Abstract
Aim
Serous carcinoma of the uterine cervix (USCC) is a very rare malignant tumor, while this histological subtype is common in the ovary, fallopian tube, uterine corpus and peritoneum. Because of its rarity, details of the clinicopathological features of USCC are largely unknown. We retrospectively analyzed the clinicopathological characteristics of five cases of pure USCC.
Methods
We reviewed the medical records and pathological specimens of five USCC cases who were treated at the Gynecology Service of the National Hospital Organization Kyushu Cancer Center, Japan, between 2000 and 2017. The clinicopathological features were also compared with those of serous carcinomas of the endometrium and ovary who were treated during the same period.
Results
Five patients were treated at our hospital between 2000 and 2017. Three tumors were stage IB1, one was stage IIB, and one was stage IVB. The median follow‐up time was 104 months (range 26–210). Four patients other than stage IVB were treated with radical hysterectomy and have been free of relapse. One patient with stage IVB tumor was treated with platinum‐based combination chemotherapy and is currently on maintenance therapy with bevacizumab and remains free of relapse.
Conclusion
USCC has a distinctive clinicopathological feature that differentiates it from serous carcinomas of other female organs. USCC had been thought to be a poor prognostic disease; however, it could be curable if it is not accompanied by lymph node metastasis or peritoneal dissemination. We might conquer USCC even if it is accompanied by lymph node metastasis with the use of multimodal therapy.
Keywords: cervical cancer, pathology, prognostic factor, serous carcinoma, therapy
Introduction
Serous carcinoma of the uterine cervix (USCC) is a very rare malignant tumor, while this histological subtype is common in the ovary, fallopian tube, uterine corpus and peritoneum.1, 2 Although it is recognized as an aggressive neoplasm, details of the clinicopathological features of USCC are largely unknown because of its rarity. Few studies on USCC have been reported, with the largest to date including 17 patients.3 We report five cases with USCC treated at our hospital and compare these with cases of serous carcinoma of the endometrium (USCE) and high‐grade serous carcinoma of the ovary (OSC). We also include a literature review.
Methods
We reviewed the medical records and pathological specimens of USCC cases who were treated at the Gynecology Service of the National Hospital Organization Kyushu Cancer Center, Japan, between 2000 and 2017. All definitive diagnosis was made based on excised specimens. This study included patients who had had whole lesion fulfilling the histological criteria of World Health Organization International Histological Classification (2014). Two pathologists re‐examined all pathological specimens independently. Five cases were diagnosed as pure USCC. Pathological features were examined by hematoxylin and eosin staining and immunohistochemistry. These slides were used to perform chromogenic in situ hybridization (ISH), according to standard protocols. The probe sets used for assay included were well validated to identify high‐risk human papillomavirus (HPV; Ventana Medical Systems). The high‐risk HPV genotypes included type 16, 18, 31, 33, 35, 45, 52, 56, 58 and 66. The slides were examined for the presence of integrated HPV using light microscopy.
The therapeutic strategy was determined according to usual cervical cancer. We also reviewed the clinicopathological findings of USCE and OSC patients who underwent surgery in our institute during the same period. The clinicopathological factors of USCC, USCE and OSC included age, symptoms, pretreatment diagnosis, tumor size, international federation of gynecology and obstetrics (FIGO) stage (USCC in FIGO 2008; USCE in FIGO 2008; OSC in FIGO 2014), treatment, lymph node metastasis, distant metastasis and so on. All the patients were periodically followed up at our outpatient clinic for at least 10 years. The ethics committee of National Hospital Organization Kyushu Cancer Center approved the study protocol.
