Abstract
Axons in the adult mammalian central nervous system (CNS) fail to regenerate inside out due to intrinsic and extrinsic neuronal determinants. During CNS development, axon growth, synapse formation and function are tightly regulated processes allowing immature neurons to effectively grow an axon, navigate towards target areas, form synaptic contacts and become part of information processing networks that control behavior in adulthood. Not only immature neurons are able to precisely control the expression of a plethora of genes necessary for axon extension and pathfinding, synapse formation and function, but also non-neuronal cells such as astrocytes and microglia actively participate in sculpting the nervous system through refinement, consolidation and elimination of synaptic contacts. Recent evidence indicates that a balancing act between axon regeneration and synaptic function may be crucial for rebuilding functional neuronal circuits after CNS trauma and disease in adulthood. Here we review the role of classical and new intrinsic and extrinsic neuronal determinants in the context of CNS development, injury and disease. Moreover, we discuss strategies targeting neuronal and non-neuronal cell behaviors, either alone or in combination, to promote axon regeneration and neuronal circuit formation in adulthood.
Keywords: Axon growth and regeneration, branching and synapse formation, astrocyte, microglia
INTRODUCTION
Axon regeneration failure following central nervous system (CNS) trauma and disease often leads to permanent functional disability. Promoting axon sprouting and regeneration of spared and injured axons as well as refinement of existing or de novo formation of neuronal circuits can attain neurological recovery (Hutson & Di Giovanni, 2019; Maier & Schwab, 2006). The development of neuronal circuits in the mammalian central nervous system (CNS) is a dynamic process that requires coordinated epigenetic regulation of gene expression (Henikoff, 2008; Jaenisch & Bird, 2003). Genome-wide remodeling of the epigenetic landscape allows axon elongation, membrane expansion and navigation towards target areas as well as axon branching, synapse formation and refinement to be spatially and temporally ordered (Kolodkin & Tessier-Lavigne, 2011; J. Li et al., 2020; Mahar & Cavalli, 2018; Palmisano et al., 2019; Venkatesh, Mehra, Wang, Califf, & Blackmore, 2018; Williams, de Wit, & Ghosh, 2010). Non-neuronal cells including astrocytes and microglia also actively participate in CNS development and function (Allen & Lyons, 2018; Barres, 2008; Nayak, Roth, & McGavern, 2014; Salter & Beggs, 2014). From worms to mammals, the ability to regenerate injured axons and rebuild functional neuronal circuits sharply declines with age (Byrne et al., 2014; Geoffroy, Hilton, Tetzlaff, & Zheng, 2016; Verdu, Ceballos, Vilches, & Navarro, 2000). Axon regeneration failure has been attributed to the presence of both neuronal intrinsic and extrinsic determinants (Fawcett & Verhaagen, 2018; He & Jin, 2016; Schwab & Strittmatter, 2014). Recent progress has furthered our understanding of the intrinsic molecular brakes to axon growth and regeneration (Barber et al., 2019; Bray et al., 2019; Chauhan et al., 2020; Koseki et al., 2017; Y. Liu et al., 2017; Sekine et al., 2018; Song et al., 2019; Tedeschi et al., 2016; Tedeschi & Popovich, 2019; C. Yang et al., 2020; Zhang et al., 2019). It is now possible to reprogram adult mammalian neurons into a growth-competent state by recapitulating, at least in part, developmental programs (Blackmore et al., 2012; Hilton & Bradke, 2017; K. Liu et al., 2010; Moore et al., 2009; O’Donovan et al., 2014). However, recapitulation of early developmental programs may proceed at the expense of synaptic specificity, synapse formation and functional connectivity (Carlin et al., 2018; Carlin, Halevi, Ewan, Moore, & Cavalli, 2019; Tedeschi & Bradke, 2017; Z. Wang, Reynolds, Kirry, Nienhaus, & Blackmore, 2015), negatively impacting formation and consolidation of neuronal circuits. Thus, intrinsic neuronal roadblocks must be overcome with temporal precision to maximize CNS repair strategies after trauma and disease. Here we discuss recent evidence that suggests that axon regeneration, synapse formation and function may be at odds with one another.
1. Neuronal intrinsic mechanisms of axon growth and synaptic targeting
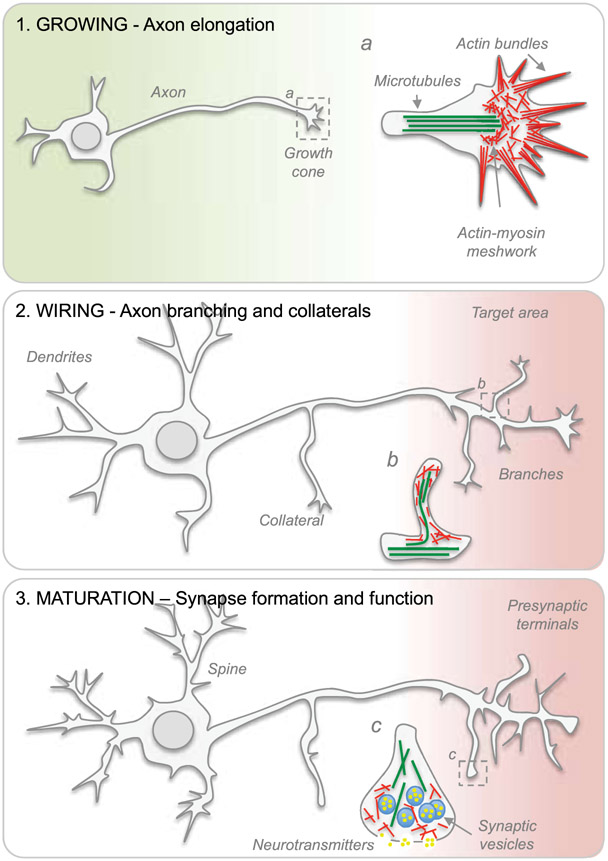
The establishment of intricate connectivity patterns where functional modules are interlinked by hub regions enables complex nervous system function. How is such a complex wiring scheme achieved in the nervous system? During early stages of development, electrically silent immature neurons rapidly extend axons towards target areas (Figure 1). This initial phase of axon growth is characterized by axon elongation with no branching and the presence of a growth cone situated on the tip of the growing axon (Gribnau, de Kort, Dederen, & Nieuwenhuys, 1986; Mason & Gregory, 1984). On the one hand, the production of building blocks including newly synthesized lipids for membrane expansion that are anterogradely transported to the distal portion of the axon via vesicles, cholesterol- and sphingolipid-rich rafts enables rapid extension of growing axons (Maday, Twelvetrees, Moughamian, & Holzbaur, 2014; Quiroga, Bisbal, & Caceres, 2018; Vance, Campenot, & Vance, 2000). On the other, cytoskeletal mechanisms, receptor arrays, cell adhesive molecules and transsynaptic signals allow growth cones to navigate with accuracy to appropriate target regions (Dickson, 2002; Giger, Hollis, & Tuszynski, 2010; Mosca, Hong, Dani, Favaloro, & Luo, 2012; Roig-Puiggros et al., 2020; Xie et al., 2019; Zipursky & Sanes, 2010).
Figure 1. The developmental transition from a growing to a transmitting phase.
Schematic representation of axon elongation, branching, synaptic transmission and synaptogenesis during neuronal maturation. Cytoskeletal arrangements present in (a) growth cones and (b) axon branches. (c) Detailed view of a few structural elements found within presynaptic terminals.
1.1. Lipid metabolism and membrane resealing
Large amount of lipids are also required during axon regeneration (Chauhan et al., 2020; de Chaves, Rusinol, Vance, Campenot, & Vance, 1997; Vance et al., 2000). Secretion and accumulation of apolipoprotein E (ApoE), a plasma protein acting as a ligand for low-density lipoprotein (LDL) receptors (Mahley, 1988), increases during regeneration of peripheral axons (Comley et al., 2011; Jimenez et al., 2005; F. Q. Li, Fowler, Neil, Colton, & Vitek, 2010; Rozenbaum et al., 2018). The tip of these regenerating axons contains high concentration of LDL receptors (Boyles et al., 1989), providing evidence for the presence of a cellular mechanism allowing cholesterol supply for membrane biogenesis during axon regeneration. By combining an axonal transection model with ex vivo postnatal day (P)1 cortical explants, another study has found that cerebral cortices from ApoE null mice show reduced axonal growth and regeneration when compared to the control condition (Yin, Guo, He, Wang, & Sun, 2019). Exogenous application of ApoE is sufficient to rescue regeneration defects in ApoE null cortices (Yin et al., 2019). Of particular interest, a recent study has shown that fatty acid synthesis in satellite glial cells actively participates in axon elongation during peripheral nerve regeneration presumably by paracrine lipid transfer to dorsal root ganglia (DRG) neurons (Avraham et al., 2019). Adult neuronal membranes are composed by a variety of lipids, but the lipids that may be necessary for membrane insertion during axon elongation and regeneration are not known. A recent study has reported that neuronal depletion of the phosphatidic acid phosphatase enzyme lipin 1 promotes axon regeneration after optic nerve injury in mice by regulating glycerolipid metabolism and, more specifically, triglyceride hydrolysis and phospholipid synthesis (C. Yang et al., 2020). Of note, lipin 1 catalyzes the conversion of phosphatidic acid to diglycerides (Carman & Han, 2019). Triglyceride hydrolysis is also required during regeneration of peripheral nerves (C. Yang et al., 2020), further suggesting triglycerides may provide lipid precursors for phospholipid synthesis necessary for membrane biosynthesis during nerve regeneration. Understanding the impact of specific classes of lipids and their metabolism on early versus late stages of neuronal maturation as well as axon regeneration versus functional connectivity will be an important topic for future investigation.
Mechanical damage to plasma membranes occurs following CNS trauma and disease. Therefore, promoting membrane resealing represents a crucial step in re-equilibrating the concentration of ions and small molecules in neurons and along axons for growth cone formation and axon regeneration to occur (Bradke, Fawcett, & Spira, 2012). A large screening for genes with growth-promoting or –inhibiting functions in Caenorhabditis elegans has identified annexins, calcium sensitive phospholipid-binding proteins (Draeger, Monastyrskaya, & Babiychuk, 2011), as plasma membrane repair factors for axon regeneration. Indeed, axon regeneration is reduced in annexin nex-1 mutants compared to wild-type worms (Nix et al., 2014). Although a growth cone may form after axotomy in nex-1 mutants, it collapses or fails to extend (Nix et al., 2014), suggesting damaged membranes that fail to reseal may interfere with growth cone formation and dynamics.
1.2. Actin dynamics and axon branching
Growth cone motility and protrusion rely on actin dynamics. Specifically, the assembly of actin filaments at the leading edge of the growth cone and disassembly at its central domain, as well as actin treadmilling and retrograde flow enable growth cone exploration, integration of directional cues and protrusion (Lowery & Van Vactor, 2009; Van Goor, Hyland, Schaefer, & Forscher, 2012). Actin depolymerizing factor (ADF)/cofilin (AC) controls actin turnover through its actin-severing activity (Bamburg & Wiggan, 2002; Pollard, Blanchoin, & Mullins, 2000). Overexpression of the transcription factor serum response factor promotes axon regeneration through cytoplasmic localization and cofilin-mediated reactivation of actin dynamics in stalled retraction bulbs (Stern et al., 2013). By genetic loss- and gain-of-function, we have recently demonstrated that elevated actin turnover fuels regeneration of sensory axons after spinal cord injury (SCI) in adult mice (Tedeschi et al., 2019). Strategies engineering neuronal growth cones by pharmacological inhibition or genetic silencing of nonmuscle myosin II have proven similarly effective in promoting axon regeneration over growth-inhibitory substrates including chondroitin sulfate proteoglycans and myelin-associated inhibitors (Hur et al., 2011). Calcium influences growth cone motility through remodeling of the actin cytoskeleton (Henley & Poo, 2004; Kater, Mattson, Cohan, & Connor, 1988; Rehder & Kater, 1992; Welnhofer, Zhao, & Cohan, 1999). Extracellular guidance cues including proteoglycans, cell adhesion molecules and myelin-associated proteins use calcium to transduce cell surface signals that affect growth cone exploratory behavior (Bandtlow, Schmidt, Hassinger, Schwab, & Kater, 1993; Nicol, Hong, & Spitzer, 2011; Snow, Atkinson, Hassinger, Letourneau, & Kater, 1994). Interestingly, the frequency of intracellular calcium transients at the growth cone has been shown to inversely correlate with the rate of axon outgrowth in the embryonic Xenopus spinal cord (Gomez & Spitzer, 1999). In particular, high frequencies of calcium transients associate with growth-cone stalling and axon retraction, also known as hallmarks of axon regeneration failure (Gomez & Spitzer, 1999). Whereas mimicking transients with photorelease of calcium slows rapid axonal extension, suppression of calcium transients effectively accelerates axon extension (Gomez & Spitzer, 1999). Mechanistically, calcium transients inhibit axon extension via the calcium-dependent phosphatase calcineurin acting on the growth cone actin cytoskeleton in Xenopus spinal neurons (Lautermilch & Spitzer, 2000).
As axons approach target areas in late development, axon elongation halts and extensive axon branching and formation of collaterals take place (Gallo, 2011; Gibson & Ma, 2011) (Figure 1), thereby maximizing terminal surface area for synaptic transmission and synapse formation. At this time, electrically silent neurons switch to a transmitting phase. In rats, the outgrowth of the pioneer axon of the corticospinal tract (CST) is completed by postnatal day (P)9 and later developing CST axon branches and collaterals are continuously added beyond P9 (Gribnau et al., 1986). During axon branching, plasmalemma expansion is thought to primarily occur via exocytosis (Futerman & Banker, 1996; Pfenninger, 2009; Pfenninger & Friedman, 1993). Not only does exocytosis promote secretion of signaling molecules and enzymes, but also the insertion of lipids and membrane proteins into the plasma membrane. Membrane fusion is a universal process in eukaryotic cells. The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and Sec1/Munc18-like (SM) proteins mediate membrane fusion of vesicles in a constitutive manner for cargos delivery or for neurotransmitter release upon calcium influx (Sudhof & Rothman, 2009). At synapses, synaptotagmin as well as Munc13 and RIM proteins localize at the active zone to control the membrane fusion machinery (Sudhof & Rizo, 2011). The SNARE proteins were first identified as targets of neurotoxins (e.g., clostridial botulinum and tetanus toxins), proteases that selectively block presynaptic membrane fusion (Link et al., 1992; Schiavo et al., 1992). The SNARE complex is constituted by three or four SNARE proteins including syntaxin, VAMP (synaptobrevin) and SNAP-25 homologs (Poirier et al., 1998; Sollner, Bennett, Whiteheart, Scheller, & Rothman, 1993; Sollner, Whiteheart, et al., 1993; Sutton, Fasshauer, Jahn, & Brunger, 1998). In humans, 35 SNARE proteins have been identified (Bock, Matern, Peden, & Scheller, 2001). SNARE proteins localize to vesicle (also called v-SNARE) and target (also called t-SNARE) membranes. To avoid inappropriate fusion events, SNARE proteins are maintained in inactive conformations. Only upon proper stimulation are SNARE proteins released to bind to each other. Axon branching requires constitutive SNARE-mediated exocytosis (Winkle et al., 2014). By taking advantage of computer vision software for automated detection of VAMP-mediated exocytosis, Urbina et al. has described spatiotemporal changes in exocytosis and membrane expansion in developing neurons (Urbina, Gomez, & Gupton, 2018). Interestingly, VAMP2-mediated vesicle fusion occurs near the site of a growing branch process (Urbina et al., 2018), thereby supplying excess material for plasma membrane expansion during morphogenesis.
