This cohort study investigates the long-term risk of overall and severe cardiovascular events in patients previously treated with recombinant human growth hormone in childhood and assesses whether these events are associated with treatment duration or dose.
Key Points
Question
Is childhood growth hormone treatment associated with an increased long-term risk of cardiovascular morbidity?
Findings
In this nationwide population-based cohort study of 3408 patients treated with growth hormone in childhood and followed up for 25 years, risk of cardiovascular events later in life was increased compared with 50 036 age-, sex-, and region-matched control individuals adjusted for possible confounders. Longer duration of treatment and higher cumulative dose further increased this risk.
Meaning
These findings suggest that childhood growth hormone treatment is associated with an increased risk of cardiovascular events in early adulthood, although conclusions of causality are limited and the absolute risks are low.
Abstract
Importance
Concerns about the cardiovascular safety of recombinant human growth hormone (rhGH) treatment in childhood have recently been raised; however, long-term studies are limited.
Objective
To investigate the long-term risk of overall and severe cardiovascular events in patients previously treated with rhGH in childhood and whether there is an association with treatment duration or dose.
Design, Setting, and Participants
This nationwide population-based cohort study included patients treated with rhGH during childhood from January 1, 1985, to December 31, 2010, in Sweden, with follow-up through December 31, 2014. Included patients were treated with rhGH owing to isolated growth hormone deficiency (GHD), small for gestational age (SGA), and idiopathic short stature (ISS). For each patient, 15 age-, sex-, and region-based matched control individuals were randomly selected from the general population as a comparison group. Data on cardiovascular outcomes and covariates including gestational age, birth weight, birth length, socioeconomic status, and height were obtained through linkage with several health care and population-based registers. Data were analyzed from January 1, 1985, to December 31, 2014.
Exposures
Treatment with rhGH during childhood and adolescence (aged 0-18 years).
Main Outcomes and Measures
The primary outcome was the first cardiovascular event recorded after the start of follow-up, and the secondary outcome was the first severe cardiovascular event.
Results
A total of 53 444 individuals (3408 patients and 50 036 controls; 67.7% men; mean [SD] age at study end, 25.1 [8.2] years) were followed up for as long as 25 years (median follow-up, 14.9 [range, 0-25] years; total, 795 125 person-years). Among 1809 recorded cardiovascular events, the crude incidence rates were 25.6 events per 10 000 person-years for patients and 22.6 events per 10 000 person-years for controls. The adjusted hazard ratio (HR) for all cardiovascular events was higher in patients compared with controls (HR, 1.69; 95% CI, 1.30-2.19), especially for women (HR, 2.05; 95% CI, 1.31-3.20) compared with men (HR, 1.55; 95% CI, 1.12-2.13). All subgroups had increased HRs (SGA, 1.97 [95% CI, 1.28-3.04]; GHD, 1.66 [95% CI, 1.21-2.26]; and ISS, 1.55 [95% CI, 1.01-2.37]). Longer duration of rhGH treatment (HR, 2.08; 95% CI, 1.35-3.20) and total cumulative dose (HR, 2.05; 95% CI, 1.18-3.55) were associated with higher risk for overall cardiovascular disease. The adjusted HR for severe cardiovascular disease was 2.27 (95% CI, 1.01-5.12).
Conclusions and Relevance
In this cohort study, treatment with rhGH during childhood due to GHD, SGA, or ISS was associated with increased risks of cardiovascular events in early adulthood, particularly in women; however, conclusions of causality are still limited and the absolute risk remains low.
Introduction
Treatment with recombinant human growth hormone (rhGH) has been in clinical use since 1985, with treatment indications now extending beyond growth hormone deficiency (GHD) to include an increasing number of conditions in which childhood short stature is not primarily due to deficient endogenous growth hormone secretion, such as small for gestational age (SGA) without catch-up growth or idiopathic short stature (ISS).1 Apart from regulating linear growth during childhood, growth hormone and its prime mediator, insulinlike growth factor 1, have several effects on the metabolism of fat, protein, and carbohydrates2 as well as on the cardiovascular system.3,4 Moreover, both excess levels of growth hormone and GHD have been associated with increased cardiovascular morbidity and mortality.5,6,7
The overall safety profile of rhGH treatment has been considered favorable, as reported in several reviews and consensus statements.8,9,10,11,12 The data underlying these conclusions, however, are predominantly from postmarketing surveillance studies with limitations such as incomplete enrollment and outcome ascertainment, limited information on potential confounders, and suboptimal comparison groups.13,14,15 In addition, the patients were mostly followed up during their ongoing treatment, which precludes the possibility of determining the long-term safety profile and discovering events appearing later in life.
Concerns regarding long-term safety were raised in 2012 by a study of a French cohort of children treated previously with rhGH for GHD, SGA, and ISS,16 in which increased risks of overall cardiovascular mortality were reported; subsequent follow-up reported increased risk of cerebrovascular morbidity.17 However, owing to several methodological limitations,18,19,20 robust long-term studies are needed.10,21,22 As previously assessed for mortality,23 our present study aimed to further address these limitations and investigate long-term cardiovascular morbidity, such as ischemic heart disease, stroke, cardiomyopathy, and aneurysms, in patients with GHD, ISS, and SGA who were treated with rhGH during childhood.
