Abstract
The need for extended second field high‐resolution (2F‐HR) HLA genotyping in kidney transplantation is debated. In a cohort of 1000 kidney transplants, we evaluated the impact of different HLA genotyping levels on the assignment of donor‐specific anti‐HLA antibodies (DSA) and investigated whether inference of 2F‐HR genotypes from low‐resolution (LR) genotypes could be used to correctly assign DSA. Based on LR genotypes, 224 pretransplant DSAs were present in 140 patients and absent in 860 patients (DSAneg group). With extended 2F‐HR HLA genotyping, we confirmed 173 DSA (77.2%) in 108 (77.1%) patients (2F‐HRposLRposDSA group) and excluded DSA in 32 patients (22.9%) (2F‐HRnegLRposDSA group). Kaplan‐Meier curves showed that 10‐year graft survival rates were similar between the DSAneg and 2F‐HRnegLRposDSA groups (82.4% vs 93.8%; P = .27) and confirmed that DSA determined using LR typing but not confirmed using 2F‐HR typing were indeed misclassified. By inferring 2F‐HR genotypes using HaploStats, DSA still could not be correctly assigned in 23.3% of cases. We conclude that extended 2F‐HR typing of the donor‐recipient pairs is relevant for the correct assessment of DSA. Although inference of 2F‐HR genotypes may improve the assessment of DSA in some cases, significant misclassification occurs, and warrants caution in using inferred HLA results for clinical and research purposes.
Keywords: basic (laboratory) research/science, clinical decision‐making, clinical research/practice, histocompatibility, kidney transplantation/nephrology, major histocompatibility complex (MHC), organ procurement and allocation, panel reactive antibody (PRA), rejection: antibody‐mediated (ABMR), risk assessment/risk stratification
The authors evaluate the impact of different HLA genotyping levels on the accuracy of donor‐specific antibody assignment and show that misclassified antibodies are not associated with either graft histology or survival. See the editorial from Baxter‐Lowe on page 3277.

Abbreviations
- ABMRh
histological phenotype of antibody‐mediated rejection
- DSA
donor‐specific anti‐HLA antibodies
- ENIS
Eurotransplant Network Information System
- HLA
human leukocyte antigen
- HR
high‐resolution
- LR
low‐resolution
- MFI
median fluorescence intensity
- NGS
next‐generation sequencing
- 2F‐HR
second field high‐resolution
- SAB
single antigen bead
1. INTRODUCTION
Human leukocyte antigen (HLA) mismatches influence long‐term graft outcome, 1 by the associated risk of T cell–mediated rejection. 2 Moreover, the presence of antibodies that react with the mismatched donor's HLA type cause antibody‐mediated rejection (ABMR) and premature graft failure. 3 , 4 , 5 Therefore, in most transplant organizations, an HLA matching strategy was introduced. Organ allocation is still based on partial HLA‐A,‐B, and ‐DR antigen matching, and on the evaluation of anti‐HLA antibodies against the mismatched donor's HLA type, defined at the low‐resolution (LR) HLA antigen level.
For decades, the complement‐dependent cytotoxicity test was the main test used to investigate the antibody reactivity pattern of the patient serum and to define unacceptable HLA mismatches. Currently, however, HLA laboratories use more sensitive solid‐phase single antigen bead (SAB) assays with precise determination of HLA antibody specificities, at the second field high‐resolution (2F‐HR) HLA level. 6 These antibodies sometimes react only with 1 allele that encodes 1 HLA molecule, or with a set of alleles that encode the same HLA molecule (2nd field HLA resolution). However, for allocation purposes, these reactive 2F‐HR detected antibodies are converted into corresponding LR antigens and recorded in registries as unacceptable HLA antigens. SAB panels often have 2 or more 2F‐HR HLA variants corresponding to the same HLA antigen. When not all 2F‐HR HLA variants of a certain antigen share the same reactivity, this creates a dilemma in the interpretation of HLA compatibility at the antigen level, in the determination of unacceptable antigens and in the decisions on organ allocation.
As the number of unacceptable HLA antigens increases, the patient's chance to receive an organ offer decreases. To avoid this and to be more specific on the definition of unacceptable HLA mismatches, the United Network for Organ Sharing registry has the possibility to report unacceptable 2F‐HR HLA variants instead of unacceptable HLA antigens. However, donor genotyping is currently still reported at the LR antigen level, which calls into question the real patient benefit of this strategy. Because no standard strategy is currently available for the exclusion of 2F‐HR unacceptable HLA variants, each transplant center decides whether an antigen mismatch is unacceptable or not when second field HLA‐specific antibodies are detected. 7
Donor HLA genotyping at the 2F‐HR HLA level could resolve these clinical dilemmas and more accurately assess the HLA compatibility of transplant pairs. Although technologies, like RealTime PCR or the most powerful next‐generation sequencing (NGS), offer the possibility to genotype the transplant pair at the 2F‐HR HLA level for 11 HLA loci, its clinical usefulness is still a matter of debate within the transplant community. 8 , 9 To date, no studies have assessed the added clinical value of 2F‐HR extended HLA typing over LR HLA‐A, ‐B, ‐DR, and ‐DQ typing in solid organ transplantation, and the LR genotyping techniques remain the cornerstone for determining the HLA compatibility between donor‐recipient pairs.
To better risk‐stratify patients on their likelihood of experiencing immune‐mediated injuries and guiding personalized immunosuppression, investigating the eplet incompatibility is gaining momentum in the field of kidney transplantation. Also, the eplet analysis requires 2F‐HR genotyping data, but many studies use statistical approaches to obtain untyped HLA data. Two different concepts exist to estimate untyped HLA data based on the linkage disequilibrium between HLA alleles of different loci. (1) Imputation tools estimate the missing untyped genotypes from neighboring typed single nucleotide polymorphisms using a reference panel. These imputation tools have become the primary tools used in case‐control association studies to search for genetic determinants of complex diseases. 10 (2) Inference tools infer the 2F‐HR genotypes from the LR results based on the observed HLA allelic frequencies and haplotypes in different ethnic populations and are often used in the transplant field. 11 , 12 , 13 , 14 The possibility to estimate the 2F‐HR HLA data brings additional confusion in the discussion about the clinical value of 2F‐HR genotyping methods in the field of solid organ transplantation. 15 Although it is possible to obtain the 2F‐HR genotypes correctly from the LR genotypes for some individuals, 16 it remains unknown whether such inference affects calculations of donor‐recipient eplet incompatibility.
