Summary
Amphibious plants thrive in areas with fluctuating water levels, partly as a result of their capacity to make specialized leaves when submerged or emerged. The tailor‐made leaves improve gas exchange underwater or prevent aerial desiccation. Aquatic leaves are thin with narrow or dissected forms, thin cuticles and fewer stomata. These traits can combine with carbon‐concentrating mechanisms and various inorganic carbon utilization strategies. Signalling networks underlying this plasticity include conserved players like abscisic acid and ethylene, but closer inspection reveals greater variation in regulatory behaviours. Moreover, it seems that amphibious leaf development overrides and reverses conserved signalling pathways of their terrestrial counterparts. The diversity of physiology and signalling makes plant amphibians particularly attractive for gaining insights into the evolution of signalling and crop improvement.
Keywords: amphibious, bicarbonate, carbon‐concentrating mechanisms, drought, flooding, leaf development, plasticity
Short abstract
See also the Editorial on this article by Sasidharan et al., 229: 5–7.
| Contents | ||
|---|---|---|
| Summary | 79 | |
| I. | Introduction | 79 |
| II. | Leaf morphological transitions | 80 |
| III. | Underwater photosynthesis | 82 |
| IV. | Conclusions and future challenges | 83 |
| Acknowledgements |
83 |
|
| References | 83 | |
I. Introduction
Hydrological gradients are a strong determinant of plant species distribution, and species occupying the riparian side of these gradients experience fluctuating water levels and high flooding risks (Silvertown et al., 2015; Sarneel et al., 2019). For plants that are used to terrestrial life, inundation has dramatic consequences (Loreti et al., 2016). In the aquatic environment, gas diffusion is c. 10 000 times slower, which has grave consequences for oxygen (O2) and carbon dioxide (CO2) availability (Nobel 2009). Combined with potential reductions in light availability underwater, photosynthesis will be severely hampered. The resulting energy and carbon crisis is perhaps the greatest challenge for flooded plants. In illuminated conditions, the reduction in photosynthesis also generates oxidative stress as a result of an imbalance between light‐harvesting and diffusion‐limited carbon fixation (Horiguchi et al., 2019). For plants that typically inhabit the aquatic niche, the sudden aerial exposure is also not without risk. The lack of a thick cuticle makes their leaves prone to desiccation and the sudden exposure to light and high amounts of O2 lead to excessive reactive oxygen species formation (Yeung et al., 2018).
Amphibious plants can successfully occupy the terrestrial–aquatic environmental interface. They often propagate via tubers and rhizomes, and/or time their life cycle to coincide with periods of favourable water levels (Sosnova et al., 2010). During shallow flooding, elongation of shoot organs can facilitate re‐establishment of aerial contact and is typically combined with aerenchyma formation to improve internal aeration (Pierik et al., 2008; Herzog & Pedersen 2014).
Despite possessing leaves with a slightly higher specific leaf area (Box 1), species from the water’s edge do not have better underwater photosynthesis than those from higher elevation levels (Winkel et al., 2016). However, many species living in this transition zone do have the capacity to form new leaves adapted to either the new aquatic or aerial conditions. This drastic alteration of leaf form in response to environmental changes is termed heterophylly.
Box 1. Glossary of specialist terms used in this article.
Specific leaf area (SLA): The amount of leaf area per unit leaf mass (m2 kg−1). It is considered a major factor associated with plant growth variation. As high SLA is associated with thin leaves, it is considered an important trait for underwater photosynthesis. In this case, diffusion distance is lower. Thus high‐SLA leaves typically have better gas exchange.
Boundary layer: A stationary fluid layer immediately covering the surface of submersed objects (e.g. flooded plant organs). No bulk flow of the liquid is observed in this layer, so movement of all compounds is driven solely by diffusion. This layer therefore severely impairs underwater gas exchange. The thickness of the diffusive boundary layer is dependent on the flow rate of the water and the surface topography of the submerged object. In the context of submerged plants, thicker boundary layers are expected in still or slow‐moving water and especially on large leaves.