Methods
Study Design and Setting
We conducted a nationwide register-based cohort study of cardiovascular events in Swedish patients treated with rhGH during childhood from January 1, 1985, to December 31, 2010. Prospectively collected data on outcomes from January 1, 1985, to December 31, 2014, and covariates of interest were retrieved through data linkage from Swedish national health and population registers. This study was approved by the Regional Ethics Review Board in Stockholm, which waived the need for informed consent for the use of registry data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
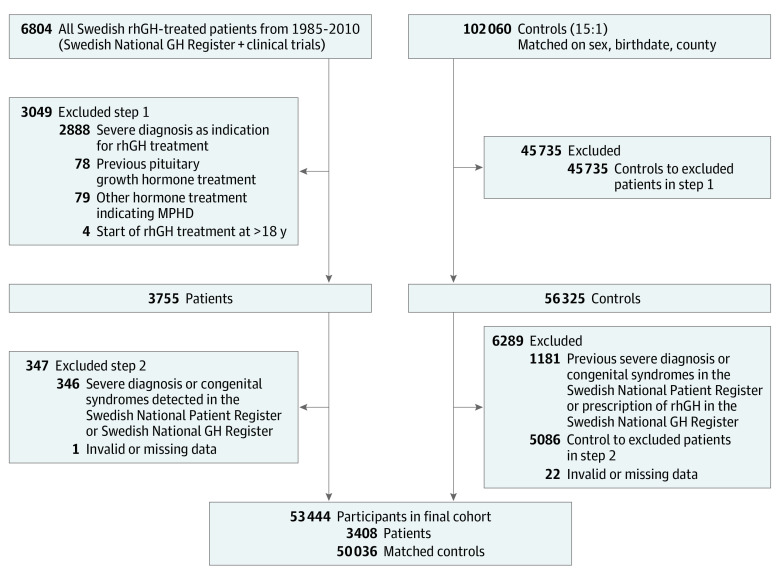
The patients originated from the Swedish National GH Register for Children and from clinical rhGH trials to form the joint GH-SAFETY cohort, as described previously (eMethods in the Supplement).23 For each patient, 15 control individuals matched for sex, birth year, and geographical region (county) were randomly selected by Statistics Sweden from the Swedish Total Population Register.24 The coverage of the register is essentially complete for the entire Swedish population, and linkage of individual information from the various registers was performed through the individuals’ unique personal identity numbers.25 Information from the Swedish National Patient Register was also used to exclude severe diagnoses or congenital syndromes for both patients and their matched controls (eTable 1 in the Supplement and the Figure).
Figure. Flowchart of Study Inclusion.
MPHD indicates multiple pituitary hormone deficiency; rhGH, recombinant human growth hormone.
Study Outcomes
The primary outcome was the first cardiovascular event recorded after the start of follow-up (start of rhGH treatment or corresponding date for matched controls). Outcome information about cardiovascular disease (CVD) was obtained from the Swedish National Patient Register and the Swedish Cause of Death Register, both with high coverage rates (eMethods in the Supplement) and defined according to codes in the International Classification of Diseases, Eighth Revision, International Classification of Diseases, Ninth Revision, or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). The secondary outcome was the first occurrence of a severe cardiovascular event, including aneurysms, ischemic heart disease, cardiomyopathy, heart failure, and cerebrovascular diseases. The specific codes for all CVD (most of chapter 9 in ICD-10) and severe CVD are listed in eTable 2 in the Supplement.
Primary Exposure
Growth hormone treatment variables, including mean dose, duration of treatment, and cumulative dose, were collected from the GH-SAFETY database. Information on adult treatment of rhGH was collected from the Swedish Prescribed Drug Register.26
Covariates
Information on birth characteristics was collected from the Swedish Medical Birth Register, which includes information on more than 98% of all births in Sweden since 1973.27 Socioeconomic data were obtained from the Swedish Income and Taxation Register as well as from the Swedish Register of Education. Through the Swedish Multi-Generation Register, it was possible to link socioeconomic data of the parents for each patient and control. The total household income at the year of study inclusion was calculated. Parental educational level was defined as the highest educational level achieved by either parent (eMethods in the Supplement).
Information about height at the start of the study was obtained from several sources. For patients, it was collected from the GH-SAFETY database. For controls, height data were obtained from the Swedish Passport Register, the Swedish Military Conscription Register, and the Swedish Medical Birth Register (height of mothers). A total of 207 091 height measurements were available, with a median of 4 (range, 1-14) measurements per control.
Statistical Analysis
Data were analyzed from January 1, 1985, to December 31, 2014. Descriptive statistics were calculated for patients and controls. The crude incidence rates of CVD events were calculated for each subgroup of baseline characteristics and presented as events per 10 000 person-years with 95% CIs. The proportions of events within each International Classification of Diseases category of CVD events between the patients and controls were tested with Fisher exact tests.
The primary and secondary outcomes of time to first CVD event (all and severe) were analyzed with Cox proportional hazard regressions and are presented as crude hazard ratios (HRs) and adjusted HRs from restricted and full models. The full model included sex, birth length, birth weight, gestational age, age and height at study start, and parental educational level and income. The restricted model included only sex, age, and height at study start. After adjusting for sex, stratified analyses by sex were also performed. The SEs were estimated with the cluster sandwich estimator taking the within-matched-group dependence into account. The proportional hazard assumption was tested using Schoenfeld residuals and visual inspection of the estimated hazard functions. No significant violations were observed. The time to first CVD event (all or severe) was calculated from the date of study inclusion (start of rhGH treatment for the patients and corresponding date for each patient’s matched control) until the date of first CVD event (all or severe), death, loss to follow-up (eg, emigration), or end of study (December 31, 2014). All participants, including those with a prior nonsevere CVD, contributed to the analysis of time to first severe CVD event. All HRs are presented with 95% CIs.
The estimation of height at study start for the control group was performed using a mixed-effects model based on multiple height measurements of each control (eMethods in the Supplement). All continuous variables were treated as such in the analyses except for family income level, which was categorized in quintiles as described in the eMethods in the Supplement, and parental educational level, which was coded and thus treated as an ordinal variable. A sensitivity analysis on a subset of patients and controls more similar in height at study start was also performed (eTables 5 and 6 in the Supplement). A 2-sided P < .05 was considered statistically significant. All analyses were performed using Stata statistical software, version 14.2 (StataCorp LLC).
Results
A total of 6804 rhGH-treated patients and 102 060 matched controls were identified, and after the predefined exclusions, the final study population included 53 444 individuals (3408 patients and 50 036 controls; 67.7% men and 32.3% women) (Figure). Mean (SD) age at the start of rhGH treatment was 9.3 (3.2) years for patients, corresponding to 9.4 (3.2) years for controls. The baseline characteristics of the patients and controls are presented in Table 1, with missing values described in eTable 4 in the Supplement.
