Summary
Low oxygen availability often is associated with soil waterlogging or submergence, but may occur also as hypoxic niches in otherwise aerobic tissues. Experimental evidence assigns a role in Botrytis cinerea resistance to a group of oxygen‐unstable Ethylene Response Factors (ERF‐VII). Given that infection by B. cinerea often occurs in aerobic organs such as leaves, where ERF‐VII stability should be compromised, we explored the possibility of local leaf hypoxia at the site of infection.
We analyzed the expression of hypoxia‐responsive genes in infected leaves. Confocal microscopy was utilized to verify the localization of the ERF‐VII protein RAP2.12. Oxygen concentration was measured to evaluate the availability of oxygen (O2).
We discovered that infection by B. cinerea induces increased respiration, leading to a drastic drop in the O2 concentration in an otherwise fully aerobic leaf. The establishment of a local hypoxic area results in stabilization and nuclear relocalization of RAP2.12. The possible roles of defence elicitors, ABA and ethylene were evaluated.
Local hypoxia at the site of B. cinerea infection allows the stabilization of ERF‐VII proteins. Hypoxia at the site of pathogen infection generates a nearly O2‐free environment that may affect the stability of other N‐degron‐regulated proteins as well as the metabolism of elicitors.
Keywords: Arabidopsis thaliana, Botrytis cinerea, Ethylene Response Factors, hypoxia, N‐degron pathway
Short abstract
See also the Editorial on this article by Sasidharan et al., 229: 5–7.
Introduction
Plants, during their life, have to face several biotic and abiotic stresses that can compromise their growth. Although environmental conditions such as drought, flooding, salt and excessive heat represent abiotic constraints to plant growth and development, pathogens are biotic factors potentially influencing plant survival. Plant pathogens are divided into two main groups depending on their lifestyle. Biotrophs invade the host cells maintaining host viability and deriving nutrients from living cells, whereas necrotrophs derive their nutrients by killing the host plant. Among the latter group, Botrytis cinerea is one of the most invasive pathogens, causing grey mould disease on several crop plant species (Williamson et al., 2007). Due to the huge damage that B. cinerea causes in terms of productivity, there is increasing interest in the mechanism(s) utilized by plants to counteract infection by this fungus. Plants rely on a sophisticated network of signal transduction pathways to respond to pathogen attacks, leading to metabolic and transcriptional reprogramming. In Arabidopsis the response against biotrophic pathogens predominantly is associated to salicylic acid (Wildermuth et al., 2001), whereas the response to necrotrophic pathogens is typically mediated by jasmonate (JA) and ethylene (ET) signalling pathways that are transduced by APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) transcription factors (Thaler et al., 2004; Broekgaarden et al., 2015; Huang et al., 2015). One of the best characterized ERF transcription factors involved in B. cinerea resistance is OCTADECANOID‐RESPONSIVE ARABIDOPSIS 59 (ORA59). Plants overexpressing ORA59 (35S:ORA59) showed enhanced resistance to B. cinerea, whereas ORA59‐silenced plants were more strongly affected, indicating that this transcription factor is involved in the plant response to this pathogen (Pré et al., 2008). Moreover, ORA59 gene expression is induced synergistically by JA and ET confirming that ORA59 is an important player in the JA and ET signalling pathway (Pré et al., 2008).
It was demonstrated recently that ORA59 interacts with RAP2.3 (Kim et al., 2018), a transcription factor that, together with RAP2.12, RAP2.2, Hypoxia Responsive ERF (HRE)1 and 2, belongs to the subgroup VII of the ethylene transcription factors (ERF‐VII). ERF‐VII are master regulator genes for the tolerance to hypoxia through a complex mechanism of oxygen (O2)‐dependent destabilization representing a sophisticated method for O2 and nitric oxide (NO) sensing (Licausi et al., 2011; Gibbs et al., 2011, 2014). Under normoxia ERF‐VIIs are degraded via the N‐degron pathway (Varshavsky, 2011). The N‐terminal methionine (Met) is cleaved leading to an exposed cysteine (Cys) which is oxidized by PLANT CYSTEINE OXIDASEs (PCOs; Weits et al., 2014; White et al., 2018) that convert Cys to Cys‐sulfinic acid in a reaction requiring molecular O2. Arginylation catalyzed by ARGINYL tRNA TRANSFERASE (ATE) follows, inducing ubiquitination by the ligase PROTEOLYSIS 6 (PRT6), and subsequent proteasomal degradation (26S proteasome). Under hypoxia, PCOs cannot oxidize the exposed Cys, and ERF‐VII transcription factors are stabilized and drive transcriptional activation of the hypoxia‐responsive genes (Bailey‐Serres et al., 2012; Gasch et al., 2016; Gibbs et al., 2011; Licausi et al., 2011). All five ERF‐VII transcription factors are involved in plant response to low O2 conditions (Giuntoli & Perata, 2018). Intriguingly, it was demonstrated recently that RAP2.2 plays an important role against the infection to B. cinerea. Plants overexpressing RAP2.2 (35S:RAP2.2) and a mutant line (rap2.2‐3) showed higher and lower resistance, respectively, suggesting that RAP2.2 is important for Arabidopsis tolerance to B. cinerea (Zhao et al., 2012).
