Abstract
Aim
To analyse the effects of different propofol starting doses as premedication for endotracheal intubation on blood pressure in neonates.
Methods
Neonates who received propofol starting doses of 1.0 mg/kg (n = 30), 1.5 mg/kg (n = 23) or 2.0 mg/kg (n = 26) as part of a previously published dose‐finding study were included in this analysis. Blood pressure in the 3 dosing groups was analysed in the first 60 minutes after start of propofol.
Results
Blood pressure declined after the start of propofol in all 3 dosing groups and was not restored 60 minutes after the start of propofol. The decline in blood pressure was highest in the 2.0 mg/kg dosing group. Blood pressure decline was mainly dependent on the initial propofol starting dose rather than the cumulative propofol dose.
Conclusion
Propofol causes a dose‐dependent profound and prolonged decrease in blood pressure. The use of propofol should be carefully considered. When using propofol, starting with a low dose and titrating according to sedative effect seems the safest strategy.
Keywords: blood pressure, hypotension, neonate, premedication, propofol
Abbreviations
- IQR
interquartile range
- MBP
mean blood pressure
- PMA
postmenstrual age
Key notes.
Propofol as premedication for neonatal endotracheal intubation causes a profound and long‐lasting decrease in blood pressure, which is mainly dependent on the starting dose and not on the cumulative dose.
Higher initial propofol starting doses cause a greater decrease in blood pressure compared to lower initial doses.
Start low and titrate according to the sedative effect causes the least decrease in blood pressure and seems the safest strategy.
1. INTRODUCTION
It is known that awake endotracheal intubation in newborns causes multiple harmful effects. 1 , 2 , 3 Therefore, in 2001 consensus was reached that only in the delivery room and in life‐threating situations associated with the unavailability of intravenous access, tracheal intubation should be performed without the use of analgesia or sedation. 4 Almost twenty years later, the most effective and safe premedication strategy in the newborn population is still to be determined. Propofol is considered one of the acceptable options 5 and is shown to be very easy in use. 6 Therefore, propofol has been implemented into clinical practice. 7 , 8 , 9 , 10 , 11
In the past decade, several studies have appeared reporting on the use of propofol for neonatal intubation, with somewhat conflicting results about the sedative effect related to dose. 6 , 9 , 10 , 12 , 13 Results regarding the hypotensive effect of propofol are probably even more conflicting, varying from no or only a slight decrease in blood pressure, 6 , 11 to a more pronounced decrease in blood pressure and a high incidence of hypotension. 9 , 10 , 13 Therefore, questions have been raised about the effectiveness and safety of propofol. In our recently performed propofol dose‐finding trial (NEOPROP‐2), we showed that propofol starting doses of 1.0 and 1.5 mg/kg were less effective in providing effective sedation compared to a propofol starting dose of 2.0 mg/kg. However, independent of the starting dose, propofol carried an unpredictable high risk of hypotension. 14
The aim of the current study was to further analyse the effects of different propofol starting doses on blood pressure. With this in‐depth analysis of the effects of propofol on blood pressure, we aimed to provide guidelines for the use of propofol in clinical practice.
2. METHODS
2.1. Participants
Neonates from the previously published NEOPROP‐2 trial were considered for inclusion. 14 The NEOPROP‐2 trial was a prospective multicentre dose‐finding trial conducted at three level III Neonatal Intensive Care Units in the Netherlands. Neonates with a postnatal age of less than 28 days who needed nonemergency endotracheal intubation were eligible for inclusion. Exclusion criteria were major congenital anomalies or neurologic disorders, upper airway anomalies, sedative or opioid administration in the preceding 24 hours and previous inclusion in the trial. Haemodynamic instability and underlying illnesses that are accompanied with a greater risk of haemodynamic instability were no specific exclusion criteria. The haemodynamic status and risk of haemodynamic insufficiency were judged by the attending physician. If the attending physician judged the patient to be haemodynamically stable enough to receive propofol, the patient could be included in the trial. The study was registered at ClinicalTrials.gov (NCT02040909; EudraCT number 2013‐005572‐17) and approved by the local medical ethics committee (NL47607.078.14, MEC‐2014‐0.68). For further details concerning patient stratification, dose‐finding approach and the assessment of effective sedation, we refer to the initial publication. 14 In summary, dose‐finding was performed by using a step‐up‐step‐down approach, starting with a propofol dose of 1.0 mg/kg in 5 consecutive patients and adjusting the dose with steps of 0.5 mg/kg for the next 5 patients based on sedative effect and side effects of the previous dose. For this analysis, all patients from the NEOPROP‐2 trial who received a propofol starting dose of 1.0, 1.5 and 2.0 mg/kg were included. Patients who received a different starting dose were excluded.