Results
Clinical courses of five cases
Five patients among 1144 cases with cervical cancer (0.4%) were treated at the Gynecology Service of the National Hospital Organization Kyushu Cancer Center, Japan, between 2000 and 2017. During the same period, 49 USCE cases (among 899 cases with endometrial cancer [5.5%]) and 161 OSC cases (among 528 [30.5%]) patients were treated, respectively. The five patients' characteristics are summarized in Table 1. The median age was 54 years old (range 34–64) and median follow‐up time was 104 months (range 26–210). No patient was lost to follow‐up. All five patients presented with abnormal genital bleeding as the primary symptom. The results of cervical cytology of four patients other than case 3 were adenocarcinoma. A cervical smear of case 3 demonstrated severe dysplasia. Punch biopsies of cervix of five patients demonstrated adenocarcinoma. A biopsy specimen of case 3 revealed adenocarcinoma and cervical intraepithelial neoplasm 3. Tumors were staged according to the 2014 International Federation of Gynecologists and Obstetricians staging system for carcinoma of the uterine cervix. Three tumors were stage IB1 and the others stage IIB and stage IVB. Four patients other than the one with the stage IVB tumor were treated with radical hysterectomy, bilateral salpingo‐oophorectomy, pelvic lymphadenectomy and para‐aortic lymphadenectomy. These four patients had no visible residual lesion after surgery completion.
Table 1.
Clinicopathological characteristics of five serous carcinoma of the uterine cervix cases
| No | Age | FIGO stage† | (p)TNM† | Stromal invasion | LN metastasis | Peritoneal cytology | Therapy | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 64 | IB1 | pT1b1N1M1‡ | Full thickness | PLN+, PAN+ | Negative | Ope + CT + CCRT | 17 years 6 months NED |
| 2 | 61 | IB1 | pT1b1N0M0 | 1/8 mm | No metastasis | Negative | Ope | 10 years 5 months NED |
| 3 | 36 | IB1 | pT1b1N0M0 | 4/14 mm | No metastasis | Negative | Ope | 8 years 8 months NED |
| 4 | 54 | IIB | pT2bN1M1‡ | 24/26 mm | PLN+, PAN+ | Negative | Ope + CT + CCRT | 4 years 8 months NED |
| 5 | 47 | IVB | T2bN1M1 | ND | PLN+ (image) | Positive | CT | 2 years 2 months NED |
FIGO 2008 and TNM classification 8th edition.
Positive para‐aortic lymph node metastasis.
CCRT, concurrent chemoradiotherapy; CT, chemotherapy; LN, lymph node; ND, no data; NED, no evidence of disease; Ope, radical hysterectomy; PAN, paraaortic lymph node; PLN, pelvic lymph node; TNM, tumor‐node‐metastasis classification.
Case 1
The final pathological diagnosis of her surgical specimen revealed a 3‐cm‐diameter tumor with full‐thickness stromal invasion, pelvic lymph node metastasis and para‐aortic lymph node metastasis. She received systemic chemotherapy (cisplatin, ifomide and 5‐fluorouracil) followed by concurrent chemoradiotherapy (CCRT; cisplatin administration and whole pelvis and para‐aortic lesion irradiation to 40.5Gy/27Fr). She suffered left breast cancer 12 years later, for which she underwent breast surgery followed by adjuvant hormonal therapy. She is alive and remains free of relapse 17 years and 6 months after initial treatment.
Case 2
Gross examination revealed a 2‐cm‐diamiter with infiltration of 1/8 mm. There was no lymph node metastasis. She also developed left breast cancer 6 years later, for which she underwent breast surgery followed by adjuvant hormonal therapy. She remains free of relapse after 10 years and 5 months follow‐up.
Case 3
Although colposcopy showed acetowhite epithelium, atypical vessels and micropapillary lesion in the cervical canal, the resected specimen revealed no gross tumor. There was a 1.5‐cm USCC lesion with infiltration of 4/14 mm associated with high‐grade squamous intraepithelial lesion and microinvasive squamous cell carcinoma. The squamous intraepithelial lesion extended to the vaginal wall. She underwent intravaginal irradiation (30Gy/10Fr). She remains free of relapse after 8 years and 8 months of follow‐up.
Case 4
There was a 2‐cm ulcerative lesion in the endocervix. The tumor deeply invaded the stroma (24/26 mm) with the right parametrium invasion, lymphovascular invasion, pelvic lymph node metastasis and para‐aortic lymph node metastasis. She received systemic chemotherapy (paclitaxel and carboplatin) followed by CCRT (cisplatin administration and whole pelvis and para‐aortic lesion irradiation to 45Gy/25Fr). She remains free of relapse 4 years and 8 months after initial treatment.
