Abstract
Aims
We compared long‐term clinical outcomes between patients treated with Orsiro sirolimus‐eluting stent (O‐SES) and those treated with durable biocompatible polymer Resolute Integrity zotarolimus‐eluting stent (R‐ZES).
Methods and Results
The ORIENT trial was a randomized controlled noninferiority trial to compare angiographic outcomes between O‐SES and R‐ZES. We performed a post hoc analysis of 3‐year clinical outcomes and included 372 patients who were prospectively enrolled and randomly assigned to O‐SES (n = 250) and R‐ZES (n = 122) groups in a 2:1 ratio. The primary endpoint was target lesion failure defined as a composite of cardiac death, nonfatal myocardial infarction, and target lesion revascularization. At 3 years, target lesion failure occurred in 4.7% and 7.8% of O‐SES and R‐ZES groups, respectively (hazard ratio, 0.58; 95% confidence intervals, 0.24–1.41; p = .232 by log‐rank test). Secondary endpoints including cardiac death, myocardial infarction, and target lesion revascularization showed no significant differences between the groups. Stent thrombosis occurred in two patients in R‐ZES group (0.0% vs. 1.6%, p = .040).
Conclusion
This study confirms long‐term safety and efficacy of the two stents. We found a trend for lower target lesion failure with O‐SES compared to R‐ZES, although statistically insignificant.
Keywords: biodegradable polymer, coronary artery disease, drug‐eluting stents, percutaneous coronary intervention
Abbreviations
- DES
drug‐eluting stents
- EES
everolimus‐eluting stent
- LLL
late lumen loss
- MI
myocardial infarction
- O‐SES
Orsiro sirolimus‐eluting stent
- PCI
percutaneous coronary intervention
- R‐ ZES
Resolute Integrity zotarolimus‐eluting stent
- TLF
target lesion failure
- TLR
target lesion revascularization
- TVR
target vessel revascularization
1. INTRODUCTION
Current‐generation drug‐eluting stents (DES) have shown high efficacy and safety, 1 , 2 , 3 and contemporary guidelines recommend DES as the preferred treatment option over bare metal stents, regardless of clinical situations. 4 , 5 Although DES have reduced the need for repeat revascularization in the short term, late stent failure remains a concern. First‐generation DES have shown be associated with a continuous accumulation of late adverse events including target lesion revascularization (TLR) and very late stent thrombosis. 6 , 7 , 8 In second‐generation DES, despite the improved performance and better patient outcomes with lower risk of late thrombotic events, stent failure still occurs. 9 , 10 Previous studies have suggested that in‐stent neoatherosclerosis contributes to late vascular complications. 11 , 12 However, there are still limited long‐term data on newer‐generation DES. 13
Orsiro Hybrid sirolimus‐eluting stent (O‐SES; Biotronik AG, Bulach, Switzerland) and Resolute Integrity zotarolimus‐eluting stent (R‐ZES; Medtronic Cardiovascular, Santa Rosa, CA) are among the widely used second‐generation DES with their good performance. 14 , 15 , 16 , 17 O‐SES has an ultrathin strut (60 μm) and unique hybrid coating of passive and active bioabsorbable polymer components. R‐ZES has a relatively thick strut (91 μm) and durable polymer, but has the advantage of good flexibility and conformability with round strut and continuous sinusoid technology. We previously reported the 9‐month angiographic outcomes of the two stents in a prospective randomized controlled trial of all‐comers with coronary artery disease. 18 , 19 In‐stent late loss at 9 months was 0.06 and 0.12 mm for O‐SES and R‐ZES respectively, which did not differ significantly. Adverse clinical event rates were low for both stents at 12 months. In the present study, we report 3‐year clinical outcomes of the trial to assess long‐term safety and efficacy of contemporary second‐generation DES.
2. METHODS
The ORIENT (the Orsiro Hybrid sirolimus‐eluting stent and Resolute Integrity zotarolimus‐eluting stent in a prospective randomized controlled trial of all‐comers with coronary artery disease) was a multicenter, randomized, open‐label, all‐comer, noninferiority trial. The design and results have been reported previously in detail. 18 , 19 In brief, the study participants were enrolled in eight centers in the Republic of Korea between October 2013 and June 2014. A total of 372 patients undergoing percutaneous coronary intervention for coronary artery disease were randomly assigned to O‐SES or R‐ZES in a 2:1 ratio, via a web‐based online randomization system. Coronary artery disease included stable angina as well as acute coronary syndrome. Percutaneous coronary intervention (PCI) was performed using standard techniques and routine protocols. Dual antiplatelet therapy was recommended for at least 12 months unless contraindicated. The study protocol was approved by the review boards of the participating institutions. All participating patients provided written informed consent for enrollment. The study complied with the provisions of the Declaration of Helsinki. After the index PCI, clinical follow‐up data were obtained from outpatient clinics visit or telephone at 1, 3, 9, and 12 months and extended annually thereafter up to 3 years.
