Abstract
Autoreactive inflammatory CD4+ T cells, such as T helper (Th)1 and Th17 subtypes, have been found to associate with the pathogenesis of autoimmune disorders. On the other hand, CD4+ Foxp3+ T regulatory (Treg) cells are crucial for the immune tolerance and have a critical role in the suppression of the excessive immune and inflammatory response promoted by these Th cells. In contrast, dendritic cells (DCs) and macrophages are immune cells that through their inflammatory functions promote autoreactive T‐cell responses in autoimmune conditions. In recent years, there has been increasing attention to exploring effective immunomodulatory or anti‐inflammatory agents from the herbal collection of traditional medicine. Berberine, an isoquinoline alkaloid, is one of the main active ingredients extracted from medicinal herbs and has been shown to exert various biological and pharmacological effects that are suggested to be mainly attributed to its anti‐inflammatory and immunomodulatory properties. Several lines of experimental study have recently investigated the therapeutic potential of berberine for treating autoimmune conditions in animal models of human autoimmune diseases. Here, we aimed to seek mechanisms underlying immunomodulatory and anti‐inflammatory effects of berberine on autoreactive inflammatory responses in autoimmune conditions. Reported data reveal that berberine can directly suppress functions and differentiation of pro‐inflammatory Th1 and Th17 cells, and indirectly decrease Th cell‐mediated inflammation through modulating or suppressing other cells assisting autoreactive inflammation, such as Tregs, DCs and macrophages.
Keywords: autoimmunity, berberine, dendritic cell, inflammation, macrophage, T helper cell
1. INTRODUCTION
1.1. Autoreactive Th1 and T17 cells
Autoreactive CD4+ T cells, such as T helper (Th)1 and Th17 subtypes, have been found to involve in the pathogenesis of several autoimmune disorders, including multiple sclerosis (MS), inflammatory bowel disease (IBD) and rheumatoid arthritis (RA). Th1 cells, which predominantly produce interferon gamma (IFNγ), participate in the elimination of intracellular pathogens and are involved in cell‐mediated and delayed‐type hypersensitivity responses. There are several lines of evidence that Th1 cells producing IFNγ are closely correlated with the clinical severity of autoimmune diseases and can independently transfer diseases into naïve mice. In mice with experimental autoimmune encephalomyelitis (EAE) as a model of human MS, IFNγ levels within the central nervous system (CNS) have been found to associate with disease severity, with high levels detected at the peak of disease that fall as the disease spontaneously subsides into remission. 1 , 2 Infiltrating CD4+ T cells were found as the source of this IFNγ, 3 and the adoptive transfer of IFNγ‐producing T‐cell lines has been demonstrated to promote autoimmune pathologies. 2 , 4 The role of Th1 in autoimmune disorders is further confirmed by findings that mice lacking the Th1 lineage‐specific transcription factors, T‐bet and signal transducer and activator of transcription 4 (STAT4), are protected from the disease. 5 The pathogenic role of Th1 cells has been also declared in other models of autoimmunity, such as adjuvant‐induced arthritis (AIA) as a model of human RA, 6 experimental autoimmune uveitis (EAU) 7 , 8 and experimental autoimmune myocarditis (EAM). 9 , 10
In parallel to Th1, some reports show that Th17 cells, a subtype of interleukin (IL)‐17 secreting CD4+ Th cells, and their relevant cytokines play important role in the severity and progression of several autoimmune diseases. The pathogenic role of Th17 cells in autoimmune diseases has emerged from studies that indicate IL‐17 expression is elevated at the inflamed sites in patients with RA, MS, uveitis, and psoriasis. 11 , 12 , 13 , 14 , 15 , 16 IL‐17 is a pro‐inflammatory cytokine that affects various cell types, including endothelial cells, fibroblasts, keratinocytes, epithelial cells, and macrophages, and promotes the generation of several cytokines including IL‐6, IL‐1, tumour necrosis factor alpha (TNF‐α), transforming growth factor beta (TGF‐β), granulocyte colony‐stimulating factor (G‐CSF) and granulocyte‐macrophage CSF (GM‐CSF), and many chemokines such as macrophage inflammatory protein 2 (MIP‐2), Cytokine‐induced neutrophil chemoattractant (CINC) and monocyte chemoattractant protein 1 (MCP‐1), as well as prostaglandins like PGE2. 17 , 18 , 19 , 20 , 21 A crucial result of such effects is the promotion and recruitment of neutrophils to the inflamed sites. 18 , 22 Moreover, IL‐17 is found to induce the generation of matrix metalloproteinases (MMPs) that act to degrade target tissue during the inflammation. 18 In addition to IL‐17, Th17 cells secret IL‐6, TNF‐α, IL‐21 and IL‐22 cytokines that are attributed to the destructive pro‐inflammatory function of these cells. 18 , 23 , 24 Of note, the impact of the Th17 response on autoimmunity has been evaluated within the various experimental models. Th17/IL‐17 deficient mice were found to has low sensitivity to AIA and EAE, 25 and treatment with IL‐17R antagonist or IL‐17 neutralizing antibody ameliorated the severity of AIA, EAE and EAU. 22 It is further supported by findings that show when Th17 regulatory factors, such as IL‐6 and retinoic acid‐related orphan receptor gamma t (RORγt), were knocked out in experimental animals, tissue infiltrating Th17 cells were significantly decreased and autoimmune inflammation was attenuated. 26
On the other hand, CD4+ Foxp3+ T regulatory (Treg) cells are crucial for immune tolerance and have a critical role in the suppression of the excessive immune and inflammatory response promoted by autoreactive Th cells. 27 , 28 Foxp3+ Treg cells can inhibit Th1 and Th17 differentiation and function. The nuclear transcription factor Foxp3, known as a specific marker for Treg cells, plays an essential role in the development and function of Treg cells, 29 which can suppress differentiation of Th1/Th17 cells by antagonizing the function of the transcription factors RORγt and ROR. 30 However, IL‐6 overcame this suppressive effect of Foxp3 on Th17 differentiation. 30 Excessive IL‐6 induces Th17, but suppresses differentiation of Treg cells, shifting the balance of Tregs towards inflammatory Th17 cells in patients with autoimmune disorders. Treg/Th17 imbalance and diminished numbers of Foxp3+ Treg cells in patients with various autoimmune diseases are associated with disease severity and activity. 31 On the contrary, DCs and macrophages, as discussed in the following sections, are immune cells that through their inflammatory functions promote autoreactive T‐cell responses in autoimmune conditions. 32 , 33
1.2. Berberine: a natural compound possessing immunomodulatory and anti‐inflammatory properties
In recent years, there has been increasing attention to exploring effective immunomodulatory or anti‐inflammatory agents from the herbal collection of traditional medicine. Herbal medicines introduce a rich source of natural compounds for the identification of new therapeutic agents having novel mechanisms of action and for providing valuable insight into new targets involved in the inflammatory process. Among medicinal plants and herbs, numerous plants of the genera Berberis and Coptis have been widely employed in traditional medicine to treat patients with abdominal pain, diarrhoea or gastroenteritis. 34 Berberine, an isoquinoline alkaloid, is one of the main bioactive ingredients in these herbs (Table 1) and has been shown to exhibit anti‐inflammatory, anti‐oxidation, anti‐atherosclerotic, antimicrobial, antidiabetic, antitumour and neuroprotective effects. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 Such pleiotropic biological and pharmacological properties of berberine have been suggested to be mainly attributed to its anti‐inflammatory and immunomodulatory properties. 35 , 43 Berberine has been found to modulate and/or suppress inflammation through suppressing the production of TNF‐α, IL‐6 and MCP‐1, down‐regulating the expression of cyclooxygenase‐2 (COX‐2), reducing generation of PGE2 and formation of exudates, and inhibiting the expression of MMP‐2 and MMP‐9 through nuclear factor‐kB (NF‐kB) and mitogen‐activated protein kinase (MAPK) signalling cascades. 35 , 44 , 45 , 46
TABLE 1.