Therefore, we aimed to evaluate the clinical utility of LR vs 2F‐HR HLA genotyping on the assignment of donor‐specific anti‐HLA antibodies (DSA) from SAB test results. Additionally, we investigated whether the inference of the donor 2F‐HR genotypes from the LR genotyping data could be used to correctly assign the presence of DSA and accurately estimate the eplet mismatch repertoire.
2. METHODS
2.1. Patients
All consecutive adult single kidney transplantations performed at the University Hospitals‐Leuven between March 1, 2004 and February 6, 2013 were eligible for this study (N = 1000). All transplantations were ABO‐compatible and were performed with negative complement‐dependent cytotoxicity crossmatches on T and B lymphocytes. No patient received preconditioning HLA antibody desensitization. All clinical data were prospectively collected during routine clinical follow‐up. This study was approved by the Ethics Committee of the University Hospitals Leuven (S53364 and S61788).
2.2. HLA genotyping data
Recipient and donor LR HLA genotypes for HLA‐A,‐B and ‐DR were obtained from the Eurotransplant Network Information System (ENIS), at split antigen level. Available donor and recipient DNA samples, prospectively collected in the Biobank Renal Transplantation of the University Hospitals Leuven, were retrospectively genotyped at the LR and 2F‐HR levels. For patients with HLA‐C,‐DQB1, and/or DP antibodies, the donors were further genotyped for HLA‐C,‐DQB1 or ‐DPB1 if the typing data were not available in ENIS. Molecular typing was performed for C and DQB1 loci by polymerase chain reaction (PCR) amplification with sequence‐specific primers (Olerup SSP) or for DPB1 (exon2) by Sanger sequencing (SBT, Protrans GmbH). We used these data to assign DSA at the LR level. In addition, all recipients (N = 1000) and donors (N = 932) with available DNA samples were genotyped retrospectively at the 2F‐HR level for HLA‐A,‐B,‐C,‐DRB1,‐DRB3,‐DRB4,‐DRB5,‐DQA1,‐DQB1,‐DPA1, and ‐DPB1 loci using NGS. Donors were genotyped using the commercially available MIA FORA NGS FLEX 11 HLA Typing Kit (Immucor) on the MiSeq sequencing instrument (Illumina Inc) or using the HiSeq sequencing system for typing exon 2, 3, and 4 for HLA ‐A,‐B,‐C and exon 2 and 3 for ‐DRB1,‐DRB3,‐DRB4,‐DRB5,‐DQA1,‐DQB1,‐DPA1, and ‐DPB1. The latter was also used to retype all recipients. These NGS genotyping results were used to define DSA at the 2F‐HR HLA level. The 2F‐HR HLA typing level discriminates all the coding differences of the extracellular HLA domains between the HLA alleles. These extracellular HLA domains define a unique HLA protein composition presented on the cell membrane.
2.3. Methods for inferring 2F‐HR HLA results
We evaluated 2 different methods to infer the 2F‐HR HLA results of the donors. Using the HLAMatchmaker 4‐digit allele converter program v1 (file name: 6ABCDRB1DQBconverterVs1), we obtained the 2F‐HR genotypes for A,‐B,‐C,‐DRB1 and ‐DQB1 loci from the LR data at split antigen level for HLA‐A,‐B,‐DR. 16 The HaploStats (www.haplostats.org) tool allowed us to estimate the 2F‐HR results for HLA‐A,‐B,‐C,‐DRB1,‐DRB3,‐DRB4,‐DRB5 and ‐DQB1 from the LR HLA‐A,‐B,‐C,‐DRB1 and ‐DQB1 genotypes. 17 In both methods, we selected the most likely 2F‐HR HLA genotypes according to the highest haplotype frequency in European whites.
2.4. Detection of circulating anti‐HLA antibodies and assignment of DSA
Anti‐HLA antibodies were systematically monitored in 1 histocompatibility laboratory (HILA‐Red Cross‐Flanders). In case this was not done in the clinical routine with the Luminex technology, we routinely retested bio‐banked sera (n = 435 pretransplant day 0 samples) for the presence of HLA antibodies and circulating DSA in the same laboratory. All sera were first screened using a LIFECODES LifeScreen Deluxe kit (Immucor), and in case of positive screening, the donor specificity was assessed using LIFECODES SAB kits (Immucor). Ethylenediaminetetraacetic acid was added to serum (1:10) to avoid the prozone effect. The cutoff for the presence of circulating HLA antibodies and DSA was a median fluorescence intensity (MFI) value ≥500 if the background‐corrected ratio or antigen density background‐corrected ratio was ≥4.
2.5. Kidney allograft biopsies and histological scoring
We included all posttransplant renal allograft biopsies, performed up to 5 years after transplantation (N = 3647). Timing and scoring of the biopsies were described in detail previously. 18 The diagnosis of the histological phenotype of ABMRh was based on the first 2 criteria for active ABMR, as defined by the Banff 2017 consensus. 19
2.6. Statistical analysis
When comparing 2 groups, Pearson's χ2 test was used for categorical data, the 2‐sample t test for the continuous variables, and the Wilcoxon test for the comparison of medians. Survival curves were plotted using the Kaplan‐Meier method and groups were compared by log‐rank testing. In the case of death with a functioning graft, we censored graft survival at the time of death. The log‐rank test was also used for comparing the cumulative incidence of developing ABMRh and all patients were censored at the last performed biopsy. All P < .05 were considered to indicate statistical significance. We used SAS (v9.4; SAS Institute) and GraphPadPrism software (v8.4; GraphPad Software Inc) for the statistical analyses.
3. RESULTS
3.1. Study population
The main clinical characteristics of this study cohort are summarized in Table 1. The median follow‐up time of this cohort was 7.5 years (interquartile range, 4.9‐10.0). During this period, 211 patients died, and 154 patients lost their graft. The overall death‐censored graft survival of the cohort at 1, 5, and 10 years was 95.2%, 89.1%, and 80.7%, respectively. Pretransplant anti‐HLA antibodies were detected in 262 (26.2%) recipients, of which the majority were against HLA class I. Ninety‐four recipients had only class I antibodies, 60 had only class II antibodies, and 104 had both class I and class II antibodies (Table 1). When we analyzed the antibody specificity based on LR HLA genotyping for HLA‐A,‐B,‐C,‐DRB1,‐DQB1 and exon 2 for DPB1, we suspected the presence of 224 different pretransplant DSAs in 140/1000 patients (LRposDSA group), and we excluded the presence of any DSA in 860/1000 patients (DSAneg group) (Figure 1).
