Abstract
Healthy older adults commonly report increased difficulties with language production. This could reflect decline in the language network, or age-related declines in other cognitive abilities that support language production, such as executive function. To examine this possibility, we conducted a whole-brain resting-state functional connectivity (RSFC) analysis in older and younger adults using two seed regions—the left posterior superior temporal gyrus and left inferior frontal gyrus. Whole-brain connectivities were then correlated with Stroop task performance to investigate the relationship between RSFC and executive function. We found that overall, younger adults had stronger RSFC than older adults. Moreover, in older, but not younger, adults stronger RSFC between left IFG and right hemisphere executive function regions correlated with better Stroop performance. This suggests that stronger RSFC among older adults between left IFG and right hemisphere regions may serve a compensatory function.
Keywords: Aging, Language production, Executive function, Resting-state functional connectivity
1. Introduction
Healthy older adults commonly exhibit age-related decline in a variety of cognitive functions, including memory, attention, executive function, and language production (Buckner, 2004; Burke & Light, 1981; Burke & Shafto, 2004; Burke & Shafto, 2008; Craik, 1994; Park et al., 2002; Rogers, 2000). Additionally, both brain structure and function decline with age, including declines in white matter integrity (Stamatakis, Shafto, Williams, Tam, & Tyler, 2011; Troutman & Diaz, 2019), as well as age-related differences in activation and functional connectivity. For example, the brain’s functional networks during rest (e.g., default mode network, executive control network) become weaker and less segregated with age, and this has been associated with cognitive decline in episodic memory, working memory, and attention (Andrews-Hanna et al., 2007; Chan, Park, Savalia, Petersen, & Wig, 2014; Tian, Ren, & Zang, 2012; Tomasi & Volkow, 2012; Zou et al., 2013). However, the extent to which these brain differences are observed in regions involved in language processing and their relationship with age-related differences in language abilities remains unknown.
In addition to age-related neural and network differences, several aspects of language production suggest there is age-related decline. For instance, older adults generally experience more tip-of-the-tongue states (Burke, MacKay, Worthley, & Wade, 1991), produce slower (Burke & Shafto, 2004; Burke & Shafto, 2008), more disfluent, and syntactically simpler speech (Kemper, Herman, & Lian, 2003; Kemper, Thompson, & Marquis, 2001), and have more slips of the tongue (MacKay & James, 2004). In addition to older adults citing these declines in production as being highly frustrating (Ossher, Flegal, & Lustig, 2013), they have the potential to cause older adults to withdraw from social interactions (Hummert, Garstka, Ryan, & Bonnesen, 2004). Although language production abilities decline with age, language comprehension abilities remain largely intact in older adults as semantic knowledge generally increases with age (Burke & Shafto, 2008; Park & Reuter-Lorenz, 2009; Salthouse, 2010). While older adults often have more semantic knowledge than younger adults, older adults perform worse on tasks requiring semantic control consistent with declines in executive aspects of language (Hoffman, 2018; Krieger-Redwood et al., 2019). One possibility, as described by the Inhibition Deficit Hypothesis (Hasher & Zacks, 1988; Lustig, Hasher, & Zacks, 2007) suggests that with increased age, inhibiting information becomes more difficult and the excess information can become distracting to older adults. Since older adults have more semantic knowledge, this may lead to increased competition among lexical items, particularly when semantic selection demands are high. Others have noted phonological aspects of age-related production failures (i.e., tip-of-the-tongue incidences) and have suggested that production declines arise from phonological deficits (Burke, MacKay, & James, 2000; Burke et al., 1991). It may be the case that both inhibitory and phonological deficits contribute to language production declines, with the impact of each varying according to the demands of the situation.
While age-related deficits in language production are commonly reported (e.g., Burke & Shafto, 2008; Hummert et al., 2004; Kemper et al., 2001), how this maps on to patterns of functional activation is less clear. Although age-related increases in task-based functional activation are commonly observed, there is considerable debate about whether such increases are compensatory or reflect neural decline (Dennis & Cabeza, 2011; Park & Bischof, 2013). According to the hemispheric asymmetry reduction in older adults (HAROLD) hypothesis, increased bilateral frontal activation in older adults may serve a compensatory function if this increased activation is associated with improved or maintained behavioral performance (Cabeza, 2002); however, if this overactivation is associated with behavioral declines it is often interpreted as evidence for dedifferentiation, in which desegregation of brain networks results in performance declines (Li & Lindenberger, 1999). In looking specifically at functional activation associated with language, there have been variable reports about the nature and extent of age-related differences in the engagement of the core left-hemisphere language network (e.g., Shafto & Tyler, 2014). For example, some have suggested that age-related differences in activation may reflect the increased cognitive demands of the task as opposed to age-related changes in language (Davis, Zhuang, Wright, & Tyler, 2014). Davis and colleagues (2014) observed minimal age-related differences during naturalistic speech comprehension. In contrast, during speech comprehension with an explicit task, there were large age-related increases in activation in bilateral prefrontal cortex, suggesting that age-related differences in fMRI activation may be due to external task demands. Other studies examining language production have found that task difficulty modulated functional activation during language production, and that older adults were more sensitive to task difficulty, and less neurally responsive to increases in task demands (Zhang, Eppes, Beatty-Martínez, Navarro-Torres, & Diaz, 2018; Zhang, Eppes, & Diaz, 2019). Moreover, Zhang and colleagues (2019) noted that brain-behavior relationships changed as a function of task difficulty, becoming weaker with increased task difficulty. Critically, task modulation of neural activity may confound interpretations of age-related differences in functional activation because these observed age-related differences in fMRI activation may be due to the task demands themselves.
