Key Points
Question
Is add-on cannabidiol superior to placebo in reducing the number of seizures associated with tuberous sclerosis complex?
Findings
In this randomized clinical trial, 224 patients with tuberous sclerosis complex were treated with cannabidiol (25 or 50 mg/kg/day) or matched placebo for 16 weeks. The percentage reduction in the type of seizures regarded as the primary end point was 27% for placebo, 49% for 25 mg/kg/day of cannabidiol, and 48% for 50 mg/kg/day of cannabidiol; a dosage of 25 mg/kg/day led to fewer adverse events than the 50-mg/kg/day dosage.
Meaning
In this study, both cannabidiol dosages were equally efficacious in reducing tuberous sclerosis complex–associated seizures compared with placebo, but the smaller dosage led to fewer adverse events.
This double-blind, placebo-controlled, randomized clinical trial evaluates the safety of 2 dosages of cannabidiol for treatment of seizures in patients with tuberous sclerosis complex.
Abstract
Importance
Efficacy of cannabidiol has been demonstrated in seizures associated with Lennox-Gastaut and Dravet syndromes but appears not yet to have been established in conditions with primarily focal seizures, such as tuberous sclerosis complex (TSC).
Objective
To evaluate efficacy and safety of 25-mg/kg/day and 50-mg/kg/day cannabidiol dosages vs placebo against seizures associated with TSC.
Design, Setting, and Participants
This double-blind, placebo-controlled randomized clinical trial (GWPCARE6) enrolled patients between April 6, 2016, and October 4, 2018; follow-up was completed on February 15, 2019. The trial was conducted at 46 sites in Australia, Poland, Spain, the Netherlands, United Kingdom, and United States. Eligible patients (aged 1-65 years) were those with a clinical diagnosis of TSC and medication-resistant epilepsy who had had at least 8 TSC-associated seizures during the 4-week baseline period, with at least 1 seizure occurring in at least 3 of the 4 weeks, and were currently taking at least 1 antiepileptic medication.
Interventions
Patients received oral cannabidiol at 25 mg/kg/day (CBD25) or 50 mg/kg/day (CBD50) or a matched placebo for 16 weeks.
Main Outcomes and Measures
The prespecified primary outcome was the change from baseline in number of TSC-associated seizures for cannabidiol vs placebo during the treatment period.
Results
Of 255 patients screened for eligibility, 31 were excluded and 224 were randomized. Of the 224 included patients (median [range] age, 11.4 [1.1-56.8] years; 93 female patients [41.5%]), 75 were randomized to CBD25, 73 to CBD50, and 76 to placebo, with 201 completing treatment. The percentage reduction from baseline in the type of seizures considered the primary end point was 48.6% (95% CI, 40.4%-55.8%) for the CBD25 group, 47.5% (95% CI, 39.0%-54.8%) for the CBD50 group, and 26.5% (95% CI, 14.9%-36.5%) for the placebo group; the percentage reduction from placebo was 30.1% (95% CI, 13.9%-43.3%; P < .001) for the CBD25 group and 28.5% (95% CI, 11.9%-42.0%; nominal P = .002) for the CBD50 group. The most common adverse events were diarrhea (placebo group, 19 [25%]; CBD25 group, 23 [31%]; CBD50 group, 41 [56%]) and somnolence (placebo group, 7 [9%]; CBD25 group, 10 [13%]; CBD50 group, 19 [26%]), which occurred more frequently with cannabidiol than placebo. Eight patients in CBD25 group, 10 in CBD50 group, and 2 in the placebo group discontinued treatment because of adverse events. Twenty-eight patients taking cannabidiol (18.9%) had elevated liver transaminase levels vs none taking placebo.
Conclusions and Relevance
Cannabidiol significantly reduced TSC-associated seizures compared with placebo. The 25-mg/kg/day dosage had a better safety profile than the 50-mg/kg/day dosage.
Trial Registration
ClinicalTrials.gov Identifier: NCT02544763
Introduction
Tuberous sclerosis complex (TSC) is a disorder caused by autosomal-dominant sequence variations in the TSC1 and/or TSC2 genes, resulting in upregulation of the mechanistic target of rapamycin (mTOR) pathway with subsequent excessive cell growth and proliferation.1,2,3,4 Tuberous sclerosis complex is characterized by the occurrence of benign hamartomas in multiple organ systems, most frequently in brain, skin, kidneys, lungs, heart, and eyes.1,5 Incidence of TSC is estimated at 1 in 6000 live births, affecting 1 to 2 million individuals worldwide.6,7
Epilepsy is the most common neurologic manifestation of TSC, affecting approximately 85% of patients, with onset often during infancy.8,9,10,11,12 Patients experience focal seizures and infantile spasms as infants and a variety of other seizures during their lifetime.10,13 Despite several treatment options for TSC-associated seizures—including antiepileptic drugs such as vigabatrin, the mTOR inhibitor everolimus, surgical procedures, and dietary therapy14—more than 60% of patients have treatment-resistant epilepsy,13 which is associated with neurodevelopmental disorders, including autism and intellectual disability.11,15
Cannabidiol is approved as Epidiolex in the US for treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, or TSC in patients 1 year and older, and as Epidyolex in the European Union in conjunction with clobazam for Lennox-Gastaut syndrome and Dravet syndrome in patients 2 years and older.16,17 Efficacy of cannabidiol was first demonstrated against Dravet syndrome–associated and Lennox-Gastaut syndrome–associated seizures.18,19,20,21 On the basis of data from patients with TSC in an expanded-access program,22 we conducted a placebo-controlled randomized clinical trial to assess efficacy and safety of add-on cannabidiol for the treatment of TSC-associated seizures (primarily focal seizures) in children and adults.