TABLE 1.
General characteristics and follow‐up of the study population (N = 1000)
| Characteristics | Entire cohort (N = 1000) |
|---|---|
| Recipient characteristics at transplantation | |
| Age (y), mean ± SD (range) | 53.7 ± 13.3 (16.2‐82.6) |
| Recipient BMI at time of transplantation (kg/m2), mean ± SD (range) | 25.4 ± 4.5 (15.5‐42.0) |
| Sex (male), n (%) | 609 (60.9) |
| White, n (%) | 984 (98.4) |
| Repeat transplantation, n (%) | 154 (15.4) |
| Diabetes mellitus, n (%) | 166 (16.6) |
| Donor characteristics at transplantation | |
| Age (y), mean ± SD (range) | 47.7 ± 14.8 (5.2‐79.0) |
| Sex (male), n (%) | 535 (53.5) |
| Deceased donor, n (%) | 941 (94.1) |
| Donation after brain death, n (%) | 780 (78.0) |
| Transplant characteristics, treatment at transplantation and follow‐up | |
| Cold ischemia time (h), mean ± SD (range) | 14.2 ± 5.7 (0.17‐36.0) |
| Immunosuppression regimen: TAC‐MPA‐CS, n (%) | 874 (87.4) |
| Induction therapy, n (%) | 416 (41.6) |
| Basiliximab, n (%) | 355 (35.5) |
| Thymoglobulin, n (%) | 16 (1.6) |
| Other, n (%) | 45 (4.5) |
| Patients with anti‐HLA antibodies, n (%) | 262 (26.2) |
| HLA class I, n (%) | 94 (9.4) |
| HLA class II, n (%) | 60 (6.0) |
| HLA class I & II, n (%) | 104 (10.4) |
| Patients with DSA at LR level, n (%) | 140 (14.0) |
| Posttransplant data | |
| Follow‐up time (y), median (IQR) | 7.5 (4.9‐10.0) |
| Death‐censored graft survival | |
| At 1 y (%) | 95.2 |
| At 5 y (%) | 89.1 |
| At 10 y (%) | 80.7 |
Abbreviations: BMI, body mass index; CS, corticosteroids; DSA, donor‐specific anti‐HLA antibodies; HLA, human leukocyte antigen; IQR, interquartile range; LR, low‐resolution; MPA, mycophenolic acid; SD, standard deviation; TAC, tacrolimus.
FIGURE 1.
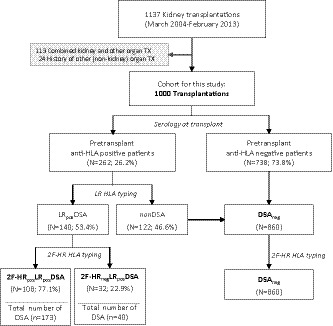
Flow chart of patient enrollment and group definition according to the presence of pretransplant HLA‐DSA, assessed at different HLA genotyping levels. DSA, donor‐specific HLA antibodies; LR, low‐resolution genotyping level; 2F‐HR, high‐resolution genotyping level; neg, negative; pos, positive; TX, transplantation
3.2. Differences in assigning DSA between LR vs 2F‐HR HLA genotype levels
Next, using the 2F‐HR HLA genotypes of each transplant pair, we reassessed the donor‐specificity of all 224 pretransplant‐suspected DSAs based on the LR data. Six transplant pairs of the LRposDSA group did not have sufficient DNA material to perform complete genotyping of all 11 HLA loci. Therefore, we genotyped only the loci with suspected DSA at the 2F‐HR level. In the analysis of the complete HLA reactivity towards 11 donor loci at 2F‐HR level, including DQA1,‐DPA1,‐DRB345, and excluding any reactivity to the recipient 2F‐HR genotypes, we confirmed the donor specificity of the HLA antibodies in 173/224 (77.2%) cases. In the remaining 51 (22.8%) cases of suspected DSA at the LR genotyping level, we disproved the donor specificity by 2F‐HR genotyping and assigned these as misclassified DSAs at the LR level. The majority of these misclassified DSAs (n = 51) were against HLA class II (70.6%), especially against the HLA‐DQ molecule (N = 22; 43.1%). The other misclassified DSA were suspected against HLA‐A (N = 6), HLA‐B (B = 7), HLA‐C (N = 2), HLA‐DRB1345 (N = 8), and HLA‐DP (N = 6) (Figure 2A). The main reasons for misclassification of DSA by LR genotyping were (1) lack of donor typing at the 2F‐HR level (29 cases, 56.9%); (2) lack of donor typing of DQA1/DPA1 loci (14 cases, 27.5%); and (3) incomplete typing of the recipient at 2F‐HR level (9 cases, 15.7%) (Figure 2B).
FIGURE 2.