This evidence highlighted the possibility that RAP2.2, RAP2.3 and possibly other ERF‐VIIs, may represent key regulators both in the low‐O2 and plant pathogen responses. Remarkably, it was shown that the N‐degron pathway positively regulates the biosynthesis of plant‐defence metabolites such as glucosinolates, as well as the biosynthesis and response to JA in response to Pseudomonas syringae (de Marchi et al., 2016). Additionally, the ERF‐VII transcription factors enhance pathogen‐induced stomatal closure (Vicente et al., 2019). Vicente et al. (2019) results also indicated, differently from de Marchi et al., (2016), that N‐degron pathway mutants are more tolerant to P. syringae infection.
The involvement of the O2‐destabilized ERF‐VII proteins in the response of plants to biotic stresses raises the question about the mechanism by which they get stabilized during pathogen infection. Pathogens often attack fully aerobic tissues such as leaves and, additionally, they often induce the synthesis of NO that can further destabilize the ERF‐VII proteins (Gibbs et al., 2014). Given that these proteins are potentially highly unstable during pathogen infection, it is tempting to speculate that pathogen infection locally induces hypoxic conditions in an otherwise fully aerobic plant tissue such as leaves. Alternatively, a mechanism independent from O2 might stabilize the ERF‐VII proteins.
In this paper, we investigated the effects of B. cinerea on the hypoxic status of the infected leaves.
Materials and Methods
Plant material
Arabidopsis (Arabidopsis thaliana, Col‐0 ecotype) was used. Other genotypes used were a line constitutively expressing a version of the RAP2.12 protein that also is stable in air (35S:Δ‐RAP2.12; Licausi et al., 2011); a line expressing β‐glucuronidase (GUS) under the control of the PLANT CYSTEINE OXIDASE (PCO)1 promoter (pPCO1:GUS; Weits et al., 2014), and the quintuple erfVII mutant (ERF‐VII, subgroup VII of ETHYLENE RESPONSE FACTOR) (Marín‐de la Rosa et al., 2014) which is a null mutant for RAP2.2, RAP2.3, RAP2.12, Hypoxia Responsive ERF (HRE) 1 and 2, and a knock‐down for RAP2.2 (Giuntoli et al., 2017). The phytoalexin deficient 3 (pad3) mutant was obtained from the Nottingham Arabidopsis Stock Center (N3805). Plants were grown in pots for 3–4 wk at 23°C with a 12 h : 12 h, light : dark photoperiod at 120 μmol photons m–2 s–1 intensity before being utilized for experiments.
