Abstract
Objective
The purpose of this study was to determine the relationship between patient‐reported symptoms of oropharyngeal dysphagia (OD) using the Eating Assessment Tool (EAT)‐10 and the swallowing function using a standardized fiberoptic endoscopic evaluation of swallowing (FEES) protocol in head and neck cancer (HNC) patients with confirmed OD.
Methods
Fifty‐seven dysphagic HNC patients completed the EAT‐10 and a FEES. Two blinded clinicians scored the randomized FEES examinations. Exclusion criteria consisted of presenting with a concurrent neurological disease, scoring below 23 on a Mini‐Mental State Examination, being older than 85 years, having undergone a total laryngectomy, and being illiterate or blind. Descriptive statistics, linear regression, sensitivity, specificity, and predictive values were calculated.
Results
The majority of the dysphagic patients (N = 38; 66.7%) aspirated after swallowing thin liquid consistency. A large number of patients showed postswallow pharyngeal residue while swallowing thick liquid consistency. More specifically, 42 (73.0%) patients presented postswallow vallecular residue, and 39 (67.9%) patients presented postswallow pyriform sinus residue. All dysphagic patients had an EAT‐10 score ≥ 3. Linear regression analyses showed significant differences in mean EAT‐10 scores between the dichotomized categories (abnormal vs. normal) of postswallow vallecular (P = .037) and pyriform sinus residue (P = .013). No statistically significant difference in mean EAT‐10 scores between the dichotomized categories of penetration or aspiration was found (P = .966).
Conclusion
The EAT‐10 questionnaire seems to have an indicative value for the presence of postswallow pharyngeal residue in dysphagic HNC patients, and a value of 19 points turned out to be useful as a cutoff point for the presence of pharyngeal residue in this study population.
Level of Evidence: 2b
Keywords: Dysphagia, deglutition, deglutition disorders, EAT‐10, head and neck cancer
INTRODUCTION
Despite the development of organ‐saving therapies for head and neck cancer (HNC), early and late toxicities of (chemo)radiotherapy or surgery cannot be avoided, and full function preservation of the upper aerodigestive tract is usually not possible. 1 , 2 , 3 , 4 , 5 , 6 Oropharyngeal dysphagia (OD) is a common symptom after HNC treatment and it is often chronic in nature, with a prevalence ranging from 23% to 100%, whereas tube‐feeding dependency ranges from 5% to 60%. 6 , 7 , 8 , 9 , 10 , 11 Furthermore, silent aspiration as a more severe expression of OD has been reported up to 45% in this population and is accompanied by a higher risk of grave consequences such as aspiration pneumonia, malnutrition, dehydration, and death. 12 , 13 The high prevalence of OD and its consequences on health‐related quality of life in this population asks for early detection of this condition in order to facilitate early implementation of swallowing rehabilitation and nutritional support. 14 , 15 , 16 Often, it remains unclear which patients should be monitored for OD and at what time points. 17 It can be expected that fewer complications of OD will arise if the nature of OD and the nutrition status are systematically monitored during the HNC follow‐up visits. 18 , 19 , 20 A reliable tool to identify and assess OD, which can be easily implemented in daily clinical practice, can help monitoring OD in HNC patients.
The Eating Assessment Tool‐10 (EAT‐10) is a patient self‐report questionnaire that documents a symptom‐specific outcome for OD. It was developed to report the initial dysphagia severity based on clinically relevant OD symptoms and is also used to monitor treatment response in patients with a variety of swallowing disorders due to, for example, HNC, esophageal abnormalities, and neurodegenerative diseases. 21 The EAT‐10 questionnaire is commonly used in daily clinical practice for various OD etiologies and it has a high test–retest reliability. 21 The purpose of this study was to determine the relationship between patient‐reported symptoms of OD using the EAT‐10 and the swallowing function using a standardized fiberoptic endoscopic evaluation of swallowing (FEES) protocol in dysphagic HNC patients. In other words: can the EAT‐10 be used as an indicator of the nature or phenotype of OD in dysphagic HNC patients?
MATERIALS AND METHODS
Patients
For this cross‐sectional cohort study, dysphagic HNC patients were recruited from the outpatient clinic for OD of the department of otorhinolaryngology at a tertiary university referral hospital between 2013 and 2016. Individuals were enrolled in the study if they had completed the HNC treatment at least 6 months prior to recruitment and their disease was in a stable period (total remission, absence of radiation mucositis). The exclusion criteria were presenting with a concurrent neurological disease (e.g. stroke, Parkinson disease), scoring below 23 on a Mini‐Mental State Examination (MMSE), 22 being older than 85 years, having undergone a total laryngectomy, having recurrent HNC or a second primary head‐and‐neck tumor, having osteoradionecrosis of the maxilla or mandible (severe pain), and being illiterate or blind. Cancer staging was performed according to the tumor, nodes, and metastasis (TNM) classification system. 23 Informed consent was obtained from all participants, and the study protocol was approved as non‐wet maatschappelijke ondersteuning (WMO) research by the institutional medical ethics committee in compliance with the WMO Medical Research Involving Human Subjects Act. 24
Swallowing Protocol
All patients underwent a standardized examination protocol used in daily clinical practice. The protocol consisted of a clinical ear, nose, and throat examination, including integrity of cranial nerves performed by a laryngologist, body mass index (BMI) measurement, FEES examination, 25 the Functional Oral Intake Scale (FOIS), 26 and the EAT‐10 questionnaire.
