Abstract
To evaluate continuous diffusion of oxygen therapy (CDO) on cytokines, perfusion, and bacterial load in diabetic foot ulcers we evaluated 23 patients for 3 weeks. Tissues biopsies were obtained at each visit to evaluate cytokines and quantitative bacterial cultures. Perfusion was measured with hyperspectral imaging and transcutaneous oxygen. We used paired T tests to compare continuous variables and independent T tests to compare healers and nonhealers. There was an increase from baseline to week 1 in TGF‐β (P = .008), TNF‐α (P = .014), VEGF (P = .008), PDGF (P = .087), and IGF‐1 (P = .058); baseline to week 2 in TGF‐β (P = .010), VEGF (P = .051), and IL‐6 (P = .031); and baseline to week 3 with TGF‐β (P = .055) and IL‐6 (P = .054). There was a significant increase in transcutaneous oxygen after 1 week of treatment on both medial and lateral foot (P = .086 and .025). Fifty‐three percent of the patients had at least a 50% wound area reduction (healers). At baseline, there were no differences in cytokines between healers and nonhealers. However, there was an increase in CXCL8 after 1 week of treatment (P = .080) and IL‐6 after 3 weeks of treatment in nonhealers (P = .099). There were no differences in quantitative cultures in healers and nonhealers.
Keywords: cytokines, diabetic foot ulcer, infection, oxygen
1. INTRODUCTION
Foot ulcers are one of the most common underlying component causes of lower extremity infections and amputations in people with diabetes. 1 Approximately 6% to 10% of people with diabetes will develop a foot ulcer each year. 2 Foot infections are distinctly uncommon when there is not a wound. The break in the skin (cutaneous barrier) provides a portal for bacterial pathogens to enter the foot and proliferate. Up to 60% of DFUs will develop foot infections during the course of treatment. In patients with faster healing times (thus shorter periods of exposure to bacteria), the risk of infection can be cut in half. 3 This effectively interrupts one of the most common factors in the pathway to diabetes‐related lower extremity amputations.
One of the unmet needs in the treatment of DFUs is improved oxygen delivery to the wound. Oxygen (O2) plays an important role in nearly every step of the wound healing process. 4 O2 is crucial for collagen synthesis, cell proliferation, antimicrobial activities, tissue repair, differentiation of fibroblasts, and angiogenesis. 5 , 6 , 7 O2 exposure has been shown to upregulate vascular endothelial growth factor (VEGF), and through formation of reactive oxygen species (ROS), act as a signalling molecule for other cytokines and growth factors such as transforming growth factor‐β (TGF‐β), tumour necrosis factor‐α (TNF‐α), platelet derived growth factor (PDGF), insulin‐like growth factor‐β (IGF‐1β), and interleukin‐8 (IL‐8). 7 Specifically, O2‐derived H2O2 is known to signal PDGF, regulating cell growth and division. 8 , 9 , 10 , 11 , 12
There are two clinically tested methods for delivery of oxygen to a wound. Hyperbaric Oxygen Therapy (HBOT) and topical oxygen therapy (TOT) and a continuous form of topical oxygen therapy termed Continuous Diffusion of Oxygen (CDO). 6 HBOT requires a hyperbaric oxygen chamber and the patient must travel to clinic for treatment every day. 13 In contrast, topical oxygen technologies do not involve high atmospheric pressure and are not a form of systemic treatment. Traditional topical oxygen therapy is applied intermittently, similar to HBOT for 90 minutes therapy time per day. A newer form of topical oxygen therapy in which oxygen is delivered continuously through a dressing, CDO therapy, is the form investigated here. Topical oxygen equipment is relatively inexpensive compared with HBOT, and treatment can be provided at home. Several clinical trials report increased rates of healing and decreased time to heal in patients with DFUs. 14 , 15 , 16 , 17 The primary purpose of this project was to evaluate the effectiveness of continuous diffusion of oxygen therapy (CDO) on cutaneous circulation, with secondary outcomes measures of change in growth factors and inflammatory cytokines, quantitative bacterial cultures, and wound healing in patients with DFUs in order to better understand the mechanism of action.
2. MATERIALS AND METHODS
This study was approved by the University of Texas Southwestern Medical Center Institution Review Board (STU 012015–051) and reported in clinicaltrials.gov (NCT02501538). This was a prospective cohort study of 23 patients treated with Continuous Diffusion of Oxygen Treatment (TransCu O2, EO2 Concepts, San Antonio, Texas). The device is a thin rectangular box measuring 5.5 × 3.0 in. that is lightweight (9 oz), silent, and rechargeable. It is worn on the patient's hip and allows for normal ambulation. It generates continuous moist O2 from room air and provides a flow rate and pressure monitor to ensure constant O2 through a plastic cannula connected to a wound dressing (OxySpur, EO2 Concepts, San Antonio, Texas). The contact layer of the dressing is two layers of hydrophilic foam which allows O2 delivery through perforations in the cannula. The dressing fully covered the wounds and affixed to the skin with a hydrocolloid border. 18
The study population was comprised of patients who were treated in clinics with diabetic foot ulcers. Study inclusion criteria were age 18 to 89, with a diagnosis of diabetes mellitus based on ADA criteria 19 with a full thickness ulcer below the ankle. The study excluded patients with end‐stage renal disease (ESRD), HIV, hepatitis, autoimmune diseases, systemic lupus erythematous (SLE), Raynaud's disease, Ankle‐Brachial Index (ABI) < 0.4, and patients unable or unwilling to provide informed consent.