The 9‐month angiographic results, in‐stent late lumen loss (LLL), have been published previously. 18 The main outcome of the present study was target lesion failure (TLF) at 3 years, a composite of cardiac death, TLR, and target vessel‐related myocardial infarction (MI). The secondary endpoints included target vessel failure, patient‐oriented composite endpoint, a composite of cardiac death and nonfatal MI, all‐cause and cardiac death, nonfatal MI (target or nontarget vessel‐related), clinically driven TLR, clinically driven target vessel revascularization (TVR), stroke, bleeding, and definite or probable stent thrombosis. Target vessel failure was defined as a composite of cardiovascular death, target vessel MI, or TVR. Patient‐oriented composite endpoint was defined as a composite of all‐cause mortality, any stroke, MI, or revascularization. The definitions of clinical events followed the recommendations of the Academic Research Consortium and the Forth Universal Definition of MI. 20 , 21 Only spontaneous MI was included, while index procedure‐related type 4a MI was not considered. Bleeding events were defined according to the PLATO (Platelet Inhibition and Patient Outcomes) criteria. 22 All clinical events were adjudicated by an independent clinical event adjudication committee.
Categorical variables were compared using the chi‐square or Fisher's exact test, and continuous variables were compared using the independent t‐test or Wilcoxon's signed‐rank test as appropriate. The study outcomes were assessed throughout 3 years since enrolment. The survival curves were constructed for time‐to‐event variables using Kaplan–Meier estimates and were compared using the log‐rank test.
Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using Cox proportional‐hazards models for between‐group comparison of clinical outcomes.
All probability values were two‐sided, and p < .05 was considered statistically significant. Statistical analyses were performed using R programming, version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
3. RESULTS
As shown in Figure 1, 98.4%, 96.2%, and 94.1% (350 out of 372) completed clinical follow‐up at 1, 2, and 3 years after coronary stent implantation, respectively. The baseline characteristics have been reported previously 18 and are reproduced in Table 1. There were no significant differences between the two groups. The mean age was 65 years, and male sex accounted for 71.5% of the study population. The proportion of hypertension, diabetes, and current smoking was 65.5%, 26.1%, and 27.4%, respectively. Approximately half of the study population (47%) was clinically diagnosed with acute coronary syndrome. Dual antiplatelet therapy was maintained in 61.7%, 36.9%, and 27.5% at 1, 2, and 3 years, respectively (Table 2).
FIGURE 1.
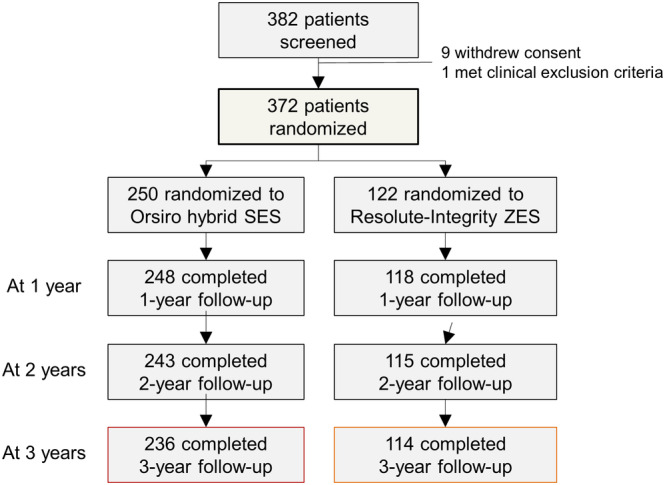
Flow diagram of the study population. SES, sirolimus‐eluting stent; ZES, zotarolimus‐eluting stent [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Baseline characteristics
| Total | Orsiro Hybrid SES (N = 250) | Resolute Integrity ZES (N = 122) | |
|---|---|---|---|
