Abstract
Merkel cell carcinoma (MCC) is a highly aggressive neuroendocrine tumor of the skin with an estimated disease‐associated mortality of 15–33%. Australia has a higher incidence of MCC compared to the rest of the world, thought to be due to a higher ultraviolet index. The Australian MCC population is distinct from the MCC population of the Northern hemisphere, characterized by a predominantly viral negative etiology with high tumor mutational burden. The optimal management of MCC and the choice of treatment modality vary significantly across the world and even between institutions within Australia. Historically, the treatment for MCC has been resection followed by radiotherapy (RT), though definitive RT is an alternative treatment used commonly in Australia. The arrival of immune checkpoint inhibitors and the mounting evidence that MCC is a highly immunogenic disease is transforming the treatment landscape for MCC. Australia is playing a key role in the further development of treatment options for MCC with two upcoming Australian/New Zealand investigator‐initiated clinical trials that will explore the interplay of RT and immunotherapy in the treatment of early and late stage MCC.
Keywords: clinical trials, immunotherapy, management, Merkel cell carcinoma, radiotherapy
1. INTRODUCTION
Merkel cell carcinoma (MCC) is a cutaneous neuroendocrine malignancy that usually appears as a rapidly growing pink/purple nodule in sun‐exposed skin. MCC typically arises in elderly Caucasians (median age 70–75) and the immunocompromised (10% of patients), and is more common in men. MCC has an annual incidence of 1.3 per 100 000 people in Australia 1 and 0.7 per 100 000 people in the United States. 2 Furthermore, these numbers have been steadily increasing year‐on‐year, with the incidence in the United States rising by greater than 95% since the year 2000. 2 The higher incidence rate in Australia is believed to be due to the higher ultraviolet (UV) index compared with other geographic regions of a similar ethnic background. 3 , 4
Clonal integration of the Merkel cell polyomavirus (MCPyV) DNA into the host genome 5 has been implicated in approximately 80% of MCC cases in the northern hemisphere. 6 , 7 In MCPyV‐positive MCC, the potent MCPyV antigen proteins drive tumorigenesis and are required for maintaining tumor cell survival. 8 However, as MCPyV infection often occurs at a young age and is generally ubiquitous in the population, other co‐operative risk factors are important for disease development. MCPyV‐negative MCC appears to be driven by UV‐carcinogenesis, 9 and this is reflected by the enrichment of MCPyV‐negative MCC in Australia and New Zealand, more frequent presentation on sun‐exposed sites, and a unique mutational pattern in these cancers. 10 , 11 , 12
DNA sequencing studies of MCC samples reflect the two distinct etiologies for MCC, which may suggest two different cells of origin. 13 MCPyV‐negative MCC is hypermutated and has the hallmark mutational signature associated with UV damage, whereas MCPyV‐positive MCC has a very low number of mutations. 14 , 15 This bimodal mutational profile could suggest that MCPyV‐positive tumors arise from a more UV‐protected precursor cell in dermis. Despite these biological differences, the clinical significance of the tumor viral status is currently unclear 16 , 17 , 18 , 19 and is not used to guide treatment decisions.
Given the rarity of MCC, there is a lack of prospective randomized clinical trials (RCTs) comparing treatment approaches and most of the information is from retrospective, single institutional or population‐based registry studies. As a result, it is difficult to reach a consensus on the “optimal management” of MCC and the choice of treatment varies between institutions. Current National Comprehensive Cancer Network (NCCN) guidelines for MCC recommend wide local excision (WLE) of the primary tumor as the standard approach to initial management. 20 Australian institutes typically favor an approach of definitive radiotherapy (RT) or less extensive resection followed by postoperative RT.
MCC is a highly aggressive malignancy with an estimated 5‐year disease‐associated mortality of 15‐33%. 21 , 22 , 23 MCC tends to grow quickly and metastasizes at an early stage; survival is improved with earlier detection and treatment. Until recently, distant metastatic MCC (mMCC) had no effective treatment. However, the arrival of immune checkpoint inhibitors (ICIs) and the evidence that MCC is an immunogenic disease is transforming the treatment landscape for MCC.
This manuscript reviews the current treatment options for locoregional and distant metastatic MCC and also discusses some of the upcoming Australian/New Zealand investigator‐initiated clinical trials that may help shape further treatment options for MCC.
2. METHODS
A literature search was conducted on PubMed and Medline databases, with language limitations (English only) but no time limitations. There are no specific Australian guidelines for the treatment of MCC; Australian clinicians have provided their insights into the treatment of MCC, often based on their own published research.
Definitive RT was defined as RT delivered to the nonexcised primary lesion and/or involved lymph nodes. Adjuvant therapy was defined as therapy delivered after resection of the primary lesion and/or lymph nodes.
