INTRODUCTION
Growth and feed efficiency of cattle are improved by supplementation with the beta-adrenergic agonists (βAA), ractopamine hydrochloride (RH; β 1AA) or zilpaterol hydrochloride (ZH; β 2AA) (Elam et al., 2009). βAA supplementation alters adipose deposition by inhibiting fatty acid biosynthesis and promoting lipolysis of stored triacylglycerols into free fatty acids (FFAs) (Johnson et al., 2014). However, β 2 adrenoceptors (βAR) desensitize with chronic activation (Re et al., 1997); supplementation is thus limited to the last 20 to 40 d of feeding.
The annual economic impact of heat stress (HS) has been estimated to exceed $2.4 billion (St-Pierre et al., 2003). Heat-stressed livestock have reduced growth rates, dry matter intake, and average daily gain (Mitlöhner et al., 2001; St-Pierre et al., 2003). In response to acute stress, signaling pathways for lipolysis of circulating and stored triglycerides are activated, while chronic stress increases lipogenesis and adipogenesis (Campbell et al., 2009; Peckett et al., 2011). In cattle, HS also increases the responsiveness of adipocytes to lipolytic signals, increasing lipolysis (Faylon et al., 2015).
The objective of this study was to understand how HS and βAA independently and interactively affect adipose tissue. Prior work identified minimal impact of RH on metabolic properties (Barnes et al., 2019) and on the transcriptome of skeletal muscle (Kubik et al., 2018). We therefore hypothesized that RH may be primarily affecting adipose; specifically, that lipolytic activity is increased due to heat and βAA in an additive fashion. We tested this hypothesis in RH-supplemented lambs and ZH-supplemented cattle exposed to HS for 30 and 21 d, respectively.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln, which is accredited by AAALAC International.
For study 1, wether lambs were fed a high-energy feedlot diet for 30 d under thermal neutral (TN; Temperature Humidity Index [THI] = 65; n = 14) or HS (THI = 80; n = 12) conditions and supplemented with ractopamine HCl (RH; 60 mg/head/d) or none (NS) in a 2 × 2 factorial. Thermal neutral lambs were pair-fed to the average intake of HS lambs. At harvest, subcutaneous fat was flash frozen. Poly-A+ selected libraries were sequenced from isolated RNA using 150 bp, paired-end reads to a minimum depth of 20 million reads/sample (Michigan State University). After quality control, transcripts as annotated in Oar_rambouillet_v1.0 were quantified (STAR; Dobin et al., 2013). Differential expression (DE) analyses were performed in DESeq2 (Love et al., 2014) with a significance threshold False Discovery Rate (FRD) of 0.05. Exploration of data was performed with DAVID (Huang et al., 2009a, 2009b). To study the main effects of environment and supplement, loci with a significant interaction were removed and the remaining loci reevaluated. Pathway analysis (Qiagen) was conducted on all loci with raw P < 0.05 for the main effects.
For study 2, Red Angus-based steers were fed a high-energy diet for 21 d under TN (n = 12; pair-fed to HS average) or HS (THI = 83; n = 12) conditions and supplemented with zilpaterol hydrochloride (ZH; 8.38 mg/kg DM/d) or none (NS) in a 2 × 2 factorial. At harvest, visceral adipose was flash frozen to determine fatty acid mobilization in a method adapted from Raclot and Groscolas (1993). Briefly, modified Krebs Ringer buffer (MKRB) was made (9 g of KRB [Sigma], 900 mL ddH2O, 15 mM NaHCO3, 2.5 mM CaCl2 dihydrate, and 4% fatty acid free BSA, pH adjusted to 7.4 and sterilized). Visceral fat was minced, strained (200 µM), washed (37 °C MKRB), and minced again. Adipose (400 ± 10 mg) was added to 5 mL MKRB containing 0 or 1 µM epinephrine. Samples were incubated in a shaking water bath (2 h, 37 °C), and the media filtered (2.4 cm glass microfiber filter) and stored at −80 °C. Free fatty acids were quantified by colorimetric detection (Sigma Aldrich Free Fatty Acid Quantification Kit), read at 570 nm (BioTek EPOCH). Concentrations were determined using a 0 to 4 nmol/µL standard curve for palmitic acid and analyzed using the PROC MIXED procedure (SAS Institute Inc., Cary, NC). P < 0.05 were considered significant.