Methods
Study Design and Participants
This was a phase 3, international, double-blind, parallel-group randomized clinical trial of add-on cannabidiol vs placebo in patients with TSC and drug-resistant epilepsy. The trial consisted of a 4-week baseline period, a 16-week treatment period (4 weeks for dose escalation [titration period] followed by 12 weeks of stable dosing [maintenance period]), a taper period of up to 10 days, and a 4-week safety follow-up (eFigure 1 in Supplement 1). The protocol was approved by an institutional review board or ethics committee at each participating site and conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice. All patients or caregivers provided written informed consent, and patients developmentally mature enough to understand the trial provided assent. All authors vouch for the accuracy of the reported results and adherence to the protocol. The manuscript was written by all authors, 3 of whom are GW Pharmaceuticals employees (F.S., D.C., and V.K.).
Eligible patients (aged 1-65 years) with a definite clinical diagnosis of TSC1 and medication-resistant epilepsy had at least 8 TSC-associated seizures during the 4-week baseline period with at least 1 seizure occurring in at least 3 of the 4 weeks and were taking at least 1 antiepileptic medication. Key exclusion criteria were a history of nonepileptic seizures, clinically significant illness other than epilepsy, epilepsy surgery in the 6 months before screening, felbamate use for less than 1 year before screening, and use of oral mTOR inhibitors. Details of eligibility criteria are provided in eTable 1 in Supplement 1.
Randomization, Concealment, and Masking
After screening and the baseline period, eligible patients were randomized equally to receive a pharmaceutical formulation of highly purified cannabidiol derived from Cannabis sativa L. (100 mg/mL oral solution; Epidiolex in the US; Epidyolex in the European Union [GW Research Ltd]) at 25 mg/kg of body weight per day (CBD25 group), 50 mg/kg/day (CBD50 group), or placebo. Patients in the placebo group were subdivided to receive a placebo matching either the 25-mg/kg/day or 50-mg/kg/day dosage of CBD. The placebo groups were pooled for reporting efficacy and safety results. The randomization schedule was created by an independent statistician and stratified by age group (1-6, 7-11, 12-17, and 18-65 years). Cannabidiol solutions and placebo solutions (excipients alone) were provided in identical 100-mL amber glass bottles.
Procedures
Study drugs were administered twice daily in equally divided doses with a faster titration schedule than prior studies: dosages started at 5 mg/kg/day and reached 25 mg/kg/day on day 9 and 50 mg/kg/day on day 29 (eFigure 2 in Supplement 1). The number and type of seizures and status epilepticus episodes were reported daily using an interactive voice-response system, and adverse events and medications were recorded using a paper-based diary. Details of the trial procedures are available in the protocol (Supplement 2). Patients who completed treatment were eligible to enter an open-label extension phase (NCT02544750). Patients who withdrew from the study or did not enter the open-label extension were seen at 4 weeks after the last dose. An independent safety monitoring committee approved the higher dosages used in this trial and monitored patient safety. An adjudication committee evaluated any potential signs of abuse.
Outcome Measures
The primary end point was the change from baseline in the number of TSC-associated seizures in patients taking add-on cannabidiol vs placebo during the 16-week treatment period. The primary end point, TSC-associated seizures, included countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic-clonic, tonic, clonic, or atonic); this excluded absence, myoclonic, and focal sensory seizures and infantile/epileptic spasms. A mean of 94% of patients’ baseline seizures were classified as TSC-associated seizures. This functional definition and classification of the primary–end-point, TSC-associated seizures was reviewed and approved by the US Food and Drug Administration, the European Medicines Agency, and the Epilepsy Study Consortium independent committee of experts.
Key secondary outcomes included the proportion of patients who had at least a 50% reduction from baseline in primary–end-point seizures; the participants’ or caregivers’ global impressions of change from baseline in overall condition, as assessed on a 7-point Likert scale that included 3 categories for improvement (ie, slightly improved, much improved, and very much improved), 3 for worsening (slightly worse, much worse, and very much worse), and an option to indicate no change; and the change from baseline in total seizures (ie, sum of all individual seizure types). Other secondary outcomes are described in the eMethods in Supplement 1. We also conducted a prespecified analysis to explore the effect of concomitant clobazam on the change in primary–end-point seizures.
Statistical Analysis
We assumed a reduction from baseline in seizures of 15% for placebo and 50% for cannabidiol with a common SD of 60%, leading to a sample size of 70 patients per treatment group with 90% power to detect a difference in response distributions. The primary analyses for all outcomes used the intention-to-treat data set, which included all randomized patients. The per-protocol data set, including patients who completed the study without major protocol deviations, was used in sensitivity analyses for the primary and key secondary outcomes. All statistical tests were 2-sided with a 5% significance level.
Negative binomial regression on the sum of the seizure counts during the treatment period was used for the primary outcome analysis and is described in the eMethods in Supplement 1, along with a description of statistical analyses used for all secondary outcomes. Type I error was controlled by a hierarchical gatekeeping procedure (eTable 2 in Supplement 1), wherein each successive end point was tested for inferential statistical significance only if the preceding comparison was statistically significant; otherwise, resultant P values were designated as nominal and used descriptively. All statistical analyses were done using SAS version 9.3 or higher (SAS Institute), and the threshold of significance was P < .05.