Kranz anatomy: Distinctive leaf anatomy associated with C4 photosynthesis. Typical ‘Kranz’ (German for ‘wreath’) structure includes an outer ring of mesophyll cells and an inner ring of bundle sheath cells surrounding the vascular tissues.
Heterophylly: The formation of extremely different leaf forms on a single plant. These leaf forms are created early in the development of a leaf. The extreme variation can be induced by a variety of factors such as age, temperature and humidity. In this paper, we refer to alterations caused by transition between flooded and aerial conditions.
True aquatics: Plant species that are adapted to submerged conditions and can only survive underwater.
Aquatic leaves, compared with those formed aerially, usually show a higher amount of dissection or they retain the simple leaf shape with a more narrow, elongated form. Additional changes in aquatic leaves include a minimal or even absent cuticle, and fewer or absent stomata. Besides the remarkable display of leaf plasticity, some amphibious plants utilize carbon‐concentrating mechanisms (CCMs) and/or bicarbonate (HCO3 –) uptake systems to improve underwater photosynthesis and facilitate the amphibious dual life. A multitude of internal and external signals are used to sense air–water transitions and trigger these dramatic changes. Here we highlight the current understanding of shoot plasticity and photosynthesis physiology, and their adaptive significance for an amphibious lifestyle. We also call for increased leveraging of wild species for broadening our knowledge on mechanisms of plasticity in variable environments.
II. Leaf morphological transitions
When confronted with a sudden change in environment, existing leaves have limited capacity to undergo drastic morphological changes. Therefore, the initiation of the development of either a terrestrial or an aquatic leaf is established early on in development at the shoot apex. Here we consider three main developmental changes from the terrestrial to aquatic transition perspective (Fig. 1), either the formation of narrow leaves or the formation of dissected leaves. Both leaf forms typically lack stomatal development.
Figure 1.
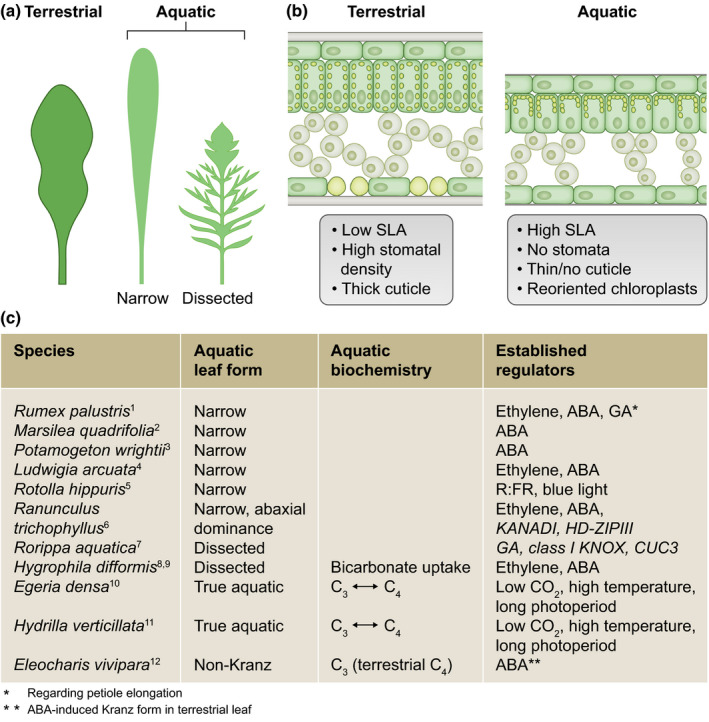
Shoot adaptive plasticity in amphibious plants. (a) General illustration of distinct terrestrial and aquatic leaf forms of amphibious species. Aquatic leaves tend either to be slightly elongated and narrow or to have a strongly dissected form. (b) Cross‐section of a typical terrestrial and aquatic leaf. Terrestrial leaves are thicker, with a thick cuticle and predominantly abaxial localized stomata. Aquatic leaves have features to enhance underwater gas exchange capacity. They tend to be much thinner (high specific leaf area, SLA) (Box 1), with a reduced or absent cuticle, larger air spaces, little or no stomata and chloroplasts reoriented towards the epidermis. (c) Overview of species mentioned in this review, together with their aquatic leaf morphology, biochemistry and established regulators. Superscript numbers in column 1 of (c) refer to the following: 1, van Veen et al. (2013); 2, Hsu et al. (2001); 3, Iida et al. (2016); 4, Kuwabara et al. (2003); 5, Momokawa et al. (2011); 6, Kim et al. (2018); 7, Nakayama et al. (2014); 8, Horiguchi et al. (2019); 9, Li et al. (2017); 10, Casati et al. (2000); 11, Rao et al. (2002); 12, Ueno (1998). ABA, abscisic acid; GA, gibberellin.