Various sources of berberine
| Family | Scientific name | Common name | Tissue source | Reference |
|---|---|---|---|---|
| Berberidaceae | Berberis amurensis Rupr. | Barberry | Stem and roots | 154 |
| Berberis concinna Hook.f. | Stem bark | |||
| Berberis aquifolium Pursh | Oregon grape | Roots | 155 | |
| Berberis aristata DC. | Tree turmeric | Bark, roots, raw herb, fruit, stem | 156, 157 | |
| Berberis asiatica Roxb. ex DC. | Chutro, rasanjan, marpyashi, daruharidra, darbi | Roots, stem, bark | 159, 163, 164 | |
| Berberis beaniana C. K. Schneid. | Kang song xiao bo | — | 166 | |
| Berberis aetnensis C.Presl | — | Leaves and roots | 167, 168 | |
| Berberis chitria Buch.‐Ham. ex Lindl. | Chitra and indian barberry | Whole plant and roots | 170, 171 | |
| Berberis congestiflora Gay | Michay | Leaves and stem | 133 | |
| Berberis croatica Mart. ex Schult. & Schult.f. | Croatian barberry | Roots | 173 | |
| Berberis floribunda Wall. ex G.Don | Nepal barberry | Roots | 174 | |
| Berberis fortunei Lindl. | Fortune's Mahonia | Wood | 164 | |
| Berberis japonica R.Br | Japanese Mahonia | Wood, root | 164 | |
| Berberis koreana Palib. | Korean barberry | Bark of the stem and roots, seeds, stem, roots | 175 | |
| Berberis lycium Royle | Boxthorn barberry | Roots | 165 | |
| Berberis microphylla G. Forst. |
Patagonian barberry, magellan barberry, calafate |
Roots | 176 | |
| Berberis umbellata Wall. ex G.Don | Himalayan barberry | Roots | 177 | |
| Berberis vulgaris L. | Barberry | Stems and roots | 178 | |
| Annonaceae | Annickia chlorantha (Oliv.) | African whitewood | Bark | 179, 180 |
| Annickia polycarpa (DC.) | African yellow wood | Bark | 180 | |
| Rollinia mucosa (Jacq.) Baill. | Biriba, wild sweet sop, wild cashina | Fruit | 181 | |
| Xylopia macrocarpa A.Chev. | Jangkang | Stem bark | 164 | |
| Xylopia polycarpa (DC.) Oliv. | — | Stem bark | 164 | |
| Papaveraceae | Argemone albiflora Hornem | White prickly poppy, Bluestem pricklypoppy | Aerial part and roots | 182 |
| Argemone mexicana L. | Prickly poppy | Apigeal parts, seeds, leaves, roots | 183, 184 | |
| Argemone ochroleuca Sweet | Chicalote | Seeds | 188 | |
| Argemone platyceras L. | Chicalote poppy, crested poppy | Leaves and stem | 189 | |
| Argemone squarrosa Greene | Hedgehog pricklypoppy | Aerial part | 190 | |
| Bocconia frutescens L. | Plume poppy, tree poppy, tree celandine, parrotweed, sea oxeye daisy, john crow bush | Leaves, roots, stalks | 183, 191 | |
| Chelidonium majus L. | Celandine poppy | Roots | 192 | |
| Corydalis chaerophylla DC. | Fitweed | Roots | 193 | |
| Corydalis solida subsp brachyloba | Fitweed | Aerial parts | 194, 195 | |
| Glaucium corniculatum (L.) Rud. subsp corniculatum | Blackspot Hornpoppy | Aerial parts | 196 | |
| Macleaya microcarpa (Maxim.) Fedde | Poppy | Roots | 197 | |
| Ranunculaceae | Coptis chinensis Franch. | Chinese goldthread | Roots | 175 |
| Coptis japonica (Thunb.) Makino | Japanese goldthread | Rhizome | 198 | |
| Coptis teeta Wall. | Gold thread | Rhizome and roots | 175, 199 | |
| Hydrastis canadensis L. | Goldenseal | — | 200 | |
| Xanthorhiza simplicissima Marshall | Yellowroot | Root, stem, leaves | 201 | |
| Rutaceae | Phellodendron amurense Rupr. | Amur cork tree | Bark, root bark, trunk bark, perennial Branch bark, annual branches, leaves | 199, 202 |
| Phellodendron chinense C. K. Schneid | Chinese cork tree | Bark | 203 | |
| Phellodendron chinense var. glabriusculum C. K. Schneid. | Chinese cork tree | Bark, branch, leaf, bark, heartwood | 175, 204, 205 | |
| Phellodendron lavallei Dode | Lavalle corktree | Bark | 206 | |
| Zanthoxylum monophyllum (Lam.) P. Wilson | Palo rubio | Stem and branches | 207 |
Several lines of experimental study have recently investigated the therapeutic potential of berberine for treating autoimmune conditions in animal models of MS, RA, IBD and autoimmune uveitis. In the present review article, we seek mechanisms underlying immunomodulatory and anti‐inflammatory effects of berberine on autoreactive inflammatory responses. Based on the reported information, we discuss direct and indirect effects of berberine on autoreactive Th1 and T17 cells, which indirect effects cover pathways by which berberine through affecting Treg cells, DCs and macrophages could suppress inflammatory responses of Th1/Th17 cells in vitro and in vivo in various experimental models of autoimmune diseases (Table 2).
TABLE 2.
Effects of berberine on cytokine production in various autoimmune diseases
| Cytokine | Source of cytokine | Changes of mRNA/protein expression | Effect information | Ref. |
|---|---|---|---|---|
| Colitis | ||||
| IL‐1β |
‐ Colon tissue ‐ Macrophage ‐ Serum |
Decreased protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation ‐ Adjusting the M2/M1 ratio |
73 |
| IL‐6 |
‐ Colon tissue ‐ Macrophage ‐ Serum |
Decreased protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation ‐ Adjusting the M2/M1 ratio |
73 |
| IL‐17 |
‐ T cells of colon tissue ‐ Serum |
Decreased mRNA and protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation ‐ Improving Treg/Th17 Balance |
73, 85, 95 |
| IFN‐γ |
‐ T cells of colon tissue ‐ Sera |
Decreased mRNA and protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation ‐ Improving Treg/Th17 Balance |
73, 95 |
| TNF‐α |
‐ Colon tissue ‐ Macrophage ‐ Serum |
Decreased protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation |
73 |
| IL‐10 | ‐ Colon tissue | Increased mRNA and protein expression |
‐ Improving Treg/Th17 Balance ‐ Adjusting the M2/M1 ratio |
73, 85, 95 |
| IL‐22 |
‐ Colon tissue ‐ Serum |
Increased protein expression |
‐ Reducing inflammatory responses ‐ Inhibiting Th1/Th17 differentiation |
73 |
| Autoimmune Encephalomyelitis | ||||
| IL‐6 | CD4+ T cells | Decreased protein expression | ‐ Inhibiting Th1/Th17 differentiation | 72 |
| IL‐17 | ||||
| IFN‐γ | ||||
| Autoimmune Hepatitis | ||||
| IL‐1β |
‐ Hepatic tissue ‐ Serum |
Decreased protein expression | ‐ Reducing hepatic injury | 208 |
| IL‐2 | ||||
| IFN‐γ | ||||
| TNF‐α | ||||
| IL‐10 |
‐ Hepatic tissue ‐ Serum |
Increased protein expression | ‐ Reducing hepatic injury | 208 |
| Autoimmune Myocarditis | ||||
| IL‐17 | Serum | Decreased protein expression |
‐ Inhibiting Th1/Th17 response ‐ Ameliorating autoimmune myocarditis |
70 |
| IFN‐γ | ||||
| Autoimmune Uveoretinitis | ||||
| IL‐1β | Dendritic cells | Decreased protein expression | ‐ Inhibiting Th17‐ mediated autoimmune response | 68 |
| IL‐6 | ||||
| IL‐23 | ||||
2. SUPPRESSIVE EFFECTS OF BERBERINE ON AUTOREACTIVE TH1/TH17 CELLS
Anomalous autoreactive responses of CD4+ T helper cells, such as Th1 cells producing IFNγ 2 and Th17 cells producing IL‐17, 47 , 48 , 49 , 50 , 51 , 52 are tightly correlated with the clinical severity and progression of several autoimmune diseases, clinically and experimentally. 11 , 12 , 53 , 54 Th1 and Th17 cells can also recruit other inflammatory cells into inflamed tissues through the secretion of several pro‐inflammatory cytokines, such as IFNγ, IL‐12, IL‐1β, IL‐2, IL‐17A, IL‐17F, IL‐21, IL‐22, IL‐23 and IL‐25 and GM‐CSF. 55 , 56 Th1 and Th17 cells differentiate from naïve CD4+ T cells through regulation by a complex network of transcription factors and cytokines. Janus kinase‐signal transducers and activators of transcription (JAK‐STAT) signalling is an important signalling transduction pathway regulating differentiation and function of Th1 and Th17 cells. 57 The key members of the JAK/STAT family are STAT1 and STAT4 that, after IL‐12 stimulation, are activated by JAK2 and Tyrosine Kinase 2 (Tyk2) and participate in differentiation of Th1 cells. 58 , 59 On the other hand, STAT3 is an essential signalling mediator for the commitment of Th17 lineage and is provoked by TGF‐β, IL‐6 and IL‐23 cytokines (21‐23). The expression of these STAT‐signalling mediators is promoted by T‐bet transcription factor for STAT1 and STAT4 and RORγt transcription factor for STAT3. 30 , 60 , 61
An early study on mice model of autoimmune tubulointerstitial nephritis showed that berberine could reduce increased levels of Th1 cells, which was associated with an improvement in renal function. 62 Experimental autoimmune neuritis (EAN) is a model of human Guillain‐Barre syndrome characterized by infiltration of the peripheral nervous system by autoreactive T cells promoting demyelination and axon damage. 63 , 64 , 65 Berberine treatment was shown to ameliorate EAN severity by inhibiting the proliferation of CD4+ T cells and down‐regulating Th1 (TNF‐α) cytokine. 66 Results from an ex vivo study on human CD4+ T cells isolated from patients with ocular Behcet's disease 67 and Vogt‐Koyanagi‐Harada disease 68 indicate that berberine can suppress Th17 responses through reducing the frequency of IL‐17 producing CD4+ T cells and inhibiting IL‐17 production. It is further confirmed by an in vivo study on AIA rats that showed berberine administration could significantly reduce the blood levels of Th17 population and the serum levels of IL‐17, which was accompanied by decreased expression of IL‐17 in synovium and Th17 transcription factor RORγt in the spleen. 69 The further experimental study revealed that berberine treatment significantly attenuated the excessive response of Th1/Th17 cells through reducing elevated levels of Th1/Th17 cells and their cytokines IL‐17/IFNγ in rats with EAM, which was along with marked reduction in the impaired cardiac function and the pathophysiological severity. 70 An in vitro study on naïve T cells isolated from the spleen of AIA rats indicated that berberine treatment could significantly decrease differentiation and survival of Th17 cells, in a concentration‐dependent manner, through down‐regulating surface marker CD196 and RORγt transcription factor. 71 In mice with EAE, as a model of MS, treatment with berberine ameliorated the encephalitogenic autoreactive T cells by suppressing differentiation of naive CD4+ T cells into Th1 and Th17 cells. 72 Suppressive effects of berberine on Th1/Th17 differentiation has been further confirmed by the other study conducted on mice with experimentally induced colitis in which such inflammatory cells are involved in the progression and severity of the disease. 73 Mechanistically, berberine can decrease phosphorylation of STAT3 and expression of RORγt transcription factor during the differentiation of Th17 cells and down‐regulate phosphorylation of STAT4 and STAT1 and expression of T‐bet in differentiating Th1 cells (Figure 1). 70 , 71 , 72 In sum, berberine can directly inhibit differentiation and function of Th1/Th17 cells and thereby decrease inflammation‐mediated severity and progression of autoimmunity disease. As discussed in the following sections, berberine can also suppress inflammatory responses of T cells through indirect effect via affecting function of Treg cells, DCs, and macrophages.
FIGURE 1.