FOIS scores range from 1 to 7, where 1 corresponds to no oral diet, and 7 corresponds to total oral diet with no restrictions. The Dutch translated version of the EAT‐10 was used in this study. 27 , 28 Similar to the English version, the Dutch translation consists of a 10‐item questionnaire with a maximum total score of 40 points. All items are rated on a 5‐point scale in which 0 indicates no problem, and 4 indicates a severe problem in swallowing function. An EAT‐10 score of ≥ 3 is abnormal and indicates a higher self‐perception of the presence of OD. 21 In the present study, the EAT‐10 questionnaire was not used as a screening tool because the HNC population was already diagnosed with OD.
During FEES examination, patients were offered three trials of thin and three trials of thick liquid. Each trial contained 10 cc of water (thin) or applesauce (One 2 fruit, van Oordt, Oud‐Beijerland) (thick) dyed with 5% methylene blue. 29 , 30 The viscosity of the bolus consistencies was measured at 25 °C and 50 s‐1 of shear rate resulting in 1 mPa.s for thin liquid and 1,200 mPa.s for thick liquid. Following the flow test instructions, thick liquid met the criteria for moderately thick according to the International Dysphagia Diet Standardisation Initiative (IDDSI). 31 The tip of the flexible fiberoptic endoscope Pentax FNL‐10RP3 (Pentax Canada Inc., Mississauga, Ontario, Canada) was positioned just above the epiglottis in what is called the high position. 25 FEES images were obtained with a Xion SD camera, XionEndoSTROBE camera control unit (PAL 25 fps), and Matrix DS datastation with DIVAS software (Xion Medical, Berlin, Germany). Neither a nasal vasoconstrictor nor a topical anesthetic was administered.
FEES Outcome Variables
Three reliable visuoperceptual ordinal variables were scored as described in previous studies: penetration or aspiration, postswallow vallecular residue, and postswallow pyriform sinus residue (Table 1). 29 , 32 , 33 Aspiration was defined as bolus passing below the level of the vocal folds entering the trachea or bolus on the true vocal folds secondarily leaking in the trachea. Three‐point ordinal scales (range 0–2), based on a visuoperceptual estimate of the amount of the bolus in the valleculae and/or pyriform sinuses, were used to capture residue severity. The term residue was defined as the amount of bolus remaining in the valleculae and/or pyriform sinuses after spontaneous clearing swallows. 29 , 32 Severe residue in the valleculae means residue up to the free edge of the epiglottis. For pyriform sinus residue, severe residue was up to the level of the arytenoids. All variables were scored for each FEES swallow at varying speed (slow motion, normal speed, and up to frame‐by‐frame). Before assessment of the swallows, two observers underwent consensus training for these measurements, as described in previous studies. 29 , 30 , 32 The observers were blinded to patient identity and medical history and to each other's scores. To determine interobserver agreement, 20% of the FEES swallows were rated twice (repeated measurements). All three swallow trials of both consistencies were rated to forestall an underestimation of the outcome. 32
TABLE 1.
Frequency Distribution of HNC Patients per Category of the Different FEES Variables Given as Absolute Numbers and Percentages.
| FEES† Variable‡ | No. of Patients (%) | Dichotomized Outcome | No. of Patients (%) |
|---|---|---|---|
| Postswallow vallecular residue§ | Postswallow vallecular residue§ | ||
| Category 0 | 9 (18%) | Category 0: “normal” | 9 (18%) |
| Category 1 | 28 (55%) | Category 1: “abnormal” | 42 (82%) |
| Category 2 | 14 (28%) | ||
| Postswallow pyriform sinus residueǁ | Postswallow pyriform sinus residueǁ | ||
| Category 0 | 23 (46%) | Category 0: “normal” | 23 (46%) |
| Category 1 | 17 (34%) | Category 1: “abnormal” | 27 (54%) |
| Category 2 | 10 (20%) | ||
| Penetration/aspirationǁ | Penetration/aspirationǁ | ||
| Category 0 | 12 (24%) | Category 0: “normal” | 12 (24%) |
| Category 1 | 11 (22%) | Category 1: “abnormal” | 38 (76%) |
| Category 2 | 27 (54%) |
Fiberoptic endoscopic evaluation of swallowing.
Lower scores refer to normal functioning; higher scores refer to more severe disability.
Six patients (10.5%) had a missing value.
Seven patients (12.3%) had a missing value.
FEES = fiberoptic endoscopic evaluation of swallowing; HNS = head and neck cancer.
Due to several patient characteristics, such as extreme postradiation xerostomia, oropharyngeal tissue fibrosis, or severe OD for specific consistencies (severe aspiration for thin liquid with increased pulmonary risk), not all patients were able to complete all swallow trials.