2.2. Blood pressure assessment
Blood pressure was measured invasively if an indwelling arterial catheter was present. Data were collected every minute from 5 minutes before until 30 minutes after the start of propofol administration, every 5 minutes from 30 to 60 minutes and every hour thereafter up to 24 hours. When no arterial catheter was present, blood pressure was measured noninvasively by an appropriately sized cuff from 5 minutes before until 60 minutes after propofol administration and every hour thereafter until 24 hours. Propofol‐induced hypotension was defined as a mean blood pressure (MBP) below postmenstrual age (PMA) detected in the first hour after propofol administration. Treatment of hypotension was left to the discretion of the treating physician.
2.3. Primary outcome measure
The primary outcome measure was the course of blood pressure over time in the first hour after start of propofol infusion relative to baseline blood pressure in three different initial propofol starting doses (1.0, 1.5 and 2.0 mg/kg). Blood pressure measured within 5 minutes before start of the propofol infusion was considered as baseline. Blood pressure data were obtained every 5 minutes from 5 minutes to 60 minutes after the start of propofol infusion.
2.4. Secondary outcome measures
Since the haemodynamic status of the patient could influence the patients' tolerability for propofol, we evaluated the incidence of hypotension and the change in MBP after start of propofol relative to the baseline MBP in relation to the haemodynamic status of the patient. For this purpose, we included all patients in whom sufficient information regarding baseline MBP and MBP in the first hour after propofol was available, and divided these patients into three groups: group 1, haemodynamically stable patients (no baseline hypotension and no sepsis/NEC); group 2, patients with baseline hypotension; and group 3, patients with a high risk of haemodynamic failure because of sepsis or NEC as indication for intubation. To elucidate the influence of cumulative propofol doses on blood pressure, we also performed a secondary analysis into the maximum decrease in MBP after different cumulative propofol doses.
2.5. Statistical analysis
Data analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 22.0. Armonk, NY, USA) and Stata (Stata, version 15, StataCorp LLC, TX, USA). Baseline characteristics were described by numbers and percentages for qualitative variables and median and interquartile range (IQR) for quantitative variables. Comparison between groups was performed with the Mann‐Whitney U test for continuous variables and the Fisher's exact test for categorical variables. Development of MBP over time‐epochs was expressed as absolute change compared to baseline. Comparison of MBP development between groups was determined using a linear mixed model analysis to take into account the dependency of observations within patients. The linear mixed models included time (added to the model as a categorical variable represented by dummy variables), dose group and the interaction between time and dose group. Besides a crude analysis, also analyses adjusted for volume resuscitation and the administration of additional doses of propofol were performed. This was done by adding volume resuscitation and the administration of additional doses of propofol to the linear mixed models as time‐dependent covariates. In addition, a linear mixed model analysis was performed with the cumulative dose of propofol as independent variable and the repeatedly measured MBP values as outcome.
3. RESULTS
3.1. Study population
Of the 91 patients in the NEOPROP‐2 study, 79 patients received a starting dose of either 1.0, 1.5 or 2.0 mg/kg of propofol and were included in this analysis. Median gestational age was 27.71 weeks (IQR 25.86‐30.71), median birthweight was 1065 g (IQR 860‐1560), and the median postnatal age at intubation was 33.53 hours (IQR 8.37‐279.53). Fifty‐two patients (66%) were boys, and 18 patients (23%) had a birthweight below the 10th percentile. Thirty patients (38%) received a propofol starting dose of 1.0 mg/kg, 23 patients (29%) received a propofol starting dose of 1.5 mg/kg, and 26 patients (33%) received a propofol starting dose of 2.0 mg/kg. Patient characteristics, sedative effect of propofol and need for extra propofol doses in these 3 dosing groups are presented in detail in the initial publication. 14 A summary of these findings relevant to the purpose of this analysis is presented in Table 1.
TABLE 1.