Case 5
Pelvic examination showed that a 5–6‐cm mass replaced the cervix and spread to vagina and left parametrium. Computed tomography scan showed ascites retention, multiple peritoneal dissemination, pelvic lymph node swelling and bilateral ovarian enlargement. Magnetic resonance imaging showed large cervical mass and smaller bilateral ovarian tumors (Fig. 1). The cervical mass was biopsied and diagnosed as USCC. The patient was diagnosed with cervical cancer with bilateral ovarian metastasis and peritoneal spread, stage IVB (T4N1M0) according to imaging findings. We selected paclitaxel and carboplatin combination chemotherapy for the primary treatment. All tumors including that in the cervix disappeared after 10 courses of chemotherapy. Bevacizumab was added to the chemotherapy on the ninth course. She is currently undergoing maintenance therapy with bevacizumab and remains free of relapse for 2 years and 2 months.
Figure 1.
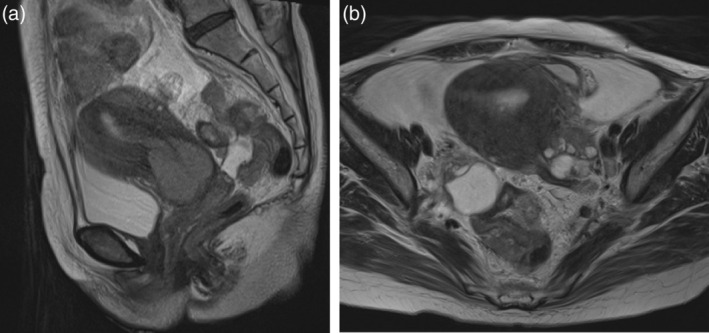
(a) T2‐weighted sagittal magnetic resonance imaging of case 5 showed an enlarged uterine cervical mass. (b). T2‐weighted axial magnetic resonance imaging of case 5 showed bilateral ovarian swelling.
Pathological findings and high‐risk HPV status of five cases
Microscopic findings were similar to OSC in morphology in all five cases (Fig. 2a–c). In the low‐power field, all cases had a complex papillary pattern and tufting with cellular buds. Nests of tumor cells invaded the cervical stroma in an irregular pattern, with clefts around the tumor cell nests. In the high‐power field, pleomorphic and macronucleated atypical cells were seen. Case 3 had high‐grade squamous intraepithelial lesion and microinvasive squamous cell carcinoma associated with USCC.
Figure 2.
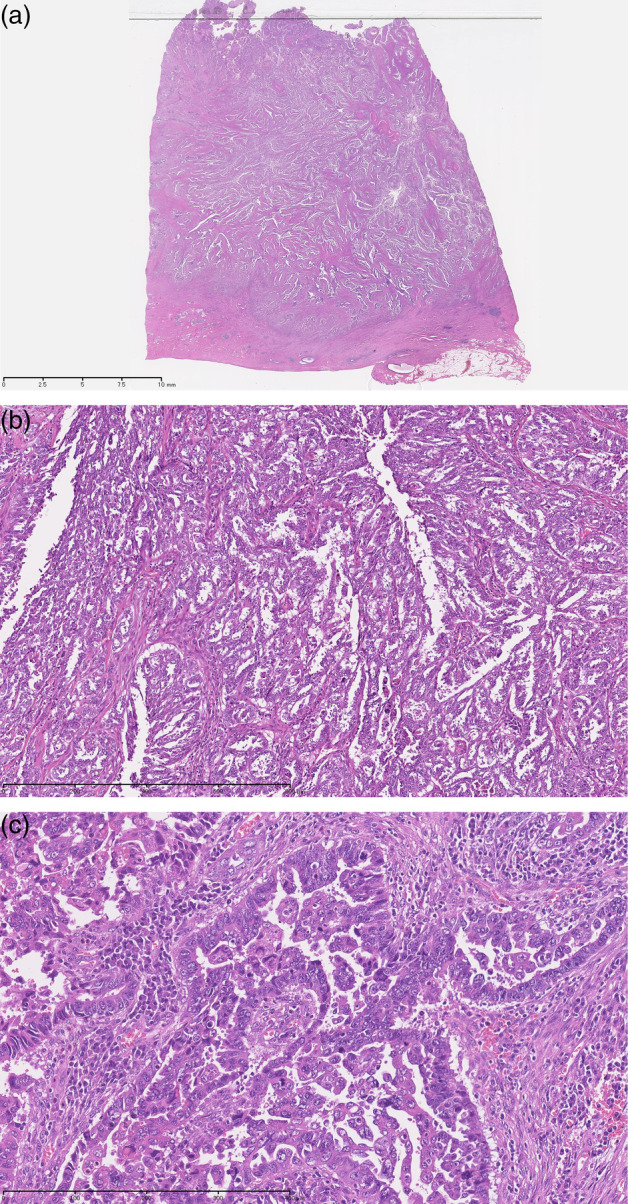
(a) Loupe image: the tumor deeply invaded the muscle layer of the cervix. HE stain. (b). Tumor had a complex papillary pattern with epithelial stratification. Hematoxylin and eosin stain, ×40. (c) Pleomorphic and macronucleated atypical cells were observed. Hematoxylin and eosin stain, ×100.
Immunohistochemistry was positive for p16, focally positive for p53 and ER, and negative for progesteron receptor, wilms tumor 1 (WT‐1) and β‐catenin in case 4 (Fig. 3). High‐risk HPV DNA ISH analyses were all negative in our five cases (data not shown).