Actin dynamic is critical for axon branching. In filopodia and lamellipodia, the balance between actin polymerization and depolymerization determines the rate of membrane protrusion and withdrawal for branching control (Kalil & Dent, 2014). In order to stabilize axon branches, dynamic microtubules enter into filopodia (Dent, Callaway, Szebenyi, Baas, & Kalil, 1999; Kornack & Giger, 2005). Actin associated proteins including enabled/vasodilator-stimulated phosphoprotein (ENA/VASP), diaphanous related formin 3 (DIAPH3) and actin-related proteins-2/3 (ARP2/3) regulate formation of actin arrays and branches (Campellone & Welch, 2010). The ENA/VASP family of proteins enhances actin filament elongation by counteracting the capping proteins that inhibit F‑actin elongation (M. Krause, Dent, Bear, Loureiro, & Gertler, 2003). Depletion of ENA/VASP proteins by sequestering them to the mitochondria surface results in a reduction of filopodia formation and branching in frog retinotectal axons (Dwivedy, Gertler, Miller, Holt, & Lebrand, 2007). Moreover, all cortical tracts including major forebrain commissures and the internal capsule in the brain of ENA/VASP null mice are lost (Kwiatkowski et al., 2007). In vitro, the vast majority of cortical neurons from null mice lack filopodia and fail to extend an axon (Kwiatkowski et al., 2007). Filopodia formation can be restored in these neurons by ectopic expression of the formin DIAPH3 (Dent et al., 2007). ARP2/3 is required for the formation of lamellipodia and branched actin networks (Korobova & Svitkina, 2008; Q. Yang, Zhang, Pollard, & Forscher, 2012). Whereas Arp2/3 blockade using N-WASP CA or Wave-1 VCA peptides in cultured primary neurons significantly enhances axon elongation, it causes defects in growth cone guidance (Strasser, Rahim, VanderWaal, Gertler, & Lanier, 2004). Arp2/3 inhibition using small molecules causes a reduction in barbed end actin assembly at the leading edge, actin veils disruption and retraction (Q. Yang et al., 2012). Depletion of Arp2/3 complex in primary neurons by RNA interfering leads to formation of multiple neurites, aberrant pattern of neurite extension and excessive formation of focal adhesions (Korobova & Svitkina, 2008). Terminal branching and neuronal wiring is also controlled by activity dependent mechanisms. In mice, expression of the inwardly rectifying potassium channel Kir2.1 in callosal projection neurons reduces firing rate of cortical neurons and suppresses their branching pattern (Mizuno, Hirano, & Tagawa, 2007). Whereas suppression of spontaneous neuron firing or synaptic activity decreased axon branching, increased spontaneous neuronal activity parallels branch formation of thalamocortical projections in organotypic cocultures of the thalamus and cortex (Uesaka, Hayano, Yamada, & Yamamoto, 2007). In vivo time-lapse imaging of Xenopus retinal axons shows how visual experience directly controls axon branch dynamics for the development of topographic maps (Ruthazer, Akerman, & Cline, 2003).
Looking at the variety of studies focused on axon elongation and branching during development, after CNS trauma and as a result of disease, it is clear that these complex mechanisms of axon growth and regeneration need to be controlled with precision to allow selection of appropriate pathways and specific targets for neurons to integrate and process information from different regions of the nervous system.
1.3. α2δ subunits, synaptic transmission and function
The transition from a dynamic growth cone to a presynaptic terminal specialized for neurotransmitter release may represent one of the first key steps in the gradual loss of axon growth and regeneration ability (Tedeschi & Bradke, 2017) (Figure 1). In search for putative gene switches controlling this transition, we have sequenced the whole transcriptome of mouse DRG neurons in both growth competent and incompetent states at different developmental stages, in diverse culture and in vivo experimental conditions. In these neurons, the developmental transition from a growth competent to a transmitting phase is associated with a marked increase in the expression of genes that control axon branching, synaptic transmission and synapse formation (Sudhof, 2018; Tedeschi et al., 2016). Similarly, a cell-surface proteomic profiling in the fly brain has discovered a global downregulation of neural development molecules and upregulation of synaptic transmission molecules in the transition from developing to mature olfactory projection neurons (J. Li et al., 2020). Our search has found Cacna2d2, the gene encoding the α2δ2 subunit of voltage-gated calcium channels (VGCC) (Dolphin, 2018), which acts as a neuron intrinsic switch limiting axon growth during development and axon regeneration in adulthood (Sun et al., 2020; Tedeschi et al., 2016). α2δ subunits positively regulate synaptic transmission by increasing plasma membrane expression of VGCC and vesicle release probability (Hoppa, Lana, Margas, Dolphin, & Ryan, 2012). However, these subunits may also play a pathological role during development and following injury. Presynaptic overexpression of α2δ2 leads to aberrant wiring of glutamatergic presynaptic boutons with GABAergic postsynaptic positions (Geisler et al., 2019), contributing to an excitatory-inhibitory imbalance that is often associated with neuropsychiatric disorders. Expression of α2δ1 increases following axonal injury, resulting in aberrant neuron activities associated with chronic pain and posttraumatic epilepsy (C. Y. Li et al., 2006; H. Li et al., 2012). Overexpression of Cacna2d1, the gene encoding α2δ1, potentiates presynaptic and postsynaptic N-methyl-D-aspartate receptor activity of spinal dorsal horn neurons to trigger pain hypersensitivity in mice (Chen et al., 2018). In rodent hippocampal neuron cultures, Cacna2d1 overexpression enhances spontaneous neuronal network activity (Bikbaev et al., 2020). Hence, increased pathological expression of α2δ subunits may hijack the self-repair mechanisms of the CNS by forcing aberrant plasticity after trauma and disease. In adult DRG neurons, Cacna2d2 forced expression leads to the formation of short and highly branched neurites in vitro. In contrast, genetic deletion or silencing of Cacna2d2 promotes the formation of long and sparsely branched neurites (Tedeschi et al., 2016). Mechanistically, Cacna2d2 forced expression in adult DRG neurons leads to an increase expression of Cav2.1 (Tedeschi et al., 2016), which is responsible for initiation of synaptic transmission (Catterall, 2011). By providing an entry point for calcium to trigger vesicle fusion, presynaptic VGCC clustered at the active zone play a crucial role in neurotransmitter exocytosis (Dolphin & Lee, 2020; Sudhof, 2013). Interestingly, recent studies have demonstrated that i) alternative splicing of Cav2.1 channels controls synapse-specific release probability (Heck et al., 2019) and ii) ultrastructural analysis of Cav2.1 and Munc13-1 shows strong synapses are comprised of synaptic vesicles that are tightly coupled (10 nm) to VGCC clusters (Rebola et al., 2019). Deletion of α2δ3 in cultured spinal ganglion neurons leads to a reduction of P/Q-type current by 60% (Stephani et al., 2019). Axon growth defects in Cacna2d2 overexpressing DRG neurons can be rescued by incubation with N- and P/Q-type channel blockers and by chelating calcium (Tedeschi et al., 2016). Interestingly, treating control neurons with the calcium-independent secretagogue Ruthenium Red and P/Q-type channel agonists can mimic similar defects in axon elongation (Tedeschi et al., 2016). This suggests that Cacna2d2 inhibits axon elongation in adult neurons by forcing synaptic transmission and branching. Munc18/nSec1 interacts with monomeric syntaxin 1, preventing its interaction with SNAP-25 and VAMP (Pevsner et al., 1994). Calcium-dependent vesicle exocytosis is markedly reduced in mouse chromaffin cells lacking Munc18-1, (Voets et al., 2001). In the absence of UNC-18, the size of the readily releasable pool is severely reduced in Caenorhabditis elegans (Weimer et al., 2003). Given that SM proteins are key partners for SNARE proteins in vesicle fusion (Sudhof & Rothman, 2009), how is axon elongation, branching and nerve regeneration regulated in the absence of Munc18 and Munc13? More recently we have found that α2δ2 also negatively regulates axon growth and regeneration of corticospinal neurons (Sun et al., 2020), the cells that originate the corticospinal tract (CST). There, upregulation of α2δ2 expression parallels the increased spontaneous firing of corticospinal neurons at times when the CST growth program is near completion and synaptogenesis starts (Bregman, Kunkel-Bagden, McAtee, & O’Neill, 1989; Gribnau et al., 1986). Gabapentinoids (e.g., gabapentin and pregabalin), drugs used clinically to treat neurological disorders (Goodman & Brett, 2017), bind with high affinity and selectivity to α2δ1/2 subunits (Gee et al., 1996; Gong, Hang, Kohler, Li, & Su, 2001). Pharmacological blockade of α2δ2 through systemic administration of gabapentinoids dampens excitatory synaptic transmission and promotes structural plasticity and regeneration after SCI in adult mice (Sun et al., 2020; Tedeschi et al., 2016). Moreover, we have shown that mice administered gabapentinoids recover upper extremity function after cervical SCI (Sun et al., 2020) and a multi-center cohort study has found that motor recovery is improved in SCI individuals receiving gabapentinoids (Warner et al., 2017). Together, these data highlight the need to consider repurposing gabapentinoids as a novel treatment for CNS repair.
In addition to the strategies discussed above, a number of target gene manipulations that promote axon regeneration in the adult CNS by recapitulating early developmental programs have been recently developed (Carlin et al., 2018; K. Liu et al., 2010; Park et al., 2008; Romero et al., 2007; D. Wu et al., 2015). Despite promoting anatomical regeneration, many of these strategies impair synaptic function including learning deficits (Costa et al., 2002), reduction in synaptic transmission (Fraser, Bayazitov, Zakharenko, & Baker, 2008), dendritic spine deficits and synaptic impairments (Goorden, van Woerden, van der Weerd, Cheadle, & Elgersma, 2007; Nie et al., 2015; Sugiura et al., 2015; Yasuda et al., 2014).
Recent evidence suggests that a spared source of synaptic activity may be sufficient to harness the activation of intrinsic regenerative programs after injury. Using highly localized laser axotomy, Lorenzana et al. has demonstrated how sensory axons respond to injuries at different locations relative to the main bifurcation point in the spinal cord. Results clearly show that a surviving intact branch suppresses the regeneration of the damaged branch (Lorenzana, Lee, Mui, Chang, & Zheng, 2015). In Caenorhabditis elegans, axon branching also influences axon regeneration. Mechanosensory axons regenerate when severed proximal to their collateral synaptic branch, but not when severed distal to the branch point (Z. Wu et al., 2007), and dendrites actively suppress axon outgrowth of adult sensory neurons (Chung et al., 2016). Following dorsal root crush injury in adult mice, regenerating sensory axons are stabilized as they penetrate into the CNS territory by formation of synaptic-like contacts on non-neuronal cells (Di Maio et al., 2011), thereby contributing to axon regeneration failure. Similarly, dystrophic sensory endings intimately associate with nerve/glial antigen 2 (NG2) positive cells in the penumbra after injury to the spinal cord (Filous et al., 2014). When stabilized, dystrophic end bulbs may persist for decades in SCI individuals (Ruschel et al., 2015).
Thus, accumulating evidence suggests that axon regeneration, synaptic transmission and function may be at odds with one another. Further testing of the developmental-dependent and adult self-repair mechanisms that stabilize neuronal connectivity at the expense of axon elongation and nerve regeneration could help the development of novel strategies and therapeutic opportunities to treat a variety of CNS trauma and disease conditions.
1.4. Lipid rafts, actin remodeling and exocytosis
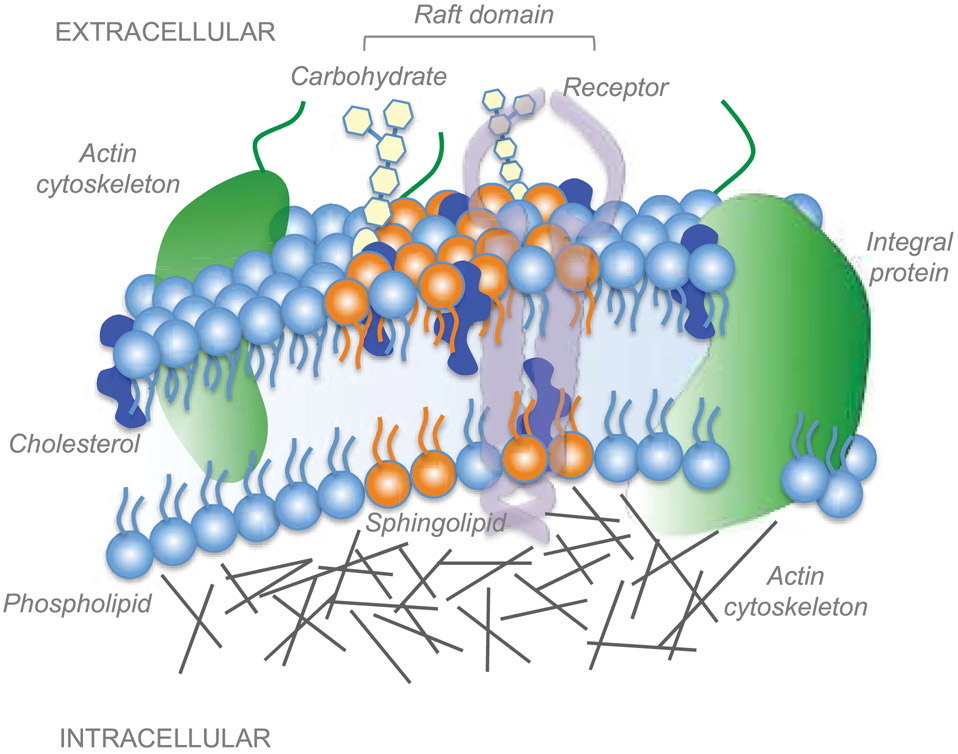
The plasma membrane features different subcompartments that differ in their composition and thus physical properties such as thickness, curvature or bending rigidity (McIntosh & Simon, 2006). Cholesterol and glycosphingolipids are important structural components of highly specialized subdomains (10-200 nm) of the plasma membrane called lipid rafts (Munro, 2003; Pike, 2006; Sezgin, Levental, Mayor, & Eggeling, 2017) (Figure 2). By recruiting other lipids and proteins, cholesterol-rich subdomains and glia-derived cholesterol play a crucial role in trafficking, signal transduction, presynaptic differentiation, evoked transmitter release and synapse formation (Goritz, Mauch, & Pfrieger, 2005; Lingwood & Simons, 2010; Mauch et al., 2001; Simons & Ikonen, 1997). Lipid rafts are decorated with a variety of signaling receptors and ion channels (Brown & London, 1998), serving as docking stations to integrate extracellular signals into intracellular pathways. The limited size (<200 nm) (Pralle, Keller, Florin, Simons, & Horber, 2000) makes lipid rafts difficult to resolve using conventional microscopy. With the development of super resolution microscopy and single particle tracking, it is now possible to study nano-scale structures and dynamics of raft in cells (Owen, Williamson, Magenau, & Gaus, 2012; Saka et al., 2014). Thus far, the molecular code for selective incorporation of proteins into raft domains remains unknown. Whereas prenyl groups associate with non-raft regions, saturated lipid anchors including glycophosphatidylinositol-anchored (GPI) proteins prefer ordered membranes (Goswami et al., 2008; Levental, Grzybek, & Simons, 2010). Trafficking and integration to raft domains is subjected to regulation through N- and O-linked glycosylation, transmembrane elements and GPI anchor (Hanzal-Bayer & Hancock, 2007). Indeed, protein N-glycosylation is one of the most abundant post-translational modifications, contributing to protein folding, stability, trafficking and localization (Freeze, Chong, Bamshad, & Ng, 2014). For example, α2δ subunits of VGCC are glycosylated extracellular proteins that localize within cholesterol-rich raft domains via GPI anchor (Dolphin, 2012). Interestingly, we have recently shown that α2δ2 glycosylation in corticospinal neurons positively correlates with increased spontaneous firing, network activity and synaptogenesis, but negatively with CST regeneration ability (Sun et al., 2020). Comprehensive analysis of rat synaptic membranes during postnatal development has shown remarkable lipidomic remodeling during the first 8 weeks with progressive accumulation of sphingolipids and cholesterol-rich raft domains to synaptic sites (Tulodziecka et al., 2016). Interestingly, blocking Neogenin raft association is sufficient to promote neuron survival and axon regeneration after both optic nerve crush and compression of the thoracic spinal cord in rats (Tassew et al., 2014). Similarly, cholesterol depletion favors axon growth in vitro and peripheral nerve regeneration in vivo by disrupting lipid raft (Rosello-Busquets et al., 2019). Of interest, the stemness-associated gene Prom1 has been recently identified as positive regulator of peripheral regeneration in adulthood (Lee et al., 2020). Prom1 encodes for the membrane glycoprotein Prominin-1. By inhibiting the expression of genes related to cholesterol biosynthesis, Prom1 forced expression enhances axon regeneration via Smad2-dependent signaling (Lee et al., 2020). It is important to note that a reduction of membrane cholesterol has been shown to alter activity of transient receptor potential cation channels in trigeminal neurons (Saghy et al., 2015), synaptic vesicle biogenesis and increased transmitter packing (Rodrigues et al., 2013). Nanoscale organization at the synaptic active zone is highly heterogeneous. In fact, presynaptic VGCC and postsynaptic ligand-gated ion channels are confined to nanodomains of the plasma membrane where individual molecules are only transiently trapped (Heine & Holcman, 2020).