3. CLINICAL PRESENTATION
The diagnosis of MCC can be challenging and is often missed or delayed as the primary tumor lacks any pathognomic features. 20 Most often, MCC presents as a rapidly growing violaceous nodule from either dermal, subcutaneous or intraepidermal layers of the skin (Figure 1). Primary MCC is most frequently located on sun‐exposed areas, particularly the head and neck.
FIGURE 1.
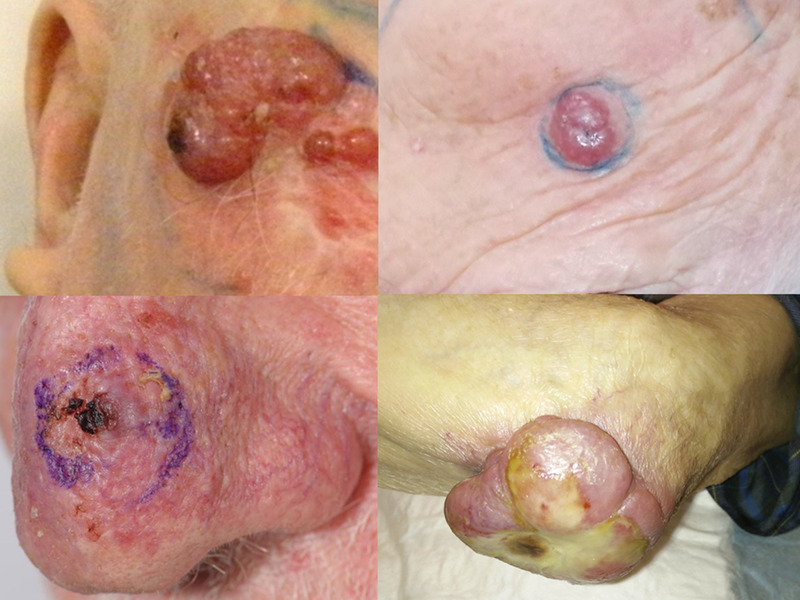
Four examples of Merkel cell carcinomas. Note the tendency for red/violaceous coloration, nodular appearance, and distribution on sun‐exposed areas [Color figure can be viewed at wileyonlinelibrary.com]
There are no “classical” clinical features of the primary tumor that distinguish it from other common skin lesions and MCC cannot be diagnosed based on the clinical examination alone. Some clinical features of MCC denoted by the acronym AEIOU are (a) asymptomatic, (b) expanding rapidly in less than 3 months, (c) immunosuppression, (d) older than 50 years, and (e) UV exposed locations of the body. The presentation of these clinical features alongside a lesion that may look benign in nature, should warrant a biopsy and histopathological analysis in order to confirm a clinical diagnosis of MCC. MCC is staged according to the eighth edition of the American Joint Committee on Cancer (AJCC). 24
MCC usually metastasizes to the draining lymph nodes first and a lymph node examination in addition to a full skin survey is recommended. Paradoxically, patients who present with metastatic nodal disease without a known cutaneous primary may have a more favorable outcome compared to patients presenting with a clear cutaneous primary. 25 , 26
4. DIAGNOSTIC WORKUP
Though MCC has a characteristic immunohistological profile, diagnosis should be confirmed by an experienced dermatopathologist. 20 In most cases, morphological features, positive staining for cytokeratin 20 (CK20), and neuroendocrine markers and negative staining for thyroid transcription factor‐1, CK7, and lymphoid markers 27 are sufficient to confirm a diagnosis of MCC. Detection of viral DNA/protein can be used to distinguish between MCPyV‐positive versus negative patients; however, this is not routinely performed as it does not alter management.
Sentinel lymph node biopsy is considered the most reliable staging tool to identify subclinical nodal disease. 20 Between 30% and 50% of patients with a primary MCC will harbor subclinical nodal metastases, and these patients should undergo pathological investigation or elective treatment of nearby lymph nodes. 28 , 29
MCC is prone to both locoregional and distant recurrences. The majority of relapses will occur within the first 2 years. Close follow‐up after initial treatment is important, including imaging. Unfortunately, there is a lack of evidence to guide the choice and timing of post‐treatment imaging and no widely accepted guidelines for imaging of MCC. Recommended imaging techniques include computed tomography (CT), ultrasound, and 18‐fluorodeoxyglucose positron emission tomography (FDG PET/CT). 20
5. TREATMENT PARADIGMS: LOCOREGIONAL DISEASE
After the confirmation of a diagnosis of MCC, patients should be promptly referred to a specialist center with experience in the management of MCC as delays between diagnosis and treatment can lead to poorer outcomes. 30 Furthermore, the management of MCC requires multidisciplinary and personalized care for optimal patient outcomes. 20
5.1. Surgical excision
There is a lack of consensus regarding the ideal first‐line approach to MCC. Resection with WLE has traditionally been recommended as a primary treatment modality 20 based on the evidence primarily sourced from retrospective studies and case reports. 31 , 32 , 33 Generally, there has been a shift in the literature toward utilizing combined treatment modalities in the management of MCC with increasing evidence for the use of limited resection/biopsy supplemented with postoperative RT.