Elongated narrow aquatic leaves
When submerged, many amphibious species form new leaves that are longer and narrower. Sometimes the leaves are also pointed at the proximal end, forming an oblanceolate shape. Additionally, these leaves have a higher SLA, are thinner, lack stomata and have minimal cuticle development (Nakayama et al., 2017). The advantage of producing a thin leaf without a cuticle is the reduction of the distance for inward diffusion of O2 and CO2 required for respiration and underwater photosynthesis. The exact importance of the aquatic leaf shape remains unclear, but a narrower leaf would have a thinner diffusive boundary layer (Box 1), further enhancing gas exchange with the environment. Detailed investigations in Rumex palustris estimated a 38‐fold reduction in CO2 diffusion resistance in aquatic leaves (compared with unacclimated terrestrial leaves) associated with higher photosynthesis rates and reduced photorespiration (Mommer et al., 2005, 2006).
Amongst amphibious plants, abscisic acid (ABA) has emerged as a major regulator of leaf morphological alterations (Nakayama et al., 2017). In Marsilea quadrifolia, the elongated aquatic leaf form requires low ABA conditions, and terrestrial leaves were created when submerged in water containing ABA. Subsequently, specific transcriptional ABA responses could already be observed at the shoot apex (Hsu et al., 2001). The correlation between elevated ABA concentrations and the terrestrial leaf form was also found in Potamogeton wrightii. Here, even salinity stress‐induced ABA triggered terrestrial leaf formation underwater (Iida et al., 2016). Such ABA‐dependent heterophyllous changes were also observed in Ludwigia arcuata where ABA concentrations were downregulated by ethylene accumulating in submerged tissues, as frequently observed in wetland species (Kuwabara et al., 2003; Benschop et al., 2005) .
The amphibious Ranunculus trichophyllus produces extremely thin, rounded aquatic leaves with enhanced abaxial and retarded adaxial development, in contrast to the thick, wide terrestrial leaf form (Kim et al., 2018). Interestingly, its terrestrial relative, Ranunculus sceleratus, does not display such heterophylly. A transcriptome analyses of R. trichophyllus aquatic and terrestrial leaves revealed strong repression of genes associated with wax biosynthesis required for cuticle formation, and secondary cell wall and vascular development. Heterophyllic leaf development was determined by hormonal regulation of gene families involved in leaf polarity control, namely HD‐ZIPIIIs and KANADI. Submergence‐induced ethylene accumulation stimulated KANADIs required for abaxial development (Kerstetter et al., 2001), whilst HD‐ZIPIII‐mediated adaxial development (McConnell et al., 2001) was retarded via a submergence‐induced loss of ABA stimulation. Ranunculus sceleratus lacked these hormonal and transcriptional responses, suggesting that the changes in ABA/ethylene signalling and leaf polarity control are key evolutionary steps for aquatic adaptation (Kim et al., 2018).