Figure 2. Lipid raft microdomains of the plasma membrane.
Lipid rafts are highly organized subdomains of the plasma membrane enriched in cholesterol and glycosphingolipids. Rafts serve as a platform for the recruitment, insertion and interaction of membrane receptors and biomolecules involved in a variety of cellular processes including signal transduction, membrane trafficking and cytoskeletal organization. Membrane proteins are either free to move or immobilized to the actin cytoskeleton.
Cholesterol reduction inhibits exocytosis of synaptic vesicles in cultured hippocampal neurons (Linetti et al., 2010). This suggests that lipid rafts are key in the regulation of exocytosis. SNARE proteins localize at defined sites of the plasma membrane including raft domains (Lafont et al., 1999), thereby allowing exocytic proteins and protein complexes to be spatially regulated. Syntaxin 1A and SNAP-25 concentrate in cholesterol-dependent clusters in PC12 cells thereby restricting docking and fusion sites for exocytosis discrete clusters (Lang et al., 2001). Importantly, these clusters disperse following cholesterol extraction from the plasma membrane (Lang et al., 2001). In addition, about 20% of syntaxin 1A and SNAP-25 copurified with detergent resistant (e.g., sphingolipid/cholesterol-rich domains) raft membranes in PC12 cells (Chamberlain, Burgoyne, & Gould, 2001). To a variable degree, SNAP23 and 25 associations to rafts is directly linked to palmitoylation of cysteine residues (Melkonian, Ostermeyer, Chen, Roth, & Brown, 1999; Salaun, Gould, & Chamberlain, 2005).
The interaction between membrane rafts and cytoskeletal components such as actin plays an important role for raft assembly and clustering (Gowrishankar et al., 2012; Head, Patel, & Insel, 2014; Kusumi, Koyama-Honda, & Suzuki, 2004; Ritchie, Iino, Fujiwara, Murase, & Kusumi, 2003; Simons & Gerl, 2010; Viola & Gupta, 2007; Whitehead, Gangaraju, Aylsworth, & Hou, 2012). Along this line, the cortical actin cytoskeleton has been shown to influence exocytosis, organization, diffusion and mechanical properties of cell membranes (Fritzsche, Erlenkamper, Moeendarbary, Charras, & Kruse, 2016; Koster & Mayor, 2016). Indeed, the integrity of actin filaments is crucial for the delivery of a variety of polypeptides to the apical membrane. Cytochalasin D, a cell permeable fungal toxin, binds to the barbed end of actin filaments inhibiting both the association and dissociation of subunits thereby disrupting the subcellular architecture of the actin network and consequently, the surface delivery of vesicles in polarized cells (Jacob, Heine, Alfalah, & Naim, 2003; Maples, Ruiz, & Apodaca, 1997; Ojakian & Schwimmer, 1988; Schliwa, 1982; Valentijn, Gumkowski, & Jamieson, 1999). The actin nucleation factors Arp2/3 are critical for the establishment of actin coats, also called ring structures, at the boundary between the vesicle and the plasma membranes (P. Li, Bademosi, Luo, & Meunier, 2018; Sokac, Co, Taunton, & Bement, 2003; Yu & Bement, 2007). As myosin II drives the contractility of the actin coats, inhibition of Myosin II causes defects in the final stages of exocytosis (Nightingale et al., 2011), more specifically a delay between vesicle fusion and release. In a reconstituted fluid bilayer, actomyosin dynamics drive local membrane component organization (Koster et al., 2016). In addition, changes in actin patterning are known to alter membrane architecture (Fritzsche et al., 2017).
Together, experimental evidence suggests that highly specialized membrane microdomains enriched in cholesterol and glycosphingolipids regulate signal transduction and pathways influencing the transition from a growing to a transmitting phase. Whether manipulating the expression and localization of regulators and components of the SNARE complex promotes axon elongation and regeneration remains to be determined.
2. Astrocyte-dependent mechanisms of circuit formation and refinement
Along with neurons, astrocytes participate in coordinating and sustaining electrical activity in the CNS. Astrocytes are widely recognized as one of the most ubiquitous glial cell type in the brain and spinal cord. Astrocytes derive their namesake when their star-shaped morphology was revealed by metal-impregnation techniques (Garcia-Marin, Garcia-Lopez, & Freire, 2007). It is now appreciated that the cellular structures of astrocytes are much more complex than when first characterized by Ramón y Cajal. Intracellular injection of fluorescent dye in rat hippocampal protoplasmic astrocytes reveals extensive ramification of fine processes that compose the majority of the astrocyte cellular domain, allowing the establishment of minimally overlapping territories among adjacent protoplasmic astrocytes (Bushong, Martone, Jones, & Ellisman, 2002). Such structural complexity is further expanded by heterogeneous morphology of astrocytes in other brain regions, such as velate astrocytes and Bergmann glia in the cerebellum and fibrous astrocytes in brain white matter. These unique structures are even more intricate and complex in nonhuman primates where astrocytes possess varicose processes or processes that extend across multiple cortical layers, which are structural elements not seen in rodent astrocytes (Oberheim, Goldman, & Nedergaard, 2012).
Astrocytes perform a multitude of homeostatic functions like maintaining extracellular potassium levels, regulating extracellular pH, water transport, control of CNS blood circulation, and neurotransmitter clearance (Kimelberg, 2010). These fundamental capabilities astrocytes possess has led to the proposal that astrocytes are functionally defined as homeostatic cells and maintain CNS homeostasis (Verkhratskiĭ & Butt, 2013). However, the extent to which astrocytes control neuron function and participate in synaptic transmission remains controversial.
2.1. Astroglial network and gap junctions
Astrocytes possess a prominent characteristic in that they couple with neighboring astrocytes through gap junctional coupling to form extensive syncytial networks, which are also referred to as the astrocyte syncytium (Brightman & Reese, 1969; Giaume et al., 1991; Kuffler, Nicholls, & Orkand, 1966; Kuffler & Potter, 1964; Rash, Yasumura, Dudek, & Nagy, 2001). Astrocyte syncytial coupling mediates the exchange of ions, metabolites, and calcium that play critical roles in neuron homeostatic and signaling functions (Kuga, Sasaki, Takahara, Matsuki, & Ikegaya, 2011; Langer, Stephan, Theis, & Rose, 2012; Lin et al., 1998; Nagy & Rash, 2000; Orkand, Nicholls, & Kuffler, 1966; Rose & Ransom, 1997; Rouach, Koulakoff, Abudara, Willecke, & Giaume, 2008; Simard, Arcuino, Takano, Liu, & Nedergaard, 2003; F. Wang et al., 2012). The importance of this syncytial network is revealed when two major astrocytic gap junctions subunits, connexin 43 (Cx43) and 30 (Cx30) are genetically deleted from mice. The loss of syncytial coupling results in disruption of potassium homeostasis: potassium clearance decelerates and higher potassium accumulates during synchronized neuronal firing in 1-3 month old mice (Wallraff et al., 2006). Also, this results in altered neuronal function as shown by a decrease in synaptic long-term potentiation (LTP) in P16-25 mice (Pannasch et al., 2011) and aberrant hippocampal network activity in P17-25 ex vivo brain slices (Chever, Dossi, Pannasch, Derangeon, & Rouach, 2016). The loss of syncytial coupling also impairs spatial memory tasks in novel object recognition in 4 months old mice (Lutz et al., 2009). Altogether, the evidence indicates the importance of the astrocyte syncytial network to facilitate neuronal function and synaptic transmission. It is enticing to envision that the functional state of the astrocyte syncytial network may be involved in neural circuit formation, in particular, synaptogenesis and synaptic plasticity. A mouse model with astrocytic Cx43 and Cx30 deletion did not show any alterations in the expression of synaptic markers synaptophysin and PSD-95 (Pannasch et al., 2011). This suggests that postnatal synaptogenesis may be astrocyte network-independent, and may be governed at the single cell level. However, it isn’t clear if the syncytial network plays any role in synaptic formation and plasticity in the adult CNS. For example, can astrocytes in one area of the brain or spinal cord influence astrocytes in another area via long-range astrocyte signaling through gap junctions thereby affecting synaptic formation and activity? Or is astrocytic influence constrained to spatiotemporal ‘hotspots’ within an astrocyte domain?
2.2. Astrocyte heterogeneity across the CNS
Astrocytes are not simply a homogenous class of glial cells, but heterogeneous and functionally specialized (Ben Haim & Rowitch, 2017; Farmer & Murai, 2017; Khakh & Deneen, 2019). Intrinsic variation and regional specificity may allow astrocytes to refine and optimize neuronal function locally. Evidence of astrocyte specialization to specific circuits is observed in a study where astrocytes were depleted in the ventral spinal cord. This resulted in altered motorneuron synaptogenesis that could not be resolved by tangential migration of neighboring astrocytes from adjacent regions (Tsai et al., 2012). This intriguing finding not only underscores critical interactions between astrocytes and neurons in synaptogenesis and circuit formation but also demonstrates that astrocytes are purposefully allocated to specific regions of the CNS. In addition, neuronal factors dictate astrocyte specialization to generate heterogenous astrocyte populations. Sonic hedgehog (Shh) signaling from neurons is necessary to confer and maintain specialized functional astrocyte properties (Farmer et al., 2016). This was demonstrated first in the cerebellum that contained velate astrocytes and Bergmann glia, which are two types of astrocytes that are morphologically and functionally distinct from each other. The perturbance of the Shh pathway using viral-delivery of Cre to genetically delete Purkinje cell-derived Shh or its receptor Patched on Bergmann glia causes Bergmann glia to take on the functional phenotype of the neighboring velate astrocytes. Conversely, constitutive activation of Smoothened, a transmembrane protein immediately downstream from Shh/Patched signaling pathway, in velate astrocytes causes the acquisition of some molecular characteristics associated with Bergmann glia, such as higher expression of AMPA receptor GluA1 and annexin A7. Interestingly, the cellular morphology appears not to be affected, suggesting Shh signaling is not involved in the diversification of astrocyte cellular structures. An interesting point of this study is that persistent Shh signaling is necessary to maintain the functional profiles of these astrocytes. This has potential implications in scenarios where Shh signaling is disrupted such as after CNS injury (Allahyari, Clark, Shepard, & Garcia, 2019). It isn’t known if the disruptions of such signaling pathways affect astrocyte contributions to synaptic formation and function as a result of an altered functional phenotype.
2.3. Tripartite Synapse: astrocyte processes as the third component of a synapse
It is increasingly recognized that astrocytes are important contributors to synapse formation and transmission. The active participation of astrocytes in synaptic function has led to its inclusion as the third component of a synapse to form the tripartite synapse (Araque, Parpura, Sanzgiri, & Haydon, 1999). The intricate cellular morphology enables a single astrocyte to cover over an estimated 100,000 synapses through its dense ramification of fine processes positioned next to synaptic terminals (Bushong et al., 2002). This direct association between presynaptic axons, postsynaptic dendritic spine, and perisynaptic astrocyte processes (PAPs) is visualized at the ultrastructural level using electron microscopy (Spacek, 1985; Ventura & Harris, 1999). Using electron microscopy to examine the association of hippocampal astrocytes with synaptic type, another study has found that PAPs preferentially associate with larger synapses and that these processes are typically less than 200 nm wide (Witcher, Kirov, & Harris, 2007). The ramification of these fine processes increases during development, which is seen in rat hippocampal protoplasmic astrocytes between 1-4 weeks of age (Bushong, Martone, & Ellisman, 2004). While these studies reveal important ultrastructural details at the synaptic level, tissue samples require chemical fixation that negates the ability to observe these processes in a living astrocyte. To circumvent this, the activity of PAPs in living astrocytes can be observed using in situ or in vivo confocal or 2-photon microscopy. In acute brainstem slices from transgenic mice expressing GFP in astrocytes, PAPs display a high degree of motility (Hirrlinger, Hulsmann, & Kirchhoff, 2004). Another study has used hippocampal slice cultures from P4-5 mice and time-lapse 2-photon imaging to correlate PAP motility to lifetime and maturation of dendritic spines (Nishida & Okabe, 2007). This dynamic process is also seen in vivo in the adult mouse barrel cortex when a whisker is stimulated and motile PAPs increase dendritic spine coverage, suggesting neuronal activity controls PAP motility in a calcium-dependent manner (Bernardinelli et al., 2014). Thus, astrocytes are structurally active with motile processes that respond to synaptic transmission, emphasizing the significant crosstalk occurring between synapses and astrocyte processes. A recent study used innovative live-cell 3D-STED microscopy to obtain nanoscale details of the astrocyte spongiform domain that contains a meshwork of shafts, loops, and nodes. Interestingly, the majority of synaptic spines form stable contacts with nodes, and these nodes exhibit spatially-constrained calcium transients (Arizono et al., 2020). While PAPs are commonly referred to as the fine processes on an astrocyte, it isn’t clear if the entire astrocyte can contribute to the same synaptic formation and function as the fine processes. In essence, are these mechanisms confined to the fine processes, or can the astrocyte soma and large processes contribute to synaptic formation and function as well? In vivo analysis should be in position to answer this question.