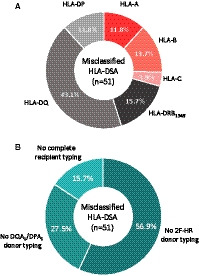
Distribution per HLA molecule/locus and reasons for DSA misclassification based on LR genotyping (n = 51). A, The majority of misclassified DSA were directed against class II (70.6%), especially against the DQ molecule (43.1%). B, The reasons for DSA misclassification were the following: not typing the donor at the second field HR level (56.9%); not typing DQA1/DPA1 loci of the donor (27.5%); and incomplete recipient typing at the second field HLA level (15.7%). 2F‐HR, second field high‐resolution; DSA, donor‐specific anti‐HLA antibodies; HLA, human leukocyte antigen; LR, low‐resolution genotyping level [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Comparison between HRnegLRposDSA and HRposLRposDSA groups
To examine whether the misclassifications by using LR genotyping were clinically relevant, we analyzed the impact of the misclassified antibodies on long‐term graft survival and graft histology. We categorized the patients in 3 groups: (1) no DSA (DSAneg); (2) presence of only misclassified DSA (according to LR genotyping; 2F‐HRnegLRposDSA); and (3) the presence of at least 1 confirmed DSA by 2F‐HR genotyping (2F‐HRposLRposDSA). One hundred eight of 140 (77.1%) patients had at least 1 confirmed DSA by 2F‐HR genotyping (2F‐HRposLRposDSA group), and the remaining 32/140 (22.9%) patients had only misclassified DSA (2F‐HRnegLRposDSA group). Kaplan‐Meier survival analysis showed that 10‐year graft survival rates, censored for recipient death, were similar between the DSAneg and 2F‐HRnegLRposDSA groups (82.4% vs 93.8%; P = .27 by log‐rank test), which were both significantly better than graft survival in the 2F‐HRposLRposDSA group (62.3%; P < .0001 and P = .0007, respectively) (Figure 3A). Also, when we investigated the histology data of posttransplant biopsies performed in this cohort, we found that none of the 32 2F‐HRnegLRposDSA patients developed the histological phenotype of ABMR (ABMRh) within the first 5 years during the biopsy follow‐up (Figure 3B). Therefore, we could not identify an association between misclassified DSA at LR level and ABMR or graft survival, confirming that these antibodies were also misclassified from the clinical perspective.
FIGURE 3.
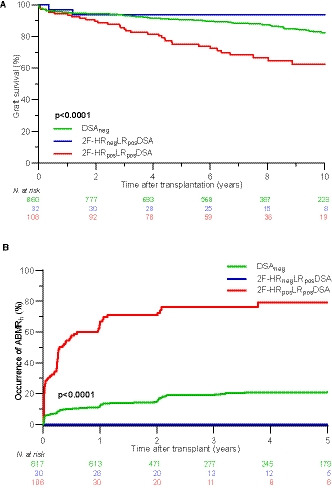
Survival analysis and occurrence of ABMRh, stratified according to the presence of DSA determined by LR vs HR genotyping. A, Ten‐year death‐censored graft survival was inferior only for patients with confirmed DSA by HR genotypes (2F‐HRposLRposDSA) when compared to patients with DSA defined only by LR and not confirmed by HR typing (2F‐HRnegLRposDSA), and when compared to DSA‐negative patients (62.3% vs 93.8% vs 82.4%, P < .0001 by log‐rank test). B, The cumulative incidence of ABMRh within the first 5 years was 21.0%, 0.0%, and 79.3% for DSAneg, 2F‐HRposLRposDSA, and 2F‐HRposLRposDSA group, respectively. Forty‐seven patients without biopsy follow‐up were excluded from this analysis. ABMRh, histological phenotype of antibody‐mediated rejection; DSA, donor‐specific anti‐HLA antibodies; 2F‐HR, second field high‐resolution; LR, low‐resolution; neg, negative; pos, positive [Color figure can be viewed at wileyonlinelibrary.com]
Next, by comparing the HLA mismatches on LR and 2F‐HR levels, the overall HLA mismatches differed between the 2F‐HRnegLRposDSA and 2F‐HRposLRposDSA group (Table 2). In terms of the DSA, although the MFI values were not statistically different, there was a higher percentage of DSA ≥ 2000 MFI in the 2F‐HRposLRposDSA compared with the 2F‐HRnegLRposDSA group (59.5% vs 42.5%, P = .05).
TABLE 2.
Immunological profiles of patients with pretransplant DSA, according to the accuracy of the DSA estimated using LR genotyping (N = 140 individual transplantations and N = 224 different DSA)
| Transplant characteristics | 2F‐HRposLRposDSA | 2F‐HRnegLRposDSA | P value |
|---|---|---|---|
| N = 108 | N = 32 | ||
| HLA mismatches | |||
| At split antigen HLA level | |||
| HLA‐A/B/DR mismatches (0‐6), mean ± SD | 2.9 ± 1.2 | 2.2 ± 1.3 | .01 |
| A antigen, mean ± SD | 0.9 ± 0.7 | 0.7 ± 0.6 | .06 |
| B antigen, mean ± SD | 1.1 ± 0.6 | 0.9 ± 0.7 | .17 |
| DR antigen, mean ± SD | 0.8 ± 0.6 | 0.6 ± 0.6 | .09 |
| At 2F‐HR HLA level a | 103 | 31 | |
| Total 2F‐HR HLA mismatches (0‐16), mean ± SD | 8.7 ± 2.3 | 7.6 ± 2.6 | .03 |
| A locus, mean ± SD | 1.7 ± 0.7 | 1.4 ± 0.8 | .11 |
| B locus, mean ± SD | 1.3 ± 0.6 | 1.2 ± 0.8 | .35 |
| C locus, mean ± SD | 1.4 ± 0.6 | 1.1 ± 0.8 | .06 |
| DRB1 locus, mean ± SD | 1.2 ± 0.7 | 1.0 ± 0.7 | .37 |
| DQB1 locus, mean ± SD | 1.0 ± 0.6 | 0.8 ± 0.5 | .11 |
| DQA1 locus, mean ± SD | 0.9 ± 0.6 | 0.9 ± 0.6 | .80 |
| DPB1 locus, mean ± SD | 1.1 ± 0.7 | 1.0 ± 0.7 | .71 |
| DPA1 locus, mean ± SD | 0.3 ± 0.6 | 0.2 ± 0.4 | .39 |
| DSA characteristics b | |||
| Total number of DSA at LR level ≥500 MFI | 184 | 40 | |
| Patients with multiple DSAs, n (%) | 53 (49.1) | 7 (21.9) | .006 |
| Misclassified DSA compared to 2F‐HR, n | 11 | 40 | |
| Total number of assigned DSA per group | 173 | 40 | |
| DSA class II, n (%) | 93 (53.8) | 26 (65.0) | .20 |
| DSA ≥ 1000 MFI, n (%) | 144 (83.2) | 31 (77.5) | .39 |
| DSA ≥ 2000 MFI, n (%) | 103 (59.5) | 17 (42.5) | .05 |
| MFI value of DSA, median (IQR) | 2593 (1328‐5146) | 1507 (1039‐3678) | .08 |
Significant differences are highlighted in bold.
Abbreviations: 2F‐HR, second field high‐resolution; DSA, donor‐specific HLA antibodies; HLA, human leukocyte antigens; HR, high‐resolution; IQR, interquartile range; LR, low‐resolution; MFI, median fluorescence intensity; SD, standard deviation.
