Scope for significant research. | 57, 58 |
CT‐pCT registration
While RIR of a planning CT (pCT) with other (diagnostic) CT images is routine practice in treatment planning, the use of DIR between CT images is still in the early stages of adoption. 6 , 7 Due to the similar nature of the information in each image, it may be the best performing of DIR applications. DIR between CT sets may be useful, for instance, when registering images acquired with the patient in different positions such as arms‐up or arms‐down positions, or flat and round couch tops.
CBCT‐pCT registration
Online and offline CBCT image‐guided treatments are routinely performed by all clinics. There is variation in how advanced tools and matches are performed. Most departments now have at least one linear accelerator (linac) couch able to correct 6 degrees of freedom, with use limited to select cases. Matching low‐contrast soft tissue information in CBCT is considered an important skill for RIR and IGRT, 7 and necessary for assessing DIR.
When using CBCT for image registration applications, it is important to remember accuracy is impacted by limited field of view, limited image length, decreased image quality of CBCT and artefacts inherent to CBCT. 8 The accuracy of CBCT HU is complex and changes with image dose, size and geometry of the subject and beam spectrum. The achievable accuracy of dose calculation on CBCT can be within 2% in some simple geometries or up to 20% in a region straddling the shoulders and neck. 9 It is important to consider the above factors when evaluating dose calculated on CBCT and consider using tissue, air and bone override regions. 10
MR‐pCT registration
Few attendees had experience with attempting DIR with MR images (13%). The tools available in commercial packages varied, but typically apply a mutual information similarity metric. DIR of MR‐pCT is often unsuccessful, and RIR is ultimately used in most cases. 11 , 12 , 13 , 14 , 15 DIR of MR‐pCT to correct MR distortion in controlled situations such as cranial stereotactic radiotherapy scans acquired in the same position may be successful with careful review. For most scenarios, workshop attendees considered RIR as the best approach to register MR imaging for radiotherapy planning. Current DIR algorithms struggle with dissimilar image information. 16 , 17 , 18 With the advent of MR‐linacs and MR‐guided adaptive treatments, there is a range of workflows reported using both rigid and deformable registration processes. 19 , 20 , 21 , 22
Direct planning on MR images may be considered where soft tissue definition is not sufficient from CT images (e.g. bilateral hip prostheses). In such a case, registration should be performed on the high‐contrast features that are visible between CT and MR, avoiding CT artefacts. In all cases where MR is to be used for planning, it is imperative that routine QA of MR image spatial distortion is performed. 23 , 24 , 25
It is considered that MR‐pCT DIR is not used routinely with the current tools available, except in individual cases where results are evaluated as suitable by multiple users on both technical and clinical grounds.
PET‐pCT registration
Some attendees had experience with DIR for PET/CT‐pCT registration (26%). Within that group, there was agreement that in many cases, the uncertainties in DIR were equivalent or not significantly more than using RIR, given the innate uncertainties of using PET images. 26 , 27 , 28 This is disputed in some literature, which indicates it provides minimal value. 15 , 29 , 30
Validation is needed for the consistent frame of reference between the PET and its attenuation correction CT before coupling other registrations, in case there is patient movement between scans or if the two bores of the PET/CT scanner are not well aligned. PET‐pCT DIR should only be performed by making use of the intermediate registration between the attenuation correction CT and pCT. 15
Contour propagation (same subject, e.g. pCT to rCT)
Deformable image registration is commonly used for contour propagation tools in replanning, atlas segmentation or adaptive planning for translating delineated structures defined on one image to another. The opinion on time and resource efficiency using DIR methods compared to recontouring from scratch depended on the clinical/anatomical site, accuracy required and individual patient anatomy.
Any structure derived from another (Boolean combinations or margin expansions) should not be automatically propagated, but instead re‐created from the corrected propagated anatomical structures. 31 Use caution when deforming tumour structures during a treatment course, as the deformation algorithm may not change the shape of the structure the same way that the actual cells are behaving. 32
Rigid or deformable propagated isodose contours (e.g. for retreatments) are not correctable with editing but require assessment as to the accuracy level achieved. All propagated structures should be reviewed and any errors considered prior to further use.
Automatic segmentation
A majority of attendees had developed or implemented atlas‐based automatic segmentation (54%). A range of anatomical site atlases is in use, with head and neck and pelvis being the most common. Setting up atlases requires significant resources and agreement on nomenclature. 33 Machine learning methods for automatic segmentation, particularly deep learning, are showing promising developments. 34
Some atlases were ultimately not routinely used clinically after development, often due to lack of stakeholder consultation, differences in delineation between clinicians or efficiency gains not eventuating in practice. Robust agreement on structures between all users of an atlas is a key starting point, or differences in practices must be accounted for in the atlas creation.