Table 1. Baseline Characteristics of Study Cohorta.
| Characteristic | Study group, No. (%) of participants (n = 53 444) | SMD | |
|---|---|---|---|
| Patients (n = 3408) | Controls (n = 50 036) | ||
| Sex | |||
| Male | 2305 (67.6) | 33 861 (67.7) | <0.001 |
| Female | 1103 (32.4) | 16 175 (32.3) | |
| Gestational age, wk | |||
| <37 | 458 (14.6) | 2596 (5.7) | 0.30 |
| 37-41 | 2475 (79.0) | 39 097 (86.1) | |
| ≥42 | 199 (6.4) | 3721 (8.2) | |
| Birth length SDSb | |||
| SGA (<–2) | 1075 (35.1) | 3263 (7.3) | 0.76 |
| AGA (–2 to <2) | 1969 (64.3) | 39 643 (88.3) | |
| LGA (≥2) | 18 (0.6) | 1966 (4.4) | |
| Birth weight SDSb | |||
| SGA (<–2) | 662 (21.2) | 1884 (4.2) | 0.55 |
| AGA (–2 to <2) | 2441 (78.2) | 42 299 (93.5) | |
| LGA (≥2) | 19 (0.6) | 1062 (2.3) | |
| Age at study start, y | |||
| 0-4 | 369 (10.8) | 5517 (11.0) | 0.02 |
| 5-9 | 1501 (44.0) | 21 750 (43.5) | |
| 10-14 | 1433 (42.0) | 21 109 (42.2) | |
| ≥15 | 105 (3.1) | 1660 (3.3) | |
| Height at study start, cm | |||
| <100 | 446 (13.8) | 1195 (2.5) | 0.93 |
| 100-149 | 2698 (83.6) | 30 984 (64.8) | |
| ≥150 | 84 (2.6) | 15 600 (32.7) | |
| Family income levelc | |||
| 1 | 627 (18.5) | 9942 (20.0) | 0.07 |
| 2 | 638 (18.8) | 9816 (19.7) | |
| 3 | 679 (20.0) | 9963 (20.0) | |
| 4 | 660 (19.4) | 10 013 (20.1) | |
| 5 | 792 (23.3) | 10 051 (20.2) | |
| Parental educational leveld | |||
| 1 | 50 (1.5) | 929 (1.9) | 0.06 |
| 2 | 149 (4.4) | 2077 (4.2) | |
| 3 | 864 (25.4) | 13 228 (26.5) | |
| 4 | 600 (17.6) | 9274 (18.6) | |
| 5 | 576 (16.9) | 8872 (17.8) | |
| 6 | 1059 (31.1) | 14 229 (28.5) | |
| 7 | 108 (3.2) | 1273 (2.6) | |
Abbreviations: AGA, appropriate for gestational age; LGA, large for gestational age; SDS, standard deviation score; SGA, small for gestational age; SMD, standardized mean difference.
Percentages have been rounded and may not total 100. Percentage of missing values for each baseline characteristic is given in eTable 4 in the Supplement.
Reference for calculation is given in the eMethods in the Supplement.
For quintiles of total disposable income within the family household at study inclusion, 1 indicates lowest and 5 the highest family income quintiles.
Reported as highest achievable educational level for parents collected from the Swedish Register of Education; 1 indicates primary school less than 9 years; 2, primary school for 9 years; 3, secondary school for 2 years or less; 4, secondary school for more than 2 years; 5, higher education of less than 3 years; 6, higher education of 3 or more years; and 7, postgraduate/doctoral studies.
The median follow-up time was 14.9 (range, 0-25) years, the mean (SD) age at study end was 25.1 (8.2) years, and the total time at risk of an event was 795 125 person-years. During the follow-up, a total of 1809 CVD events were recorded, with crude incidence rates of 25.6 (95% CI, 21.6-30.4) events per 10 000 person-years among the patients and 22.6 (95% CI, 21.5-23.7) events per 10 000 person-years among the controls (Table 2). The rate was higher in female patients (31.2 [95% CI, 23.4-41.5] events/10 000 person-years) than in female controls (23.2 [95% CI, 21.3-25.3] events/10 000 person-years), but incidence rates were similar between male patients (23.3 [95% CI, 18.8-28.9] events/10 000 person-years) and male controls (22.3 [95% CI, 21.0-23.6] events/10 000 person-years). The crude incidence rates for all subgroups of baseline characteristics are presented in Table 2.
Table 2. Number of Events, Person-Years, and Incidence Rates for Overall Cardiovascular Events.
| Characteristic | Study group (n = 53 444) | |||||
|---|---|---|---|---|---|---|
| Patients (n = 3408) | Controls (n = 50 036) | |||||
| No. of events | Person-years | Incidence rate (95% CI)a | No. of events | Person-years | Incidence rate (95% CI)a | |
| Total cohort | 130 | 50 702 | 25.6 (21.6-30.4) | 1679 | 744 423 | 22.6 (21.5-23.7) |
| Male | 83 | 35 618 | 23.3 (18.8-28.9) | 1165 | 523 333 | 22.3 (21.0-23.6) |
| Female | 47 | 15 084 | 31.2 (23.4-41.5) | 514 | 221 090 | 23.2 (21.3-25.3) |
| Gestational age, wk | ||||||
| <37 | 15 | 6085 | 24.7 (14.9-40.9) | 103 | 37 927 | 27.2 (22.4-32.9) |
| 37-41 | 97 | 37 597 | 25.8 (21.1-31.5) | 1272 | 583 212 | 21.8 (20.6-23.0) |
| ≥42 | 8 | 3154 | 25.4 (12.7-50.7) | 139 | 58 035 | 24.0 (20.3-28.3) |