5.2. Adjuvant radiotherapy or definitive radiotherapy
MCC is a highly radiosensitive malignancy. 34 , 35 The NCCN guidelines recommend RT as an adjuvant therapy following excision of the primary tumor and this is currently the most common role of RT in the management of MCC.
Several groups have reported the use of definitive RT in the management of MCC (Table 1). Given the relatively high local and regional control rates observed in these studies, definitive RT is a reasonable noninvasive alternative to resection in select patients and is used as such in many Australian institutions.
TABLE 1.
Loco‐regional control rates for radiotherapy alone
| Author | Year | N | Control | OS | Median dose (Gy) |
|---|---|---|---|---|---|
| Koh and Veness 60 | 2009 | 8 | 87.5% in‐field control | 12.5% 1‐year OS | 50 |
| Veness et al 61 | 2010 | 43 | 75% in‐field control | 37% 5‐year OS | 51 |
| Pape et al 62 | 2011 | 25 | 100% LC; 92% RC | NA | 65 |
| Sundaresan et al 63 | 2012 | 18 | 89% in‐field control | NA | 50 |
| Harrington and Kwan 64 | 2014 | 57 | 89% LC; 79% RC | NA | NA |
| Veness and Howle 49 | 2015 | 41 | 85% in‐field control | 40% 5‐year OS | 51 |
Abbreviations: LC, local control; NA, not available; OS, overall survival; RC, regional control.
Where a resection has been undertaken as the initial treatment, a number of studies have shown excellent local control rates with the addition of adjuvant RT (Table 2). Retrospective reviews have concluded that the use of adjuvant RT after resection improved local control rates and was significantly associated with improved survival, regardless of tumor size. 36 , 37 However, whether adjuvant RT is necessary for all stages of disease is unclear, with an RCT reporting no improvement in overall survival (OS) and progression‐free survival (PFS) with adjuvant RT in stage I MCC, though there was a significant decrease in the risk of regional recurrence. 38 However, low recurrence rates have also been reported after surgical excision alone in early stage patients who have not received adjuvant RT. 32 , 39 Similarly, when MCC has spread to regional lymph nodes, there is a lack of prospective data comparing the use of RT, nodal dissection, or a combination of the two. 20 However, in Australian practice, definitive RT is generally advocated to the involved nodes and surrounding nodal basin in this setting. 66
TABLE 2.
Published reports of loco‐regional control rates and overall survival for resection and adjuvant radiotherapy
| Author | Year | N | Control rate | Overall survival |
|---|---|---|---|---|
| Gillenwater et al 65 | 2001 | 26 |
88% (3‐year LC) 95% (3‐year RC) |
77.4% (3‐year OS) |
| Lewis et al 66 | 2006 | 169 |
88% (5‐year LRFS) 77% (5‐year RRFS) |
57.3% (5‐year OS) |
| Clark et al 67 | 2006 | 66 |
84% (5‐year LC) 69% (5‐year RC) |
49% (5‐year OS) |
| Pape et al 62 | 2011 | 25 | 88% (in‐field control) | NA |
| Hui et al 68 | 2011 | 165 |
81% (in‐field control) 76% (5‐year actuarial LRC) |
45% (5‐year actuarial OS) |
| Ghadjar et al 69 | 2011 | 118 |
94% (5‐year LRFS) 76% (5‐year RRFS) |
56% (5‐year OS) |
| Fields et al 70 | 2012 | 75 |
97% (LC) 88% (RC) |
NA |
| Kang et al 71 | 2012 |
32 43 |
89% (2‐year LRFS; primary site RT) 84% (2‐year RRFS; regional RT) |
No improvement in OS |
| Bishop et al 72 | 2016 | 106 |
96% (5‐year actuarial LC) 96% (5‐year actuarial RC) |
58% (5‐year OS) |
Abbreviations: LC, local control; LRC, loco‐regional control; LRFS, loco‐regional recurrence‐free survival; NA, not available; OS, overall survival; RC, regional control; RRFS, regional recurrence‐free survival.
5.3. Chemoradiation trials
Given that MCC is chemosensitive, there has been interest utilizing it in the concurrent and adjuvant setting for patients who are perceived to be at high risk of relapse. A prospective study by the Trans‐Tasman Radiation Oncology Group (TROG) investigated the use of synchronous carboplatin, etoposide, and radiation therapy in 53 patients with high‐risk MCC. The 3‐year OS, loco‐regional control, and distant control were 76%, 75%, and 76%, respectively, indicating that chemoradiation (CRT) offered high levels of locoregional control and survival. 40 However, a multivariate analysis that compared the results of the patients from the above study to a historic cohort indicated that postoperative CRT did not improve outcomes compared with postoperative RT. 41 Yet, another retrospective study found that adjuvant CRT provided a survival benefit (HR, 0.62, 95% CI 0.47‐0.81) over resection alone and may help improve survival in patients with high‐risk MCC. 42 Given the significant toxicity and a lack of definitive benefit, adjuvant CRT has fallen out of fashion, especially with the advent of more effective systemic therapies for MCC in the form of programmed death‐1 (PD1)‐based immunotherapy.