Leaf morphology is also strongly regulated by light quality cues (Momokawa et al., 2011). The red : far‐red ratio (R : FR) rises with increasing water depth. Accordingly, low R : FR triggered terrestrial leaf formation in submerged Rotolla hipuris, with the converse being true for aquatic leaves upon emersion. Interestingly, R : FR values indicative of proximity to the water surface or aerial conditions required high blue light to facilitate underwater terrestrial leaf formation. At high R : FR values typical of deep flooding, blue light had no effect. Thus the integration of light quantity and quality can be critical in detecting water‐level fluctuations (Momokawa et al., 2011).
Dissected aquatic leaves
An extreme form of heterophylly is the formation of highly dissected leaves underwater, with reduced stomatal density and cuticle thickness. A narrow and dissected leaf might also facilitate better water flow around and through it, and so prevent mechanical stress. An increase in dissection index is found in many species across a wide range of phylogenetic lineages. The underlying mechanisms have recently been extensively investigated for species such as Hygrophila difformis and Rorippa aquatica (Nakayama et al., 2014; Li et al., 2017; Horiguchi et al., 2019). While enhanced dissections are triggered by many cues (e.g. temperature and humidity), it can also be the default leaf shape. Common among all types of compound leaves and dissections identified thus far is the fact that leaves originate as simple primordia from the shoot meristem. In simple leaves, the primordia enter a differentiated state via a reduction in class I KNOX gene expression and an increase in class II KNOX gene expression. However, compound and dissected leaves can re‐enter an undifferentiated state by transient reactivation of class I KNOX genes (Bharathan et al., 2002). This undifferentiated state then allows leaflet initiation, instigated by PIN1‐mediated auxin maxima (Barkoulas et al., 2008). The separation of these leaflets requires CUP SHAPED COTYLEDON (CUC3)‐mediated suppression of growth between the auxin maxima, a process that is conserved across eudicots (Blein et al., 2008). Among the Brassicaceae, leaf dissection is further determined by the presence of REDUCED COMPLEXITY (RCO) which locally suppresses growth at the sides of leaves to enhance dissection (Sicard et al., 2014; Vlad et al., 2014).
In R. aquatica, the submergence‐induced formation of the dissected leaf coincides with a decrease of class I KNOX and CUC3 expression, analogous to the existing knowledge of compound leaf formation. Moreover, a drop in gibberellin (GA) concentrations was observed, and KNOXI genes are known to suppress GA biosynthesis. Subsequently, exogenous GA application or biosynthesis inhibition led to the reversal or exaggeration of leaf dissection, respectively (Nakayama et al., 2014). However, hormonal investigation of submergence‐induced leaf dissection in H. difformis found contrasting effects of GA compared with R. aquatica (Li et al., 2017). Here leaf dissection was predominantly driven by ethylene and low ABA concentrations. Although the molecular machinery of leaf dissection is considered conserved across species, two contrasting signalling behaviours were identified here.
Development of stomatal density
The aquatic leaf has a strongly reduced stomatal density and cuticle thickness. Indeed, detailed molecular investigation in R. trichophyllus identified a strong downregulation of stomatal developmental genes underwater, some of which have been lost altogether in aquatic plants (Olsen et al., 2016; Kim et al., 2018). The underwater regulation of stomatal density and cuticle typically goes hand in hand with that of leaf shape, namely via ethylene, low ABA and/or high R : FR (Kuwabara et al., 2003; Momokawa et al., 2011; Iida et al., 2016; Kim et al., 2018) . A thick cuticle in terrestrial leaves, which have higher ABA concentrations than their aquatic counterparts, agrees with the role of ABA in mediating drought responses, which includes strengthening the cuticle (Cui et al., 2016). However, the signals linked to stomatal development of amphibious heterophylly do not always align with patterns commonly observed. Stomatal density increases with high light and low CO2 availability, and is signalled through systemic leaves (Casson & Hetherington 2010). Although light intensities follow the same trend for heterophylly, the low CO2 availability underwater is not translated into high stomatal density. Likewise, low ABA concentrations and high R : FR are also known to increase stomatal density (Boccalandro et al., 2009; Tanaka et al., 2013; Jalakas et al., 2018), whereas in amphibious heterophyllous plants, low ABA and high R : FR decrease stomatal density (Kuwabara et al., 2003; Momokawa et al., 2011; Iida et al., 2016; Kim et al., 2018). Thus, the aquatic developmental programme appears to override routine terrestrial regulatory networks determining leaf formation and stomatal density.
