Abstract
The ab initio method for prediction of the enthalpies of formation for CHON-containing organic compounds proposed earlier (J. Chem. Theory Comput. 2018, 14, 5920-5932) has been extended to their fluorinated derivatives. A single experimental ΔfHom is typically available for compounds in this scope. Thus, a priori evaluation of the data quality was found to be inefficient despite all available experimental data for C1─C3 hydrofluorocarbons and 34 data points for medium-size organofluorine compounds being considered. The training set was derived by analyzing consistency of the experimental and predicted values and removal of outliers. Significant issues in the experimental data, including inconsistency across different laboratories, were identified and potential causes for these problems were discussed. A conservative estimate of uncertainty for the experimental ΔfHom of organofluorine compounds was proposed.
1. Introduction
Fluorinated organic compounds are an important class of chemicals used in the pharmaceutical industry, agriculture, production of polymers, refrigerants, surfactants, and oil-repellants.1,2,3 Some of these compounds are also considered pollutants due to their toxicity, bioaccumulation, and contribution to global warming. Knowledge of their thermodynamic properties is critical for industrial applications and development of pollution prevention strategies. In this work, we focus on evaluation of their ideal-gas enthalpies of formation, a property essential to process modeling in a variety of applications.
The experimental methods used to determine enthalpies of formation for the compounds of interest include oxygen and fluorine combustion calorimetries, reaction calorimetry, primarily, with alkali metals and hydrogen, and mass spectrometry. Some enthalpies of formation were also derived from complex thermodynamic cycles involving different types of measurements.
As demonstrated below, oxygen combustion calorimetry is the most reliable and widespread method for determination of ΔfHom for the considered compounds. Two principal techniques have been used in combustion calorimetry. The most popular one involves combustion in a rotating bomb, in which a certain amount of water has been added.4,5 To use this approach, the studied or auxiliary compound should have sufficient hydrogen content to yield aqueous HF as a main fluorine product. In the second technique, the combustion is performed in a bomb with no water added.6 This approach has been used for fluorocarbons. To our knowledge, presently, combustion calorimetry measurements for fluorinated compounds are only conducted at the Center of Chemical Research at the University of Porto. The number of fluorinated compounds containing, C, H, O, or N atoms, for which experimental enthalpies of formation have been determined, is well below 200.
The combustion calorimetry experiments are conducted at a high pressure of oxygen (around 3 MPa). A multi-step procedure4 is used to transform the experimental combustion energies into the standard-state energies of combustion at T = 298.15 K. To derive ΔfHom, the reference enthalpies of formation for water, CO2, and HF(aq) are required. One should note that values for the suggested enthalpy of formation for the latter evolved significantly over time, and the latest recommendations are to a large extent based on the results of Johnson et al.7,8 Consequently, the results reported in the literature need to be reevaluated with the use of updated reference values.
Another challenge of the method is that the combustion energies of organofluorine compounds are significantly lower than those of the non-fluorinated counterparts. Thus, the calorimetric results are highly sensitive to the incompletely fluorinated impurities.4 In many cases, the resulting values are based on the results of chemical analyses of products for HF, CO2, HNO3, and less frequently for CF4 and F2, which adds complexity to the procedure. As demonstrated below, consistency of the results for different laboratories and repeatability-based uncertainties are often worse than those for CHON compounds. All factors listed above make a reliable predictive procedure highly desirable. The existing approaches involving high-level ab initio calculations are mostly focused on small (C1 and C2) molecules (e.g., Refs. 9, 10).
Historically, the main contributors to the field are
Thermodynamics Laboratory of the Bartesville Petroleum Research Center, Bureau of Mines (Bartesville, OK, U.S.A.);
Chemical Thermodynamics Group of the National Chemical Laboratory (Teddington, U.K.)
Thermochemistry Laboratory of the Moscow State University (Soviet Union);
Department of Chemistry, University of Porto (Portugal);
Institute of Organic Chemistry and Biochemistry at the University of Freiburg (Germany);
Department of Chemistry, University of Windsor (Canada).
Some reliable values originate from the Chemistry Departments of the University of Wisconsin and the Rice University, the National Bureau of Standards (now NIST), the Argonne National Laboratory, and Thermal Research Laboratory of the Dow Chemical Company (all U.S.A). For brevity, the laboratories will be referred to by location in our discussion (e.g., the Moscow lab).
The goal of this work is to extend the ab initio method we proposed earlier11,12 to organofluorine compounds and evaluate the reliability of the predicted and available experimental gas-phase enthalpies of formation for these compounds.
2. Calculations
We considered conformational equilibria in the studied compounds because, in our previous work, they were shown to have a significant effect even for some moderate-size molecules.12 The model was adopted that assumed the ideal-gas equilibrium mixture of individual conformers with the entropy component of the standard Gibbs energy computed using the same rigid rotor-harmonic oscillator model as was used for the thermal correction to the enthalpy, , term. Enthalpy of formation of a given compound was computed as the Gibbs-energy average for the conformer population. The generation of conformers was performed as described earlier.13,14,15,16 An initial pool of conformer candidates was produced via systematic search using molecular mechanics based on the MMFF94 force field.17 The resulting conformer candidates were further optimized, and their vibrational spectra were computed with B3LYP/def2-TZVP-D3(BJ).18,19,20,21 This combination is expected to provide a compromise between computational cost and accuracy for complex molecules.12 The final set of conformers was established by eliminating duplicated structures and transition states identified from the vibrational analysis. The frequencies were scaled by 0.990 for zero-point vibrational energies (ZPVE). In the “rigid rotor – harmonic oscillator” calculations of the thermal correction Δ0TH, the scaling factors of 0.96 for hydrogen stretches and 0.985 for all other modes were applied. The rotational symmetry numbers needed for the entropy evaluation were obtained using the libmsym library.22 The conformer geometries for the single-point energy calculations were optimized with the density-fitted (resolution-of-identity) second-order Møller-Plesset perturbation theory (DF-MP2) with the aug-cc-pvqz basis set.23,24 Single-point energy calculations were conducted with the 2016 version of local CCSD(T) by Kállay et al.25,26,27 and the aug-cc-pvqz basis set. DFT calculations were performed with Gaussian 0928, local CCSD(T) was carried out with MRCC (release of September 25, 2017)27, and DF-MP2 was done with Psi4 v1.1.29 The choice of computational tools was motivated by the performance of the methods on the hardware used.12
The enthalpies of formation at T = 298.15 K were evaluated as12:
| (1) |
where E is the total electronic energy from the single-point calculations, ZPVE is the zero-point vibrational energy, and is the thermal correction from 0 to 298.15 K. The effective enthalpies, hi in eq 1, for C, H, O, and N atomic types where fixed to the values determined in the previous study12: h(C saturated or aromatic) = −99910.32 kJ·mol−1, h(C unsaturated) = −99909.44 kJ·mol−1, h(H) = −1524.23 kJ·mol−1, h(O) = −197138.05 kJ·mol−1, and h(N) = −143612.32 kJ·mol−1. Determination of the contribution h(F) is one of the goals of the present study. The h(F) values for individual compounds were determined from eq 1 using their experimental ΔfHom, and the final recommendation was derived via averaging of the individual values over the selected training set of molecules.
Reported uncertainties are the expanded uncertainties for a 0.95 level of confidence (k ≈ 2). For the experimental works, these are based on the authors’ uncertainty estimates (mainly repeatabilities) and uncertainties in the reference values.
3. Results
3.1. Experimental data
The enthalpies of formation considered in this work were derived using the original experimental data, reference enthalpies of formation presented in Table 1, and ΔfH(HF(aq)) from Johnson et al.7 shifted by 0.33 kJ·mol−1, as recommended by CODATA.8 The enthalpies of formation for hydrofluorocarbons are often determined from their enthalpies of combustion which are, in turn, based on the results of chemical analysis for CO2 and HF. If the average values based on those two analyses differed by more than 4 kJ·mol−1 and could not be explained by formation of CF4, the enthalpy of formation was calculated from the results based on the CO2 determination, since the latter was suggested to be more reliable.30 The heat capacities of compounds required to reduce the experimental ΔrH to T = 298.15 K were taken from NIST ThermoData Engine v.10.3 31 unless specified otherwise.
Table 1.