5.4. Adjuvant immunotherapy
Viral antigens are expressed in MCPyV‐positive tumor cells, which make them immunogenic. MCPyV‐negative MCCs may also be immunogenic, based on a high tumor mutation burden and neoantigens created as a result of UV exposure. As such, the PD1‐programmed death‐ligand 1 (PDL‐1) immune‐checkpoint pathway is a key therapeutic target for MCC, 43 and ICIs have been approved for use in patients with advanced‐stage MCC.
There is a great interest in moving immunotherapy to the treatment of earlier stage MCC in the curative setting. Several northern hemisphere trials are currently underway to investigate the efficacy of ICI in the adjuvant setting in MCC. The ADMEC‐O trial (NCT02196961) is investigating the efficacy of adjuvant nivolumab monotherapy in patients with completely resected MCC. The ADAM trial (NCT03271372) is investigating the relapse‐free survival benefits of adjuvant avelumab compared to placebo in patients with stage IIIB MCC who have undergone resection with or without adjuvant radiation therapy. The NCI ECOG‐ACRIN trial (NCT03712605) is comparing 12 months of pembrolizumab to standard of care for patients with resected stage I‐III MCC.
The Immunotherapy Merkel Adjuvant Trial (I‐MAT), coordinated by the Melanoma and Skin Cancer (MASC) trials group, is an Australian investigator initiated study, which will aim to investigate the recurrence‐free survival of avelumab as an adjuvant treatment in stage I‐III MCC. This trial differs from the adjuvant immunotherapy clinical trials in the northern hemisphere as it will be in an Australian population who are likely biologically distinct from patients in the northern hemisphere, with a greater proportion of viral negative cancers with high tumor mutation burden. This trial will feature a shorter 6 months duration of adjuvant immunotherapy, which is likely to be more acceptable and convenient for patients; as well as being more cost‐effective for health services. In addition, this trial will evaluate the safety and synergy of avelumab given concurrently with RT, which is more in‐line with current Australian practice.
6. TREATMENT PARADIGMS: DISTANT METASTATIC DISEASE
MCC has a high risk of distant spread and outcomes in patients with metastatic disease are poor with a historical 5‐year survival of 13.5%. 44 The NCCN guidelines recommend that patients with distant mMCC have either systemic therapy alone or a combination of systemic and local therapies, such as resection and/or RT, depending on the clinical situation. Recently, immunotherapy has become a standard of care first‐line therapy for advanced MCC. However, due to the rapidly evolving treatment landscape, where possible, MCC patients should always be considered for enrollment in clinical trials.
6.1. Chemotherapy and radiotherapy
Prior to the advent of immunotherapy, cytotoxic chemotherapy was the treatment of choice. Though overall response rates ranged from 40% to 60%, the responses were fairly short‐lived with a median PFS of 3‐5 months, and a median OS of 10 months. 45 , 46 Chemotherapy is currently considered to only have a palliative role 47 and should only be reserved for cases of immunotherapy failure or where immunotherapy is contraindicated.
RT is used in the metastatic setting primarily for palliating symptomatic sites, such as skeletal metastases or large lymph nodes, but may also have a role in altering and augmenting the immune response. Due to the high radiosensitivity of MCC, even relatively low‐dose regimens, such as 8 Gy in a single treatment, have been demonstrated to be effective in reducing tumor burden and providing durable palliation, with limited side‐effects. 48 Hypofractionated schedules of 20 Gy in five fractions are also commonly used in the palliative treatment setting. 49
Treatment with RT or chemotherapy does not increase survival in patients with advanced MCC, and novel, effective agents that can induce durable responses in patients with metastatic or recurrent disease and that have a good safety and tolerability profile are needed.
6.2. Immunotherapy
Anti‐PDL‐1 and anti‐PD1 agents, such as avelumab and pembrolizumab, have been studied in advanced MCC patients and have durable responses, with favorable PFS and OS trends compared to chemotherapy treatment. 50 , 51 , 52 , 53 The Javelin Merkel 200 trial evaluated avelumab as second‐line and first‐line treatment in patients with mMCC. After more than 2 years follow up of 88 patients, an objective response rate (ORR) of 33% (95% CI 23.3‐43.8) was obtained, the PFS was 26% at 2 years, and the median OS was 12.6 months (95% CI 7.5‐17.1) in the second‐line setting. 54 Clinical activity was observed across all patient subgroups, irrespective of MCPyV status. Avelumab demonstrated a good safety profile with 11.4% of patients with a grade 3 or above treatment‐related adverse event and no treatment‐related deaths. 54 In the first‐line setting, the ORR after at least 3 and 6 months of follow‐up was 62.1% (n = 29; 95% CI 42.3‐79.3) and 71.4% (n = 14; 95% CI 41.9‐91.6), respectively.