III. Underwater photosynthesis
In air, gaseous CO2 diffuses relatively easily through the leaf. But when submerged, plants need to access the dissolved inorganic carbon (DIC). Between pH 7 and 10, CO2 availability is limited and HCO3 – is the dominant DIC form (Pedersen et al., 2013). This poses an additional problem for submerged plants, as HCO3 –, unlike CO2, does not easily cross lipid membranes. It is not surprising, therefore, that many aquatic plants have HCO3 – uptake mechanisms (Maberly & Madsen, 2002; Yin et al., 2017). Various HCO3 – uptake strategies and CCMs have been characterized in cyanobacteria, algae, seagrasses and other higher plants (Poschenrieder et al., 2018). In angiosperms, three forms can generally be distinguished (Fig. 2). First, the conversion of HCO3 – to CO2 by apoplastic carbonic anhydrases (CAs). Second, an H+‐ATPase‐mediated acidification of the apoplast and diffusive boundary layer, which pushes the CO2/HCO3 – equilibrium towards CO2. Third, a symporter‐mediated cotransport of HCO3 –/H+, and subsequent HCO3 – dehydration to CO2 via cytosolic CAs. A variety of metabolic routes have been identified in aquatic plants to subsequently fix the acquired HCO3 –/CO2. For example, the true aquatics (Box 1) Hydrilla verticillata and Egeria densa can switch between C3 and C4 photosynthesis underwater and can do so even in a single cell (Casati et al., 2000; Rao et al., 2002).
Figure 2.
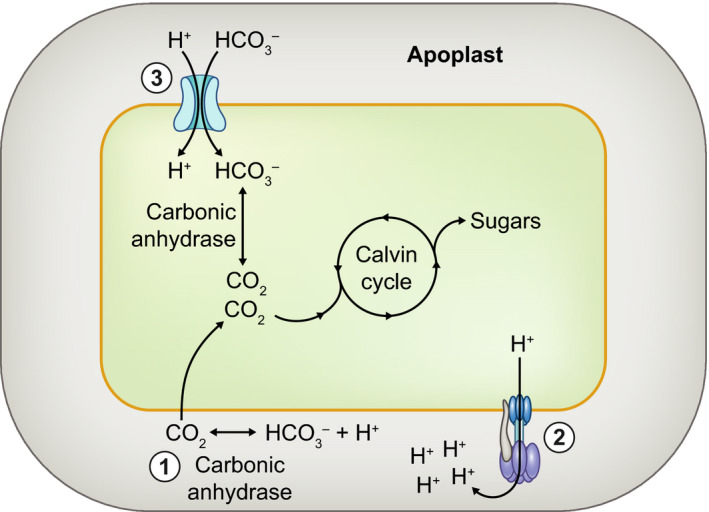
Bicarbonate utilization routes in angiosperms. In water, the predominant form of inorganic carbon is the membrane‐impermeable bicarbonate (HCO3 –) ion. Successful underwater photosynthesis therefore requires bicarbonate uptake mechanisms, broadly categorized into three routes, depicted in the schematic: (1) an apoplastic carbonic anhydrase converting HCO3 – to CO2; (2) apoplastic acidification by H+‐ATPases, which shifts the chemical equilibrium towards CO2; and (3) symporters facilitating HCO3 – import into the cytosol. CO2 produced via these routes can ultimately be fixed via the Calvin cycle, eventually resulting in carbohydrate biosynthesis.