Statistical Analysis
Numerical variables were reported in terms of mean with standard deviation (SD) or median with interquartile range where appropriate. The categorical variables were presented by number and percentage. The intra‐ and interobserver agreement was determined using a linearly weighted kappa coefficient of agreement (κ) for all visuoperceptual ordinal FEES variables. 34 The maximum score (indicating more severe impairment) of each FEES variable, independent of the consistency, was used in the statistical analysis. The given scores for postswallow vallecular and pyriform sinus residue, as well as for the variable penetration and/or aspiration, were subsequently dichotomized as normal function if the given score was 0 and as abnormal function if the scoring was ≥ 1. Dichotomization was carried out following the observer agreement analysis and was done to increase the small group sizes if possible (Table 1). To evaluate the relationship between the outcome of the EAT‐10 questionnaire and the scored FEES variables, linear regression analyses were performed. All assumptions of linear regression analysis were checked using histograms, residual plots, and Cook's distances (> 1 indicates influential outlier). Two‐sided P values ≤ .05 were considered to be statistically significant. In addition, the effect of the mathematically composed variable postswallow pharyngeal residue (= postswallow vallecular residue and/or postswallow pyriform sinus residue) on the EAT‐10 outcome was assessed to evaluate the impact of the presence of postswallow pharyngeal residue on EAT‐10 scores. The same procedure was done to determine the effect of the FEES variable penetration or aspiration on the EAT‐10 outcome. Subsequent statistical correction for residue location (vallecula vs. pyriform sinus) and variable penetration/aspiration was performed. The (adjusted) differences in means with corresponding 95% confidence intervals (CI) and P values were reported. The means and 95% CI were also plotted to visualize the association between the FEES outcome variables and the EAT‐10 scores (Figs. 1, 2, 3). All dysphagic HNC patients scored more than 3 points on the EAT‐10 questionnaire; therefore, the cutoff value of 3 was not specifically used in the linear regression model. 21 Instead, the whole range of scores (0–40 points) on the EAT‐10 was used to explore the entire severity range of patient‐reported OD symptoms. The diagnostic values (sensitivity, specificity, predictive values, and area under the receiver operating characteristic (ROC) curve) of the EAT‐10 for postswallow pharyngeal residue at any location were calculated using the cutoff point derived from the ROC curve, which ensured a sensitivity ≥ 0.90 (Fig. 4). The Youden index for computing the optimal EAT‐10 cutoff point for the sensitivity and specificity of postswallow pharyngeal residue was explored, but this technique was not chosen to forestall an underestimation of the presence of residue in the present dysphagic HNC population. 35
Fig 1.
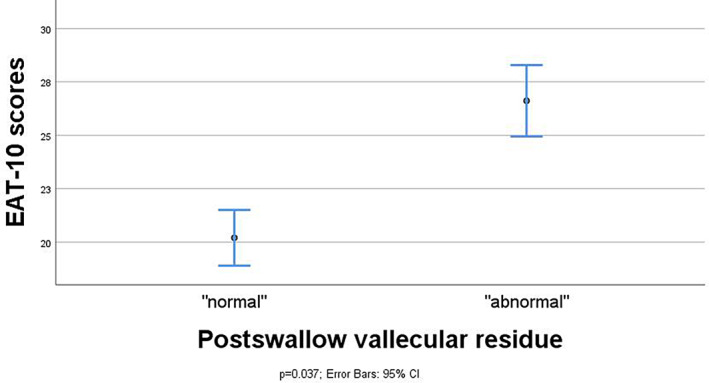
Association between FEES outcome variables and EAT‐10 scores in means and 95% CI and maximum spread of EAT‐10 scores for the FEES variable postswallow vallecular residue (n = 48). CI = confidence interval; EAT = Eating Assessment Tool; FEES = fiberoptic endoscopic evaluation of swallowing.
Fig 2.
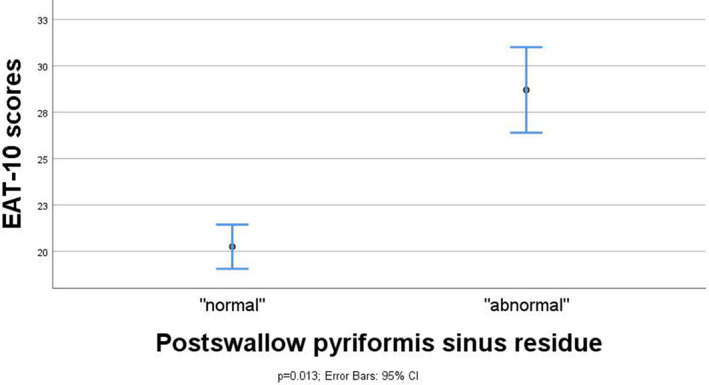
Association between FEES outcome variables and EAT‐10 scores in means and 95% CI and maximum spread of EAT‐10 scores for the FEES variable postswallow pyriform sinus residue (n = 47). CI = confidence interval; EAT = Eating Assessment Tool; FEES = fiberoptic endoscopic evaluation of swallowing.
Fig 3.