After we obtained informed consent, study subjects all received treatment with continuous topical oxygen therapy. As part of standard wound therapy, ulcers were surgically debrided at every visit, and patients were offloaded with either a boot (DH Offloading Walker, Össur, Reykjavík, Iceland) or a healing sandal (Med‐Surg Post‐Operative Shoe, Darco, Huntington, WV) based on the ulcer location and the postural stability or fall risk of the subject. We evaluated patients in clinic every 7 days for a total of 21 days. Data collected during the study included the following: demographics, comorbidities, history of drug, alcohol, tobacco use, wound location and aetiology, and wound duration. We evaluated sensory neuropathy with a 10‐g Semmes Weinstein monofilament and Vibration Perception Threshold Testing (VPT) (Vibration Perception Threshold Meter, Xilas Medical Inc., San Antonio, Texas) at the great toe and medial malleolus. We defined sensory neuropathy as either VPT > 25 or any site missed with 10 g monofilament. We evaluated perfusion with Ankle Brachial Indices (ABI) from the dorsalis pedis and posterior tibial arteries in the treated foot. We used the lowest systolic pressure to define ABI. In addition, we used hyperspectral imaging (HyperView, Hypermed, Memphis, TN) and transcutaneous oxygen measurements (TCOM) (PeriFlux 5000, Perimed, Järfälla, Sweden) to evaluate perfusion. Wound size was evaluated using a 3D measurement device (inSight, eKare, Fairfax, VA), and percent wound area reduction (PWAR) was calculated as the percent change from baseline. We used a 50% wound area reduction as a surrogate marker for healing with healing being defined as complete epithelialisation with no drainage.
We obtained tissue biopsy at each visit to evaluate cytokines and quantitative bacterial cultures. Specimens were snap frozen immediately at −80°C until RNA extraction. We used a regional reference lab for quantitative polymerase chain reaction (qPCR). Their procedures have been previously cited (Pathogenius Laboratories, Lubbock, TX).
The expression of human genes was analysed as described previously with modification. 20 Briefly, total RNA was extracted using microRNeasy kit (Qiagen, Hilden, Germany) and treated with DNase‐I (Ambion, Invitrogen, Carlsbad, CA). cDNA synthesis was performed using 1 μL of total RNA volume iScript Reverse Transcription Supermix according to manufacturer protocol (Bio‐Rad, Hercules, CA). Quantitative real‐time PCR was performed using Taqman primers and a PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Aliquots of amplified cDNA were used for each reaction and were run in triplicate. Each gene was normalised to the expression of the housekeeping genes human ActinB. Gene expression was calculated according to the 2–ΔΔCt method, as described by the manufacturer (Applied Biosystems).
We summarised study variables as median, means, and standard deviations (SD) for continuous variables and proportions or percentages for categorical variables. We used paired T tests for comparison to baseline values in continuous variables and independent t tests to compare groups at time points. (SPSS, IBM, Chicago). For categorical variables, we used Chi square and Fisher's exact test to compare the proportion of outcomes. Because this was an exploratory, pilot study we used an alpha of 0.10.
3. RESULTS
During the 3‐week evaluation, there was a significant difference in transcutaneous oxygen measurement from baseline after 1 week of treatment on both the medial and lateral foot (mean ± SD, 53.91 ± 13.97, P = .086 and 57.81 ± 13.59, P = .025, respectively) but no significant differences after 2 and 3 weeks of treatment (medial P = .121 and .497 and lateral P = .232 and .559). Shown in Figure 1. There was a single point of significance in the hyperspectral imaging; dorsal oxyhaemoglobin concentration was increased after 1 week of treatment (64.50 ± 33.94, P = .006). No differences were seen in the dorsal deoxyhaemoglobin, plantar oxyhaemoglobin, or plantar deoxyhaemoglobin (Figure 2).
FIGURE 1.