1.1. Resting-State Functional Connectivity
As discussed in section 1. above, some research suggests that behavioral and fMRI age-related differences in language production are, at least in part, due to task modulation. One method that can help disentangle the influences of age and task on brain activity is resting state functional connectivity (RSFC). RSFC examines how activation correlates across brain regions while participants are not performing an explicit task (Biswal, Yetkin, Haughton, & Hyde, 1995). Interestingly, brain regions that function together during tasks, also demonstrate synchronous correlations while participants are at rest (Rosazza & Minati, 2011). Importantly, studies have demonstrated that brain activity elicited while not actively engaged during a task can be used to predict off-line behavioral task performance. For example, a study using resting-state fMRI by Tian, Ren, and Zang (2012) observed that positive correlations between bilateral inferior frontal cortex (IFC) and the Default Mode Network (DMN) and negative correlations between bilateral middle occipital cortex, left inferior temporal cortex (ITC), and posterior insula activity reliably predicted shorter stop signal reaction times. Others have shown that resting-state activity predicted performance on an N-back working memory task (Zou et al., 2013), in which increased resting-state brain connectivity between the middle frontal gyrus and parietal regions and decreased connectivity between the medial prefrontal cortex and posterior cingulate was correlated with improved working memory. Additionally, studies have shown that age-related alterations during RSFC exist in a variety of networks including the default mode, executive control, and dorsal attentional networks, and these age-related declines have been associated with cognitive deficits in older adults (Andrews-Hanna et al., 2007; Chan et al., 2014; Sala-Llonch, Bartrés-Faz, & Junqué, 2015). However, few studies have examined RSFC within language regions (Zhu et al., 2014) or how such connectivity relates to task performance (Ferre et al., 2019; Miró-Padilla, Bueichekú, Ventura-Campos, Palomar-García, & Ávila, 2017). Additionally, to our knowledge, only two RSFC studies measured the extent to which RSFC in a language network related to behavioral performance (Ferre et al., 2019; Miró-Padilla et al., 2017). Miró-Padilla et al. (2017) found that among younger adults, improved performance on the verbal fluency task was associated with higher functional connectivity between the thalamus and cerebellum and lower functional connectivities between the left inferior frontal gyrus (IFG) and right insula and between the left supplementary motor area (SMA) and right insula (Miró-Padilla et al., 2017). In another study, Ferre and colleagues examined both task-based functional connectivity and resting-state functional connectivity in language and default mode regions (Ferre et al., 2019). Of particular relevance to the present study, they found that overall older adults had weaker resting-state connectivities compared to younger adults. They also observed age-related increases in connectivity during language tasks, particularly in the left inferior frontal, left occipital, and bilateral parietal regions. Although Ferre and colleagues examined the relation between functional connectivity, age, and vocabulary, there were no significant interactions, suggesting that although vocabulary increases with age, the functional networks that support this ability remain intact across the lifespan. These studies provide important insights into how functional connectivity relates to language production in younger adults and vocabulary in both younger and older adults; however, the effect of age and the role that domain general resources, such as cognitive control, may have on language-related differences in older adults remains less clear.
1.2. The Present Study
Few studies have looked at whole-brain functional connectivity with key language regions during resting-state in younger and older adults and its relationship with behavioral performance (Ferre et al., 2019; Miró-Padilla et al., 2017). Davis et al. (2014) highlighted the notion that age-related declines observed in language may not be due to declines in the language network itself, but to declines in executive function, and more specifically the relationship between language and executive function regions. Additionally, conducting resting-state functional connectivity analyses allowed us to further investigate the whole-brain RSFC in language processing regions while removing the potential confound of task demands. The present study examined whole-brain functional connectivity with two language regions—left posterior superior temporal gyrus (pSTG) and the left inferior frontal gyrus (IFG), pars triangularis division. The left pSTG, was chosen for its role in lexical selection and phonological retrieval and encoding (Graves, Grabowski, Mehta, & Gordon, 2007). The left IFG was selected for its role in executive function processes, including semantic control, and for its involvement in lexical selection during language processing (Sowell, Thompson, Tessner, & Toga, 2001; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997). Consistent with prior reports (e.g., Ferre et al., 2019), we predicted that compared to younger adults, older adults would show weaker functional connectivity overall.
We were also interested in the relationship between RSFC and behavior. To examine this, we correlated whole-brain functional connectivity using the language seed regions with a task involving both language processing and executive function—the Stroop task—to better understand the relationship between language and executive function regions, and to examine how RSFC may predict age-related differences in behavioral performance. Because prior research has found that increased task-based functional activation in right hemisphere regions was associated with improved word retrieval performance in older adults (e.g., Wierenga et al., 2008), and others have observed increased age-related functional connectivity between left frontal and bilateral parietal regions, we predicted that in older adults, stronger functional connectivity between our seed language regions and right hemisphere regions involved in executive function would correlate with better Stroop performance. Moreover, given well-documented age-related decline in the frontal cortex, age-related differences in RSFC and RSFC-behavior relations may be greatest in frontal regions.
2. Methods
2.1. Participants
Forty younger adults between the ages of 18 and 32 (mean age = 23.18, females = 20) and 40 older adults participated in the study. Two older adults were excluded from further data analysis—one for an incidental finding and one for excessive head motion, resulting in the number of TRs censored preventing the calculation of a reliable correlation (Van Dijk et al., 2010)—resulting in 38 older adults between the ages 60 and 79 (mean age = 67.26, females = 28), remaining in the study. All participants were healthy, right-handed, native English speakers with no reported history of neurological or physiological disorders and who did not report having any major medical conditions (e.g., cancer, diabetes, heart disease). Additionally, no participants were taking any psychotropic medications that might affect the brain or cerebral blood flow. All participants had normal or corrected-to-normal vision, which was measured using the Freiburg Visual Acuity and Contrast Test (Bach, 1996), as well as normal color vision, which was measured using Ishihara Plates (Clark, 1924). Additionally, all participants scored at least a 27 on the Mini Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), had at least 12 years of education, and scored under 5 on the Geriatric Depression Scale (GDS; Yesavage et al., 1982). See Table 1 for detailed participant demographic information. All participants provided written informed consent and all experimental procedures were approved by the Institutional Review Board of The Pennsylvania State University.
Table 1.
Participant Demographics and Neuropsychological Testing Information
| Younger Adults | Older Adults | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Demographics | ||
| N | 40 | 38 |
| Age (years) | 23.18 (4.28) | 67.26 (5.99) |
| Sex (F/M) | 20/20 | 28/10 |
| Education | 16.35 (2.53) | 16.51 (2.38) |
| MMSE | 29.15 (0.95) | 28.5 (1.08) |
| GDS | 0.78 (1.12) | 0.57 (0.73) |
| Language and Reading | ||
| Vocabulary | 51.53 (8.95) | 54.70 (6.10) |
| Author Recognition* | 13.85 (8.62) | 32.81 (14.58) |
| Magazine Recognition* | 12.70 (7.12) | 25.57 (5.91) |
| Verbal Fluency | 61.58 (17.57) | 61.62 (18.92) |
| Memory | ||
| Immediate Recall | 11.63 (2.02) | 10.89 (2.14) |
| Delayed Recall* | 10.60 (2.33) | 8.79 (2.64) |
| Forward Digit Span | 7.08 (1.00) | 7.11 (1.13) |
| Backward Digit Span | 5.25 (1.21) | 4.89 (1.25) |
| Processing | ||
| Digit Symbol* | 1295.06 (212.20) | 1831.67 (310.48) |
| Simple Speed* | 259.12 (32.13) | 280.38 (44.94) |
| Complex Speed* | 276.20 (25.26) | 329.69 (60.86) |
| MRI Data Quality | ||
| Motion* | 0.12 (0.05) | 0.17 (0.07) |
| Invalid Scans* | 6.38 (6.99) | 9.73 (7.26) |
Note. Tasks included the Wechsler Adult Intelligence Scale (WAIS-III) vocabulary and digit symbol subtests (Tombaugh, Kozak, & Rees, 1999; Wechsler, 1997); phonemic (F, A, S) and categorical (animals) fluency; the Califorina Verbal Learning Test to assess immediate and delayed recall (Alexander, Stuss, & Fansabedian, 2003); forward and backward digit span to test working memory; simple and choice reaction time tests to assess speed; and the author recognition and magazine recognition tests to assess reading habits (Acheson, Wells, & MacDonald, 2008).
Scores for which there was a significant age difference, p < .05
2.2. Cognitive Assessment
Participants completed a neuropsychological battery to assess cognition prior to the MRI scanning session. The assessment measured executive function, language, processing speed, and working memory using paper/pencil and computer-based tasks. The results from the neuropsychologcial battery serve to characterize the current sample and are summarized in Table 1.