Reference enthalpies of formation used in this worka
| Compound | ΔfHom/kJ·mol−1 | Reference |
|---|---|---|
| CO2(g) | −393.51 ±0.13 | 8 |
| H2O(l) | −285.83 ± 0.04 | 8 |
| F−(aq) | −335.35 ± 0.65 | 8 |
| HF(l) | −303.55 ± 0.25 | 7 |
| HF(g) | −273.30 ± 0.70 | 8 |
| HCl(g) | −92.31 ± 0.10 | 8 |
| CF4(g) | −933.2 ± 0.8b | 32 |
| NF3 | −131.5 ± 1.0 | 33, 34 |
| NaF(cr) | −576.6 ± 0.7 | 35 |
| KF(cr) | −569.9 ± 0.7 | 35 |
| NaCl(cr) | −411.26 ± 0.12 | 35 |
| propane | −104.6 ± 0.2 | 36 |
Reported uncertainties are the expanded uncertainties for 0.95 level of confidence
see discussion in the text
When fluorinated organic compounds are burnt in oxygen, CF4 sometimes appears as a product, and larger fluorine content results in a higher yield of CF4. Consequently, the enthalpy of formation of tetrafluoromethane is an important reference quantity affecting experimental ΔfHom for many compounds. The enthalpy of formation ΔfHom = −(933.2 ± 0.8) kJ·mol−1 32 was derived by Greenberg and Hubbard from the experiments on graphite combustion in fluorine. The values obtained by Domalski and Armstrong using a similar technique37 and in references therein are consistent with this result. On the other hand, the enthalpy of reaction CF4(g) + 2H2O = CO2(g) + 4HF(·20H2O), ΔrHom = −(173.2 ± 1.3) kJ·mol−1 38,39,40, can be derived from the results of experiments in which poly(tetrafluoroethylene) and perfluorobicyclohexyl were burnt in oxygen with some water present. This value implies ΔfHom = −(936.2 ± 2.9) kJ·mol−1 for CF4, if the reference data above are used. The difference between this value and the direct result32 is relatively large, although the corresponding error bars slightly overlap. The apparent inconsistency should be resolved by replacing the enthalpy of formation of either CF4 or HF(aq) (or both) with more accurate value(s). In the current Active Thermochemical Tables36, the statistical analysis yielded ΔfHom(HF·20H2O) = −(321.03 ± 0.16) kJ·mol−1, which is 0.86 kJ·mol−1 less negative than the CODATA value. On the other hand, Ganyecz et al.41 computationally analyzed the thermochemical network for fluorinated and chlorinated methanes and ethanes using the diet-HEAT-F12 protocol42 and concluded that ΔfHom(CF4) by Greenberg and Hubbard is not consistent with the ab initio results and needs further studies. Overall, the CODATA ΔfHom(HF(aq)) is supported by a larger number of experimental studies as compared to that for ΔfHom(CF4), but it is still insufficient to make a conclusive assessment.
In this work, we used ΔfHom(CF4) = −(933.2 ± 0.8) kJ·mol−1 if CF4 was one of the major combustion products. If a small amount of CF4 was formed and reduction of the experimental data implied its hydrolysis, the enthalpy change ΔrHom = −(173.2 ± 1.3) kJ·mol−1 was used.
Fluorinated organic compounds, for which the enthalpies of formation are available, can be tentatively divided into three groups. The first group consists of relatively large molecules typically containing more than five carbon atoms. This group has been most studied over the past decades. The second group includes small (up to three carbon atoms) hydrofluorocarbons, most of which are gases at ambient conditions. The third group consists of inorganic species such as HF or NF3. In this work, 22 compounds (34 ΔfHom values) from group 1 (Table 2) were selected to provide reasonable variability with respect to the molecular structural features and the sources of information (i.e., the labs where the measurements were performed). Consideration of the data origins was important to avoid any bias due to a potential systematic error originating from a laboratory. The best effort was made to provide a comprehensive review of the experimental data for small hydrofluorocarbons (Table 3). The considered inorganic species included F2, HF, OF2, and NF3.
Table 2.
Experimental thermochemical data at T = 298.15 K for organofluorine compounds composed of medium-size moleculesa
| Compound | Formula | CASRN | Phase | ΔfHom(cond) | ΔcondgHom | Lab.b | Ref. | ΔcondgCop,m | ΔfHom,exp | ΔfHom,calc |
|---|---|---|---|---|---|---|---|---|---|---|
| at T | kJ·mol−1 | kJ·mol−1 | J·K−1·mol−1 | kJ·mol−1 | kJ·mol−1 | |||||
| 5-fluorouracil (1) | C4H3FN2O2 | 51-21-8 | cr | 132.1 ± 2.8 | 43 | −19c | ||||
| 153.6 ± 5.0 | 44 | |||||||||
| −581.9 ± 1.2 | A | 45 | ||||||||
| −586.8 ± 1.1 | 131.9 ± 1.8 | A | 46 | |||||||
| −586.8 ± 1.1 | 132.0 ± 1.5 | −454.8 ± 1.9 | −456.1 | |||||||
| perfluoropiperidine (2) | C5F11N | 836-77-1 | l | −2076.2 ± 3.9 | 30.0 ± 0.2 | B | 47 | −2046.2 ± 3.9 | −2058.2 | |
| (trifluoroacetyl)acetone (3) | C5H5F3O2 | 367-57-7 | l | 37.2 ± 0.2 | 48 | |||||
| −1039.4 ± 3.3 | C | 49 | ||||||||
| −1034.2 ± 2.3 | A | 50 | ||||||||
| −1034.9 ± 2.3 | −997.7 ± 2.3 | −996.6 | ||||||||
| hexafluorobenzene (4) | C6F6 | 392-56-3 | l | −991.1 ± 4.2 | D | 51 | ||||
| −982.7 ± 8.4 | E | 6 | ||||||||
| −991.1 ± 4.2 | 35.6 ± 0.3d | −955.5 ± 4.2 | −952.1 | |||||||
| decafluorocyclohexene (5) | C6F10 | 355-75-9 | l | −1966.6 ± 4.5 | 30.8 | D | 51 | |||
| −1938.0 ± 7.2 | 31.0 | E | 52 | |||||||
| −1966.6 ± 4.5 | 30.9 ± 0.1 | −1935.7 ± 4.5 | −1942.1 | |||||||
| perfluoro(2-methyl-2-pentene) (6) | C6F12 | 1584-03-8 | l | −2522.4 ± 8.8e | 23.4 ± 0.4 | C | 53 | −2499.0 ± 8.8 | −2499.3 | |
| pentafluorophenol (7) | C6HF5O | 771-61-9 | cr | −1024.2 ± 4.5 | f | D | 54 | |||
| 67.8 ± 0.5 | 55, 56g | |||||||||
| −1024.2 ± 4.5 | 67.8 ± 0.5 | −956.4 ± 4.5 | −954.2 | |||||||
| 4-fluoronitrobenzene (8) | C6H4FNO2 | 350-46-9 | l | −189.6 ± 1.4 | 56.0 ± 0.2 | A | 57 | −133.6 ± 1.4 | −129.8 | |
| 1,4-difluorobenzene (9) | C6H4F2 | 540-36-3 | l | −342.2 ± 1.6 | 35.8 ± 0.2h | B | 58 | −306.4 ± 1.6 | −302.5 | |
| 2-trifluoroacetylpyrrole (10) | C6H4F3NO | 2557-70-2 | cr | −776.6 ± 2.8 | 72.8 ± 1.4 | A | 59 | −50 | −703.8 ± 3.1 | −694.7 |
| Fluorobenzene (11) | C6H5F | 462-06-6 | l | −150.6 ± 1.6 | 34.7 ± 0.1d | B | 40 | −115.9 ± 1.6 | −112.2 | |
| 4-fluoroaniline (12) | C6H6FN | 371-40-4 | l | −164.0 ± 1.4 | 54.8 ± 0.8 | A | 60 | −109.2 ± 1.6 | −103.5 | |
| 5-fluoro-1,3-dimethyluracil (13) | C6H7FN2O2 | 3013-92-1 | cr | 121 ± 3 | 44 | |||||
| −575.0 ± 1.2 | 96.8 ± 1.6 | A | 46 | −30 | ||||||
| 98.0 ± 0.9 | 61 | |||||||||
| 97.7 ± 0.8 | −477.3 ± 1.5 | −473.5 | ||||||||
| fluorocyclohexane (14) | C6H11F | 372-46-3 | l | −373.5 ± 2.0 i | 36.9 ± 1.5 | F | 62 | −54j | −336.7 ± 2.5 | −330.4 |
| 4-fluorobenzonitrile (15) | C7H4FN | 1194-02-1 | cr | −53.5 ± 1.6 | 67.6 ± 0.5 | A | 63 | −25 | 14.1 ± 1.7 | 24.0 |
| 4-fluorobenzoic acid (16) | C7H5FO2 | 456-22-4 | cr | −586.7 ± 1.6 | B | 40 | ||||
| −584.9 ± 1.4 | D | 51 | ||||||||
| 93.7 ± 1.7 | 54 | −15k | ||||||||
| −585.2 ± 1.3 | G | 64 | ||||||||
| −584.4 ± 1.4 | G | 64 | ||||||||
| −585.2 ± 1.3 | F | 62 | ||||||||
| −584.4 ± 1.2 | A | 65 | ||||||||
| 92.7 ± 1.6 | 66 | |||||||||
| −584.8 ± 1.2 | 93.1 ± 1.1 | −491.7 ± 1.6 | −490.7 | |||||||
| (trifluoromethyl)benzene (17) | C7H5F3 | 98-08-8 | l | −635.0 ± 2.7 | B | 40 | ||||
| −636.9 ± 2.2 | B | 67 | ||||||||
| −640.6 ± 3.4 | C | 68 | ||||||||
| −635.8 ± 2.3 | C | 49 | ||||||||
| −636.6 ± 2.2 | 37.9 ± 0.9d | −598.7 ± 2.4 | −596.1 | |||||||
| 4-fluoromethylbenzene (18) | C7H7F | 352-32-9 | l | −186.9 ± 1.3 | B | 58 | ||||
| 39.4 ± 0.1 | 69 | −147.5 ± 1.3 | −142.6 | |||||||
| 1-fluoro-4-methoxybenzene (19) | C7H7FO | 459-60-9 | l | −308.9 ± 1.6 | 48.7 ± 1.1 | A | 70 | −260.2 ± 2.0 | −260.2 | |
| 5-fluoro-2-methylbenzoxazole (20) | C8H6FNO | 701-16-6 | l | −260.2 ± 3.8 | 57.2 ± 1.0 | A | 71 | −203.0 ± 3.9 | −211.3 | |
| (2,2,2-trifluoroethyl)benzene (21) | C8H7F3 | 21249-93-4 | l | −669.0 ± 2.8 | 46.1 ± 0.5 | F | 62 | l | −622.9 ± 2.8 | −633.8 |
| 1,1'-(1,1,2,2-tetrafluoro-1,2-ethanediyl)bis-benzene (22) | C14H10F4 | 425-32-1 | cr | −783.0 ± 4.6 | 101.9 ± 3.7 | F | 62 | l | −681.1 ± 5.9 | −691.8 |
Standard molar quantities: ΔfHom(cond), the enthalpy of combustion of a compound in the condensed state; ΔcondgHom, the enthalpy of sublimation or vaporization; ΔcondgCop,m, the heat capacity difference between the ideal-gas and condensed phases; ΔfHom,exp(g) and ΔfHom,calc(g), the experimental and calculated ideal-gas enthalpies of formation, respectively. All experimental enthalpies of combustion and formation are recalculated using the reference values from Table 1. Values selected for generation of the training data set are given in bold; the values not used for averaging are italicized. Reported uncertainties are the expanded uncertainties for 0.95 level of confidence
Source of the condensed-state enthalpy of formation: A, University of Porto; B, Bartesville Petroleum Research Center; C, Moscow State University; D, National Chemical Laboratory, Teddington; E, University of Windsor; F, University of Freiburg; G, National Bureau of Standards, Washington, DC
evaluated by NIST ThermoData Engine31 based on multiple sources
based on CO2 analysis
the sublimation pressure data54 include the solid-to-solid phase transition region and are not used
calorimetric data by Paukov et al.73 are inconsistent with the publications from the Teddington lab. The latter are used to keep consistency between different properties.