Patient characteristics and sedative effect in 3 different propofol starting doses (see original report 14 for details)
| Dosing groups | Comparison between groups | |||||
|---|---|---|---|---|---|---|
|
1.0 mg/kg (n = 30) |
1.5 mg/kg (n = 23) |
2.0 mg/kg (n = 26) |
1.0 vs 1.5 | 1.0 vs 2.0 | 1.5 vs 2.0 | |
| Gestational age (wk), median (IQR) | 27.5 (25.86‐30.93) | 26.86 (25.57‐30.14) | 29.07 (26.43‐31.71) | P = .37 | P = .66 | P = .20 |
| Birthweight (g), median (IQR) | 1075 (784‐1410) | 908 (780‐1600) | 1215 (895‐1568) | P = .46 | P = .51 | P = .19 |
| Postnatal age (h), median (IQR) | 125 (12‐397) | 37.35 (21‐387) | 19.58 (8‐43) | P = .68 | P = .01 | P = .04 |
| Male gender, n (%) | 22 (73) | 12 (52) | 18 (69) | P = .16 | P = .77 | P = .25 |
| Reason for intubation, n (%) | P = .53 | P = .27 | P = .35 | |||
| RDS | 12 (40) | 8 (34.8) | 17 (65.4) | |||
| Apnoea | 6 (20) | 8 (34.8) | 4 (15.4) | |||
| Sepsis/NEC | 4 (13.3) | 3 (13) | 2 (7.7) | |||
| Respiratory insufficiency | 7 (23.3) | 2 (8.7) | 2 (7.7) | |||
| Elective | 1 (3.3) | 1 (4.3) | 0 | |||
| Other | 0 | 1 (4.3) | 1 (3.8) | |||
| Effective sedation, n (%) | 1/28 (4) | 3/23 (13) | 18/24 (86) | P = .21 | P < .001 | P < .001 |
| Extra propofol administered, n (%) | 25 (83) | 20 (87) | 11 (42) | P = 1.0 | P = .002 | P = .002 |
| Cumulative propofol dose (mg/kg), median (IQR) | 3.0 (1.9‐4.0) | 3.4 (2.5‐4.5) | 2.0 (2.0‐3.0) | P = .06 | P = .97 | P = .03 |
Abbreviations: IQR, interquartile range; NEC, necrotising enterocolitis; RDS, respiratory distress syndrome.
3.2. Primary outcome measure
3.2.1. Occurrence of hypotension and lowest MBP in 3 dosing groups
In Table 2, data on MPB before administration of propofol, the definition of hypotension and data on MBP after start of propofol in the 3 dosing groups are presented. These data show that the incidence of hypotension was not significantly different between the 3 groups. In the 2.0 mg/kg group, more patients were treated with volume resuscitation (75%) compared to the 1.0 mg/kg (47%) and 1.5 mg/kg (36%) groups, but this difference did not reach statistical significance. The maximum decrease in MBP as percentage from baseline was equal in all 3 groups. However, this maximum decrease was reached significantly earlier in the 1.0 mg/kg group (18.2 minutes) compared to the 2.0 mg/kg group (28.6 minutes; P = .04).
TABLE 2.
Blood pressure data in 3 different dosing groups
| Dosing groups | Comparison between groups | |||||
|---|---|---|---|---|---|---|
|
1.0 mg/kg (n = 30) |
1.5 mg/kg (n = 23) |
2.0 mg/kg (n = 26) |
1.0 vs 1.5 | 1.0 vs 2.0 | 1.5 vs 2.0 | |
| Baseline MBP (mm Hg), mean (SD) | 40.5 (11.2) | 44.4 (9.7) | 42.4 (12.3) | P = .22 | P = .73 | P = .30 |
| Hypotension before propofol, n (%) | 4/29 (14) | 0/21 | 3/26 (12) | P = .13 | P = 1.0 | P = .24 |
| Hypotension at any time point after start of propofol, n (%) | 15/24 (63) | 11/21 (52) | 16/26 (62) | P = .56 | P = 1.0 | P = .57 |
| Treatment of hypotension with volume resuscitation, n (%) | 7/15 (47) | 4/11 (36) | 12/16 (75) | P = .70 | P = .15 | P = .06 |
| Lowest MBP (mm Hg) after start of propofol, mean (SD) | 27.8 (9.5) | 27.8 (6.9) | 27.1 (5.5) | P = .85 | P = .73 | P = .88 |
| Time after start of propofol (min) of lowest MBP, mean (SD) | 18.2 (12.5) | 22.6 (14.3) | 28.6 (18.2) | P = .24 | P = .04 | P = .25 |
| Maximum decrease in MBP as % from baseline, mean (SD) | −30 (16.5) | −36.4 (14.0) | −32.6 (18.7) | P = .07 | P = .15 | P = .73 |
Abbreviations: MBP, mean blood pressure; SD, standard deviation.