Lexical competition and selection were evaluated via a computerized color Stroop task presented in E-Prime (E-Prime 2.0, Psychology Software Tools, 2012). During the Stroop task, words were presented in either red or blue text and participants were instructed to press different keys on a computer keyboard depending on the color that a word was presented in, as quickly and accurately as possible. Response handness was counterbalaned across participants. The task included three conditions with 40 trials in each condition: congruent, incongruent, and neutral. In the congruent condition, the text color was the same as the word meaning (e.g., the word “red” printed in red-colored text). In the incongruent condition, the text color was different from the word meaning (e.g., the word “red” printed in blue-colored text). The neutral condition consisted of non-color words printed in red or blue text. Stroop effect scores were calcuated as the reaction time difference between the incongruent and congruent conditions.
2.3. Acquisition of fMRI Data
Imaging data were collected at The Pennsylvania State University, University Park campus using a 3T Siemens Prisma Fit MRI scanner with a 20-channel head coil. Prior to the resting-state scan, T1 weighted anatomical images were collected using a magnetization-prepared rapid acquisition gradient echo (MP RAGE) sequence (repetition time [TR] = 2300 ms; echo time [TE] = 2.28 ms; Inversion Time [TI] = 900 ms; flip angle = 8°; echo spacing = 7 ms; acceleration factor = 2; field of view [FOV] = 256 mm2; voxel size = 1 mm3; 160 contiguous slices). Resting-state images were collected using an echoplanar imaging (EPI) sequence (TR = 2500 ms; TE = 25.0 ms; flip angle = 90°; echo spacing = 0.49 ms; FOV = 240 mm2; voxel size = 3 mm3; 41 interleaved contiguous slices; 142 volumes; phase encoding = anterior to posterior, fat saturation = on; resting-state scan duration = ~ 6 minutes). Two additional volumes were acquired and deleted at the start of the scan to reach steady state equilibrium. During the resting-state scan, participants were instructed to relax in the scanner with their eyes open and to look at a fixation cross presented in the center of the screen.
2.4. Resting-State Preprocessing
Functional and anatomical images were visually inspected for artifacts and signal drop-out. Processing and analyses were conducted using the CONN functional connectivity toolbox version 18.a (Whitfield-Gabrieli & Nieto-Castanon, 2012). Specifically, functional realignment and unwarping was done to estimate and correct for participant motion, followed by slice-timing correction, which corrected for maturation of the BOLD signal over time (Huettel, Song, & McCarthy, 2004). Functional outliers were detected with an ART (Artifact Detection Tools)-based identification method using the conservative setting (95th percentiles in a normative sample). Segmentation was done on all anatomical and functional images to segment images into white matter WM, gray matter (GM), and cerebrospinal fluid (CSF) and all images were normalized to standard Montreal Neurological Institute (MNI) space. During registration, functional images were aligned to anatomical images and both were normalized to standard space, allowing for group comparisons. A smoothing kernel of 8 mm was used to increase the signal to noise ratio, as well as to reduce spurious activation of single voxels. A band-pass filter of 0.008 – 0.09 Hz was used to reduce non-neural sources of noise, such as motion, respiration, and cardiac pulsation (Davey, Grayden, Egan, & Johnston, 2013; Gohel & Biswal, 2015; Hallquist, Hwang, & Luna, 2013). The following quality assurance parameters were included as second level covariates during data preprocessing: number of outlier and non-outlier scans (outlier threshold = 0.5 mm), max and mean motion, and max and mean global BOLD signal changes (outlier threshold = global-signal z-value of 3). The total average numbers of invalid scans were 6.38 (6.99) for Younger Adults and 9.74 (7.26) for Older Adults, p = .041. The Anatomical CompCor program within CONN was used for denoising. During denoising, representative noise signal from WM (5 components) and CSF (5 components) was extracted, and any signal correlated with these components was removed from the BOLD signal. We also modeled any signal correlated with any realignment parameters, which represented participant motion (Mean Motion: Younger Adults = 0.12 (0.05) mm, Older Adults = 0.17 (0.07) mm, p < .001), as additional nuisance regressors. The analyses removing variance associated with all of the variables described above occurred in a single linear regression step, and the residualized BOLD signal was used for further statistical analyses.
2.5. Resting-State Data Analyses
Using the CONN functional connectivity toolbox, we conducted seed-to-voxel functional connectivity analysis using bivariate correlations without weighting, using two seeds: the left pSTG and the left IFG pars triangularis. The two seeds were identified using the Harvard-Oxford atlas for cortical and subcortical areas, with a threshold of 0.25 or larger (Desikan et al., 2006). To investigate age-related differences in functional connectivity, contrasts between two groups were conducted on the overall functional connectivity. Significant functional connections were determined in a two-step process. Statistically significant voxels were first identified using a voxel-wise threshold of p < .001. Then, identified clusters were corrected for multiple comparisons using false discovery rate (FDR) estimation, such that only clusters with a significance of p < .05 were retained (Benjamini & Hochberg, 1995; Genovese, Lazar, & Nichols, 2002).
We were also interested in whether RSFC predicted behavioral performance on the Stroop task. Therefore, Stroop effect scores were added to the model as a covariate and were correlated with the functional connectivity between each seed region and the whole brain across all participants. Next, to examine if there were significant age group differences in RSFC-Stroop effect correlations, we conducted an interaction analysis. To further explore the interaction results, we examined the within-group RSFC-Stroop effect score correlations. These interaction results were further masked by the within-group analyses to limit significant differences to those regions showing significant within group correlations. All RSFC-behavior analyses were also conducted using the CONN toolbox. All reported figures and results reflect corrections for multiple comparisons.
3. Results
3.1. Stroop Task Behavioral Results
We conducted a linear regression on reaction time (RT) to assess overall performance where Condition (incongruent, congruent, neutral) and Age Group (younger, older) were examined. There was a significant effect of Age Group—older adults performed significantly slower across conditions compared to the younger adults, β = 154.00, F(5, 228) = 17.72, p < .001. Follow up t-tests showed that there were significant Age Group differences across all three conditions: Incongruent, t(73.9) = 3.77, p < .001; Congruent, t(75.9) = 2.34, p = .022; Neutral, t(67.3) = 2.65, p = .010. In the full linear regression analysis described above, the effect of Condition and the Age Group by Condition interaction were not significant. However, when examining the Stroop effect scores (calculated as Incongruent RTs – Congruent RTs), younger adults had significantly smaller Stroop effect scores than the older adults, indicating that younger adults had better performance (See Table 2).
Table 2.