| Age | 65.1 ± 11.6 | 65.2 ± 11.9 | 64.8 ± 11.0 |
| Sex (male) | 266 (71.5) | 180 (72.0) | 86 (70.5) |
| Body mass index (kg/m2) | 24.7 ± 3.4 | 24.8 ± 3.5 | 24.5 ± 3.1 |
| Hypertension | 244 (65.6) | 163 (65.2) | 81 (66.4) |
| Diabetes | 97 (26.1) | 63 (25.2) | 34 (27.9) |
| Dyslipidemia | 200 (53.8) | 134 (53.6) | 66 (54.1) |
| Current smoker | 102 (27.4) | 67 (26.8) | 35 (28.7) |
| Chronic renal failure | 10 (2.7) | 7 (2.8) | 3 (2.5) |
| History of stroke | 33 (8.9) | 25 (10.0) | 8 (6.6) |
| Peripheral artery disease | 8 (2.2) | 4 (1.6) | 4 (3.3) |
| Previous PCI | 52 (14.0) | 34 (13.6) | 18 (14.8) |
| Previous bypass surgery | 2 (0.5) | 2 (0.8) | 0 (0.0) |
| Chronic lung disease | 12 (3.2) | 9 (3.6) | 3 (2.5) |
| Clinical diagnosis | |||
| Stable angina | 200 (53.8) | 133 (53.2) | 67 (54.9) |
| Unstable angina | 87 (23.4) | 62 (24.8) | 25 (20.5) |
| NSTEMI | 53 (14.2) | 33 (13.2) | 20 (16.4) |
| STEMI | 32 (8.6) | 22 (8.8) | 10 (8.2) |
| Discharge medications | |||
| Aspirin | 363 (97.6) | 243 (97.2) | 120 (98.4) |
| Clopidogrel | 361 (97.0) | 243 (97.2) | 118 (96.7) |
| ACE inhibitors | 138 (37.1) | 92 (36.8) | 46 (37.7) |
| Angiotensin receptor blockers | 122 (32.8) | 82 (32.8) | 40 (32.8) |
| β‐Blockers | 245 (65.9) | 158 (63.2) | 87 (71.3) |
| Calcium channel blockers | 118 (31.7) | 76 (30.4) | 42 (34.4) |
| Statins | 342 (91.9) | 224 (89.6) | 118 (96.7) |
Abbreviations: ACE, angiotensin‐converting enzyme; NSTEMI, Non‐ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; SES, sirolimus‐eluting stent; STEMI, ST‐elevation myocardial infarction; ZES, zotarolimus‐eluting stent.
TABLE 2.
Medications at 1, 2, and 3 years
| Total | Orsiro Hybrid SES (N = 250) | Resolute Integrity ZES (N = 122) | p values | |
|---|---|---|---|---|
| At 1 year | (n = 363) | (n = 246) | (n = 117) | |
| Aspirin | 82.9 (301/363) | 83.7 (206/246) | 81.2 (95/117) | .599 |
| Clopidogrel | 74.9 (272/363) | 76.0 (187/246) | 72.6 (85/117) | .785 |
| Dual antiplatelet therapy | 61.7 (224/363) | 64.2 (158/246) | 56.4 (66/117) | .316 |
| At 2 years | (n = 350) | (n = 238) | (n = 112) | |
| Aspirin | 73.7 (258/350) | 75.6 (180/238) | 69.6 (78/112) | .167 |
| Clopidogrel | 54.0 (189/350) | 52.9 (126/238) | 56.2 (63/112) | .726 |
| Dual antiplatelet therapy | 36.9 (129/350) | 37.8 (90/238) | 34.8 (39/112) | .671 |
| At 3 years | (n = 349) | (n = 237) | (n = 112) | |
| Aspirin | 66.8 (233/349) | 68.8 (163/237) | 62.5 (70/112) | .085 |
| Clopidogrel | 47.6 (166/349) | 46.0 (109/237) | 50.9 (57/112) | .689 |
| Dual antiplatelet therapy | 27.5 (96/349) | 27.8 (66/237) | 26.8 (30/112) | .812 |
Abbreviations: SES, sirolimus‐eluting stent; ZES, zotarolimus‐eluting stent.
TLF occurred in 4.7% and 7.8% at 3 years in the O‐SES and R‐ZES groups, respectively (log‐rank p = .227) (Figure 2a). The occurrence of patient‐oriented composite endpoint did not differ between the two groups (15.6% and 11.3%; log‐rank p = .313) (Figure 2b). Table 3 summarizes the cumulative event rates at 1, 2, and 3 years. No significant differences were observed between the two groups in terms of death, MI, repeat revascularization, stroke, and bleeding.
FIGURE 2.