Complete 2F‐HR HLA typing was not available for 6 transplant pairs.
Some of the patients have multiple DSA.
3.4. Impact of inferred 2F‐HR HLA genotypes on DSA assignment
We then used HLAMatchmaker and HaploStats to infer the donor 2F‐HR HLA genotypes from the LRposDSA group (N = 134). Six transplant pairs of this group did not have sufficient DNA material and not all 11 loci could be genotyped at the 2F‐HR level. With HLAMatchmaker, donors’ 2F‐HR genotypes were correctly obtained in 87.9%, 71.5%, 50.9%, 49.5%, and 57.8% for HLA‐A,‐B,‐C,‐DRB1, and ‐DQB1 loci, respectively (Figure 4A). By using the Haplostat tool, donors’ 2F‐HR genotypes were correctly obtained in 97.5%, 90.0%, 89.6%, 83.3%, 70.7%, and 84.0% for HLA‐A,‐B,‐C,‐DRB1,‐DRB345 and ‐DQB1 loci, respectively (Figure 4B). Because of the higher accuracy of the HaploStats tool, we used only these inferred high‐resolution (HR) donors’ genotypes in further analysis.
FIGURE 4.
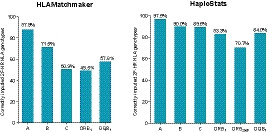
Accuracy of inferred 2F‐HR HLA typing results using HaploStats (A,‐B,‐C,‐DR,‐DQ input) and HLAMatchmaker (A,‐B,‐DR input) programs in the LRposDSA cohort with available full 2F‐HR genotypes (N = 134). Bars on the graph represent the percentage of correctly inferred second field HLA genotypes per locus. DSA, donor‐specific anti‐HLA antibodies; 2F‐HR, second field high‐resolution; LR, low‐resolution; HLA, human leukocyte antigen; pos, positive [Color figure can be viewed at wileyonlinelibrary.com]
Using the Haplostats‐inferred donor 2F‐HR HLA genotypes of transplants with LR misclassified DSA (the 2F‐HRnegLRposDSA group), DSA could be correctly excluded in 18/21 (85.7%) of the patients who required 2F‐HR genotyping results, thus were wrongly classified in 3/21 (14.3%) cases. In the remaining 11 patients, DSA could not be excluded as DQA1, DPB1 and DPA1 loci could not be inferred by Haplostats. However, when we used the inferred donors’ HR HLA genotypes to reassess the presence of DSA in the 2F‐HRposLRposDSA group, we found that DSA would be wrongly excluded in 20 (18.5%) patients (2F‐HRposInferrednegDSA) and correctly confirmed in 88 (81.5%) of patients (2F‐HRposInferredposDSA). We confirmed the clinical implications of DSA misclassification based on the inference of 2F‐HR HLA genotyping in survival analysis. Patients with wrongly excluded DSA (2F‐HRposInferrednegDSA) had the lowest 10‐year graft survival rate of 44.9% and the highest cumulative incidence of ABMRh (91.2%) within the first 5 years posttransplant (Figure 5).
FIGURE 5.
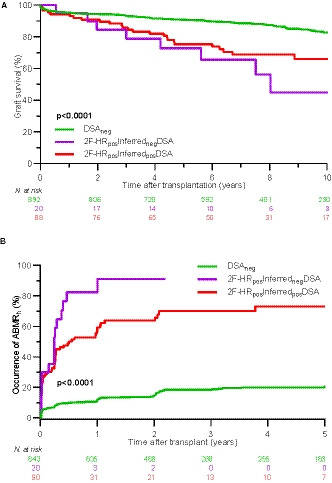
Survival analysis and occurrence of ABMRh, stratified according to the presence of DSA assessed by inferred vs true 2F‐HR genotypes. A, Both the 2F‐HRposInferredposDSA group and the 2F‐HRposInferrednegDSA group had inferior 10‐year death‐censored graft survival compared to DSAneg patients (66.0% vs 44.9% vs 82.8%, P < .0001 by log‐rank test). B, The cumulative incidence of ABMRh within the first 5 years was 20.4%, 73.1%, and 91.2% for DSAneg, 2F‐HRposInferredposDSA, and 2F‐HRposInferrednegDSA, respectively (P < .0001 by log‐rank test). Forty‐seven patients without biopsy follow‐up were excluded from this analysis. ABMRh, the histological phenotype of antibody‐mediated rejection; DSA, donor‐specific anti‐HLA antibodies; 2F‐HR, second field high‐resolution; neg, negative; pos, positive [Color figure can be viewed at wileyonlinelibrary.com]
From this, we evaluated the accuracy of the 3 different donor HLA genotyping levels for assigning DSA in HLA‐sensitized patients (N = 262). Using the 2F‐HR genotyping results as the criterion standard for comparison to the LR and inferred 2F‐HR genotyping, the specificity was higher for the inferred donor typing data (89.6%) than for the LR donor data (81.8%). However, the accuracy of LR genotyping was 79.8%, even better than the accuracy of 76.7% for the inferred 2F‐HR donor data due to the lower sensitivity of the inferred genotypes (Table 3).
TABLE 3.
Comparison of the accuracy of DSA assignment with LR and inferred 2F‐HR genotyping with HaploStats. Donor‐recipient 2F‐HR HLA genotyping was considered the criterion standard (N = 262 patients with anti‐HLA antibodies)
| Donor genotyping HLA level | ||||
|---|---|---|---|---|
| LR split antigen level | Inferred 2F‐HR level | |||
| DSA ≥ 500 | pos | neg | pos | neg |
| Final DSA positive | 83 | 25 | 63 | 45 |
| Final DSA negative | 28 | 126 | 16 | 138 |
| Accuracy (%) | 79.8 | 76.7 | ||
| Sensitivity (%) | 76.9 | 58.3 | ||
| Specificity (%) | 81.8 | 89.6 | ||
| PPV (%) | 74.8 | 79.8 | ||
| NPV (%) | 83.4 | 75.4 | ||
Abbreviations: 2F‐HR, second field high‐resolution; DSA, donor‐specific anti‐HLA antibodies; HLA, human leukocyte antigens; LR, low‐resolution; neg, negative; NPV, negative predictive value; pos, positive; PPV, positive predictive value.