Pembrolizumab was investigated as a first systemic therapy for 50 patients with metastatic or recurrent locally advanced MCC. 51 After a median follow up of 14.9 months, an ORR of 56% (95% CI 41.3‐70.0) was obtained with ORRs of 59% in virus positive and 53% in virus negative tumors. The 2‐year PFS rate was 48.3% and a median OS had not been reached. 51 Pembrolizumab had a manageable safety profile with grade 3 or 4 adverse events occurring in 28% of patients and led to treatment discontinuation in 14% of patients, including one treatment‐related death. 51 These response rates in advanced MCC cases support the use of ICIs in standard clinical practice. Avelumab, an anti‐PD‐L1 monoclonal antibody, is an approved treatment option for mMCC in several countries, including the United States, Europe, and Australia. Pembrolizumab, has recently been approved for recurrent locally advanced MCC or mMCC in the United States. Nivolumab is a fully human IgG4 anti‐PD‐1 antibody with clinical activity in advanced MCC still under investigation in a phase I/II trial (CheckMate 358, NCT02488759). Initial data showed that 11 out of 17 patients (65%) with resectable stage IIA‐IV MCC who received two doses of neoadjuvant nivolumab had a major pathologic response. The majority of operated patients remained tumor free at 12 months. 55
Ultimately, immune‐checkpoint blockade does result in responses but only in around half of patients treated. 50 , 52 Moreover, of those who do respond, a substantial number of patients acquire secondary resistance. Clearly, more needs to be done to understand the underlying mechanisms of primary and secondary resistance to overcome these issues.
6.3. Other experimental treatments
Alternative treatments to immunotherapy are required for patients with advanced stage MCC who do not respond to or have contraindications to immunotherapy. Fifty to seventy percent of patients with MCC express somatostatin receptors (SSTRs) 56 and somatostatin analogues are being investigated for MCC. Peptide receptor radionuclide therapy (PRRT) is a novel approach that uses a tumor‐targeting peptide to deliver a payload of radiation to sites of disease while sparing normal surrounding tissue. Specifically, using a radio‐labeled somatostatin analogue 177Lu‐DOTATATE to target sites expressing the SSTR may be an effective treatment for MCC. The TarGeted THerapy and Avelumab in MCC (GoTHAM) clinical trial (NCT04261855), run by the MASC trials group, will investigate antitumor activity, measured by ORR, for the combination of avelumab plus PRRT and avelumab plus RT in patients with unresectable or mMCC. It is expected that there may be a synergistic benefit from the combination of radiation and immunotherapy, which may stem from interactions between radiation and the immune system. 57
6.4. Post‐treatment surveillance
Currently, there are no standardized national post‐treatment surveillance guidelines for MCC. As such, follow up is individualized depending on patient and institutional factors. Regular skin examination and palpation of nodal areas is performed routinely, and imaging with ultrasound, CT, or PET/CT is utilized at many institutions.
Recently, a serological assay has been developed that detects the presence of antibodies against the MCPyV oncoprotein and is clinically available in the United States. 58 Rising antibody titres have proven to be correlated with MCC recurrence with a 66% positive predictive value. Given the high proportion of MCPyV‐negative patients in Australasia and the costs involved for samples to be shipped and analyzed in the United States, the MCPyV antibody test is currently not routinely used at any Australian institution.
7. CONCLUSION
Most MCCs diagnosed in Australia are viral negative and genetically distinct from those arising in other parts of the world. This may have implications for future treatment options for Australian patients, though at this stage, there is insufficient evidence that viral status should alter management in MCC. 59
Historically, the treatment for MCC has been resection followed by RT or chemotherapy and this is still often the case in the United States and Europe. MCC is highly sensitive to RT and several studies have shown the success of definitive RT in the treatment of MCC. Definitive RT for early stage MCC is widely adopted in Australian practice.
Immunotherapy is changing the treatment landscape for MCC and is now established as the standard of care in the metastatic setting. Australia is playing a key role in the further development of treatment options as demonstrated by the upcoming MASC GoTHAM and IMAT clinical trials. Ongoing international collaboration will result in further advances in the diagnosis and management of this rare disease.