assuming that the reported combustion energies refer to HF·20H2O.
ΔvapCp estimated by NIST ThermoData Engine31
Ref. 75
the correction to T = 298.15 K was small compared to the uncertainty and not applied
Table 3.
Experimental thermochemical data for small hydrofluorocarbons in the ideal-gas statea
| Compound | CASRN | Ref. | Lab.b | Reaction | T / K | ΔrHom(T) |
ΔfHom,expc |
ΔfHom,calcc |
|---|---|---|---|---|---|---|---|---|
| kJ·mol−1 | kJ·mol−1 | kJ·mol−1 | ||||||
| CH2F2 | 75-10-5 | 76 | A | CH2F2(g) + O2(g) = CO2(g) + 2HF(·22H2O) | 298.15 | −585.1 ± 0.9 | −452.3 ± 1.6 | −452.2 |
| CHF3 | 75-46-7 | 76 | A | CHF3(g) + 1/2O2(g) + H2O(l) = CO2(g) + 3HF(·24H2O) | 298.15 | −696.6 ± 3.3 | −698.2 | |
| CF4 | 75-73-0 | 32 | B | C(gr) + 2F2(g) = CF4(g) | 298.15 | −933.2 ± 0.8 | −933.2 ± 0.8 | −934.9 |
| CHF2CH3 | 75-37-6 | 77 | C | C2H4F2(g) + 5/2 O2(g) = 2CO2(g) + H2O(l) + 2HF(·20H2O) | 298.15 | −1212.6 ± 2.0 | −504.0 ± 2.4 | −504.5 |
| CF3CH3 | 420-46-2 | 78, 79 | C | CF3CH3 + 2O2 = 2CO2 + 3HF(·27H2O) | 298.15 | −1004.2 ± 2.6 | −748.6 ± 3.3 | −753.8 |
| CHF2CH2F | 430-66-0 | 80, 81, 82, 83 | C,D | Multiple reactions involving CF2=CFCl | 298.15 | −696.0 ± 3.2 | −669.5 | |
| CF3CF2H | 354-33-6 | See text | −1115.2 | |||||
| CF3CF3 | 76-16-4 | 84 | E | C2F6(g) + 6K = 2C(soot) + 6KF | 298.15 | −2088 | −1311 ± 17d | |
| 85 | F | C2F6 + 2/3NF3 = 2CF4 + 1/3N2 | 298.15 | −434.7 ± 2.5 | −1344.0 ± 3.0 | −1345.5 | ||
| CH2FCH2CH3 | 460-13-9 | 86 | D | C3H7F(g) + H2 = C3H8(g) + HF(g) | 521 | −95.2 ± 1.0 | −284.8 ± 1.3 | −293.3 |
| CH3CHFCH3 | 420-26-8 | 86 | D | C3H7F(g) + H2 = C3H8(g) + HF(g) | 521 | −88.3 ± 0.7 | −292.2 ± 1.1 | −313.9 |
| 87 | G | CH2=CHF(g) + CH4(g) = C3H7F(g) | 298.15 | −112.9 ± 6.1 | ||||
| CH3CF2CH3 | 420-45-1 | 87 | G | CH2=CF2(g) + CH4(g) = CH3CF2CH3(g) | 298.15 | −123.5 ± 6.1 | −554.6 | |
| CF3CH2CF3 | 690-39-1 | 88,89 | C | C3H2F6(g) + 2O2(g) + 2H2O(l) = 3CO2(g) + 6HF(aq) | 298.15 | −1406.1 ± 8.1 | −1409.2 | |
| CF3CF2CF3 | 76-19-7 | 90 | C | C3F8(g) + 8Na(cr) = 8NaF(cr) + 3C(amorph) | 298.15 | −2761.3 ± 4.9 | −1783.7 ± 7.8 i | −1757.7 |
| HFC=CH2 | 75-02-5 | 30 | C | C2H3F(g) + 5/2O2(g) = 2CO2(g) + H2O(l) + HF(·40H2O) | 298.15 | −1256.1 ± 1.9 | −138.7 +± 2.0 | −143.6 |
| F2C=CH2 | 75-38-7 | 91 | A | C2H2F2(g) + 2O2(g) = 2CO2(g) + 2HF(·50H2O) | 298.15 | −1096.9 ± 7.0 | −334.2 ± 7.1 | −351.1 |
| 92 | C | C2H2F2(g) + 2O2(g) = 2CO2(g) + 2HF(·30H2O) | 298.15 | −1086.8 ± 8.2 | −344.1 ± 8.3 | |||
| F2C=CHF | 359-11-5 | 92 | C | C2HF3(g) + 3/2O2(g) = 2CO2(g) + 3HF(·30H2O) | 298.15 | −980.5 ± 3.9 | −486.5 ± 4.3 | −498.6 |
| F2C=CF2 | 116-14-3 | 84 | E | C2F4(g) + 4K = 2C(soot) + 4KF(cr) | 298.15 | −706 ± 8d | −673.0 | |
| 93 | H | C2F4(g) + 4K = 2C(gr) + 4KF(cr) | 298.15 | −715 ± 11e | ||||
| 94 | l | C2F4(g) = C(soot) + CF4(g) | 297.31 | −257.0 ± 6.4 | −676.2 ± 6.5e | |||
| 94 | l | C2F4(g) + 2H2(g) = 2C(soot) + 4HF(l) | 297.16 | −555.3 ± 5.6 | −658.9 ± 5.7e | |||
| 91 | A | C2F4(g) + 2H2(g) = 2C(amorph) + 4HF(·18H2O) | 298.15 | −618.1 ± 4.5 | −657.0 ± 5.4f | |||
| 91 | A | C2F4(g) = C(amorph) + CF4(g) | 298.15 | −266.0 ± 1.6 | −659.5 ± 2.0g | |||
| 95 | C | C2F4(g) + 4Na = 2C(amorph) + 4NaF(cr) | 298.15 | −1611.3 ± 5.3 | −662.1 ± 6.0h | |||
| CH2FCH=CH2, E-CH3CH=CHF, Z-CH3CH=CHF | 818-92-820327-65-519184-10-2 | 96 | J | CH2FCH=CH2 = (Z and E)-CH3CH=CHF | 484.0 | −22.0 ± 3.4 | ||
| 97 | K | CH2FCH=CH2(g) = (Z)-CH3CH=CHF(g) | 658.6 | −13.6 ± 0.6 | ||||
| 97 | K | CH2FCH=CH2(g) = (E)-CH3CH=CHF(g) | 658.6 | −11.0 ± 0.6 | ||||
| 96 | J | (E)-CH3CH=CHF(g) = (Z)-CH3CH=CHF(g) | 470.5 | −2.9 ± 0.5 | ||||
| 97 | K | (E)-CH3CH=CHF(g) = (Z)-CH3CH=CHF(g) | 658.6 | −2.6 ± 0.6 | ||||
| CHF2CH=CH2, CH3CH=CF2 | 430-62-6430-63-7 | 97 | K | CH3CH=CF2(g) = CHF2CH=CH2(g) | 672.6 | −10.7 ± 1.2 | ||
| CF3CH=CH2 | 677-21-4 | 98 | C | C3H3F3(g) + 3O2(g) = 3CO2(g) + 3HF(·xH2O), x = 13 or 45 | 298.15 | −621.7 ± 1.4 | −629.7 | |
| CF3CF=CF2 | 116-15-4 | 99,100 | C,D | Multiple reactions involving CF3CFClCF2Cl | −1151.8 ± 4.4 | −1150.6 | ||
| 94 | l | C2F4(g) = 2/3 CF3CF=CF2(g) | 299 | −86 ± 11 |
Standard molar quantities: ΔrHom, the enthalpy of a reaction shown; ΔfHom,exp(g) and ΔfHom,calc(g), the experimental and calculated ideal-gas enthalpies of formation, respectively. All experimental enthalpies of combustion and formation are recalculated using the reference values from Table 1. Values used in the training set are shown in bold. Reported uncertainties are the expanded uncertainties for 0.95 level of confidence
A, University of Wisconsin; B, Argonne National Laboratory; C, Moscow State University; D, University of Colorado at Boulder; E, Imperial Chemical Industries; F, Dow Chemical Company; G, California Institute of Technology; H, University of Göttingen; I, E. I. du Pont de Nemours & Co.; J, University of Rhode Island; K, University of Florida
at T = 298.15 K
assuming ΔfHom(soot) = 10.5 kJ·mol−1
assuming ΔfHom(soot) = 0 kJ·mol−1
ΔfHom(soot) = 6.2 kJ·mol−1 determined experimentally
ΔfHom(soot) = 7.7 kJ·mol−1 determined experimentally
ΔfHom(soot) = 16.5 kJ·mol−1 determined experimentally
ΔfHom(soot) = 22.6 kJ·mol−1 determined experimentally.