3.2.2. Absolute changes in MBP after propofol in the 3 dosing groups
The absolute changes in blood pressure compared to baseline at different time intervals after the start of propofol infusion for the 3 dosing groups are presented in Figure 1. These data show that MBP declined in all 3 groups compared to baseline and that this decline was highest in the 2.0 mg/kg dosing group. In the 1.0 mg/kg group, the decline in MBP from baseline was significant at 20, 25, 35 and 45 minutes after start of propofol administration. In the 1.5 mg/kg group, the decline from baseline was significant at time points 5 up to and including 30 minutes and 55 minutes after start of propofol. Finally, in the 2.0 mg/kg group the decline in MBP from baseline was significant at all time points with the exception of 5 minutes after the start of propofol. Correcting for volume resuscitation and the administration of extra doses of propofol did not alter the results from the initial analysis (Figure 2).
FIGURE 1.
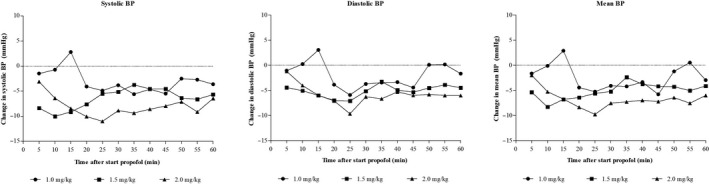
Changes of systolic, diastolic and mean blood pressure after start of propofol in 3 dosing groups
FIGURE 2.
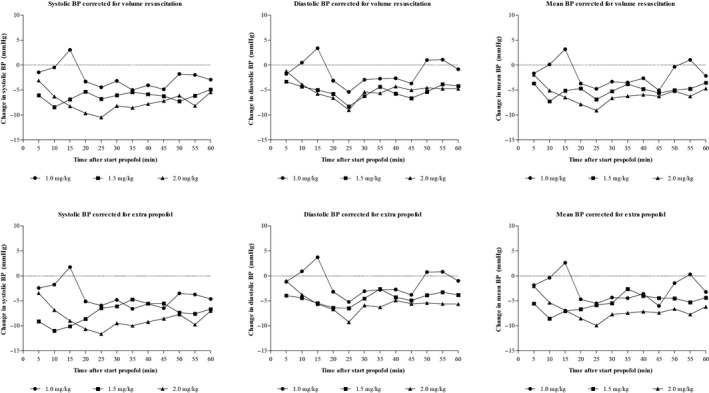
Changes in MBP after correcting for volume resuscitation and extra propofol administration
3.3. Secondary outcome measures
3.3.1. Changes in MBP in relation to haemodynamic status
For this analysis, we included 69 patients of whom sufficient data regarding baseline MBP and MBP in the first hour after propofol were available. The results of the analysis are presented in Table 3. The incidence of hypotension was, as expected, significantly higher in group 2, and the lowest MBP after start of propofol was significantly lower in this group. The incidence of hypotension, the lowest MBP after start of propofol and the maximum decrease in MBP after propofol were equal between group 1 and group 3.
TABLE 3.
Analysis of blood pressure in relation to haemodynamic stability
|
Group 1 N = 53 |
Group 2 N = 7 |
Group 3 N = 9 |
P‐value | |
|---|---|---|---|---|
| Propofol starting dose, n (%) | .35 | |||
| 1.0 mg/kg | 16 (30.2) | 4 (57.1) | 4 (44.4) | |
| 1.5 mg/kg | 16 (30.2) | 0 | 3 (33.3) | |
| 2.0 mg/kg | 21 (39.6) | 3 (42.9) | 2 (22.2) | |
| Baseline MBP (mm Hg), mean (SD) | 44.5 (10.2) | 25.3 (3.1) | 42.7 (10.5) | <.001 |
| Absolute difference between baseline MBP and MBP indicating hypotension (mm Hg), mean (SD) | 15.0 (9.8) | −4.1 (4.0) | 14.9 (9.8) | <.001 |
| Relative difference between baseline MBP and MBP indicating hypotension (%), mean (SD) | 54 (35.9) | −13.6 (12.4) | 53 (34.2) | <.001 |
| Hypotension at any time point after propofol administration, n (%) | 28 (52.8) | 7 (100) | 6 (66.7) | .05 |
| Treatment of hypotension with volume resuscitation, n (%) | 10/28 (36) | 3/7 (43) | 2/6 (33) | .28 |
| Lowest MBP (mm Hg) after start of propofol, mean (SD) | 28.5 (7.2) | 20.9 (4.3) | 27 (7.8) | .01 |
| Time after start of propofol (min) of lowest MBP, mean (SD) | 23.5 (16.2) | 19.9 (12.5) | 24.8 (17.7) | .88 |
| Maximum decrease in MBP as % from baseline, mean (SD) | −34.3 (16.6) | −16.9 (16.1) | −35.9 (11.7) | .05 |
Group 1 = haemodynamically stable (no baseline hypotension and no sepsis/NEC; group 2 = baseline hypotension; group 3 = high risk of haemodynamic failure based on sepsis or NEC as underlying morbidity.