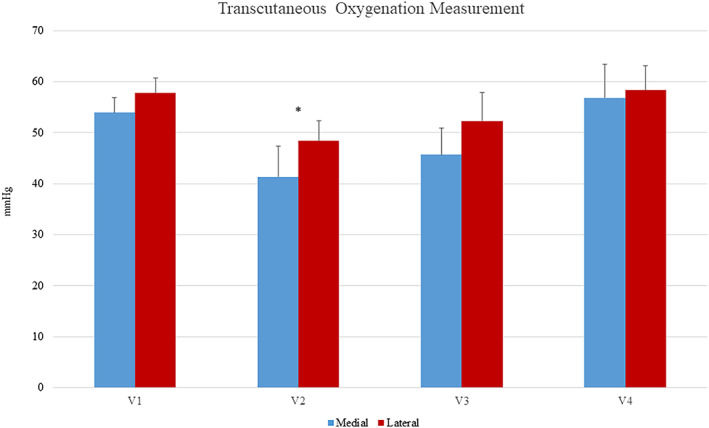
Transcutaneous oxygen measurements of medial and lateral foot at each visit. The asterisk indicates significance difference between baseline and 1 week of treatment
FIGURE 2.
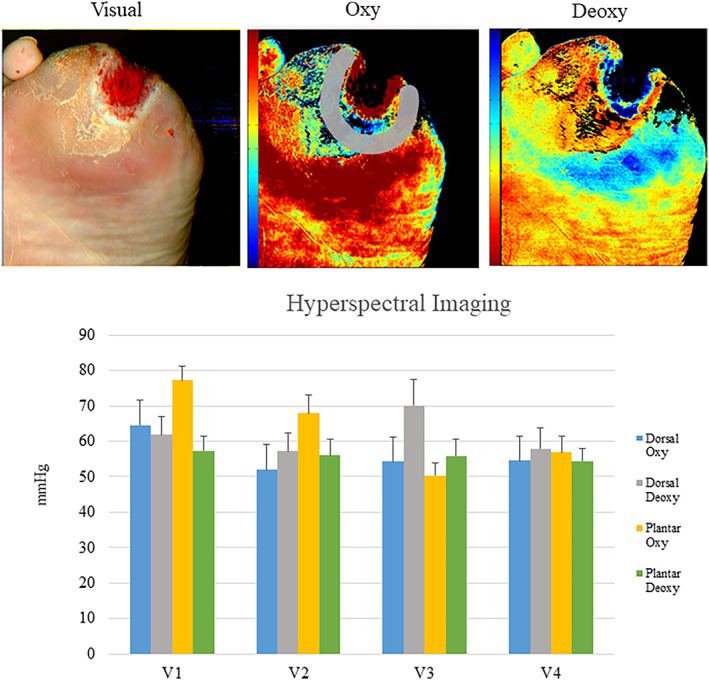
Above: Hyperspectral images of oxyhemoglobin and deoxyhaemoglobin concentrations. Below: Hyperspectral measurements over four visits. There were no significant differences
After the initiation of therapy, there were significant changes in several cytokines or growth factors. There was a significant increase in TGF‐β (mean = 10.42, SEM = 3.05, P = .008), TNF‐α (8.17 ± 2.26, P = .014) and VEGF (5.85 ± 1.40, P = .008), PDGF (3.92 ± 1.31, P = .087), and IGF‐1 (19.91 ± 9.10, P = .058) after 1 week of therapy. There was a significant increase in TGF‐β (4.65 ± 1.22, P = .010), VEGF (4.35 ± 1.42, P = .051), and IL‐6 (18.24 ± 6.48, P = .031) compared with baseline after 2 weeks, and an increase in TGF‐β (3.96 ± 1.26, P = .055) and IL‐6 (9.12 ± 2.53, P = .54) after 3 weeks (Figure 3). There were no significant differences in quantitative cultures when baseline was compared with topical oxygen therapy after 1, 2, and 3 weeks of therapy. During the study, 13% of patients healed and 53% of subjects had at least a 50% wound area reduction.
FIGURE 3.
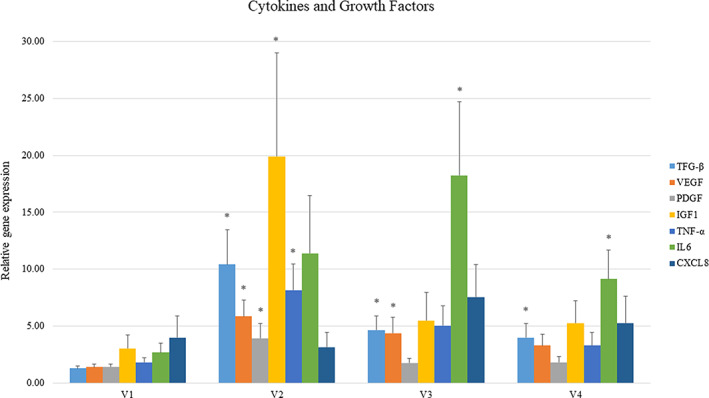
Cytokines and growth factors at each visit. Asterisks indicate significant increase from baseline
A post‐hoc analysis was performed to compare healers and nonhealers. Healers were defined as patients with a 50% wound area reduction at the end of the study. In this analysis, there were no differences in sex, age, BMI, foot ulcer history, length of diabetes, and wound area (Table 1). Percent wound area reduction over time is shown in Figure 4 for healers and nonhealers. The healers had a 59.91% reduction in wound area at 3 weeks post treatment, and the nonhealer group's average PWAR increased by 11.21% by 3 weeks post treatment (P = .007). At baseline, there were no differences in any cytokines when healers and nonhealers were compared. However, after initiating therapy, there was a significant increase in CXCL8 after 1 week of treatment (P = .080) and a significant increase in IL6 after 3 weeks of treatment in nonhealers (P = .099, Figures 5 and 6). There was a significant increase between healers and nonhealers on the medial transcutaneous oxygen measurement after 3 weeks of treatment (P = .054). There were no differences in perfusion measured with hyperspectral imaging or quantitative bacterial cultures at any time point when comparing healers and nonhealers (Figure 7).