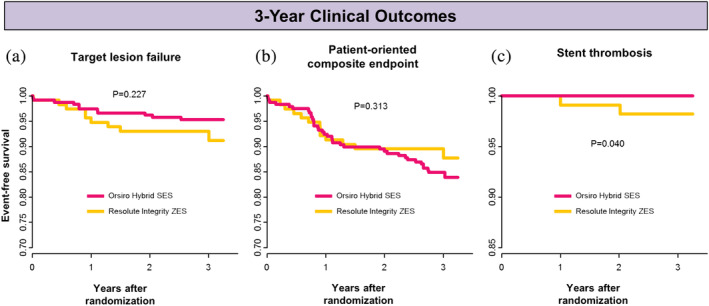
Kaplan–Meier time‐to‐event curves for 3‐year clinical outcomes: (a) target lesion failure, (b) patient‐oriented composite endpoint and death, and (c) stent thrombosis. SES, sirolimus‐eluting stent; ZES, zotarolimus‐eluting stent [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Clinical outcomes up to 3 years
| Orsiro Hybrid SES | Resolute Integrity ZES | OR (95% CI) | p values | |
|---|---|---|---|---|
| Events at 1 year | ||||
| All‐cause death | 4 (1.6) | 3 (2.5) | 0.63 (0.11–4.37) | .686 |
| Cardiovascular death | 2 (0.8) | 2 (1.7) | 0.47 (0.03–6.59) | .597 |
| Myocardial infarction | 0 (0.0) | 2 (1.7) | – | .103 |
| Repeat revascularization | 19 (7.7) | 7 (6.0) | 1.31 (0.51–3.81) | .666 |
| Target lesion revascularization | 6 (2.4) | 4 (3.4) | 0.71 (0.16–3.47) | .733 |
| Target vessel revascularization | 9 (3.6) | 5 (4.2) | 0.85 (0.25–3.31) | .776 |
| Stroke | 1 (0.4) | 0 (0.0) | – | 1.000 |
| Bleeding | 7 (2.8) | 5 (4.2) | 0.66 (0.17–2.68) | .534 |
| Major, life‐threatening | 0 (0.0) | 1 (0.9) | – | .320 |
| Major, others | 1 (0.4) | 1 (0.9) | 0.47 (0.01–37.5) | .542 |
| Minor | 6 (2.5) | 3 (2.6) | 0.94 (0.20–5.92) | 1.000 |
| Cardiac death or myocardial infarction | 2 (0.8) | 3 (2.6) | 0.31 (0.03–2.77) | .334 |
| TLF (cardiac death, MI, TLR) | 8 (3.2) | 6 (5.0) | 0.63 (0.19–2.25) | .395 |
| TVF (cardiac death, MI, TVR) | 11 (4.4) | 8 (6.7) | 0.64 0.23–1.90) | .450 |
| POCE (death, MI, RR) | 23 (9.3) | 10 (8.4) | 1.11 (0.49–2.72) | .848 |
| Events at 2 years | ||||
| All‐cause death | 5 (2.0) | 3 (2.0) | 0.78 (0.15–5.14) | .715 |
| Cardiovascular death | 2 (0.8) | 2 (1.7) | 0.47 (0.03–6.56) | .596 |
| Myocardial infarction | 0 (0.0) | 3 (2.6) | – | .032 |
| Repeat revascularization | 22 (9.0) | 9 (7.8) | 1.17 (0.50–2.99) | .841 |
| Target lesion revascularization | 8 (3.3) | 6 (5.2) | 0.62 (0.18–2.22) | .391 |
| Target vessel revascularization | 11 (4.5) | 7 (6.0) | 0.73 (0.25–2.29) | .605 |
| Stroke | 2 (0.8) | 0 (0.0) | – | 1.000 |
| Bleeding | 7 (2.9) | 6 (5.2) | 0.54 (0.15–1.99) | .363 |
| Major, life threatening | 0 (0.0) | 1 (0.9) | – | .319 |
| Major, others | 1 (0.4) | 1 (0.9) | 0.47 (0.01–37.3) | .540 |
| Minor | 6 (2.5) | 4 (3.6) | 0.70 (0.16–3.42) | .732 |
| Cardiac death or myocardial infarction | 2 (0.8) | 4 (3,5) | 0.23 (0.02–1.64) | .087 |
| TLF (cardiac death, MI, TLR) | 10 (4.1) | 8 (6.9) | 0.58 (0.20–1.74) | .302 |
| TVF (cardiac death, MI, TVR) | 14 (5.8) | 10 (8.6) | 0.65 (0.26–1.69) | .366 |
| POCE (death, MI, RR) | 28 (11.5) | 12 (10.3) | 1.13 (0.53–2.54) | .858 |
| Events at 3 years | ||||
| All‐cause death | 9 (3.8) | 4 (3.5) | 1.09 (0.30–4.95) | 1.000 |
| Cardiovascular death | 2 (0.8) | 3 (2.6) | 0.32 (0.03–2.86) | .336 |