Results
Patients
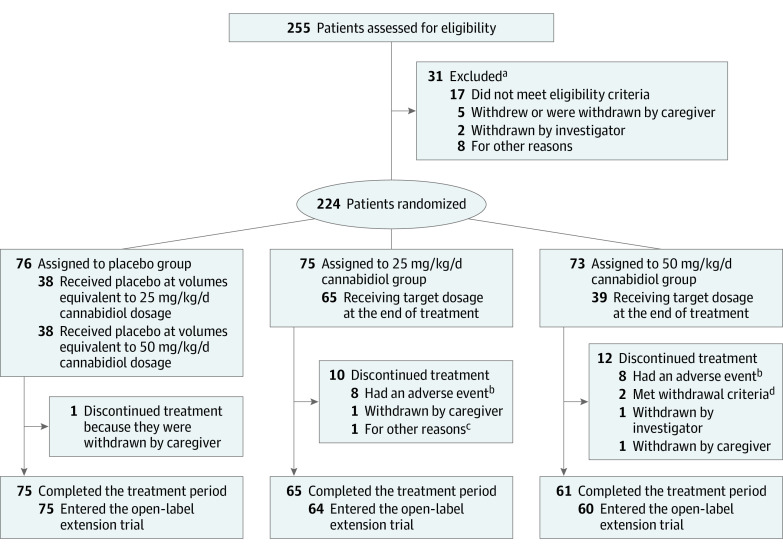
Between April 6, 2016, and October 4, 2018, 255 patients were assessed for eligibility at 46 sites; 224 patients (median [range] age, 11.4 [1.1-56.8] years; 93 female patients [41.5%]) underwent randomization (US, 112; Poland, 61; Australia, 24; Spain, 11; the Netherlands, 9; UK, 7) to the CBD25 group (n = 75), the CBD50 group (n = 73), and the placebo group (n = 76) (Figure 1). Not all patients reached or remained at their assigned dosage (eTable 3 in Supplement 1); 87% (65 of 75 patients) in the CBD25 group and 53% (39 of 73 patients) in the CBD50 group were receiving their target dosage at treatment end. The mean of each patient’s modal dosage was 24 mg/kg/day in the CBD25 group and 36 mg/kg/day in the CBD50 group.
Figure 1. Screening, Randomization, and Treatment Period.
aPatients may have more than 1 reason for exclusion.
bPatients had adverse event as the primary reason for discontinuation.
cOne patient in the 25–mg/kg/d cannabidiol group discontinued treatment because of difficulty taking the study medication, problems with eating and drinking, occurrence of constipation, and increase in seizures.
dTwo patients in the 50–mg/kg/d cannabidiol group met the withdrawal criteria via elevations in alanine aminotransferase or aspartate aminotransferase levels.
Overall, the following 23 patients (10.3%) discontinued treatment and were excluded from the per-protocol analysis set: 12 (16%) in the CBD50 group, 10 (13%) in the CBD25 group, and 1 (1%) in the placebo group. Sixteen of 22 patients taking cannabidiol had an adverse event as the primary reason for discontinuation. Overall, 201 patients completed treatment; of those, 199 (99%) entered the open-label extension trial.
Baseline characteristics were similar between the treatment groups (Table 1). Patients had previously discontinued a median (range) of 4 (0-15) antiepileptic drugs and were concurrently taking a median (range) of 3 (0-5) antiepileptic drugs. The median (interquartile range [IQR]) number of primary–end-point seizures was 56.9 (28.5-107.4) in the 4-week baseline period.
Table 1. Demographic and Clinical Characteristics of Patients at Baseline.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Treatment group | |||
| Placebo (n = 76) | CBD25 (n = 75) | CBD50 (n = 73) | |
| Age, median (range), y | 10.9 (1.2-55.8) | 11.6 (1.1-56.8) | 10.2 (1.8-34.9) |
| Age, y | |||
| 1-6 | 22 (29) | 21 (28) | 21 (29) |
| 7-11 | 18 (24) | 18 (24) | 18 (25) |
| 12-17 | 16 (21) | 16 (21) | 16 (22) |
| 18-65 | 20 (26) | 20 (27) | 18 (25) |
| Male | 45 (59) | 43 (57) | 43 (59) |
| Median No. of prior antiepileptic drugs (range) | 4 (0-15) | 4 (0-13) | 4 (0-13) |
| No. of concomitant antiepileptic drugs | |||
| Median (range) | 3 (1-5) | 3 (0-4) | 3 (1-5) |
| 1 | 8 (11) | 9 (12) | 7 (10) |
| 2 | 27 (36) | 20 (27) | 24 (33) |
| ≥3 | 41 (54) | 45 (60) | 42 (58) |
| Median No. of prior and current antiepileptic drugs (range) | 7 (2-18) | 7 (1-15) | 7 (1-15) |
| Concomitant antiepileptic drugs | |||
| Valproate | 35 (46) | 29 (39) | 36 (49) |
| Vigabatrin | 17 (22) | 28 (37) | 29 (40) |
| Levetiracetam | 24 (32) | 19 (25) | 22 (30) |
| Clobazam | 25 (33) | 17 (23) | 19 (26) |
| Prior antiepileptic drugs not currently taken | |||
| Valproate | 23 (30) | 28 (37) | 25 (34) |
| Vigabatrin | 42 (55) | 26 (35) | 29 (40) |
| Levetiracetam | 36 (47) | 39 (52) | 33 (45) |
| Clobazam | 22 (29) | 24 (32) | 16 (22) |
| Everolimus | 7 (9) | 7 (9) | 7 (10) |
| Median No. of seizures during the 28-d baseline period (IQR) | |||
| Primary–end-point seizuresa | 54.1 (26.4-102.0) | 56.0 (21.2-101.0) | 61.0 (36.0-117.0) |
| Total seizuresb | 56.5 (27.5-138.1) | 56.0 (22.6-101.0) | 70.0 (38.0-130.0) |
| Seizure subtypes during the 28-d baseline period | |||
| Focal seizures without impaired awareness | 33 (43) | 29 (39) | 39 (53) |
| Focal seizures with impaired awareness | 50 (66) | 46 (61) | 54 (74) |
| Focal to bilateral motor seizures | 24 (32) | 17 (23) | 24 (33) |
| Tonic-clonic | 14 (18) | 22 (29) | 16 (22) |
| Tonic | 15 (20) | 27 (36) | 23 (32) |
| Clonic | 2 (3) | 3 (4) | 3 (4) |
| Atonic | 13 (17) | 10 (13) | 5 (7) |
| Otherc | 15 (20) | 12 (16) | 24 (33) |
Abbreviations: CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day; IQR, interquartile range; TSC, tuberous sclerosis complex.