Plant inoculation with B. cinerea, A. brassicicola and P. syringae
The Botryitis cinerea strain (Ferrari et al., 2003) was cultured on MEP plates (maltose extract 20 g l−1, peptone 10 g l−1 and agar 15 g l−1) for 15–20 d at 23°C in the dark. Conidia were collected by washing the plates with potato dextrose broth (PDB, Formedium™) medium and the spore suspension was adjusted to the appropriate concentration (~ 1 × 106). Inoculation with B. cinerea was conducted on 3–4‐wk‐old plants by placing 5 µl droplets of a spore suspension (1 × 106 conidia ml−1) in 12 g l−1 PDB on leaves (placing the drops on the sides of the rib). Alternaria brassicicola was cultured on potato dextrose agar (PDA, Formedium™) plates for 7–10 d at 23°C in the dark. Conidia were collected by washing the plates with potato dextrose broth (PDB) medium and the spore suspension was adjusted to the appropriate concentration (~ 1 × 106). Inoculation with B. cinerea and A. brassicicola was conducted on 3–4‐wk‐old plants by placing 5 µl droplets of a spore suspension (1 × 106 conidia ml−1) in 12 g l−1 potato dextrose broth on leaves (placing the drops on the sides of the rib). Pseudomonas syringae (Pst DC3000) was cultured on nutrient agar (NAG) plates (Oxoid) for 2 d at 28°C in the dark. Plates were washed with 10 mM MgCl2 containing Silwet L77 (0.05%). Plants were dipped in the P. syringae‐containing solution with OD600 of 0.2 (c. 108 colony forming units (CFU) ml−1). The inoculated plants were placed in plastic tanks with lids and the humidity was maintained by placing a steam source inside. Plants were incubated in a growth chamber (Percival Scientific, Perry, IA, USA) at 23°C with a 12 h : 12 h, light : dark photoperiod at 80 μmol photons m–2 s–1 intensity for 24 h.
Plant treatment with flagellin 22, OGs, 1‐MCP and ABA)
Plants were treated with flagellin 22 peptide (flg22; Genscript) at 1 or 10 μM, with oligogalacturonides (OGs) (DP 10‐15; Elicityl, Crolles, France) at 200 μg ml–1, and abscisic acid (ABA) at 0.1 or 1 μM by placing two 5‐µl droplets on leaves which are incubated for 24 h, unless differently shown in figures. Plants were treated with 1‐methylcyclopropene (1‐MCP) to inhibit ethylene (ETH) signalling as follows. Plants were closed in a plastic box (4 l) in which 150 mg 1‐MCP was placed in a beaker. Water (50 ml) was added rapidly and the box closed immediately. Plants were kept in the box for 1 h and subsequently utilized for the experiments.
Total RNA extraction and qPCR
Total RNA was extracted as described previously (Perata et al., 1997) with a minor modification (omission of aurintricarboxylic acid) to make the protocol compatible with the subsequent PCR procedures. Electrophoresis using a 1% agarose gel was performed for all RNA samples to check for RNA integrity, followed by spectrophotometric quantification. cDNA was synthesized from 1 µg of total RNA using the Maxima Reverse Transcriptase kit (Life Technologies). Quantitative (q)PCR amplification was performed on 30 ng of cDNA with the ABI Prism 7300 sequence detection system (Applied Biosystems, Waltham, MA, USA), using the PowerUp SYBRGreen Master Mix (Applied Biosystems). UBIQUITIN10 (At4g053290) was exploited as the housekeeping gene. Relative expression levels were calculated using genorm (https://genorm.cmgg.be/). A full list of the primers used for qPCR is provided in Supporting Information Table S1.
GUS staining
Histochemical GUS staining was carried out according to Jefferson et al. (1987). Briefly, plant material was fixed immediately after sampling in ice‐cold 90% acetone for 1 h, rinsed several times in 100 mM phosphate buffer (pH 7.2), and then stained in a freshly prepared reaction solution (0.2% Triton X‐100, 2 mM potassium ferrocyanide, 2 mM potassium ferricyanide and 2 mM X‐Gluc (5‐bromo‐4‐chloro‐3‐indolyl β‐d‐glucuronide, sodium salt dissolved in DMSO) in 100 mM phosphate buffer, pH 7.2). Plants were stained overnight and chlorophyll was eliminated from green tissues by washing them with absolute ethanol.
Laser scanning confocal microscopy and quantification of fluorescence intensity
RAP2.12‐GFP fluorescence was imaged post‐infection with B. cinerea using a Zeiss Airyscan 800 confocal microscope (GFP, green fluorescent protein). Zen blue software (Carl Zeiss, Oberkochen, Germany) was used to quantify RAP2.12‐GFP fluorescence intensity. Five individual biological replicates were used for each treatment. Each data point represents the average fluorescence intensity at the nuclei or plasma membrane of all analyzed cells in one biological replicate. Boxplot limits represent the 25th and 75th percentiles of each dataset. The whiskers extend to the lowest and highest data point within the 1.5 × interquartile range of the 25th and 75th quartile. The central line represents the median.