3.5. Impact of the inferred or incomplete HLA type on the donor‐recipient eplet incompatibility
Finally, we investigated the impact of the inferred 2F‐HR donor genotypes on the eplet mismatch load repertoire in the patients with available LR HLA‐A,‐B,‐C,‐DR and ‐DQ donor typing data (N = 471). By using the HaploStats tool, we obtained inferred 2F‐HR results for 469 donors. In this cohort, donors’ 2F‐HR genotypes were correctly estimated in 96.3%, 91.3%, 95.4%, 81.7%, 67.3%, and 81.5% for HLA‐A,‐B,‐C,‐DRB1,‐DRB345 and ‐DQB1 loci, respectively. First, we evaluated the impact of using inferred donors’ genotypes in the assessment of eplet mismatch load. For this, we calculated differences in the eplet(s) between the eplet load of the actual 2F‐HR genotypes and the eplet load of the inferred donors 2F‐HR genotypes for each transplant pair, for both class I and class II molecules (N = 469). The agreement in the total number of mismatched eplets was 91.3% for class I (A, B, and C) and 53.9% for class II (DRB1345 and DQB1) (Figure 6A). Considering the differences in the mismatched eplet repertoire instead of the total number of mismatched eplets, the agreement between the inferred and the real 2F‐HR genotypes was 75.3% for class I and 35.4% for class II. This illustrates that the currently used methods for inferring second field HLA results do not allow correct evaluation of the eplet mismatch repertoire, especially of class II molecules.
FIGURE 6.
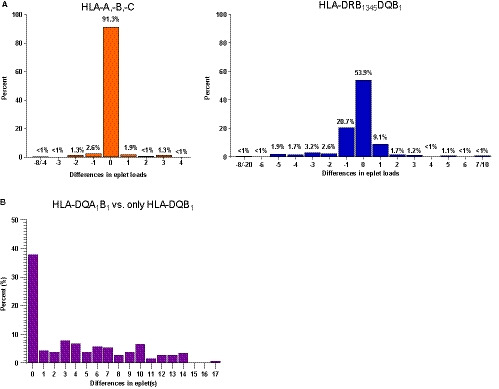
Differences in the eplet mismatch repertoire when using incomplete or inferred donor HLA genotypes. The differences are calculated in eplet(s) and the x‐axis shows the difference in eplets between the eplet mismatch loads. A, Differences in the eplet mismatch loads between true vs inferred HLA genotypes for HLA‐A/‐B/‐C and DRB1345/DQB1. The differences refer only to the mismatch load, not to the eplet mismatch repertoire. Considering the differences in the mismatched eplet repertoire instead of the total number of mismatched eplets, the agreement between the inferred and the real 2F‐HR genotypes was 75.3% for class I and 35.4% for class II. B, Differences in the eplet mismatch loads between the eplet load of the complete DQ (DQA1/DQB1) molecule vs the eplet mismatch load calculated only for DQB1 locus. 2F‐HR, second field high‐resolution; HLA, human leukocyte antigen [Color figure can be viewed at wileyonlinelibrary.com]
Because the DQ molecule is currently extensively studied at the eplet mismatch level for risk stratification due to the high incidence of de novo DSA formation, several studies focus on DQB1 data. 11 , 12 , 13 , 14 Therefore, we evaluated the differences in the mismatch eplet repertoires when the whole DQA1B1 molecule is assessed compared to the DQB1 only. We found that only in 37.8% of the cases, the eplet mismatch load is the same between the whole DQA1B1 molecule and the DQB1 only, while in 62.2% the eplet repertoire in the eplet mismatch load differs from 1 to 17 eplets (Figure 6B).
4. DISCUSSION
In the current study, we investigated how 2F‐HR HLA genotyping impacts the characterization of HLA antibodies detected by solid‐phase SAB, and whether inference of the 2F‐HR genotypes from the LR genotypes could be used to correctly assign the presence of DSA and accurately estimate the eplet mismatch repertoire. We showed that in one‐fifth of the pretransplant DSA suspected by LR HLA genotypes, the donor specificity could not be confirmed after 2F‐HR genotyping, primarily related to misclassifications of HLA‐DQ antibodies. We also showed that although inference of the 2F‐HR genotypes from LR data improved the assessment of DSA of misclassified cases, significant misclassifications still occurred in ≈20%, calling into question the relevance of such inference for clinical purposes. The accuracy for assigning DSA in sensitized patients was 79.8% for LR genotyping and 76.7% for inferred 2F‐HR donor HLA genotyping using HaploStats. Finally, due to inaccuracies in the eplet compatibility estimation, mainly for HLA class II, complete 2F‐HR HLA genotype measurement is recommended for clinical and research purposes.