| Birth length SDSb | ||||||
| SGA (<−2) | 53 | 16 216 | 32.7 (25.0-42.8) | 122 | 51 405 | 23.7 (19.9-28.3) |
| AGA (−2 to <2) | 63 | 29 441 | 21.4 (16.7-27.4) | 1297 | 59 1219 | 21.9 (20.8-23.2) |
| LGA (≥2) | 1 | 275 | 36.4 (5.1-258.2) | 79 | 29 649 | 26.6 (21.4-33.2) |
| Birth weight SDSb | ||||||
| SGA (<−2) | 27 | 9973 | 27.1 (18.6-39.5) | 72 | 30 261 | 23.8 (18.9-30.0) |
| AGA (−2 to <2) | 93 | 36 432 | 25.5 (20.8-31.3) | 1400 | 631 209 | 22.2 (21.0-23.4) |
| LGA (≥2) | 0 | 269 | NA | 38 | 15 266 | 24.9 (18.1-34.2) |
| Age at study start, y | ||||||
| 0-4 | 8 | 4905 | 16.3 (8.2-32.6) | 114 | 73 251 | 15.6 (13.0-18.7) |
| 5-9 | 52 | 20 650 | 25.2 (19.2-33.0) | 586 | 299 076 | 19.6 (18.1-21.2) |
| 10-14 | 68 | 23 190 | 29.3 (23.1-37.2) | 885 | 342 103 | 25.9 (24.2-27.6) |
| ≥15 | 2 | 1956 | 10.2 (2.6-40.9) | 94 | 29 993 | 31.3 (25.6-38.4) |
| Height at study start, cm | ||||||
| <100 | 12 | 6236 | 19.2 (10.9-33.9) | 25 | 17 839 | 14.0 (9.5-20.7) |
| 100-149 | 114 | 41 464 | 27.5 (22.9-33.0) | 885 | 441 444 | 20.0 (18.8-21.4) |
| ≥150 | 3 | 1494 | 20.1 (6.5-62.3) | 727 | 263 088 | 27.6 (25.7-29.7) |
| Family income levelc | ||||||
| 1 | 23 | 9336 | 24.6 (16.4-37.1) | 319 | 143 688 | 22.2 (19.9-24.8) |
| 2 | 24 | 9552 | 25.1 (16.8-37.5) | 323 | 147 153 | 22.0 (19.7-24.5) |
| 3 | 31 | 9995 | 31.0 (21.8-44.1) | 365 | 148 589 | 24.6 (22.2-27.2) |
| 4 | 27 | 10 102 | 26.7 (18.3-39.0) | 347 | 151 304 | 22.9 (20.6-25.5) |
| 5 | 25 | 11 610 | 21.5 (14.6-31.9) | 320 | 151 739 | 21.1 (18.9-23.5) |
| Parental educational leveld | ||||||
| 1 | 3 | 934 | 32.0 (10.3-99.3) | 42 | 16 834 | 24.9 (18.4-33.8) |
| 2 | 14 | 2528 | 55.4 (32.8-93.5) | 103 | 34 405 | 29.9 (24.7-36.3) |
| 3 | 46 | 14 087 | 32.7 (24.5-43.6) | 519 | 213 317 | 24.3 (22.3-26.5) |
| 4 | 16 | 8358 | 19.1 (11.7-31.2) | 289 | 130 480 | 22.1 (19.7-24.9) |
| 5 | 22 | 8616 | 25.5 (16.8-38.8) | 276 | 129 943 | 21.2 (18.9-23.9) |
| 6 | 26 | 14 553 | 17.9 (12.2-26.2) | 410 | 199 911 | 20.5 (18.6-22.6) |
| 7 | 3 | 1593 | 18.8 (6.1-58.4) | 36 | 18 383 | 19.6 (14.1-27.1) |
| Treatment indication subgroups | ||||||
| SGA (n = 672) | 27 | 9559 | 28.2 (19.4-41.2) | NA | NA | NA |
| GHD (n = 1837) | 76 | 28 449 | 26.7 (21.3-33.4) | NA | NA | NA |
| ISS (n = 899) | 27 | 12 694 | 21.3 (14.6-31.0) | NA | NA | NA |
Abbreviations: AGA, appropriate for gestational age; GHD, growth hormone deficiency; ISS, idiopathic short stature; LGA, large for gestational age; NA, not applicable; SDS, standard deviation score; SGA, small for gestational age.
Calculated as number of events per 10 000 person-years.
Reference for calculation is given in the eMethods in the Supplement.
For quintiles of total disposable income within the family household at study inclusion, 1 indicates lowest and 5 the highest family income quintiles.
Reported as highest achievable educational level for parents collected from the Swedish Register of Education; 1 indicates primary school less than 9 years; 2, primary school for 9 years; 3, secondary school for 2 years or less; 4, secondary school for more than 2 years; 5, higher education of less than 3 years; 6, higher education of 3 or more years; and 7, postgraduate/doctoral studies.
The main analysis of time to first CVD event revealed a crude HR of 1.13 (95% CI, 0.95-1.36), which increased in the restricted model (HR, 1.58; 95% CI, 1.23-2.01) and in the full model (HR, 1.69; 95% CI, 1.30-2.19), especially in female patients (HR, 2.05; 95% CI, 1.31-3.20) compared with male patients (HR, 1.55; 95% CI, 1.12-2.13) (Table 3). In subgroups of patients, increased HRs were most evident in the SGA group (HR, 1.97; 95% CI, 1.28-3.04) but were also seen in GHD, defined as stimulated growth hormone levels of less than 10 ng/mL (to convert to micrograms per liter, multiply by 1) (HR, 1.66; 95% CI, 1.21-2.26), and ISS (HR, 1.55; 95% CI, 1.01-2.37). The HRs for the patients with GHD and stimulated growth hormone levels of 0 to 4 and 5 to 9 ng/mL were 1.79 (95% CI, 1.12-2.87) and 1.60 (95% CI, 1.12-2.28), respectively. In the patient group, 15 of 130 events (11.5%) occurred during ongoing childhood rhGH treatment. The Kaplan-Meier curve of the overall CVD events during the follow-up period in the patients and controls, and separated by sex, is presented in the eFigure in the Supplement.