While Dice similarity coefficient (DSC) is commonly used to assess atlas performance, it should be used in combination with other metrics such as volume, location and surface measures such as mean distance to agreement (MDA) or Hausdorff distance. 35 Ideally, the clinical impact of automatically generated contours should be evaluated through determination of the dosimetric differences when using automatic versus manual segmentation. 36 . That is, the accuracy of the automatically generated contours should be evaluated based on the eventual use of those contours.
To reduce editing time and improve computational performance, use pre‐ and post‐processing steps. 37 For example, build atlases with smoothed and cleaned contours. Reducing atlas contours to every third slice can be more efficient for correcting and then interpolating to all slices as a final step.
Sharing of atlases was viewed favourably by attendees. Infrastructure and governance factors can be solved (privacy requirements, data transfer, storage and effort required). Adopting an atlas across multiple departments would most likely require changing local practice to conform to the atlas contours. Large cooperative trials or professional groups may be well placed to test shared atlases.
Adaptive radiation therapy (ART)
Most attendees stated performing some form of ART (93%), typically utilising a replan CT triggered by image guidance during treatment or patient set‐up variations, for example mask not fitting. Precisely, combining and accounting for adaptations often require DIR. Other types of ART not commonly in routine use include adaptive dose monitoring, daily adaptation and online replanning. Replanning is specifically addressed below.
Consensus indicated that offline adaptation (scheduled replans, adaptive dose monitoring and regular replans between treatments) is feasible with current tools. However, it is resource‐intensive and should be undertaken with care to ensure it is feasible within a specific working departmental environment. Online adaptation tools are often available, but workflows and expertise are not necessarily developed sufficiently in many cases. This is likely to improve in the near future as vendors provide more integrated solutions. This will bring challenges for the radiotherapy community to cope with the additional information and decisions in an optimal manner 38 .
Replanning
Deformable image registration is often used as part of replanning processes, including for registration between pCT and rCT (rescan planning CT), propagating the original contours to the rCT (manually or as an atlas). Anecdotally, increased image guidance increases replan rates amongst attendees, and typical rates were 5‐10% of head and neck plans and up to 30% of lung plans. Breast and prostate plans were also occasionally replanned during treatment. The turnaround of a replan was typically 3 days.
The introduction of DIR to replanning workflows can increase the efficiency and improve plan turnaround time, as well as reduce the number of manual tasks required. However, automated DIR processes still need careful review as would normally be performed for manual replans 39 . Investigations that ultimately do not result in replanning take considerable department resources, and DIR can be utilised to make these more efficient also.
Dose warping
Dose warping is rarely used clinically at present, but may be used for example to evaluate historical dose for retreatments. Another application is to compare total dose for nearby irradiated regions when the planning images were acquired in different positions (such as one arms‐up and one arms‐down). Some scenarios where it may be beneficial to use dose warping include between images in a 4DCT set, 40 treatment dose accumulation, retreatment (local and distant) and for assessing dose–response relationships to functional imaging. Other applications of dose warping were limited to research settings.
Dose warping (or dose deformation) is a purely mathematical tool and does not always directly relate to physical processes, 41 but it can be valuable in some scenarios, especially as dose–volume histograms cannot be summed between plans calculated on different underlying anatomy.
For retreatment scans in different patient positions, it may be better to use DIR instead of the traditional practice of rigidly registering two images and assessing overlap, and accept the uncertainties present.
Dose deformation should be rigorously reviewed by a RT and ROMP together to consider the accuracy of the resulting dose, and the radiation oncologist (RO) involved or informed of the processes taken to inform approval of the process. 1 , 42
Retreatment
Patients receiving more than one course of radiotherapy are a significant part of radiotherapy department workloads – a multi‐institutional study reported an average retreatment rate for 16% of patients and 25% of courses. 43 With improved treatment techniques and better data on normal tissue dose–volume relationships, 44 , 45 there is demand for improved accuracy of historical dose in subsequent courses of treatment (distant or local). DIR can be used to warp previous treatment dose to the new scan, to indicate overlap or response to treatment. In such cases, the DIR uncertainties may be deemed minor compared to other clinical uncertainties in decisions for retreatments.
There was general agreement that DIR for retreatment has a positive benefit/risk ratio, particularly when there is (1) significant time lapse between courses and already high uncertainties in tissue changes, forgotten dose, etc., or (2) simulation images acquired in different positions (high uncertainty in correspondence of dose due to anatomy deformation, difficult to indicate range of dose overlap).