CONFLICTS OF INTEREST
MV, JH, AW, MC, and MP have no conflicts of interest to declare. DK is a travel grant recipient from Merck Healthcare Australia Pty Ltd. SS is on the advisory boards and has received funding for grants for clinical research from Merck Sharp and Dohme, Bristol Meyers Squibb, Amgen, Endocyte, and Astra Zeneca. WX is on advisory boards and has received speaker fees and research funding from Merck Serono. He has also received speaker fees from Merck Sharp and Dohme and has received educational travel support from Roche and Astra Zeneca. RT has received honoraria and travel sponsorship from Merck Serono. AG has received fees for participating in advisory boards for Regeneron, SunPharma, Merck Serono, Sanofi, MSD, Tilray, Pfizer, and Eisai. AG has also received conference travel support from BMS, Merck Serono, and SunPharma and clinical trial funding from SunPharma. GK served as an unpaid advisory board member for Merck Pharma. GF is a member of advisory boards and is a grant recipient (2018 and 2019) from Merck Healthcare Australia Pty Ltd. He is also a Shareholder of Genesis Care.
ACKNOWLEDGMENTS
The authors thank Theresa Wade, PhD, of WriteSource Medical Pty Ltd, Sydney, Australia, for providing editorial support and collating and incorporating author comments. We gratefully acknowledge the infrastructure support through Cancer Australia's “Support for Cancer Clinical Trials Program” for editorial and administrative support provided by Melanoma and Skin Cancer (MASC) Trials. We acknowledge the support of Merck Serono alliance in the development of this resource.
Kok DL, Wang A, Xu W, et al. The changing paradigm of managing Merkel cell carcinoma in Australia: an expert commentary. Asia-Pac J Clin Oncol. 2020;16:312–319. 10.1111/ajco.13407
REFERENCES
- 1. Australian Institute for Health and Welfare . AIHW Australian Cancer Database 2010.
- 2. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457‐463.e452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agelli M, Clegg LX, Becker JC, Rollison DE. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer. 2010;34(1):14‐37. [DOI] [PubMed] [Google Scholar]
- 4. Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol. 2013;68(3):425‐432. [DOI] [PubMed] [Google Scholar]
- 5. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez‐Vela MDC, Curiel‐Olmo S, Derdak S, et al. Shared oncogenic pathways implicated in both virus‐positive and UV‐induced Merkel cell carcinomas. J Invest Dermatol. 2017;137(1):197‐206. [DOI] [PubMed] [Google Scholar]
- 7. Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV‐positive and MCPyV‐negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7(3):3403‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeCaprio JA. Merkel cell polyomavirus and Merkel cell carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732):20160276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paik JY, Hall G, Clarkson A, et al. Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum Pathol. 2011;42(10):1385‐1390. [DOI] [PubMed] [Google Scholar]
- 11. Dabner M, McClure RJ, Harvey NT, et al. Merkel cell polyomavirus and p63 status in Merkel cell carcinoma by immunohistochemistry: Merkel cell polyomavirus positivity is inversely correlated with sun damage, but neither is correlated with outcome. Pathology. 2014;46(3):205‐210. [DOI] [PubMed] [Google Scholar]
- 12. Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938‐945. [DOI] [PubMed] [Google Scholar]
- 13. Sunshine JC, Jahchan NS, Sage J, Choi J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene. 2018;37(11):1409‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus‐negative Merkel cell carcinoma. Cancer Res. 2015;75(18):3720‐3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong SQ, Waldeck K, Vergara IA, et al. UV‐associated mutations underlie the etiology of MCV‐negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228‐5234. [DOI] [PubMed] [Google Scholar]
- 16. Hall BJ, Pincus LB, Yu SS, Oh DH, Wilson AR, McCalmont TH. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol. 2012;39(10):911‐917. [DOI] [PubMed] [Google Scholar]
- 17. Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus‐negative Merkel cell carcinoma: a more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol. 2017;137(4):819‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Handschel J, Muller D, Depprich RA, et al. The new polyomavirus (MCPyV) does not affect the clinical course in MCCs. Int J Oral Maxillofac Surg. 2010;39(11):1086‐1090. [DOI] [PubMed] [Google Scholar]
- 19. Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131(8):1631‐1638. [DOI] [PubMed] [Google Scholar]
- 20. Bichakjian CK, Olencki T, Aasi SZ, et al. Merkel cell carcinoma, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(6):742‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 22. Farley CR, Perez MC, Soelling SJ, et al. Merkel cell carcinoma outcomes: does AJCC8 underestimate survival? Ann Surg Oncol. 2020;27(6):1978‐1985. [DOI] [PubMed] [Google Scholar]