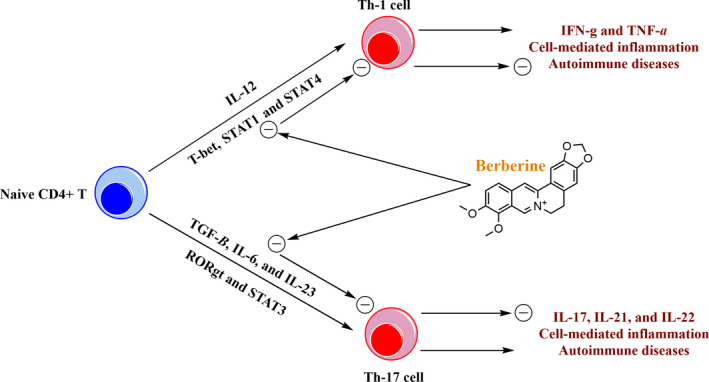
Suppressive effects of berberine on the differentiation and inflammatory function of Th‐1 and Th‐17 cells. Berberine can inhibit differentiation of naive CD4+ T cells into Th‐1 cells through down‐regulating the expression of IL‐12, STAT1, STAT4 and T‐bet, and into Th‐17 cells through down‐regulating the expression of TGF‐β, IL‐6, IL‐23, RORγt and STAT3. Suppressive effects of berberine on the differentiation of Th‐1 and Th‐17 cells leads to reduced levels of pro‐inflammatory cytokines and decreased autoimmune inflammation
3. BENEFICIAL MODULATORY EFFECTS OF BERBERINE ON TREG/TH17 BALANCE
Treg cells are anti‐inflammatory cells that secrete inhibitory cytokines including IL‐10, IL‐35 and TGF‐β and, thereby, suppress the function of inflammatory Th1/Th17 cells. 27 , 74 Treg cells are often functionally defective and indicate only mild expansion in autoimmune disorders and are far from reaching numbers that can counterbalance the inflammatory immune responses. 27 , 28 , 75 Treg/Th17 imbalance is an important hallmark of autoimmune disorders. In a model of autoimmune uveitis, berberine treatment was found to regulate Treg/Th17 balance, which was associated with an alteration in the composition of the intestinal microbiota, including an increase in the gut levels of Akkermansia genera, Oscillibacter, as well as Lachnospiraceae and Ruminococcaceae, and a decrease in Lactobacilli bacterial. 76 The genera Akkermansia is known to suppress the negative effects of IFNγ and thereby decrease Th17 responses. 77 Lachnospiraceae and Ruminococcaceae are butyrate‐producing bacteria that can increase the Treg/Th17 ratio. 78 , 79 Butyrate, a physiologically abundant short‐chain fatty acid (SCFA), has been suggested to involve in the regulation of T‐cell differentiation through several mechanisms, including the induction of G‐protein‐coupled receptor (GPCR) signalling, inhibition of de novo fatty acid synthesis through the deactivation of acetyl‐CoA carboxylase 1 and epigenetic regulation through inhibition of histone deacetylase activity, which are known to limit Th17 cell differentiation and promote Treg development. 79 Likewise, Oscillibacter is a known producer of pentanoate and capable of enhancing the differentiation of Treg cells. 79 , 80 Pentanoate, another SCFA, through mechanisms similar to butyrate can promote IL‐10 production on Treg cells and inhibit Th17 generation. 81 Moreover, Lactobacillaceae, a family of lactic acid bacteria, were shown to cause an increased type I IFN gene expression in the spleen and to worsen autoimmune manifestations. 82 , 83 , 84
Modulation of Treg/Th17 responses by berberine through gut microbiota‐dependent regulation is also further supported in a model of ulcerative colitis in which berberine treatment decreased levels of gut bacteria including Bacteroides. 85 Bacteroides are known to produce metabolites that induce Treg to produce IL‐10. Bacteroides have been reported to protect against experimental colitis through the release of polysaccharide A. This anti‐inflammatory effect is mediated by a decreased production of IL‐17 in the intestine and through the promotion of CD4+ T‐cell differentiation to IL‐10‐producing Treg. 86 , 87 However, mechanisms underlying the effects of berberine on the gut microbiota remain unclear yet and are needed to be taken into account in forthcoming studies.
Improving effects of berberine on Treg/Th17 balance has been also confirmed in the other study that showed berberine could modulate differentiation of splenic naïve T cells of AIA rats; berberine treatment could shift differentiation of naïve CD4+ T cells into CD4+ Foxp3+ Treg cells, instead Th17 cells, through activating AhR/CYP1A1/Foxp3 axis. 88 The differentiation and survival of Treg cells rely on the expression of Foxp3, which is induced by aryl hydrocarbon receptor (AhR) transcription factor and elevation in levels of cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1); a downstream element of AhR. 71 In mechanism, berberine activates AhR transcription factor by which up‐regulates CYP1A1 levels and subsequently increases Foxp3 expression, leading to the differentiation of Treg cells (Figure 2). 88 These findings can be further supported by reports that show berberine treatment can modulate Th17/Treg responses in other autoimmune diseases, such as colitis, 73 , 85 , 89 type 1 diabetes, 90 as well as EAE 72 and myocarditis. 70
FIGURE 2.
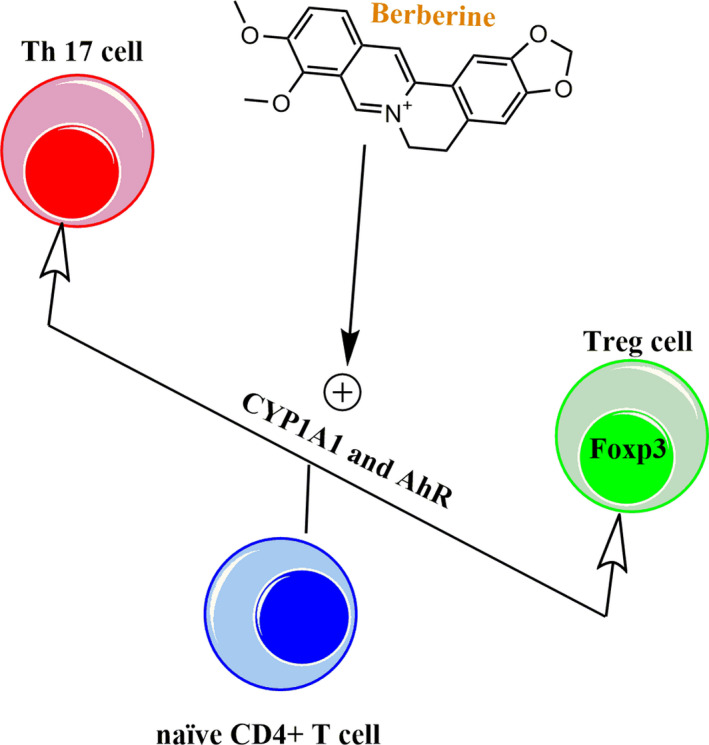
Mechanism underlying improving effects of berberine on Treg/Th17 balance. Berberine can shift differentiation of naïve CD4+ T cells into CD4+ Foxp3+ Treg cells through activating AhR transcription factor and up‐regulating CYP1A1
Taken together these findings suggest that berberine can beneficially modulate Treg/Th17 balance in autoimmune conditions, through two distinct mechanisms, directly by modulation of naïve CD4+ T cells’ differentiation and indirectly by affecting pattern of the gut microbiota.
4. MODULATORY EFFECTS OF BERBERINE ON DCS
The important players in T cell autoreactive responses are antigen‐presenting cells (APCs) like DCs, which provoke the complete activation of T cells by antigen presentation through the tri‐complex of antigen, MHC and TCR and exposing co‐stimulatory molecules, such as CD80 and CD86. 91 These co‐stimulatory molecules interact with cognate ligands on Th1 and Th17 cells to derive a positive signal that is needed for T‐cell activation. 47 , 92 Moreover, APCs, particularly DCs, secrete pro‐inflammatory cytokines TNF‐α and IL‐12 or IL‐6 and TGF‐β that through activating the STAT signalling can induce differentiation of T cell lineage and thereby promote the generation of Th1 or Th17 cells, respectively. 93 , 94 There is evidence that shows the modulatory effects of berberine on T cells are, in part, APC‐dependent. Berberine was found to hamper APC function through reducing expression of co‐stimulatory molecules CD80 and CD86 as well as down‐regulating production of IL‐6 and IL‐12 cytokines. 72 , 91 In EAE mice, the modulatory effect of berberine on Th1 and Th17 cells was shown to be dependent on the inhibitory impact on APC‐derived IL12 and IL‐6, respectively. 72 Another study on mice with IBD indicated that berberine reduced the production of TNF‐α, IL‐12, IL‐6 and TGF‐β in the maturated DCs and thereby decreased the population of Th1/Th17 cells in the mesenteric lymph nodes (MLNs), resulting in the amelioration of colon inflammation in colitis‐induced mice. 95 In mechanism, berberine was shown to act as an antagonist at dopamine D1‐ and D2‐like receptors and whereby modulate cytokine production in DCs. 95 Dopamine functions both as a hormone and a neurotransmitter, and there is evidence that dopamine involves in IBD development. 95 , 96 , 97 During the development of IBD, the condition of the colon impairs the intracellular storage of dopamine in enteroendocrine cells and the enteric nervous system, which affects the associated inflammatory process. 96 As for genetic evidence, the frequency of dopamine D2 receptor polymorphisms in IBD was similar in different groups of disease localization, behaviour and age of disease onset, supporting the involvement of dopamine receptors in IBD. 97 Dopamine receptor subtypes are also known to be expressed on the surface of immune cells, such as DCs, where dopamine can bind during the development of IBD. 95 Further supporting the involvement of dopamine receptor‐mediated signalling within immune cells in the progression of IBD is evidence that antagonists targeting dopamine D1‐like receptor and/or dopamine D2‐like receptor can prevent lipopolysaccharide (LPS)‐induced inflammation in lymphocytes and modulate cytokine secretion of DCs. 95
Furthermore, the timely elimination of mature DCs is important to prevent aberrant activation of the inflammatory immune responses. Apoptosis deficiency in DCs leads to the accumulation and prolonged activity of DCs that, in turn, result in long‐last activation of lymphocytes and progression of autoimmunity responses. 98 Berberine has been shown to exert anti‐apoptotic effects on DCs in in vitro and in vivo models of RA. 99 Berberine could time‐ and dose‐dependently induce apoptosis in murine bone marrow(BM)‐derived DCs. 99 Freshly isolated BM cells were found to be insensitive to berberine, and the susceptibility to berberine‐promoted apoptosis was increased during DC differentiation, in which mature IL‐12‐producing DCs showed higher sensitivity to berberine than immature DCs. Thus, berberine can selectively trigger apoptosis in mature DCs and whereby restrict DC maturation and shorten their lifespan. 99 As mature DCs play the crucial roles in pathogenic inflammation and immune responses in autoimmune diseases, berberine‐induced apoptosis in mature DCs provides a major mechanism of immunomodulation that can be accounted, at least in part, for its immunosuppressive impacts observed in animal models of autoimmune diseases. Although the exact intracellular mechanisms underlying selective pro‐apoptotic effect in DCs remain unknown, it has been shown that the production of reactive oxygen species (ROS) and mitochondrial depolarization, as well as caspase 3 activation, are involved in berberine‐mediated apoptosis induction. 99 In accordance with the aforementioned in vitro findings, berberine was indicated to markedly reduce the ratio of mature to immature DCs in spleens, confirming its selective pro‐apoptotic effect in mature DCs in vivo. 99 In this regard, berberine treatment could cause a considerable loss of DCs and an elevation in the apoptosis of DCs within spleens and lymph nodes in AIA mice, which was accompanied by the antiarthritic and immunosuppressive effects in these mice. 99
In sum, berberine has the potential to decrease survival and inflammatory functions of autoreactive APCs, mainly DCs, through inducing apoptosis and inhibiting co‐stimulatory molecules and inflammatory cytokine secretion, which is accompanied with reducing Th1/Th17 population and ameliorating severity and progression of autoimmune disorders.