| Myocardial infarction | 1 (0.4) | 3 (2.6) | 0.16 (0.00–2.03) | .106 |
| Repeat revascularization | 28 (12.0) | 9 (7.8) | 1.59 (0.70–3.98) | .271 |
| Target lesion revascularization | 9 (3.8) | 6 (5.2) | 0.73 (0.22–2.55) | .580 |
| Target vessel revascularization | 15 (6.3) | 7 (6.0) | 1.04 (0.39–3.11) | 1.000 |
| Stroke | 2 (0.9) | 1 (0.9) | 0.97 (0.05–57.9) | 1.000 |
| Bleeding | 8 (3.4) | 6 (5.2) | 0.64 (0.19–2.31) | .402 |
| Major, life threatening | 1 (0.4) | 1 (0.9) | 0.49 (0.01–38.4) | .548 |
| Major, others | 1 (0.4) | 1 (0.9) | 0.49 (0.01–37.3) | .551 |
| Minor | 6 (2.6) | 4 (3.6) | 0.72 (0.17–3.56) | .734 |
| Cardiac death or myocardial infarction | 3 (1.3) | 5 (4.4) | 0.29 (0.04–1.50) | .121 |
| TLF (cardiac death, MI, TLR) | 11 (4.7) | 9 (7.8) | 0.59 (0.21–1.66) | .327 |
| TVF (cardiac death, MI, TVR) | 18 (7.6) | 11 (9.6) | 0.79 (0.34–1.93) | .543 |
| POCE (death, MI, RR) | 37 (15.6) | 13 (11.3) | 1.45 (0.72–3.11) | .330 |
Abbreviations: CI, confidence interval; MI, myocardial infarction; OR, odd ratio; POCE, patient‐oriented clinical endpoint; RR, repeat revascularization; SES, sirolimus‐eluting stent; TLF, target lesion failure; TLR, target lesion revascularization; TVF, target vessel failure; TVR, target vessel revascularization; ZES, zotarolimus‐eluting stent.
No cases of stent thrombosis were reported in the O‐SES group, while two patients experienced stent thrombosis in the R‐ZES arm (log‐rank p = .040) (Figure 2c), which were confirmed as definite thrombosis on angiography. One of them developed thrombosis at 365 days since the index procedure, while the patient discontinued the dual antiplatelet therapy on his own for 7 days. Regarding the other case, the index lesion was chronic total occlusion of the right coronary artery, and long stenting was performed. Dual antiplatelet therapy was switched to aspirin alone at 1 year, and stent thrombosis developed at 736 days. Subgroup analysis showed no significant effect modification across subgroups (Figure 3).
FIGURE 3.
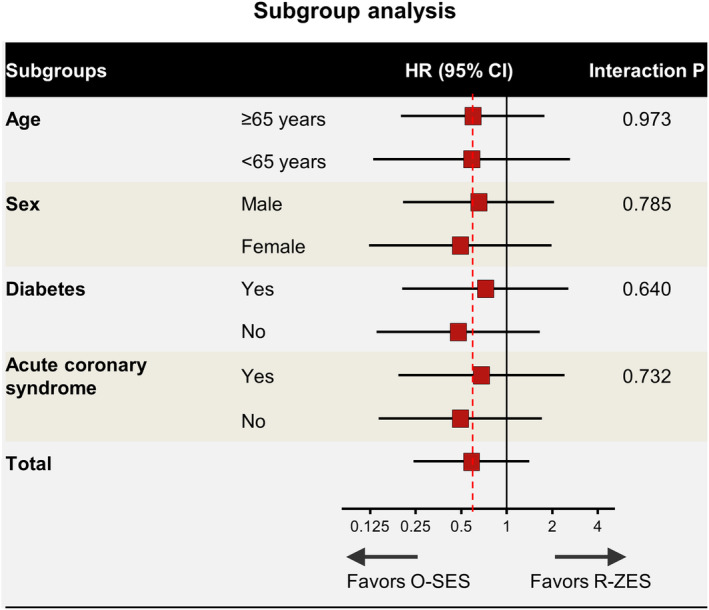
Subgroup analysis for target lesion failure. Stratified analyses for several subgroups of target lesion failure. Horizontal lines represent 95% confidence intervals. HR, hazard ratio; O‐SES, Orsiro sirolimus‐eluting stent; R‐ZES, Resolute Integrity zotarolimus‐eluting stent [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, we report 3‐year clinical outcomes of patients who were randomly assigned to O‐SES and R‐ZES. The risk of adverse events including target lesion failure did not differ significantly between the two stents up to 3 years. Two patients developed very late stent thrombosis, both of them were in the R‐ZES arm.