Increased uncertainty in a retreatment may result in practical impacts such as larger target volumes, increased toxicity or changing from radical to palliative intent. The best estimate of previous dose possible should be used, with the tools available.
For retreatments with different fractionations, radiobiological equivalent dose scaling might be considered. In the case of summation of photon dose and proton or ion treatment dose, care should be taken to consider the differences in charged particle deliveries: the PTV concept does not translate well, and robust optimisation strategies may be applied to ensure coverage. 46 Doses may be reported in a DVH band, for a range of delivery scenarios. This uncertainty, as well as radiobiological effectiveness (RBE), increases the difficulty of dose summation.
Treated dose accumulation
It is possible to calculate dose using a CBCT, but to do this in a meaningful way requires careful analysis. 32 It should not be considered validated as a general solution. 3 The result can be warped to the planning CT to build a cumulative treated dose distribution. Alternatively, the previous day’s cumulative dose can be warped to the current day’s image and calculated dose for summation. Refer to above section CBCT‐pCT Registration for the issues and corrections. Some reports of calculating daily accumulated dose have required a full‐time staff member to perform it;47 however, improvements to workflow and automation may make this viable.
Considerable experience and understanding of the local treatment systems are required to be able to make meaningful decisions based on dose accumulation results. 48 RT, ROMP and RO should assess as a team before clinical decisions are made. The value of dose accumulation is not yet proven, and it is unclear if treated dose correlates with response in the same manner as the current planned dose evidence base. 49 , 50 At present, any dose accumulation should be rigorously reviewed due to inherent uncertainties before being used for clinical decision‐making.
Brachytherapy
Brachytherapy may rely on registration of multiple CT, MR and US images. These images may be acquired in different positions and in the presence of differing applicators, seeds and probes. These changing parameters make the task non‐trivial; however, DIR in brachytherapy is typically only used in research applications. Despite these uncertainties, DIR may have an important role in accumulating dose between sequential brachytherapy insertions and in adding external beam to brachytherapy dose. 51 , 52 , 53 DIR in brachytherapy is not used routinely with the current tools available, except in individual cases where results are evaluated as suitable by multiple users on both technical and clinical grounds.
Response assessment
Deformable image registration can create a common reference for assessing images prior to, during and after treatment. There is potential for significant advances in quantitative response assessment, beyond the Response Evaluation Criteria In Solid Tumours (RECIST) rules. 54 Combining functional imaging with DIR methods is creating new opportunities such as mapping changes in lung function with perfusion and ventilation imaging. 55 , 56 , 57 Future advances in radiomics will also need to work with or alongside DIR. 58 , 59
Implementation of DIR
Commissioning
Using DIR in the clinic brings enhanced functionality and responsibility to commission and QA appropriately. The TG‐132 report provides a framework for commissioning DIR, which this report endorses. It consists of commissioning tests for data integrity, collection of baselines for periodic testing after upgrades and performing end‐to‐end tests for each new application in the clinic. Ideally, the performance of DIR should be evaluated for all possible clinical scenarios using local clinical data sets prior to clinical implementation. However, this may not be feasible in practice, and a pragmatic approach can be used that covers a range of example data sets representing desired clinical applications or use cases, and a risk‐based approach assessing DIR as part of the overall radiotherapy treatment chain. Similarly, routine ongoing QA should follow from baselines acquired during commissioning and reflect clinical usage.
Patient‐specific QA and documentation
There was strong consensus agreement for adoption of the TG‐132 Request and Report forms to be used as a transparent and documented method for patient‐specific QA of both RIR and DIR. The request allows the RO detail areas of importance, registration technique and what a registration will be used for. The report form details what was performed, quality assurance results and an estimate of accuracy to guide decision‐making. Using the forms for communication is important for both clinical efficiency and effectiveness of the IR. While experience and understanding are being built, it is advisable to have multiple redundant checks of all uses of DIR.