5. MODULATORY EFFECTS OF BERBERINE ON INFLAMMATORY MACROPHAGES
Macrophages are the major innate immune cells present in almost every tissue or organ system and mainly act as phagocytic cells that engulf and digest cellular debris resulted from apoptosis, foreign substances, microbes and pathogens. 100 In addition to acting as professional phagocytic cells, macrophages produce various cytokines, chemokines and growth factors whereby exhibit broad immunomodulatory, inflammatory and tissue‐repairing capabilities and actively promote the development of several autoimmune diseases. 100 , 101 For example, abnormally activated intestinal macrophages in IBD patients and experimental models of colitis produce various inflammatory cytokines, such as IFNγ, IL‐1β, IL‐6, IL‐17, IL‐23 and TNF‐α necessary for T‐cell differentiation, specifically inducing the production of Th1 and Th17 cells (191‐194). In berberine‐administrated mice with colitis, levels of such pro‐inflammatory cytokines in the colon and sera were significantly decreased, which was accompanied by a reduction in colonic macrophages and percentages of IL‐6+, IL‐1β+ and TNF‐α+ secreting macrophages among splenocytes. 73 , 102 Moreover, macrophage infiltration in the inflamed lesions is one of the most important hallmarks of many autoimmune disorders such as IBD, 103 , 104 and berberine was shown to decrease macrophage infiltration into the colon by promoting apoptosis and inhibit signalling pathways involved in the stimulation of pro‐inflammatory cytokine production, including MAPK and NF‐κB, in colonic macrophages of mice with colitis. 102 Macrophages differentiate into two different polarization states serving opposite functions: classical M1 phenotype, which generates pro‐inflammatory cytokines; participate as inducer and effector cells in polarized Th1 responses; derive resistance against intracellular pathogens and tumours; and promote tissue destruction, and alternative M2 subsets, which generate anti‐inflammatory cytokines and contribute to tissue repair and remodelling as well as tumour progression. 105 , 106 Of note, it was indicated that anti‐inflammatory M2 macrophages become the more dominant macrophage population after berberine treatment in colitis mice. 73 In conclusion, the inhibitory effect of berberine on inflammatory macrophages can be considered as another mechanism through which ameliorates autoreactive T‐cell responses in autoimmune disorders.
6. BERBERINE‐MEDIATED ATTENUATING DEMYELINATION AND AUTOIMMUNE INFLAMMATION IN THE CENTRAL NERVOUS SYSTEM
As berberine can cross the blood‐brain barrier (BBB), evaluating the beneficial effects of berberine on neurodegenerative diseases has attracted extensive attention. 107 , 108 Berberine treatment has been indicated to effectively ameliorate the severity of EAE in C57 BL/6 mice either when evaluated clinically or by neuropathological criteria. Berberine significantly decreased the severity of clinical symptoms including the loss of tail tonicity, flaccid tail, ataxia and/or paresis of hindlimbs, complete paralysis of hindlimbs, as well as moribund or death in EAE mice. Neuropathological manifestations including the demyelination in the lumbar spinal cords and infiltration of inflammatory cells such as macrophages, T and B lymphocytes into CNS white matter in the lumbar spinal cords were markedly alleviated in berberine‐treated EAE mice. 109 , 110 Elevated permeability of BBB is mainly responsible for the infiltration of leucocytes into CNS and plays a key role in the initiation and progression of MS and EAE, 111 whereas preventing BBB alterations limits the severity and progression of the disease. 112 , 113 Interestingly, berberine‐mediated reduction of leucocyte infiltration and CNS inflammation in treated EAE mice was indicated to be due to reduced BBE permeability. 109 Of note, BBB permeability is known to be elevated by MMPs, 114 , 115 particularly the gelatinases, MMP‐2 and MMP‐9, 115 , 116 which exist in the brain and the cerebrospinal fluid (CSF) and govern migration of cells across the BBB through degrading type IV collagen and disrupting other components of the extracellular matrix surrounding blood vessels, resulting in disruption of the BBB integrity. 117 , 118 , 119 Inhibitory effect of berberine on BBB permeability was shown to be in part due to reducing the activity and expression of MMP‐9 in the brain and CSF of treated EAE mice. 109 , 110 MMPs also degrade laminins that are the major components of the extracellular matrix participating in neuronal development, survival, and regeneration. 120 Matrix proteins such as laminins are also widely disseminated throughout the brain parenchyma, and loss of parenchymal laminins may affect cell‐matrix interactions and cell survival. 121 , 122 , 123 , 124 The destruction of laminins around nerve cells by MMP‐9 can disrupt cell‐matrix interactions and further contribute to neuronal cell death. 125 , 126 Berberine administration could exert a neuroprotective effect on the brain following EAE through up‐regulating laminin activity simultaneously accompanied with the diminished MMP‐9 activity, which resulted in the decreased neuronal apoptosis. 127
In response to CNS pathologies, astrocytes are activated and the degree of their reactivity positively associates with the severity of MS and EAE. 128 , 129 Sphingosine‐1‐phosphate (S1P) is a lipid that binds to S1P1 in astrocytes and promotes essential steps in the pathogenesis of EAE through inducing the release of interleukins and other cytokines that mediate inflammatory responses. 130 Sphingosine kinase 1 (SphK1) is a kinase that phosphorylates and activates S1P, 131 and up‐regulated SphK1/S1P signalling is one of the key factors involves in astrocytes‐mediated inflammatory responses in MS pathogenesis. 131 , 132 Mechanistical study indicates that berberine can decrease demyelination and loss of neurophysiological function in EAE mice by suppressing the SphK1/S1P signalling pathway in astrocytes. 110 To sum up, berberine can attenuate clinical and pathological parameters of EAE in mice through reducing the demyelination in the lumbar spinal cords and alleviating leucocyte infiltration and CNS inflammation, together with neuroprotective effect, by maintaining BBB integrity and increasing parenchymal laminins via inhibiting MMP‐9 and SphK1/S1P signalling in the CSF and brain.
7. BERBERINE‐MEDIATED ATTENUATING AUTOIMMUNE INFLAMMATION IN THE PERIPHERAL NERVOUS SYSTEM
Guillain‐Barre syndrome (GBS) is an autoimmune disease attacking the peripheral nervous system (PNS) and characterized by inflammatory demyelination and axon damage. Experimental autoimmune neuritis (EAN) is a commonly‐used animal model recapitulating clinical symptoms and pathological features of human GBS 63 which is promoted by immunization with PNS myelin proteins or corresponding neurogenic peptides, such as P0 peptide 180‐199, combined with Freund's complete adjuvant. 64 , 133
The hallmark of EAN is PNS infiltration by inflammatory cells, particularly Th1 cells and macrophages, which secrete pro‐inflammatory cytokines such as TNF‐α at local sites of inflammation. Interestingly, berberine treatment was shown to significantly ameliorate EAN by suppressing both cellular and humoural immunity that are implicated in GBS/EAN. 66 In berberine‐treated EAN rats, clinical symptoms, including flaccid or dragging tail and hind limb paraparesis, were detected to be significantly alleviated. 66 The ameliorating effect of berberine on EAN was found to be accompanied by an inhibited proliferation of CD4+ T cells, down‐regulated both Th1 (TNF‐α) and Th2 (IL‐10) cytokines and decreased anti‐P0 peptide IgG1 and IgG2a. 66
Pro‐inflammatory cytokines, such as TNF‐α, have a destructive role in several autoimmune diseases, such as GBS, 134 , 135 , 136 Crohn's disease, 137 RA 138 and MS. 139 TNF‐α further promotes and recruits inflammatory cells 140 and decreases the permeability of the blood‐nerve barrier (BNB), through inducing MMPs to facilitate the infiltration. 141 In addition, TNF‐α suppresses the proliferation of Schwann cells (SC) and promotes SC death. 142 , 143 Therefore, the ameliorating effect of berberine on clinical symptoms of EAN can, in part, stem from inhibitory effects on TNF‐α secretion by Th1 cells. 66 Mitogen‐activated protein kinase (MAPK) signalling, a regulator of TNF‐α production, is known to be suppressed by berberine and can be a possible mechanism for the inhibitory effect of berberine on TNF‐α secretion by Th1 cells in EAN. 66
Besides, growing evidence shows that IL‐10, commonly known as an anti‐inflammatory cytokine, plays a key role in both the initiation and progression of autoimmune diseases, through activating proliferation and antibody production of B cells. 144 , 145 , 146 In GBS and EAN, IL‐10 secretion is elevated and positively associated with axonal nerve damage and antiganglioside antibodies. 146 This can explain the protective effects of berberine against neuropathy in EAN mice; however, underlying mechanisms remain largely unknown. 66
8. AMELIORATING EFFECTS OF BERBERINE ON OCULAR MANIFESTATIONS AND AUTOIMMUNE INFLAMMATION OF UVEITIS
Uveitis is a blinding intraocular inflammatory disorder caused by an autoimmune response implicated the uveal layers, the retina and vitreous. 147 , 148 Autoreactive retina‐specific T cells that secrete IFN‐γ or IL‐17A are generated in lymph nodes and spleen and cross the blood‐retinal barrier (BRB), whereafter inflammatory cells are recruited into the retina that eventually leads to full‐blown uveoretinal inflammation. 149 , 150 EAU is a widely used model of autoimmune uveitis in humans, possessing an acute and severe inflammation involving both the anterior and posterior segments of the eye. 151 , 152
Of note, berberine has been found to ameliorate ocular manifestations of EAU in the experimental model. 76 , 153 Berberine‐treated EAU mice showed alleviated anterior chamber inflammation and attenuated clinical manifestations including corneal oedema, ciliary injection of the cornea, and cells in the aqueous humour as well as posterior synechiae. 76 Berberine treatment could also attenuate BRB breakdown and decrease histological characteristics of uveitis, including massive inflammatory cells in the choroid and retina, retinal folds and damage of photoreceptor cells. 76 Such inhibitory effect of berberine on ocular manifestations of EAU was found to be accompanied by a decreased frequency of pathogenic Th1 and Th17 cells and a small elevation of Tregs as well as a remarkable alteration in intestinal microbial composition (as discussed with detail in the previous section). 76 Similar results were also reported by another study on berberine‐treated EAU rats. 153 These findings are further supported by clinical studies showing blocking effects of berberine on inflammatory T cells in patients with Ocular Behcet's disease 67 and/or Vogt‐Koyanagi‐Harada, 68 the most common causes of uveitis.