For all compounds from the first group (Table 2), the enthalpies of formation were derived from their energies of combustion in oxygen in a rotating bomb calorimeter. In most cases, the well-established technique of combustion in the presence of water4,5 was used. A detailed discussion of these results is presented below.
Multiple experimental techniques, including oxygen and fluorine combustion calorimetry, reaction calorimety with alkaline metals and hydrogen, chemical equilibrium studies, and mass spectrometry were used to obtain ΔfHom for small hydrofluorocarbons.
Fluoromethanes.
Enthalpies of formation for difluoromethane and trifluoromethane were determined by Neugebauer and Margrave76 from the results of combustion calorimetry. The recommended ΔfHom of CF4 is based on the results of direct combustion of graphite in fluorine.32
1,1-Difluoroethane.
The enthalpy of formation was reported by Kolesov et al.77 The authors analyzed the CO2 and HF content in the combustion products. The enthalpies of combustion based on these two values differed by about 13 kJ·mol−1. The enthalpy of combustion accepted in this paper was calculated from the CO2 analysis.
1,1,1-Trifluoroethane.
The enthalpy of formation was determined by Kolesov et al.78 from the results of combustion calorimetry. Later, Kolesov and Papina79 revised the experimental value by adding a correction for reduction to the standard state missing in the original work. Analysis of the kinetic data by Rodgers and Ford101 provides a similar value if ΔfH(CH3) and ΔfH(CF3) from Ref. 35 are used. However, the expanded uncertainty exceeds 10 kJ·mol−1 in this case.
1,1,2-Trifluoroethane.
The enthalpy of formation was calculated from the enthalpies of chlorotrifluoroethylene hydrogenation ΔrHom(298 K) = −(271.2 ± 2.3) kJ·mol−1 83 and its enthalpy of formation ΔfHom(CF2CFCl) = −(517.1 ± 2.2) kJ·mol−1 found as weight-average of the results from Refs. 80,81,82.
Pentafluoroethane.
The enthalpy of formation was reported by Buckley et al.102 who studied chemical equilibria:
CF3CF2Br(g) + I2(g) = CF3CF2I(g) + IBr(g)
Br2(g) + C2F5H(g) = HBr(g) + CF3CF2Br(g)
The equilibrium constants for the second reaction were determined by Coomber and Whittle.103 However, the reported ΔfHom(CF3CF2H) was, in turn, based on ΔfHom(CF3CF2I) estimated by Wu et al.104 as ΔfHom(CF3CF2I) = ½(ΔfHom(CF3CF3) + ΔfHom(CF2ICF2I)). Thus, this compound was excluded from further data analysis.
Hexafluoroethane.
The most precise value was obtained by Sinke85 by the reaction bomb calorimetry. The result strongly depends on reference ΔfHom of CF4 and NF3.
1-fluoropropane, 2-fluoropropane, 2,2-difluoropropane.
The enthalpies of formation for monofluoropropanes were obtained using the high-temperature enthalpies of hydrogenation determined in a flow calorimeter by Lacher et al.86 Enthalpies of the formal reactions
CH2=CHF + CH4 = CH3CHFCH3
CH2=CF2 + CH4 = CH3CF2CH3
could be found from results of the mass-spectrometric study.87
1,1,1,3,3,3-Hexafluoropropane.
The enthalpy of combustion and formation for this compound was reported by Kolesov.88 The concentration of the resulting HF solution was not specified. In the review89, the enthalpy of formation was recalculated with respect to the updated enthalpies of formation for the reference compounds.
Octafluoropropane.
ΔfHom was found90 from the enthalpy of its reaction with sodium. Detailed analysis of the combustion products was performed.
Fluoroethylene, 1,1-difluoroethylene, trifluoroethylene.
Kolesov et al. conducted combustion calorimetry studies for these compounds.30,92 ΔfHom of 1,1-difluoroethylene was also reported by Neugebauer and Margrave.91
Tetrafluoroethylene.
The enthalpy of formation for this compound was reported by many researchers. Neugebauer and Margrave91 and Kolesov et al.95 measured the energy of combustion for the amorphous carbon product.
Equilibria of mono- and difluoropropenes.
We are aware of two works reporting these equilibria.96,97 While the enthalpies of Z/E-isomerization of 1-propenes are consistent, the enthalpies of formation for 3-fluoropropene differ by 8 kJ·mol−1. That cannot be explained by their temperature difference.
3,3,3-Trifluoropropene.
The enthalpy of formation for this compound was determined98 from its enthalpy of combustion in oxygen in a bomb calorimeter. The average values of the enthalpy of combustion derived from the results of chemical analysis for HF and CO2 differ by about 5 kJ·mol−1. The enthalpy of formation was recalculated based on the amount of CO2. The ΔfHom values obtained in experiments 1 and 3 of the original publication are over 20 kJ·mol−1 too high relative to the other experiments, while the standard deviation calculated for experiments 2 and (4 to 12) is only 1.7 kJ·mol−1. Thus, the enthalpy of formation was calculated using the data from experiments 2 and 4 to 12.
Hexafluoropropene.
Following Papina and Kolesov99, the enthalpy of formation for this compound can be determined using the enthalpies of the processes
CF2ClCFClCF3(l) + O2(g) + 4H2O(l) = 3CO2(g) + 6HF(·20H2O) + 2HCl(·600H2O) ΔHmo(298 K) = −(925.6 ± 2.0) kJ·mol−1 99
CF3CF=CF2(g) + Cl2(g) = CF2ClCFClCF3(g) ΔrHmo(363 K) = −(197.3 ± 0.5) kJ·mol−1 100
CF2ClCFClCF3(l) = CF2ClCFClCF3(g) ΔlgHmo(298 K) = (26.93 ± 0.03) kJ·mol−1 105
The temperature dependence of ΔrHmo for the chlorination reaction can be neglected.
The enthalpy of reaction
C2F4(g) = 2/3 CF3CF=CF2(g) ΔrHmo(298 K) = −86 kJ·mol−1
was estimated by Duus94 using combustion experiments.
Four inorganic compounds were considered: F2, HF, NF3, and OF2. The enthalpies of formation of HF and NF3 are given in Table 1, for OF2, ΔfHom = (24.5 ± 1.6) kJ·mol−1.106
3.2. Consistency of experimental data and derivation of the training set
For generation of the CHON training set11, we used only those compounds for which the experimental data were available from multiple consistent sources and the resulting expanded uncertainty was under 2 kJ·mol−1. If these criteria were applied to the fluorinated compounds, a training set of sufficient size could not be compiled. Thus, all available data were analyzed. The effective enthalpy of fluorine, h(F), was derived for each data point using eq 1 and the experimental ΔfHom. The resulting h(F) were used to assess the consistency among the considered data points and identification of outliers.
The effective enthalpies h(F) for the compounds of group 1 are compared in Figure 1. The value h(F) = −261711.5 kJ·mol−1 used in the figure is a preliminary estimate based on all available experimental data. Most of the data are spread within (−4 to 6) kJ·mol−1 of this estimate. One would expect h(F) values to congregate near a certain value. This consistency should be better for compounds with similar fluorine functionality (e.g., para-substituted fluorinated benzene derivatives). Considering the uncertainty of the predicted ΔfHom was (2.5 to 3.0) kJ·mol−1 for CHON-containing compounds12, variations below 1 kJ·mol−1 due to computational model limitations are expected.