Figure 3.

Immunohistochemistry showed positivity for p16, focal positivity for p53 and estrogen receptor, and negativity for WT‐1 in case 4.
Comparison of clinicopathological features with USCE and OSC
Forty‐nine USCE cases and 161 OSC cases were diagnosed and underwent primary treatment between 2000 and 2017 in our institute. The results are summarized in comparison with USCC (Table 2). The median ages at USCE, USCC and OSC onsets were 67 years old (range 47–83), 54 years old (range 36–64) and 57 years old (range 26–84), respectively. USCE seems to occur in older patients. More cases had positive peritoneal (washing) cytology in OSC and USCE than USCC. The positive ratios of peritoneal (washing) cytology were 79.5% in OSC, 44.9% in USCE and 20% in USCC. The numbers of peritoneal dissemination increased with the increasing positive cytology (80.1% in OSC, 32.7% in USCE and 20% in USCC). The rates of lymph node metastasis in USCE or OSC were lower than that of USCC (42.6% in USCE, 41.0% in OSC and 60% in USCC). Follow‐up information was available in all cases. The mortality rate associated with USCE was 34.7% (the median follow‐up period was 41 months [range 10 days to 156 months]) and that for OSC was 44.7% (the median follow‐up period was 48 months [range 1–227 months]), whereas none of the five USCC patients died (the median follow‐up period was 104 months [range 26–210 months]).
Table 2.
Comparison of clinicopathological characteristics of serous carcinoma of the uterine cervix (USCC), serous carcinoma of the endometrium (USCE) and serous carcinoma of the ovary (OSC)
| Cases (%) | |||
|---|---|---|---|
| USCC (5) | USCE (49) | OSC (161) | |
| Peritoneal cytology positive | 1 (20) | 22 (44.9) | 128 (79.5) |
| Peritoneal dissemination | 1 (20) | 16 (32.7) | 129 (80.1) |
| Lymph node metastasis | 3 (60) | 20 (42.6) | 66 (41.0) |
| Ovarian metastasis | 1 (20) | 14 (28.6) | NA |
| Extra‐abdominal metastasis | 0 | 6 (12.2) | 32 (19.9) |
| Cases of DOD | 0 | 17 (34.7) | 72 (44.7) |
| Median age (years old) | 54 (36–64) | 67 (47–83) | 57 (26–84) |
| Median follow‐up period (months) | 104 (26–210) | 41 (0–156) | 48 (1–227) |
DOD, dead of disease.