Department‐specific workflows should ensure trained staff are assigned clear tasks at each part of the process for image registration as well as upstream and downstream processes. Guidelines on what constitutes a satisfactory registration should be based on metrics determined from commissioning data sets. Despite many attempts in the literature, no robust quantitative measure for individual DIR accuracy has been developed, and the required evaluation and QA are dependent on application. 3 As such, a range of quantitative QA metrics and qualitative QA (e.g. visual inspection) should be used in all clinical applications. Examples of QA for various clinical applications are given in Table 6. For example, contour propagation may require visual inspection only, with all contours to be reviewed and adjusted by the RO before further use. However, a retreatment dose summation may require qualitative review of the deformation vector field (DVF) for plausibility, quantitative measures such as mean distance to agreement (MDA) and Dice similarity coefficient (DSC) applied to structures of interest in the summation, an independent user check of the correct images and structures being propagated and resampled, as well as any isodoses from the resampled dose that might then be used for input to a new plan optimisation. Depending on the accuracy required, point‐to‐point landmark correspondence may need to be evaluated. There is a range of metrics and methods proposed in the literature that can also supplement these methods or be useful in specific situations 60 , 61 .
Table 6.
Suggested patient‐specific QA tasks by clinical DIR application. This is an example list, and each case may have its own requirements. Replanning, retreatment and adaptive processes may include multiple of the below applications
| Clinical Application | QA tasks |
|---|---|
| Multimodal image registration for contouring as input to a treatment plan |
|
| Anatomical contour propagation pCT/rCT |
|
|
Dose‐derived contour propagation pCT/rCT (e.g. isodoses) |
|
| Atlas Segmentation |
|
| Dose warping |
|
| Dose Accumulation |
|
Clinical implementation, workflows and automation
Consideration of the appropriate model for clinical roll‐out is needed. Identify which aspects/techniques are to be prioritised (e.g. retreatments). It may be beneficial to choose a single anatomical site as a pilot to begin, driven by need. Develop communication channels and common language for discussing image registration. Roles, responsibilities and QA mechanisms should also be defined upfront between RO, RT and ROMP, including clear and consistent communication and interfacing between and across the different group tasks. Multidisciplinary review of DIR, at least during early stages of implementation, can be highly beneficial. Each profession brings a different expertise to the problem, and shared experience will improve understanding and skills across the board. Ultimately, as the tools and experience in each department will be different, local implementation strategies and protocols are needed that are specific to equipment, expertise and clinical needs.
Data management policies are useful for tracking data, performing tasks in the correct order and deciding where each task is performed if multiple systems are used (e.g. contouring may be performed in multiple workspaces, but consider use of one system where contours are finalised for consistency). Standard operating procedures and automated workflows in image registration toolkits can be created in parallel. Naming conventions, approval processes and version control all need to be considered. 62 , 63
Deformable image registration can quickly become a complex process with many steps which are laborious and error‐prone. Using automated workflows is encouraged to reduce the chance of simple errors, but enough checkpoints are needed to allow for manual review and validation of the automated results. Education and experience become critical as automation is introduced. When the automation fails at a task, manual processing will be required, and so, expertise and a fundamental understanding of the algorithms need to be maintained within a department for this situation.
The resources required to perform and quality assure DIR need to be considered. Adding delays in the patient’s planning processes should be avoided. Inevitably, careful introduction of this new technology will put increased pressure on the time required to plan a patient’s treatment; however, with increased adoption and user experience/confidence, this will ease. DIR has the potential to reduce time, for example atlas‐based segmentation and contour propagation, and is integral to fast automated plan adaptation.
Education and training
Training for RIR and DIR is important, and consideration needs to be made for the appropriate model of training for a department. Example models include all staff trained to minimum level in both theory and software, or a small group of expert users to support the wider group. The minimum level of understanding for an application or software package will vary but should include a basic understanding of algorithms, comparable to IMRT optimisation for radiotherapy planning. Vendors should be able to provide material describing specific algorithms. Training amongst all staff groups involved is required. Differences in training needs between disciplines should be considered. Traditionally, roles in performing image registration have been segregated, with RTs performing the majority of image registration in ANZ. However, DIR requires a collaborative approach as uncertainties, technical limitations and clinical decisions associated with using DIR need to be understood by all groups. Site visits and discussions with experienced departments are encouraged. Training should cover ‘how‐to’ training for new software, as well as background theory to develop critical analysis to identify and rectify suboptimal results.
There is limited formal training available in this area beyond vendor‐specific application courses, and further opportunities for teaching in DIR theory and application should be considered in the future by the professional organisations. While the workshops gave general advice for DIR in various clinical applications, participants in the workshops expressed a desire for more detailed help to perform DIR tasks. Software differences make it difficult to provide universal solutions, but there is scope for future workshops, training and credentialing packages to address this for a particular software.
Utilising a risk‐based framework
Risk‐based approaches allow for assessment of relative risks of all processes that are unique to departments, enabling quality controls that target vulnerabilities. To enable effective, feasible, and practical quality control, quality measures in place can target the root causes, combined with appropriate data and models 64 .