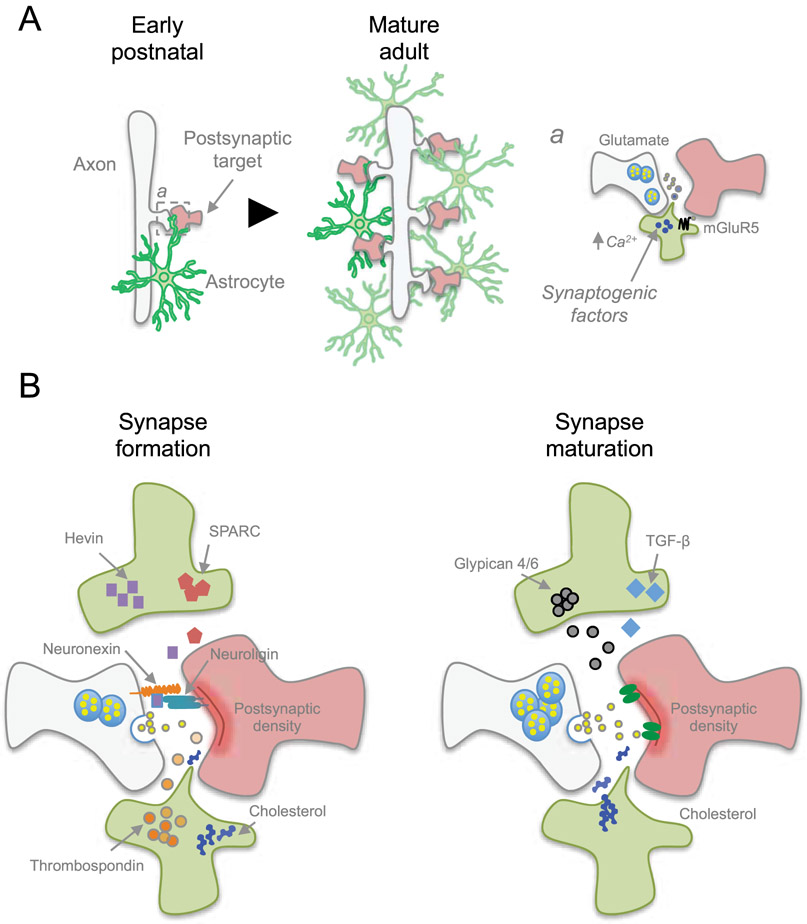
The importance of astrocytes in synaptogenesis, synapse maturation, and synaptic activity is evident in experiments where murine neurons cocultured with astrocytes exhibit an increased number of mature and functional synapses, whereas neurons cultured without astrocytes form only a few functionally immature synapses (Pfrieger & Barres, 1997; Ullian, Sapperstein, Christopherson, & Barres, 2001). The first study showing a connection between astrocytes and synaptic function was performed in vitro using retinal ganglion cells (RGC) and glia cocultures. When glial cells are present, RGC exhibit a large increase in the frequency and amplitude of postsynaptic currents. They also decrease the failure rate of evoked synaptic transmission (Pfrieger & Barres, 1997). This has been followed by another study using the same model in vitro system to show that astrocytes also increase the number of functional synapses between neurons using immunoblot and immunofluorescence imaging of synaptic markers synaptotagmin, synaptophysin, and SV2 (Ullian et al., 2001). These studies suggest that astrocytes are signaling to neurons to generate functional synapses. The notion that astrocytes facilitate synapse formation and maturation is underscored by the fact that synaptogenesis coincides with peak astrogenesis during development (Reemst, Noctor, Lucassen, & Hol, 2016) (Figure 3). This strongly suggests that astrocytes play a critical role in the formation and maturation of synapses during this developmental time window.
Figure 3. Astrocytes regulate formation and function of synapses.
A) During early postnatal development, astrocytes begin participating in synaptogenesis while proliferating to expand the astrocyte network. This rapid expansion of astrocytes coincides with the increase in the number of synapses as the nervous system matures. a) Astrocytes control synaptogenesis by releasing synaptogenic factors in a calcium-dependent response after sensing neurotransmitters, such as through Gq-metabotropic glutamate receptor mGluR5. B) Astrocytes release factors to facilitate structural formation of synapses. Cholesterol provides building material for new membrane production, while other factors such as Hevin and Thrombospondins promote synapse formation, or opposing synapse formation via SPARC. Additional factors are involved in synapse maturation and function, such as Glypican 4/6 and TGF-β that induces functional synapses.
2.4. Astrocyte-derived factors and synaptogenesis
Astrocytes secrete substances that facilitate the formation and maturation of synapses (Figure 3). One of the first discovered astrocyte-derived soluble factors is cholesterol (Mauch et al., 2001). Of note, cholesterol is an essential structural component of cell membranes that acts as a bidirectional regulator of membrane fluidity. Whereas cholesterol intercalation between the phospholipids prevents membrane stiffening at low temperatures, it stabilizes membranes and raises their melting point at high temperatures (M. R. Krause & Regen, 2014). This study used glial conditioned culture media and gel filtration chromatography and initially identified apolipoprotein E (ApoE). Afterwards, cholesterol was identified as the active substrate and ApoE as the cholesterol carrier. As synaptogenesis requires the production of new cell membranes, astrocytes provide cholesterol as building material to allow synapses to form.
Of the multiple classes of molecules that astrocytes release to induce synapse formation, matricellular protein thrombospondin has been identified in filtered astrocyte conditioned media to promote synaptogenesis (Christopherson et al., 2005). The addition of thrombospondin to RGC increases the number of synaptic puncta for synaptotagmin and PSD-95 (Christopherson et al., 2005). Thrombospondin synaptogenic effect is mediated via α2δ1 (Eroglu et al., 2009). However, measurements of miniature excitatory post synaptic currents (mEPSC) show that thrombospondin only facilitates the formation of postsynaptically-silent synapses (Christopherson et al., 2005). Gene expression profiling of astrocytes has uncovered matricellular proteins hevin and SPARC as highly expressed genes that persist through adulthood (Kucukdereli et al., 2011). The addition of hevin to cultured RGC increases the number of synapses in vitro, while SPARC exhibits an antagonistic effect to hevin. A genetic knockout (KO) of hevin and SPARC in mice creates defects in synaptogenesis in the superior colliculus, whereas hevin KO resulted in a decrease of synapses and SPARC KO had an increase in synapses in vivo. Similarly to thrombospondin, the synapses formed by hevin are postsynaptically silent. This study identified hevin and SPARC as positive and negative regulators of synapse formation, respectively. Hevin was later determined to induce synaptogenesis by acting as a bridge between presynaptic neurexin1α and postsynaptic neuroligin1 (Singh et al., 2016). The formation of silent synapses suggests that there may be an astrocyte-derived signal to induce a silent synapse to become functionally active. Heparan sulfate proteoglycans glypicans 4 and 6 have been identified through biochemical fractionation of astrocyte conditioned media, and are sufficient to induce synaptic activity (Allen et al., 2012). The addition of glypicans to RGCs in vitro induces an increase in amplitude and frequency of mEPSC events. This regulation of synaptic activity by Glypican 4 and 6 is achieved by inducing increased surface level expression and clustering of AMPA glutamate receptor subunit GluA1 on postsynaptic terminals. Astrocyte-released cytokines such as transforming growth factor beta (TGF-β) is also necessary for synaptogenesis. In both human and murine astrocyte conditioned media, TGF-β induces the formation of functional excitatory synapses in cortical neurons and increases levels of NMDA co-agonist D-serine in vitro (Diniz et al., 2012). Similarly, TGF-β promotes inhibitory synaptic formation by activating calcium/calmodulin-dependent protein kinase II in neurons, which in turn assembles neuroligin 2 on inhibitory postsynaptic terminals (Diniz et al., 2014).
There are some studies that show direct astrocyte contact also plays a role in synaptogenesis. Astrocytic expression of γ-Protocadherin, a cell adhesion membrane protein, promotes both excitatory and inhibitory synapses in the neonatal mouse spinal cord and contact-dependency has been shown in vitro (Garrett & Weiner, 2009). Selective KO of membrane protein neuroligin 2 in murine cortical astrocytes in vivo causes a reduction in synaptic puncta for excitatory synapses (Stogsdill et al., 2017).
2.5. Astrocytic release of synaptogenic factors
It is well established that astrocytes play an important role in building neuronal circuits. The body of evidence has revealed that astrocytes utilize an assortment of extrinsic factors to facilitate synaptogenesis during CNS development. The number of secreted- and contact-factors raises a number of intriguing questions about how astrocytes perform their role in forming a neural circuit. If astrocytes regulate these factors, through what mechanisms do they achieve this? One mechanism may be through sensing synaptic activity through astrocytic neurotransmitter receptors. A study using neuron-astrocyte feeder cultures reported increased production of SPARC in astrocytes when neuronal firing is increased using bicuculline. This astrocytic response acts through Group I Gq-linked metabotropic glutamate receptors (mGluR), which was determined using a Group I mGluR agonist 3,5-dihydroxyphenylglycine (DHPG), and elicits an astrocytic cytosolic calcium response and increased SPARC expression (Jones et al., 2011). Group I mGluRs consists of mGluR1 and 5, and astrocytes express only mGluR5 during early development that peaks at P7 in mice and drops rapidly thereafter at the transcript level (Cahoy et al., 2008). In adult mice, cortical astrocytes fail to elicit a calcium response to Group I agonists, which is in agreement with the transcriptional profile (Sun et al., 2013). This developmental timing coincides with synaptogenesis, which suggests one mechanism is that astrocytes sense synaptic activity and release synaptogenic factors through a calcium-dependent response (Figure 3). However, mGluR5 is downregulated by the end of the third postnatal week (Sun et al., 2013). This raises the question if mature astrocytes deliver synaptogenic factors by utilizing other calcium-responding mechanisms, such as transient receptor channels, purinergic P2X receptors, sodium/calcium exchangers and mitochondrial calcium efflux through its permeability transition pore (Agarwal et al., 2017; Palygin, Lalo, Verkhratsky, & Pankratov, 2010; Pankratov, Lalo, Krishtal, & Verkhratsky, 2009; Shigetomi, Tong, Kwan, Corey, & Khakh, 2011; Verkhratsky, Reyes, & Parpura, 2014; Verkhratsky, Rodriguez, & Parpura, 2012). Is there any spatiotemporal specificity in the release of astrocytic factors? The previous studies have performed the critical task of identifying the multitude of astrocyte factors involved in synaptic formation. It isn’t clear how all these factors identified so far work together in vivo to form synapses. Does the astrocyte release a cocktail of factors at once, or are these factors segregated according to the specific signaling mechanisms regulating its release? In addition, are these secreted factors spatially targeted towards a single synapse, or is release more volumetric in nature?
While the physiological mechanisms remain to be fully elucidated, the ability of astrocytes to regulate synapse formation and function under injury and disease conditions is unclear. The role of astrocyte participation in forming neural circuits is generally considered in the context of CNS development. How are the astrocytes’ roles in synaptic formation altered in the context of injury and disease? Astrocytes become reactive in response to injury and disease with morphological and molecular changes, such as the characteristic upregulation of glial fibrillary acidic protein (GFAP) (Sofroniew & Vinters, 2010). It is currently proposed that this astroglial reactivity is heterogeneous in nature that varies depending on the context and severity of the insult, ranging from mild to severe reactive astrogliosis (Anderson, Ao, & Sofroniew, 2014). In certain insults such as SCI, newly proliferated elongated astrocytes intertwine to form an astrocyte scar border that surrounds the lesion area (Wanner et al., 2013). Scar-forming astrocytes have traditionally been viewed as a physical and chemical barrier to axon regeneration and functional recovery (Silver & Miller, 2004), though recent reports challenge that notion. Attenuation or ablation of scar-forming astrocytes resulted in exacerbation of neuronal loss and did not result in increased axon regrowth or recovery, suggesting a protective role of the glial scar in preserving the surrounding neural tissue (Anderson et al., 2016; Gu et al., 2019). It isn’t clear what specific changes and functional consequences occur in facilitating synaptic formation and function, in particular during the initial and post-injury processes. Are astrocyte synaptogenic and homeostatic functions impacted as a result of astrocyte reactivity? Does the presence or absence of astrocyte synaptogenic factors contribute to failures in axon regeneration and synapse reformation? Astrocyte homeostatic functions and synaptogenic factors may represent an attractive target to manipulate for therapeutic purposes.
3. Microglia-dependent mechanisms of circuit formation and refinement
Microglia is another class of non-neuronal cells that are commonly described as the phagocytic immune cells of the CNS. These cells are mesodermal in origin that migrate into the brain parenchyma early during development (Ginhoux & Prinz, 2015). Many of the studies of microglial function have focused in the context of CNS injury and disease. However, recent work has shed light on microglial function in a healthy and uninjured CNS, in particular to microglia’s role in synaptic refinement and elimination.
3.1. Microglial actively monitor the neural environment
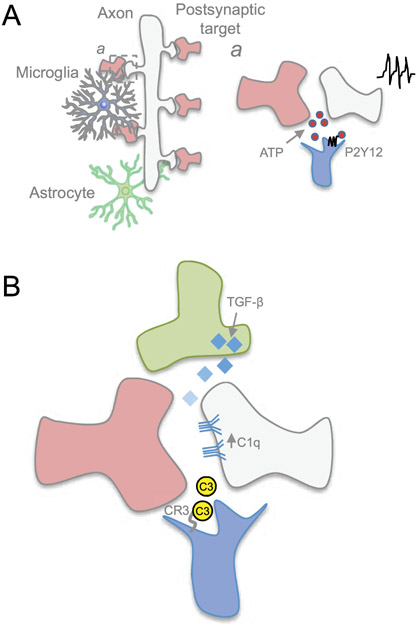
The development of chronic in vivo imaging techniques and genetic strategies to observe microglia ‘at play’ has led to findings that microglial processes constantly extend and contract to actively inspect the brain parenchyma (Wake, Moorhouse, Jinno, Kohsaka, & Nabekura, 2009). Such dynamic process allows microglia to rapidly survey the cellular environment and physically contact other cellular structures such as axons and synapses (Davalos et al., 2005; Jung et al., 2000; Nimmerjahn, Kirchhoff, & Helmchen, 2005) (Figure 4). In the mouse somatosensory and visual cortex, microglia processes contact synapses about once per hour (Wake et al., 2009). Implementation of cutting edge super resolution microscopy combined with fluorescent labeling of the extracellular fluid has demonstrated microglia move rapidly in an amoeboid fashion across bundles of axons and displacing neural structures in living organotypic mouse brain slices. This restructuring of the extracellular space by motile microglia may also effect changes in extracellular matrix structures. In 2-photon induced lesion conditions, microglia move in a curved path towards and around the lesion area, while other microglia rips through the neuropil and leaving an empty space from its path (Tonnesen, Inavalli, & Nagerl, 2018). A number of studies has demonstrated that microglia motility is regulated by neuronal activity. In vivo time lapse calcium imaging in the optic tectum of larval zebrafish has found that neuronal activity instructs microglia motility and promote microglia contact with active neurons through activation of pannexin-1 hemichannels expressed in neurons (Y. Li, Du, Liu, Wen, & Du, 2012). Microglial motility and dendritic spine contacts are regulated by neuronal activity, and is increased in awake mice compared to anesthetized mice in vivo (Nebeling et al., 2019). Conversely, dampening neuronal activity causes a decrease in the frequency of microglia contacts and motility (Nebeling et al., 2019; Wake et al., 2009), further underscoring a reciprocal regulation between microglia dynamics and neuronal activity in vivo. While synapses can be targeted for pruning, microglia requires a mechanism that enables a cellular response to changes in synaptic activity. Microglia accomplishes this through P2Y12 purinergic signaling (Figure 4). By altering their morphology, motility, phagocytic behavior and interaction at synapses, microglia actively respond to monocular deprivation during the critical period in the visual system (Sipe et al., 2016). Monocular deprivation during a critical period of brain development leads to changes of neuronal firing (Wiesel & Hubel, 1963). Of note, P2Y12 disruption causes alteration in microglia response following monocular deprivation in mice, effectively abrogating ocular dominance plasticity (Sipe et al., 2016).
Figure 4. Microglia refines the circuit by eliminating synapses.
A) Microglia constantly surveils the neural environment, and a) its motile processes respond to synaptic activity through P2Y12 receptor by sensing ATP released during neurotransmission. B) Microglia, through its C3 receptor, identifies synapses to be pruned through complement molecules C1q and C3. C1q expression on neurites may be regulated through astrocyte-secreted TGF-β.