Discussion
USCC was first described as a subtype of cervical adenocarcinoma by Young and Scully in 1990.1 Gilks and Clement reported it in detail in 1992 and noted the aggressive nature of this rare neoplasm.2 USCC microscopically resembles OSC, fallopian tube, peritoneum and endometrium. USCC is a very rare tumor and no large‐scale multicenter study has been performed. Reports in the literature are on limited numbers of patients, with the largest to date including 17 patients3 and the second largest including 12 patients.6
We report five cases of USCC. Four cases underwent radical hysterectomy, and an advanced case was treated by combination chemotherapy. Four cases who were treated with radical hysterectomy have now been free of relapse for between 56 and 210 months. An advanced case treated by chemotherapy is undergoing treatment with bevacizumab for maintenance therapy. She has also been disease‐free for 26 months. The prognosis was unexpectedly favorable.
USCC has a complex papillary architecture with slit‐like lumina on microscopic examination. In the high‐power field, neoplastic cells had pleomorphic nuclei with numerous mitotic figures.1, 2, 3 Although previous reports defined at least 10–20% of the tumor area had to be of papillary serous type to be classified USCC, our cases were pure USCC, except for case 3 coexisting micro invasive squamous cell carcinoma antigen.
Immunohistochemistry is extremely helpful in diagnosis and differentiation. Previous reports stated that p53, p16 and CA125 were expressed in USCC and serous carcinoma of other origins. WT‐1 and estrogen receptor (ER) were positive in OSC whereas USCE and USCC showed weak or negative expression. Carcinoembryonic antigen was sometimes positive in USCC but OSC and USCE rarely showed positive expression.17, 18, 19 Our case (case 4) was positive for p16, p53 and ER and negative for WT‐1 and progesterone receptor.
Although the overall prevalence of HPV was reported to be between 63% and 94% in cervical adenocarcinoma, reports on the HPV status of USCC are limited. HPV DNA was detected in four of 12 USCC and six of 24 USCC cases by Togami et al. and Pirog et al., respectively.17, 20 Others also reported that some USCC cases were related HPV infection and most of them were HPV type 16 and 18. We examined human high‐risk HPV status of our five USCC cases that were all negative. USCC may be induced by HPV infection, but not always.
Regarding treatment for USCC, most patients underwent surgery with or without adjuvant therapy in previous reports. While radiotherapy was often used as an adjuvant therapy, chemotherapy was sometimes selected. The regimens of chemotherapy were all platinum‐based combination therapy.2, 3, 6, 10, 11, 14 Cases 2 and 3, whose pathological status was pT1b1, underwent radical hysterectomy without adjuvant therapy. Cases 1 and 4 who had full‐thickness stromal invasion (parametrium invasion), and lymph node metastasis underwent radical hysterectomy, postoperative CCRT and systemic chemotherapy.
However, only a few cases were reported who were inoperable with advanced disease. Ueda et al. reported that combination therapy with paclitaxel and carboplatin was effective for advanced USCC.12 USCC might be sensitive to platinum‐based combination therapy. Case 5 also had advanced disease, for which she received systemic chemotherapy. The patient achieved complete remission after chemotherapy with paclitaxel, carboplatin and bevacizumab. She is now under remission‐maintenance treatment with bevacizumab. Although there is no report on chemosensitivity of USCC, bevacizumab may be effective against USCC.
There are few articles about radiotherapy as an initial therapy. Rose and Reale reported a patient with T2b disease who had a small (3 mm) left uterosacral ligament implant. She had been treated with CCRT after an exploratory laparotomy and were free of disease after 32 months.4 Zhou et al. reported the results of primary radiotherapy for six patients with T1b‐T3 disease.3 A patient with T1b disease was free of relapse for more than 6 months. Conversely, five patients died from USCC. The decision on initial treatment might depend on the disease status including lymph node metastasis and primary lesions. It is inadequate to conclude that primary radiotherapy is not effective for USCC.