TABLE 1.
Patient demographics, comorbidities, and past medical history
| Healers, N = 12 | Nonhealers, N = 11 | P value | |
|---|---|---|---|
| Male | 8 (66.7) | 7 (63.6) | .88 |
| Age | 58.5, 58.2 (9.4) | 58.0, 54.1 (10.8) | .3 |
| BMI (kg/m3) | 33.0, 34.80 (10.6) | 36.20, 36.49 (8.37) | .70 |
| Race | |||
| Caucasian | 7 (58.3) | 4 (36.4) | .29 |
| African American | 2 (16.7) | 3 (27.3) | .54 |
| Hispanic | 3 (25.0) | 4 (36.4) | .55 |
| Substance use history | |||
| Tobacco | 4 (33.3) | 3 (27.3) | .75 |
| Alcohol | 1 (8.3) | 4 (36.4) | .10 |
| Offloading | |||
| Boot | 9 (75.0) | 7 (63.6) | .55 |
| Shoe | 3 (25.0) | 4 (36.4) | .55 |
| Foot ulcer history | 8 (66.7) | 5 (45.5) | .31 |
| Amputation history | 8 (66.7) | 7 (63.6) | .88 |
| Type II diabetes | 11 (92.0) | 11 (100) | 1.0 |
| Diabetes duration (years) | 13.5, 13.5 (8.4) | 15.5, 14.8 (8.8) | .73 |
| Coronary artery disease | 3 (25.0) | 0 (0) | .22 |
| Congestive heart failure | 1 (8.3) | 0 (0) | 1.0 |
| Retinopathy | 3 (25.0) | 0 (0) | 1.0 |
| Chronic kidney disease | 4 (33.3) | 3 (27.3) | .75 |
| Index wound area (cm2) | 4.8, 5.2 (4.0) | 1.8, 3.8 (4.3) | .44 |
| Glycated haemoglobin (%) | 8.1, 8.2 (2.6) | 10.9, 9.7 (3.3) | .32 |
| Albumin (g/dL) | 3.9, 3.8 (0.5) | 3.6, 3.6 (0.6) | .40 |
| Sensory neuropathy | |||
| Abnormal 10‐g monofilament | 11 (91.7) | 8 (72.7) | .23 |
| Vibration perception forefoot (volt) | 70.7, 65.4 (33.2) | 50.5, 50.8 (31.9) | .31 |
| Vibration perception ankle (volt) | 36.5, 47.1 (23.0) | 42.2, 43.7 (21.3) | .73 |
| Ankle brachial index | 1.2, 1.3 (0.2) | 1.1, 1.1 (0.3) | .06 |
| Hyperspectral imaging | |||
| Dorsal oxygenated haemoglobin | 59.5, 63.6 (38.7) | 62.0, 61.7 (30.3) | .91 |
| Dorsal deoxygenated haemoglobin | 59.0, 58.8 (27.5) | 65.0, 77.1 (42.2) | .23 |
| Dorsal oxygen saturation | 49.0, 49.7 (21.1) | 52.0, 43.5 (15.4) | .44 |
| Dorsal area | 559.5, 500.3 (182.1) | 562.0, 688.7 (474.5) | .22 |
| Plantar oxygenated haemoglobin | 83.5, 76.3 (16.8) | 77.0, 77.5 (19.6) | .88 |
| Plantar deoxygenated haemoglobin | 48.0, 55.7 (23.5) | 58.0, 58.3 (12.3) | .75 |
| Plantar oxygen saturation | 60.0, 58.4 (9.0) | 57.0, 56.7 (3.7) | .57 |
| Plantar area | 612.0, 569.3 (169.4) | 683.0, 720.0 (285.0) | .13 |
| Skin perfusion pressures (mmHg) | |||
| Medial foot | 55.1, 53.3 (14.9) | 54.5, 53.6 (13.2) | .96 |
| Lateral foot | 53.0, 55.2 (12.0) | 62.3, 59.3 (15.5) | .48 |
Note: Dichotomous variables are presented as N (%). Continuous variables are presented as median, mean (SD).
FIGURE 4.