Numerous studies have been performed through the innovation of stent design and platform for better outcomes. 23 , 24 Biodegradable polymer has been adopted owing to concerns that durable polymer may trigger local inflammation and contribute to the development of late stent thrombosis. 14 , 25 , 26 Histopathological analysis in a porcine model of biodegradable polymer sirolimus stent implantation demonstrated favorable vascular healing, reductions in neointimal area, and low inflammatory responses. 27 Thinner struts, however, may be associated with more contribution to the lower risk of stent thrombosis by reducing arterial damage and facilitating reendothelialization. 28 , 29 , 30
Recent studies have confirmed the efficacy and safety of O‐SES, which is coated with biodegradable polymer and has ultra‐thin strut. Several randomized controlled trials with O‐SES showed comparable clinical outcomes with contemporary durable‐polymer DES. 14 , 16 , 31 , 32 The recently published 5‐year outcomes of the BIOSCIENCE (Sirolimus‐eluting Stents With Biodegradable Polymer Versus an Everolimus‐eluting Stents) trial showed that the long‐term risk of TLF was similar for O‐SES and durable‐polymer everolimus‐eluting stent (EES). 33 In the meanwhile, O‐SES outperformed durable‐polymer EES in a complex patient population undergoing percutaneous coronary intervention in terms of TLF and target‐vessel‐related MI in the BIOFLOW V (Biotronik Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus‐Eluting Coronary Stent System in the Treatment of Subjects with Up to Three De Novo or Restenotic Coronary Artery Lesions V) trial. 34 , 35 A recent meta‐analysis of 10 randomized trials suggested a marginally lower risk of TLF and a significantly lower risk of MI with O‐SES than second‐generation thicker strut DES at 1 year. 30
In this study, the results regarding stent thrombosis need judicious interpretation. Although the difference was statistically significant, there were only two cases of stent thrombosis. However, current studies have shown similar trends in the risk of stent thrombosis of O‐SES. The 5‐year outcomes of the BIOFLOW‐II trial showed a marginally lower risk of stent thrombosis in patients treated with O‐SES than in those treated with EES (0.7% vs. 2.8%; p = .088). In the BIOFLOW V trial, late/very late rates of both definite and definite/probable stent thrombosis were shown to be significantly lower in the O‐SES cohort. 34 , 35 The BIOSCIENCE trial also showed a marginal interaction of higher risk of stent thrombosis within 1 year and a lower risk of very late stent thrombosis between 1 and 5 years with O‐SES (interaction p = .080), although the overall cumulative incidence was similar in both O‐SES and durable‐polymer EES. 33
However, caution is required as the BIONYX (Bioresorbable polymer‐coated Orsiro versus durable polymer‐coated Resolute Onyx stents) trial showed results contradictory. 36 O‐SES was compared with the Resolute Onyx stent, which is the next iteration of Resolute Integrity ZES. Resolute Onyx has a very similar design as the previous version, except a metallic platform, which includes a platinum‐iridium core intended to improve radiographic visibility. The overall 1‐year risk of target vessel failure was similar between the two groups. However, definite or probable stent thrombosis occurred in a significantly lower rate in the Resolute Onyx group than that in the O‐SES group (0.1% vs. 0.7%; p = .011). Further clinical studies with longer‐term follow‐up are needed.
5. LIMITATIONS
The trial was originally powered for the primary endpoint of 9‐month in‐stent restenosis. This is a report of a post hoc analysis; thus, the sample size is not adequately powered for comparison of clinical outcomes. Therefore, the results of this study should be considered as hypothesis generating. Second, there was a small number of events, especially regarding stent thrombosis. Even the lost in follow‐up rate reached close to 6%. Third, medical treatment, including antiplatelet regimen was left at the discretion of the treating physicians. Lastly, while Resolute Integrity ZES was tested in this study, a newer version of Resolute Onyx™ (Medtronic) is being used in clinical practice.
6. CONCLUSION
We report favorable outcomes of O‐SES and R‐ZES throughout 3 years after implantation. The present study confirmed the safety and efficacy profiles of the current generation DES. The rate of TLF did not significantly differ between the two stents.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Kim S‐H, Kang S‐H, Lee JM, et al. Three‐year clinical outcome of biodegradable hybrid polymer Orsiro sirolimus‐eluting stent and the durable biocompatible polymer Resolute Integrity zotarolimus‐eluting stent: A randomized controlled trial. Catheter Cardiovasc Interv. 2020;96:1399–1406. 10.1002/ccd.28654
EDITORIAL COMMENT: Expert Article Analysis for: Times up to demonstrate a difference is current DES platforms
Soo‐Hyun Kim and Si‐Hyuck Kang contributed equally to this study.