Stroop Task Performance
| Younger Adults | Older Adults | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Stroop Effect Score** | 26.30 (58.64) | 101.11 (104.99) |
| Incongruent Condition (ms)** | 502.23 (199.77) | 656.22 (159.61) |
| Congruent Condition (ms)* | 475.93 (155.97) | 555.11 (143.32) |
| Neutral Condition (ms)* | 494.65 (178.39) | 584.32 (115.88) |
p < .05,
p < .001
3.2. Left pSTG Connectivity
3.2.1. Overall Connectivity
Comparisons were conducted to determine if there were any age-related differences in the whole-brain functional connectivity with the left pSTG (see Figure 1A and Table 3). Results showed that younger adults had stronger functional connectivity than older adults in bilateral frontal pole, left posterior middle temporal gyrus (pMTG), left cingulate gyrus, and left lateral occipital cortex. There were no regions in which older adults had stronger functional connectivity with the left pSTG compared to younger adults.
Figure 1.
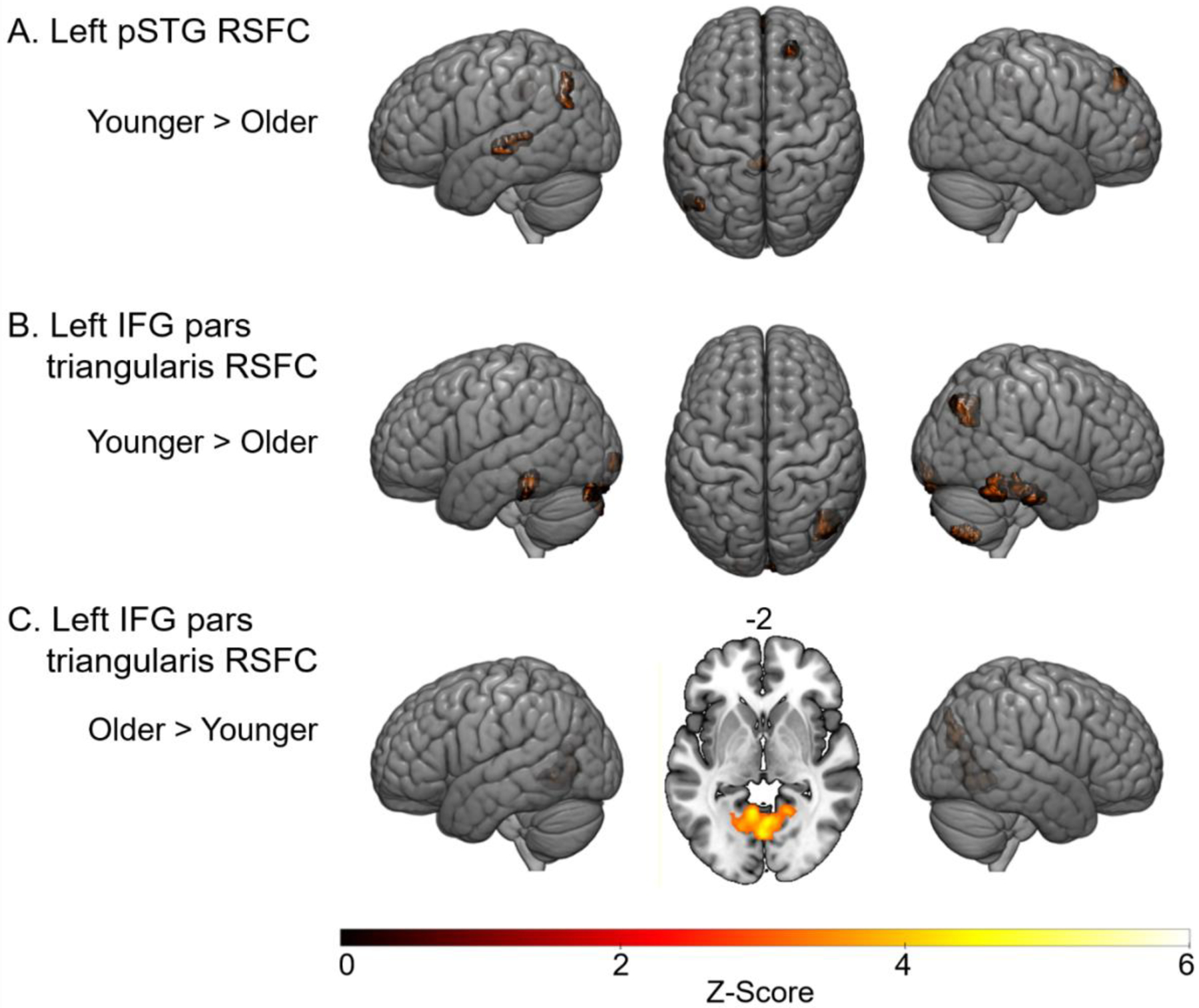
A. Whole brain connectivity with the left STG—regions in which younger adults elicited stronger connectivities than older adults. B. Whole brain connectivity with the left IFG—regions in which younger adults elicited stronger connectivities than older adults. C. Whole brain connectivity with the left IFG—regions in which older adults had stronger connectivities than younger adults. Voxel-wise threshold = p < .001. FDR corrected cluster threshold = p < .05.
Table 3.
Coordinates for Regions with Significant RSFC to the left pSTG from the Seed-to-Voxel Analysis
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
|---|---|---|---|---|---|---|
| Younger > Older | ||||||
| Frontal pole*** | — | 113 | 4.22 | 0 | 60 | −2 |
| Frontal pole | Left | 3.78 | −2 | 62 | −4 | |
| Frontal pole | Right | 3.59 | 4 | 62 | 0 | |
| Frontal pole*** | Right | 157 | 3.97 | 18 | 42 | 48 |
| Posterior Superior temporal gyrus*** | Left | 492 | 5.57 | −54 | −24 | 0 |
| Posterior Middle temporal gyrus | Left | 3.55 | −62 | −23 | −5 | |
| Planum temporale | Left | 4.25 | −54 | −28 | 4 | |
| Cingulate gyrus*** | Left | 138 | 4.31 | −2 | −36 | 38 |
| Lateral occipital cortex*** | Left | 110 | 3.79 | −54 | −68 | 32 |
| Older > Younger | ||||||
| No Significant functional connectivity | ||||||
| Stroop Correlation: All Participants | ||||||
| Parietal operculum cortex*** | Right | 139 | −4.27 | 50 | −30 | 16 |
| Planum temporale | Right | −3.81 | 49 | −27 | 12 | |
| Age Group X RSCF Interaction on Stroop Effect Score | ||||||
| No Significant Interaction |
Note. Voxel-wise threshold = p < .001. FDR corrected cluster threshold = p < .05. Significant levels for the peak cluster:
p < .001.
3.2.2. Correlation with Stroop Effect Score
A correlation was run to examine the relationship between Stroop performance and whole-brain functional connectivity with the left pSTG across all participants (see Figure 2A and Table 3). Results indicated significant negative correlations with one significant cluster, where smaller Stroop effect scores (i.e., better performance) were correlated with stronger functional connectivity. The cluster peaked in the right parietal operculum cortex and extended to the right planum temporale. An age group by functional connectivity interaction on Stroop effect scores was then conducted to determine whether there were significant group differences observed in RSFC to the left pSTG as they related to Stroop task performance. Results of this analysis revealed that although the RSFC-Stroop correlation with left pSTG functional connectivity was significant across participants, the interaction between age group and RSFC on Stroop effect scores was not significant, suggesting that the RSFC between the left pSTG and right parietal operculum cortex and right planum temporale that covaried as a function of Stroop effect scores was not significantly different between younger and older adults.