Few studies report photosynthetic adaptation to submergence in amphibious plants. The aquatic leaves of R. palustris clearly had better photosynthetic capacity underwater than did terrestrial leaves (Mommer et al., 2005). In the heterophyllous amphibian H. difformis, a combination of biochemical and anatomical leaf adaptations facilitates underwater photosynthesis (Horiguchi et al., 2019). Submergence triggered the formation of highly dissected aquatic leaves with substantial O2 production underwater. By contrast, submerged terrestrial leaves struggled to capture inorganic carbon, regardless of illumination. The decreased photosynthesis underwater and subsequent excess energy were linked to high oxidative stress in these leaves. Aquatic leaves had a high capacity to utilize HCO3 –, which was absent in their terrestrial counterparts. Specific inhibitors were used to discern the mechanism for HCO3 – uptake in aquatic leaves. Interestingly, neither the inhibition of the apoplastic CA nor the HCO3 –/H+ symport affected underwater photosynthesis. Significant photosynthesis impairment was observed only when intracellular CA activity was blocked. These observations imply that submerged leaves of H. difformis can import HCO3 – into the cell without H+ cotransport (Horiguchi et al., 2019). Although common amongst true aquatic species, the extent of HCO3 – utilization amongst amphibious plants is currently unknown.
In true aquatics, CCMs such as the C4 system, can be induced by a several factors, such as photoperiod, low CO2 availability and ABA (Casati et al., 2000; Rao et al., 2002). The amphibious sedge Eleocharis vivipara exhibits an aquatic Kranz‐less C3 form and terrestrial C4‐like traits with Kranz anatomy (Box 1). The terrestrial form can be imposed on the aquatic leaf by ABA application and is considered as a stress signal (Ueno 1998) . Interestingly, in H. difformis biochemical HCO3 – usage, could be mimicked by application of ethylene or prevented by blocking ethylene perception. Even existing terrestrial leaves were sensitive to ethylene and submergence and achieved an intermediary capacity of HCO3 – usage (Horiguchi et al., 2019). The importance of flooding‐specific cues, such as ethylene, is also apparent from work on R. palustris, where the morphological adaptations to submergence can also be induced by low light conditions. However, these do not yield any photosynthetic benefit, as does a true aquatic leaf (Mommer et al., 2005).
IV. Conclusions and future challenges
Amphibious plants are truly shape shifters, adjusting their morphology and physiology to adapt to fluctuating environments. They have provided crucial insights into developmental regulatory networks underlying leaf plasticity. However, while some consistent regulatory factors (e.g. ABA and ethylene) are recognized, there have also been contradictions (e.g. GA regulation of leaf dissection), and much remains to be discovered regarding other cues such as light, temperature and abaxial dominance in narrow leaves. This will require a greater use of amphibious species for exploring the molecular regulation of adaptive plasticity to water extremes. Given the increased fluctuations in water stress associated with climate change, understanding such adaptations will be important if we are to engineer resilient crops (Voesenek et al., 2014). The plant’s current capacity to enhance underwater photosynthesis in existing terrestrial leaves of H. difformis is a good sign that such traits might, at some point, be transferable to crop species.
Acknowledgements
This work was funded by Dutch Research Council (NWO) grants 016.VIDI.171.006 and 867.15.031 to RS, and TTW 14700 and ALWOP.419 to HvV and RS. The authors thank Rens Voesenek for comments on a final draft of this paper.