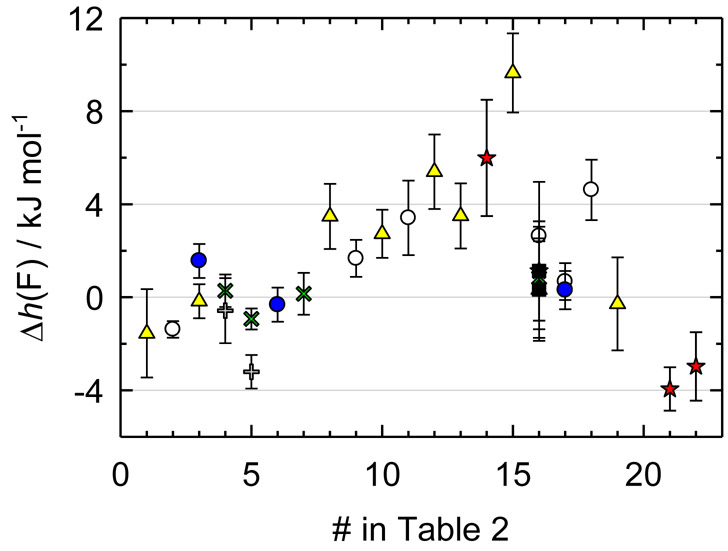
Figure 1.
Relative effective enthalpy, Δh(F) / (kJ·mol−1) = h(F) / (kJ·mol−1) + 261711.5, of a fluorine atom for the medium-size organic molecules as a function of the sequential number of a compound in Table 2: blue circles, Moscow lab; yellow triangles, Porto lab (1997-2014); green crosses, Teddington lab; gray pluses, Windsor lab; red stars, Freiburg lab; empty circles, Bartesville; black squares, Gaithersburg. The value for 5-fluoro-2-methylbenzoxazole (#20) is not shown due to a large deviation (−8.6 kJ·mol−1). For 5-fluorouracil (#1) and (trifluoromethyl)benzene (#17) only the most recent values for laboratories are shown.
The results from the Moscow and Teddington labs do demonstrate reasonable consistency. On the other hand, the values from Freiburg exhibit a scatter of about ±6 kJ·mol−1 around the average value for this laboratory. These data as well as those from Windsor where a less precise technique was used were excluded from the training set. Four out of six values from the Bartesville lab and five out of nine values from the Porto lab show significant positive deviation from the average. It implies that the experimental ΔfHom is more negative than the expected computed counterpart. Two reasons may be responsible for the observed behavior. First, the samples could contain incompletely fluorinated impurities. This may also explain why not all results from these laboratories are biased. Second, the reduction to the standard state could be based on obsolete reference values. The samples of 4-fluorobenzoic acid (#16) from the Bartesville lab were further studied in the Teddington lab and at the National Bureau of Standards and (1.5 to 2.3) kJ·mol−1 more positive values were obtained. At the same time, the results from these and two other laboratories are consistent within ±0.5 kJ·mol−1 (Table 2). The enthalpies of formation for non-fluorinated compounds from the Porto and Bartesville labs are normally consistent with the results from the other laboratories and the predicted values. Thus, it seems highly unlikely that the problem is due to the instrumental error such as incorrect calibration, etc. Further clarification of this problem clearly goes beyond the scope of this paper. All results from these two laboratories exceeding +2 kJ·mol−1 deviation were excluded from the training data set. If several consistent ΔfHom were available for a compound, the weight-average was calculated with the weights determined from uncertainties of the enthalpies of combustion. The resulting values used in the training set are shown in bold in Table 2.
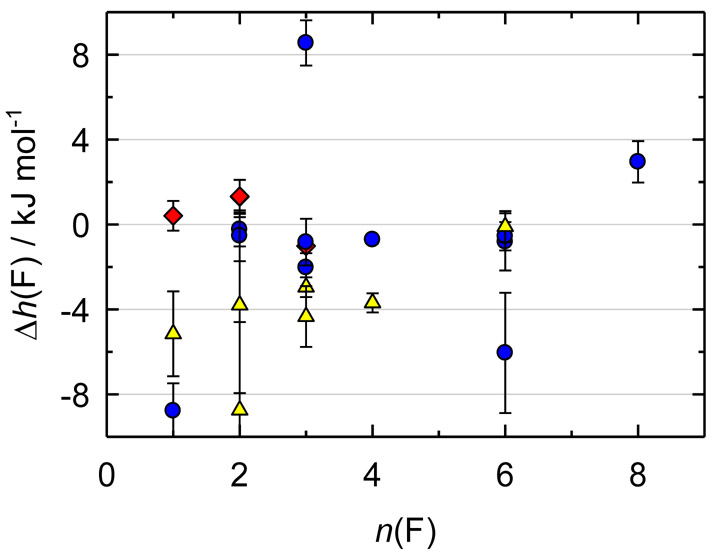
The data scatter for small fluorinated organics (Figure 2) is larger than that for the compounds of group 1, with Δh(F) generally within ±9 kJ·mol−1. Seven out of twelve saturated compounds are grouped in the range −(0.2 to 2.0) kJ·mol−1. Six of these values were obtained from combustion calorimetry and one from reaction calorimetry. Combustion calorimetry was used to derive only one outlying value, where a thermodynamic cycle also involved the results from a high-temperature hydrogen reaction calorimetry. Therefore, for the saturated compounds, combustion calorimetry was the most reliable technique despite all difficulties associated with quantification of the combustion process for gaseous species. Unlike the condensed-phase compounds, the exact amount of a gas introduced to the bomb is unknown and only chemical analysis of the products allows one to determine the burnt amount of the sample.
Figure 2.
Relative effective enthalpy, Δh(F) / (kJ·mol−1) = h(F) / (kJ·mol−1) + 261711.5, of a fluorine atom for fluoroalkanes (blue circles), fluoroalkenes (yellow triangles), and inorganic molecules (red diamonds). The value for 2-fluoropropane (−22 kJ·mol−1) is not shown. For tetrafluoroethylene, the value is a weight-average of the results91,95
Five values for the fluorinated alkenes lie close to −4 kJ·mol−1. Four of these values are based on the combustion calorimetry results from the Moscow lab. Different methods were used for tetrafluoroethylene (n = 4), which is the fifth consistent compound. However, based on the high-level ab initio calculations, ΔfHom of the latter has been demonstrated to have a large error (see, for example, Ref. 9). The combination of this fact with the results in Figure 2 leads to a conclusion that the remaining four experimental values have a similar systematic error per fluorine atom. A possible explanation of this problem might be either incorrect characterization of the combustion products or incorrect reduction to the standard state. The value for perfluoropropene is consistent with those of the saturated compounds. This h(F) was derived in a different way: it is based on a thermodynamic cycle involving the results of combustion calorimetry from the Moscow lab and high-temperature chlorination calorimetry.
Based on the analysis above, we selected the consistent experimental ΔfHom of the saturated compounds and perfluoropropene for further analysis. The results for inorganic compounds are close to those of small fluorinated alkanes (Figure 2).
3.3. Effective enthalpy of the fluorine atom for ab initio calculations
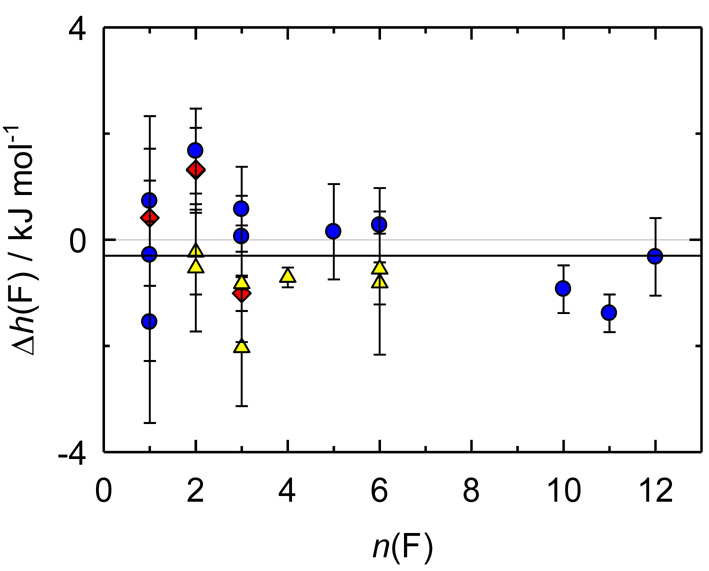
Data consistency for the training dataset is demonstrated in Figure 3. At a small number of fluorine atoms, the results for group 1 are typically higher than those for group 2. For perfluorinated compounds of group 1 except perfluorobenzene, h(F) values are close to those of group 2. The exact reason of this behavior is unknown; however, based on our limited tests, we believe that this is mainly due to the insufficient theory level for ZPVE calculations.
Figure 3.
Relative effective enthalpy, Δh(F) / (kJ·mol−1) = h(F) / (kJ·mol−1) + 261711.5, of a fluorine atom for group 1 (blue circles), small hydrofluorocarbons (yellow triangles), and inorganic molecules (red diamonds). The black line shows the resulting h(F) value.
The resulting h(F) = −(261711.80 ± 0.37) kJ·mol−1 was found by unweighted averaging all h(F) values. The enthalpies of formation calculated with this effective enthalpy are presented in Tables 2 and 3. The uncertainty (in kJ·mol−1) for compounds can be estimated as follows:
| (2) |
The first term in the right-hand side of eq 2 is a contribution associated with C, H, O, and N atoms, which can be calculated as described earlier.12 This contribution considers uncertainty of the model as well as uncertainties of the effective enthalpies of atoms. For the considered compounds, U(CHON) is close to (2.5 to 3.0) kJ·mol−1. Eq 2 does not consider uncertainties in the reference enthalpies of formation for CO2, H2O, HF(aq), etc. The uncertainty of h(F) could be decreased if repeated consistent measurements for some reference compounds were available, which is unlikely with current experimental infrastructure. Also, predictions could be improved through the development of efficient and reliable computational procedures for anharmonic ZPVE, which appears to be a limiting factor for the considered group of compounds.