Analyzing the patients’ sera reactivity of the HLA antibodies detected by solid‐phase SAB is a challenging task when the traditional LR donor genotyping approaches are applied. Our finding that one‐fifth of the cases with DSA were misclassified when using LR donor genotypes has important clinical consequences. Patients with DSA at LR level that are disproved by 2F‐HR typing could be wrongly classified as having high immunological risk and receive more aggressive immunosuppression and specific antibody‐targeting therapies, while the outcome is good in such cases even without therapeutic intervention. Although inferring the 2F‐HR genotypes can improve the assessment of DSA in these patients, by excluding a relevant percent of misclassified DSA at LR level, clinically significant misclassification remains in the total DSA cohort. Our data thus suggest that HLA compatibility and mismatch acceptability need to be determined at the 2F‐HR level, in order to allow correct assessment of the donor‐specificity of the HLA antibodies. These findings echo the results of a recent case series, where 2F‐HR HLA genotypes, obtained from routine clinical practice, helped in the correct assessment of the presence of DSA. 20
Our findings also have important implications for the allocation of kidneys in HLA‐sensitized patients. We found that one‐fifth of the patients with DSA assessed at the antigen level do not have a higher risk of rejection and graft failure than the DSA‐negative patients. Suitable organs at the 2F‐HR level might be declined for these patients due to the wrongly assumed high immunological risk evaluated at the antigen level. By better characterization of the donor genotypes at the 2F‐HR HLA level, and thus ruling out any DSA misclassification, the chances of receiving a transplant offer would not decrease as one could fear. 9 On the contrary, by not excluding the whole HLA antigen groups, the donor pool for finding compatible donors will increase. Finally, having second field HLA variants available for donor‐recipient pairs would also optimize and unify the listing of unacceptables for the sensitized renal transplant candidates, and would allow correct assessment of the broadness of HLA sensitization as quantified by panel reactive antibodies. 21
In our cohort, HLA‐DQ was the molecule most needing 2F‐HR genotyping for correct evaluation. Of all misclassified DSAs at the LR level, 43% were directed against the DQ molecule. There were 3 main issues that complicated the correct interpretation of the DQ antibody reactivity based on LR genotyping: (1) insufficient LR genotyping level of the donor DQB1 locus, which level does not allow accurate determination of the DQB1 reactivity patterns; (2) absence of genotyping information for DQA1 locus, and therefore wrong attribution of anti‐DQA1 reactivity to DQB1 in some cases; 22 and (3) inability to analyze the sera reactivity toward the physiological donors' DQ molecules as not all DQA1/DQB1 donor dimers are present in the SAB assays. Because the DQ molecule is associated with the highest incidence of de novo DSA occurrence and poor graft outcome, further studying of DQ immunogenicity at the molecular level should be the main focus of research. 23
Due to the high inaccuracy of the eplet mismatch repertoire, especially for HLA class II, our results warrant caution in the interpretation of studies that use inferred HLA genotypes, as was also concluded in another recent study. 24 HLA genotype inference may impact the conclusions of such studies by influencing the accuracy of the DSA evaluation, and also by including substantial inaccuracies in the eplet mismatch load calculation. When studying molecular HLA mismatches between organ donors and recipients, genotyping at the 2F‐HR level seems crucial, especially for the DQ locus.
It may be argued that the threshold used for assigning DSA positivity in our cohort of 500 background‐corrected MFI value is low. Although this may well be true for some cases of misclassified DSA, it should be noted that increasing the MFI threshold could lead to neglecting pathogenic DSAs due to the known phenomenon of artificial reduction of the MFI in the SAB assay. 25 This phenomenon is caused by a common epitope that is recognized by a single antibody but present on multiple beads in the SAB assay. Therefore the measured MFI value of a single bead sometimes does not always accurately represent the titer of a specific anti‐HLA antibody. For this, the complete donor 2F‐HR genotyping data can help in the data interpretation by identifying common epitopes or amino acid residues on different beads that are involved in antibody reactivity patterns. Also, the complete recipient 2F‐HR genotyping, used as additional locus‐specific negative controls, helps excluding unspecific reactivities when going above the cutoff for DSA positivity in the final DSA, as was the case in 15.7% of the misclassified DSAs in our study. This also provides an opportunity to avoid the use of fixed cutoff MFI values for DSA positivity and to determine more specifically the anti‐HLA sera reactivity.
Our study has several limitations. First, we do not have data on the donor ethnicity, which may have impacted the inference of the 2F‐HR HLA. However, as the Eurotransplant allocation policy does not allow the recording of donor ethnicity, this HLA inference accuracy reflects the accuracy that would be achieved if HLA inference were used for clinical purposes. Also, we did not test the second vendor of SAB tests to investigate the correct donor molecules that were not present in our reagents. In these few cases, we analyzed the sera reactivity towards the HLA molecules present in the SAB assay that have the same sequences in the extracellular domains (exons 2, 3, 4, for class I and exons 2, 3 for class II) as the donor molecule. This was the case mainly for the HLA‐DP molecule. Therefore, the possibility of missed DSA in the DSA‐negative group is very low. Also, for a few patients, the donors' DQA1B1 dimers were not present in our LSA2 kit. Therefore, we assigned DSA only against DQA1 or DQB1 molecules. It can be anticipated that the lack of the correct donor dimer can underestimate the real strength of the DSA. Finally, we did not apply specific antibody‐targeting desensitization therapies in this population. While this allowed evaluation of the natural presentation of the DSA‐positive patients, outcome data could be different in more recent years with the advent of desensitization options for patients with pretransplant DSA. Re‐evaluation of our conclusions in such recent cohorts is therefore necessary.
In summary, our study provides clinical support for extended 2F‐HR HLA genotyping of transplant pairs to correctly determine the presence of DSA. Extended 2F‐HR HLA genotyping also seems relevant in more accurately defining the HLA compatibility in a given donor/recipient combination, to eventually adopt preventative strategies for allograft rejection, to guide follow‐up intensity, and to improve decisions on DSA‐targeting therapies. Therefore, for all living donor kidney transplantations in sensitized recipients, NGS (or another methodology able to provide 2F‐HR typing) should be done. The currently available NGS methods (or other methods for 2F‐HR typing) do not meet the turnaround time needed for deceased donor typing. The real‐time quantitative PCR or HR sequence‐specific oligonucleotide methods should be used for rapid deceased donor typing as they increase the number of targeted HLA loci and discriminate most of the common second field HLA variants present in the SAB kits. If the 2F‐HR typing level cannot be achieved in the pretransplant setting, donor‐retyping with NGS (or another 2F‐HR typing method) needs to be performed as soon as possible posttransplant in sensitized recipients. Such posttransplant retyping at the 2F‐HR typing obviously cannot help in allocation decisions but allows optimization of posttransplant patient management by more correct evaluation of the donor specificities of the HLA antibodies. Further efforts should be made to shorten the turnaround time of the 2F‐HR typing methods, to make the 2F‐HR genotyping results available in the allocation process also for deceased donor transplantation. Finally, due to inaccuracies in the eplet mismatch estimation of the inferred 2F‐HR genotypes, mainly for HLA class II, complete 2F‐HR HLA genotyping, including DRB345, DQA1 and DPA1, should be mandatory for clinical use and research on eplet mismatch calculations.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
AS, MPE, and MN conceived and designed the study. AS, MPE, VVS, EL, MC, BS, DK, and MN were involved in data collection. AS analyzed the data and prepared the figures and tables. AS and MN drafted the manuscript and all coauthors revised and approved the final version.
ACKNOWLEDGMENTS
This project is funded by the Research Foundation ‐ Flanders (FWO) and the Flanders Innovation & Entrepreneurship agency (VLAIO), with a TBM project (grant no. IWT.150199; "TEMPLATE"). MN and BS are senior clinical investigators of the Research Foundation Flanders (FWO) (1844019N and 1842919N, respectively). MN is also funded by a C3 internal grant from the KU Leuven (grant no. C32/17/049).