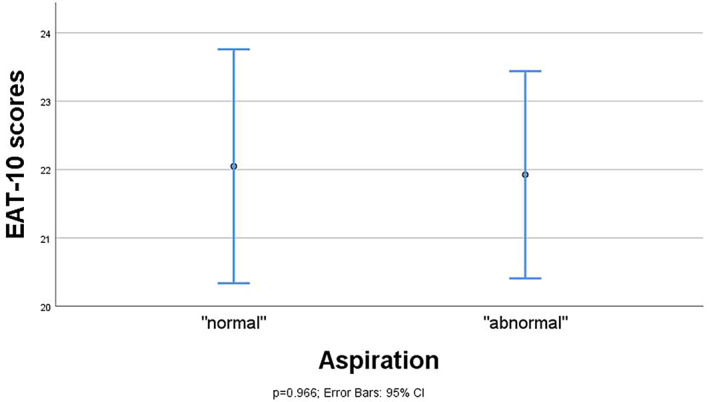
Association between FEES outcome variables and EAT‐10 scores in means and 95% CI and maximum spread of EAT‐10 scores for the FEES variable penetration/aspiration (n = 47). CI = confidence interval; EAT = Eating Assessment Tool; FEES = fiberoptic endoscopic evaluation of swallowing.
Fig 4.
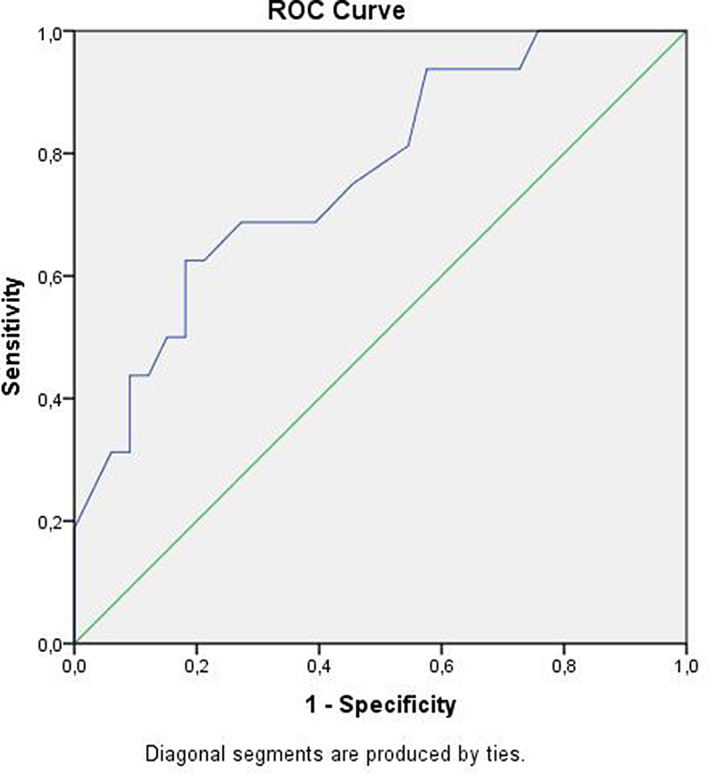
ROC curve of the EAT‐10 outcome score. AUC (AUC 0.719, 95% CI 0.641, 0.797) of the mathematically composed FEES variable postswallow pharyngeal residue at any location (= postswallow vallecular and/or pyriform sinus residue). AUC = area under the curve; CI = confidence interval; EAT = Eating Assessment Tool; FEES = fiberoptic endoscopic evaluation of swallowing; ROC = receiver operating characteristic.
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY).
RESULTS
Participants
Fifty‐seven patients were enrolled in this study. The mean (SD) age of the patients was 64.8 (10.8) years, and the FOIS showed a modified texture diet for all patients. The mean (SD) score of the EAT‐10 and BMI was 22.2 (9.3) and 24.9 (4.9), respectively. Patient characteristics are presented in Table 2.
TABLE 2.
Frequency Distribution of HNC Patient Characteristics (Total Number of Patients = 57).
| Characteristic | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 39 (68) |
| Female | 18 (32) |
| T classification † , ‡ | |
| Tis | 1 (2) |
| T1 | 8 (17) |
| T2 | 16 (35) |
| T3 | 10 (22) |
| T4 | 10 (22) |
| Tx | 1 (2) |
| N classification † , ‡ | |
| N0 | 24 (52) |
| N1 | 6 (13) |
| N2 | 15 (33) |
| N3 | 1 (2) |
| Therapy § | |
| Definitive radiotherapy | 20 (36) |
| Definitive chemoradiotherapy | 10 (18) |
| Surgery | 8 (15) |
| Surgery and adjuvant radiotherapy | 16 (29) |
| Surgery and adjuvant chemoradiotherapy | 1 (2) |
| Type of surgery ǁ | |
| Local resection primary tumor | 7 (28) |
| Local resection primary tumor with neck dissection | 13 (52) |
| Local resection primary tumor, neck dissection, and free flap reconstruction | 4 (16) |
| Neck dissection | 1 (4) |
| Tumor location ¶ | |
| Nasopharynx | 4 (7) |
| Oropharynx | 13 (23) |
| Hypopharynx | 2 (4) |
| Larynx | 20 (36) |
| Oral cavity | 9 (16) |
| Nasal (sinus) cavity | 1 (2) |
| Other (skin cancer with head and neck treatment, salivary gland cancer) | 7 (13) |
| Tumor histopathology # | |
| Squamous cell carcinoma | 40 (83) |
| Adenocarcinoma | 2 (4) |
| Verrucous carcinoma | 1 (2) |
| Other | 5 (10) |
(Primary) tumor and node classification (TNM Classification of Malignant Tumours 7th edition).
Eleven patients (19%) had a missing value.
Two patients (4%) had a missing value.
Two patients (4%) had a missing value.