4. Conclusion
An ab initio method for prediction of the enthalpies of formation has been extended to fluorinated compounds. Additional uncertainty associated with the presence of fluorine was estimated to be about 1 kilojoule per mole per F atom. Analysis of the experimental data with the use of the computed results revealed significant problems in the former. These included systematic errors of ΔfHom in some laboratories and a shortage of reliable ΔfHom values for fluoroalkenes. The observed agreement between the experimental and computed values was somewhat worse than for CHON compounds. If no additional information is available, 5n(F) kJ·mol−1 should be considered as a conservative estimate of the expanded uncertainty for the experimental values of ΔfHom of medium-size fluorinated organic compounds. For small hydrofluorocarbons, this uncertainty should be close to 9n(F) kJ·mol−1.
Acknowledgement
Contribution of the U.S. National Institute of Standards and Technology and not subject to copyright in the United States. Trade names are provided only to specify procedures adequately and do not imply endorsement by the National Institute of Standards and Technology. Similar products by other manufacturers may be found to work as well or better.
Footnotes
The authors declare no competing financial interest.
References
- 1.Organofluorine Chemistry: Principles and Commercial Applications. Banks RE, Smart BE, Tatlow JC, Eds. Plenum Press: New York, 1994. [Google Scholar]
- 2.Maienfisch P; Hall RG The Importance of Fluorine in the Life Science Industry. Chimia 2004, 58, 93–99. [Google Scholar]
- 3.Okazoe T Overview on the history of organouorine chemistry from the viewpoint of material industry. Proc. Jpn. Acad., Ser. B 2009, 85, 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Good WD; Scott DW Combustion in a Bomb of Organic Fluorine Compounds In: Experimental Thermochemistry. Vol. II; Skinner HA, Ed.; Interscience Publishers: New York, 1962; pp 15–39. [Google Scholar]
- 5.Head AJ; Good WD Combustion of Liquid/Solid Organic Compounds with Non-Metallic Hetero-Atoms. Compounds Containing Fluorine In: Experimental Chemical Thermodynamics. Vol. 1. Combustion Calorimetry; Sunner S, Månsson M, Eds.; Pergamon Press: New York, 1979, pp 9-5–9-7. [Google Scholar]
- 6.Krech M; Price SJW; Yared WF Determination of the Heat of Formation of Hexafluorobenzene. Can. J. Chem 1972, 50, 2935–2938. [Google Scholar]
- 7.Johnson GK; Smith PN; Hubbard WN The Enthalpies of Solution and Neutralization of HF(l); Entahlpies of Dilution and Derived Thermodynamic Properties of HF(aq). J. Chem. Thermodyn 1973, 5, 793–809. [Google Scholar]
- 8.Cox JD; Wagman DD; Medvedev VA CODATA Key Values for Thermodynamics; Hemisphere Publishing Corp, New York, 1989. [Google Scholar]
- 9.Feller D; Peterson KA; Dixon DA Ab Initio Coupled Cluster Determination of the Heats of Formation of C2H2F2, C2F2, and C2F4. J. Phys. Chem. A 2011, 115, 1440–1451. [DOI] [PubMed] [Google Scholar]
- 10.Csontos J; Rolik Z; Das S; Kállay M High-Accuracy Thermochemistry of Atmospherically Important Fluorinated and Chlorinated Methane Derivatives. J. Phys. Chem. A 2010, 114, 13093–13103. [DOI] [PubMed] [Google Scholar]
- 11.Paulechka E; Kazakov A Efficient DLPNO–CCSD(T)-Based Estimation of Formation Enthalpies for C-, H-, O-, and N-Containing Closed-Shell Compounds Validated Against Critically Evaluated Experimental Data. J. Phys. Chem. A 2017, 121, 4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulechka E; Kazakov A Efficient Estimation of Formation Enthalpies for Closed-Shell Organic Compounds with Local Coupled-Cluster Methods. J. Chem. Theory Comput 2018, 14, 5920–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazakov A; Muzny CD; Diky V; Chirico RD; Frenkel M Predictive correlations based on large experimental datasets: Critical constants for pure compounds. Fluid Phase Equilib. 2010, 298, 131–142. [Google Scholar]
- 14.Kazakov A; McLinden MO; Frenkel M Computational Design of New Refrigerant Fluids Based on Environmental, Safety, and Thermodynamic Characteristics. Ind. Eng. Chem. Res 2012, 51, 12537–12548. [Google Scholar]
- 15.Paulechka E; Diky V; Kazakov A; Kroenlein K; Frenkel M Reparameterization of COSMO-SAC for Phase Equilibrium Properties Based on Critically Evaluated Data. J. Chem. Eng. Data 2015, 60, 3554–3561. [Google Scholar]
- 16.Carande WH; Kazakov A; Muzny C; Frenkel M Quantitative Structure-Property Relationship Predictions of Critical Properties and Acentric Factors for Pure Compounds. J. Chem. Eng. Data 2015, 60, 1377–1387. [Google Scholar]
- 17.Halgren TA Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem 1996, 17, 490–519. [Google Scholar]
- 18.Becke AD Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys 1993, 98, 5648–5652. [Google Scholar]
- 19.Stephens PJ; Devlin FJ; Chabalowski CF; Frisch MJ Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem 1994, 98, 11623–11627. [Google Scholar]
- 20.Weigend F; Ahlrichs R Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys 2005, 7, 3297–3305. [DOI] [PubMed] [Google Scholar]
- 21.Grimme S; Ehrlich S; Goerigk L Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem 2011, 32, 1456–1465. [DOI] [PubMed] [Google Scholar]
- 22.Johansson M; Veryazov V Automatic procedure for generating symmetry adapted wavefunctions. J. Cheminf 2017, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunning TH Jr Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys 1989, 90, 1007–1023. [Google Scholar]
- 24.Kendall RA; Dunning TH Jr; Harrison RJ Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys 1992, 96, 6796–6806. [Google Scholar]
- 25.Nagy PR; Kállay M Optimization of the linear-scaling local natural orbital CCSD(T) method: Redundancy-free triples correction using Laplace transform. J. Chem. Phys 2017, 146, 214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy PR; Samu G; Kállay M An Integral-Direct Linear-Scaling Second-Order Møller-Plesset Approach. J. Chem. Theory Comput 2016, 12, 4897–4914. [DOI] [PubMed] [Google Scholar]
- 27.Kállay M; Rolik Z; Csontos J; Nagy P; Samu G; Mester D; Csóka J; Szabó B; Ladjánszki I; Szegedy L; Ladóczki B; Petrov K; Farkas M; Mezei PD; Hégely B Mrcc, a quantum chemical program suite, 2017. https://www.mrcc.hu, accessed: April 6, 2019. [Google Scholar]
- 28.Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA et al. Gaussian 09 Revision D.01. Gaussian Inc, Wallingford CT, 2013. [Google Scholar]
- 29.Parrish RM; Burns LA; Smith DGA; Simmonett AC; DePrince AE; Hohenstein EG; Bozkaya U; Sokolov AY; Di Remigio R; Richard RM et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput 2017, 13, 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolesov VP; Papina TS Standard Enthalpy of Formation of Vinyl Fluoride. Russ. J. Phys. Chem 1970, 44, 611–613. [Google Scholar]
- 31.NIST ThermoData Engine Version 10.3 - Pure Compounds, Binary Mixtures and Reactions NIST Standard Reference Database 103b, Gaithersburg, MD, 2016. https://www.nist.gov/srd/nist-standard-reference-database-103b, accessed: April 6, 2019. [Google Scholar]
- 32.Greenberg E; Hubbard WN Fluorine Bomb Calorimetry. XXIII. The Enthalpy of Formation of Carbon Tetrafloride. J. Phys. Chem 1968, 72, 222–227. [Google Scholar]
- 33.Sinke GC Heat of Reaction of Hydrogen and Nitrogen Trifluoride. J. Chem. Eng. Data 1965, 10, 295–296. [Google Scholar]