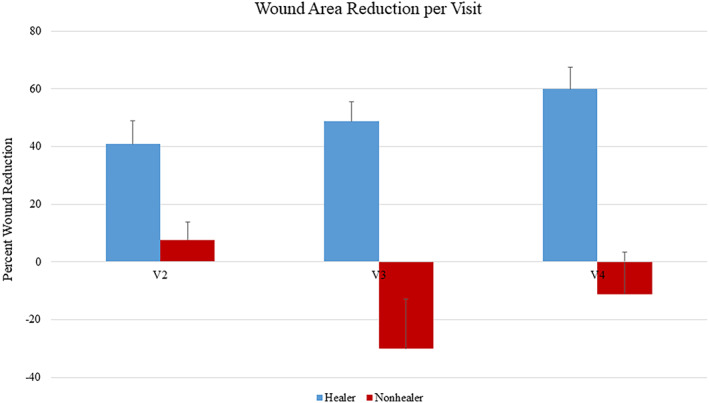
Percent wound area reduction per visit with SEM error bars. There was no significant difference in wound size between groups at initiation of study
FIGURE 5.
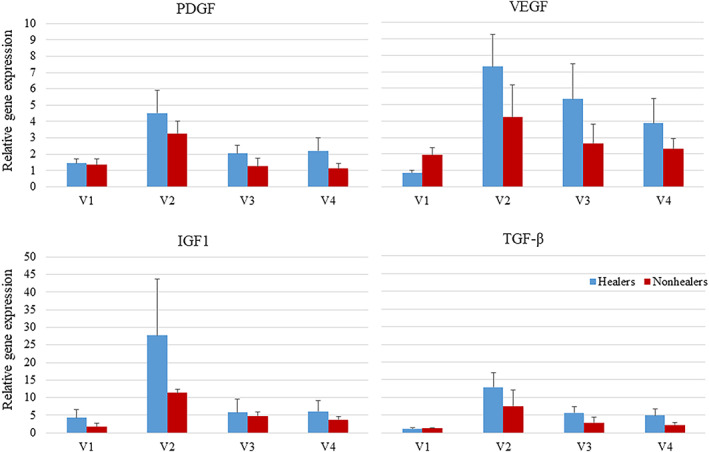
Gene expression of growth factors. There was no significant difference between healers and nonhealers at any time point
FIGURE 6.
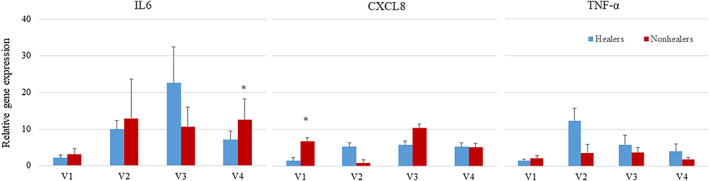
Gene expression of cytokines. Asterisks indicate significant difference between healers and nonhealers at that time point
FIGURE 7.
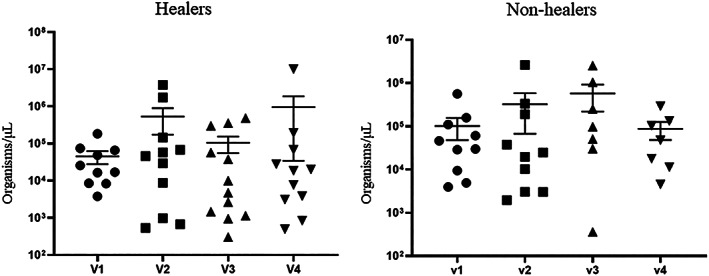
Quantitative culture with SEM error bars. There were no significant differences between groups at any time point
4. DISCUSSION
Improved O2 delivery to the wound has been a great challenge for the management of DFUs as O2 plays a vital role throughout the wound healing process including collagen synthesis, cell proliferation, cell differentiation, and neovascularisation, along with limiting bacterial infection. 5 , 6 , 7 , 21 Previous studies suggest that O2 delivery to the chronic wound can be achieved by exposure to hyperbaric oxygen therapy with modest improvements. 22 , 23 Here we evaluated an alternative O2 therapy that provides continuous diffusion of oxygen (CDO) in a home setting. The results of this study demonstrate a significant increase in tissue cytokines after the application of CDO in healers compared with nonhealers. However, this therapy did not improve bioburden.