3.2. Microglia shape neural circuitry by pruning synapses
During development and maturation of the CNS, neural circuits are sculpted and refined through elimination of synapses also known as synaptic pruning. It is now recognized that microglia plays a major role in synaptic pruning by engulfing synaptic structures (Paolicelli et al., 2011), and accomplishes this function through several mechanisms. C1q/C3 complement cascade signaling mediates microglia phagocytosis of synapses (Figure 4). C1q and C3 are members of the classical complement cascade that may be used as a molecular “eat me” tag for microglia, through its C3 receptor (CR3), to recognize and prune the synapse during development in the lateral geniculate nucleus (Schafer et al., 2012; Stevens et al., 2007). Whereas neurons express C1q during circuit refinement, the extracellular signals controlling C1q expression have remained obscure. Astrocyte-secreted transforming growth factor beta (TGF-β) has been found to regulate neuronal C1q expression, where genetic ablation of TGF-β receptor II in retinal neurons reduces C1q expression and synaptic localization of complement, thereby causing defects in subsequent pruning (Bialas & Stevens, 2013). Advanced light-sheet microscopy and correlative light and electron microscopy have further characterized microglia behavior in association with synapses. Rather than eliminating the entire synaptic apparatus, microglia prune presynaptic structures via partial phagocytosis or trogocytosis in developing postnatal organotypic mouse hippocampal cultures (Weinhard et al., 2018). C1q tagging of a synapse requires specific alteration in the synaptic proteome. Along this line, a recent study has demonstrated the presence of apoptotic-like mechanisms underlying C1q tagging of synapses in the mouse cerebral cortex (Gyorffy et al., 2018). While the classical complement cascade is used to eliminate synapses, inhibition of the complement cascade can protect synapses against removal. Sushi domain protein SRPX2 is shown to bind directly to C1q, preventing the initiation and activation of the complement cascade, thereby preventing complement-mediated synapse elimination (Cong, Soteros, Wollet, Kim, & Sia, 2020).
Microglia production of C1q and C3 and regulation of lysosomal function are mediated by expression of progranulin. Deficiencies in progranulin in microglia lead to upregulation of complement and lysosomal genes, as well as excessive synaptic pruning (Lui et al., 2016). Another receptor essential for microglia-mediated synaptic refinement and pruning is triggering receptor expressed on myeloid cells 2 (TREM2). Hence, mice lacking Trem2 display neurodevelopmental defects due to microglia dysfunction. At P18-20, Trem2 null hippocampal CA1 pyramidal neurons show higher frequency of miniature excitatory postsynaptic currents when compared to control littermates (Filipello et al., 2018). Similarly, an increase in synaptic contacts is also confirmed by the higher density of pre and postsynaptic specialization in Trem2 null mice compared to the control condition (Filipello et al., 2018). The analysis of microglia morphology in the CA1 region of P18-20 Trem2 null mice highlights a more complex, ramified structure (Filipello et al., 2018). TREM2 is required for synapse engulfment as microglia originating from null mice display reduced engulfment of synaptosomes compared to controls (Filipello et al., 2018), further supporting the relevance of the neuro-immune crosstalk for normal CNS development.
Microglial phagocytosis is also regulated by cytokine signaling transmitted from other cells. During CNS development, astrocyte-derived Interleukin-33 (IL-33) signals to microglia to promote synaptic pruning and circuit remodeling through synaptic engulfment in the thalamus and spinal cord (Vainchtein et al., 2018). Interestingly, neuron-derived IL-33 promotes microglial phagocytosis of the extracellular matrix in an activity-dependent manner in the adult brain. This clearance and remodeling of the extracellular matrix thereby promotes synaptic formation and dendritic spine plasticity (Nguyen et al., 2020).
Genetic regulations of microglia phagocytic function in healthy and uninjured CNS are beginning to be uncovered. A study has identified the TAR DNA-binding protein 43 (TDP-43) as a critical regulator of microglial phagocytic activity. TDP-43 is encoded by the Tardbp gene and acts as transcriptional repressor, splicing factor and mRNA binding protein (Buratti & Baralle, 2001; Lagier-Tourenne, Polymenidou, & Cleveland, 2010). Depletion of TDP-43 induces excessive phagocytosis and loss of synapses as shown by the decrease in the pre and postsynaptic markers Vglut1 and PSD95 in the cerebral cortex of null mice (Paolicelli et al., 2017).
The cumulative evidence demonstrates that microglia play an important role in refining synaptic circuitry, in particular through partial phagocytosis of synaptic structures. The aforementioned studies have taken place predominantly in the brain, where detailed connectivity maps have been characterized in select areas. In addition, brain structures such as the hippocampus and cortex are readily accessible for chronic in vivo imaging studies. While the role and mechanisms of microglia synaptic engulfment have been characterized in select brain regions, it remains unknown if these same mechanisms governing microglia synaptic engulfment are implicated in other unexplored brain regions and spinal cord of other species with different neuronal populations and connectivity. The development of new technologies, powerful algorithms and more specific genetic tools will reveal in more detail the microglia-synapse interactions and microglia’s role in synapse refinement and elimination.
DISCUSSION
Here we have presented and discussed recent evidence that suggests axon regeneration, synapse formation and function may be at odds with one another. The correct assembly, refinement and consolidation of intricate neuronal networks that constitute the mammalian CNS represent an arduous task during development. The same task is far more challenging after injury and disease in adulthood where the chaotic and metabolically disrupted environment adds on to the age dependent decline in axon regeneration ability (Byrne et al., 2014; Geoffroy et al., 2016; Verdu et al., 2000). In adulthood, the gap that separates injured axons from their target field is significantly larger than what the very same axons are programmed to cover during early stages of development (He & Jin, 2016). Building functional neuronal circuits requires a coordinated series of steps including axon elongation, branching, formation and consolidation of synaptic contacts in specific target areas (Lewis, Courchet, & Polleux, 2013). Not only neurons, but also non-neuronal cells including astrocytes and microglia actively participate in synapse formation and remodeling (Allen & Eroglu, 2017; Clarke & Barres, 2013; Kettenmann, Kirchhoff, & Verkhratsky, 2013; Y. Wu, Dissing-Olesen, MacVicar, & Stevens, 2015). Such an ordered series of steps is accomplished through spatial and temporal dynamics of gene expression (Delile et al., 2019). Pioneer transcription factors are able to unmask chromatin domains during development to jump start different cellular programs in a timely fashion (Mayran & Drouin, 2018; Zaret & Carroll, 2011). Following trauma, however, epigenetic constraints prevent proper tuning of the transcriptional landscape (Cho, Sloutsky, Naegle, & Cavalli, 2015; Loh et al., 2017; Puttagunta et al., 2014; Tedeschi, 2011; Weng et al., 2017). Promoting axon regeneration and new circuit formation using uncoordinated strategies can have deleterious outcomes such as aberrant synaptogenesis, spasticity, hyperexcitability and excitation-inhibition imbalance of neuronal circuits, all of which obstruct functional recovery (Carlin et al., 2018; Ding & Hammarlund, 2018; Z. Wang et al., 2015). Further dissection of the cellular and molecular mechanisms controlling the transition from a growing to a transmitting phase as well as the role of the genetic background (Omura et al., 2015; Tedeschi, Omura, & Costigan, 2017) in coordinating non-neuronal cell behavior may pave the way for the development of novel treatment strategies for CNS repair.
ACKNOWLEDGEMENTS
We would like to thank all members of the laboratory for providing insightful comments on the manuscript. The National Institute of Neurological Disorders and Stroke (R01NS110681 and R21NS109787), Chronic Brain Injury Discovery Theme at The Ohio State University support research in the Tedeschi laboratory.
Footnotes
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, … Bergles DE (2017). Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron, 93(3), 587–605 e587. doi: 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahyari RV, Clark KL, Shepard KA, & Garcia ADR (2019). Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury. Sci Rep, 9(1), 565. doi: 10.1038/s41598-018-37555-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, & Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature, 486(7403), 410–414. doi: 10.1038/nature11059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, & Eroglu C (2017). Cell Biology of Astrocyte-Synapse Interactions. Neuron, 96(3), 697–708. doi: 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, & Lyons DA (2018). Glia as architects of central nervous system formation and function. Science, 362(6411), 181–185. doi: 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, & Sofroniew MV (2014). Heterogeneity of reactive astrocytes. Neurosci Lett, 565, 23–29. doi: 10.1016/j.neulet.2013.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, … Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature, 532(7598), 195–200. doi: 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, & Haydon PG (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci, 22(5), 208–215. doi: 10.1016/s0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- Arizono M, Inavalli V, Panatier A, Pfeiffer T, Angibaud J, Levet F, … Nagerl UV (2020). Structural basis of astrocytic Ca(2+) signals at tripartite synapses. Nat Commun, 11(1), 1906. doi: 10.1038/s41467-020-15648-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham O, Deng P-Y, Jones S, Kuruvilla R, Semenkovich CF, Klyachko VA, & Cavalli V (2019). Fatty acid synthesis in satellite glial cell promotes regenerative growth in sensory neurons. bioRxiv, 2019.2012.2013.874669. doi: 10.1101/2019.12.13.874669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, & Wiggan OP (2002). ADF/cofilin and actin dynamics in disease. Trends Cell Biol, 12(12), 598–605. doi: 10.1016/s0962-8924(02)02404-2 [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Schmidt MF, Hassinger TD, Schwab ME, & Kater SB (1993). Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science, 259(5091), 80–83. doi: 10.1126/science.8418499 [DOI] [PubMed] [Google Scholar]
- Barber AC, Evans RS, Nieuwenhuis B, Pearson CS, Fuchs J, MacQueen AR, … Eva R (2019). PI 3-kinase delta enhances axonal PIP3 to support axon regeneration in the adult CNS. bioRxiv, 787994. doi: 10.1101/787994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA (2008). The mystery and magic of glia: a perspective on their roles in health and disease. Neuron, 60(3), 430–440. doi: 10.1016/j.neuron.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Ben Haim L, & Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci, 18(1), 31–41. doi: 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Randall J, Janett E, Nikonenko I, Konig S, Jones EV, … Muller D (2014). Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol, 24(15), 1679–1688. doi: 10.1016/j.cub.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Bialas AR, & Stevens B (2013). TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci, 16(12), 1773–1782. doi: 10.1038/nn.3560 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bikbaev A, Ciuraszkiewicz-Wojciech A, Heck J, Klatt O, Freund R, Mitlohner J, … Heine M (2020). Auxiliary alpha2delta1 and alpha2delta3 Subunits of Calcium Channels Drive Excitatory and Inhibitory Neuronal Network Development. J Neurosci, 40(25), 4824–4841. doi: 10.1523/JNEUROSCI.1707-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, … Bixby JL (2012). Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A, 109(19), 7517–7522. doi: 10.1073/pnas.1120684109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, & Scheller RH (2001). A genomic perspective on membrane compartment organization. Nature, 409(6822), 839–841. doi: 10.1038/35057024 [DOI] [PubMed] [Google Scholar]
- Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, … et al. (1989). A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest, 83(3), 1015–1031. doi: 10.1172/JCI113943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, & Spira ME (2012). Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci, 13(3), 183–193. doi: 10.1038/nrn3176 [DOI] [PubMed] [Google Scholar]
- Bray ER, Yungher BJ, Levay K, Ribeiro M, Dvoryanchikov G, Ayupe AC, … Park KK (2019). Thrombospondin-1 Mediates Axon Regeneration in Retinal Ganglion Cells. Neuron, 103(4), 642–657 e647. doi: 10.1016/j.neuron.2019.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, McAtee M, & O’Neill A (1989). Extension of the critical period for developmental plasticity of the corticospinal pathway. J Comp Neurol, 282(3), 355–370. doi: 10.1002/cne.902820304 [DOI] [PubMed] [Google Scholar]
- Brightman MW, & Reese TS (1969). Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol, 40(3), 648–677. doi: 10.1083/jcb.40.3.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, & London E (1998). Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol, 14, 111–136. doi: 10.1146/annurev.cellbio.14.1.111 [DOI] [PubMed] [Google Scholar]
- Buratti E, & Baralle FE (2001). Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem, 276(39), 36337–36343. doi: 10.1074/jbc.M104236200 [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, & Ellisman MH (2004). Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci, 22(2), 73–86. doi: 10.1016/j.ijdevneu.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, & Ellisman MH (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci, 22(1), 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AB, Walradt T, Gardner KE, Hubbert A, Reinke V, & Hammarlund M (2014). Insulin/IGF1 signaling inhibits age-dependent axon regeneration. Neuron, 81(3), 561–573. doi: 10.1016/j.neuron.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, … Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci, 28(1), 264–278. doi: 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, & Welch MD (2010). A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol, 11(4), 237–251. doi: 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin D, Golden JP, Mogha A, Samineni VK, Monk KR, Gereau R. W. t., & Cavalli V (2018). Deletion of Tsc2 in Nociceptors Reduces Target Innervation, Ion Channel Expression, and Sensitivity to Heat. eNeuro, 5(2). doi: 10.1523/ENEURO.0436-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin D, Halevi AE, Ewan EE, Moore AM, & Cavalli V (2019). Nociceptor Deletion of Tsc2 Enhances Axon Regeneration by Inducing a Conditioning Injury Response in Dorsal Root Ganglia. eNeuro, 6(3). doi: 10.1523/ENEURO.0168-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GM, & Han GS (2019). Fat-regulating phosphatidic acid phosphatase: a review of its roles and regulation in lipid homeostasis. J Lipid Res, 60(1), 2–6. doi: 10.1194/jlr.S087452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA (2011). Voltage-gated calcium channels. Cold Spring Harb Perspect Biol, 3(8), a003947. doi: 10.1101/cshperspect.a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, & Gould GW (2001). SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A, 98(10), 5619–5624. doi: 10.1073/pnas.091502398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan MZ, Arcuri J, Park KK, Zafar MK, Fatmi R, Hackam AS, … Bhattacharya SK (2020). Multi-Omic Analyses of Growth Cones at Different Developmental Stages Provides Insight into Pathways in Adult Neuroregeneration. iScience, 23(2), 100836. doi: 10.1016/j.isci.2020.100836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, … Pan HL (2018). The alpha2delta-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions. Cell Rep, 22(9), 2307–2321. doi: 10.1016/j.celrep.2018.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chever O, Dossi E, Pannasch U, Derangeon M, & Rouach N (2016). Astroglial networks promote neuronal coordination. Sci Signal, 9(410), ra6. doi: 10.1126/scisignal.aad3066 [DOI] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, & Cavalli V (2015). Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell, 161(3), 691. doi: 10.1016/j.cell.2015.04.019 [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, … Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell, 120(3), 421–433. doi: 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Chung SH, Awal MR, Shay J, McLoed MM, Mazur E, & Gabel CV (2016). Novel DLK-independent neuronal regeneration in Caenorhabditis elegans shares links with activity-dependent ectopic outgrowth. Proc Natl Acad Sci U S A, 113(20), E2852–2860. doi: 10.1073/pnas.1600564113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, & Barres BA (2013). Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci, 14(5), 311–321. doi: 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comley LH, Fuller HR, Wishart TM, Mutsaers CA, Thomson D, Wright AK, … Gillingwater TH (2011). ApoE isoform-specific regulation of regeneration in the peripheral nervous system. Hum Mol Genet, 20(12), 2406–2421. doi: 10.1093/hmg/ddr147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Q, Soteros BM, Wollet M, Kim JH, & Sia GM (2020). The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development. Nat Neurosci, 23(9), 1067–1078. doi: 10.1038/s41593-020-0672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, … Silva AJ (2002). Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature, 415(6871), 526–530. doi: 10.1038/nature711 [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, … Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci, 8(6), 752–758. doi: 10.1038/nn1472 [DOI] [PubMed] [Google Scholar]