One patient (2%) had a missing value.
Nine patients (16%) had a missing value.
FEES = fiberoptic endoscopic evaluation of swallowing; HNS = head and neck cancer; TNM = tumor, node, metastasis.
FEES Variables
The intra‐ and interobserver agreement levels were substantial‐to‐almost perfect for all FEES variables (i.e., κ ≥ 0.7) (Table 3). 34 All patients showed an impaired swallowing function during the FEES examination. Of all patients presenting postswallow vallecular residue, 31 (54.4%) patients presented penetration and/or aspiration. Of the patients presenting postswallow pyriform sinus residue, 23 (40.4%) showed penetration and/or aspiration. A large number of patients showed postswallow pharyngeal residue while swallowing thick liquid consistency, that is, postswallow vallecular residue in 42 (73.0%) patients and postswallow pyriform sinus residue in 39 patients (67.9%), respectively. The majority of the patients (38; 66.7%) aspirated while swallowing thin liquid consistency.
TABLE 3.
Description and Observer Agreement Levels for the FEES Outcome Variables.
| FEES† Ordinal Outcome Variable | Definition | Ordinal Scale | Interobserver Agreement (Linearly Weighted Kappa)‡ | Intraobserver Agreement (Linearly Weighted Kappa)‡ (Observer 1; Observer 2) |
|---|---|---|---|---|
| Postswallow vallecular residue | Residue in the valleculae after the swallow |
3‐point scale (0–2) 0 = no residue 1 = mild to intermediate residue 2 = severe residue up to complete filling of the valleculae |
0.73 | 0.76; 0.87 |
| Postswallow pyriform sinus residue | Residue in the pyriform sinuses after the swallow |
3‐point scale (0–2) 0 = no residue 1 = mild to intermediate residue 2 = severe residue up to complete filling of the sinuses (up to the level of the arytenoids) |
0.71 | 0.81; 0.84 |
| Penetration/aspiration | Penetration or aspiration |
3‐point scale (0–2) 0 = normal (no penetration/aspiration) 1 = penetration with bolus in the larynx above the level of the vocal folds 2 = aspiration with bolus on and below the level of the vocal folds |
0.76 | 0.81; 0.71 |
Fiberoptic endoscopic evaluation of swallowing.
Kappa agreement (linearly weighted kappa coefficient of agreement.
<0 less than chance agreement. 0.01–0.20 slight agreement. 0.21–0.40 fair agreement. 0.41–0.60 moderate agreement. 0.61–0.80 substantial agreement. 0.81–0.99 almost perfect agreement.
FEES = fiberoptic endoscopic evaluation of swallowing.
Swallowing Function and EAT‐10 Outcome
Linear regression analyses showed significant differences in mean EAT‐10 scores between the dichotomized categories (presence vs. absence) of postswallow vallecular residue (difference 6.4, 95% CI 0.4, 12.4; P = .037 [n = 48]) and postswallow pyriform sinus residue (difference 8.5, 95% CI 1.9, 15.0; P = .013 [n = 47]). Contrarily, there was no statistically significant difference in mean EAT‐10 scores between the dichotomized outcomes scores of aspiration (difference −0.1, 95% CI −5.9, 5.7; P = .966, [n = 47]). The mean EAT‐10 score was significantly higher for patients with postswallow pharyngeal residue compared to those without any residue (difference 8.9, 95% CI 3.7, 14.3; P = .001 [n = 49]), which remained significant after correction for aspiration in the regression models (adjusted difference 9.5, 95% CI 3.8, 15.3; P = .002 [n = 47]). Also, subsequent correction for residue location (vallecula vs. pyriform sinus) showed no difference in the significant relationship between postswallow pharyngeal residue and the EAT‐10 scores.
The diagnostic values (sensitivity, specificity, predictive values, and area under the ROC curve) of the EAT‐10 for postswallow pharyngeal residue were calculated (Table 4). The area under the ROC curve showed a result of 0.76 (95% CI 0.71, 0.82), which is considered a fair test for the discrimination between the presence or absence of postswallow pharyngeal residue. 36
TABLE 4.
Assessment of the Diagnostic Accuracy of the EAT‐10 Questionnaire for Postswallow Pharyngeal Residue at Any Location (Yes/No), Where EAT‐10 ≥ 19 is Considered as an Increased Symptom‐Specific Outcome for OD.
| Pharyngeal Residue | No Pharyngeal Residue | Total | |
|---|---|---|---|
| EAT‐10 ≥ 19 | 15 (a) | 20 (b) | 35 |
| EAT‐10 < 19 | 1 (c) | 13 (d) | 14 |
| Total | 16 | 33 | 49 |
Values represent number of patients. Sensitivity: 100%*a / (a + c) = 93.8%. Specificity: 100%*d / (b + d) = 39.4%. Positive predictive value: 100%*a / (a + b) = 42.9%. Negative predictive value: 100%*d / (c + d) = 92.9%.
EAT = Eating Assessment Tool; OD = oropharyngeal dysphagia.