9. CONCLUSION
Growing evidence witnessed by the in vitro and in vivo experimental studies reveals that berberine has the potential to ameliorate destructive autoreactive inflammation in autoimmune conditions (Figure 3). Berberine can directly suppress pro‐inflammatory responses of Th1 and Th17 cells by inhibiting the function and differentiation of these cells, mechanistically, through hampering STAT and RORγt signalling pathways. Berberine is also found to indirectly decrease Th cell‐mediated inflammation through modulating or suppressing other cells assisting autoreactive inflammation, such as Tregs, DCs and macrophages. Imbalance of Treg/Th17 cells is an important hallmark of autoimmune disorders, and berberine has been found to induce differentiation of Tregs in autoimmune conditions through two distinct mechanisms, directly by modulation of naïve CD4+ T cells’ differentiation and indirectly by affecting pattern of the gut microbiota. Also, berberine can decrease survival and inflammatory functions of DCs through inducing apoptosis and inhibiting co‐stimulatory molecules and inflammatory cytokine secretion, which is accompanied with a reduction of Th1/Th17 population and amelioration of the severity and progression of autoimmune complications. Likewise, berberine treatment can elevate the population of anti‐inflammatory M2 macrophages and suppress M1 macrophages producing pro‐inflammatory cytokines, resulting in amelioration of autoreactive T‐cell responses in autoimmune disorders. To our knowledge, all reported ameliorating effects of berberine on T cell‐mediated autoimmune inflammation are based on preclinical and cell culture investigations. Hence, further investigations are required to determine the clinical efficiency of berberine in patients with autoimmunity.
FIGURE 3.
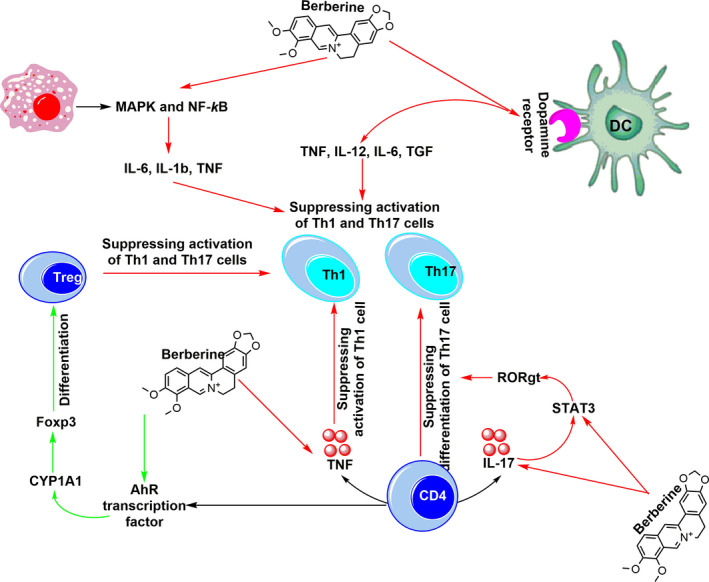
The direct and indirect inhibitory effects of berberine on autoreactive Th17 and Th1 cells. Through the direct route, berberine suppresses differentiation of CD4 cells into Th17 and Th1 cells and inhibits activation of these cells through decreasing expression of TNF and IL17 cytokines via inhibiting STAT3 and RORgt. Through the indirect route, berberine suppresses the activation of both Th17 and Th1 cells via modulating the activity of macrophages and DCs via suppressing the production of inflammatory cytokine. Further suppressive effect of berberine on Th17 and Th1 cells is achieved through its promoting impact on Treg differentiation and activation via inducing activity of AhR transcription factor, CYP1A1 and Foxp3. Green and red arrows reflect promoting and inhibiting effects of berberine, respectively
CONFLICT OF INTEREST
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
AUTHOR CONTRIBUTIONS
Seyed‐Morteza Ehteshamfar: Writing‐original draft (equal). Masoume Akhbari: Writing‐review & editing (lead). Jalil Tavakol Afshari: Validation (lead). Motahareh Seyedi: Software (lead). Banafsheh Nikfar: Investigation (equal). Abbas Shapouri‐Moghaddam: Supervision (equal); Validation (equal). Erfan Ghanbarzadeh: Investigation (equal); Project administration (equal). Amir Abaas Momtazi‐Borojeni: Conceptualization (lead); Project administration (supporting); Supervision (equal); Validation (equal).
Ehteshamfar S‐M, Akhbari M, Afshari JT, et al. Anti‐inflammatory and immune‐modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J Cell Mol Med. 2020;24:13573–13588. 10.1111/jcmm.16049
Seyed‐Morteza Ehteshamfar and Masoume Akhbari are contributed equally to this work and should be considered co‐first authors.
Contributor Information
Abbas Shapouri‐Moghaddam, Email: abbasmomtazi@yahoo.com.
Erfan Ghanbarzadeh, Email: shapourimoghaddama@gmail.com, Email: GhahremaniA3@mums.ac.ir, Email: momtaziaa921@mums.ac.ir, Email: abbasmomtazi@yahoo.com.
Amir Abbas Momtazi‐Borojeni, Email: abbasmomtazi@yahoo.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Merrill JE, Kono DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide‐induced disease of experimental allergic encephalomyelitis in SJL and B10. PL mice. Proc Natl Acad Sci USA. 1992;89:574‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL‐12–and IL‐23–modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Renno T, Krakowski M, Piccirillo C, Lin J, Owens T. TNF‐alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944‐953. [PubMed] [Google Scholar]
- 4. Pettinelli C, McFarlin D. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2‐T lymphocytes. J Immunol. 1981;127:1420‐1423. [PubMed] [Google Scholar]
- 5. Panitch HS. Interferons in multiple sclerosis. Drugs. 1992;44:946‐962. [DOI] [PubMed] [Google Scholar]
- 6. Germann T, Hess H, Szeliga J, Rüde E. Characterization of the adjuvant effect of IL‐12 and efficacy of IL‐12 inhibitors in type II collagen‐induced arthritis. Ann N Y Acad Sci. 1996;795:227‐240. [DOI] [PubMed] [Google Scholar]
- 7. Caspi RR, Silver PB, Chan C‐C, et al. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol. 1996;157:2668‐2675. [PubMed] [Google Scholar]
- 8. Tarrant TK, Silver PB, Chan C‐C, Wiggert B, Caspi RR. Endogenous IL‐12 is required for induction and expression of experimental autoimmune uveitis. J Immunol. 1998;161:122‐127. [PubMed] [Google Scholar]
- 9. Zhang Y‐Y, Li J‐N, Xia HH‐X, et al. Protective effects of losartan in mice with chronic viral myocarditis induced by coxsackievirus B3. Life Sci. 2013;92:1186‐1194. [DOI] [PubMed] [Google Scholar]
- 10. Tajiri K, Imanaka‐Yoshida K, Matsubara A, et al. Suppressor of cytokine signaling 1 DNA administration inhibits inflammatory and pathogenic responses in autoimmune myocarditis. J Immunol. 2012;189:2043‐2053. [DOI] [PubMed] [Google Scholar]
- 11. Lock C, Hermans G, Pedotti R, et al. Gene‐microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500‐508. [DOI] [PubMed] [Google Scholar]
- 12. Matusevicius D, Kivisäkk P, He B, et al. Interleukin‐17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Multi Scler J. 1999;5:101‐104. [DOI] [PubMed] [Google Scholar]
- 13. Teunissen MB, Bos JD, Koomen CW, de Waal MR, Wierenga EA. Interleukin‐17 and interferon‐γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645‐649. [DOI] [PubMed] [Google Scholar]
- 14. Aarvak T, Chabaud M, Miossec P, Natvig JB. IL‐17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246‐1251. [PubMed] [Google Scholar]
- 15. Amadi‐Obi A, Yu C‐R, Liu X, et al. T H 17 cells contribute to uveitis and scleritis and are expanded by IL‐2 and inhibited by IL‐27/STAT1. Nat Med. 2007;13:711. [DOI] [PubMed] [Google Scholar]
- 16. Chi W, Zhu X, Yang P, et al. Upregulated IL‐23 and IL‐17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058‐3064. [DOI] [PubMed] [Google Scholar]
- 17. Langrish CL, Chen Y, Blumenschein WM, et al. IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolls JK, Lindén A. Interleukin‐17 family members and inflammation. Immunity. 2004;21:467‐476. [DOI] [PubMed] [Google Scholar]
- 19. Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor‐β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151‐1156. [DOI] [PubMed] [Google Scholar]
- 20. Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)‐1 in the induction of IL‐17–producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kotake S, Udagawa N, Takahashi N, et al. IL‐17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Investig. 1999;103:1345‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL‐21 in the generation of inflammatory T cells. Nature. 2007;448:480‐483. [DOI] [PubMed] [Google Scholar]
- 24. Liang SC, Tan X‐Y, Luxenberg DP, et al. Interleukin (IL)‐22 and IL‐17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen‐induced arthritis in IL‐17‐deficient mice. J Immunol. 2003;171:6173‐6177. [DOI] [PubMed] [Google Scholar]
- 26. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell. 2006;126:1121‐1133. [DOI] [PubMed] [Google Scholar]
- 27. Alunno A, Manetti M, Caterbi S, et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators Inflamm. 2015;2015:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330‐336. [DOI] [PubMed] [Google Scholar]
- 30. Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally occurring CD4+ regulatory T‐cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113‐123. [DOI] [PubMed] [Google Scholar]
- 32. Ma W‐T, Gao F, Gu K, Chen D‐K. The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee KH, Ahn BS, Cha D, et al. Understanding the immunopathogenesis of autoimmune diseases by animal studies using gene therapy: a comprehensive review. Autoimmun Rev. 2020;102469. [DOI] [PubMed] [Google Scholar]
- 34. Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19:1297‐1307. [DOI] [PubMed] [Google Scholar]
- 35. Kuo C‐L, Chi C‐W, Liu T‐Y. The anti‐inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127‐137. [DOI] [PubMed] [Google Scholar]
- 36. Kumar A, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015;761:288‐297. [DOI] [PubMed] [Google Scholar]
- 37. Cicero AF, Baggioni A. Berberine and Its Role in Chronic Disease. Anti‐Inflammatory Nutraceuticals and Chronic Diseases. Berlin, Germany: Springer; 2016:27‐45. [DOI] [PubMed] [Google Scholar]
- 38. Zhang M, Feng L, Li J, Chen L. Therapeutic potential and mechanisms of berberine in cardiovascular disease. Curr Pharmacol Rep. 2016;2:281‐292. [Google Scholar]
- 39. Ayati SH, Fazeli B, Momtazi‐Borojeni AA, Cicero AF, Pirro M, Sahebkar A. Regulatory effects of berberine on microRNome in cancer and other conditions. Crit Rev Oncol Hematol. 2017;116:147‐158. [DOI] [PubMed] [Google Scholar]