- de Chaves EI, Rusinol AE, Vance DE, Campenot RB, & Vance JE (1997). Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J Biol Chem, 272(49), 30766–30773. doi: 10.1074/jbc.272.49.30766 [DOI] [PubMed] [Google Scholar]
- Delile J, Rayon T, Melchionda M, Edwards A, Briscoe J, & Sagner A (2019). Single cell transcriptomics reveals spatial and temporal dynamics of gene expression in the developing mouse spinal cord. Development, 146(12). doi: 10.1242/dev.173807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, & Kalil K (1999). Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci, 19(20), 8894–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, … Gertler FB (2007). Filopodia are required for cortical neurite initiation. Nat Cell Biol, 9(12), 1347–1359. doi: 10.1038/ncb1654 [DOI] [PubMed] [Google Scholar]
- Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, … Son YJ (2011). In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci, 31(12), 4569–4582. doi: 10.1523/JNEUROSCI.4638-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ (2002). Molecular mechanisms of axon guidance. Science, 298(5600), 1959–1964. doi: 10.1126/science.1072165 [DOI] [PubMed] [Google Scholar]
- Ding C, & Hammarlund M (2018). Aberrant information transfer interferes with functional axon regeneration. Elife, 7. doi: 10.7554/eLife.38829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, Almeida JC, Tortelli V, Vargas Lopes C, Setti-Perdigao P, Stipursky J, … Gomes FC (2012). Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem, 287(49), 41432–41445. doi: 10.1074/jbc.M112.380824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, Tortelli V, Garcia MN, Araujo AP, Melo HM, Silva GS, … Gomes FC (2014). Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia, 62(12), 1917–1931. doi: 10.1002/glia.22713 [DOI] [PubMed] [Google Scholar]
- Dolphin AC (2012). Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci, 13(8), 542–555. doi: 10.1038/nrn3311 [DOI] [PubMed] [Google Scholar]
- Dolphin AC (2018). Voltage-gated calcium channel alpha 2delta subunits: an assessment of proposed novel roles. F1000Res, 7. doi: 10.12688/f1000research.16104.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC, & Lee A (2020). Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nat Rev Neurosci, 21(4), 213–229. doi: 10.1038/s41583-020-0278-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger A, Monastyrskaya K, & Babiychuk EB (2011). Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol, 81(6), 703–712. doi: 10.1016/j.bcp.2010.12.027 [DOI] [PubMed] [Google Scholar]
- Dwivedy A, Gertler FB, Miller J, Holt CE, & Lebrand C (2007). Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development, 134(11), 2137–2146. doi: 10.1242/dev.002345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, … Barres BA (2009). Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell, 139(2), 380–392. doi: 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, … Murai KK (2016). Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science, 351(6275), 849–854. doi: 10.1126/science.aab3103 [DOI] [PubMed] [Google Scholar]
- Farmer WT, & Murai K (2017). Resolving Astrocyte Heterogeneity in the CNS. Front Cell Neurosci, 11, 300. doi: 10.3389/fncel.2017.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, & Verhaagen J (2018). Intrinsic Determinants of Axon Regeneration. Dev Neurobiol, 78(10), 890–897. doi: 10.1002/dneu.22637 [DOI] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, … Matteoli M (2018). The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity, 48(5), 979–991 e978. doi: 10.1016/j.immuni.2018.04.016 [DOI] [PubMed] [Google Scholar]
- Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, … Silver J (2014). Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci, 34(49), 16369–16384. doi: 10.1523/JNEUROSCI.1309-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MM, Bayazitov IT, Zakharenko SS, & Baker SJ (2008). Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience, 151(2), 476–488. doi: 10.1016/j.neuroscience.2007.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Chong JX, Bamshad MJ, & Ng BG (2014). Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet, 94(2), 161–175. doi: 10.1016/j.ajhg.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche M, Erlenkamper C, Moeendarbary E, Charras G, & Kruse K (2016). Actin kinetics shapes cortical network structure and mechanics. Sci Adv, 2(4), e1501337. doi: 10.1126/sciadv.1501337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsche M, Li D, Colin-York H, Chang VT, Moeendarbary E, Felce JH, … Eggeling C (2017). Self-organizing actin patterns shape membrane architecture but not cell mechanics. Nat Commun, 8, 14347. doi: 10.1038/ncomms14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, & Banker GA (1996). The economics of neurite outgrowth--the addition of new membrane to growing axons. Trends Neurosci, 19(4), 144–149. doi: 10.1016/s0166-2236(96)80025-7 [DOI] [PubMed] [Google Scholar]
- Gallo G (2011). The cytoskeletal and signaling mechanisms of axon collateral branching. Dev Neurobiol, 71(3), 201–220. doi: 10.1002/dneu.20852 [DOI] [PubMed] [Google Scholar]
- Garcia-Marin V, Garcia-Lopez P, & Freire M (2007). Cajal’s contributions to glia research. Trends Neurosci, 30(9), 479–487. doi: 10.1016/j.tins.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Garrett AM, & Weiner JA (2009). Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci, 29(38), 11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, & Woodruff GN (1996). The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem, 271(10), 5768–5776. doi: 10.1074/jbc.271.10.5768 [DOI] [PubMed] [Google Scholar]
- Geisler S, Schopf CL, Stanika R, Kalb M, Campiglio M, Repetto D, … Obermair GJ (2019). Presynaptic alpha2delta-2 Calcium Channel Subunits Regulate Postsynaptic GABAA Receptor Abundance and Axonal Wiring. J Neurosci, 39(14), 2581–2605. doi: 10.1523/JNEUROSCI.2234-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Hilton BJ, Tetzlaff W, & Zheng B (2016). Evidence for an Age-Dependent Decline in Axon Regeneration in the Adult Mammalian Central Nervous System. Cell Rep, 15(2), 238–246. doi: 10.1016/j.celrep.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, & Gros D (1991). Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron, 6(1), 133–143. doi: 10.1016/0896-6273(91)90128-m [DOI] [PubMed] [Google Scholar]
- Gibson DA, & Ma L (2011). Developmental regulation of axon branching in the vertebrate nervous system. Development, 138(2), 183–195. doi: 10.1242/dev.046441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER 2nd, & Tuszynski MH (2010). Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol, 2(7), a001867. doi: 10.1101/cshperspect.a001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, & Prinz M (2015). Origin of microglia: current concepts and past controversies. Cold Spring Harb Perspect Biol, 7(8), a020537. doi: 10.1101/cshperspect.a020537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, & Spitzer NC (1999). In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature, 397(6717), 350–355. doi: 10.1038/16927 [DOI] [PubMed] [Google Scholar]
- Gong HC, Hang J, Kohler W, Li L, & Su TZ (2001). Tissue-specific expression and gabapentin-binding properties of calcium channel alpha2delta subunit subtypes. J Membr Biol, 184(1), 35–43. doi: 10.1007/s00232-001-0072-7 [DOI] [PubMed] [Google Scholar]
- Goodman CW, & Brett AS (2017). Gabapentin and Pregabalin for Pain - Is Increased Prescribing a Cause for Concern? N Engl J Med, 377(5), 411–414. doi: 10.1056/NEJMp1704633 [DOI] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, & Elgersma Y (2007). Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol, 62(6), 648–655. doi: 10.1002/ana.21317 [DOI] [PubMed] [Google Scholar]
- Goritz C, Mauch DH, & Pfrieger FW (2005). Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci, 29(2), 190–201. doi: 10.1016/j.mcn.2005.02.006 [DOI] [PubMed] [Google Scholar]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, … Mayor S (2008). Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell, 135(6), 1085–1097. doi: 10.1016/j.cell.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar K, Ghosh S, Saha S, C R, Mayor S, & Rao M (2012). Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell, 149(6), 1353–1367. doi: 10.1016/j.cell.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Gribnau AA, de Kort EJ, Dederen PJ, & Nieuwenhuys R (1986). On the development of the pyramidal tract in the rat. II. An anterograde tracer study of the outgrowth of the corticospinal fibers. Anat Embryol (Berl), 175(1), 101–110. doi: 10.1007/bf00315460 [DOI] [PubMed] [Google Scholar]
- Gu Y, Cheng X, Huang X, Yuan Y, Qin S, Tan Z, … Su Z (2019). Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav Immun, 80, 394–405. doi: 10.1016/j.bbi.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Gyorffy BA, Kun J, Torok G, Bulyaki E, Borhegyi Z, Gulyassy P, … Kardos J (2018). Local apoptotic-like mechanisms underlie complement-mediated synaptic pruning. Proc Natl Acad Sci U S A, 115(24), 6303–6308. doi: 10.1073/pnas.1722613115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzal-Bayer MF, & Hancock JF (2007). Lipid rafts and membrane traffic. FEBS Lett, 581(11), 2098–2104. doi: 10.1016/j.febslet.2007.03.019 [DOI] [PubMed] [Google Scholar]
- He Z, & Jin Y (2016). Intrinsic Control of Axon Regeneration. Neuron, 90(3), 437–451. doi: 10.1016/j.neuron.2016.04.022 [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, & Insel PA (2014). Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta, 1838(2), 532–545. doi: 10.1016/j.bbamem.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck J, Parutto P, Ciuraszkiewicz A, Bikbaev A, Freund R, Mitlohner J, … Heine M (2019). Transient Confinement of CaV2.1 Ca(2+)-Channel Splice Variants Shapes Synaptic Short-Term Plasticity. Neuron, 103(1), 66–79 e12. doi: 10.1016/j.neuron.2019.04.030 [DOI] [PubMed] [Google Scholar]
- Heine M, & Holcman D (2020). Asymmetry Between Pre- and Postsynaptic Transient Nanodomains Shapes Neuronal Communication. Trends Neurosci, 43(3), 182–196. doi: 10.1016/j.tins.2020.01.005 [DOI] [PubMed] [Google Scholar]
- Henikoff S (2008). Nucleosome destabilization in the epigenetic regulation of gene expression. Nat Rev Genet, 9(1), 15–26. doi: 10.1038/nrg2206 [DOI] [PubMed] [Google Scholar]
- Henley J, & Poo MM (2004). Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol, 14(6), 320–330. doi: 10.1016/j.tcb.2004.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton BJ, & Bradke F (2017). Can injured adult CNS axons regenerate by recapitulating development? Development, 144(19), 3417–3429. doi: 10.1242/dev.148312 [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Hulsmann S, & Kirchhoff F (2004). Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci, 20(8), 2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x [DOI] [PubMed] [Google Scholar]
- Hoppa MB, Lana B, Margas W, Dolphin AC, & Ryan TA (2012). alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature, 486(7401), 122–125. doi: 10.1038/nature11033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Yang IH, Kim DH, Byun J, Saijilafu, Xu WL, … Zhou FQ (2011). Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci U S A, 108(12), 5057–5062. doi: 10.1073/pnas.1011258108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson TH, & Di Giovanni S (2019). The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat Rev Neurol, 15(12), 732–745. doi: 10.1038/s41582-019-0280-3 [DOI] [PubMed] [Google Scholar]
- Jacob R, Heine M, Alfalah M, & Naim HY (2003). Distinct cytoskeletal tracks direct individual vesicle populations to the apical membrane of epithelial cells. Curr Biol, 13(7), 607–612. doi: 10.1016/s0960-9822(03)00188-x [DOI] [PubMed] [Google Scholar]
- Jaenisch R, & Bird A (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet, 33 Suppl, 245–254. doi: 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- Jimenez CR, Stam FJ, Li KW, Gouwenberg Y, Hornshaw MP, De Winter F, … Smit AB (2005). Proteomics of the injured rat sciatic nerve reveals protein expression dynamics during regeneration. Mol Cell Proteomics, 4(2), 120–132. doi: 10.1074/mcp.M400076-MCP200 [DOI] [PubMed] [Google Scholar]
- Jones EV, Bernardinelli Y, Tse YC, Chierzi S, Wong TP, & Murai KK (2011). Astrocytes control glutamate receptor levels at developing synapses through SPARC-beta-integrin interactions. J Neurosci, 31(11), 4154–4165. doi: 10.1523/JNEUROSCI.4757-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, & Littman DR (2000). Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol, 20(11), 4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K, & Dent EW (2014). Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat Rev Neurosci, 15(1), 7–18. doi: 10.1038/nrn3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater SB, Mattson MP, Cohan C, & Connor J (1988). Calcium regulation of the neuronal growth cone. Trends Neurosci, 11(7), 315–321. doi: 10.1016/0166-2236(88)90094-x [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, & Verkhratsky A (2013). Microglia: new roles for the synaptic stripper. Neuron, 77(1), 10–18. doi: 10.1016/j.neuron.2012.12.023 [DOI] [PubMed] [Google Scholar]
- Khakh BS, & Deneen B (2019). The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci, 42, 187–207. doi: 10.1146/annurev-neuro-070918-050443 [DOI] [PubMed] [Google Scholar]
- Kimelberg HK (2010). Functions of mature mammalian astrocytes: a current view. Neuroscientist, 16(1), 79–106. doi: 10.1177/1073858409342593 [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, & Tessier-Lavigne M (2011). Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol, 3(6). doi: 10.1101/cshperspect.a001727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, & Giger RJ (2005). Probing microtubule +TIPs: regulation of axon branching. Curr Opin Neurobiol, 15(1), 58–66. doi: 10.1016/j.conb.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Korobova F, & Svitkina T (2008). Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell, 19(4), 1561–1574. doi: 10.1091/mbc.E07-09-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki H, Donega M, Lam BY, Petrova V, van Erp S, Yeo GS, … Fawcett JW (2017). Selective rab11 transport and the intrinsic regenerative ability of CNS axons. Elife, 6. doi: 10.7554/eLife.26956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster DV, Husain K, Iljazi E, Bhat A, Bieling P, Mullins RD, … Mayor S (2016). Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proc Natl Acad Sci U S A, 113(12), E1645–1654. doi: 10.1073/pnas.1514030113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster DV, & Mayor S (2016). Cortical actin and the plasma membrane: inextricably intertwined. Curr Opin Cell Biol, 38, 81–89. doi: 10.1016/j.ceb.2016.02.021 [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, & Gertler FB (2003). Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol, 19, 541–564. doi: 10.1146/annurev.cellbio.19.050103.103356 [DOI] [PubMed] [Google Scholar]
- Krause MR, & Regen SL (2014). The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc Chem Res, 47(12), 3512–3521. doi: 10.1021/ar500260t [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, … Eroglu C (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A, 108(32), E440–449. doi: 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, & Orkand RK (1966). Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol, 29(4), 768–787. doi: 10.1152/jn.1966.29.4.768 [DOI] [PubMed] [Google Scholar]
- Kuffler SW, & Potter DD (1964). Glia in the Leech Central Nervous System: Physiological Properties and Neuron-Glia Relationship. J Neurophysiol, 27, 290–320. doi: 10.1152/jn.1964.27.2.290 [DOI] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, & Ikegaya Y (2011). Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci, 31(7), 2607–2614. doi: 10.1523/JNEUROSCI.5319-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi A, Koyama-Honda I, & Suzuki K (2004). Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic, 5(4), 213–230. doi: 10.1111/j.1600-0854.2004.0178.x [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, … Gertler FB (2007). Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron, 56(3), 441–455. doi: 10.1016/j.neuron.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, & Simons K (1999). Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci U S A, 96(7), 3734–3738. doi: 10.1073/pnas.96.7.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Polymenidou M, & Cleveland DW (2010). TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet, 19(R1), R46–64. doi: 10.1093/hmg/ddq137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, & Jahn R (2001). SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J, 20(9), 2202–2213. doi: 10.1093/emboj/20.9.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J, Stephan J, Theis M, & Rose CR (2012). Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia, 60(2), 239–252. doi: 10.1002/glia.21259 [DOI] [PubMed] [Google Scholar]