The TSC-associated seizures for this trial were defined as countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic-clonic, tonic, clonic, or atonic).
Total seizures include all seizure types combined, including focal sensory seizures and epileptic spasms.
Other seizures include absence, myoclonic, and focal sensory seizures and infantile or epileptic spasms.
Primary Outcome
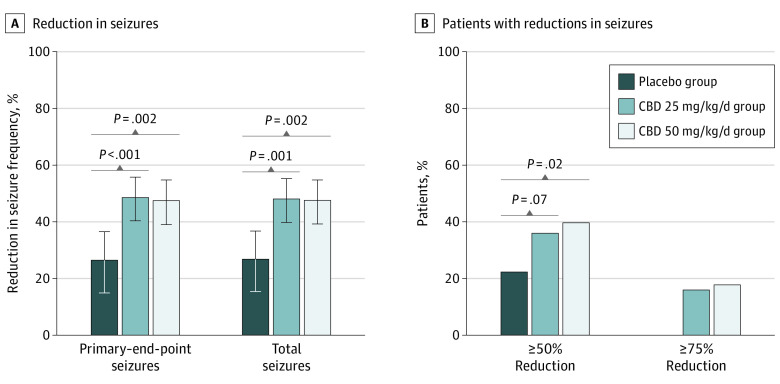
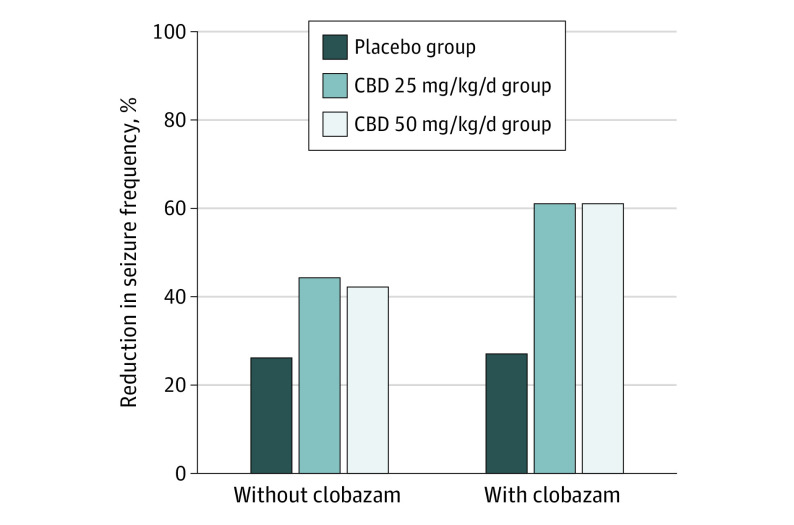
A reduction from baseline in primary–end-point seizures of 48.6% (95% CI, 40.4%-55.8%) was observed for the CBD25 group, 47.5% (95% CI, 39.0%-54.8%) for the CBD50 group, and 26.5% (95% CI, 14.9%-36.5%) for the placebo group during the treatment period (Figure 2A). The percentage reduction from placebo was 30.1% (95% CI, 13.9%-43.3%; P < .001) for the CBD25 group and 28.5% (95% CI, 11.9%-42.0%; nominal P = .002) for the CBD50 group. During the maintenance period, patients had a 36.9% reduction in primary–end-point seizures from placebo for both the CBD25 and the CBD50 groups (eFigure 3 in Supplement 1). This treatment effect on the primary end point was evident regardless of concomitant clobazam use (Figure 3). Results of the sensitivity analyses were consistent with the primary outcome (eFigure 4 in Supplement 1). In particular, other statistical methods used in sensitivity analyses of the primary outcome yielded similar results: median (IQR) percentage reductions of 43.4% (13.6%-67.8%; P = .004) for the CBD25 group and 36.6% (5.5%-67.0%; P = .009) for the CBD50 group vs 20.1% (3.1%-47.1%) with placebo using the Wilcoxon rank-sum test; geometric mean percentage reductions of 48.3% (95% CI, 32.9%-60.1%; P = .002) for CBD25 and 49.3% (95% CI, 34.4%-60.8%; P = .002) for CBD50 vs 23.9% (95% CI, 1.3%-41.3%) for placebo, using log-transformed analysis of covariance; and least square mean percentage reductions of 35.6% (95% CI, 26.1%-45.0%; P = .03) for CBD25 and 35.2% (95% CI, 25.6%-44.7%; P = .03) for CBD50 vs 20.4% (95% CI, 11.1%-29.8%) for placebo using analysis of covariance. A reduction in primary–end-point seizures was observed during the titration period and maintained throughout the treatment period (eFigure 5 in Supplement 1). Reductions in primary–end-point seizures were 47.9% (95% CI, 39.0%-55.6%) in the CBD25 group and 48.9% (95% CI, 36.7%-58.8%) in the CBD50 group vs 27.0% (95% CI, 15.9%-36.6%) in the placebo group when patients who withdrew or patients whose modal dosage was less than their randomized dosage were excluded from the analysis.
Figure 2. Seizure Outcomes During the Treatment Period.