- 40. Mortazavi H, Nikfar B, Esmaeili S‐A, et al. Potential cytotoxic and anti‐metastatic effects of berberine on gynaecological cancers with drug‐associated resistance. Eur J Med Chem. 2020;187:111951. [DOI] [PubMed] [Google Scholar]
- 41. Fatahian A, Haftcheshmeh SM, Azhdari S, Farshchi HK, Nikfar B, Momtazi‐Borojeni AA. Promising anti‐atherosclerotic effect of berberine: evidence from in vitro, in vivo, and clinical studies. Rev Physiol Biochem Pharmacol. 2020;2020:1–28. [DOI] [PubMed] [Google Scholar]
- 42. Shen P, Jiao Y, Miao L, Chen J‐H, Momtazi‐Borojeni AA. Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J Cell Mol Med. 2020. 10.1111/jcmm.15803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126:5‐17. [DOI] [PubMed] [Google Scholar]
- 44. Chen F, Yang Z, Liu Y, et al. Berberine inhibits the expression of TNFα, MCP‐1, and IL‐6 in AcLDL‐stimulated macrophages through PPARγ pathway. Endocrine. 2008;33:331‐337. [DOI] [PubMed] [Google Scholar]
- 45. Kim S, Kim Y, Kim JE, Cho KH, Chung JH. Berberine inhibits TPA‐induced MMP‐9 and IL‐6 expression in normal human keratinocytes. Phytomedicine. 2008;15:340‐347. [DOI] [PubMed] [Google Scholar]
- 46. Ho Y‐T, Yang J‐S, Li T‐C, et al. Berberine suppresses in vitro migration and invasion of human SCC‐4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF‐κB, u‐PA and MMP‐2 and‐9. Cancer Lett. 2009;279:155‐162. [DOI] [PubMed] [Google Scholar]
- 47. Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bettelli E, Oukka M, Kuchroo VK. TH‐17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345‐350. [DOI] [PubMed] [Google Scholar]
- 49. Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL‐17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821‐852. [DOI] [PubMed] [Google Scholar]
- 50. Serada S, Fujimoto M, Mihara M, et al. IL‐6 blockade inhibits the induction of myelin antigen‐specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041‐9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uyttenhove C, Sommereyns C, Theate I, Michiels T, Van Snick J. Anti‐IL‐17A autovaccination prevents clinical and histological manifestations of experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 2007;1110:330‐336. [DOI] [PubMed] [Google Scholar]
- 52. Uyttenhove C, Van Snick J. Development of an anti‐IL‐17A auto‐vaccine that prevents experimental auto‐immune encephalomyelitis. Eur J Immunol. 2006;36:2868‐2874. [DOI] [PubMed] [Google Scholar]
- 53. Komiyama Y, Nakae S, Matsuki T, et al. IL‐17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566‐573. [DOI] [PubMed] [Google Scholar]
- 54. Tzartos JS, Friese MA, Craner MJ, et al. Interleukin‐17 production in central nervous system‐infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomas K, Bettelli E, Oukka M, Kuchroo VK. IL‐17 and Th17 cells. Annu Rev Immunol. 2009;27:485‐517. [DOI] [PubMed] [Google Scholar]
- 56. Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933‐944. [DOI] [PubMed] [Google Scholar]
- 58. Kaplan MH, Sun Y‐L, Hoey T, Grusby MJ. Impaired IL‐12 responses and enhanced development of Th2 cells in Stat4‐deficient mice. Nature. 1996;382:174‐177. [DOI] [PubMed] [Google Scholar]
- 59. Thierfelder WE, van Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin‐12‐mediated responses of natural killer and T cells. Nature. 1996;382:171‐174. [DOI] [PubMed] [Google Scholar]
- 60. Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell. 2006;126:1121‐1133. [DOI] [PubMed] [Google Scholar]
- 62. Marinova EK, Nikolova DB, Popova DN, Gallacher GB, Ivanovska ND. Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine. Immunopharmacology. 2000;48:9‐16. [DOI] [PubMed] [Google Scholar]
- 63. Xia RH, Yosef N, Ubogu EE. Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain‐Barré syndrome. J Neuroimmunol. 2010;219:54‐63. [DOI] [PubMed] [Google Scholar]
- 64. Li X‐L, Dou Y‐C, Liu Y, et al. Atorvastatin ameliorates experimental autoimmune neuritis by decreased Th1/Th17 cytokines and up‐regulated T regulatory cells. Cell Immunol. 2011;271:455‐461. [DOI] [PubMed] [Google Scholar]
- 65. Zou LP, Ljunggren HG, Levi M, et al. P0 protein peptide 180–199 together with pertussis toxin induces experimental autoimmune neuritis in resistant C57BL/6 mice. J Neurosci Res. 2000;62:717‐721. [DOI] [PubMed] [Google Scholar]
- 66. Li H, Li XL, Zhang M, et al. Berberine ameliorates experimental autoimmune neuritis by suppressing both cellular and humoral immunity. Scand J Immunol. 2014;79:12‐19. [DOI] [PubMed] [Google Scholar]
- 67. Yang Y, Wang Q, Xie M, et al. Berberine exerts an anti‐inflammatory role in ocular Behcet's disease. Mol Med Rep. 2017;15:97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang Y, Qi J, Wang Q, et al. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci. 2013;54:2516‐2522. [DOI] [PubMed] [Google Scholar]
- 69. Yue M, Xia Y, Shi C, et al. Berberine ameliorates collagen‐induced arthritis in rats by suppressing Th17 cell responses via inducing cortistatin in the gut. FEBS J. 2017;284:2786‐2801. [DOI] [PubMed] [Google Scholar]
- 70. Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016;79:222‐230. [DOI] [PubMed] [Google Scholar]
- 71. Tong B, Yuan X, Dou Y, et al. Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. Int J Biochem Cell Biol. 2016;75:63‐73. [DOI] [PubMed] [Google Scholar]
- 72. Qin X, Guo BT, Wan B, et al. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185:1855‐1863. [DOI] [PubMed] [Google Scholar]
- 73. Li C, Xi Y, Li S, et al. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Mol Immunol. 2015;67:444‐454. [DOI] [PubMed] [Google Scholar]
- 74. Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323‐6327. [DOI] [PubMed] [Google Scholar]
- 75. Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191‐199. [DOI] [PubMed] [Google Scholar]
- 76. Du Z, Wang Q, Huang X, et al. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int Immunopharmacol. 2020;81:106270. [DOI] [PubMed] [Google Scholar]
- 77. Greer RL, Dong X, Moraes ACF, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat Commun. 2016;7:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vital M, Karch A, Pieper DH. Colonic butyrate‐producing communities in humans: an overview using omics data. mSystems. 2017;2:e00130‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature. 2013;504:451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li J, Sung CYJ, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA. 2016;E1306‐E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luu M, Pautz S, Kohl V, et al. The short‐chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic‐epigenetic crosstalk in lymphocytes. Nat Commun. 2019;10:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shimizu J, Kubota T, Takada E, et al. Bifidobacteria abundance‐featured gut microbiota compositional change in patients with Behcet’s disease. PLoS One. 2016;11:e0153746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Janowitz C, Nakamura YK, Metea C, et al. Disruption of intestinal homeostasis and intestinal microbiota during experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2019;60:420‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zegarra‐Ruiz DF, El Beidaq A, Iñiguez AJ, et al. A diet‐sensitive commensal Lactobacillus strain mediates TLR7‐dependent systemic autoimmunity. Cell Host Microbe. 2019;25:113‐127.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cui H, Cai Y, Wang L, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front Pharmacol. 2018;9:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T‐cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204‐12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dinesh P, Rasool M. Berberine mitigates IL‐21/IL‐21R mediated autophagic influx in fibroblast‐like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis. 2019;24:644‐661. [DOI] [PubMed] [Google Scholar]
- 89. Li Y‐H, Xiao H‐T, Hu D‐D, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium‐induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227‐239. [DOI] [PubMed] [Google Scholar]
- 90. Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420‐28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wan B, Nie H, Liu A, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844‐8850. [DOI] [PubMed] [Google Scholar]
- 92. Odobasic D, Leech MT, Xue JR, Holdsworth SR. Distinct in vivo roles of CD80 and CD86 in the effector T‐cell responses inducing antigen‐induced arthritis. Immunology. 2008;124:503‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen Y, Langrish CL, McKenzie B, et al. Anti‐IL‐23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gutcher I, Becher B. APC‐derived cytokines and T cell polarization in autoimmune inflammation. J Clin Investig. 2007;117:1119‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kawano M, Takagi R, Kaneko A, Matsushita S. Berberine is a dopamine D1‐and D2‐like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J Neuroimmunol. 2015;289:43‐55. [DOI] [PubMed] [Google Scholar]
- 96. Magro F, Vieira‐Coelho M, Fraga S, et al. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5‐hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216‐224. [DOI] [PubMed] [Google Scholar]
- 97. Magro F, Cunha E, Araujo F, et al. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig Dis Sci. 2006;51:2039‐2044. [DOI] [PubMed] [Google Scholar]
- 98. Tarbell KV, Rahman MJ. Dendritic Cells in Autoimmune Disease. The Autoimmune Diseases. Cambridge, MA: Elsevier; 2020:213‐227. [Google Scholar]