RESULTS
Seventy-one loci were DE (Padj < 0.05) due to a temperature by supplement interaction. Differential expression loci with the greatest logFC include G-protein-coupled receptor, GPRC5A, and others involved in G-protein receptor signaling (e.g., SAA1, NPW, and NGEF; Table 1). When loci DE due to interaction were removed, RBM3 and ATXN7L1 were DE (Padj < 0.1) due to temperature (Table 2). No loci were DE due to supplement. Pathway analysis predicted the “Adipogenesis Pathway” to be altered (P < 0.001) but without clear directionality of dysregulation. The top regulator effect networks included the biological functions: concentration of fatty acid, molecule transport, and cell migration (Table 3).
Table 1.
Loci DE due to an interaction of environment and supplement in adipose of wether lambs
| Gene ID | P-value | Adjusted P-value |
|---|---|---|
| GPRC5A | 1.10E−07 | <0.001 |
| FOSB | 1.43E−04 | 0.033 |
| FOS | 1.92E−06 | 0.002 |
| SAA2 | 1.09E−04 | 0.023 |
| SERPINF2 | 6.63E−08 | <0.001 |
| SAA1 | 3.31E−05 | 0.012 |
| NPW | 2.33E−04 | 0.048 |
| FMOD | 4.08E−05 | 0.014 |
| C1QTNF3 | 2.55E−06 | 0.002 |
| SFRP4 | 8.97E−07 | 0.001 |
| TPSB2 | 2.09E−05 | 0.008 |
| LTC4S | 449E−09 | <0.001 |
| NGEF | 9.53E−05 | 0.025 |
| IL34 | 3.23E−05 | 0.012 |
Table 2.
Loci DE in wether lamb adipose due to HS
| Gene ID | Log2fold change | P-value | Adjusted P-value |
|---|---|---|---|
| RBM3 | −0.888 | 2.53E−07 | 0.006 |
| ATXN7L1 | 0.550 | 1.06E−05 | 0.10 |
Table 3.
Top regulator effect networks in the adipose transcriptome due to HS
| Regulators | Disease and functions | Consistency score |
|---|---|---|
| CD28, CD3 group, LCN2, RETN | Concentration of fatty acid, transport of molecule | 7.217 |
| CD3 group | Concentration of fatty acid, migration of cells | 6.425 |
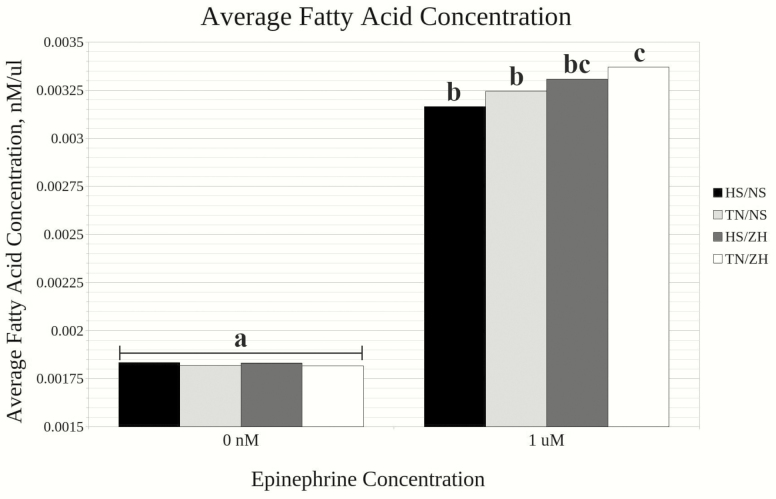
There was no interaction between environment and supplement for ex vivo FFA mobilization from steer adipose. Free fatty acid concentration did not differ among groups at 0 µM (Fig. 1). At 1 µM epinephrine, FFA was greater (P < 0.05) in TN than HS. All treatment groups responded to epinephrine, with TN/ZH having the highest concentration (Fig. 1).
Figure 1.
Mean fatty acid concentration for each treatment group. Superscripts denote a significance (P < 0.05).
DISCUSSION
We identified interacting and independent effects of HS and βAA supplementation on adipogenesis and lipolysis in sheep, as indicated by gene expression. We also found that fatty acid mobilization was impaired by HS but enhanced by βAA supplementation in cattle. Zilpaterol hydrochloride supplementation in heat-stressed cattle increased the FFA-mobilization response to epinephrine even after 21 d, but the response was less due to HS.