- Lautermilch NJ, & Spitzer NC (2000). Regulation of calcineurin by growth cone calcium waves controls neurite extension. J Neurosci, 20(1), 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shin JE, Lee B, Kim H, Jeon Y, Ahn SH, … Cho Y (2020). The stem cell marker Prom1 promotes axon regeneration by down-regulating cholesterol synthesis via Smad signaling. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1920829117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental I, Grzybek M, & Simons K (2010). Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry, 49(30), 6305–6316. doi: 10.1021/bi100882y [DOI] [PubMed] [Google Scholar]
- Lewis TL Jr., Courchet J, & Polleux F (2013). Cell biology in neuroscience: Cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol, 202(6), 837–848. doi: 10.1083/jcb.201305098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, … Luo ZD (2006). Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain, 125(1–2), 20–34. doi: 10.1016/j.pain.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FQ, Fowler KA, Neil JE, Colton CA, & Vitek MP (2010). An apolipoprotein E-mimetic stimulates axonal regeneration and remyelination after peripheral nerve injury. J Pharmacol Exp Ther, 334(1), 106–115. doi: 10.1124/jpet.110.167882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Graber KD, Jin S, McDonald W, Barres BA, & Prince DA (2012). Gabapentin decreases epileptiform discharges in a chronic model of neocortical trauma. Neurobiol Dis, 48(3), 429–438. doi: 10.1016/j.nbd.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han S, Li H, Udeshi ND, Svinkina T, Mani DR, … Luo L (2020). Cell-Surface Proteomic Profiling in the Fly Brain Uncovers Wiring Regulators. Cell, 180(2), 373–386 e315. doi: 10.1016/j.cell.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bademosi AT, Luo J, & Meunier FA (2018). Actin Remodeling in Regulated Exocytosis: Toward a Mesoscopic View. Trends Cell Biol, 28(9), 685–697. doi: 10.1016/j.tcb.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, & Du JL (2012). Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell, 23(6), 1189–1202. doi: 10.1016/j.devcel.2012.10.027 [DOI] [PubMed] [Google Scholar]
- Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, … Nedergaard M (1998). Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci, 1(6), 494–500. doi: 10.1038/2210 [DOI] [PubMed] [Google Scholar]
- Linetti A, Fratangeli A, Taverna E, Valnegri P, Francolini M, Cappello V, … Rosa P (2010). Cholesterol reduction impairs exocytosis of synaptic vesicles. J Cell Sci, 123(Pt 4), 595–605. doi: 10.1242/jcs.060681 [DOI] [PubMed] [Google Scholar]
- Lingwood D, & Simons K (2010). Lipid rafts as a membrane-organizing principle. Science, 327(5961), 46–50. doi: 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, … Jahn R (1992). Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun, 189(2), 1017–1023. doi: 10.1016/0006-291x(92)92305-h [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, … He Z (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci, 13(9), 1075–1081. doi: 10.1038/nn.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang Z, … He Z (2017). A Sensitized IGF1 Treatment Restores Corticospinal Axon-Dependent Functions. Neuron, 95(4), 817–833 e814. doi: 10.1016/j.neuron.2017.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YE, Koemeter-Cox A, Finelli MJ, Shen L, Friedel RH, & Zou H (2017). Comprehensive mapping of 5-hydroxymethylcytosine epigenetic dynamics in axon regeneration. Epigenetics, 12(2), 77–92. doi: 10.1080/15592294.2016.1264560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzana AO, Lee JK, Mui M, Chang A, & Zheng B (2015). A surviving intact branch stabilizes remaining axon architecture after injury as revealed by in vivo imaging in the mouse spinal cord. Neuron, 86(4), 947–954. doi: 10.1016/j.neuron.2015.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, & Van Vactor D (2009). The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol, 10(5), 332–343. doi: 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, … Huang EJ (2016). Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell, 165(4), 921–935. doi: 10.1016/j.cell.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, & Brosnan CF (2009). Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci, 29(24), 7743–7752. doi: 10.1523/JNEUROSCI.0341-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, & Holzbaur EL (2014). Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron, 84(2), 292–309. doi: 10.1016/j.neuron.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar M, & Cavalli V (2018). Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci, 19(6), 323–337. doi: 10.1038/s41583-018-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW (1988). Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science, 240(4852), 622–630. doi: 10.1126/science.3283935 [DOI] [PubMed] [Google Scholar]
- Maier IC, & Schwab ME (2006). Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci, 361(1473), 1611–1634. doi: 10.1098/rstb.2006.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples CJ, Ruiz WG, & Apodaca G (1997). Both microtubules and actin filaments are required for efficient postendocytotic traffic of the polymeric immunoglobulin receptor in polarized Madin-Darby canine kidney cells. J Biol Chem, 272(10), 6741–6751. doi: 10.1074/jbc.272.10.6741 [DOI] [PubMed] [Google Scholar]
- Mason CA, & Gregory E (1984). Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J Neurosci, 4(7), 1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, & Pfrieger FW (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science, 294(5545), 1354–1357. doi: 10.1126/science.294.5545.1354 [DOI] [PubMed] [Google Scholar]
- Mayran A, & Drouin J (2018). Pioneer transcription factors shape the epigenetic landscape. J Biol Chem, 293(36), 13795–13804. doi: 10.1074/jbc.R117.001232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TJ, & Simon SA (2006). Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct, 35, 177–198. doi: 10.1146/annurev.biophys.35.040405.102022 [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, & Brown DA (1999). Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem, 274(6), 3910–3917. doi: 10.1074/jbc.274.6.3910 [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, & Tagawa Y (2007). Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J Neurosci, 27(25), 6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, & Goldberg JL (2009). KLF family members regulate intrinsic axon regeneration ability. Science, 326(5950), 298–301. doi: 10.1126/science.1175737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Hong W, Dani VS, Favaloro V, & Luo L (2012). Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature, 484(7393), 237–241. doi: 10.1038/nature10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S (2003). Lipid rafts: elusive or illusive? Cell, 115(4), 377–388. doi: 10.1016/s0092-8674(03)00882-1 [DOI] [PubMed] [Google Scholar]
- Nagy JI, & Rash JE (2000). Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev, 32(1), 29–44. doi: 10.1016/s0165-0173(99)00066-1 [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, & McGavern DB (2014). Microglia development and function. Annu Rev Immunol, 32, 367–402. doi: 10.1146/annurev-immunol-032713-120240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebeling FC, Poll S, Schmid LC, Mittag M, Steffen J, Keppler K, & Fuhrmann M (2019). Microglia motility depends on neuronal activity and promotes structural plasticity in the hippocampus. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, … Molofsky AV (2020). Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell, 182(2), 388–403 e315. doi: 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Hong KP, & Spitzer NC (2011). Spatial and temporal second messenger codes for growth cone turning. Proc Natl Acad Sci U S A, 108(33), 13776–13781. doi: 10.1073/pnas.1100247108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie D, Chen Z, Ebrahimi-Fakhari D, Di Nardo A, Julich K, Robson VK, … Sahin M (2015). The Stress-Induced Atf3-Gelsolin Cascade Underlies Dendritic Spine Deficits in Neuronal Models of Tuberous Sclerosis Complex. J Neurosci, 35(30), 10762–10772. doi: 10.1523/JNEUROSCI.4796-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, … Cutler DF (2011). Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol, 194(4), 613–629. doi: 10.1083/jcb.201011119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, & Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308(5726), 1314–1318. doi: 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- Nishida H, & Okabe S (2007). Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci, 27(2), 331–340. doi: 10.1523/JNEUROSCI.4466-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix P, Hammarlund M, Hauth L, Lachnit M, Jorgensen EM, & Bastiani M (2014). Axon regeneration genes identified by RNAi screening in C. elegans. J Neurosci, 34(2), 629–645. doi: 10.1523/JNEUROSCI.3859-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan KJ, Ma K, Guo H, Wang C, Sun F, Han SB, … Zhong J (2014). B-RAF kinase drives developmental axon growth and promotes axon regeneration in the injured mature CNS. J Exp Med, 211(5), 801–814. doi: 10.1084/jem.20131780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, & Nedergaard M (2012). Heterogeneity of astrocytic form and function. Methods Mol Biol, 814, 23–45. doi: 10.1007/978-1-61779-452-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, & Schwimmer R (1988). The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol, 107(6 Pt 1), 2377–2387. doi: 10.1083/jcb.107.6.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Omura K, Tedeschi A, Riva P, Painter MW, Rojas L, … Woolf CJ (2015). Robust Axonal Regeneration Occurs in the Injured CAST/Ei Mouse CNS. Neuron, 86(5), 1215–1227. doi: 10.1016/j.neuron.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand RK, Nicholls JG, & Kuffler SW (1966). Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol, 29(4), 788–806. doi: 10.1152/jn.1966.29.4.788 [DOI] [PubMed] [Google Scholar]
- Owen DM, Williamson DJ, Magenau A, & Gaus K (2012). Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat Commun, 3, 1256. doi: 10.1038/ncomms2273 [DOI] [PubMed] [Google Scholar]
- Palmisano I, Danzi MC, Hutson TH, Zhou L, McLachlan E, Serger E, … Di Giovanni S (2019). Epigenomic signatures underpin the axonal regenerative ability of dorsal root ganglia sensory neurons. Nat Neurosci, 22(11), 1913–1924. doi: 10.1038/s41593-019-0490-4 [DOI] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Verkhratsky A, & Pankratov Y (2010). Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium, 48(4), 225–231. doi: 10.1016/j.ceca.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal OA, & Verkhratsky A (2009). P2X receptors and synaptic plasticity. Neuroscience, 158(1), 137–148. doi: 10.1016/j.neuroscience.2008.03.076 [DOI] [PubMed] [Google Scholar]
- Pannasch U, Vargova L, Reingruber J, Ezan P, Holcman D, Giaume C, … Rouach N (2011). Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A, 108(20), 8467–8472. doi: 10.1073/pnas.1016650108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, … Gross CT (2011). Synaptic pruning by microglia is necessary for normal brain development. Science, 333(6048), 1456–1458. doi: 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Jawaid A, Henstridge CM, Valeri A, Merlini M, Robinson JL, … Rajendran L (2017). TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss. Neuron, 95(2), 297–308 e296. doi: 10.1016/j.neuron.2017.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, … He Z (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science, 322(5903), 963–966. doi: 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, & Scheller RH (1994). Specificity and regulation of a synaptic vesicle docking complex. Neuron, 13(2), 353–361. doi: 10.1016/0896-6273(94)90352-2 [DOI] [PubMed] [Google Scholar]
- Pfenninger KH (2009). Plasma membrane expansion: a neuron’s Herculean task. Nat Rev Neurosci, 10(4), 251–261. doi: 10.1038/nrn2593 [DOI] [PubMed] [Google Scholar]
- Pfenninger KH, & Friedman LB (1993). Sites of plasmalemmal expansion in growth cones. Brain Res Dev Brain Res, 71(2), 181–192. doi: 10.1016/0165-3806(93)90170-f [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, & Barres BA (1997). Synaptic efficacy enhanced by glial cells in vitro. Science, 277(5332), 1684–1687. doi: 10.1126/science.277.5332.1684 [DOI] [PubMed] [Google Scholar]
- Pike LJ (2006). Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res, 47(7), 1597–1598. doi: 10.1194/jlr.E600002-JLR200 [DOI] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, & Bennett MK (1998). The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol, 5(9), 765–769. doi: 10.1038/1799 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, & Mullins RD (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct, 29, 545–576. doi: 10.1146/annurev.biophys.29.1.545 [DOI] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, & Horber JK (2000). Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol, 148(5), 997–1008. doi: 10.1083/jcb.148.5.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttagunta R, Tedeschi A, Soria MG, Hervera A, Lindner R, Rathore KI, … Di Giovanni S (2014). PCAF-dependent epigenetic changes promote axonal regeneration in the central nervous system. Nat Commun, 5, 3527. doi: 10.1038/ncomms4527 [DOI] [PubMed] [Google Scholar]
- Quiroga S, Bisbal M, & Caceres A (2018). Regulation of plasma membrane expansion during axon formation. Dev Neurobiol, 78(3), 170–180. doi: 10.1002/dneu.22553 [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, & Nagy JI (2001). Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci, 21(6), 1983–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Reva M, Kirizs T, Szoboszlay M, Lorincz A, Moneron G, … DiGregorio DA (2019). Distinct Nanoscale Calcium Channel and Synaptic Vesicle Topographies Contribute to the Diversity of Synaptic Function. Neuron, 104(4), 693–710 e699. doi: 10.1016/j.neuron.2019.08.014 [DOI] [PubMed] [Google Scholar]
- Reemst K, Noctor SC, Lucassen PJ, & Hol EM (2016). The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci, 10, 566. doi: 10.3389/fnhum.2016.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder V, & Kater SB (1992). Regulation of neuronal growth cone filopodia by intracellular calcium. J Neurosci, 12(8), 3175–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Iino R, Fujiwara T, Murase K, & Kusumi A (2003). The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques (Review). Mol Membr Biol, 20(1), 13–18. doi: 10.1080/0968768021000055698 [DOI] [PubMed] [Google Scholar]
- Rodrigues HA, Lima RF, Fonseca Mde C, Amaral EA, Martinelli PM, Naves LA, … Guatimosim C (2013). Membrane cholesterol regulates different modes of synaptic vesicle release and retrieval at the frog neuromuscular junction. Eur J Neurosci, 38(7), 2978–2987. doi: 10.1111/ejn.12300 [DOI] [PubMed] [Google Scholar]
- Roig-Puiggros S, Vigouroux RJ, Beckman D, Bocai NI, Chiou B, Davimes J, … Chedotal A (2020). Construction and reconstruction of brain circuits: normal and pathological axon guidance. J Neurochem, 153(1), 10–32. doi: 10.1111/jnc.14900 [DOI] [PubMed] [Google Scholar]
- Romero MI, Lin L, Lush ME, Lei L, Parada LF, & Zhu Y (2007). Deletion of Nf1 in neurons induces increased axon collateral branching after dorsal root injury. J Neurosci, 27(8), 2124–2134. doi: 10.1523/JNEUROSCI.4363-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, & Ransom BR (1997). Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia, 20(4), 299–307. doi: [DOI] [PubMed] [Google Scholar]
- Rosello-Busquets C, de la Oliva N, Martinez-Marmol R, Hernaiz-Llorens M, Pascual M, Muhaisen A, … Soriano E (2019). Cholesterol Depletion Regulates Axonal Growth and Enhances Central and Peripheral Nerve Regeneration. Front Cell Neurosci, 13, 40. doi: 10.3389/fncel.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, & Giaume C (2008). Astroglial metabolic networks sustain hippocampal synaptic transmission. Science, 322(5907), 1551–1555. doi: 10.1126/science.1164022 [DOI] [PubMed] [Google Scholar]
- Rozenbaum M, Rajman M, Rishal I, Koppel I, Koley S, Medzihradszky KF, … Fainzilber M (2018). Translatome Regulation in Neuronal Injury and Axon Regrowth. eNeuro, 5(2). doi: 10.1523/ENEURO.0276-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, … Bradke F (2015). Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science, 348(6232), 347–352. doi: 10.1126/science.aaa2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, & Cline HT (2003). Control of axon branch dynamics by correlated activity in vivo. Science, 301(5629), 66–70. doi: 10.1126/science.1082545 [DOI] [PubMed] [Google Scholar]
- Saghy E, Szoke E, Payrits M, Helyes Z, Borzsei R, Erostyak J, … Szolcsanyi J (2015). Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of Transient Receptor Potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol Res, 100, 101–116. doi: 10.1016/j.phrs.2015.07.028 [DOI] [PubMed] [Google Scholar]