Abbreviations: MBP, mean blood pressure; NEC, necrotising enterocolitis; SD, standard deviation.
3.3.2. Changes in MBP in relation to cumulative propofol dose
Independent of the propofol starting doses that were administered, we also analysed the average change in MBP over time for different cumulative doses of propofol, independent of the propofol starting dose. The results of this analysis are shown in Table 4. In all cumulative doses, MBP significantly declined compared to baseline, with the largest declines in the 1.0 and 2.5 mg/kg cumulative doses. These results have to be interpreted with some caution because of the small patient numbers, but could indicate that the cumulative dose of propofol did not influence the decline in MBP after propofol.
TABLE 4.
MBP changes in different cumulative propofol doses
| Cumulative propofol dose | Change in MBP relative to baseline | 95% Confidence interval | P‐value | |
|---|---|---|---|---|
| 1 mg/kg | −8.9 | −12.8 | −5.0 | <.001 |
| 1.5 mg/kg | −4.8 | −8.8 | −0.9 | .02 |
| 2 mg/kg | −2.8 | −5.2 | −0.4 | .02 |
| 2.5 mg/kg | −9.4 | −14.7 | −4.2 | <.001 |
| 3 mg/kg | −4.9 | −7.6 | −2.3 | <.001 |
| ≥3.5 mg/kg | −4.3 | −6.7 | −1.8 | .001 |
Abbreviations: MBP, mean blood pressure.
4. DISCUSSION
This post hoc analysis was performed to explore the effects of different propofol starting doses as premedication for endotracheal intubation on blood pressure. The results of this analysis show that propofol starting doses of 1.0 mg/kg, 1.5 mg/kg and 2.0 mg/kg all caused a profound and prolonged decline in blood pressure. In all three dosing groups, MBP decreased by a maximum of 30%‐35% in comparison with the baseline MBP, and MBP was not restored after one hour. The decrease in blood pressure was most pronounced with a propofol starting dose of 2.0 mg/kg. The incidence of hypotension was over 50% in all groups. The blood pressure decline was mainly dependent on the starting dose that was used and less influenced by the cumulative propofol dose that was administered to achieve successful endotracheal intubation.
To the best of our knowledge, this is the first study to evaluate the effect of different propofol doses on blood pressure. Comparison with data from the literature, therefore, is somewhat difficult. In our analysis, propofol‐induced hypotension was observed in 63%, 52% and 62% of the patients receiving a propofol starting dose of 1.0 mg/kg, 1.5 mg/kg and 2.0 mg/kg, respectively. Previous literature shows somewhat controversial effects of propofol on blood pressure in newborns. Smits et al, 13 in their dose‐finding study, found an incidence of hypotension of 64% in the entire population irrespective of the starting dose that was administered. Welzing et al and Simons et al both reported an incidence of propofol‐induced hypotension of 39%. 9 , 10 In their randomised controlled trial comparing propofol to sufentanil and atracurium, Durrmeyer et al 12 found hypotension to occur in 13.3% of the patients in the propofol group. In contrast to these findings, others reported no hypotension to occur in their study population treated with propofol for endotracheal intubation. 6 , 11
Part of these controversial results might be found in different definitions used for hypotension in preterm infants. Even in the 21st century, there is no generally accepted definition. Without any evidence to support it, the most popular criterion to define hypotension is MBP below gestational age. 15 , 16 The second most used definition is a MBP below the 10th or 5th percentile. 16 There are numerous reference ranges, often based on gestational age, birthweight and postnatal age criteria, with considerable variation among these reference ranges. 15 , 17 Finally, MBP below 30 mm Hg is used to define hypotension, because some studies found loss of cerebral autoregulation below this threshold. 16 , 18 , 19
In our study and in the study of Smits et al, 13 , 14 the MBP below gestational age criterion was used. Both studies also used this definition for infants beyond the first 72 hours of life and therefore somewhat modified the definition to MPB below postmenstrual age. Simons et al 9 also used the MBP below gestational age criterion but only reported on hypotension of a severity that required treatment. This could explain why they found a lower incidence of hypotension of 39%. Should we have only reported on hypotension that required treatment, our incidence of hypotension should have been 32%. Welzing et al 10 used a much more liberal definition of MBP less than 25 mm Hg in a study population with a gestational age of 29‐32 weeks. Should they have used the MBP below gestational age criterion, the incidence of hypotension would have been much higher.