A, Reduction from baseline in the frequency of primary–end-point and total seizures. B, Proportion of patients with a 50% or more and 75% or more reduction from baseline in primary–end-point seizures. Negative binomial regression was used to compare seizure frequency between cannabidiol groups with placebo. The treatment period (16 weeks) constituted the titration and maintenance phases. The estimated ratio of least squares means for the treatment period to baseline period was used to evaluate the reduction in seizure frequency. The P values for the testing of the null hypothesis that the estimated ratio of each cannabidiol group to placebo was 1 are presented. The primary–end-point seizures are all countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic-clonic, tonic, clonic, or atonic). Total seizures include all types combined, including focal sensory seizures and epileptic spasms. The odds ratios are presented for the comparisons in a 50% responder rate between the placebo group and the groups receiving 25–mg/kg/d and 50–mg/kg/d of cannabidiol. The P values were calculated from a Cochran–Mantel-Haenszel test stratified by age group (1-6, 7-11, 12-17, and 18-65 years). The percentage reduction in primary–end-point seizures from placebo was 30.1% (95% CI, 13.9%-43.3%; P < .001) in the cannabidiol 25 mg/kg/day (CBD25) group and 28.5% (95% CI, 11.9%-42.0%; nominal P = .002) in the cannabidiol 50 mg/kg/day (CBD50) group. The percentage reduction in total seizures from placebo was 29.1% (95% CI, 12.7% to 42.4%; nominal P = .001) in the CBD25 group and 28.4% (95% CI, 11.8% to 41.8%; nominal P = .002) in the CBD50 group. Note: the P values displayed in the Figure are nominal values.
Figure 3. Change From Baseline in Frequency of Primary–End-Point Seizures During the Treatment Period in Patients Taking Cannabidiol Without Clobazam and With Clobazam.
Negative binomial regression was used to compare seizure frequency between the cannabidiol groups with the placebo group. The treatment period (16 weeks) constituted the titration and maintenance phases. The estimated ratio of least squares means for treatment period to baseline period was used to evaluate the reduction in seizure frequency. The primary–end-point seizures are all countable focal motor seizures without impairment of awareness, focal seizures with impairment of awareness, focal seizures evolving to bilateral motor seizures, and generalized seizures (tonic-clonic, tonic, clonic, or atonic). The percentage reduction from placebo was 24.7% (95% CI, 3.7%-41.1%) between placebo and cannabidiol 25 mg/kg/day (CBD25) groups and 22.0% (95% CI, −0.1% to 39.1%) between the placebo and cannabidiol 50 mg/kg/day (CBD50) groups among patients not taking clobazam and 46.6% (95% CI, 20.0%-64.4%) between the placebo and CBD25 groups and 46.6% (95% CI, 21.1%-63.9%) between the placebo and CBD50 groups among patients taking clobazam.
Key Secondary Outcomes
Primary–end-point seizures were reduced at least 50% from baseline during the treatment period in 27 of 75 patients (36%) in the CBD25 group, 29 of 73 patients (40%) in the CBD50 group, and 17 of 76 patients (22%) in the placebo group (Figure 2B). Similar results were observed during the maintenance period (eFigure 3B in Supplement 1). Twenty-five patients (16.9%) taking cannabidiol had at least a 75% reduction in seizures, vs none taking placebo (Figure 2B). One patient in the CBD25 group was seizure free during the full treatment period; 4 patients in the CBD25 group, 2 in the CBD50 group, and none in the placebo group were seizure free during the maintenance period.
At treatment end, 48 of 70 patients (69%) in the CBD25 group, 43 of 69 patients (62%) in the CBD50 group, and 30 of 76 patients (39%) in the placebo group reported improvement from baseline in overall condition, according to the participants’ or caregivers’ global impression of change (eFigure 6 in Supplement 1). The odds ratios were 2.25 (95% CI, 1.24-4.07; nominal P = .007) for CBD25 vs placebo and 1.77 (95% CI, 0.98-3.20; nominal P = .06) for CBD50 vs placebo.
The percentage reduction from baseline in total seizures was 48.1% (95% CI, 39.8%-55.3%) for the CBD25 group, 47.6% (95% CI, 39.3%-54.8%) for the CBD50 group, and 26.9% (95% CI, 15.4%-36.8%) for the placebo group during the treatment period (Figure 2A). During the maintenance period, a 35.6% (95% CI, 17.1%-50.0%) reduction in total seizures compared with the level in the placebo group was observed for the CBD25 group; it was 37.0% (95% CI, 18.6%-51.2%) for the CBD50 group (eFigure 3 in Supplement 1).
Other Outcomes
During the 4-week baseline, patients had a mean (SD) of 7 (7) seizure-free days. During the 12-week maintenance period, patients in cannabidiol groups gained a mean of 10 (95% CI, 3-17) or 8 (95% CI, 1-15) additional seizure-free days over placebo in the CBD25 and CBD50 groups, respectively. Results of all other secondary outcomes analyses are presented in eTable 4 in Supplement 1.
Adverse Events
Adverse events were reported by 70 patients (93%) in the CBD25 group, 73 patients (100%) in the CBD50 group, and 72 patients (95%) in the placebo group; 88% of the adverse events were mild or moderate. The most common adverse events in the cannabidiol groups were diarrhea (placebo group, 19 [25%]; CBD25 group, 23 [31%]; CBD50 group, 41 [56%]), somnolence (placebo group, 7 [9%]; CBD25 group, 10 [13%]; CBD50 group, 19 [26%]), decreased appetite (placebo group, 9 [12%]; CBD25 group, 15 [20%]; CBD50 group, 17 [23%]), and liver transaminase level elevations (alanine aminotransferase level increased: placebo group: 0; CBD25 group, 9 [12%]; CBD50 group, 16 [22%]; aspartate aminotransferase level increased: placebo group, 0; CBD25 group, 8 [11%]; CBD50 group, 14 [19%]) (Table 2). In patients taking CBD with clobazam vs without clobazam, somnolence (placebo: 3 of 25 [12%] vs 4 of 51 [8%]; CBD25, 5 of 17 [29%] vs 5 of 58 [9%]; CBD50, 10 of 19 [53%] vs 9 of 54 [17%]), rash (placebo: 2 of 25 [8%] vs 0 of 51; CBD25, 2 of 17 [12%] vs 2 of 58 [3%]; CBD50, 1 of 19 [5%] vs 6 of 54 [11%]), and pneumonia (placebo, 0 of 25 vs 1 of 51 [2%]; CBD25, 2 of 17 [12%] vs 0 of 58; CBD50, 1 of 19 [5%] vs 1 of 54 [2%]) generally occurred more frequently (eTable 5 in Supplement 1).