- 99. Hu Z, Jiao Q, Ding J, et al. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Rheum. 2011;63:949‐959. [DOI] [PubMed] [Google Scholar]
- 100. Laria A, Lurati A, Marrazza M, Mazzocchi D, Re KA, Scarpellini M. The macrophages in rheumatic diseases. J Inflamm Res. 2016;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yan F, Wang L, Shi Y, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS‐treated mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G504‐G514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Magnusson MK, Brynjólfsson SF, Dige A, et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016;9:171‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015;6:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and T H 1‐T H 17 responses. Nat Immunol. 2011;12:231. [DOI] [PubMed] [Google Scholar]
- 106. Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176‐185. [DOI] [PubMed] [Google Scholar]
- 107. Cai Z, Wang C, Yang W. Role of berberine in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2016;12:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang M, Chen S, Liang Y, Guo Y. The role of berberine in the multi‐target treatment of senile dementia. Curr Top Med Chem. 2016;16:867‐873. [DOI] [PubMed] [Google Scholar]
- 109. Ma X, Jiang Y, Wu A, et al. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One. 2010;5:e13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Luo J, Chen R, Zeng S, et al. The effects of berberine on a murine model of multiple sclerosis and the SPHK1/S1P signaling pathway. Biochem Biophys Res Comm. 2017;490:927‐932. [DOI] [PubMed] [Google Scholar]
- 111. de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood‐brain barrier in neuroinflammatory diseases. Pharmacol Rev. 1997;49:143‐156. [PubMed] [Google Scholar]
- 112. Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC. Loss of blood–brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci USA. 2007;104:5656‐5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood‐central nervous system barrier integrity. J Immunol. 2000;165:6511‐6518. [DOI] [PubMed] [Google Scholar]
- 114. Avolio C, Ruggieri M, Giuliani F, et al. Serum MMP‐2 and MMP‐9 are elevated in different multiple sclerosis subtypes. J Neuroimmunol. 2003;136:46‐53. [DOI] [PubMed] [Google Scholar]
- 115. Hartung H‐P, Kieseier BC. The role of matrix metalloproteinases in autoimmune damage to the central and peripheral nervous system. J Neuroimmunol. 2000;107:140‐147. [DOI] [PubMed] [Google Scholar]
- 116. Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mun‐Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood‐brain barrier during inflammation. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1203‐R1211. [DOI] [PubMed] [Google Scholar]
- 118. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121‐125. [DOI] [PubMed] [Google Scholar]
- 119. Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP‐2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391‐398. [DOI] [PubMed] [Google Scholar]
- 120. Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618‐624. [DOI] [PubMed] [Google Scholar]
- 121. Hagg T, Muir D, Engvall E, Varon S, Manthorpe M. Laminin‐like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 1989;3:721‐732. [DOI] [PubMed] [Google Scholar]
- 122. Murtomäki S, Trenkner E, Wright JM, Saksela O, Liesi P. Increased proteolytic activity of the granule neurons may contribute to neuronal death in the weaver mouse cerebellum. Dev Biol. 1995;168:635‐648. [DOI] [PubMed] [Google Scholar]
- 123. Chen Z‐L, Strickland S. Neuronal death in the hippocampus is promoted by plasmin‐catalyzed degradation of laminin. Cell. 1997;91:917‐925. [DOI] [PubMed] [Google Scholar]
- 124. Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase‐9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401‐6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gu Z, Kaul M, Yan B, et al. S‐nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186‐1190. [DOI] [PubMed] [Google Scholar]
- 127. Jiang Y, Wu A, Zhu C, et al. The protective effect of berberine against neuronal damage by inhibiting matrix metalloproteinase‐9 and laminin degradation in experimental autoimmune encephalomyelitis. Neurol Res. 2013;35:360‐368. [DOI] [PubMed] [Google Scholar]
- 128. Correale J, Farez MF. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ludwin SK, Rao VTS, Moore CS, Antel JP. Astrocytes in multiple sclerosis. Multi Scler J. 2016;22:1114‐1124. [DOI] [PubMed] [Google Scholar]
- 130. Choi JW, Gardell SE, Herr DR, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1‐phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108:751‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Obinata H, Hla T. Sphingosine 1‐Phosphate in Coagulation and Inflammation. Seminars in Immunopathology. Berlin, Germany: Springer; 2012;73‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fischer I, Alliod C, Martinier N, Newcombe J, Brana C, Pouly S. Sphingosine kinase 1 and sphingosine 1‐phosphate receptor 3 are functionally upregulated on astrocytes under pro‐inflammatory conditions. PLoS One. 2011;6:e23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zou LP, Ljunggren HG, Levi M, et al. P0 protein peptide 180–199 together with pertussis toxin induces experimental autoimmune neuritis in resistant. J Neurosci Res. 2000;62:717‐721. [DOI] [PubMed] [Google Scholar]
- 134. Lu M‐O,Zhu J. The role of cytokines in Guillain‐Barré syndrome. J Neurol. 2011;258:533‐548. [DOI] [PubMed] [Google Scholar]
- 135. Radhakrishnan V, Sumi M, Reuben S, Mathai A, Nair MJ. Serum tumour necrosis factor‐α and soluble tumour necrosis factor receptors levels in patients with Guillain‐Barre syndrome. Acta Neurol Scand. 2004;109:71‐74. [DOI] [PubMed] [Google Scholar]
- 136. Zhang J, Dong H, Li B,Li C‐Y, Guo L. Association of tumor necrosis factor polymorphisms with Guillain‐Barre syndrome. Eur Neurol. 2007;58:21‐25. [DOI] [PubMed] [Google Scholar]
- 137. Noth R, Stüber E, Häsler R, et al. Anti‐TNF‐α antibodies improve intestinal barrier function in Crohn's disease. J Crohns Colitis. 2012;6:464‐469. [DOI] [PubMed] [Google Scholar]
- 138. Moelants EA, Mortier A, Van Damme J, Proost PJ. Regulation of TNF‐α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91:393‐401. [DOI] [PubMed] [Google Scholar]
- 139. Özenci V, Kouwenhoven M, Huang YM, Kivisäkk P, Link HJ. Multiple sclerosis is associated with an imbalance between tumour necrosis factor‐alpha (TNF‐α)‐and IL‐10‐secreting blood cells that is corrected by interferon‐beta (IFN‐β) treatment. Clin Exp Immunol. 2000;120:147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang H‐L, Hassan MY, Zheng X‐Y, et al. Attenuated EAN in TNF‐α deficient mice is associated with an altered balance of M1/M2 macrophages. PLoS One. 2012;7:e38157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFα‐induced MMP‐9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Boyle K, Azari MF, Cheema SS, Petratos S. TNFα mediates Schwann cell death by upregulating p75NTR expression without sustained activation of NFκB. Neurobiol Dis. 2005;20:412‐427. [DOI] [PubMed] [Google Scholar]
- 143. Tao T, Ji Y, Cheng C, et al. Tumor necrosis factor‐alpha inhibits Schwann cell proliferation by up‐regulating Src‐suppressed protein kinase C substrate expression. J Neurochem. 2009;111:647‐655. [DOI] [PubMed] [Google Scholar]
- 144. Howard M, O'Garra A, Ishida H, de Waal Malefyt R, De Vries J. Biological properties of interleukin. J Clin Immunol. 1992;10(12):239‐247. [DOI] [PubMed] [Google Scholar]
- 145. Huang Y, Kivisäkk P, Özenci V, Pirskanen R, Link H. Increased levels of circulating acetylcholine receptor (AChR)‐reactive IL‐10‐secreting cells are characteristic for myasthenia gravis (MG). Clin Exp Immunol. 1999;118:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Press R, Deretzi G, Zou LP, et al. IL‐10 and IFN‐γ in Guillain–Barré syndrome. J Neuroimmunol. 2001;112:129‐138. [DOI] [PubMed] [Google Scholar]
- 147. Witkowski L, Cywinska A, Paschalis‐Trela K, Crisman M, Kita J. Multiple etiologies of equine recurrent uveitis – A natural model for human autoimmune uveitis: a brief review. Comp Immunol Microbiol Infect Dis. 2016;44:14‐20. [DOI] [PubMed] [Google Scholar]
- 148. Rothova A, Buitenhuis HJ, Meenken C, et al. Uveitis and systemic disease. Br J Ophthalmol. 1992;76:137‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073‐3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Horai R, Caspi RR. Cytokines in autoimmune uveitis. J Interferon Cytokine Res. 2011;31:733‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Caspi R, Roberge F, Chan C, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490‐1495. [PubMed] [Google Scholar]
- 152. Jiang H‐R, Lumsden L, Forrester JV. Macrophages and dendritic cells in IRBP‐induced experimental autoimmune uveoretinitis in B10RIII mice. Invest Ophthalmol Vis Sci. 1999;40:3177‐3185. [PubMed] [Google Scholar]
- 153. Li M, Chen X, Liu J, et al. Treatment of experimental autoimmune uveoretinitis with different natural compounds. Mol Med Rep. 2016;13:4654‐4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tomita M, Kugo T. Alkaloids of Berberidaceous plants‐XIX: Alkaloids of B. tschonoskyana I. Isolation of bases. Yakugak Zasshi. 1956;79:317‐321. [Google Scholar]
- 155. Parsons H. Examination of the root of Berberis aquifolium, v. alpens, “oregon grape root”. Pharm J. 1882;13:46‐48. [Google Scholar]
- 156. Chakravarti K, Dhar D, Siddiqui S. Alkaloidal consistuents of the bark of Berberis aristata. J Sci Ind Res. 1950;7:161‐164. [Google Scholar]