Regarding the prognostic features of USCC, Zhou et al. suggested that USCC might not be an important prognostic factor independent of other factors, such as stage, tumor size, depth of invasion and presence of lymph node metastases. They stated that primary surgical therapy might be preferable to primary radiotherapy in early‐stage USCC. Togami et al. also reported that patients with pT1b disease might have a favorable prognosis with radical surgery, but patients with advanced‐stage disease had had a poor prognosis because of extra‐pelvic recurrence. We collected reports on outcomes of USCC according to the presence or absence of lymph node metastasis with the present study (Table 3).2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Twenty cases without lymph node metastasis with T1a1‐T2b disease were free of relapse except for one case reported by Kaplan et al. whose treatment was incomplete. However, 13 cases with lymph node metastasis were dead or alive with disease, except for five cases (one with T1b disease reported by Zhou et al., one with T1b1 disease reported by Togami et al. and three of our cases [cases 1, 4 and 5]). USCC might be a possibly curable disease without lymph node metastasis, while lymph node metastasis is likely associated with poor prognosis. USCC with lymph node metastasis should be treated using multimodal therapy because two of our cases (cases 1 and 4) have prolonged survival despite pelvic and para‐aortic lymph node metastasis.
Table 3.
Summary of reported cases of serous carcinoma of the uterine cervix
| n | (p)T | N | Extra uterine lesions | Treatment | Outcome | |
|---|---|---|---|---|---|---|
| Gilks CB et al., 19922, Zhou et al., 19983 | 3 | pT1b | 0 | RAH | NED | |
| 1 | pT1b | 0 | RAH → RT | NED | ||
| 1 | pT1b | 0 | RAH → RT + CT | NED | ||
| 1 | T1b | 0 | RT → tracherectomy | NED > 60 months | ||
| 1 | T1b | 0 | RT | NED | ||
| Rose and Reale, 19934 | 1 | pT1a1 | 0 | RAH | NED (32 months) | |
| 1 | T2b | 0 | PD | CCRT | NED (32 months) | |
| Watrowski et al., 20005 | 1 | pT1b1 | 0 | RAH | NED (38 months) | |
| Togami et al., 20126 | 5 | pT1b1 | 0 | RAH | NED (54–127 months) | |
| 2 | pT1b2 | 0 | NED (28–45 months) | |||
| Arai et al., 19967 | 1 | pT2b | 0 | TAH → RT | NED (108 months) | |
| Kaplan et al., 199814 | 1 | pT1b1 | 0 | LAVH→RT + CT | AWD (24 months) | |
| Lurie et al., 199115 | 1 | pT1b1 | 0 | RAH | ND | |
| Present study | 2 | pT1b1 | 0 | RAH | NED (104–125 months) | |
| Zhou et al., 19982, 3 | 1 | pT1b | 1 | RAH → RT | NED > 60 months | |
| 1 | pT1b | 1 | RAH → RT | DOD | ||
| 1 | pT1b | 1 | RAH → RT | AWD (36 months) | ||
| 1 | T1b | 1 | PAN | RT → CT | DOD | |
| 1 | T1b | 1 | RT | DOD | ||
| 2 | T2 | 1 | RT | DOD | ||
| 1 | T3 | 1 | RT | DOD | ||
| Togami et al., 20126 | 1 | pT1b1 | 1 | Ovary | RAH → CT | DOD (5 months) |
| 1 | pT1b1 | 1 | RAH → RT | NED (48 months) | ||
| 1 | pT2b | 1 | DOD (51 months) | |||
| 1 | pT2b | 1 | Ovary | DOD (40 months) | ||
| 1 | pT2b | 1 | RAH | DOD (28 months) | ||
| Tang et al., 20008 | 1 | pT2a1 | 1 | RAH | DOD (17 months) | |
| Shintaku et al., 19939 | 1 | pT2 | 1 | TAH → RT | DOD (8 months) | |
| Khan et al., 201610 | 1 | pT1b1 | 1 | Multiple LNs, Lung | TAH → CT | ND |
| Yuksel et al., 201111 | 1 | pT1b1 | 1 | Multiple PD | RAH → CT | DOD (18 months) |
| Ueda et al., 201212 | 1 | T3b | 1 | Multiple LNs | CT | ND |
| Batistatou et al., 200013 | 1 | pT2b | 1 | PAN | RAH → RT + CT | DOD (36 months) |
| Present study | 1 | pT1b1 | 1 | PAN | RAH → CT + CCRT | NED (210 months) |
| 1 | pT2b | 1 | PAN | NED (56 months) | ||
| 1 | T2b | 1 | Multiple PD, Ovary | CT | NED (26 months) |
AWD, alive with disease; CCRT, concurrent chemoradiotherapy; CT, chemotherapy; DOD, died of disease; LAVH, laparoscopically assisted vaginal hysterectomy; LN, lymph node; n, number of patients; NED, no evidence of disease; ND, no data; PAN, para‐aortic lymph node; PD, peritoneal dissemination; RAH, radical hysterectomy; RT, radiotherapy; TAH, total abdominal hysterectomy.