Funding information Biotronik Korea Co., Korea, Grant/Award Number: 10.13039/501100005035
REFERENCES
- 1. Nordrehaug JE, Wiseth R, Bonaa KH. Drug‐eluting or bare‐metal stents for coronary artery disease. N Engl J Med. 2016;375(26):2604‐2605. [DOI] [PubMed] [Google Scholar]
- 2. Byrne RA, Serruys PW, Baumbach A, et al. Report of a European Society of Cardiology‐European Association of Percutaneous Cardiovascular Interventions task force on the evaluation of coronary stents in Europe: executive summary. Eur Heart J. 2015;36(38):2608‐2620. [DOI] [PubMed] [Google Scholar]
- 3. Kang SH, Chae IH, Park JJ, et al. Stent thrombosis with drug‐eluting stents and bioresorbable scaffolds: evidence from a network meta‐analysis of 147 trials. JACC Cardiovasc Interv. 2016;9(12):1203‐1212. [DOI] [PubMed] [Google Scholar]
- 4. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non‐ST‐Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134(10):e123‐e155. [DOI] [PubMed] [Google Scholar]
- 5. Neumann F‐J, Sousa‐Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40(2):87‐165. [Google Scholar]
- 6. Weisz G, Leon MB, Holmes DR, et al. Five‐year follow‐up after sirolimus‐eluting stent implantation. Results of the SIRIUS (Sirolimus‐Eluting Stent in De‐Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53(17):1488‐1497. [DOI] [PubMed] [Google Scholar]
- 7. Galloe AM, Kelbaek H, Thuesen L, et al. 10‐year clinical outcome after randomization to treatment by Sirolimus‐ or paclitaxel‐eluting coronary stents. J Am Coll Cardiol. 2017;69(6):616‐624. [DOI] [PubMed] [Google Scholar]
- 8. Yamaji K, Raber L, Zanchin T, et al. Ten‐year clinical outcomes of first‐generation drug‐eluting stents: the Sirolimus‐eluting vs. paclitaxel‐eluting stents for coronary revascularization (SIRTAX) VERY LATE trial. Eur Heart J. 2016;37(45):3386‐3395. [DOI] [PubMed] [Google Scholar]
- 9. Camenzind E, Wijns W, Mauri L, et al. Stent thrombosis and major clinical events at 3 years after zotarolimus‐eluting or sirolimus‐eluting coronary stent implantation: a randomised, multicentre, open‐label, controlled trial. Lancet. 2012;380(9851):1396‐1405. [DOI] [PubMed] [Google Scholar]
- 10. Brener SJ, Kereiakes DJ, Simonton CA, et al. Everolimus‐eluting stents in patients undergoing percutaneous coronary intervention: final 3‐year results of the clinical evaluation of the XIENCE V Everolimus eluting coronary stent system in the treatment of subjects with de novo native coronary artery lesions trial. Am Heart J. 2013;166(6):1035‐1042. [DOI] [PubMed] [Google Scholar]
- 11. Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. 2015;36(32):2147‐2159. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura D, Attizzani GF, Toma C, et al. Failure mechanisms and Neoatherosclerosis patterns in very late drug‐eluting and bare‐metal stent thrombosis. Circ Cardiovasc Interv. 2016;9(9):e003785. [DOI] [PubMed] [Google Scholar]
- 13. Kang SH, Gogas BD, Jeon KH, et al. Long‐term safety of bioresorbable scaffolds: insights from a network meta‐analysis including 91 trials. EuroIntervention. 2018;13(16):1904‐1913. [DOI] [PubMed] [Google Scholar]
- 14. Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus‐eluting stent versus durable polymer everolimus‐eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single‐blind, non‐inferiority trial. Lancet. 2014;384(9960):2111‐2122. [DOI] [PubMed] [Google Scholar]
- 15. Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus‐eluting durable‐polymer‐coated stent versus a biolimus‐eluting biodegradable‐polymer‐coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non‐inferiority trial. Lancet (London, England). 2015;385(9977):1527‐1535. [DOI] [PubMed] [Google Scholar]
- 16. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus‐eluting and sirolimus‐eluting stents versus durable polymer zotarolimus‐eluting stents in allcomers with coronary artery disease (BIO‐RESORT): a three‐arm, randomised, non‐inferiority trial. Lancet (London, England). 2016;388(10060):2607‐2617. [DOI] [PubMed] [Google Scholar]
- 17. Park K‐H, Ahn Y, Koh Y‐Y, et al. Effectiveness and safety of Zotarolimus‐eluting stent (resolute™ Integrity) in patients with diffuse long coronary artery disease. Korean Circ J. 2019;49(8):709‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang SH, Chung WY, Lee JM, et al. Angiographic outcomes of Orsiro biodegradable polymer sirolimus‐eluting stents and resolute integrity durable polymer zotarolimus‐eluting stents: results of the ORIENT trial. EuroIntervention. 2017;12(13):1623‐1631. [DOI] [PubMed] [Google Scholar]