Ethylene measurement
Ethylene production was measured by enclosing a single whole plant, after having gently removed the substrate from the roots, in glass air‐tight containers (30 ml) with holed plastic screw caps provided with rubber septa. Gas samples (2 ml) were taken from the headspace of the containers after 1 h incubation at room temperature. The ETH concentration in the sample was measured by a gas chromatograph (HP5890; Agilent Technologies Italia SpA, Cernusco sul Naviglio MI, Italy) using a flame ionization detector (FID), a stainless steel column (150 × 0.4 cm I.d. packed with HaySep®; Agilent Technologies, Santa Clara, CA, USA), column and detector temperatures of 70°C and 350°C, respectively, and nitrogen carrier gas at a flow rate of 30 ml min−1. Quantification of ETH was achieved by external standard techniques according to Mensuali Sodi et al. (1992) Data represent the mean of five biological replicates and ETH was expressed as pl g−1 h−1 FW.
Oxygen measurement
Oxygen (O2) concentration was measured using a FireStingO2 high‐precision, PC‐controlled fibre‐optic oxygen meter produced by Pyro Science. The needle‐type oxygen probe used was OXR50. A MM33 micromanipulator (Unisense) was used to precisely insert the sensor into the leaf tissue. Air temperature was measured simultaneously using the TDIP15 temperature probe.
Respiration measurement
The respiration rate was measured in excised leaves as described by Kerpen et al., (2019). The fresh weight of the leaves was determined before the O2 measurements. Oxygen consumption was measured with an integrated optical oxygen sensor in respiration vials (Pyroscience) filled with sterile distilled H2O and under continuous mixing using a magnetic stirrer. The O2 consumption rate was calculated by dividing the decrease in O2 concentration by the time and corrected for the weight of each sample.
Statistics
Data were subjected to ANOVA and mean values were separated using Tukey’s post‐hoc test, P < 0.05. Values that significantly differ from each other are indicated by different letters in figures. Pairwise comparison were performed by Student’s t‐test.
Results
Botrytis cinerea infection generates local hypoxia in the leaf
Analysis of the response to B. cinerea in terms of induction of pathogen‐responsive gene expression (PDF1.2) showed that infection by the fungus occurred under our experimental conditions (Fig. 1). To explore the possibility of hypoxia at the site of infection, we analyzed the mRNA level of several anaerobic genes in leaves that were infected with B. cinerea (Fig. 1). The induction was evident (> 3‐fold induction) for most of the genes, particularly for HRE2, but it was limited for ALCOHOL DEHYDROGENASE 1 (ADH1) (Fig. 1). Dilution of RNA coming from uninfected areas with that from the infected area may influence the result, but dissection of leaves was proven to be difficult, owing to the fragility of the infected area. We thus decided to observe hypoxia‐dependent gene induction using pPCO1:GUS plants, in which the promoter of PCO1, a gene which is strongly and specifically induced by hypoxia (Weits et al., 2014), drives the expression of the GUS reporter gene. When infected with B. cinerea, leaves of pPCO1:GUS plants showed strong activation of the PCO1 promoter as blue GUS staining corresponding to the sites where B. cinerea spores were applied (Fig. 2a). The GUS staining observed in control plants was due to the expression of hypoxia‐responsive genes in the shoot apex and in young leaves (Giuntoli et al., 2014).
Fig. 1.
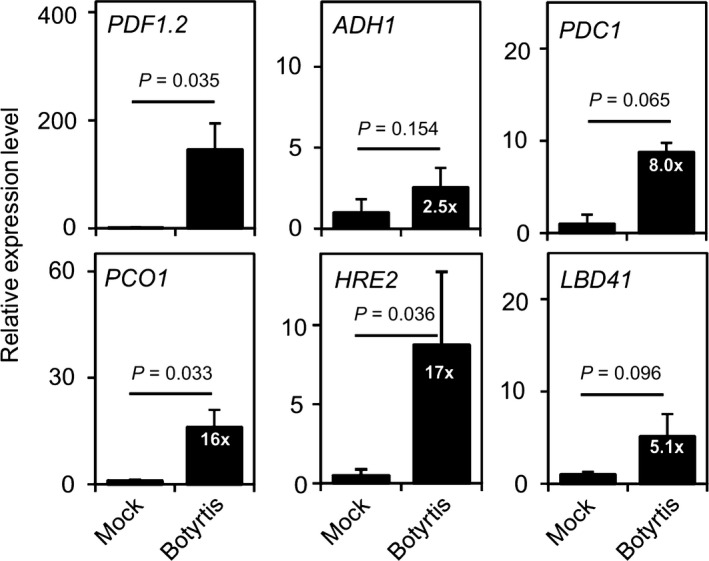
Infection with Botrytis cinerea results in induction of several anaerobic genes. Botrytis cinerea was inoculated as drops on leaves of 3–4‐wk old Arabidopsis plants grown in soil (mock: culture broth). The entire rosette was harvested, extracted for RNA, and the resulting cDNA analyzed by quantitative PCR for the mRNA of the genes indicated in the figure. Data are mean ± SD (n = 3). The P‐value resulting for pairwise comparison by Student’s t‐test is shown.