Hypotension in the preterm infant has been associated with mortality, cerebral injury such as intraventricular haemorrhage and periventricular leukomalacia, and long‐term neurologic sequelae. 16 , 20 , 21 , 22 , 23 , 24 The question arises, however, if every infant with low blood pressure needs treatment for hypotension. Blood pressure is only one aspect of cardiovascular status and may not directly correlate with tissue perfusion. Infants with hypotension in the absence of biochemical or clinical signs of shock presumably have adequate tissue oxygen delivery, a phenomenon indicated as permissive hypotension. 15 It has been shown that infants with permissive hypotension who did not receive treatment for hypotension had similar outcomes as normotensive patients. 25 A recent French population‐based cohort study, however, showed that preterm infants below 29 weeks' gestation who were treated for hypotension in the first 72 hours of life had significantly higher survival rates without major morbidity and a lower rate of severe cerebral abnormalities, compared to infants with hypotension who were untreated. 26 These conflicting results indicate that the importance of hypotension in the preterm population is still to be elucidated.
Despite the statement that the haemodynamic status of the patients needed to be sufficiently stable to administer propofol, seven patients were hypotensive before the start of propofol. Besides this, nine patients received propofol while being at risk for haemodynamic insufficiency based on sepsis or NEC as underlying illness. Inclusion of these (possible) haemodynamically instable patients could have influenced the results and could have magnified the effect of propofol on blood pressure. Our analysis on the influence of haemodynamic status on the effect of propofol in blood pressure, however, shows that the effect of propofol on blood pressure is not different between patients who are presumed to be haemodynamically stable and infants who are presumed to have an increased risk of haemodynamic failure based on sepsis or NEC. Although caution with the interpretation of these results is warranted because of the small patient numbers, these data suggest that the tolerance for propofol in haemodynamically stable patients is not different from haemodynamically compromised patients. It should also be kept in mind that these results could also indicate that the haemodynamically stable patients in group 1 were not as haemodynamically stable as they were presumed to be.
In our initial analysis, we showed that a propofol starting dose of 2.0 mg/kg provided effective sedation in 86% of patients, compared to 4% and 13% of the patients who received a starting dose of 1.0 mg/kg or 1.5 mg/kg, respectively. 14 Solely based on the sedative effect of propofol, a starting dose of 2.0 mg/kg of propofol would be the best strategy. However, despite an equal incidence of hypotension compared to the 1.0 mg/kg starting dose, a dose of 2.0 mg/kg had a much more profound decrease in blood pressure despite a lower cumulative propofol dose compared to the 1.0 mg/kg group. Therefore, when using propofol as premedication for endotracheal intubation, the safest strategy seems to start with a low dose of 1.0 mg/kg and titrating until effective sedation has been reached.