References
- Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M. 2008. A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsute . Nature Genetics 40: 1136–1141. [DOI] [PubMed] [Google Scholar]
- Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. 2002. Homologies in leaf form inferred from KNOXI gene expression during development. Science 296: 1858–1860. [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Jackson MB, Gühl K, Vreeburg RAM, Croker SJ, Peeters AJM, Voesenek LACJ. 2005. Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. The Plant Journal 44: 756–768. [DOI] [PubMed] [Google Scholar]
- Blein T, Pulido A, Vialette‐Guiraud A, Nikovics K, Morin H, Hay A, Johansen IE, Tsiantis M, Laufs P. 2008. A conserved molecular framework for compound leaf development. Science 322: 1835–1839. [DOI] [PubMed] [Google Scholar]
- Boccalandro HE, Rugnone ML, Moreno JE, Ploschuk EL, Serna L, Yanovsky MJ, Casal JJ. 2009. Phytochrome B enhances photosynthesis at the expense of water‐use efficiency in Arabidopsis. Plant Physiology 150: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casati P, Lara MV, Andreo CS. 2000. Induction of a C4‐Like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiology 123: 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Hetherington AM. 2010. Environmental regulation of stomatal development. Current Opinion in Plant Biology 13: 90–95. [DOI] [PubMed] [Google Scholar]
- Cui F, Brosché M, Lehtonen MT, Amiryousefi A, Xu E, Punkkinen M, Valkonen JP, Fujii H, Overmyer K. 2016. Dissecting abscisic acid signaling pathways involved in cuticle formation. Molecular Plant 9: 926–938. [DOI] [PubMed] [Google Scholar]
- Herzog M, Pedersen O. 2014. Partial versus complete submergence: snorkelling aids root aeration in Rumex palustris but not in R. acetosa . Plant, Cell & Environment 37: 2381–2390. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Nemoto K, Yokoyama T, Hirotsu N. 2019. Photosynthetic acclimation of terrestrial and submerged leaves in the amphibious plant Hygrophila difformis . AoB Plants 11: plz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TC, Liu HC, Wang JS, Chen RW, Wang YC, Lin BL. 2001. Early genes responsive to abscisic acid during heterophyllous induction in Marsilea quadrifolia . Plant Molecular Biology 47: 703–715. [DOI] [PubMed] [Google Scholar]
- Iida S, Ikeda M, Amano M, Sakayama H, Kadono Y, Kosuge K. 2016. Loss of heterophylly in aquatic plants: not ABA‐mediated stress but exogenous ABA treatment induces stomatal leaves in Potamogeton perfoliatus . Journal of Plant Research 129: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalakas P, Merilo E, Kollist H, Brosché M. 2018. ABA‐mediated regulation of stomatal density is OST 1‐independent. Plant Direct 2: e00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. 2001. KANADI regulates organ polarity in Arabidopsis. Nature 411: 706–709. [DOI] [PubMed] [Google Scholar]
- Kim J, Joo Y, Kyung J, Jeon M, Park JY, Lee HG, Chung DS, Lee E, Lee I. 2018. A molecular basis behind heterophylly in an amphibious plant, Ranunculus trichophyllus . PLoS Genetics 14: e1007208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara A, Ikegami K, Koshiba T, Nagata T. 2003. Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 217: 880–887. [DOI] [PubMed] [Google Scholar]
- Li G, Hu S, Yang J, Schultz EA, Clarke K, Hou H. 2017. Water‐Wisteria as an ideal plant to study heterophylly in higher aquatic plants. Plant Cell Reports 36: 1225–1236. [DOI] [PubMed] [Google Scholar]
- Loreti E, van Veen H, Perata P. 2016. Plant responses to flooding stress. Current Opinion in Plant Biology 33: 64–71. [DOI] [PubMed] [Google Scholar]
- Maberly SC, Madsen TV. 2002. Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Functional Plant Biology 29: 393–405. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. 2001. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411: 709–713. [DOI] [PubMed] [Google Scholar]
- Mommer L, De Kroon H, Pierik R, Bögemann GM, Visser EJ. 2005. A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytologist 167: 197–206. [DOI] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Wolters‐Arts M, Venema JH, Visser EJ. 2005. Submergence‐induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiology 139: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommer L, Pons TL, Visser EJW. 2006. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. Journal of Experimental Botany 57: 283–290. [DOI] [PubMed] [Google Scholar]