- 23. Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015;81(8):802‐806. [DOI] [PubMed] [Google Scholar]
- 24. Bichakjian CK, Nghiem P, Johnson T, Wright CL, Sober AJ. Merkel cell carcinoma In: Amin MB, Edge S, Greene F, eds. AJCC Cancer Staging Manual. 8th ed American Joint Commission on Cancer, Springer International Publishing; 2016, pp. 549‐562. [Google Scholar]
- 25. Haymerle G, Fochtmann A, Kunstfeld R, Pammer J, Erovic BM. Management of Merkel cell carcinoma of unknown primary origin: the Vienna Medical School experience. Eur Arch Otorhinolaryngol. 2015;272(2):425‐429. [DOI] [PubMed] [Google Scholar]
- 26. Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012;67(3):395‐399. [DOI] [PubMed] [Google Scholar]
- 27. Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140(2):427‐447. [PMC free article] [PubMed] [Google Scholar]
- 28. Gunaratne DA, Howle JR, Veness MJ. Sentinel lymph node biopsy in Merkel cell carcinoma: a 15‐year institutional experience and statistical analysis of 721 reported cases. Br J Dermatol. 2016;174(2):273‐281. [DOI] [PubMed] [Google Scholar]
- 29. Karunaratne YG, Gunaratne DA, Veness MJ. Systematic review of sentinel lymph node biopsy in Merkel cell carcinoma of the head and neck. Head Neck. 2018;40(12):2704‐2713. [DOI] [PubMed] [Google Scholar]
- 30. Tsang G, O'Brien P, Robertson R, Hamilton C, Wratten C, Denham J. All delays before radiotherapy risk progression of Merkel cell carcinoma. Australas Radiol. 2004;48(3):371‐375. [DOI] [PubMed] [Google Scholar]
- 31. Tai PT, Yu E, Tonita J, Gilchrist J. Merkel cell carcinoma of the skin. J Cutan Med Surg. 2000;4(4):186‐195. [DOI] [PubMed] [Google Scholar]
- 32. Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300‐2309. [DOI] [PubMed] [Google Scholar]
- 33. Wright GP, Holtzman MP. Surgical resection improves median overall survival with marginal improvement in long‐term survival when compared with definitive radiotherapy in Merkel cell carcinoma: a propensity score matched analysis of the National Cancer Database. Am J Surg. 2018;215(3):384‐387. [DOI] [PubMed] [Google Scholar]
- 34. Ashby MA, Jones DH, Tasker AD, Blackshaw AJ. Primary cutaneous neuroendocrine (Merkel cell or trabecular carcinoma) tumour of the skin: a radioresponsive tumour. Clin Radiol. 1989;40(1):85‐87. [DOI] [PubMed] [Google Scholar]
- 35. Leonard JH, Ramsay JR, Kearsley JH, Birrell GW. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32(5):1401‐1407. [DOI] [PubMed] [Google Scholar]
- 36. Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25(9):1043‐1047. [DOI] [PubMed] [Google Scholar]
- 37. Harrington C, Kwan W. Radiotherapy and conservative surgery in the locoregional management of Merkel cell carcinoma: the British Columbia Cancer Agency Experience. Ann Surg Oncol. 2016;23(2):573‐578. [DOI] [PubMed] [Google Scholar]
- 38. Jouary T, Leyral C, Dreno B, et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol. 2012;23(4):1074‐1080. [DOI] [PubMed] [Google Scholar]
- 39. Frohm ML, Griffith KA, Harms KL, et al. Recurrence and survival in patients with Merkel cell carcinoma undergoing surgery without adjuvant radiation therapy to the primary site. JAMA Dermatol. 2016;152(9):1001‐1007. [DOI] [PubMed] [Google Scholar]
- 40. Poulsen M, Rischin D, Walpole E, et al. High‐risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans‐Tasman Radiation Oncology Group Study–TROG 96:07. J Clin Oncol. 2003;21(23):4371‐4376. [DOI] [PubMed] [Google Scholar]
- 41. Poulsen MG, Rischin D, Porter I, et al. Does chemotherapy improve survival in high‐risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006;64(1):114‐119. [DOI] [PubMed] [Google Scholar]
- 42. Chen MM, Roman SA, Sosa JA, Judson BL. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: an analysis of 4815 patients. JAMA Otolaryngol Head Neck Surg. 2015;141(2):137‐141. [DOI] [PubMed] [Google Scholar]
- 43. Chan IS, Bhatia S, Kaufman HL, Lipson EJ. Immunotherapy for Merkel cell carcinoma: a turning point in patient care. J Immunother Cancer. 2018;6(1):23‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11):3564‐3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cowey CL, Mahnke L, Espirito J, Helwig C, Oksen D, Bharmal M. Real‐world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13(19):1699‐1710. [DOI] [PubMed] [Google Scholar]