The above‐mentioned advice answers the question which propofol strategy for endotracheal intubation in preterm neonates is the safest. The question if this is safe enough and if it is justified to continue using propofol as premedication for endotracheal intubation in newborns still needs to be answered. The statement on hypotension without clinical and biochemical signs of poor perfusion being permissive concerns the spontaneous course of blood pressure of extremely preterm infants in the first 72 hours of life. 17 Although most of the patients in our analysis were within their first 72 hours of life, the occurrence of hypotension was not spontaneous but induced by the administration of propofol. Although one‐third of patients in each of our 3 study groups did not fulfil our criteria of hypotension, MBP significantly decreased relative to baseline in almost all patients and this decrease was not restored 60 minutes after the start of propofol administration. Thewissen et al 27 showed that cerebral autoregulation stayed intact during episodes of hypotension caused by propofol. Two other reports also could not demonstrate an important correlation between blood pressure and cerebral oxygenation in the neonatal population. 28 , 29 Although these data are somewhat reassuring, the possible negative effects on short‐ and long‐term outcomes of hypotension induced by the use of propofol are not known and possibly by far not as permissive as we might think. Neonatologists should ask themselves if they would expose the most vulnerable (extremely preterm) neonates to this side effect with unknown consequences on the short and on the long term. In our opinion, the effect of propofol on blood pressure is a safety concern and the use of propofol should be carefully considered in every individual patient. Studies into the short‐term and long‐term effects of propofol‐induced hypotension and comparison to alternative premedication strategies are warranted if propofol is continued to be used for this purpose in this population.
There are some limitations to our study. At first, not all patients in our study population had indwelling arterial catheters, and therefore, invasively and noninvasively measured blood pressure data were combined. Secondly, data of near‐infrared spectroscopy monitoring were missing on a large scale, and consequently, we have no data on cerebral oxygenation during propofol treatment.
5. CONCLUSION
Propofol used as premedication to sedate neonates for endotracheal intubation causes a profound and prolonged decrease in MBP which is more pronounced with a higher starting dose. It also causes a high incidence of propofol‐induced hypotension, irrespective of the starting dose that is used. Although premedication for endotracheal intubation is essential, propofol might not be the preferred drug. When propofol is used in neonates, starting with a low dose and titrating according to sedative effect seems the safest strategy with the least pronounced effect on blood pressure.
CONFLICT OF INTEREST
All authors have no potential conflict of interest to disclose.
ETHICAL APPROVAL
The study was registered at ClinicalTrials.gov (NCT02040909; EudraCT number 2013‐005572‐17) and approved by the local medical ethics committee (NL47607.078.14, MEC‐2014‐0.68).
Funding information
This study was financially supported by a grant of fondsNutsOhra (grant number 1201‐020) and a personal grant (SS) of the Netherlands Organization for Health Research and Development (ZonMw 90713494).
ACKNOWLEDGEMENTS
We would like to thank the research nurses Annemieke de Lange, Tinneke Jonckers and Marieke Vervoorn for their help in performing this trial.
de Kort EHM, Twisk JWR, van t Verlaat EPG, Reiss IKM, Simons SHP, van Weissenbruch MM. Propofol in neonates causes a dose‐dependent profound and protracted decrease in blood pressure. Acta Paediatr. 2020;109:2539–2546. 10.1111/apa.15282
REFERENCES
- 1. Maheshwari R, Tracy M, Badawi N, Hinder M. Neonatal endotracheal intubation: how to make it more baby friendly. J Paediatr Child Health. 2016;52(5):480‐486. [DOI] [PubMed] [Google Scholar]
- 2. Byrne E, MacKinnon R. Should premedication be used for semi‐urgent or elective intubation in neonates? Arch Dis Child. 2006;91:79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carbajal R, Eble B, Anand KJS. Premedication for tracheal intubation in neonates: confusion or controversy? Semin Perinatol. 2007;31:309‐317. [DOI] [PubMed] [Google Scholar]
- 4. Anand KJ, International evidence‐based group for neonatal pain . Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173‐180. [DOI] [PubMed] [Google Scholar]
- 5. Kumar P, Denson SE, Mancuso TJ, Committee on Fetus and Newborn, Section on Anesthesiology and pain Medicine . Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010;125(3):608‐615. [DOI] [PubMed] [Google Scholar]