Transcriptome analyses in wethers did not clearly predict whole-pathway changes due to the interaction of HS and βAA. Several loci implicated however are components of G-protein receptor pathways, through which βAA signal. Others such as FOS and FOSB are associated with inflammation (Wagner and Efrel, 2005). Additional data from this study demonstrated an inflammatory response due to HS that was moderated by RH (Swanson et al., 2020). Heat stress alone altered expression of two loci in adipose. RBM3 encodes for a protein induced in response to hypoxia and cold shock (Wellmann et al., 2010); it was downregulated in HS lambs. ATXN7L1 is associated with neurological disorders (Carlson et al., 2009), but Komolka et al. (2016) reported it is downregulated in the longissimus dorsi of cattle with greater intramuscular fat. Increased ATXN7L1 expression in HS lambs could therefore contribute to the observed decrease in fat (Swanson et al., 2020). Pathway analyses predicted adipogenesis to be dysregulated due to HS. This was not surprising, considering that there are presumably mechanisms whereby HS affects adipose tissue not apparent from RNA analyses alone. Two regulator effect networks identified affected fatty acid concentrations and movement, supporting our hypothesis that HS impairs fat homeostasis. Conversely, we did not identify transcriptome changes in subcutaneous adipose attributable solely to RH. It is worth noting that samples were collected after 30 d on RH, and thus it is possible that βAR had been desensitized. The chronic effect βAA on adipose can also be difficult to observe from a single time-point, as βAA enhance lipid mobilization but increase fatty acid synthesis (Yang and McElligott, 1989).
In cattle, HS and ZH supplementation each modified fatty acid mobilization of adipose due to epinephrine independently, although the two factors did not have interacting effects. Zilpaterol hydrochloride preferentially binds β 2AR, and thus the heightened effect could be due to epinephrine acting on β 1AR with greater frequency than it would in the absence of ZH. It is more likely, however, that exposure to both epinephrine and ZH had an additive effect. Decreased FFA mobilization due to HS was possibly an effect of chronic exposure. In study 1, HS caused increased circulating epinephrine (Swanson et al., 2020). If steers in study 2 responded likewise, it is reasonable to speculate that their βAR became desensitized and less responsive to epinephrine. Acute HS increases adipocyte responses to lipolytic signals (Faylon et al., 2015), but we postulate that chronic HS decreased responsiveness due to downregulated βAR.
IMPLICATIONS
Loci in adipose have altered expression due to the combined impact of HS and βAA supplementation. Specific loci associated with inflammation were observed, warranting additional investigation in order to fully elucidate their role in adipose HS. We also provide evidence that increased lipolysis is a mechanism by which ZH can reduce carcass fat and promote feed efficiency. Conversely, HS impaired fatty acid mobilization, presumably via βAR desensitization resulting from chronic stimulation. Finally, no interacting effects of HS and βAA supplementation on mechanisms that would impact animal wellbeing were apparent. Building a better understanding of the mechanisms by which animals respond to HS and βAA supplementation will aid in generating improved management practices to improve sustainability of livestock production.
Conflict of interest statement. None declared.
Footnotes
This project is based on research that was partially supported by the Nebraska Agricultural Experiment Station with funding from the Hatch Multistate Research capacity funding program (accession number 1011055) from the USDA National Institute of Food and Agriculture. A portion of this work was completed utilizing the Holland Computing Center of the University of Nebraska, which receives support from the Nebraska Research Initiative.