- 19. Lee JM, Park SD, Lim SY, et al. Angiographic and clinical comparison of novel Orsiro hybrid sirolimus‐eluting stents and resolute integrity zotarolimus‐eluting stents in all‐comers with coronary artery disease (ORIENT trial): study protocol for a randomized controlled trial. Trials. 2013;14:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia‐Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the academic research Consortium‐2 consensus document. Circulation. 2018;137(24):2635‐2650. [DOI] [PubMed] [Google Scholar]
- 21. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231‐2264. [DOI] [PubMed] [Google Scholar]
- 22. James S, Akerblom A, Cannon CP, et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient outcomes (PLATO) trial. Am Heart J. 2009;157(4):599‐605. [DOI] [PubMed] [Google Scholar]
- 23. Stefanini GG, Taniwaki M, Windecker S. Coronary stents: novel developments. Heart. 2014;100(13):1051‐1061. [DOI] [PubMed] [Google Scholar]
- 24. Capodanno D. Bioresorbable scaffolds in coronary intervention: unmet needs and evolution. Korean Circ J. 2018;48(1):24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joner M, Finn AV, Farb A, et al. Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193‐202. [DOI] [PubMed] [Google Scholar]
- 26. Windecker S, Serruys PW, Wandel S, et al. Biolimus‐eluting stent with biodegradable polymer versus sirolimus‐eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non‐inferiority trial. Lancet. 2008;372(9644):1163‐1173. [DOI] [PubMed] [Google Scholar]
- 27. Koppara T, Joner M, Bayer G, Steigerwald K, Diener T, Wittchow E. Histopathological comparison of biodegradable polymer and permanent polymer based sirolimus eluting stents in a porcine model of coronary stent implantation. Thromb Haemost. 2012;107(6):1161‐1171. [DOI] [PubMed] [Google Scholar]
- 28. Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high‐risk interventional settings is driven by stent design and deployment and protected by polymer‐drug coatings. Circulation. 2011;123(13):1400‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen Lisette O, Thayssen P, Maeng M, et al. Randomized comparison of a biodegradable polymer ultrathin strut sirolimus‐eluting stent with a biodegradable polymer biolimus‐eluting stent in patients treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9(7):e003610. [DOI] [PubMed] [Google Scholar]
- 30. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer‐generation ultrathin strut drug‐eluting stents versus older second‐generation thicker strut drug‐eluting stents for coronary artery disease. Circulation. 2018;138(20):2216‐2226. [DOI] [PubMed] [Google Scholar]
- 31. Windecker S, Haude M, Neumann FJ, et al. Comparison of a novel biodegradable polymer sirolimus‐eluting stent with a durable polymer everolimus‐eluting stent: results of the randomized BIOFLOW‐II trial. Circ Cardiovasc Interv. 2015;8(2):e001441. [DOI] [PubMed] [Google Scholar]
- 32. Teeuwen K, van der Schaaf RJ, Adriaenssens T, et al. Randomized multicenter trial investigating angiographic outcomes of hybrid sirolimus‐eluting stents with biodegradable polymer compared with everolimus‐eluting stents with durable polymer in chronic total occlusions: The PRISON IV Trial. JACC Cardiovasc Interv. 2017;10(2):133‐143. [DOI] [PubMed] [Google Scholar]
- 33. Pilgrim T, Piccolo R, Heg D, et al. Ultrathin‐strut, biodegradable‐polymer, sirolimus‐eluting stents versus thin‐strut, durable‐polymer, everolimus‐eluting stents for percutaneous coronary revascularisation: 5‐year outcomes of the BIOSCIENCE randomised trial. Lancet. 2018;392(10149):737‐746. [DOI] [PubMed] [Google Scholar]
- 34. Kandzari DE, Mauri L, Koolen JJ, et al. Ultrathin, bioresorbable polymer sirolimus‐eluting stents versus thin, durable polymer everolimus‐eluting stents in patients undergoing coronary revascularisation (BIOFLOW V): a randomised trial. Lancet. 2017;390(10105):1843‐1852. [DOI] [PubMed] [Google Scholar]
- 35. Kandzari DE, Koolen JJ, Doros G, et al. Ultrathin bioresorbable polymer sirolimus‐eluting stents versus thin durable polymer everolimus‐eluting stents. J Am Coll Cardiol. 2018;72(25):3287‐3297. [DOI] [PubMed] [Google Scholar]
- 36. von Birgelen C, Zocca P, Buiten RA, et al. Thin composite wire strut, durable polymer‐coated (resolute onyx) versus ultrathin cobalt‐chromium strut, bioresorbable polymer‐coated (Orsiro) drug‐eluting stents in allcomers with coronary artery disease (BIONYX): an international, single‐blind, randomised non‐inferiority trial. Lancet (London, England). 2018;392(10154):1235‐1245. [DOI] [PubMed] [Google Scholar]