Figure 2.
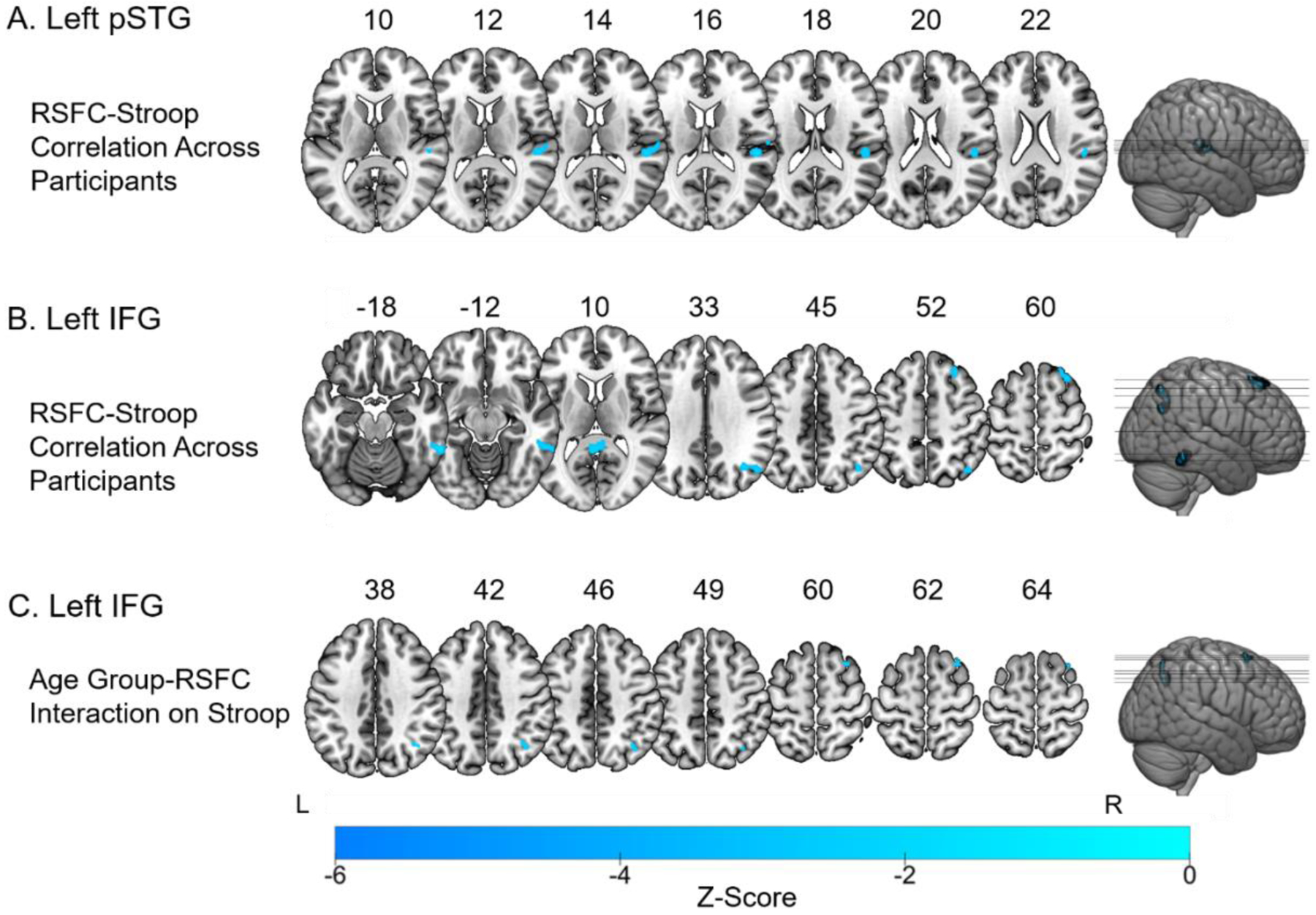
A. Stroop effect scores correlated with RSFC between the left pSTG to the rest of the brain similarly across all participants. Significant regions included the right parietal operculum cortex and right planum temporale. B. Stroop effect scores correlated with whole-brain RSFC from the left IFG pars triangularis in all participants. Significant regions included the right superior and middle frontal gyri, the left cingulate gyrus, the right inferior temporal gyrus, and the right lateral occipital cortex. C. The Age Group by RSFC interaction on Stroop effect scores using the left IFG as the seed region. Significant regions included the right middle frontal gyrus and right lateral occipital cortex. Older adults with stronger left IFG RSFC had smaller Stroop effect scores. Voxel-wise threshold = p < .001. FDR corrected cluster threshold = p < .05.
3.3. Left IFG pars triangularis Connectivity
3.3.1. Overall Connectivity
Comparisons were conducted to determine if there were any age-related differences in the whole-brain functional connectivity with the left IFG pars triangularis (see Figure 1B and Table 4). Compared to older adults, younger adults had stronger connectivity in the bilateral inferior temporal gyri, which extended to bilateral middle temporal gyri, right lateral occipital cortex, bilateral occipital pole, and bilateral cerebellum, which extended into the left lingual gyrus. Older adults showed stronger functional connectivity with the left lingual gyrus, which extended to the right lingual gyrus, bilateral intracalcarine cortex, left cerebellum, and bilateral precuneus. Older adults also exhibited stronger RSFC with the right lateral occipital cortex compared to younger adults (Figure 1C).
Table 4.