- 46. Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016;5(9):2294‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nghiem P, Kaufman HL, Bharmal M, Mahnke L, Phatak H, Becker JC. Systematic literature review of efficacy, safety and tolerability outcomes of chemotherapy regimens in patients with metastatic Merkel cell carcinoma. Future Oncol. 2017;13(14):1263‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iyer JG, Parvathaneni U, Gooley T, et al. Single‐fraction radiation therapy in patients with metastatic Merkel cell carcinoma. Cancer Med. 2015;4(8):1161‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veness M, Howle J. Radiotherapy alone in patients with Merkel cell carcinoma: the Westmead Hospital experience of 41 patients. Australas J Dermatol. 2015;56(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 50. Nghiem PT, Bhatia S, Lipson EJ, et al. PD‐1 blockade with pembrolizumab in advanced Merkel‐cell carcinoma. N Engl J Med. 2016;374(26):2542‐2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first‐line therapy. J Clin Oncol. 2019;37(9):693‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy‐refractory metastatic Merkel cell carcinoma: a multicentre, single‐group, open‐label, phase 2 trial. Lancet Oncol. 2016;17(10):1374‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D'Angelo SP, Russell J, Lebbe C, et al. Efficacy and safety of first‐line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9):e180077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nghiem P, Bhatia S, Brohl AS, et al. Two‐year efficacy and safety update from JAVELIN Merkel 200 part A: a registrational study of avelumab in metastatic Merkel cell carcinoma progressed on chemotherapy. Am J Clin Oncol. 2018;36(15_suppl):9507‐9507. [Google Scholar]
- 55. Topalian SL, Bhatia S, Kudchadkar RR, et al. Nivolumab (Nivo) as neoadjuvant therapy in patients with resectable Merkel cell carcinoma (MCC) in CheckMate 358. Am J Clin Oncol. 2018;36(Suppl 15):9505‐9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gardair C, Samimi M, Touze A, et al. Somatostatin receptors 2A and 5 are expressed in Merkel cell carcinoma with no association with disease severity. Neuroendocrinology. 2015;101(3):223‐235. [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Deng W, Li N, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paulson KG, Lewis CW, Redman MW, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer. 2017;123(8):1464‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harms PW, Harms KL, Moore PS, et al. The biology and treatment of Merkel cell carcinoma: current understanding and research priorities. Nat Rev Clin Oncol. 2018;15(12):763‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Koh CS, Veness MJ. Role of definitive radiotherapy in treating patients with inoperable Merkel cell carcinoma: the Westmead Hospital experience and a review of the literature. Australas J Dermatol. 2009;50(4):249‐256. [DOI] [PubMed] [Google Scholar]
- 61. Veness M, Foote M, Gebski V, Poulsen M. The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients. Int J Radiat Oncol Biol Phys. 2010;78(3):703‐709. [DOI] [PubMed] [Google Scholar]
- 62. Pape E, Rezvoy N, Penel N, et al. Radiotherapy alone for Merkel cell carcinoma: a comparative and retrospective study of 25 patients. J Am Acad Dermatol. 2011;65(5):983‐990. [DOI] [PubMed] [Google Scholar]
- 63. Sundaresan P, Hruby G, Hamilton A, et al. Definitive radiotherapy or chemoradiotherapy in the treatment of Merkel cell carcinoma. Clin Oncol (R Coll Radiol). 2012;24(9):e131‐e136. [DOI] [PubMed] [Google Scholar]
- 64. Harrington C, Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann Surg Oncol. 2014;21(11):3401‐3405. [DOI] [PubMed] [Google Scholar]
- 65. Gillenwater AM, Hessel AC, Morrison WH, et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001;127(2):149‐154. [DOI] [PubMed] [Google Scholar]
- 66. Lewis KG, Weinstock MA, Weaver AL, Otley CC. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142(6):693‐700. [DOI] [PubMed] [Google Scholar]
- 67. Clark JR, Veness MJ, Gilbert R, O'Brien CJ, Gullane PJ. Merkel cell carcinoma of the head and neck: is adjuvant radiotherapy necessary? Head Neck. 2007;29(3):249‐257. [DOI] [PubMed] [Google Scholar]
- 68. Hui AC, Stillie AL, Seel M, Ainslie J. Merkel cell carcinoma: 27‐year experience at the Peter MacCallum Cancer Centre. Int J Radiat Oncol Biol Phys. 2011;80(5):1430‐1435. [DOI] [PubMed] [Google Scholar]
- 69. Ghadjar P, Kaanders JH, Poortmans P, et al. The essential role of radiotherapy in the treatment of Merkel cell carcinoma: a study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2011;81(4):e583‐e591. [DOI] [PubMed] [Google Scholar]
- 70. Fields RC, Busam KJ, Chou JF, et al. Five hundred patients with Merkel cell carcinoma evaluated at a single institution. Ann Surg. 2011;254(3):465‐473. Discussion 473‐465. [DOI] [PubMed] [Google Scholar]
- 71. Kang SH, Haydu LE, Goh RY, Fogarty GB. Radiotherapy is associated with significant improvement in local and regional control in Merkel cell carcinoma. Radiat Oncol. 2012;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bishop AJ, Garden AS, Gunn GB, et al. Merkel cell carcinoma of the head and neck: favorable outcomes with radiotherapy. Head Neck. 2016;38(Suppl 1):E452‐E458. [DOI] [PubMed] [Google Scholar]