Table 3. Crude and Adjusted HRs for All Cardiovascular Events Between Patients and Matched Controls.
| Characteristic | No. of patients | HR (95% CI) | ||
|---|---|---|---|---|
| Crude | Adjusted simple modela | Adjusted full modelb | ||
| All patients | 3408 | 1.13 (0.95-1.36) | 1.58 (1.23-2.01) | 1.69 (1.30-2.19) |
| Male | 2305 | 1.04 (0.84-1.30) | 1.39 (1.03-1.89) | 1.55 (1.12-2.13) |
| Female | 1103 | 1.34 (0.99-1.81) | 2.05 (1.36-3.08) | 2.05 (1.31-3.20) |
| Patients with SGA | 672 | 1.27 (0.87-1.85) | 1.84 (1.21-2.80) | 1.97 (1.28-3.04) |
| Male | 423 | 1.14 (0.70-1.88) | 1.60 (0.93-2.77) | 1.78 (1.02-3.09) |
| Female | 249 | 1.47 (0.81-2.68) | 2.40 (1.24-4.63) | 2.39 (1.17-4.88) |
| Patients with GHD | 1837 | 1.16 (0.92-1.46) | 1.60 (1.19-2.13) | 1.66 (1.21-2.26) |
| Male | 1312 | 1.07 (0.81-1.42) | 1.41 (0.99-2.01) | 1.55 (1.06-2.25) |
| Female | 525 | 1.38 (0.92-2.07) | 2.10 (1.28-3.47) | 1.94 (1.11-3.39) |
| Patients with GHD (maximum growth hormone level, 0-4 ng/mL)c | 485 | 1.28 (0.86-1.90) | 1.77 (1.15-2.73) | 1.79 (1.12-2.87) |
| Male | 349 | 1.13 (0.69-1.85) | 1.51 (0.88-2.58) | 1.64 (0.92-2.92) |
| Female | 136 | 1.70 (0.87-3.32) | 2.52 (1.22-5.21) | 2.16 (0.94-4.94) |
| Patients with GHD (maximum growth hormone level, 5-9 ng/mL)c | 1352 | 1.11 (0.84-1.47) | 1.52 (1.09-2.12) | 1.60 (1.12-2.28) |
| Male | 963 | 1.05 (0.74-1.48) | 1.37 (0.91-2.05) | 1.51 (0.98-2.31) |
| Female | 389 | 1.26 (0.77-2.06) | 1.94 (1.09-3.44) | 1.84 (0.97-3.47) |
| Patients with ISS | 899 | 0.97 (0.67-1.42) | 1.34 (0.89-2.01) | 1.55 (1.01-2.37) |
| Male | 570 | 0.90 (0.56-1.45) | 1.20 (0.72-2.01) | 1.38 (0.81-2.35) |
| Female | 329 | 1.13 (0.61-2.12) | 1.69 (0.87-3.28) | 2.00 (1.00-4.02) |
| Duration of treatment, yd | ||||
| 0-2 | 925 | 0.71 (0.43-1.18) | 0.98 (0.57-1.69) | 1.05 (0.59-1.88) |
| 3-6 | 1522 | 0.99 (0.74-1.32) | 1.35 (0.95-1.91) | 1.58 (1.10-2.28) |
| ≥7 | 961 | 1.45 (1.01-2.08) | 1.94 (1.29-2.93) | 2.08 (1.35-3.20) |
| P value for trend | NA | .02 | .01 | .01 |
| Mean growth hormone dose, μg/kg/dd | ||||
| 0-29 | 402 | 1.10 (0.53-2.31) | 1.49 (0.70-3.19) | 1.76 (0.82-3.77) |
| 30-39 | 2383 | 1.09 (0.85-1.40) | 1.50 (1.10-2.05) | 1.64 (1.18-2.28) |
| 40-49 | 337 | 0.73 (0.36-1.47) | 0.99 (0.48-2.05) | 1.25 (0.60-2.59) |
| ≥50 | 279 | 0.94 (0.50-1.75) | 1.28 (0.68-2.47) | 1.51 (0.79-2.91) |
| P value for trend | NA | .55 | .47 | .51 |
| Cumulative dose, mgd | ||||
| 0-1499 | 1015 | 0.86 (0.54-1.37) | 1.16 (0.70-1.90) | 1.34 (0.80-2.25) |
| 1500-2999 | 954 | 1.20 (0.85-1.69) | 1.64 (1.11-2.42) | 1.76 (1.16-2.68) |
| 3000-4499 | 902 | 0.85 (0.56-1.28) | 1.16 (0.73-1.84) | 1.36 (0.84-2.18) |
| ≥4500 | 381 | 1.35 (0.82-2.21) | 1.86 (1.10-3.16) | 2.05 (1.18-3.55) |
| P value for trend | NA | .43 | .25 | .24 |
Abbreviations: GHD, growth hormone deficiency; HR, hazard ratio; ISS, idiopathic short stature; SGA, small for gestational age.
SI conversion factor: To convert growth hormone to μg/L, multiply by 1.
Adjusted only for age at start, height at start, and sex (if not stratified for sex).
Adjusted for gestational age, birth length, birth weight, age at start, height at start, parental educational level, family income, and sex (if not stratified for sex).
Maximum indicates growth hormone peak level on either provocation test (mainly arginine-insulin tolerance test) or during spontaneous 12- or 24-hour growth hormone secretion profiles.
Analysis with 2-year lag-period after end of treatment to avoid reversed causality (protopathic bias).
A longer duration of treatment and the highest cumulative dose were also associated with an increased HR (Table 3). We observed a significant increase in risk with increasing treatment duration (P = .01 for trend) but not with cumulative dose (P = .24 for trend). The highest adjusted HRs were seen in the subgroups with the longest duration (2.08; 95% CI, 1.35-3.20) and highest cumulative dose (2.05; 95% CI, 1.18-3.55). No association between higher mean daily dose and increased HR was found.
A total of 167 first severe CVD events were recorded during the follow-up period, with crude incidence rates of 3.48 (95% CI, 2.19-5.52) events per 10 000 person-years among patients and 1.97 (95% CI, 1.68-2.32) events per 10 000 person-years among controls (Table 4). The patients had an increased hazard compared with that in the controls in both the crude (HR, 1.75; 95% CI, 1.08-2.87) and full (HR, 2.27; 95% CI, 1.01-5.12) models, but differences did not reach significance in the restricted model (HR, 1.56; 95% CI, 0.73-3.34). The adjusted HRs (full model) were 1.92 (95% CI, 0.72-5.11) and 3.68 (95% CI, 0.84-16.08) for male and female patients, respectively (Table 4).