The overall image registration process can be evaluated for residual risk and uncertainty with departmental consensus on acceptable risk, uncertainty and trade‐offs based on available solutions available (e.g. side‐by‐side images, rigid only, deformable only, re‐image, do not use). For an example approach, see the paper by Yuen et al 65 .
The workshop identified that there are varying risks in the use of DIR, depending on the application of the DIR results. For example, the risk of DIR for propagating deformed contours is far lower than the use of DIR for propagating deformed images and/or doses. Validation of deformed contours can be done with existing expertise and software by fixing contours and does not require DIR‐specific evaluation such as DVF analysis. Validation of deformed images and/or doses requires more DIR‐specific quality assurance with validation requirements depending on the intended use and accuracy level required. Departmental DIR commissioning and experience will guide understanding of risk factors relating to DIR which can be used to modulate the quality assurance required.
DIR research packages
Open source and research tools are not recommended for routine clinical use. They require specialised expertise and, if used clinically, should be within a well‐documented protocol, for example clinical trials.
They can, however, supplement existing practices as tools for training, benchmarking or extending clinical systems. All data going in and out of an oncology information systems and treatment planning systems should be parsed through, for example, an Australian Therapeutic Goods Administration (TGA)‐approved software first to ensure integrity. These packages generate virtual phantoms, perform cross‐validation with different DIR algorithms and test advanced concepts like masking, multi‐algorithm registrations and prototype pipelines for workflows. If used in the context of clinical trials, research tools should be validated/commissioned carefully to ensure the outcome of the trial is not invalidated.
Conclusions
The applications of DIR are still maturing without a set of definite practice standards. The workshops provided an avenue for knowledge sharing and constructive discussion on both theory and practice in image registration. Conclusions reached by the majority of active participants in the discussions resulted in a set of agreed best practices for clinics integrating DIR.
It is impossible to provide standardised instructions for all DIR cases. An understanding of the processes involved is important to assess and revise the results obtained. When viewed in a risk framework, DIR has many applications with a positive benefit/risk ratio if implemented with care. However, this is not so for all applications. There is no single quantitative measure that can fully evaluate DIR for different applications and use cases, and so, professional judgement and multidisciplinary input are normally required for evaluation. Different DIR algorithms will behave differently; therefore, users need to be aware of specifics of their software before clinical use. The TG‐132 Request and Report forms are an example tool for combining relevant information for all parties to improve the benefit/risk ratio.
Education and training are vital for high‐quality utilisation of image registration and effective implementation of DIR. There is a scarcity of resources and training events at present. RIR may be a mature and routine task in many departments; however, it is a fundamental requirement to performing DIR tasks optimally. It should not be ignored when training for DIR. Automation should be utilised where possible, but a general understanding of the processes and what can realistically be achieved needs to be conveyed to all users.
The SMIRF group responsible for the workshop and this report has transformed into the Medical Image Registration Special Interest Group (MIRSIG) of the Australasian College of Physical Sciences and Engineering in Medicine (ACPSEM). Future developments, research and guidance from this group will follow in this rapidly developing area.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank all the workshop participants who provided robust and thoughtful discussion that has been aggregated into this report, as well as all members of SMIRF who have provided discussion and ideas.