Based on the ROC curve, an EAT‐10 cutoff point of 19 was determined. This cutoff value clearly demonstrated the presence of postswallow pharyngeal residue considering that a higher sensitivity (≥ 0.90) of the EAT‐10 is more desirable than a higher specificity to forestall an underestimation of postswallow pharyngeal residue and its potential related risk of secondary aspiration. 37 For this EAT‐10 cutoff point 19, the sensitivity was 93.9% (95% CI 0.68, 0.99); the specificity was 39.4% (95% CI 0.23, 0.58); the positive predictive value was 42.9% (95% CI 0.27, 0.60); and the negative predictive value was 92.9% (95% CI 0.64, 0.99). The mean (SD) EAT‐10 score of the patients with postswallow pharyngeal residue versus patients without pharyngeal residue was 28.1 (7.6) and 19.2 (8.9), respectively.
DISCUSSION
In this cross‐sectional observational study, the relationship between the OD‐symptom‐specific questionnaire EAT‐10 and the characteristics of OD identified using FEES in dysphagic HNC patients was described. There is a growing need to have an easy‐to‐use OD assessment tool that is not only measuring OD‐burden but that can also disclose information on the nature or phenotype of OD in dysphagic HNC patients. FEES was selected as instrumental swallowing assessment tool because it enables an extensive evaluation of the pharyngeal phase of swallowing, which is often compromised following HNC treatment. 38 FEES is a safe, widely used, and well‐known instrument to diagnose OD, and because there is no exposure to radiation, it is highly recommended for this already intensively radiation exposed group of patients. 39 However, a carefully conducted FEES examination takes time, which makes its implementation in the regular and busy HNC outpatient clinic very difficult. Therefore, a reliable self‐report assessment tool for OD can help clinicians to quickly identify the nature of OD complaints and indicate which patient would benefit from a more extensive swallowing evaluation.
The preliminary data show that the EAT‐10 questionnaire seems to have an indicative value at a score of 19 points to demonstrate the presence of postswallow pharyngeal residue as a dominant OD phenotype in HNC. This finding encourages further research to confirm that an EAT‐10 cutoff point can be used to better characterize the nature of OD in HNC patients during their oncological follow‐up visits.
Although several studies reported the relationship between the EAT‐10 score and the presence of OD, only two studies investigated this relationship in HNC patients; of these, neither used FEES to evaluated swallowing. 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47
Arrese et al. enrolled 44 HNC patients and compared the EAT‐10 scores with the presence of OD using videofluoroscopie (VFS) examination. 46 OD was determined using the penetration‐aspiration scale and the modified barium swallow impairment profile. The results showed a significant relationship between the EAT‐10 score and the presence of OD in the group comprising patients in the period pretreatment up to 1 year post‐HNC treatment. No significant relationship was found in the groups comprising patients longer than 1 year post‐HNC treatment. The mean EAT‐10 score (24.4, SD 8.3) of the patients who aspirated in this study, is comparable to the mean EAT‐10 score (24.0, SD 9.3) of the patients who aspirated in the present study.
Cheney et al. studied 360 dysphagic patients with different OD etiologies who underwent VFS. 47 Of this population, 79 (22%) patients developed OD following radiotherapy, and 32 (9%) patients were classified as other etiologies of OD, including among others postsurgical HNC patients. The mean (SD) EAT‐10 score was 16.1 (10.2) for nonaspirators and 23.2 (10.9) for aspirators, similar to the values from the present study. Furthermore, Cheney et al. found a statistically significant correlation between the EAT‐10 scores on the one hand and the risk of aspiration and a prolonged total pharyngeal transit time on the other hand. Patients with an EAT‐10 score >15 were 2.2 times more likely to aspirate. The sensitivity of an EAT‐10 score >15 in case of aspiration was 71%; the specificity was 53%. The study of Cheney et al. described that the EAT‐10 questionnaire can be used to predict aspiration in a general OD population. However, no group‐specific analysis was performed, and thus HNC‐specific data was missing. The present study did not find a significant relationship between the EAT‐10 and the presence of aspiration using a standardized FEES protocol. A possible explanation for this finding might be that the dysphagic HNC population has a higher incidence of post(chemo)radiation neuropathy with impaired sensibility in the upper aerodigestive tract, resulting in silent aspiration or a reduced subjective perception of aspiration. 48 This might cause an underestimation of the presence of OD in the EAT‐10 scores.
In conclusion, the preliminary data of the present study suggests that the EAT‐10 questionnaire seems to have an indicative value for the presence of the OD phenotype postswallow pharyngeal residue in dysphagic HNC patients.
Limitations of the Study
Stratification of the data for tumor subsites, oncological treatment modalities, time after treatment, and tumor characteristics was not possible due to the limited sample size. Due to the limited sample size and the lack of matching healthy control subjects or nondysphagic HNC patients with a similar TNM classification and oncological treatment history, it is not possible to compute an EAT‐10 cutoff point that can be used for OD assessment in the general HNC population. In addition, in an advanced TNM stage, the majority of the patients will have OD, especially following multimodality HNC treatment.
CONCLUSION
The preliminary results of the present study showed that the EAT‐10 questionnaire seems to have an indicative value (cutoff point) for the presence of the OD phenotype postswallow pharyngeal residue in dysphagic HNC patients. However, for the time being it remains recommended to perform a multidimensional swallowing assessment in HNC patients with OD complaints or at risk for OD until the generalization of the results can be confirmed.
ACKNOWLEDGMENT
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Editor's Note: This Manuscript was accepted for publication on March 02, 2020.