Senev A, Emonds M‐P, Van Sandt V, et al. Clinical importance of extended second field high‐resolution HLA genotyping for kidney transplantation. Am J Transplant. 2020;20:3367–3378. 10.1111/ajt.15938
See also: Baxter‐Lowe.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Williams RC, Opelz G, McGarvey CJ, Weil EJ, Chakkera HA. The risk of transplant failure with hla mismatch in first adult kidney allografts from deceased donors. Transplantation. 2016;100(5):1094‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nankivell BJ, Alexander SI. Rejection of the kidney allograft. Schwartz RS, ed. N Engl J Med. 2010;363(15):1451‐1462. [DOI] [PubMed] [Google Scholar]
- 3. Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody‐mediated rejection: current status and novel approaches. Am J Transplant. 2014;14(2):255‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor‐specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21(8):1398‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Senev A, Lerut E, Van SV, et al. Specificity, strength and evolution of pretransplant donor‐specific HLA antibodies determine outcome after kidney transplantation. Am J Transplant. 2019;19(11):3100‐3113. [DOI] [PubMed] [Google Scholar]
- 6. Amico P, Hönger G, Mayr M, Steiger J, Hopfer H, Schaub S. Clinical relevance of pretransplant donor‐specific HLA antibodies detected by single‐antigen flow‐beads. Transplantation. 2009;87(11):1681‐1688. [DOI] [PubMed] [Google Scholar]
- 7. Wissing KM, Abramowicz D. Unacceptable human leucocyte antigens: how to navigate between increased immunological risk and waiting time? Nephrol Dial Transplant. 2017;32(5):745‐747. [DOI] [PubMed] [Google Scholar]
- 8. Duquesnoy RJ, Kamoun M, Baxter‐Lowe LA, et al. Should HLA mismatch acceptability for sensitized transplant candidates be determined at the high‐resolution rather than the antigen level? Am J Transplant. 2015;15(4):923‐930. [DOI] [PubMed] [Google Scholar]
- 9. Cecka JM, Reed EF, Zachary AA. HLA high‐resolution typing for sensitized patients: a solution in search of a problem? Am J Transplant. 2015;15(4):855‐856. [DOI] [PubMed] [Google Scholar]
- 10. Lin DY, Hu Y, Huang BE. Simple and efficient analysis of disease association with missing genotype data. Am J Hum Genet. 2008;82(2):444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snanoudj R, Kamar N, Cassuto E, et al. Epitope load identifies kidney transplant recipients at risk of allosensitization following minimization of immunosuppression. Kidney Int. 2019;95(6):1471‐1485. [DOI] [PubMed] [Google Scholar]
- 12. Lachmann N, Niemann M, Reinke P, et al. Donor‐recipient matching based on predicted indirectly recognizable HLA epitopes independently predicts the incidence of de novo donor‐specific HLA antibodies following renal transplantation. Am J Transplant. 2017;17(12):3076‐3086. [DOI] [PubMed] [Google Scholar]
- 13. Philogene MC, Amin A, Zhou S, et al. Correction to: Eplet mismatch analysis and allograft outcome across racially diverse groups in a pediatric transplant cohort: a single‐center analysis. Pediatr Nephrol. 2020;35(4):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sapir‐Pichhadze R, Zhang X, Ferradji A, et al. Epitopes as characterized by antibody‐verified eplet mismatches determine risk of kidney transplant loss. Kidney Int. 2019:1‐8. 10.1016/j.kint.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 15. Kumru Sahin G, Unterrainer C, Süsal C. Critical evaluation of a possible role of HLA epitope matching in kidney transplantation. Transplant Rev. 2020;34(2):100533 10.1016/j.trre.2020.100533. [DOI] [PubMed] [Google Scholar]
- 16. Gragert L, Madbouly A, Freeman J, Maiers M. Six‐locus high resolution HLA haplotype frequencies derived from mixed‐resolution DNA typing for the entire US donor registry. Hum Immunol. 2013;74(10):1313‐1320. [DOI] [PubMed] [Google Scholar]
- 17. Duquesnoy RJ. HLA epitope based matching for transplantation. Transpl Immunol. 2014;31(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 18. Senev A, Coemans M, Lerut E, et al. Histological picture of antibody‐mediated rejection without donor‐specific anti‐HLA antibodies: clinical presentation and implications for outcome. Am J Transplant. 2019;19(3):763‐780. [DOI] [PubMed] [Google Scholar]
- 19. Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell–mediated rejection, antibody‐mediated rejection, and prospects for integrative endpoints for next‐generation clinical trials. Am J Transplant. 2018;18(2):293‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Y, Dinh A, Heron S, et al. Assessing the utilization of high‐resolution 2‐field HLA typing in solid organ transplantation. Am J Transplant. 2019;19(7):1955‐1963. [DOI] [PubMed] [Google Scholar]
- 21. Tambur AR, Audry B, Antoine C, Suberbielle C, Glotz D, Jacquelinet C. Harnessing scientific and technological advances to improve equity in kidney allocation policies. Am J Transplant. 2017;17(12):3149‐3158. [DOI] [PubMed] [Google Scholar]
- 22. Tambur AR, Leventhal JR, Zitzner JR, Carlin Walsh R, Friedewald JJ. The DQ barrier: improving organ allocation equity using HLA‐DQ information. Transplantation. 2013;95(4):635‐640. [DOI] [PubMed] [Google Scholar]
- 23. Tambur AR, McDowell H, Hod‐Dvorai R, Casillas Abundis MA, Pinelli DF. The quest to decipher HLA immunogenicity: telling friend from foe. Am J Transplant. 2019;19(10):2910‐2925. [DOI] [PubMed] [Google Scholar]
- 24. D'Souza Y, Ferradji A, Saw CL, et al. Inaccuracies in epitope repertoire estimations when using multilocus allele‐level HLA genotype imputation tools. HLA. 2018;92(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 25. Tambur AR, Campbell P, Claas FH, et al. Sensitization in transplantation: assessment of Risk (STAR) 2017 Working Group Meeting Report. Am J Transplant. 2018;18(7):1604‐1614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