- 6. Ghanta S, Abdel‐Latif ME, Lui K, Ravindranathan H, Awad J, Oei J. Propofol compared with the morphine, atropine and suxamethonium regimen as induction agent for neonatal endotracheal intubation: a randomized, controlled trial. Pedriatics. 2007;119(6):e1248‐e1255. [DOI] [PubMed] [Google Scholar]
- 7. Carbajal R, Eriksson M, Courtois E, et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Resp Med. 2015;3:796‐812. [DOI] [PubMed] [Google Scholar]
- 8. Flint RB, Van Beek F, Andriessen P, et al. Large differences in neonatal drug use between NICUs are common practice: time for consensus? Br J Clin Pharmacol. 2018;84:1313‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simons SHP, van der Lee R, Reiss IKM, van Weissenbruch MM. Clinical evaluation of propofol as sedative for endotracheal intubation in neonates. Acta Paediatr. 2013;102(11):e487‐e492. [DOI] [PubMed] [Google Scholar]
- 10. Welzing L, Kribs A, Eifinger F, Huenseler C, Oberthuer A, Roth B. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth. 2010;20(7):605‐611. [DOI] [PubMed] [Google Scholar]
- 11. Nauta M, Onland W, De Jaegere A. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm infants. Paediatr Anaesth. 2011;21:711‐712. [DOI] [PubMed] [Google Scholar]
- 12. Durrmeyer X, Breinig S, Claris O, et al. Effect of atropine with propofol versus atropine with atracurium and sufentanil on oxygen desaturation in neonates requiring nonemergency intubation. A randomized clinical trial. JAMA. 2018;319(17):1790‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smits A, Thewissen L, Caicedo A, Naulaers G, Allegaert K. Propofol dose‐finding to reach optimal effect for (semi‐)elective intubation in neonates. J Pediatr. 2016;179:54‐60. [DOI] [PubMed] [Google Scholar]
- 14. De Kort EHM, Prins SA, Reiss IKM, et al. Propofol for endotracheal intubation in neonates: a dose‐finding trial. Arch Dis Child Fetal Neonatal Ed. 2020. 10.1136/archdischild-2019-318474 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dempsey EM, Barrington KJ. Evaluation and treatment of hypotension in the preterm infant. Clin Perinatol. 2009;36:75‐85. [DOI] [PubMed] [Google Scholar]
- 16. Peter D, Gandy C, Hoffman SB. Hypotension and adverse outcomes in prematurity: comparing definitions. Neonatology. 2017;111:228‐233. [DOI] [PubMed] [Google Scholar]
- 17. Dempsey EM. What should be do about low blood pressure in preterm infants? Neonatology. 2017;111:402‐407. [DOI] [PubMed] [Google Scholar]
- 18. Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;13:16‐23. [DOI] [PubMed] [Google Scholar]
- 19. Borch K, Lou HC, Greisen G. Cerebral white matter blood flow and arterial blood pressure in preterm infants. Acta Paediatr. 2010;99:1489‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miall‐Allen VM, De Vries LS, Whitelaw AL. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child. 1987;62:1068‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bada HS, Korones SB, Perry EH, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr. 1990;117:607‐613. [DOI] [PubMed] [Google Scholar]
- 22. Watkins AMC, West CR, Cooke RWI. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early Hum Dev. 1989;19:103. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein RF, Thompson RJ Jr, Oehler JM, Crazy JE. Influence of acidosis, hypoxemia, and hypotension on neurodevelopmental outcome in very low birth weight infants. Pediatrics. 1995;95:238‐243. [PubMed] [Google Scholar]
- 24. Faust K, Härtel C, Preuß M, et al. Short‐term outcome of very‐low‐birthweight infants with arterial hypotension in the first 24 h of life. Arch Dis Child Fetal Neonatal Ed. 2015;100:F388‐F392. [DOI] [PubMed] [Google Scholar]
- 25. Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:F241‐F244. [DOI] [PubMed] [Google Scholar]
- 26. Durrmeyer X, Marchand‐Martin L, Porcher R, et al. Abstinention or intervention for isolated hypotension in the first 3 days of life in extremely preterm infants: association with short‐term outcomes in the EPIPAGE 2 cohort study. Arch Dis Child Fetal Neonatal Ed. 2017;102:F490‐F496. [DOI] [PubMed] [Google Scholar]
- 27. Thewissen L, Caicedo A, Dereymaeker A, et al. Cerebral autoregulation and activity after propofol for endotracheal intubation in preterm neonates. Pediatr Res. 2018;84(5):719‐725. [DOI] [PubMed] [Google Scholar]
- 28. Vedrenne‐Cloquet M, Breinig S, Dechartres A, et al. Cerebral oxygenation during neonatal intubation – ancillary study of the Prettineo‐study. Front Pediatr. 2019;7:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanderhaegen J, Naulaers G, Van Huffel S, Vanhole C, Allegaert K. Cerebral and systemic hemodynamic effects of intravenous bolus administration of propofol in neonates. Neonatology. 2010;98:57‐63. [DOI] [PubMed] [Google Scholar]