Coordinates for Regions with Significant RSFC to the left IFG, pars triangularis from the Seed-to-Voxel Analysis
| Region | Hemisphere | Voxels | Max Z | X | Y | Z |
|---|---|---|---|---|---|---|
| Younger > Older | ||||||
| Posterior Inferior temporal gyrus*** | Right | 301 | 4.68 | 56 | −22 | −22 |
| Posterior Middle temporal gyrus | Right | 3.72 | 60 | −32 | −14 | |
| Posterior Inferior temporal gyrus*** | Left | 275 | 5.26 | −56 | −38 | −24 |
| Posterior Middle temporal gyrus | Left | 4.73 | −55 | −36 | −15 | |
| Inferior temporal gyrus temporoocciptal part | Left | 3.22 | −54 | −45 | −14 | |
| Inferior temporal gyrus, temporooccipital part*** | Right | 216 | 4.63 | 58 | −48 | −28 |
| Cerebellum | Right | 3.29 | 54 | −46 | −29 | |
| Cerebellum*** | Right | 202 | 5.35 | 36 | −60 | −56 |
| Lateral occipital cortex*** | Right | 446 | 4.74 | 42 | −72 | 36 |
| Cerebellum*** | Left | 344 | 4.51 | −42 | −84 | −22 |
| Occipital pole | Left | 3.96 | −7 | −92 | −17 | |
| Lingual gyrus | Left | 3.56 | −5 | −88 | −17 | |
| Occipital pole*** | Right | 248 | 4.42 | 12 | −94 | −12 |
| Occipital pole*** | Left | 136 | 4.27 | −14 | −102 | −2 |
| Older > Younger | ||||||
| Lingual gyrus*** | Left | 1336 | 5.58 | −8 | −54 | −4 |
| Lingual gyrus | Right | 4.53 | 8 | −62 | −6 | |
| Intracalcarine cortex | Right | 4.14 | 4 | −65 | 12 | |
| Cerebellum | Left | 5.26 | −8 | −54 | −6 | |
| Intracalcarine cortex | Left | 3.87 | −4 | −65 | 10 | |
| Precuneus | — | 4.18 | 0 | −63 | 14 | |
| Lateral occipital cortex*** | Right | 235 | 4.40 | 20 | −76 | 22 |
| Stroop Correlation: All Participants | ||||||
| Superior frontal gyrus*** | Right | 138 | −4.39 | 26 | 32 | 58 |
| Middle frontal gyrus | Right | −3.66 | 29 | 25 | 58 | |
| Cingulate gyrus*** | Left | 153 | −4.04 | −2 | −46 | 10 |
| Inferior temporal gyrus, temporooccipital part*** | Right | 150 | −4.67 | 64 | −48 | −18 |
| Middle temporal gyrus | Right | −3.73 | 59 | −44 | −11 | |
| Lateral occipital cortex*** | Right | 198 | −4.17 | 36 | −62 | 34 |
| Age Group X RSCF Interaction on Stroop Effect Score | ||||||
| Middle frontal gyrus*** | Right | 24 | −4.96 | 32 | 20 | 62 |
| Superior frontal gyrus | Right | −3.94 | 28 | 18 | 60 | |
| Lateral Occipital Cortex*** | Right | 71 | −4.25 | 36 | −66 | 54 |
Note. Voxel-wise threshold = p < .001. FDR corrected cluster threshold = p < .05. Significant levels for the peak cluster:
p < .001.
3.3.2. Correlation with Stroop Effect Score
A correlation was run to examine the relationship between Stroop performance and whole-brain functional connectivity with the left IFG pars triangularis across all participants (see Figure 2C and Table 4). Results indicated significant negative correlations with four significant clusters, where smaller Stroop effect scores (i.e., better performance) correlated with stronger functional connectivity. These regions included the right superior frontal gyrus which extended into right middle frontal gyrus, right posterior cingulate gyrus, right inferior temporal gyrus (temporooccipital part), which extended to the middle temporal gyrus, and right lateral occipital cortex. An age group by functional connectivity interaction analysis on Stroop effect scores was then conducted to determine whether there were significant group differences observed in RSFC to the left IFG as they relate to Stroop task performance. Results of this analysis confirmed that there were significant age group differences in the Stroop-RSFC correlations between the left IFG pars triangularis seed region, and right hemisphere regions including the right middle frontal gyrus, which extended into right superior frontal gyrus, and right lateral occipital cortex (Table 4 and Figure 2C). Follow up analyses on the interaction, revealed that older adults had significantly stronger RSFC-Stroop correlations compared to younger adults in these right frontal and occipital regions. Moreover, these significant RSFC-Stroop correlations in older adults were negative, with smaller Stroop effect scores correlated with better connectivity in the right middle and superior frontal gyri, and the right lateral occipital cortex. Within the younger adults, there were no significant correlations between Stroop effect scores and left IFG functional connectivity.
4. Discussion
Although older adults often report experiencing increased language production difficulties, how this corresponds to age-related differences in the language network is not well understood. For example, some have argued that age-related task differences in language may be due to age-related declines in executive function (Shafto & Tyler, 2014) because experimental tasks place additional demands on cognitive control (Zhang et al., 2018; Zhang et al., 2019). Moreover, age-related performance differences have been shown to disappear during more naturalistic language processing (Davis et al., 2014). Therefore, the present study used resting state fMRI to examine age-related differences in whole-brain RSFC between left hemisphere language regions and examined how this related to performance on the Stroop task. RSFC avoids the confound of external task demands, while incorporating offline performance on the Stroop task allows us to examine the intersection of language processing and executive function.
In this study, we examined whole-brain resting-state functional connectivity differences in younger and older adults in the left pSTG—selected for its role in lexical selection, phonological retrieval and encoding (Graves et al., 2007; Wilson, Isenberg, & Hickok, 2009)— and the left IFG pars triangularis division—selected for its role in executive aspects of language, such as semantic control (Sowell et al., 2001; Thompson-Schill et al., 1997). Specifically, to examine how executive function ability interacts with language regions and age, we correlated Stroop task performance with resting-state functional connectivity and examined the data for age-related differences in both RSFC and RSFC-Stroop effects. Overall, we found that younger adults demonstrated stronger whole-brain connectivity with our two language seed regions (left pSTG & IFG) compared to older adults. Additionally, significant age-group differences were found in RSFC-Stroop correlations where older, but not younger, adults had stronger connectivity between left IFG and right frontal and occipital regions that was associated with better performance on the Stroop task.
Looking at the results in more detail, we found that overall, younger adults demonstrated stronger RSFC with the left pSTG and regions in bilateral frontal, left superior and middle temporal gyri, left cingulate, and left occipital cortices compared to the older adults, suggesting that the overall connectivity between the left pSTG region and frontal, temporal, and occipital regions decreases with age. Similarly, when we examined whole-brain connectivity with the left IFG pars triangularis, again we found that overall younger adults showed stronger connectivity with several regions in bilateral inferior and middle temporal gyri, bilateral occipital cortices, and bilateral cerebellum. In contrast to our left pSTG results in which there were no regions where older adults had stronger RSFC, in left IFG, older adults had stronger connectivity compared to younger adults in right lateral occipital cortex and bilateral lingual gyrus, which spread to bilateral intracalcarine cortex, left cerebellum, and bilateral precuneus. While the stronger RSFC in the right hemispheres was somewhat unexpected for younger adults, the increased RSFC for older adults is consistent with existing research. Prior RSFC studies have found that older adults often have more desegregated brain networks (Chan et al., 2014; Tomasi & Volkow, 2012) and while the language network is largely left-lateralized in younger adults, increased connectivity with areas outside of the traditional language network within older adults has been previously observed (e.g., Ferre et al., 2019). Additionally, while the lingual gyrus is involved in visual processing, as well as the recognition and identification of words (Mechelli, Humphreys, Mayall, Olson, & Price, 2000), this region may become less lateralized with age (Agcaoglu, Miller, Mayer, Hugdahl, & Calhoun, 2015).
To more conclusively link RSFC differences to behavioral performance, we correlated RSFC with Stroop task performance across participants, and found that functional connectivity with the left IFG pars triangularis and several right hemisphere regions correlated with improved performance on the Stroop task. Of most central interest were age group differences. An Age Group x Condition interaction demonstrated that the left IFG RSFC-Stroop correlations significantly differed across age groups, with older adults having stronger left IFG RSFC-behavior correlations in right middle and superior frontal gyri and right lateral occipital cortex. These stronger correlations were associated with improved Stroop task performance in older adults. The right middle and superior frontal gyri are both important for executive function (Alvarez & Emory, 2006; Hu, Ide, Zhang, & Chiang-shan, 2016). The middle frontal gyrus is involved in several aspects of executive function including working memory, attention allocation, motor planning, and response inhibition (Alvarez & Emory, 2006). Increased activation in the middle frontal gyrus has previously been related to improved Stroop task performance (Banich et al., 2000; Bush et al., 1998). Similarly, the right superior frontal gyrus also supports executive function. For example, activation in the right superior frontal gyrus has been linked to better response inhibition and less motor urgency suggesting that it supports better impulse control and action restraint (Hu et al., 2016).