J Med Radiat Sci 67(2020) 318–332
References
- 1. Brock KK, Mutic S, McNutt TR, Li H, Kessler ML. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM radiation therapy committee task group no. 132. Med Phys 2017; 44: e43–e76. [DOI] [PubMed] [Google Scholar]
- 2. Oh S, Kim S. Deformable image registration in radiation therapy. Radiat Oncol J 2017; 35: 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rigaud B, Simon A, Castelli J, et al. Deformable image registration for radiation therapy: principle, methods, applications and evaluation. Acta Oncol (Madr) 2019; 58: 1225–37. [DOI] [PubMed] [Google Scholar]
- 4. Brouwers MC, Kerkvliet K, Spithoff K. AGREE next steps consortium ANS. The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016; 352: i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Yang K, Marušic A, et al. A reporting tool for practice guidelines in health care: The RIGHT statement. Ann Intern Med 2017; 166: 128. [DOI] [PubMed] [Google Scholar]
- 6. Yuen J, Ralston A, Gray A, et al. O126 Rigid and deformable image registration practice pattern: Australasian results. Australas Phys Eng Sci Med 2019; 10.1007/s13246-019-00724-x [DOI] [Google Scholar]
- 7. Batumalai V, Holloway LC, Kumar S, et al. Survey of image‐guided radiotherapy use in Australia. J Med Imaging Radiat Oncol 2017; 61: 394–401. [DOI] [PubMed] [Google Scholar]
- 8. Remeijer P, Deurloo K, Eenink M, et al. NCS Report 32: Quality assurance of cone‐beam CT [Internet]. 2019. 10.25030/ncs-032 [DOI]
- 9. Rosen B, Lee C, Brock K. SU‐F‐J‐15: The use of CBCT for dose calculations in H/N patients: impact of limited FOV. Med Phys 2016; 43: 3409. [Google Scholar]
- 10. Poludniowski GG, Evans PM, Webb S. Cone beam computed tomography number errors and consequences for radiotherapy planning: An investigation of correction methods. Int J Radiat Oncol Biol Phys 2012; 84: e109–e114. [DOI] [PubMed] [Google Scholar]
- 11. Speight R. MRI to CT image registration In: Liney G, van der Heide U. (eds). MRI for Radiotherapy: Planning, Delivery, and Response Assessment. Springer Nature, Switzerland AG, 2019. 21–42. [Google Scholar]
- 12. Liney GP, Moerland MA. Magnetic resonance imaging acquisition techniques for radiotherapy planning. Semin Radiat Oncol 2014; 24(3): 160–168. [DOI] [PubMed] [Google Scholar]
- 13. Gustafsson C, Sohlin M, Filipsson L. Method book for the use of MRI in radiotherapy, Version 3 [Internet]. 2016.
- 14. Brock KK, Dawson LA. Point: Principles of magnetic resonance imaging integration in a computed tomography‐based radiotherapy workflow. Semin Radiat Oncol 2014; 24: 169–74. [DOI] [PubMed] [Google Scholar]
- 15. Guckenberger M, Hugo G, Weiss E. Multimodality imaging for planning and assessment in radiation therapy In: Kim S, Wong J. (eds). Advanced and Emerging Technologies in Radiation Oncology Physics. CRC Press, Boca Raton, Florida, 2018; 83–120. [Google Scholar]
- 16. van der Put RW, Kerkhof EM, Raaymakers BW, Jürgenliemk‐Schulz IM, Lagendijk JJW. Contour propagation in MRI‐guided radiotherapy treatment of cervical cancer: the accuracy of rigid, non‐rigid and semi‐automatic registrations. Phys Med Biol 2009; 54: 7135–50. [DOI] [PubMed] [Google Scholar]
- 17. Zhong H, Wen N, Gordon JJ, Elshaikh MA, Movsas B, Chetty IJ. An adaptive MR‐CT registration method for MRI‐guided prostate cancer radiotherapy. Phys Med Biol 2015; 60: 2837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nix MG, Prestwich RJD, Speight R. Automated, reference‐free local error assessment of multimodal deformable image registration for radiotherapy in the head and neck. Radiother Oncol 2017; 125: 478–84. [DOI] [PubMed] [Google Scholar]
- 19. Hunt A, Hansen VN, Oelfke U, Nill S, Hafeez S. Adaptive radiotherapy enabled by MRI guidance. Clin Oncol 2018; 30: 711–9. [DOI] [PubMed] [Google Scholar]
- 20. Xing A, Holloway L, Arumugam S, et al. Commissioning and quality control of a dedicated wide bore 3T MRI simulator for radiotherapy planning. Int J Cancer Ther Oncol 2016; 4: 421. [Google Scholar]
- 21. Devic S. MRI simulation for radiotherapy treatment planning. Med Phys 2012; 39: 6701–11. [DOI] [PubMed] [Google Scholar]