- Saka SK, Honigmann A, Eggeling C, Hell SW, Lang T, & Rizzoli SO (2014). Multi-protein assemblies underlie the mesoscale organization of the plasma membrane. Nat Commun, 5, 4509. doi: 10.1038/ncomms5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, Gould GW, & Chamberlain LH (2005). Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem, 280(20), 19449–19453. doi: 10.1074/jbc.M501923200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, & Beggs S (2014). Sublime microglia: expanding roles for the guardians of the CNS. Cell, 158(1), 15–24. doi: 10.1016/j.cell.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, … Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 74(4), 691–705. doi: 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, & Montecucco C (1992). Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature, 359(6398), 832–835. doi: 10.1038/359832a0 [DOI] [PubMed] [Google Scholar]
- Schliwa M (1982). Action of cytochalasin D on cytoskeletal networks. J Cell Biol, 92(1), 79–91. doi: 10.1083/jcb.92.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, & Strittmatter SM (2014). Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol, 27, 53–60. doi: 10.1016/j.conb.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Lin-Moore A, Chenette DM, Wang X, Jiang Z, Cafferty WB, … Strittmatter SM (2018). Functional Genome-wide Screen Identifies Pathways Restricting Central Nervous System Axonal Regeneration. Cell Rep, 23(2), 415–428. doi: 10.1016/j.celrep.2018.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, & Eggeling C (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol, 18(6), 361–374. doi: 10.1038/nrm.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, & Khakh BS (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci, 15(1), 70–80. doi: 10.1038/nn.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, & Miller JH (2004). Regeneration beyond the glial scar. Nat Rev Neurosci, 5(2), 146–156. doi: 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, & Nedergaard M (2003). Signaling at the gliovascular interface. J Neurosci, 23(27), 9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, & Gerl MJ (2010). Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol, 11(10), 688–699. doi: 10.1038/nrm2977 [DOI] [PubMed] [Google Scholar]
- Simons K, & Ikonen E (1997). Functional rafts in cell membranes. Nature, 387(6633), 569–572. doi: 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, … Eroglu C (2016). Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1alpha and NL1 via Hevin. Cell, 164(1–2), 183–196. doi: 10.1016/j.cell.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, & Majewska AK (2016). Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun, 7, 10905. doi: 10.1038/ncomms10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, & Kater SB (1994). Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol, 166(1), 87–100. doi: 10.1006/dbio.1994.1298 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, & Vinters HV (2010). Astrocytes: biology and pathology. Acta Neuropathol, 119(1), 7–35. doi: 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, Co C, Taunton J, & Bement W (2003). Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol, 5(8), 727–732. doi: 10.1038/ncb1025 [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, & Rothman JE (1993). A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell, 75(3), 409–418. doi: 10.1016/0092-8674(93)90376-2 [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, & Rothman JE (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature, 362(6418), 318–324. doi: 10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, … Jan YN (2019). The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron, 102(2), 373–389 e376. doi: 10.1016/j.neuron.2019.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J (1985). Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berl), 171(2), 245–252. doi: 10.1007/bf00341419 [DOI] [PubMed] [Google Scholar]
- Stephani F, Scheuer V, Eckrich T, Blum K, Wang W, Obermair GJ, & Engel J (2019). Deletion of the Ca(2+) Channel Subunit alpha2delta3 Differentially Affects Cav2.1 and Cav2.2 Currents in Cultured Spiral Ganglion Neurons Before and After the Onset of Hearing. Front Cell Neurosci, 13, 278. doi: 10.3389/fncel.2019.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S, Haverkamp S, Sinske D, Tedeschi A, Naumann U, Di Giovanni S, … Knoll B (2013). The transcription factor serum response factor stimulates axon regeneration through cytoplasmic localization and cofilin interaction. J Neurosci, 33(48), 18836–18848. doi: 10.1523/JNEUROSCI.3029-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, … Barres BA (2007). The classical complement cascade mediates CNS synapse elimination. Cell, 131(6), 1164–1178. doi: 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, … Eroglu C (2017). Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature, 551(7679), 192–197. doi: 10.1038/nature24638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, & Lanier LM (2004). Arp2/3 is a negative regulator of growth cone translocation. Neuron, 43(1), 81–94. doi: 10.1016/j.neuron.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Sudhof TC (2013). Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron, 80(3), 675–690. doi: 10.1016/j.neuron.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2018). Towards an Understanding of Synapse Formation. Neuron, 100(2), 276–293. doi: 10.1016/j.neuron.2018.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, & Rizo J (2011). Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol, 3(12). doi: 10.1101/cshperspect.a005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, & Rothman JE (2009). Membrane fusion: grappling with SNARE and SM proteins. Science, 323(5913), 474–477. doi: 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Yasuda S, Katsurabayashi S, Kawano H, Endo K, Takasaki K, … Yamagata K (2015). Rheb activation disrupts spine synapse formation through accumulation of syntenin in tuberous sclerosis complex. Nat Commun, 6, 6842. doi: 10.1038/ncomms7842 [DOI] [PubMed] [Google Scholar]
- Sun W, Larson MJ, Kiyoshi CM, Annett AJ, Stalker WA, Peng J, & Tedeschi A (2020). Gabapentinoid treatment promotes corticospinal plasticity and regeneration following murine spinal cord injury. J Clin Invest, 130(1), 345–358. doi: 10.1172/JCI130391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, … Nedergaard M (2013). Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science, 339(6116), 197–200. doi: 10.1126/science.1226740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, & Brunger AT (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature, 395(6700), 347–353. doi: 10.1038/26412 [DOI] [PubMed] [Google Scholar]
- Tassew NG, Mothe AJ, Shabanzadeh AP, Banerjee P, Koeberle PD, Bremner R, … Monnier PP (2014). Modifying lipid rafts promotes regeneration and functional recovery. Cell Rep, 8(4), 1146–1159. doi: 10.1016/j.celrep.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Tedeschi A (2011). Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci, 4, 60. doi: 10.3389/fnmol.2011.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, & Bradke F (2017). Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol, 42, 118–127. doi: 10.1016/j.conb.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Curcio M, Laskowski CJ, Schaffran B, Flynn KC, … Bradke F (2019). ADF/Cofilin-Mediated Actin Turnover Promotes Axon Regeneration in the Adult CNS. Neuron, 103(6), 1073–1085 e1076. doi: 10.1016/j.neuron.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, … Bradke F (2016). The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron, 92(2), 419–434. doi: 10.1016/j.neuron.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Omura T, & Costigan M (2017). CNS repair and axon regeneration: Using genetic variation to determine mechanisms. Exp Neurol, 287(Pt 3), 409–422. doi: 10.1016/j.expneurol.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, & Popovich PG (2019). The Application of Omics Technologies to Study Axon Regeneration and CNS Repair. F1000Res, 8. doi: 10.12688/f1000research.17084.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen J, Inavalli V, & Nagerl UV (2018). Super-Resolution Imaging of the Extracellular Space in Living Brain Tissue. Cell, 172(5), 1108–1121 e1115. doi: 10.1016/j.cell.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, … Rowitch DH (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science, 337(6092), 358–362. doi: 10.1126/science.1222381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulodziecka K, Diaz-Rohrer BB, Farley MM, Chan RB, Di Paolo G, Levental KR, … Levental I (2016). Remodeling of the postsynaptic plasma membrane during neural development. Mol Biol Cell, 27(22), 3480–3489. doi: 10.1091/mbc.E16-06-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka N, Hayano Y, Yamada A, & Yamamoto N (2007). Interplay between laminar specificity and activity-dependent mechanisms of thalamocortical axon branching. J Neurosci, 27(19), 5215–5223. doi: 10.1523/JNEUROSCI.4685-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, & Barres BA (2001). Control of synapse number by glia. Science, 291(5504), 657–661. doi: 10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- Urbina FL, Gomez SM, & Gupton SL (2018). Spatiotemporal organization of exocytosis emerges during neuronal shape change. J Cell Biol, 217(3), 1113–1128. doi: 10.1083/jcb.201709064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, … Molofsky AV (2018). Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science, 359(6381), 1269–1273. doi: 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn KM, Gumkowski FD, & Jamieson JD (1999). The subapical actin cytoskeleton regulates secretion and membrane retrieval in pancreatic acinar cells. J Cell Sci, 112 ( Pt 1), 81–96. [DOI] [PubMed] [Google Scholar]
- Van Goor D, Hyland C, Schaefer AW, & Forscher P (2012). The role of actin turnover in retrograde actin network flow in neuronal growth cones. PLoS One, 7(2), e30959. doi: 10.1371/journal.pone.0030959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE, Campenot RB, & Vance DE (2000). The synthesis and transport of lipids for axonal growth and nerve regeneration. Biochim Biophys Acta, 1486(1), 84–96. doi: 10.1016/s1388-1981(00)00050-0 [DOI] [PubMed] [Google Scholar]
- Venkatesh I, Mehra V, Wang Z, Califf B, & Blackmore MG (2018). Developmental Chromatin Restriction of Pro-Growth Gene Networks Acts as an Epigenetic Barrier to Axon Regeneration in Cortical Neurons. Dev Neurobiol, 78(10), 960–977. doi: 10.1002/dneu.22605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, & Harris KM (1999). Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci, 19(16), 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, & Navarro X (2000). Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst, 5(4), 191–208. doi: 10.1046/j.1529-8027.2000.00026.x [DOI] [PubMed] [Google Scholar]
- Verkhratskiĭ AN, & Butt A (2013). Glial physiology and pathophysiology. Chichester, West Sussex, UK ; Hoboken, NJ, USA: Wiley-Blackwell. [Google Scholar]
- Verkhratsky A, Reyes RC, & Parpura V (2014). TRP channels coordinate ion signalling in astroglia. Rev Physiol Biochem Pharmacol, 166, 1–22. doi: 10.1007/112_2013_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, & Parpura V (2012). Calcium signalling in astroglia. Mol Cell Endocrinol, 353(1–2), 45–56. doi: 10.1016/j.mce.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Viola A, & Gupta N (2007). Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol, 7(11), 889–896. doi: 10.1038/nri2193 [DOI] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, … Verhage M (2001). Munc18–1 promotes large dense-core vesicle docking. Neuron, 31(4), 581–591. doi: 10.1016/s0896-6273(01)00391-9 [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, & Nabekura J (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci, 29(13), 3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, & Steinhauser C (2006). The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci, 26(20), 5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, … Nedergaard M (2012). Astrocytes modulate neural network activity by Ca(2)+-dependent uptake of extracellular K+. Sci Signal, 5(218), ra26. doi: 10.1126/scisignal.2002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Reynolds A, Kirry A, Nienhaus C, & Blackmore MG (2015). Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J Neurosci, 35(7), 3139–3145. doi: 10.1523/JNEUROSCI.2832-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, … Sofroniew MV (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci, 33(31), 12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FM, Cragg JJ, Jutzeler CR, Rohrich F, Weidner N, Saur M, … Kramer JK (2017). Early Administration of Gabapentinoids Improves Motor Recovery after Human Spinal Cord Injury. Cell Rep, 18(7), 1614–1618. doi: 10.1016/j.celrep.2017.01.048 [DOI] [PubMed] [Google Scholar]
- Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, & Jorgensen EM (2003). Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci, 6(10), 1023–1030. doi: 10.1038/nn1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, … Gross CT (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun, 9(1), 1228. doi: 10.1038/s41467-018-03566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welnhofer EA, Zhao L, & Cohan CS (1999). Calcium influx alters actin bundle dynamics and retrograde flow in Helisoma growth cones. J Neurosci, 19(18), 7971–7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YL, An R, Cassin J, Joseph J, Mi R, Wang C, … Ming GL (2017). An Intrinsic Epigenetic Barrier for Functional Axon Regeneration. Neuron, 94(2), 337–346 e336. doi: 10.1016/j.neuron.2017.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SN, Gangaraju S, Aylsworth A, & Hou ST (2012). Membrane raft disruption results in neuritic retraction prior to neuronal death in cortical neurons. Biosci Trends, 6(4), 183–191. doi: 10.5582/bst.2012.v6.4.183 [DOI] [PubMed] [Google Scholar]
- Wiesel TN, & Hubel DH (1963). Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol, 26, 1003–1017. doi: 10.1152/jn.1963.26.6.1003 [DOI] [PubMed] [Google Scholar]
- Williams ME, de Wit J, & Ghosh A (2010). Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron, 68(1), 9–18. doi: 10.1016/j.neuron.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, McClain LM, Valtschanoff JG, Park CS, Maglione C, & Gupton SL (2014). A novel Netrin-1-sensitive mechanism promotes local SNARE-mediated exocytosis during axon branching. J Cell Biol, 205(2), 217–232. doi: 10.1083/jcb.201311003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher MR, Kirov SA, & Harris KM (2007). Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia, 55(1), 13–23. doi: 10.1002/glia.20415 [DOI] [PubMed] [Google Scholar]
- Wu D, Klaw MC, Connors T, Kholodilov N, Burke RE, & Tom VJ (2015). Expressing Constitutively Active Rheb in Adult Neurons after a Complete Spinal Cord Injury Enhances Axonal Regeneration beyond a Chondroitinase-Treated Glial Scar. J Neurosci, 35(31), 11068–11080. doi: 10.1523/JNEUROSCI.0719-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dissing-Olesen L, MacVicar BA, & Stevens B (2015). Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol, 36(10), 605–613. doi: 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, & Chisholm AD (2007). Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci U S A, 104(38), 15132–15137. doi: 10.1073/pnas.0707001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Wu B, Li J, Xu C, Li H, Luginbuhl DJ, … Luo L (2019). Transsynaptic Fish-lips signaling prevents misconnections between nonsynaptic partner olfactory neurons. Proc Natl Acad Sci U S A, 116(32), 16068–16073. doi: 10.1073/pnas.1905832116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Wang X, Wang J, Wang X, Chen W, Lu N, … Liu K (2020). Rewiring Neuronal Glycerolipid Metabolism Determines the Extent of Axon Regeneration. Neuron, 105(2), 276–292 e275. doi: 10.1016/j.neuron.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhang XF, Pollard TD, & Forscher P (2012). Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J Cell Biol, 197(7), 939–956. doi: 10.1083/jcb.201111052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Sugiura H, Katsurabayashi S, Shimada T, Tanaka H, Takasaki K, … Yamagata K (2014). Activation of Rheb, but not of mTORC1, impairs spine synapse morphogenesis in tuberous sclerosis complex. Sci Rep, 4, 5155. doi: 10.1038/srep05155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Guo ZD, He ZZ, Wang ZY, & Sun XC (2019). Apolipoprotein E Affects In Vitro Axonal Growth and Regeneration via the MAPK Signaling Pathway. Cell Transplant, 28(6), 691–703. doi: 10.1177/0963689718808736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HY, & Bement WM (2007). Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat Cell Biol, 9(2), 149–159. doi: 10.1038/ncb1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, & Carroll JS (2011). Pioneer transcription factors: establishing competence for gene expression. Genes Dev, 25(21), 2227–2241. doi: 10.1101/gad.176826.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Williams PR, Jacobi A, Wang C, Goel A, Hirano AA, … He Z (2019). Elevating Growth Factor Responsiveness and Axon Regeneration by Modulating Presynaptic Inputs. Neuron, 103(1), 39–51 e35. doi: 10.1016/j.neuron.2019.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, & Sanes JR (2010). Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell, 143(3), 343–353. doi: 10.1016/j.cell.2010.10.009 [DOI] [PubMed] [Google Scholar]