Several authors have identified significant differences in cytokines in diabetic foot ulcers among healers and nonhealers that receive standard wound care. 24 , 25 , 26 In our study, there was no difference in cytokines in people that responded to therapy and those that did not respond at baseline, but after initiation of therapy, there was an increase in two cytokines, CXCL8 and IL‐6 in nonhealers after 1 and 3 weeks of therapy. There are only a few studies in humans that demonstrate a change in cytokines associated with oxygen therapies. Gordillo and colleagues evaluated 32 patients treated with HBOT and 25 treated with topical oxygen therapy. They identified a significant increase in VEGF in patients treated with topical oxygen therapy and no changes with HBOT. 9 Driver and colleagues evaluated a transdermal continuous topical oxygen device in an RCT to treat DFUs. They reported significant changes in IL‐6 and IL‐8, matrix metalloproteinase‐1 (MMP‐1), MMP‐2, MMP‐3, and tissue inhibitor of matrix metalloproteinase (TIMP‐1) after 2 and 4 weeks of therapy. 27
We hypothesised that topical oxygen therapy would affect cutaneous perfusion and bioburden, because other therapies have reported changes in both factors. For instance, there are several studies in humans that show significant improvement in perfusion with treatment modalities such as electrical stimulation 28 and HBOT. 29 , 30 The improvement in both TCOM and hyperspectral imaging were expected to be part of the mechanism of action of therapy. There is some debate about the validity of hyperspectral imaging for predicting ulcer healing. In a study of 43 patients with diabetes who were imaged once at the beginning of treatment, Jeffcoate et al found a negative correlation between oxygenated haemoglobin and wound healing at 12 weeks. 31 Nouvong and colleagues performed hyperspectral imaging on 66 patients 11 times over 24 weeks, showing significantly more patients healed who had higher oxygenated haemoglobin at the border of their ulcers with sensitivity 80% and specificity 74%. 32
Treatments such as iodine paste, 33 silver dressings, 34 and maggot therapy 35 have been reported to reduce bioburden in wounds. However, we did not identify any changes in quantitative bacterial cultures during the course of therapy with topical oxygen therapy or in healers and nonhealers in this study. Other authors have reported a change in the microbiome with continuous topical oxygen. 36 It is important to note, that reduction of quantitative bacterial cultures has not clearly been associated with DFU healing. And while bioburden reduction is the focus of many therapies, there is little clinical evidence that therapies such as silver dressings reduce the clinical risk of infection or improve the proportion of DFUs that heal. 37 , 38 Gardner and colleagues evaluated a cohort of patients with DFUs that received serial debridement and offloading in a total contact cast. A very high proportion of ulcers healed (84%). In the regression analysis, quantitative bacterial cultures with RNA sequencing was not associated with healing. 39 Like Gardner, we did not identify any differences in quantitative bacterial cultures in healers and nonhealers at any time point of the study (Figure 7).
Due to the short timeframe of this study, we used 50% wound area reduction as a surrogate marker for healing as described by Margolis et al. 40 Fifty three percent of patients had at least a 50% wound area reduction over 3 weeks of therapy. This compares favourably to the results of randomised clinical studies of various modalities to treat diabetic foot ulcers. 41 , 42 There are a number of studies that evaluate various devices and approaches to deliver topical oxygen therapy to patients with diabetic foot ulcers. 14 , 16 , 17 Niederauer and colleagues evaluated the devices used in our study in a multicentre randomised clinical trial (RCT). They reported that patients treated with continuous topical oxygen therapy had a significantly higher proportion of healed ulcers compared with patients that received standard wound care (32.4% versus 16.7%). 14 In addition, there are several successful RCTs that use other applications of intermittent and continuous topical oxygen therapy to heal DFUs. 16 , 17
There are several limitations of this study. This was a small prospective cohort study with only 23 patients and no control arm. The duration of the study was only 3 weeks. We, therefore, relied on surrogate markers for wound healing and only had four time points to evaluate and compare changes in cytokines, perfusion, and bioburden. Because this was an exploratory study, we were only able to evaluate a limited number of cytokines. In addition, we did not measure the duration of therapy in minutes or hours. The device is intended to be used continuously; however, there were inevitably times the device came off while the patient was sleeping or during the day, and because all of our study subjects had severe diabetic sensory neuropathy, the problem was often not identified immediately. Our results should not be generalised to other products or approaches that deliver topical oxygen or other types of wounds. 43 The device used in this study delivered continuous topical oxygen. There are several products that provide intermittent topical oxygen therapy, usually once a day. The duration of some therapies is relatively short, and all the topical oxygen therapy devices use different doses. A large RCT that evaluates changes in wound area reduction and cytokines in active and sham treatment groups would give us better insights about the effect of continuous topical oxygen on and timing and the expression of cytokines.
In conclusion, the study evaluated a novel application of topical oxygen therapy that has had a successful randomised clinical trial in patients with diabetic foot ulcers. Growth factors, cytokines, and perfusion were elevated following therapy, similar to HBOT, although there was no difference in quantitative bacterial cultures More studies with larger patient population are required to demonstrate the effectiveness of this therapy in chronic wound healing, and its potential to be used as an alternative to HBOT.
CONFLICT OF INTEREST
LAL has funding from Osiris Therapeutics, Integra, Cardinal Health, Medimmune, Avazzia, and Pluristem. ALK has funding from Osiris Therapeutics, Integra, Medimmune, Avazzia, and Pluristem. DF has no relevant conflicts of interest. YA has no relevant conflicts of interest. PAC has participated in research funded by the American Diabetes Association, Cardinal Health, EO2 Concepts, Avazzia, Pluristem Therapeutics, Inc. MM has funding from Medline Industries. KED has funding from Osiris Therapeutics, Integra, Cardinal Health, Medimmune, Avazzia, and Pluristem.