- 22. Tijssen RHN, Paulson ES, Rai R. Implementation and acquisition protocols In: Liney G, van der Heide U. (eds). MRI in Radiotherapy: Planning, Delivery, and Response Assessment. Springer Nature, Switzerland AG, 2019; 3–20. [Google Scholar]
- 23. Metcalfe P, Liney GP, Holloway L, et al. The potential for an enhanced role for mri in radiation‐therapy treatment planning. Technol Cancer Res Treat 2013; 12: 429–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt MA, Payne GS. Radiotherapy planning using MRI. Phys Med Biol 2015; 60: R323–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chandarana H, Wang H, Tijssen RHN, Das IJ. Emerging role of MRI in radiation therapy. J Magn Reson Imaging 2018; 48: 1468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delikgoz Soykut E, Ozsahin EM, Yukselen Guney Y, et al. The use of PET/CT in radiotherapy planning: contribution of deformable registration. Front Oncol 2013; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hwang AB, Bacharach SL, Yom SS, et al. Can positron emission tomography (PET) or PET/computed tomography (CT) acquired in a nontreatment position be accurately registered to a head‐and‐neck radiotherapy planning CT? Int J Radiat Oncol Biol Phys 2009; 73: 578–84. [DOI] [PubMed] [Google Scholar]
- 28. Kovalchuk N, Jalisi S, Subrama‐Niam RM, Truong MT. Deformable registration of preoperative PET/ CT with postoperative radiation therapy planning CT in head and neck cancer. Radiographics 2012; 32: 1329–41. [DOI] [PubMed] [Google Scholar]
- 29. Specht L, Berthelsen AK. PET/CT in radiation therapy planning. Semin Nucl Med 2018; 48: 67–75. [DOI] [PubMed] [Google Scholar]
- 30. Fortin D, Basran PS, Berrang T, Peterson D, Wai ES. Deformable versus rigid registration of PET/CT images for radiation treatment planning of head and neck and lung cancer patients: a retrospective dosimetric comparison. Radiat Oncol 2014; 9: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonke J‐J, Aznar M, Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol 2019; 29: 245–57. [DOI] [PubMed] [Google Scholar]
- 32. Chetty IJ, Rosu‐Bubulac M. Deformable registration for dose accumulation. Semin Radiat Oncol 2019; 29: 198–208. [DOI] [PubMed] [Google Scholar]
- 33. Sharp G, Fritscher KD, Pekar V, et al. Vision 20/20: Perspectives on automated image segmentation for radiotherapy. Med Phys 2014; 41: 50902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB. Advances in auto‐segmentation. Semin Radiat Oncol 2019; 29: 185–97. [DOI] [PubMed] [Google Scholar]
- 35. Roach D, Jameson MG, Dowling JA, et al. Correlations between contouring similarity metrics and simulated treatment outcome for prostate radiotherapy. Phys Med Biol 2018; 63: 35001. [DOI] [PubMed] [Google Scholar]
- 36. Eldesoky AR, Francolini G, Thomsen MS, et al. Dosimetric assessment of an Atlas based automated segmentation for loco‐regional radiation therapy of early breast cancer in the Skagen Trial 1: A multi‐institutional study. Clin Transl Radiat Oncol 2017; 2: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu Y, Byrne M, Archibald‐Heeren B, et al. Implementing user‐defined atlas‐based auto‐segmentation for a large multi‐centre organisation: the Australian Experience. J Med Radiat Sci 2019; 66: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brock KK. Adaptive radiotherapy: moving into the future. Semin Radiat Oncol 2019; 29: 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rowbottom C. SP‐0394: The practical “costs” of adaptive radiotherapy. Radiother Oncol 2016; 119: S184–5. [Google Scholar]
- 40. Yeo UA, Taylor ML, Supple JR, et al. Evaluation of dosimetric misrepresentations from 3D conventional planning of liver SBRT using 4D deformable dose integration. J Appl Clin Med Phys 2014; 15: 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeo UJ, Taylor ML, Supple JR, et al. Is it sensible to “deform” dose? 3D experimental validation of dose‐warping. Med Phys 2012; 39: 5065–72. [DOI] [PubMed] [Google Scholar]
- 42. RANZCR . Imaging in Radiation Oncology ‐ A RANZCR consensus white paper [Internet] 2014.