Table 2. Common Adverse Events Among Patients in the Safety Analysis Seta.
| Adverse event | Treatment group, No. (%) | ||
|---|---|---|---|
| Placebo (n = 76) | CBD25 (n = 75) | CBD50 (n = 73) | |
| Diarrhea | 19 (25) | 23 (31) | 41 (56) |
| Mild | 16 (21) | 20 (27) | 35 (48) |
| Moderate | 3 (4) | 3 (4) | 5 (7) |
| Severe | 0 | 0 | 1 (1) |
| Somnolence | 7 (9) | 10 (13) | 19 (26) |
| Mild | 6 (8) | 10 (13) | 12 (16) |
| Moderate | 1 (1) | 0 | 6 (8) |
| Severe | 0 | 0 | 1 (1) |
| Decreased appetite | 9 (12) | 15 (20) | 17 (23) |
| Mild | 9 (12) | 9 (12) | 13 (18) |
| Moderate | 0 | 6 (8) | 3 (4) |
| Severe | 0 | 0 | 1 (1) |
| Alanine aminotransferase level increasedb | 0 | 9 (12) | 16 (22) |
| Mild | 0 | 7 (9) | 6 (8) |
| Moderate | 0 | 2 (3) | 10 (14) |
| Aspartate aminotransferase level increasedb | 0 | 8 (11) | 14 (19) |
| Mild | 0 | 7 (9) | 6 (8) |
| Moderate | 0 | 1 (1) | 8 (11) |
| Vomiting | 7 (9) | 13 (17) | 13 (18) |
| Mild | 7 (9) | 8 (11) | 9 (12) |
| Moderate | 0 | 4 (5) | 4 (6) |
| Severe | 0 | 1 (1) | 0 |
| Pyrexia | 6 (8) | 14 (19) | 12 (16) |
| Mild | 4 (5) | 13 (17) | 9 (12) |
| Moderate | 2 (3) | 1 (1) | 3 (4) |
| Nasopharyngitis | 12 (16) | 11 (15) | 11 (15) |
| Mild | 12 (16) | 11 (15) | 10 (14) |
| Moderate | 0 | 0 | 1 (1) |
| γ-Glutamyltransferase level increasedb | 0 | 12 (16) | 10 (14) |
| Mild | 0 | 11 (15) | 8 (11) |
| Moderate | 0 | 1 (1) | 2 (3) |
| Seizure | 5 (7) | 5 (7) | 8 (11) |
| Mild | 4 (5) | 2 (3) | 7 (10) |
| Moderate | 1 (1) | 2 (3) | 1 (1) |
| Severe | 0 | 1 (1) | 0 |
| Upper respiratory tract infection | 10 (13) | 7 (9) | 7 (10) |
| Mild | 8 (11) | 6 (8) | 7 (10) |
| Moderate | 2 (3) | 1 (1) | 0 |
| Constipation | 6 (8) | 8 (11) | 5 (7) |
| Mild | 6 (8) | 6 (8) | 4 (6) |
| Moderate | 0 | 2 (3) | 1 (1) |
| Cough | 5 (7) | 8 (11) | 3 (4) |
| Mild | 5 (7) | 7 (9) | 3 (4) |
| Moderate | 0 | 1 (1) | 0 |
Abbreviations: CBD25, cannabidiol 25 mg/kg/day; CBD50, cannabidiol 50 mg/kg/day.
Adverse events occurring in at least 10% of patients in any of the treatment groups are reported.
Liver enzyme level elevations include only those reported as an adverse event; see eTable 8 in Supplement 1 for all elevations regardless of adverse event status. Severity of an adverse event was determined by the investigators and did not involve independent adjudication.
An adverse event was listed as one of the reasons for treatment discontinuation in 20 patients (CBD25, 8 [11%]; CBD50, 10 [14%]; placebo, 2 [3%]); most common adverse events leading to discontinuation were rash (CBD25 group, 2 patients [3%]; CBD50 group, 2 patients [3%]), alanine aminotransferase level elevations, somnolence, and urticaria (2 patients [3%] each in the CBD50 group). Nine patients (12%) in the CBD25 group, 21 (29%) in the CBD50 group, and 4 (5%) in the placebo group had permanent dosage reductions because of an adverse event, most commonly diarrhea (placebo group, 1 [1%]; CBD25 group, 2 [3%]; CBD50 group, 7 [10%]) (eTable 6 in Supplement 1).
Serious adverse events were reported in 28 patients (CBD25, 16 [21%]; CBD50, 10 [14%]; placebo, 2 [3%]); liver enzyme level elevations were the most frequent serious adverse events (eTable 7 in Supplement 1). No deaths were reported.
Serum aminotransferase level elevations greater than 3 times the upper limit of the normal range occurred in 28 of 148 patients (18.9%) taking cannabidiol (CBD25, 9 of 75 [12%]; CBD50, 19 of 73 [26%]) and no patient taking a placebo; 22 of 28 affected patients (79%) were taking concomitant valproate (eTable 8 in Supplement 1). Most elevations occurred within 30 days of starting treatment and resolved either spontaneously, following treatment discontinuation, or after cannabidiol or antiepileptic drug dosage reduction (eTable 9 in Supplement 1). No patient met the Hy’s Law criteria for drug-induced liver injury. Additional safety, tolerability, and laboratory parameters are included in the eResults in the Supplement 1.