LITERATURE CITED
- Barnes T. L., Cadaret C. N., Beede K. A., Schmidt T. B., Petersen J. L., and Yates D. T.. 2019. Hypertrophic muscle growth and metabolic efficiency were impaired by chronic heat stress, improved by zilpaterol supplementation, and not affected by ractopamine supplementation in feedlot lambs1. J. Anim. Sci. 97:4101–4113. doi: 10.1093/jas/skz271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Hawke T., and Riddell M.. 2009. Glucocorticoids are capable of stimulating both lipolysis and adipogenesis in 3T3-L1 adipocytes. Can. J. Diabetes 33:302–303. doi: 10.1016/S1499-2671(09)33296-7 [DOI] [Google Scholar]
- Carlson K. M., Andresen J. M., and Orr H. T.. 2009. Emerging pathogenic pathways in the spinocerebellar ataxias. Curr. Opin. Genet. Dev. 19:247–253. doi: 10.1016/j.gde.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., and Gingeras T. R.. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21. doi: 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam N. A., Vasconcelos J. T., Hilton G., VanOverbeke D. L., Lawrence T. E., Montgomery T. H., Nichols W. T., Streeter M. N., Hutcheson J. P., Yates D. A., et al. 2009. Effect of zilpaterol hydrochloride duration of feeding on performance and carcass characteristics of feedlot cattle. J. Anim. Sci. 87:2133–2141. doi: 10.2527/jas.2008-1563 [DOI] [PubMed] [Google Scholar]
- Faylon M. P., Baumgard L. H., Rhoads R. P., and Spurlock D. M.. 2015. Effects of acute heat stress on lipid metabolism of bovine primary adipocytes. J. Dairy Sci. 98:8732–8740. doi: 10.3168/jds.2015-9692 [DOI] [PubMed] [Google Scholar]
- Huang D. W., Sherman B. T., and Lempicki R. A.. 2009a. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 4:44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Huang D. A. W., Sherman B. T., and Lempicki R. A.. 2009b. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37:1–13. doi: 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. J., Smith S. B., and Chung K. Y.. 2014. Historical overview of the effect of β-adrenergic agonists on beef cattle production. Asian-Australas. J. Anim. Sci. 27:757–766. doi: 10.5713/ajas.2012.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komolka K., Ponsuksili S., Albrecht E., Kühn C., Wimmers K., and Maak S.. 2016. Gene expression profile of Musculus longissimus dorsi in bulls of a Charolais × Holstein F2-cross with divergent intramuscular fat content. Genom. Data 7:131–133. doi: 10.1016/j.gdata.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S.. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik R. M., Tietze S. M., Schmidt T. B., Yates D. T., and Petersen J. L.. 2018. Investigation of the skeletal muscle transcriptome in lambs fed β adrenergic agonists and subjected to heat stress for 21 d1. Transl. Anim. Sci. 2(Issue Suppl. 1):S53–S56. doi: 10.1093/tas/txy053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitlöhner F. M., Morrow J. L., Dailey J. W., Wilson S. C., Galyean M. L., Miller M. F., and McGlone J. J.. 2001. Shade and water misting effects on behavior, physiology, performance, and carcass traits of heat-stressed feedlot cattle. J. Anim. Sci. 79:2327–2335. doi: 10.2527/2001.7992327x [DOI] [PubMed] [Google Scholar]
- Peckett A. J., Wright D. C., and Riddell M. C.. 2011. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 60:1500–1510. doi: 10.1016/j.metabol.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Raclot T., and Groscolas R.. 1993. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J. Lipid Res. 34:1515–1526. [PubMed] [Google Scholar]
- Re G., Badino P., Novelli A., and Girardi G.. 1997. Effects of clenbuterol as a repartitioning agent on beta-adrenoceptor concentrations in heart, bronchi and brain of veal calves. Vet. J. 153:63–70. doi: 10.1016/s1090-0233(97)80009-3 [DOI] [PubMed] [Google Scholar]
- St-Pierre N., Cobanov B., and Schnitkey G.. 2003. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Swanson R. M., Tait R. G., Galles B. M., Duffy E. M., Schmidt T. B., Petersen J. L., and Yates D. T.. 2020. Heat stress-induced deficits in growth, metabolic efficiency, and cardiovascular function coincided with chronic systemic inflammation and hypercatecholaminemia in ractopamine-supplemented feedlot lambs. J. Anim. Sci. 98. doi: 10.1093/jas/skaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., and Eferl R.. 2005. Fos/AP-1 proteins in bone and the immune system. Immunol. Rev. 208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x [DOI] [PubMed] [Google Scholar]
- Wellmann S., Truss M., Bruder E., Tornillo L., Zelmer A., Seeger K., and Bührer C.. 2010. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr. Res. 67:35–41. doi: 10.1203/PDR.0b013e3181c13326 [DOI] [PubMed] [Google Scholar]
- Yang Y. T., and McElligott M. A.. 1989. Multiple actions of β-adrenergic agonists on skeletal muscle and adipose tissue. Biochem. J. 261(1):1–10. doi: 10.1042/bj2610001 [DOI] [PMC free article] [PubMed] [Google Scholar]