- 34.Sinke GC The Enthalpy of Dissociation of Nitrogen Trifluoride. J. Phys. Chem 1967, 71, 359–360. [Google Scholar]
- 35.Gurvich LV; Veits IV; Medvedev VA; Bergman GA; Yungman VS; Khachkuruzov GA; Iorish VS; Dorofeeva OV; Osina EL; Tolmach PI et al. Thermodynamic Properties of Individual Substances. Vol. 4, Parts 1 and 2; Glushko VP, Ed.; Nauka: Moscow, 1981–1982. [Google Scholar]
- 36.Ruscic B Active Thermochemical Tables (ATcT) values based on ver. 1.122d of the Thermochemical Network (2018). https://atct.anl.gov, accessed: April 6, 2019. [Google Scholar]
- 37.Domalski ES; Armstrong GT The Heats of Combustion of Polytetrafluoroethylene (Teflon) and Graphite in Elemental Fluorine. J. Res. Natl. Bur. Stand 1967, 71A, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox JD; Gundry HA; Head AJ Thermodynamic Properties of Fluorine Compounds. Part 4. – Enthalpy of Formation of Carbon Tetrafluoride. Trans. Faraday Soc 1965, 61, 1594–1600. [Google Scholar]
- 39.Scott DW; Good WD; Waddington G Heat of Formation of Tetrafluoromethane from Combustion Calorimetry of Polytetrafluoroethylene. J. Am. Chem. Soc 1955, 77, 245–246. [Google Scholar]
- 40.Good WD; Scott DW; Waddington G Combustion Calorimetry of Organic Fluorine Compounds by a Rotating-Bomb Method. J. Phys. Chem 1956, 60, 1080–1089. [Google Scholar]
- 41.Ganyecz Á; Kállay M; Csontos J High Accuracy Quantum Chemical and Thermochemical Network Data for the Heats of Formation of Fluorinated and Chlorinated Methanes and Ethanes. J. Phys. Chem. A 2018, 122, 5993–6006. [DOI] [PubMed] [Google Scholar]
- 42.Ganyecz Á; Kállay M; Csontos J Moderate-Cost Ab Initio Thermochemistry with Chemical Accuracy. J. Chem. Theory Comput 2017, 13, 4193–4204. [DOI] [PubMed] [Google Scholar]
- 43.Szterner P; Kaminski M; Zielenkiewicz A Vapour Pressures, Molar Enthalpies of Sublimation and Molar Enthalpies of Solution in Water of Five Halogenated Derivatives of Uracil. J. Chem. Thermodyn 2002, 34, 1005–1012. [Google Scholar]
- 44.Brunetti B; Portalone G; Piacente V Sublimation Thermodynamic Parameters for 5-Fluorouracil and Its 1-Methyl and 1,3-Dimethyl Derivatives from Vapor Pressure Measurements. J. Chem. Eng. Data 2002, 47, 17–19. [Google Scholar]
- 45.Ribeiro da Silva MAV; Amaral LMPF; Szterner P Experimental Thermochemical Study of Fluoro-, Chloro-, and Bromo-Derivatives of Uracil. J. Chem. Thermodyn 2012, 52, 30–35. [Google Scholar]
- 46.Amaral LMPF; Szterner P; Miranda MS; Ribeiro da Silva MAV Enthalpy of Formation of 5-Fluoro-1,3-Dimethyluracil: 5-Fluorouracil Revisited. J.Chem. Thermodyn 2014, 75, 106–115. [Google Scholar]
- 47.Good WD; Todd SS; Messerly JF; Lacina JL; Dawson JP; Scott DW; McCullough JP Perfluoropiperidine: Entropy, Heat of Formation, and Vapor Pressure; N─F Bond Energy; and Solid-State Transitions. J. Phys. Chem 1963, 67, 1306–1311. [Google Scholar]
- 48.Irving RJ; Ribeiro da Silva MAV Enthalpies of Vaporization of Some β-Diketones. J. Chem. Soc., Dalton Trans 1975, 798–800. [Google Scholar]
- 49.Erastov PA; Kolesov VP; Igumenov IK Standard Enthalpies of Formation of Some Fluorinated β-Diketones. Russ. J. Phys. Chem 1984, 58, 1311–1313. [Google Scholar]
- 50.Ribeiro da Silva MAV; Gonçalves JM Standard Molar Enthalpies of Formation of Nine Fluorinated β-Diketones by Rotating Bomb Calorimetry. J. Chem. Thermodyn 1997, 29, 253–260. [Google Scholar]
- 51.Cox JD; Gundry HA; Head AJ Thermodynamic Properties of Fluorine Compounds. Part 1. – Heats of Combustion of p-Fluorobenzoic Acid, Pentafluorobenzoic Acid, Hexafluorobenzene and Decafluorocyclohexene. Trans. Faraday. Soc 1964, 60, 653–665. [Google Scholar]
- 52.Price SJW; Sapiano HJ Determination of ΔHof298(C6F10,g) and ΔHof298(C6F12,g) from Studies of the Combustion of Decafluorocyclohexene and Dodecafluorocyclohexane in Oxygen and Calculation of the Resonance Energy of Hexafluorobenzene. Can. J. Chem 1979, 57, 685–688. [Google Scholar]
- 53.Zhogina EV; Papina TS; Kolesov VP; Gervits LL; Makarov KN; Vorob’eva VP Stnadard Enthalpies of Formation of Hexafluoropropene Oligomers. Russ. J. Phys. Chem 1990, 64, 1514–1517. [Google Scholar]
- 54.Cox JD; Gundry HA; Harrop D; Head AJ Thermodynamic Properties of Fluorine Compounds. 9. Enthalpies of Formation of Some Compounds Containing the Pentafluorophenyl Group. J. Chem. Thermodyn 1969, 1, 77–87. [Google Scholar]
- 55.Steele WV; Chirico RD; Knipmeyer SE; Nguyen A Vapor Pressure, Heat Capacity, and Density along the Saturation Line, Measurements for Dimethyl Isophthalate, Dimethyl Carbonate, 1,3,5-Triethylbenzene, Pentafluorophenol, 4-tert-Butylcatechol, r-Methylstyrene, and N,N′-Bis(2-hydroxyethyl)ethylenediamine. J. Chem. Eng. Data 1997, 42, 1008–1020. [Google Scholar]
- 56.Andon RJL; Counsell JF; Hales JL; Lees EB; Martin JF Thermodynamic Properties of Fluorine Compounds. Part VII. Heat Capacity and Entropy of Pentafluorochlorobenzene and Pentafluorophenol. J. Chem. Soc. A 1968, 2357–2361. [Google Scholar]
- 57.Ribeiro da Silva MAV; Monte MJS; Lobo Ferreira AIMC; Oliveira JASA; Cimas Á Experimental and Computational Thermodynamic Study of Three Monofluoronitrobenzene Isomers. J. Phys. Chem. B 2010, 114, 7909–7919. [DOI] [PubMed] [Google Scholar]
- 58.Good WD; Lacina JL; Scott DW; McCullough JP Combustion Calorimetry of Organic Fluorine Compounds. The Heats of Combustion and Formation of the Difluorobenzene, 4-Fluorotoluene and m-Trifluorotoluic Acid. J. Phys. Chem 1962, 66, 1529–1532. [Google Scholar]
- 59.Santos AFLOM; Ribeiro da Silva MAV Experimental and Computational Energetic Study of Two Halogenated 2-Acetylpyrrole Derivatives: 2-Trichloroacetylpyrrole and 2-Trifluoroacetylpyrrole. J. Chem. Thermodyn 2010, 42, 1079–1086. [Google Scholar]
- 60.Ribeiro da Silva MAV; Ferreira AIMCL; Gomes JRB Combined Experimental and Computational Study of the Thermochemistry of the Fluoroaniline Isomers. J. Phys. Chem. B 2007, 111, 2052–2061. [DOI] [PubMed] [Google Scholar]
- 61.Brunetti B; Irrera S; Portalone G Sublimation Enthalpies of 5-Haloderivatives of 1,3-Dimethyluracil. J. Chem. Eng. Data 2015, 60, 74–81. [Google Scholar]
- 62.Schaffer F; Verevkin SP; Rieger H-J; Beckhaus H-D; Rüchardt C Enthalpies of Formation of a Series of Fluorinated Hydrocarbons and Strain-Free Group Increments to Assess Polar and Anomeric Stabilization and Strain. Liebigs Ann. 1997, 7, 1333–1344. [Google Scholar]
- 63.Ribeiro da Silva MAV; Monte MJS; Rocha IM; Cimas Á Energetic Study Applied to the Knowledge of the Structural and Electronic Properties of Monofluorobenzonitriles. J. Org. Chem 2012, 77, 4312–4322. [DOI] [PubMed] [Google Scholar]
- 64.Johnson WH; Prosen EJ The Enthalpies of Combustion and Formation of Ortho- and Parafluorobenzoic Acid. J. Res. Natl. Bur. Stand 1975, 79A, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro da Silva MAV; Ferreira AIMCL; Gomes JRB Experimental and Computational Study of the Thermochemistry of the Fluoromethylaniline Isomers. J. Phys. Chem. B 2007, 111, 6444–6451. [DOI] [PubMed] [Google Scholar]
- 66.Zherikova KV; Svetlov AA; Kuratieva NV; Verevkin SP Structure-Property Relationships in Halogenbenzoic Acids: Thermodynamics of Sublimation, Fusion, Vaporization and Solubility. Chemosphere 2016, 161, 157–166. [DOI] [PubMed] [Google Scholar]
- 67.Good WD; Lacina JL; DePrater BL; McCullough JP A New Approach to the Combustion Calorimetry of Silicon and Organosilicon Compounds. Heats of Formation of Quartz, Fluorosilicic Acid, and Hexamethyldisiloxange. J. Phys. Chem 1964, 68, 579–586. [Google Scholar]