ACKNOWLEDGEMENTS
This paper was funded by a grant from EO2 Concepts.
Lavery LA, Killeen AL, Farrar D, et al. The effect of continuous diffusion of oxygen treatment on cytokines, perfusion, bacterial load, and healing in patients with diabetic foot ulcers. Int Wound J. 2020;17:1986–1995. 10.1111/iwj.13490
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Younes NA, Albsoul AM, Awad H. Diabetic heel ulcers: a major risk factor for lower extremity amputation. Ostomy Wound Manage. 2004;50(6):50‐60. [PubMed] [Google Scholar]
- 2. Lavery LA, Peters EJ, Williams JR, et al. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the international working group on the diabetic foot. Diabetes Care. 2008;31(1):154‐156. 10.2337/dc07-1302. [DOI] [PubMed] [Google Scholar]
- 3. Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix([R]) for the treatment of chronic diabetic foot ulcers: results of a multi‐Centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554‐560. 10.1111/iwj.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257‐268. [DOI] [PubMed] [Google Scholar]
- 5. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259‐263. [DOI] [PubMed] [Google Scholar]
- 6. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21(4):503‐511. [DOI] [PubMed] [Google Scholar]
- 7. Tandara AA, Mustoe TA. Oxygen in wound healing—more than a nutrient. World J Surg. 2004;28(3):294‐300. [DOI] [PubMed] [Google Scholar]
- 8. Sundaresan M, Yu Z‐X, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H$_2$O$_2$ for platelet‐derived growth factor signal transduction. Science. 1995;270(5234):296‐299. 10.2307/2888541. [DOI] [PubMed] [Google Scholar]
- 9. Gordillo GM, Roy S, Khanna S, et al. Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol. 2008;35(8):957‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopf HW, Gibson JJ, Angeles AP, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13(6):558‐564. [DOI] [PubMed] [Google Scholar]
- 11. Patel V, Chivukala I, Roy S, et al. Oxygen: from the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxid Redox Signaling. 2005;7(9–10):1377‐1387. [DOI] [PubMed] [Google Scholar]
- 12. Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg. 2000;135(11):1293‐1297. [DOI] [PubMed] [Google Scholar]
- 13. Wu SC, Marston W, Armstrong DG. Wound care: the role of advanced wound‐healing technologies. J Am Podiatr Med Assoc. 2010;100(5):385‐394. 10.7547/1000385. [DOI] [PubMed] [Google Scholar]
- 14. Niederauer MQ, Michalek JE, Liu Q, Papas KK, Lavery LA, Armstrong DG. Continuous diffusion of oxygen improves diabetic foot ulcer healing when compared with a placebo control: a randomised, double‐blind, multicentre study. J Wound Care. 2018;27(Sup9):S30‐S45. 10.12968/jowc.2018.27.Sup9.S30. [DOI] [PubMed] [Google Scholar]
- 15. Nataraj M, Maiya AG, Karkada G, et al. Application of topical oxygen therapy in healing dynamics of diabetic foot ulcers—a systematic review. Rev Diabetes Stud. 2019;15:74‐82. 10.1900/RDS.2019.15.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frykberg RG, Franks PJ, Edmonds M, et al. A multinational, multicenter, randomized, double‐blinded, placebo‐controlled trial to evaluate the efficacy of cyclical topical wound oxygen (TWO2) therapy in the treatment of chronic diabetic foot ulcers: the TWO2 study. Diabetes Care. 2020;43(3):616‐624. 10.2337/dc19-0476. [DOI] [PubMed] [Google Scholar]
- 17. Driver VR, Reyzelman A, Kawalec J, French M. A prospective, randomized, blinded, controlled trial comparing transdermal continuous oxygen delivery to moist wound therapy for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2017;63(4):12‐28. [PubMed] [Google Scholar]
- 18. Niederauer MQ, Michalek JE, Armstrong DG. A prospective, randomized, double‐blind multicenter study comparing continuous diffusion of oxygen therapy to sham therapy in the treatment of diabetic foot ulcers. J Diabetes Sci Technol. 2017;11(5):883‐891. 10.1177/1932296817695574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumar R, Nandhini LP, Kamalanathan S, Sahoo J, Vivekanadan M. Evidence for current diagnostic criteria of diabetes mellitus. World J Diabetes. 2016;7(17):396‐405. 10.4239/wjd.v7.i17.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kislevitz M, Lu KB, Wamsley C, Hoopman J, Kenkel J, Akgul Y. Novel use of non‐invasive devices and microbiopsies to assess facial skin rejuvenation following laser treatment. Lasers Surg Med. 2020;1–9. 10.1002/lsm.23233. [DOI] [PubMed] [Google Scholar]
- 21. Lam G, Fontaine R, Ross FL, Chiu ES. Hyperbaric oxygen therapy: exploring the clinical evidence. Adv Skin Wound Care. 2017;30(4):181‐190. 10.1097/01.ASW.0000513089.75457.22. [DOI] [PubMed] [Google Scholar]