Discussion
This is the first randomized clinical trial to assess add-on cannabidiol in a disorder with primarily focal seizures, and the efficacy results are consistent with those from the 4 prior phase 3 trials of cannabidiol in the treatment of Lennox-Gastaut and Dravet syndromes.18,20,21,23 Our trial included patients younger than 2 years and used a much higher dosage of cannabidiol, with an accelerated titration than previously tested. Patients in this study had frequent and highly treatment-resistant seizures; cannabidiol was their eighth attempted medication on average. Nonetheless, cannabidiol led to meaningful reductions in seizures vs placebo observed as early as the titration period and maintained throughout the study. Importantly, patients and caregivers perceived meaningful improvement in overall condition, evident by participants’ and caregivers’ global impressions of change scores and enrollment in the open-label extension phase.
Given the bidirectional drug-drug interaction between cannabidiol and clobazam, in which exposure of the active metabolite of each agent is increased,24,25 it is important to understand the clobazam-independent efficacy of cannabidiol. This trial supports cannabidiol’s independent efficacy, because most patients (73%) were not taking clobazam, and cannabidiol was significantly more efficacious than placebo. Furthermore, although subgroup analyses from individual studies should be interpreted with caution, the treatment effect remained evident in the subgroup without clobazam. Cannabidiol administration has been shown to increase levels of mTOR inhibitors everolimus and sirolimus26 and calcineurin inhibitor tacrolimus.27 Therefore, use of cannabidiol as an adjunctive treatment in patients taking these medications may necessitate dosage adjustments; no patient in this study was taking concomitant mTOR inhibitors.
The cannabidiol dosage range of 25 to 50 mg/kg/day used in this study was informed by data from a cohort of patients with TSC (N = 18) in an expanded access program with the same cannabidiol formulation. In this study, both dosages showed similar efficacy but differing safety profiles. The safety profile of the 25 mg/kg/day dosage was consistent with the highest dosage (20 mg/kg/day) tested in prior trials; however, dosages greater than 25 mg/kg/day were associated with higher incidences of certain adverse and serious adverse events, with half of the patients randomized to 50 mg/kg/day unable to reach or maintain that dosage. Given the high proportion of patients in the CBD50 group who were not actually taking 50 mg/kg/day, we performed multiple sensitivity analyses excluding these patients, and there was still no difference in efficacy between the CBD25 and the CBD50 groups.
The most frequent adverse event leading to withdrawal were transient liver enzyme level elevation. The known potential for drug-induced liver injury with cannabidiol, as defined by an alanine aminotransferase level elevation greater than 5 times the upper limit of the normal range,28 especially with concomitant valproate, was confirmed in this study. Transaminase level elevations were more pronounced in patients taking concomitant valproate and/or 50 mg/kg/day of cannabidiol and those with baseline alanine aminotransferase elevations. All patients with drug-induced liver injury recovered, and none of the elevations in bilirubin levels met the Hy’s Law criteria. Overall, cannabidiol had an acceptable safety profile, with the clinical trial physicians managing adverse events associated with the accelerated titration scheme in this study by dosage reduction or treatment discontinuation.
Limitations
This trial is not without limitations. Because most patients in this trial were taking multiple medications, the potential for drug-drug interactions and their effect on safety and efficacy should be explored further. Although seizure type classification in this trial was confirmed by the Epilepsy Study Consortium, no video-electroencephalographic confirmation of the individual seizure subtypes was obtained. Potentially because of enhanced expectations for cannabidiol treatment, a higher than expected placebo effect was observed; however, this did not affect statistical significance of the treatment effect. Finally, long-term evaluation of cannabidiol in patients with TSC is needed and will be conducted in the ongoing open-label extension trial.
Conclusions
In patients with TSC and a high baseline burden of treatment-resistant, primarily focal seizures, add-on cannabidiol significantly reduced seizure frequency compared with placebo. The safety profile in this study is consistent with prior Lennox-Gastaut and Dravet syndrome studies, with confirmation of cannabidiol-associated risks of transaminase level elevations (especially in the presence of valproate) and somnolence and sedation (especially in the presence of clobazam).
eMethods. Study Design, Outcome Measures, Statistical Analysis.
eResults. Additional Safety, Tolerability, and Laboratory Parameters, and Pharmacokinetics.
eTable 1. Eligibility Criteria.
eTable 2. Hierarchical Sequential Procedure − Type I Error Control by Gatekeeping.
eTable 3. Summary of Cannabidiol Dosage During the Treatment Period.
eTable 4. Other Secondary Outcomes.
eTable 5. Adverse Events of Special Interest in Patients Taking Cannabidiol With Clobazam.
eTable 6. Adverse Events Leading to Treatment Discontinuation and Dose Reduction.
eTable 7. Serious Adverse Events in ≥1 Patient.
eTable 8. Treatment-Emergent ALT/AST Elevations in Patients by Valproate Use and ALT Levels at Baseline.
eTable 9. Onset and Resolution of ALT/AST Elevations.
eFigure 1. Trial Schematic.
eFigure 2. Dosage Titration Schedule.
eFigure 3. Seizure Outcomes during the Maintenance Period.
eFigure 4. Sensitivity Analyses of Outcome.
eFigure 5. Reduction From Baseline in the Primary End Point Seizures During the Titration Period and the 4-Week Treatment Windows During the Maintenance Period.
eFigure 6. Combined Participant/Caregiver Global Impression of Change in Overall Condition.
Protocol.
Nonauthor Collaborators. GWPCARE6 Study Group members.
Data Sharing Statement.