Table 4. Incidence Rates and HRs of First Severe Cardiovascular Event in Patients Compared to Matched Controls.
| Group | No. of participants | No. of events | Person-years | Incidence rate (95% CI)a | HR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Crude | Adjusted, simple modelb | Adjusted, full modelc | |||||
| All | |||||||
| Controls | 50 036 | 149 | 755 755 | 1.97 (1.68-2.32) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Patients | 3408 | 18 | 51 781 | 3.48 (2.19-5.52) | 1.75 (1.08-2.87) | 1.56 (0.73-3.34) | 2.27 (1.01-5.12) |
| Male | |||||||
| Controls | 33 861 | 114 | 531 469 | 2.15 (1.79-2.58) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Patients | 2305 | 13 | 36 391 | 3.57 (2.07-6.15) | 1.66 (0.93-2.94) | 1.21 (0.48-3.03) | 1.92 (0.72-5.11) |
| Female | |||||||
| Controls | 16 175 | 35 | 224 287 | 1.56 (1.12-2.17) | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Patients | 1103 | 5 | 15 391 | 3.25 (1.35-7.81) | 2.08 (0.81-5.35) | 3.40 (0.93-12.45) | 3.68 (0.84-16.08) |
Abbreviation: HR, hazard ratio.
Calculated as number of events per 10 000 person-years.
Adjusted for age and height at study start and sex.
Adjusted for gestational age, birth length, birth weight, age and height at study start, parental educational level, family income, and sex.
The sensitivity analysis, which retained controls more similar in height at study start, found similar results as our overall analysis (eTable 6 in the Supplement). For overall CVD, the crude HR was 1.66 (95% CI, 1.07-2.58), with adjusted HRs of 1.42 (95% CI, 0.88-2.31) in the restricted model and 1.56 (95% CI, 0.89-2.71) in the full model. For severe CVD, crude HR was 3.21 (95% CI, 1.04-9.95), with adjusted HRs of 2.13 (95% CI, 0.67-6.81) in the restricted model and 4.33 (95% CI, 0.81-23.25) in the full model (eTable 6 in the Supplement). The point estimates for the HRs were similar to those in our main analysis, but the 95% CIs were wider owing to less statistical power with fewer controls and patients retained in this analysis.
The 3 most common overall CVD groups were unspecified diseases of the circulatory system, arrhythmias, and hypertensive disease; for severe CVD, they were ischemic heart disease, cardiomyopathy, and stroke (eTable 2 in the Supplement). All numbers of events for the patients and controls within each subcategory of cardiovascular event are presented in eTable 2 in the Supplement together with a comparison of proportions using Fisher exact tests.
Discussion
This nationwide population-based cohort study, with 25 years of follow-up, found that treatment with rhGH for GHD, SGA, or ISS during childhood was associated with an increased risk of CVD events. The association was most clearly seen in women and patients treated for SGA, but with overlapping 95% CIs with the other subgroups. Although the absolute risk of a CVD event was relatively low in patients and controls, a longer duration of rhGH treatment and the highest cumulative dose in childhood were associated with the highest CVD risk. The association between childhood rhGH treatment and CVD events was also seen when assessing only severe CVD outcomes, but with even lower absolute risks.
Few studies have investigated cardiovascular morbidity in patients previously treated with rhGH. The limited number of studies that exist have primarily focused on cardiovascular mortality, reported unadjusted standard mortality ratios in treated adults, and compared them with the rates in the general population.16,28,29 Nonideal comparison groups have also been used in other studies of patients treated with rhGH in childhood, such as the previously mentioned study by Poidvin et al,17 in which data from 2 background populations in France and the United Kingdom were used to calculate the standardized incidence ratios.30 In all these studies, data on confounding variables have also been lacking. Furthermore, several of the reports referenced above are based on a mix of different types of patients with sometimes severe underlying conditions, making it difficult to draw definite conclusions regarding the effect of rhGH treatment.
The increased risk of CVD in female patients reported in previous studies28,29 was also observed in our study. Differences in estrogen levels or responsiveness to rhGH treatment have previously been hypothesized as possible explanations, but the underlying mechanism for this sex difference still remains unclear and merits further investigation.28,31 In general, children born SGA have higher risks of CVD as adults, and in particular if coupled with a rapid postnatal weight gain.32,33,34 However, children born SGA who are eligible for rhGH treatment lack a catch-up growth, at least for height, which could in theory put them in a group with a relatively lower baseline risk of CVD. In this study, we included information on birth characteristics, such as gestational age, birth weight, and birth length, for both patients and controls to take these parameters into account when investigating CVD morbidity in rhGH-treated patients with SGA. A recent study35 reported that compared with children with untreated SGA (with or without catch-up growth) and children born appropriate for gestational age, no negative association with CVD risk factors up to 5 years after treatment cessation was observed for rhGH-treated children with SGA. Although these results are reassuring regarding short-term effects on CVD risk factors, how these results transfer to actual CVD morbidity later in life is uncertain. In the present study of actual cardiovascular outcomes with longer follow-up, we found increased risks for both overall and severe CVD in the rhGH-treated group with SGA.
There are several challenges in addressing the issue of long-term safety in childhood rhGH treatment, and the current evidence is largely built on studies with methodological limitations and short median follow-up times. The main strengths of our study are the long follow-up time, completeness of follow-up, independent and prospective collection of outcome data, and linkage to several health and population-based registries for data on important and potential confounders. The alternative, setting up a randomized clinical trial with a follow-up of 25 years, would not be a viable option for economical and logistical reasons and would be ethically questionable in depriving patients of an approved treatment deemed to be effective. However, by matching on sex, birth year, and geographical region and including important covariates, such as birth characteristics, socioeconomic status, and height at treatment start, we tried to overcome some of the limitations of previous studies.
To isolate the possible effects of childhood rhGH treatment, we investigated the potential dose-response association with the outcome. We found that the highest risk was among those with the longest treatment duration and the highest cumulative dose, but with no association with the mean daily dose. This finding could indicate a dose-response association but could also be caused by an underlying heterogeneity among the treated patients, wherein those with longest treatment duration and highest cumulative dose also had an underlying increased risk for the outcome for other reasons.