- Momokawa N, Kadono Y, Kudoh H. 2011. Effects of light quality on leaf morphogenesis of a heterophyllous amphibious plant, Rotala hippuris . Annals of Botany 108: 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JL, Rouzé P, Verhelst B, Lin YC, Bayer T, Collen J, Dattolo E, De Paoli E, Dittami S, Maumus F et al 2016. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530: 331–335. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Nakayama N, Seiki S, Kojima M, Sakakibara H, Sinha N, Kimura S. 2014. Regulation of the KNOX‐GA gene module induces heterophyllic alteration in North American Lake Cress. Plant Cell 26: 4733–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Sinha NR, Kimura S. 2017. How do plants and phytohormones accomplish heterophylly, leaf phenotypic plasticity, in response to environmental cues. Frontiers in Plant Science 8: 1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS. 2009. Physicochemical and environmental plant physiology, 4th edn Burlington, VT, USA: Elsevier Academic Press. [Google Scholar]
- Pierik RV, Van Aken JM, Voesenek LACJ. 2008. Is elongation‐induced leaf emergence beneficial for submerged Rumex species? Annals of Botany 103: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Colmer T, Sand‐Jensen K. 2013. Underwater photosynthesis of submerged plants – recent advances and methods. Frontiers in Plant Science 4: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschenrieder C, Fernández JA, Rubio L, Pérez L, Terés J, Barceló J. 2018. Transport and use of bicarbonate in plants: current knowledge and challenges ahead. International Journal of Molecular Sciences 19: 1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SK, Magnin NC, Reiskind JB, Bowes G. 2002. Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single‐cell, facultative C4 system of Hydrilla verticillata . Plant Physiology 130: 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarneel JM, Hefting MM, Kowalchuk GA, Nilsson C, Van der Velden M, Visser EJ, Voesenek LA, Jansson R. 2019. Alternative transient states and slow plant community responses after changed flooding regimes. Global Change Biology 25: 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard A, Thamm A, Marona C, Lee YW, Wahl V, Stinchcombe JR, Wright SI, Kappel C, Lenhard M. 2014. Repeated evolutionary changes of leaf morphology caused by mutations to a homeobox gene. Current Biology 24: 1880–1886. [DOI] [PubMed] [Google Scholar]
- Silvertown J, Araya Y, Gowing D. 2015. Hydrological niches in terrestrial plant communities: a review. Journal of Ecology 103: 93–108. [Google Scholar]
- Sosnova M, van Diggelen R, Klimešová J. 2010. Distribution of clonal growth forms in wetlands. Aquatic Botany 92: 33–39. [Google Scholar]
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. 2013. ABA inhibits entry into stomatal‐lineage development in Arabidopsis leaves. The Plant Journal 74: 448–457. [DOI] [PubMed] [Google Scholar]
- Ueno O. 1998. Induction of Kranz anatomy and C4‐like biochemical characteristics in a submerged amphibious plant by abscisic acid. Plant Cell 10: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen H, Mustroph A, Barding GA, Vergeer‐van Eijk M, Welschen‐Evertman RA, Pedersen O, Visser EJ, Larive CK, Pierik R, Bailey‐Serres J et al 2013. Two Rumex species from contrasting hydrological niches regulate flooding tolerance through distinct mechanisms. Plant Cell 25: 4691–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad D, Kierzkowski D, Rast MI, Vuolo F, Dello Ioio R, Galinha C, Gan X, Hajheidari M, Hay A, Smith RS et al 2014. Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science 343: 780–783. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Van Veen H, Sasidharan R. 2014. Learning from nature: the use of non‐model species to identify novel acclimations to flooding stress. AoB Plants 6: plu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel A, Visser EJ, Colmer TD, Brodersen KP, Voesenek LA, Sand‐Jensen K, Pedersen O. 2016. Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant, Cell & Environment 39: 1537–1548. [DOI] [PubMed] [Google Scholar]
- Yeung E, van Veen H, Vashisht D, Paiva AL, Hummel M, Rankenberg T, Steffens B, Steffen‐Heins A, Sauter M, de Vries M et al 2018. A stress recovery signaling network for enhanced flooding tolerance in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 115: E6085–E6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Li W, Madsen TV, Maberly SC, Bowes G. 2017. Photosynthetic inorganic carbon acquisition in 30 freshwater macrophytes. Aquatic Botany 140: 48–54. [Google Scholar]