Peritoneal dissemination of USCC has rarely been reported. There have been two reports by Rose and Reale and Yuksel et al. and we have reported one case (case 5) in this paper. The patient reported by Rose and Reale received CCRT and were free of disease after 32 months as previously noted. Yuksel et al. reported a case with intraperitoneal numerous metastatic implants who underwent surgery and adjuvant chemotherapy. She died 1 year and 6 months later. Case 5 was also found with multiple peritoneal dissemination and she received systemic chemotherapy. She is now free of disease and under remission‐maintenance treatment with bevacizumab.
USCE is associated with a poor prognosis because 60–70% of women present with the disease outside the uterus.21, 22 USCE is often associated with lymphatic metastasis and peritoneal dissemination. We reviewed the medical records of USCC, USCE and OSC patients who received medical treatment in our institute between 2000 and 2017 (Table 2). There were more cases with positive peritoneal cytology in USCE than USCC. The peritoneal dissemination rate increased with increasing positive cytology. However, lymph node metastasis rate in USCE was lower than that in USCC. The USCE mortality rate was 34.7%, whereas none of our five patients died from USCC. The difference in the positivity rates of peritoneal cytology (or peritoneal dissemination) between USCE and USCC might reflect prognosis other than lymph node metastasis.
USCC is a very rare tumor. Although both USCC and USCE originate in the uterus, USCC has clinicopathological features distinct from USCE, and had been thought of as a poor‐prognosis disease. However, we could have salvaged the patients with USCC by multimodality therapy. We might conquer USCC even with lymph node metastasis or peritoneal dissemination with aggressive multimodality therapy. Further accumulation of cases is warranted to clarify the nature of the disease and to develop a strategy against USCC.
Disclosure
None declared.
Acknowledgments
We thank Katsumi Takizawa (Department of Pathology, National Hospital Organization Kyushu Cancer Center) for micrographing specimens and Yukihiko Okumura (Department of Pathology, National Hospital Organization Kyushu Cancer Center) for high‐risk HPV DNA ISH.
The copyright line for this article was changed on 1 December 2020 after original online publication.