The increased risk of cardiovascular events may have been caused by the lack of continuous rhGH treatment in adulthood for those with GHD rather than treatment in childhood. Several studies36,37,38 have shown improvement in cardiovascular risk profile concerning lipid profile, body composition, and blood pressure in adults with GHD treated with rhGH, whereas others37,39,40 reported increased insulin resistance with risk of type 2 diabetes. A subanalysis of patients who continued treatment after 18 years of age revealed an even higher adjusted HR for the outcome than that of the total cohort (eTable 3 in the Supplement). This finding could indicate that these individuals constitute a subgroup with an underlying increased risk for other reasons but may also imply that continuous treatment augments later risk of CVD.
Limitations
There are several limitations of the present study, such as the possibility of confounding by indication, where the underlying reason for starting rhGH treatment might also be linked with the risk of later cardiovascular events. We tried to handle such potential bias by applying a matched cohort study design, adjusting for multiple potential confounders, and performing dose-response analyses. However, it is impossible to completely avoid the possibility of residual confounding, and the associations described should be seen in this light and not be viewed as evidence of causal relationships. Furthermore, when studying rare events that are expected to occur later in life, the issue of statistical power is always a possible limitation. To address this limitation, we included all patients in Sweden treated with rhGH in childhood during the study period, and adding more than 15 controls per patient would not have increased the statistical power. Our cohort is, however, still quite young, and the need for continuous follow-up is necessary not only to answer questions of even longer follow-up but also for statistical precision in our estimates with more events to analyze.
Conclusions
This nationwide population-based cohort study with 25 years of follow-up found that Swedish children treated with rhGH for GHD, SGA, or ISS had a higher adjusted HR for overall and severe CVD in adulthood compared with a matched control group. However, the absolute risks were low, which could be reassuring to individual patients. At the group level, and perhaps especially for female patients and those treated for SGA indication, further close monitoring and future studies of CVD safety are warranted.
eMethods. Study Population, Outcomes, and Analyses
eTable 1. ICD Codes Corresponding to Severe Diagnoses for Exclusion of Patients and Controls
eTable 2. List of ICD Codes for Cardiovascular Diseases and Number of Total Events for Each Category
eTable 3. Incidence Rate Ratios (IRRs) and Crude and Adjusted Hazard Ratios (HRs) for Overall and Severe CVD if rhGH Treatment Prescribed After 18 Years of Age in the Prescribed Drug Register (2005-2015)
eTable 4. Percentage Missing Values of Baseline Characteristics
eTable 5. Basal Characteristics for the Patients and Controls in the Sensitivity Analysis
eTable 6. Number of Events, Person-Years, Incidence Rate Ratios (IRRs), Crude and Adjusted Hazard Ratios (HRs) for Overall CVD and Severe CVD in the Sensitivity Analysis on a Subset of Patients and Controls
eFigure. Kaplan-Meier Curve of CVD Event-Free Probability in Patients vs Controls, Overall and Separated by Sex
eReferences.
References
- 1.Richmond E, Rogol AD. Current indications for growth hormone therapy for children and adolescents. Endocr Dev. 2010;18:92-108. doi: 10.1159/000316130 [DOI] [PubMed] [Google Scholar]
- 2.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. doi: 10.1210/er.2008-0027 [DOI] [PubMed] [Google Scholar]
- 3.Clemmons DR Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425-443, vii-viii. doi: 10.1016/j.ecl.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colao A The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf). 2008;69(3):347-358. doi: 10.1111/j.1365-2265.2008.03292.x [DOI] [PubMed] [Google Scholar]
- 5.Lombardi G, Di Somma C, Grasso LF, Savanelli MC, Colao A, Pivonello R. The cardiovascular system in growth hormone excess and growth hormone deficiency. J Endocrinol Invest. 2012;35(11):1021-1029. [DOI] [PubMed] [Google Scholar]
- 6.Palmeiro CR, Anand R, Dardi IK, Balasubramaniyam N, Schwarcz MD, Weiss IA. Growth hormone and the cardiovascular system. Cardiol Rev. 2012;20(4):197-207. doi: 10.1097/CRD.0b013e318248a3e1 [DOI] [PubMed] [Google Scholar]
- 7.Hallengren E, Almgren P, Engström G, et al. Fasting levels of high-sensitivity growth hormone predict cardiovascular morbidity and mortality: the Malmö Diet and Cancer study. J Am Coll Cardiol. 2014;64(14):1452-1460. doi: 10.1016/j.jacc.2014.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collett-Solberg PF, Ambler G, Backeljauw PF, et al. Diagnosis, genetics, and therapy of short stature in children: a Growth Hormone Research Society international perspective. Horm Res Paediatr. 2019;92(1):1-14. doi: 10.1159/000502231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimberg A, DiVall SA, Polychronakos C, et al. ; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society . Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361-397. doi: 10.1159/000452150 [DOI] [PubMed] [Google Scholar]
- 10.Allen DB, Backeljauw P, Bidlingmaier M, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016;174(2):1-9. doi: 10.1530/EJE-15-0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167-177. doi: 10.1210/jc.2009-0178 [DOI] [PubMed] [Google Scholar]
- 12.Stochholm K, Kiess W. Long-term safety of growth hormone: a combined registry analysis. Clin Endocrinol (Oxf). 2018;88(4):515-528. doi: 10.1111/cen.13502 [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld RG Pharmacological interventions for short stature: pros and cons. Nestle Nutr Inst Workshop Ser. 2013;71:207-217. doi: 10.1159/000342640 [DOI] [PubMed] [Google Scholar]