- 157. Amritpal S, Sanjiv D, Navpreet K, Jaswinder S. Berberine: alkaloid with wide spectrum of pharmacological activities. J Nat Prod. 2010;3:64‐75. [Google Scholar]
- 158. Kamal Y, Singh M, Tamboli E, Parveen R, Ahmad S. Quantitative analysis of berberine in Berberis aristata fruits and in a traditional anti‐inflammatory unani formulation by use of a validated HPLC method. Acta Chromatogr. 2011;23:157‐168. [Google Scholar]
- 159. Andola HC, Rawal R, Rawat M, Bhatt I, Purohit VK. Variations of berberine contents in Berberis pseudumbellata: a high value medicinal shrub of west Himalaya, India. Medicinal Plants. 2010;2:111‐115. [Google Scholar]
- 160. Rajasekaran A, Pokhriyal R, Singh Y. Quantitative estimation of berberine in roots of different provenances of Berberis aristata DC by HPLC and study of their antifungal properties. Phcog Mag. 2009;5:355. [Google Scholar]
- 161. Singh J, Kakkar P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J Ethnopharmacol. 2009;123:22‐26. [DOI] [PubMed] [Google Scholar]
- 162. Srivastava SK, Khatoon S, Rawat AKS, Mehrotra S. Pharmacognostic evaluation of the root of Berberis aristata . Pharm Biol. 2001;7:102‐106. [Google Scholar]
- 163. Srivastava SK, Singh Rawat AK, Mehrotra S. Pharmacognostic evaluation of the root of Berberis aristata . Pharm Biol. 2004;42:467‐473. [Google Scholar]
- 164. Willaman JJ, Schubert B. Alkaloid‐Bearing Plants and Their Contained Alkaloids. US Department of Agriculture; 1961. [Google Scholar]
- 165. Garhwal S. Analysis of berberine content using HPTLC fingerprinting of root and bark of three Himalayan Berberis species. Asian J Biotechnol. 2010;2:239‐245. [Google Scholar]
- 166. Steffens P, Nagakura N, Zenk MH. Purification and characterization of the berberine bridge enzyme from berberis beaniana cell cultures. Phytochemistry. 1985;24:2577‐2583. [Google Scholar]
- 167. Bonesi M, Loizzo MR, Conforti F, et al. Berberis aetnensis and B libanotica: a comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer's disease and antioxidant activity. J Pharm Pharmacol. 2013;65:1726‐1735. [DOI] [PubMed] [Google Scholar]
- 168. Musumeci R, Speciale A, Costanzo R, et al. Berberis aetnensis C. Presl. extracts: antimicrobial properties and interaction with ciprofloxacin. Int J Antimicrob Agents. 2003;22:48‐53. [DOI] [PubMed] [Google Scholar]
- 169. Hirschhorn HH. Botanical remedies of South and Central America, and the Caribbean: an archival analysis. Part I. J Ethnopharmacol. 1981;4:129‐158. [DOI] [PubMed] [Google Scholar]
- 170. Hussaini FA, Shoeb AJP. Isoquinoline derived alkaloids from Berberis chitria. Phytochemistry. 1985;24:633. [Google Scholar]
- 171. Srivastava SK, Rai V, Srivastava M, Rawat A, Mehrotra S. Estimation of heavy metals in different berberis species and its market samples. Environ Monit Assess. 2006;116:315‐320. [DOI] [PubMed] [Google Scholar]
- 172. Srivastava SK, Rawat AKS, Srivastava M, Mehrotra S. Pharmacognostic evaluation of the roots of Berberis chitria Lindl. Nat Prod Sci. 2006;12:19. [Google Scholar]
- 173. Končić MZ, Kremer D, Karlović K, Kosalec I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem Toxicol. 2010;48:2176‐2180. [DOI] [PubMed] [Google Scholar]
- 174. Chatterjee R. Plant alkaloids. Part I‐ B. Floribunda Wal. Ex. Don. J Indian Chem Soc. 1951;28:225‐228. [Google Scholar]
- 175. Neag MA, Mocan A, Echeverría J, et al. Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic and renal disorders. Front Pharmacol. 2018;9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Freile M, Giannini F, Sortino M, et al. Antifungal activity of aqueous extracts and of berberine isolated from Berberis heterophylla . Fitoterapia. 2006;25:83. [DOI] [PubMed] [Google Scholar]
- 177. Singh R, Tiwari SS, Srivastava S, Rawat A. Botanical and phytochemical studies on roots of Berberis umbellata Wall. ex G. Don. Indian J Nat Prod Resources. 2012;3:55‐60. [Google Scholar]
- 178. Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999‐1012. [DOI] [PubMed] [Google Scholar]
- 179. Mell C. Interesting sources of natural dyestuffs. Textile Colorist. 1929;58:128. [Google Scholar]
- 180. Buzas A, Egnell C. On the presence of quinidine in addition to berberine alkaloids in the barks of Enantia pilosa and Enantia polycarpa (Annonaceae). Ann Pharm Fr. 1965;23:351‐354. [PubMed] [Google Scholar]
- 181. Chen Y‐Y, Chang F‐R, Wu Y‐C. Isoquinoline alkaloids and lignans from Rollinia mucosa . J Nat Prod. 1996;59:904‐906. [Google Scholar]
- 182. Slavikova L, Shun T, Slavik J. Alkaloide der mohngewächse (Papaveraceae) XIV. Alkaloide aus Argemone alba LESTIB. Collect Czech Chem Commun. 1960;25:756‐760. [Google Scholar]
- 183. Haisova K, Slavík J. On the minor alkaloids from Argemone mexicana L. Collect Czech Chem Commun. 1975;40:1576‐1578. [Google Scholar]
- 184. Israilov I, Chelombit'ko V, Nazarova LE. Argemone alkaloids. Chem Nat Compd. 1986;22:742‐743. [Google Scholar]
- 185. Bapna S, Choudhary PK, Ramaiya M, Chowdhary A. Antiplasmodial activity of Argemone mexicana: an in vivo and in vitro study. World J Pharm Res. 2015;4:1653‐1663. [Google Scholar]
- 186. Shivlal S. Quantitative analysis of berberine in Argemone mexicana Linn. (Papaveraceae) using HPLC and HPTLC. Adv Plant Sci. 2014;27:209‐211. [Google Scholar]
- 187. Kukula‐Koch W, Mroczek TJA. Application of hydrostatic CCC–TLC–HPLC–ESI‐TOF‐MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal Bioanal Chem. 2015;407:2581‐2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Fletcher M, Takken G, Blaney B, Alberts V. Isoquinoline alkaloids and keto‐fatty acids of Argemone ochroleuca and A mexicana (Mexican poppy) seed. I. An assay method and factors affecting their concentration. Aust J Agric Res. 1993;44:265‐275. [Google Scholar]
- 189. Israilov I, Yunusov M. Alkaloids of four species of Argemone. Chem Nat Compd. 1986;22:189‐192. [Google Scholar]
- 190. Stermitz FR. Alkaloids of the Papaveraceae V. Muramine and berberine from Argemone squarrosa . J Pharm Sci. 1967;56:760‐762. [DOI] [PubMed] [Google Scholar]
- 191. Táborská E, Věžník F, Slavík J. Alkaloids from Bocconia frutescens L. Collect Czech Chem Commun. 1980;45:1301‐1304. [Google Scholar]
- 192. Jusiak L. Separation of Chelidonium majus alkaloids by countercurrent cascade extraction. II. Acta Pol Pharm. 1967;24:65‐70. [Google Scholar]
- 193. Jha R, Pandey M, Singh A, Singh S,Singh V. New alkaloids from Corydalis species. Nat Prod Res. 2009;23:250‐255. [DOI] [PubMed] [Google Scholar]
- 194. Sener B, Temizer H. Pharmacognosic investigations on Corydalis solida (L.) Swartz ssp. brachyloba (Boiss.) Cullen & Davis. II. Alkaloids of Corydalis solida (L.) Swartz ssp. brachyloba (Boiss.) Cullen & Davis. J Fac Pharm Gazi Univ. 1988;5:9‐11. [Google Scholar]
- 195. Sener B, Temizer H. Chemical studies on the minor isoquinoline alkaloids from Corydalis solida subsp. brachyloba. J Chem Soc. 1991;13:63‐66. [Google Scholar]
- 196. Doncheva T, Kostova N, Yordanova G, et al. Comparison of alkaloid profile from Glaucium corniculatum (Papaveraceae) of Algerian and Bulgarian origin. Biochem Syst Ecol. 2014;56:278‐280. [Google Scholar]
- 197. Pěnčíková K, Urbanová J, Musil P, Táborská E, Gregorová JJM. Seasonal variation of bioactive alkaloid contents in Macleaya microcarpa (Maxim.). Fedde. 2011;16:3391‐3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kubota M, Katsunori M,Miyazawa Y. Berberine contents in cultivated Coptis japonica Makino. Nagano‐ken Eisei Kogai Kenkyusho Kenkyu Hokoku. 1980;2:22‐27. [Google Scholar]
- 199. Zhang J, Cai C‐T, Cai Z‐Q, Liu G‐Z, Luo Y, Yang Z‐X. Variation patterns of Coptis teeta biomass and its major active compounds along an altitude gradient. Ying Yong Sheng Tai Xue Bao. 2008;19:1455‐1461. [PubMed] [Google Scholar]
- 200. Cometa M, Mazzanti G, Tomassini L. Sedative and spasmolytic effects of Viburnum tinus L. and its major pure compounds. Phytother Res. 1998;12:S89‐S91. [Google Scholar]
- 201. Okunade AL, Hufford CD, Richardson MD, Peterson JR, Clar AM. Antimicrobial properties of alkaloids from Xanthorhiza simplicissima . J Pharm Sci. 1994;83:404‐406. [DOI] [PubMed] [Google Scholar]
- 202. Chiang YL, Su CR, Kuo PC, Damu AG, Wu T‐S. Two isoquinolones from the roots of Phellodendron amurense var. Wilsonii. ChemInform. 2006;68:339‐345. [Google Scholar]
- 203. Chan C‐O, Chu C‐C, Mok DK‐W, Chau F‐T. Analysis of berberine and total alkaloid content in Cortex Phellodendri by near infrared spectroscopy (NIRS) compared with high‐performance liquid chromatography coupled with ultra‐visible spectrometric detection. Anal Chim Acta. 2007;592:121‐131. [DOI] [PubMed] [Google Scholar]
- 204. Chen A. Studies on the analysis of alkaloids of Phellodendron wilsonii Hay. et Kaneh. Kaneh. Kexue Fazhan Yuekan. 1981;9:398‐411. [Google Scholar]
- 205. Tan E, Luo S, Lin S, et al. Determination of five active ingredient in Phellodendron chinense var glabriusculum and P chinense by HPLC. Chin J Exp Tradition Med Formulae. 2013;19:135‐139. [Google Scholar]
- 206. Yavich P, Kakhetelidze M, Sarabunovich AJ. Quantitative determination of berberine in the Phellodendron lavalei bark. 1993:49.
- 207. Stermitz FR, Sharifi IA. Alkaloids of Zanthoxylum monophyllum and Z. punctatum. Phytochemistry. 1977;16:2003‐2006. [Google Scholar]
- 208. Wang Y, Zhou L, Li Y, et al. The effects of berberine on concanavalin A‐induced autoimmune hepatitis (AIH) in mice and the adenosine 5′‐monophosphate (AMP)‐activated protein kinase (AMPK) pathway. Med Sci Monit. 2017;23:6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