References
- 1.Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group . Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243-254. doi: 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JA, Zhang H, Roberts PS, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63(12):1236-1242. doi: 10.1093/jnen/63.12.1236 [DOI] [PubMed] [Google Scholar]
- 3.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657-668. doi: 10.1016/S0140-6736(08)61279-9 [DOI] [PubMed] [Google Scholar]
- 4.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345-1356. doi: 10.1056/NEJMra055323 [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Fallah A. Optimal management of seizures associated with tuberous sclerosis complex: current and emerging options. Neuropsychiatr Dis Treat. 2014;10:2021-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute of Neurological Disorders and Stroke . Tuberous sclerosis fact sheet. Published 2019. Accessed July 10, 2019. https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Tuberous-Sclerosis-Fact-Sheet
- 7.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125-127. doi: 10.1111/j.1749-6632.1991.tb37754.x [DOI] [PubMed] [Google Scholar]
- 8.Kingswood JC, d’Augères GB, Belousova E, et al. ; TOSCA consortium and TOSCA investigators . TuberOus SClerosis registry to increase disease Awareness (TOSCA)—baseline data on 2093 patients. Orphanet J Rare Dis. 2017;12(1):2. doi: 10.1186/s13023-016-0553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuberous Sclerosis Alliance . Diagnosis, surveillance, and management for healthcare professionals. Accessed July 10, 2019. https://www.tsalliance.org/healthcare-professionals/diagnosis/
- 10.Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449. doi: 10.1111/epi.13467 [DOI] [PubMed] [Google Scholar]
- 11.de Vries PJ, Wilde L, de Vries MC, Moavero R, Pearson DA, Curatolo P. A clinical update on tuberous sclerosis complex-associated neuropsychiatric disorders (TAND). Am J Med Genet C Semin Med Genet. 2018;178(3):309-320. doi: 10.1002/ajmg.c.31637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries PJ, Belousova E, Benedik MP, et al. ; TOSCA Consortium and TOSCA Investigators . TSC-associated neuropsychiatric disorders (TAND): findings from the TOSCA natural history study. Orphanet J Rare Dis. 2018;13(1):157. doi: 10.1186/s13023-018-0901-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51(7):1236-1241. doi: 10.1111/j.1528-1167.2009.02474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curatolo P, Nabbout R, Lagae L, et al. Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations. Eur J Paediatr Neurol. 2018;22(5):738-748. doi: 10.1016/j.ejpn.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Amin S, Lux A, Calder N, Laugharne M, Osborne J, O’callaghan F. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59(6):612-617. doi: 10.1111/dmcn.13352 [DOI] [PubMed] [Google Scholar]
- 16.Greenwich Biosciences Inc . Epidiolex (cannabidiol) oral solution. Published October 2020. Accessed November 16, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210365s008lbl.pdff
- 17.GW Pharma Ltd . Epidyolex (cannabidiol) oral solution. Published October 4, 2019. Accessed August 19, 2020. https://www.medicines.org.uk/emc/product/10781
- 18.Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011-2020. doi: 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Patel AD, Thiele EA, et al. ; GWPCARE1 Part A Study Group . Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204-e1211. doi: 10.1212/WNL.0000000000005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group . Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897. doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 21.Thiele EA, Marsh ED, French JA, et al. ; GWPCARE4 Study Group . Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096. doi: 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- 22.Hess EJ, Moody KA, Geffrey AL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(10):1617-1624. doi: 10.1111/epi.13499 [DOI] [PubMed] [Google Scholar]
- 23.Miller I, Scheffer IE, Gunning B, et al. ; GWPCARE2 Study Group . Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77(5):613-621. doi: 10.1001/jamaneurol.2020.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009-1031. doi: 10.1002/cpdd.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246-1251. doi: 10.1111/epi.13060 [DOI] [PubMed] [Google Scholar]
- 26.Ebrahimi-Fakhari D, Agricola KD, Tudor C, Krueger D, Franz DN. Cannabidiol elevates mechanistic target of rapamycin inhibitor levels in patients with tuberous sclerosis complex. Pediatr Neurol. 2020;105:59-61. doi: 10.1016/j.pediatrneurol.2019.11.017 [DOI] [PubMed] [Google Scholar]
- 27.Leino AD, Emoto C, Fukuda T, Privitera M, Vinks AA, Alloway RR. Evidence of a clinically significant drug-drug interaction between cannabidiol and tacrolimus. Am J Transplant. 2019;19(10):2944-2948. doi: 10.1111/ajt.15398 [DOI] [PubMed] [Google Scholar]
- 28.Ewing LE, Skinner CM, Quick CM, et al. Hepatotoxicity of a cannabidiol-rich cannabis extract in the mouse model. Molecules. 2019;24(9):1694. doi: 10.3390/molecules24091694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Design, Outcome Measures, Statistical Analysis.
eResults. Additional Safety, Tolerability, and Laboratory Parameters, and Pharmacokinetics.
eTable 1. Eligibility Criteria.
eTable 2. Hierarchical Sequential Procedure − Type I Error Control by Gatekeeping.
eTable 3. Summary of Cannabidiol Dosage During the Treatment Period.
eTable 4. Other Secondary Outcomes.
eTable 5. Adverse Events of Special Interest in Patients Taking Cannabidiol With Clobazam.
eTable 6. Adverse Events Leading to Treatment Discontinuation and Dose Reduction.
eTable 7. Serious Adverse Events in ≥1 Patient.
eTable 8. Treatment-Emergent ALT/AST Elevations in Patients by Valproate Use and ALT Levels at Baseline.
eTable 9. Onset and Resolution of ALT/AST Elevations.
eFigure 1. Trial Schematic.
eFigure 2. Dosage Titration Schedule.
eFigure 3. Seizure Outcomes during the Maintenance Period.
eFigure 4. Sensitivity Analyses of Outcome.
eFigure 5. Reduction From Baseline in the Primary End Point Seizures During the Titration Period and the 4-Week Treatment Windows During the Maintenance Period.
eFigure 6. Combined Participant/Caregiver Global Impression of Change in Overall Condition.
Protocol.
Nonauthor Collaborators. GWPCARE6 Study Group members.
Data Sharing Statement.