- 14.Allen DB Growth hormone post-marketing surveillance: safety, sales, and the unfinished task ahead. J Clin Endocrinol Metab. 2010;95(1):52-55. doi: 10.1210/jc.2009-2364 [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow AJ, Cooke R, Albertsson-Wikland K, et al. Description of the SAGhE cohort: a large European study of mortality and cancer incidence risks after childhood treatment with recombinant growth hormone. Horm Res Paediatr. 2015;84(3):172-183. doi: 10.1159/000435856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carel JC, Ecosse E, Landier F, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416-425. doi: 10.1210/jc.2011-1995 [DOI] [PubMed] [Google Scholar]
- 17.Poidvin A, Touzé E, Ecosse E, et al. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2014;83(9):780-786. doi: 10.1212/WNL.0000000000000737 [DOI] [PubMed] [Google Scholar]
- 18.Geffner ME, Santen R, Kopchick J. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2015;84(10):1062-1063. doi: 10.1212/WNL.0000000000001385 [DOI] [PubMed] [Google Scholar]
- 19.Linglart A, Tauber M, Bougneres P, Lebouc Y, Chatelain P. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2015;84(10):1062-1063. doi: 10.1212/WNL.0000000000001385 [DOI] [PubMed] [Google Scholar]
- 20.Sperling MA Long-term therapy with growth hormone: bringing sagacity to SAGHE. J Clin Endocrinol Metab. 2012;97(1):81-83. doi: 10.1210/jc.2011-3271 [DOI] [PubMed] [Google Scholar]
- 21.van Bunderen CC, van Varsseveld NC, Erfurth EM, Ket JC, Drent ML. Efficacy and safety of growth hormone treatment in adults with growth hormone deficiency: a systematic review of studies on morbidity. Clin Endocrinol (Oxf). 2014;81(1):1-14. doi: 10.1111/cen.12477 [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld RG, Cohen P, Robison LL, et al. Long-term surveillance of growth hormone therapy. J Clin Endocrinol Metab. 2012;97(1):68-72. doi: 10.1210/jc.2011-2294 [DOI] [PubMed] [Google Scholar]
- 23.Albertsson-Wikland K, Mårtensson A, Sävendahl L, et al. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. 2016;101(5):2149-2159. doi: 10.1210/jc.2015-3951 [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. doi: 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659-667. doi: 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726-735. doi: 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 27.Källén B, Källén K. The Swedish Medical Birth Register: A Summary of Content and Quality. Socialstyrelsen; 2003. Accessed August 23, 2018. http://lup.lub.lu.se/record/1127699
- 28.van Bunderen CC, van Nieuwpoort IC, Arwert LI, et al. Does growth hormone replacement therapy reduce mortality in adults with growth hormone deficiency? data from the Dutch National Registry of Growth Hormone Treatment in Adults. J Clin Endocrinol Metab. 2011;96(10):3151-3159. doi: 10.1210/jc.2011-1215 [DOI] [PubMed] [Google Scholar]
- 29.Gaillard RC, Mattsson AF, Akerblad AC, et al. Overall and cause-specific mortality in GH-deficient adults on GH replacement. Eur J Endocrinol. 2012;166(6):1069-1077. doi: 10.1530/EJE-11-1028 [DOI] [PubMed] [Google Scholar]
- 30.Hindmarsh PC, Peters CJ. Growth hormone treatment and stroke: should we be concerned? a case for cohort studies. Clin Endocrinol (Oxf). 2015;82(2):178-179. doi: 10.1111/cen.12611 [DOI] [PubMed] [Google Scholar]
- 31.Span JP, Pieters GF, Sweep FG, Hermus AR, Smals AG. Gender differences in rhGH-induced changes in body composition in GH-deficient adults. J Clin Endocrinol Metab. 2001;86(9):4161-4165. doi: 10.1210/jcem.86.9.7815 [DOI] [PubMed] [Google Scholar]
- 32.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802-1809. doi: 10.1056/NEJMoa044160 [DOI] [PubMed] [Google Scholar]
- 33.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301(21):2234-2242. doi: 10.1001/jama.2009.761 [DOI] [PubMed] [Google Scholar]
- 34.Ibáñez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91(6):2153-2158. doi: 10.1210/jc.2005-2778 [DOI] [PubMed] [Google Scholar]
- 35.van der Steen M, Kerkhof GF, Smeets CCJ, Hokken-Koelega ACS. Cardiovascular risk factors and carotid intima media thickness in young adults born small for gestational age after cessation of growth hormone treatment: a 5-year longitudinal study. Lancet Diabetes Endocrinol. 2017;5(12):975-985. doi: 10.1016/S2213-8587(17)30311-X [DOI] [PubMed] [Google Scholar]
- 36.Verhelst J, Abs R. Cardiovascular risk factors in hypopituitary GH-deficient adults. Eur J Endocrinol. 2009;161(suppl 1):S41-S49. doi: 10.1530/EJE-09-0291 [DOI] [PubMed] [Google Scholar]
- 37.Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P; Metaanalysis of Blinded, Randomized, Placebo-Controlled Trials . Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2004;89(5):2192-2199. doi: 10.1210/jc.2003-030840 [DOI] [PubMed] [Google Scholar]
- 38.Di Somma C, Scarano E, Savastano S, Savanelli MC, Pivonello R, Colao A. Cardiovascular alterations in adult GH deficiency. Best Pract Res Clin Endocrinol Metab. 2017;31(1):25-34. doi: 10.1016/j.beem.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 39.Luger A, Mattsson AF, Koltowska-Häggström M, et al. Incidence of diabetes mellitus and evolution of glucose parameters in growth hormone-deficient subjects during growth hormone replacement therapy: a long-term observational study. Diabetes Care. 2012;35(1):57-62. doi: 10.2337/dc11-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Claessen KM, Appelman-Dijkstra NM, Adoptie DM, et al. Metabolic profile in growth hormone-deficient (GHD) adults after long-term recombinant human growth hormone (rhGH) therapy. J Clin Endocrinol Metab. 2013;98(1):352-361. doi: 10.1210/jc.2012-2940 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Population, Outcomes, and Analyses
eTable 1. ICD Codes Corresponding to Severe Diagnoses for Exclusion of Patients and Controls
eTable 2. List of ICD Codes for Cardiovascular Diseases and Number of Total Events for Each Category
eTable 3. Incidence Rate Ratios (IRRs) and Crude and Adjusted Hazard Ratios (HRs) for Overall and Severe CVD if rhGH Treatment Prescribed After 18 Years of Age in the Prescribed Drug Register (2005-2015)
eTable 4. Percentage Missing Values of Baseline Characteristics
eTable 5. Basal Characteristics for the Patients and Controls in the Sensitivity Analysis
eTable 6. Number of Events, Person-Years, Incidence Rate Ratios (IRRs), Crude and Adjusted Hazard Ratios (HRs) for Overall CVD and Severe CVD in the Sensitivity Analysis on a Subset of Patients and Controls
eFigure. Kaplan-Meier Curve of CVD Event-Free Probability in Patients vs Controls, Overall and Separated by Sex
eReferences.