We also found that activation in lateral occipital cortex was related to improved Stroop performance in older adults. The lateral occipital cortex, while not involved in executive functioning, is involved in object recognition (Grill-Spector, Kourtzi, & Kanwisher, 2001). Stronger RSFC between the left IFG, which is involved in lexical selection and semantic control, and right hemisphere regions implicated in object recognition, attention allocation, motor planning, and response inhibition correlated with better Stroop task performance in older adults. It is possible that this increased coupling between these executive control, object recognition, and language control regions improved coordination between older adults’ ability to reconcile the words they perceived, the semantic and lexical information they had to retrieve and select, and the attention allocation, motor planning, and response inhibition necessary to improve performance on the Stroop task. Therefore, this suggests that having stronger functional connectivity between domain general executive control regions, like the right middle and superior frontal gyri, and executive language regions, like the left IFG, may serve a compensatory function in older adults.
In summary, results from both seed regions illustrated two main findings. First, resting-state functional connectivity largely declined with age, which is consistent with prior studies illustrating age-related declines in functional connectivity (Andrews-Hanna et al., 2007, H.-Y Zhang et al., 2014). Second, connectivity between left IFG and the right hemisphere executive function regions was associated with improved Stroop performance. However, we did not observe any group differences in left pSTG RSFC-Stroop correlations, suggesting an age-related stability in how this posterior language region supports Stroop task performance. In contrast, RSFC-Stroop relations in left IFG, a region involved in more executive aspects of language, revealed that older adults with stronger RSFC had improved performance on the Stroop task, a task which involves language and executive functions. These two findings of maintained RSFC in left pSTG and stronger RSFC in left IFG in older adults also supports previous studies that suggest that the declines observed in language production in older adults may be due to declines in more anterior brain regions and declines in the relationship between language and executive function, as opposed to declines in language regions per se. Additionally, finding stronger connectivities between left IFG and right hemisphere executive function regions, and their correlation with improved Stroop performance in older adults further highlights the Stroop task as involving both language processing and executive functioning.
Our results are partially consistent with the one other study investigating age-related differences in functional connectivity of language. Ferre and colleagues (2019) observed stronger task-based connectivity between left frontal, and bilateral parietal and occipital regions in older adults during vocabulary tasks. Although Ferre and colleagues found no significant brain-behavior relations during resting-state, the increased task-based functional connectivity among left frontal and right lateral occipital regions is consistent with our results. However, in contrast to the present results, previous work examining RSFC correlations with verbal fluency in younger adults found that improved performance was associated with decreased right hemisphere connectivities (Miró-Padilla et al., 2017). These differences in findings may reflect age-related differences in functional organization suggesting that improved performance in younger adults involves mainly left hemisphere regions, while successful language processing in older adults involves right hemisphere regions. Additionally, this discrepancy between our results and Miro-Padilla et al. may highlight executive function differences between the Stroop and verbal fluency tasks, with the former having a stronger executive function component and the latter having a stronger emphasis on language production. Alternatively, this difference in findings could be related to task differences. Although the Stroop task that we used involved semantic control and language processing, it did not involve overt articulation. It could be the case that increased right hemisphere activation is more beneficial when there is increased executive function, but less beneficial when overt production is involved.
Our observations of negative RSFC-Stroop effect score correlations among older adults, also relates to the broader aging literature, in which older adults often elicit increased task-based activation, particularly in right hemisphere regions (Cabeza, 2002). Although, generally, we did not observe increased functional connectivities for older compared to younger adults, within older adults increases in left IFG RSFC were associated with improved behavioral performance suggesting a compensatory function to at least some increases in functional co-activation (Cabeza et al., 2018). These findings may be specific to language tasks that involve a strong executive function component and future research should investigate whether beneficial relationships with right hemisphere activations extend to language production more broadly.
4.1. Conclusions
The present study demonstrated that stronger RSFC was generally associated with improved behavioral performance on the Stroop task. We observed age-related stability in left pSTG RSFC-Stroop relations and right hemisphere parietal regions, suggesting a preservation of brain-behavior relations in posterior language processing regions. In contrast, RSFC between left IFG pars triangularis and executive function and visual regions correlated with better Stroop task performance among older adults. This suggests that there are greater age-related differences in executive language regions, and that such increases in RSFC may serve a compensatory function. Results from this study highlight the interaction between language and executive function and the potential role of executive function in offsetting age-related differences in language production.
Acknowledgments
This publication was supported by NIH R01 AG034138 (mtd) and funding from the Social Sciences Research Institute and the Department of Psychology at Penn State. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Preliminary results were presented at the 2019 Dallas Aging and Cognition Conference. We thank the staff and scientists at the Social, Life, & Engineering Sciences Imaging Center and the Center for Language Science, where the experiment was conducted.
References
- Acheson DJ, Wells JB, & MacDonald MC (2008). New and updated tests of print exposure and reading abilities in college students. Behavior research methods, 40(1), 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcaoglu O, Miller R, Mayer AR, Hugdahl K, & Calhoun VD (2015). Lateralization of resting state networks and relationship to age and gender. Neuroimage, 104, 310–325. doi: 10.1016/j.neuroimage.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M, Stuss D, & Fansabedian N (2003). California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain, 126(6), 1493–1503. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev, 16(1), 17–42. doi: 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935. doi: 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M (1996). The Freiburg Visual Acuity Test-automatic measurement of visual acuity. Optometry and vision science, 73(1), 49–53. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Barad V, & Gullett D (2000). Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cognitive Brain Research, 10(1–2), 1–9. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, & Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208. doi: 10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Burke DM, & Light LL (1981). Memory and aging: The role of retrieval processes. Psychol Bull, 90(3), 513–546. doi: 10.1037/0033-2909.90.3.513 [DOI] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, & James LE (2000). Theoretical approaches to language and aging.