- 22. Salama SE, Eldeeb AE, Elbarbary AH, Abdelghany SE. Adjuvant hyperbaric oxygen therapy enhances healing of nonischemic diabetic foot ulcers compared with standard wound care alone. Int J Low Extrem Wounds. 2019;18(1):75‐80. 10.1177/1534734619829939. [DOI] [PubMed] [Google Scholar]
- 23. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. 10.1007/s12325-017-0478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tecilazich F, Dinh T, Pradhan‐Nabzdyk L, et al. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8(12):e83314. 10.1371/journal.pone.0083314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937‐2947. 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19‐26. [PubMed] [Google Scholar]
- 28. Gilcreast DM, Stotts NA, Froelicher ES, Baker LL, Moss KM. Effect of electrical stimulation on foot skin perfusion in persons with or at risk for diabetic foot ulcers. Wound Repair Regen. 1998;6(5):434‐441. 10.1046/j.1524-475x.1998.60505.x. [DOI] [PubMed] [Google Scholar]
- 29. Stirban A, Lentrodt S, Nandrean S, Pop A, Tschoepe D, Scherbaum WA. Functional changes in microcirculation during hyperbaric and normobaric oxygen therapy. Undersea Hyperb Med. 2009;36(5):381‐390. [PubMed] [Google Scholar]
- 30. Lin PY, Sung PH, Chung SY, et al. Hyperbaric oxygen therapy enhanced circulating levels of endothelial progenitor cells and angiogenesis biomarkers, blood flow, in ischemic areas in patients with peripheral arterial occlusive disease. J Clin Med. 2018;7(12):1–9. 10.3390/jcm7120548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeffcoate WJ, Clark DJ, Savic N, et al. Use of HSI to measure oxygen saturation in the lower limb and its correlation with healing of foot ulcers in diabetes. Diabet Med. 2015;32(6):798‐802. 10.1111/dme.12778. [DOI] [PubMed] [Google Scholar]
- 32. Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care. 2009;32(11):2056‐2061. 10.2337/dc08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malone M, Schwarzer S, Radzieta M, et al. Effect on total microbial load and community composition with two vs six‐week topical Cadexomer iodine for treating chronic biofilm infections in diabetic foot ulcers. Int Wound J. 2019;16(6):1477‐1486. 10.1111/iwj.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sibbald RG, Contreras‐Ruiz J, Coutts P, Fierheller M, Rothman A, Woo K. Bacteriology, inflammation, and healing: a study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv Skin Wound Care. 2007;20(10):549‐558. 10.1097/01.ASW.0000294757.05049.85. [DOI] [PubMed] [Google Scholar]
- 35. Tantawi TI, Gohar YM, Kotb MM, Beshara FM, El‐Naggar MM. Clinical and microbiological efficacy of MDT in the treatment of diabetic foot ulcers. J Wound Care. 2007;16(9):379‐383. 10.12968/jowc.2007.16.9.27868. [DOI] [PubMed] [Google Scholar]
- 36. Hunter P, Greco E, Cross K, Perry J. Topical oxygen therapy shifts microbiome dynamics in chronic diabetic foot ulcers. Wounds. 2020;32(3):81‐85. [PubMed] [Google Scholar]
- 37. Storm‐Versloot MN, Vos CG, Ubbink DT, Vermeulen H. Topical silver for preventing wound infection. Cochrane Database Syst Rev. 2010;3:CD006478. 10.1002/14651858.CD006478.pub2. [DOI] [PubMed] [Google Scholar]
- 38. Bergin SM, Wraight P. Silver based wound dressings and topical agents for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2006;1:CD005082. 10.1002/14651858.CD005082.pub2. [DOI] [PubMed] [Google Scholar]
- 39. Gardner SE, Haleem A, Jao YL, et al. Cultures of diabetic foot ulcers without clinical signs of infection do not predict outcomes. Diabetes Care. 2014;37(10):2693‐2701. 10.2337/dc14-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Margolis DJ, Gelfand JM, Hoffstad O, Berlin JA. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2003;26(6):1696‐1700. 10.2337/diacare.26.6.1696. [DOI] [PubMed] [Google Scholar]
- 41. Driver VR, Lavery LA, Reyzelman AM, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. 2015;23(6):891‐900. 10.1111/wrr.12357. [DOI] [PubMed] [Google Scholar]
- 42. Buchberger B, Follmann M, Freyer D, Huppertz H, Ehm A, Wasem J. The evidence for the use of growth factors and active skin substitutes for the treatment of non‐infected diabetic foot ulcers (DFU): a health technology assessment (HTA). Exp Clin Endocrinol Diabetes. 2011;119(8):472‐479. 10.1055/s-0031-1279713. [DOI] [PubMed] [Google Scholar]
- 43. Burghart K. The topical hyperbaric oxygen therapy debate. Ostomy Wound Manage. 2003;49(4):8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