Conflict of Interest: The authors declare that they have no conflict of interest.
BIBLIOGRAPHY
- 1. Mosel DD, Bauer RL, Lynch DP, Hwang ST. Oral complications in the treatment of cancer patients. Oral Dis 2011;17:550–559. [DOI] [PubMed] [Google Scholar]
- 2. van der Veen J, Nuyts S. Can intensity‐modulated‐radiotherapy reduce toxicity in head and neck squamous cell carcinoma? Cancers (Basel) 2017;9(10):E135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erkal EY, Canoglu D, Kaya A, et al. Assessment of early and late dysphagia using videofluoroscopy and quality of life questionnaires in patients with head and neck cancer treated with radiation therapy. Radiat Oncol 2014;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denaro N, Merlano MC, Russi EG. Dysphagia in head and neck cancer patients: pretreatment evaluation, predictive factors, and assessment during radio‐chemotherapy, recommendations. Clin Exp Otorhinolaryngol 2013;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dysphagia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) , Raber‐Durlacher JE, Brennan MT, et al. Swallowing dysfunction in cancer patients. Support Care Cancer 2012;20:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnhart MK, Robinson RA, Simms VA, et al. Treatment toxicities and their impact on oral intake following non‐surgical management for head and neck cancer: a 3‐year longitudinal study. Support Care Cancer 2018;26:2341–2351. [DOI] [PubMed] [Google Scholar]
- 7. Roden DF, Altman KW. Causes of dysphagia among different age groups: a systematic review of the literature. Otolaryngol Clin North Am 2013;46:965–987. [DOI] [PubMed] [Google Scholar]
- 8. Machtay M, Moughan J, Farach A, et al. Hypopharyngeal dose is associated with severe late toxicity in locally advanced head‐and‐neck cancer: an RTOG analysis. Int J Radiat Oncol Biol Phys 2012;84:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiley SG, Hargunani CA, Skoner JM, Holland JM, Wax MK. Swallowing function after chemoradiation for advanced stage oropharyngeal cancer. Otolaryngol Head Neck Surg 2006;134:455–459. [DOI] [PubMed] [Google Scholar]
- 10. Agarwal J, Palwe V, Dutta D, et al. Objective assessment of swallowing function after definitive concurrent (chemo)radiotherapy in patients with head and neck cancer. Dysphagia 2011;26:399–406. [DOI] [PubMed] [Google Scholar]
- 11. Crowder SL, Douglas KG, Yanina Pepino M, Sarma KP, Arthur AE. Nutrition impact symptoms and associated outcomes in post‐chemoradiotherapy head and neck cancer survivors: a systematic review. J Cancer Surviv 2018;12:479–494. [DOI] [PubMed] [Google Scholar]
- 12. Rosen A, Rhee TH, Kaufman R. Prediction of aspiration in patients with newly diagnosed untreated advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 2001;127:975–979. [DOI] [PubMed] [Google Scholar]
- 13. Wilson JA, Carding PN, Patterson JM. Dysphagia after nonsurgical head and neck cancer treatment: patients' perspectives. Otolaryngol Head Neck Surg 2011;145:767–771. [DOI] [PubMed] [Google Scholar]
- 14. Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003;124:328–336. [DOI] [PubMed] [Google Scholar]
- 15. Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community‐acquired pneumonia. Clin Ther 1998;20:820–837. [DOI] [PubMed] [Google Scholar]
- 16. Xinou E, Chryssogonidis I, Kalogera‐Fountzila A, Panagiotopoulou‐Mpoukla D, Printza A. Longitudinal evaluation of swallowing with videofluoroscopy in patients with locally advanced head and neck cancer after chemoradiation. Dysphagia 2018;33:691–706. [DOI] [PubMed] [Google Scholar]
- 17. Federatie Medisch Specialisten . Richtlijn Orofaryngeale Dysfagie, 2017, NVKNO, Utrecht, Available at: https://richtlijnendatabase.nl/richtlijn/orofaryngeale_dysfagie/startpagina_orofaryngeale_dysfagie.html. [Google Scholar]
- 18. Webster KT, Tippett D, Simpson M, et al. Speech‐language pathology care and short‐ and long‐term outcomes of oropharyngeal cancer treatment in the elderly. Laryngoscope 2018;128:1403–1411. [DOI] [PubMed] [Google Scholar]
- 19. Kraaijenga SAC, Molen LV, Stuiver MM, et al. Efficacy of a novel swallowing exercise program for chronic dysphagia in long‐term head and neck cancer survivors. Head Neck 2017;39:1943–1961. [DOI] [PubMed] [Google Scholar]
- 20. Kraaijenga SA, van der Molen L, Jacobi I, Hamming‐Vrieze O, Hilgers FJ, van den Brekel MW. Prospective clinical study on long‐term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur Arch Otorhinolaryngol 2015;272:3521–3531. [DOI] [PubMed] [Google Scholar]
- 21. Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT‐10). Ann Otol Rhinol Laryngol 2008;117:919–924. [DOI] [PubMed] [Google Scholar]
- 22. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 23. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, eds. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [Google Scholar]