References
- 1. Young RH, Scully RE. Invasive adenocarcinoma and related tumors of the the uterine cervix. Semin Diagn Pathol 1990; 7: 205–227. [PubMed] [Google Scholar]
- 2. Gilks CB, Clement PB. Papillary adenocarcinoma of the uterine cervix: A report three cases. Mod Pathol 1992; 5: 426–431. [PubMed] [Google Scholar]
- 3. Zhou C, Gilks CB, Hayes M, Clement PB. Papillary serous adenocarcinoma of the uterine cervix: A clinicopathologic study of 17 cases. Am J Surg Pathol 1998; 22: 113–120. [DOI] [PubMed] [Google Scholar]
- 4. Rose PG, Reale FR. Serous papillary carcinoma of the cervix. Gynecol Oncol 1993; 50: 361–364. [DOI] [PubMed] [Google Scholar]
- 5. Watrowski R, Stripecke E, Jager C, Bauknecht T, Horst C. Papillary‐serous adenocarcinoma of the uterine cervix during tamoxifen therapy after bilateral breast cancer. Anticancer Res 2012; 32: 5075–5078. [PubMed] [Google Scholar]
- 6. Togami S, Kasamatsu T, Saejima Y et al Serous adenocarcinoma of the uterine cervix: A clinicopathological study of 12 cases and review of the literature. Gynecol Obstet Invest 2012; 73: 26–31. [DOI] [PubMed] [Google Scholar]
- 7. Arai Y, Nishida M, Nishide K, Kono K, Tsunoda H, Kubo T. A case of serous adenocarcinoma of the uterine cervix. J Jpn Soc Cytol 1996; 35: 424–427. [Google Scholar]
- 8. Tang W, Zhang Z, Yao H, Zeng Z, Wan G. Papillary serous carcinoma of the cervix mixed with squamous cells: A report of the first case. Gynecol Oncol Case Rep 2013; 6: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shintaku M, Ueda H. Serous papillary adenocarcinoma of the uterine cervix. Histopathology 1993; 22: 506–507. [DOI] [PubMed] [Google Scholar]
- 10. Khan M, Gilman AD, Nizami S, Barbaryan A, Ali AM, Mirrakhimov AE. Papillary serous carcinoma of the uterine cervix with lung metastasis. Case Rep Oncol Med 2014; 2014: 683103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuksel H, Sezer SD, Kucuk M, Riza Obadasi A, Doger FK. Papillary serous adenocarcinoma of the uterine cervix: A case report. Eur J Gynaecol Oncol 2011; 32: 240–242. [PubMed] [Google Scholar]
- 12. Ueda M, Koshiyama M, Yamaguchi A et al Advanced papillary serous carcinoma of the uterine cervix: A case with a remarkable response to paclitaxel and carboplatin chemotherapy. Rare Tumors 2012; 4: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Batistatou A, Zolota V, Tzoracoleftherakis E, Scopa CD. Papillary serous adenocarcinoma of the endocervix: A rare neoplasm. Immunohistochemical profile. Int J Gynedol Cancer 2000; 10: 336–339. [DOI] [PubMed] [Google Scholar]
- 14. Kaplan EJ, Caputo TA, Shen PU, Sasson RI, Soslow RA. Familial papillary serous carcinoma of the cervix, peritoneum, and ovary: A report of the first case. Gynecol Oncol 1998; 70: 289–229. [DOI] [PubMed] [Google Scholar]
- 15. Lurie S, Dgani R, Gorbacz S, Borenstein R. Invasive papillary serous adenocarcinoma of the endocervix in pregnancy: A case report. Eur J Obstet Gynecol Reprod Biol 1991; 40: 79–81. [DOI] [PubMed] [Google Scholar]
- 16. Power DG, GP MV, Delaney DW et al Papillary serous carcinomas of the uterine cervix and paraneoplastic cerebellar degeneration: A report of two cases. Acta Oncol 2008; 47: 1590–1593. [DOI] [PubMed] [Google Scholar]
- 17. Togami S, Sasajima Y, Kasamatsu T et al Immunophenotype and human papillomavirus status of serous adenocarcinoma of the uterine cervix. Pathol Oncol Res 2015; 21: 487–494. [DOI] [PubMed] [Google Scholar]
- 18. Nofech‐Mozes S, Khalifa MA, Ismiil N et al Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol 2008; 21: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 19. Barbu I, Craitois S, Margartiescu C. Cervical adenocarcinoma: A retrospective clinicopathologic study of 16 cases. Rom J Morphol Embryol 2012; 53: 615–624. [PubMed] [Google Scholar]
- 20. Pirog EC, Lloveras B, Molijin A et al HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, worldwide analysis of 760 cases. Mod Pathol 2014; 27: 1559–1567. [DOI] [PubMed] [Google Scholar]
- 21. del Carmen MG, Bire M, Schorge JO. Uterine papillary serous cancer: A review of the litelature. Gynecol Oncol 2012; 127: 651–661. [DOI] [PubMed] [Google Scholar]
- 22. Gatius S, Matias‐Guiu X. Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod Pathol 2016; 29 (Suppl 1): S45–S58. [DOI] [PubMed] [Google Scholar]