- 43. Barton MB, Allen S, Delaney GP, et al. Patterns of retreatment by radiotherapy. Clin Oncol 2014; 26: 611–618 [DOI] [PubMed] [Google Scholar]
- 44. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010; 76: S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grimm J. Dose tolerance for stereotactic body radiation therapy. Semin Radiat Oncol 2016; 26: 87–8. [DOI] [PubMed] [Google Scholar]
- 46. Moyers M, Toth T, Sadagopan R, et al. Physical uncertainties in the planning and delivery of light ion beam treatments TG‐202.[Internet]. 2020. 10.37206/200 [DOI]
- 47. Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head‐and‐neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys 2012; 83: 986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rink A, Borg J, Simeonov A, et al. Dosimetric impact of intrafraction changes in MR‐guided high‐dose‐rate (HDR) brachytherapy for prostate cancer. Brachytherapy 2018; 17: 59–67. [DOI] [PubMed] [Google Scholar]
- 49. Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tomé WA. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys 2010; 76: S135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swaminath A, Massey C, Brierley JD, et al. Accumulated delivered dose response of stereotactic body radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys 2015; 93: 639–48. [DOI] [PubMed] [Google Scholar]
- 51. Flower EE, Do V, Sykes J, Dempsey C, Holloway L, Thwaites D. Deformable image registration (dir) for cervical cancer brachytherapy: A comparison of the reproducibility of three different methods and the effects of DIR on the anatomical stability of OAR DVH parameters. Brachytherapy 2016; 15: S101. [Google Scholar]
- 52. Flower E, Do V, Sykes J, et al. Deformable image registration for cervical cancer brachytherapy dose accumulation: Organ at risk dose–volume histogram parameter reproducibility and anatomic position stability. Brachytherapy 2017; 16: 387–92. [DOI] [PubMed] [Google Scholar]
- 53. Poder J, Yuen J, Howie A, Bece A, Bucci J. Dose accumulation of multiple high dose rate prostate brachytherapy treatments in two commercially available image registration systems. Phys Medica 2017; 43: 43–8. [DOI] [PubMed] [Google Scholar]
- 54. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 55. Hardcastle N, Hofman MS, Hicks RJ, et al. Accuracy and utility of deformable image registration in 68Ga 4D PET/CT assessment of pulmonary perfusion changes during and after lung radiation therapy. Int J Radiat Oncol Biol Phys 2015; 93: 196–204. [DOI] [PubMed] [Google Scholar]
- 56. MacManus M, Everitt S, Schimek‐Jasch T, Li XA, Nestle U, Kong F‐MS. Anatomic, functional and molecular imaging in lung cancer precision radiation therapy: treatment response assessment and radiation therapy personalization. Transl Lung Cancer Res 2017; 6: 670–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hegi‐Johnson F, Keall P, Barber J, et al. Evaluating the accuracy of 4D‐CT ventilation imaging: first comparison with technegas SPECT ventilation. Med Phys 2017; 44: 4045–55. [DOI] [PubMed] [Google Scholar]
- 58. Baumann M, Krause M, Overgaard J, et al. Radiation oncology in the era of precision medicine. Nat Publ Gr 2016; 16: 234–249. [DOI] [PubMed] [Google Scholar]
- 59. Jaffray DA. Image‐guided radiotherapy: From current concept to future perspectives. Nat Rev Clin Oncol 2012; 9: 688–99. [DOI] [PubMed] [Google Scholar]
- 60. Kierkels RGJ, Den Otter LA, Korevaar EW, et al. An automated, quantitative, and case‐specific evaluation of deformable image registration in computed tomography images. Phys Med Biol 2018; 63: 045026 10.1088/1361-6560/aa9dc2 [DOI] [PubMed] [Google Scholar]
- 61. Varadhan R, Karangelis G, Krishnan K, Hui S. A framework for deformable image registration validation in radiotherapy clinical applications. J Appl Clin Med Phys 2013; 14: 192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mayo CS, Moran JM, Bosch W, et al. American association of physicists in medicine task group 263: Standardizing nomenclatures in radiation oncology. Int J Radiat Oncol Biol Phys 2018; 100: 1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miller A. A rational Informatics‐enabled approach to standardised nomenclature of contours and volumes in radiation oncology planning. J Radiat Oncol Informatics 2014; 6: 53–70. [Google Scholar]
- 64. Huq MS, Fraass BA, Dunscombe PB Jr, et al. The report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality management The report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality management. Med Phys 2016; 43: 4209–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yuen J, Brown R, Foessel L, Barber J, Ralston A, Poder J. O101 Application of AAPM TG132 and TG100: optimisation of image registration workflow for deformable image registration. Australas Phys Eng Sci Med 2019; 42: 285–401.30805854 [Google Scholar]
- 66. Johnson PB, Padgett KR, Chen KL, Dogan N. Evaluation of the tool “Reg Refine” for user‐guided deformable image registration. Journal of Applied Clinical Medical Physics. 2016; 17: 158–170. 10.1120/jacmp.v17i3.6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Green OL, Henke LE, Hugo GD. Practical clinical workflows for online and offline adaptive radiation therapy. Seminars in Radiation Oncology. 2019; 29: 219–227. 10.1016/j.semradonc.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dong L, Yang J, Zhang Y. Image Processing in Adaptive Radiotherapy Brock K, Image Processing in Radiation Therapy. Boca Raton, FL: CRC Press; 2014. 3–20. [Google Scholar]
- 69. Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tomé WA. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. International Journal of Radiation Oncology Biology Physics. 2010; 76: S135–S139. 10.1016/j.ijrobp.2009.06.093 [DOI] [PMC free article] [PubMed] [Google Scholar]