- 24. Centrale Commissie Mensgebonden Onderzoek (CCMO) . Niet‐WMO‐onderzoek. 2018, CCMO, Den Haag, Available at: https://www.ccmo.nl/onderzoekers/wet‐en‐regelgeving‐voor‐medisch‐wetenschappelijk‐onderzoek/uw‐onderzoek‐wmo‐plichtig‐of‐niet. [Google Scholar]
- 25. Langmore SE, Schatz K, Olson N. Endoscopic and videofluoroscopic evaluations of swallowing and aspiration. Ann Otol Rhinol Laryngol 1991;100:678–681. [DOI] [PubMed] [Google Scholar]
- 26. Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–1520. [DOI] [PubMed] [Google Scholar]
- 27. Nestle Health Science . Eating Assessment Tool (EAT‐10). 2017, Nestle Health Science, Oosterhout, Available at: https://www.nestlehealthscience.nl/nl/services/screening‐tools/eat‐10. [Google Scholar]
- 28. Heijnen BJ, Speyer R, Bulow M, Kuijpers LM. 'What about swallowing?' Diagnostic performance of daily clinical practice compared with the Eating Assessment Tool‐10. Dysphagia 2016;31:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pilz W, Vanbelle S, Kremer B, et al. Observers' agreement on measurements in fiberoptic endoscopic evaluation of swallowing. Dysphagia 2016;31:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Florie M, Baijens L, Kremer B, et al. Relationship between swallow‐specific quality of life and fiber‐optic endoscopic evaluation of swallowing findings in patients with head and neck cancer. Head Neck 2016;38:E1848–E1856. [DOI] [PubMed] [Google Scholar]
- 31.Complete IDDSI Framework Detailed definitions IDDSI, Available at: https://iddsi.org/Documents/IDDSIFramework‐CompleteFramework.pdf. 2017.
- 32. Baijens LW, Speyer R, Pilz W, Roodenburg N. FEES protocol derived estimates of sensitivity: aspiration in dysphagic patients. Dysphagia 2014;29:583–590. [DOI] [PubMed] [Google Scholar]
- 33. Swan K, Cordier R, Brown T, Speyer R. Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre‐endoscopic evaluations of swallowing: a systematic review. Dysphagia 2019;34:2–33. [DOI] [PubMed] [Google Scholar]
- 34. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 35. Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut‐point estimated from observations affected by a lower limit of detection. Biom J 2008;50:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978;8:283–298. [DOI] [PubMed] [Google Scholar]
- 37. Simon SR, Florie M, Pilz W, et al. Association between pharyngeal pooling and aspiration using fiberoptic endoscopic evaluation of swallowing in head and neck cancer patients with dysphagia. Dysphagia 2020;35:42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiss SG, Postma GN. Fiberoptic endoscopic evaluation of swallowing. Laryngoscope 2003;113:1386–1393. [DOI] [PubMed] [Google Scholar]
- 39. Taylor‐Goh S. 5.8 Disorders of feeding, eating, drinking & swallowing (Dysphagia) In: Taylor‐Goh S, ed. Royal College of Speech & Language Therapists Clinical Guidelines. London, UK: Routledge; 2005:63–72. [Google Scholar]
- 40. Rofes L, Arreola V, Mukherjee R, Clave P. Sensitivity and specificity of the Eating Assessment Tool and the Volume‐Viscosity Swallow Test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil 2014;26:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kendall KA, Ellerston J, Heller A, Houtz DR, Zhang C, Presson AP. Objective measures of swallowing function applied to the dysphagia population: a one year experience. Dysphagia 2016;31:538–546. [DOI] [PubMed] [Google Scholar]
- 42. Soyer T, Yalcin S, Arslan SS, Demir N, Tanyel FC. Pediatric Eating Assessment Tool‐10 as an indicator to predict aspiration in children with esophageal atresia. J Pediatr Surg 2017;52:1576–1579. [DOI] [PubMed] [Google Scholar]
- 43. Allen J, Blair D, Miles A. Assessment of videofluoroscopic swallow study findings before and after cricopharyngeal myotomy. Head Neck 2017;39:1869–1875. [DOI] [PubMed] [Google Scholar]
- 44. Regan J, Lawson S, De Aguiar V. The Eating Assessment Tool‐10 predicts aspiration in adults with stable chronic obstructive pulmonary disease. Dysphagia 2017;32:714–720. [DOI] [PubMed] [Google Scholar]
- 45. Abdel‐Aziz M, Azab N, Lasheen H, Naguib N, Reda R. Swallowing disorders among patients with diffuse idiopathic skeletal hyperostosis. Acta Otolaryngol 2017;137:623–626. [DOI] [PubMed] [Google Scholar]
- 46. Arrese LC, Carrau R, Plowman EK. Relationship between the Eating Assessment Tool‐10 and objective clinical ratings of swallowing function in individuals with head and neck cancer. Dysphagia 2017;32:83–89. [DOI] [PubMed] [Google Scholar]
- 47. Cheney DM, Siddiqui MT, Litts JK, Kuhn MA, Belafsky PC. The ability of the 10‐item Eating Assessment Tool (EAT‐10) to predict aspiration risk in persons with dysphagia. Ann Otol Rhinol Laryngol 2015;124:351–354. [DOI] [PubMed] [Google Scholar]
- 48. Funk GF, Karnell LH, Christensen AJ. Long‐term health‐related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg 2012;138:123–133. [DOI] [PubMed] [Google Scholar]