- Burke DM, MacKay DG, Worthley JS, & Wade E (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579.doi: 10.1016/0749-596X(91)90026-G [DOI] [Google Scholar]
- Burke DM, & Shafto MA (2004). Aging and language production. Current Directions in Psychological Science, 13(1), 21–24. doi: 10.1111/j.0963-7214.2004.01301006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, & Shafto MA (2008). Language and aging In Craik F & Salthouse T (Eds.), The handbook of aging and cognition (Vol. 3, pp. 373–443). [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, & Rauch SL (1998). The counting Stroop: an interference task specialized for functional neuroimaging—validation study with functional MRI. Hum Brain Mapp, 6(4), 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. doi: 10.1037/0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FI, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, & Reuter-Lorenz PA (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, & Wig GS (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences, 111(46), E4997. doi: 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J (1924). The Ishihara test for color blindness. American Journal of Physiological Optics. [Google Scholar]
- Craik FIM (1994). Memory changes in normal aging. Current Directions in Psychological Science, 3(5), 155–158. doi: 10.1111/1467-8721.ep10770653 [DOI] [Google Scholar]
- Davey CE, Grayden DB, Egan GF, & Johnston LA (2013). Filtering induces correlation in fMRI resting state data. Neuroimage, 64, 728–740. [DOI] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, & Tyler LK (2014). Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia, 63, 107–115. doi: 10.1016/j.neuropsychologia.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, & Cabeza R (2011). Neuroimaging of healthy cognitive aging The handbook of aging and cognition (pp. 10–63): Psychology Press. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Ferre P, Benhajali Y, Steffener J, Stern Y, Joanette Y, & Bellec P (2019). Resting-state and Vocabulary Tasks Distinctively Inform On Age-Related Differences in the Functional Brain Connectome. Lang Cogn Neurosci, 34(8), 949–972. doi: 10.1080/23273798.2019.1608072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, & Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage, 15(4), 870–878. [DOI] [PubMed] [Google Scholar]
- Gohel SR, & Biswal BB (2015). Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain connectivity, 5(1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, & Gordon JK (2007). A neural signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience, 19(4), 617–631. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, & Kanwisher N (2001). The lateral occipital complex and its role in object recognition. Vision Research, 41(10), 1409–1422. doi: 10.1016/S0042-6989(01)00073-6 [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, & Luna B (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage, 82, 208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: A review and a new view In Gordon HB (Ed.), Psychology of Learning and Motivation (Vol. Volume 22, pp. 193–225): Academic Press. [Google Scholar]
- Hoffman P (2018). An individual differences approach to semantic cognition: Divergent effects of age on representation, retrieval and selection. Scientific Reports, 8(1), 8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, & Chiang-shan RL (2016). The right superior frontal gyrus and individual variation in proactive control of impulsive response. Journal of Neuroscience, 36(50), 12688–12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, & McCarthy G (2004). Functional magnetic resonance imaging (Vol. 1): Sinauer Associates Sunderland, MA. [Google Scholar]
- Hummert, Garstka, Ryan, & Bonnesen. (2004). The role of age stereotypes in interpersonal communication. Handbook of Communication and Aging Research, 2, 91–114. [Google Scholar]
- Kemper S, Herman RE, & Lian CH (2003). The costs of doing two things at once for young and older adults: Talking while walking, finger tapping, and ignoring speech of noise. Psychology and Aging, 18(2), 181. [DOI] [PubMed] [Google Scholar]
- Kemper S, Thompson M, & Marquis J (2001). Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psychology and Aging, 16(4), 600. [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K, Wang H-T, Poerio G, Martinon LM, Riby LM, Smallwood J, & Jefferies E (2019). Reduced semantic control in older adults is linked to intrinsic DMN connectivity. Neuropsychologia, 107133. [DOI] [PubMed] [Google Scholar]
- Li S-C, & Lindenberger U (1999). Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age Cognitive neuroscience of memory (pp. 103–146): Hogrefe & Huber. [Google Scholar]
- Lustig C, Hasher L, & Zacks RT (2007). Inhibitory deficit theory: Recent developments in a “new view”. Inhibition in cognition, 17, 145–162. [Google Scholar]
- MacKay DG, & James LE (2004). Sequencing, speech production, and selective effects of aging on phonological and morphological speech errors. Psychology and Aging, 19(1), 93. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Humphreys GW, Mayall K, Olson A, & Price CJ (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267(1455), 1909–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miró-Padilla A, Bueichekú E, Ventura-Campos N, Palomar-García M-Á, & Ávila C (2017). Functional connectivity in resting state as a phonemic fluency ability measure. Neuropsychologia, 97, 98–103. doi: 10.1016/j.neuropsychologia.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Ossher L, Flegal KE, & Lustig C (2013). Everyday memory errors in older adults. Aging, Neuropsychology, and Cognition, 20(2), 220–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, & Bischof GN (2013). The aging mind: Neuroplasticity in response to cognitive training. Dialogues in clinical neuroscience, 15(1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299–320. [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools, I. (2012). E-Prime (Version 2.0).
- Rogers WA (2000). Attention and aging Cognitive aging: A primer. (pp. 57–73). New York, NY, US: Psychology Press. [Google Scholar]
- Rosazza C, & Minati L (2011). Resting-state brain networks: Literature review and clinical applications. Neurological Sciences, 32(5), 773–785. doi: 10.1007/s10072-011-0636-y [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, & Junqué C (2015). Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology, 6, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society : JINS, 16(5), 754–760. doi: 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, & Tyler LK (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. doi: 10.1126/science.1254404 [DOI] [PubMed] [Google Scholar]
- Sowell, Thompson PM, Tessner KD, & Toga AW (2001). Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience, 21(22), 8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA, Shafto MA, Williams G, Tam P, & Tyler LK (2011). White matter changes and word finding failures with increasing age. PloS one, 6(1), e14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, 94(26), 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Ren J, & Zang Y (2012). Regional homogeneity of resting state fMRI signals predicts Stop signal task performance. Neuroimage, 60(1), 539–544. doi: 10.1016/j.neuroimage.2011.11.098 [DOI] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Molecular psychiatry, 17(5), 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14(2), 167–177. doi: 10.1093/arclin/14.2.167 [DOI] [PubMed] [Google Scholar]
- Troutman SBW, & Diaz MT (2019). White matter disconnection is related to age-related phonological deficits. Brain Imaging and Behavior. doi: 10.1007/s11682-019-00086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2010). Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol, 103(1), 297–321. doi: 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997) Wechsler adult intelligence scale - III. New York: Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, Conway T, Cato MA, Briggs R, & Crosson B (2008). Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging, 29(3), 436–451. doi: 10.1016/j.neurobiolaging.2006.10.024 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Isenberg AL, & Hickok G (2009). Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Hum Brain Mapp, 30(11), 3596–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Zhang H, Eppes A, Beatty-Martínez A, Navarro-Torres C, & Diaz MT (2018). Task difficulty modulates brain-behavior correlations in language production and cognitive control: Behavioral and fMRI evidence from a phonological go/no-go picture-naming paradigm. Cognitive, Affective, & Behavioral Neuroscience, 18(5), 964–981. doi: 10.3758/s13415-018-0616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Eppes A, & Diaz MT (2019). Task difficulty modulates age-related differences in the behavioral and neural bases of language production. Neuropsychologia, 124, 254–273. doi: 10.1016/j.neuropsychologia.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Fan Y, Zou Q, Wang J, Gao J-H, & Niu Z (2014). Temporal reliability and lateralization of the resting-state language network. PloS one, 9(1), e85880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Ross TJ, Gu H, Geng X, Zuo X-N, Hong LE, Gao J-H, Stein EA, Zang Y-F, & Yang Y (2013). Intrinsic resting-state activity predicts working memory brain activation and behavioral performance. Hum Brain Mapp, 34(12), 3204–3215. doi: 10.1002/hbm.22136 [DOI] [PMC free article] [PubMed] [Google Scholar]