- 68.Kolesov VP; Ivanov LS; Skuratov SM The Standard Enthalpy of Formation of 2,2,2-Trifluoroethanol. Russ. J. Phys. Chem 1971, 45, 303–305. [Google Scholar]
- 69.Scott DW; Messerly JF; Todd SS; Hossenlopp IA; Douslin DR; McCullough JP 4-Fluorotoluene: Chemical Thermodynamic Properties, Vibrational Assignment, and Internal Rotation. J. Chem. Phys 1962, 37, 867–873. [Google Scholar]
- 70.Ribeiro da Silva MAV; Lobo Ferreira AIMC Experimental and Computational Study on the Molecular Energetics of the Three Monofluoroanisole Isomers. J. Chem. Thermodyn 2009, 41, 361–366. [Google Scholar]
- 71.Silva ALR; Gonçalves JM; Ribeiro da Silva MDMC Experimental and Computational Thermochemical Study of Two Fluorobenzazoles: 2-Fluoro-2-methylbenzoxazole and 5-Fluoro-2-methylbenzothiazole. J. Chem. Thermodyn 2018, 120, 157–163. [Google Scholar]
- 72.Zielenkiewicz W; Kozbiał M; Swierzewski R; Szterner P Heat Capacities of Uracil, Thymine, and Its Alkylated, Cyclooligomethylenated, and Halogenated Derivatives by Differential Calorimetry. J. Chem. Eng. Data 2007, 52, 93–97. [Google Scholar]
- 73.Paukov IE; Lavrent’eva MN; Anisimov MP Heat Capacity, Phase Transitions, Entropy, and Enthalpy of Pentafluorophenol at Low Temperatures. Russ. J. Phys. Chem 1969, 43, 436–438. [Google Scholar]
- 74.Osborn AG; Scott DW Vapor Pressures of 17 Miscellaneous Organic Compounds. J. Chem. Thermodyn 1980, 12, 429–438. [Google Scholar]
- 75.Chirico RD; Kazakov AF; Bazyleva A; Diky V; Kroenlein K; Emel’yanenko VN; Verevkin SP Critical Evaluation of Thermodynamic Properties for Halobenzoic Acids Through Consistency Analyses for Results from Experiment and Computational Chemistry. J. Phys. Chem. Ref. Data 2017, 46, 023105. [Google Scholar]
- 76.Neugebauer CA; Margrave JL The Heats of Formation of CHF3 and CH2F2. J. Phys. Chem 1958, 62, 1043–1048. [Google Scholar]
- 77.Kolesov VP; Shtekher SN; Martynov AM; Skuratov SM Standard Enthalpy of Formation of 1,1-Difluoroethane. Russ. J. Phys. Chem 1968, 42, 975–976. [Google Scholar]
- 78.Kolesov VP; Martynov AM; Skuratov SM Standard Enthalpy of Formation of 1,1,1-Trifluoroethane. Russ. J. Phys. Chem 1965, 39, 223–225. [Google Scholar]
- 79.Kolesov VP; Papina TS Thermochemistry of Haloethanes. Russ. Chem. Rev 1983, 52, 425–439. [Google Scholar]
- 80.Erastov PA; Kolesov VP; Dityaeva LN; Golovanova Yu. G. The Enthalpy of Formation of 1,1,2-Trifluoro-1,2,2-trichloroethane. J. Chem. Thermodyn 1981, 13, 663–669. [Google Scholar]
- 81.Papina TS; Erastov PA; Kolesov VP The Enthalpies of Formation of 1,1,1-Trifluoro-2-chloro-2-bromoethane and 1,1,2-Trifluoro-2-chloro-1-bromoethane. J. Chem. Thermodyn 1981, 13, 683–689. [Google Scholar]
- 82.Erastov PA; Kolesov VP Enthalpy of Formation of Trifluorochloroethene. J. Chem. Thermodyn 1982, 14, 103–106. [Google Scholar]
- 83.Lacher JR; Kianpour A; Oetting F; Park JD Reaction Calorimetry. The Hydrogenation of Organic Fluorides and Chlorides. Trans. Farady Soc 1956, 52, 1500–1508. [Google Scholar]
- 84.Kirkbride FW; Davidson FG Heats of Formation of Gaseous Fluoro- and Fluorochloro-Carbons. Nature 1954, 174, 79–80. [Google Scholar]
- 85.Sinke GC The Heat of Reaction of Nitrogen Trifluoride and Hexafluoroethane. J. Phys. Chem 1966, 70, 1326–1327. [Google Scholar]
- 86.Lacher JR; Kianpour A; Park JD Reaction Heats of Organic Halogen Compounds. VI. The Catalytic Hydrogenation of Some Alkyl Fluorides. J. Phys. Chem 1956, 60, 1454–1455. [Google Scholar]
- 87.Williamson AD; LeBreton PR; Beauchamp JL Photoionization Mass Spectrometry of 2-Fluoropropane and 2,2-Difluoropropane. A Novel Determination of the Proton Affinity of Vinyl Fluoride and 1,1-Difluoroethylene. J. Am. Chem. Soc 1976, 98, 2705–2709. [Google Scholar]
- 88.Kolesov VP Determination of the Enthalpies of Formation of Selected Organo-fluorine and -chlorine Compounds. Proc. I Int. Conf. on Calorimetry and Thermodynamics. August 31-September 4, 1969, pp. 305–310. [Google Scholar]
- 89.Kolesov VP; Kozina MP Thermochemistry of Organic and Organohalogen Compounds. Russ. Chem. Rev 1986, 55, 912–928. [Google Scholar]
- 90.Kolesov VP; Talakin OG; Skuratov SM Standard Enthalpy of Formation of Perfluoropropane and Enthalpies of Formation of Normal Perfluoroalkanes. Vestnik Mosk. Univ., Ser. 2: Khim, 1967, 22, 60–66. [Google Scholar]
- 91.Neugebauer CA; Margrave JL The Heats of Formation of Tetrafluoroethylene, Tetrafluoromethane and 1,1-Difluoroethylene. J. Phys. Chem 1956, 60, 1318–1321. [Google Scholar]
- 92.Kolesov VP; Martynov AM; Shtekher SM; Skuratov SM Standard Entahlpies of Formation of 1,1-Difluoroethylene and of Trifluoroethylene. Russ. J. Phys. Chem 1962, 36, 1118–1120. [Google Scholar]
- 93.Wartenberg H; Schiefer J Bildungswärmen von Fluor-Chlor-Kohlenstoff-Verbindungen. Z. Anorg. Chem 1955, 278, 326–332. [Google Scholar]
- 94.Duus HC Thermochemical Studies on Fluorocarbons. Heat of Formation of CF4, C2F4, C3F6, C2F4 Dimer, and C2F4 Polymer. Ind. Eng. Chem 1955, 47, 1445–1449. [Google Scholar]
- 95.Kolesov VP; Zenkov ID; Skuratov SM The Standard Enthalpy of Formation of Tetrafluoroethylene. Russ. J. Phys. Chem 1962, 36, 45–47. [Google Scholar]
- 96.Abell PI; Adolf PK HBr Catalyzed Photoisomerization of Allyl Halides. J. Chem. Thermodyn 1969, 1, 333–338. [Google Scholar]
- 97.Dolbier WR; Medinger KS; Greenberg A; Liebman JF The Thermodynamic Effect of Fluorine as a Substituent. Vinylic Di- and Monofluoromethylene and Allylic Difluorovinyl. Tetrahedron 1982, 38, 2415–2420. [Google Scholar]
- 98.Kolesov VP; Martynov AM; Skuratov SM Standard Enthalpy of Formation of 1,1,1-Trifluoropropene. Russ. J. Phys. Chem 1967, 41, 482–484. [Google Scholar]
- 99.Papina TS; Kolesov VP; Golovanova, Yu. G. Standard Enthalpies of Formation of 1,2-Dichlorohexafluoropropane and Hexafluoropropene. Russ. J. Phys. Chem 1987, 61, 1168–1170. [Google Scholar]
- 100.Lacher JR; McKinley JJ; Walden C; Lea K; Park JD Reaction Heats of Organic Fluorine Compounds. II. The Vapor Phase Heats of Chlorination of Some Simple Fluoroölefins. J. Am. Chem. Soc 1949, 71, 1334–1337. [Google Scholar]
- 101.Rogers AS; Ford WGF Analysis of the Kinetics of the Thermally and Chemically Activated Elimination of HF from 1,1,1-Trifluoroethane: the C─C Bond Dissociation Energy and the Heat of Formation of 1,1,1-Trifluoroethane. Int. J. Chem. Kinet 1973, 5, 965–975. [Google Scholar]
- 102.Buckley GS; Ford WGF; Rodgers AS The Gas Phase Thermochemistry of the Reaction: C2F5Br + I2 = C2F5I + IBr. Thermochim. Acta 1981, 49, 199–205. [Google Scholar]
- 103.Coomber JW; Whittle E Bond Dissociation Energies from Equilibrium Studies. Trans. Faraday Soc 1967, 63, 608–619. [Google Scholar]
- 104.Wu E-C; Pickard JM; Rodgers AS Thermochemistry of the Gas-Phase Reaction CF2=CF2 + I2 = CF2ICF2I. Heat of Formation of 1,2-Diiodoperfluoroethane and of Iodoperfluoroethane. J. Phys. Chem 1975, 79, 1078–1081. [Google Scholar]
- 105.Majer V; Svoboda V; Pošta A; Pick J Determination of Heats of Vaporization and Some Other Thermodynamic Quantities for Several Fluorinated Halogen Derivatives of Ethane and Propane. Collect. Czechosolov. Chem. Commun 1979, 45, 3063–3068. [Google Scholar]
- 106.King RC; Armstrong GT Constant Pressure Flame Calorimetry with Fluorine. II. The Heat of Formation of Oxygen Difluoride. J. Res. Natl. Bur. Stand 1968, 72A, 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]