Fig. 2.
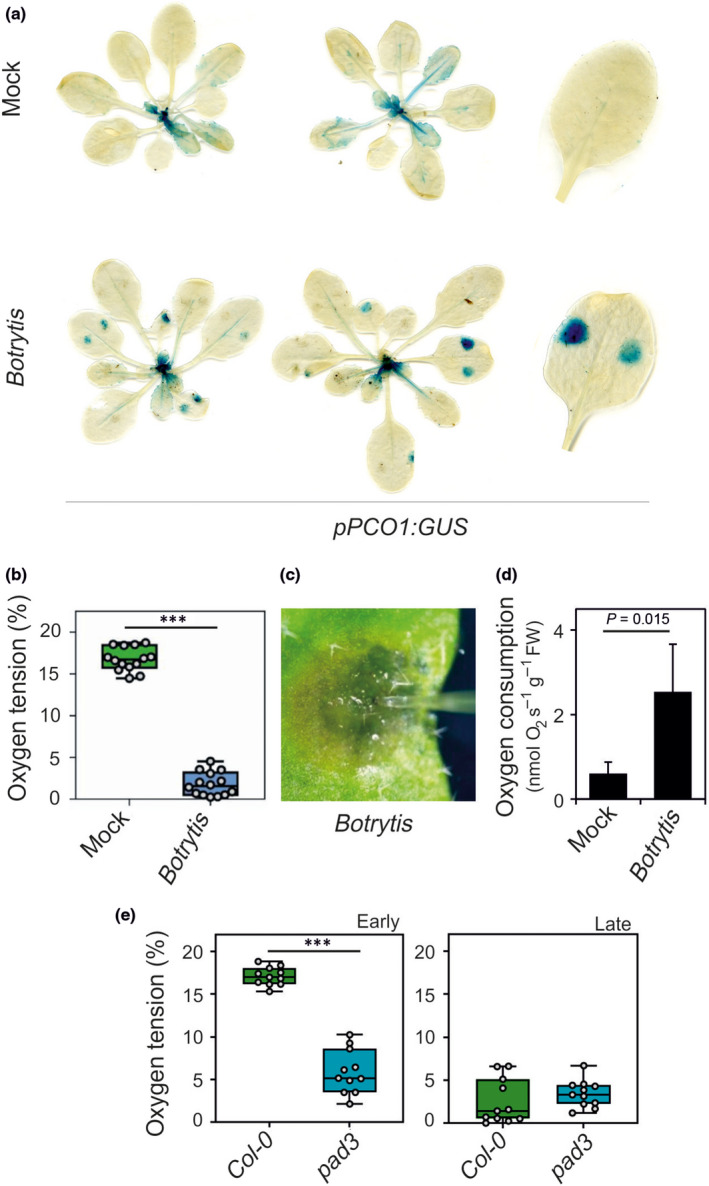
Infection by Botrytis cinerea results in localized hypoxia and expression of the transgenic line having the β‐glucuronidase (GUS) gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) reporter. Botrytis cinerea was inoculated as two drops on each leaves of Arabidopsis plants grown in soil (mock: culture broth). (a) GUS staining revealing the expression of the pPCO1:GUS reporter. (b) Oxygen tension measured using an oxygen microsensor probe (24 h post‐infection). To assess the statistical significance of the observed datasets (n = 14), one‐way ANOVA was performed followed by Tukey’s post‐hoc test (***, P < 0.001). Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. (c) Image showing the probe inserted in the centre of the infected area. (d) Oxygen consumption rate of infected leaves compared to mock‐treated leaves (culture broth). Data are mean ± SD (n = 5). The P‐value resulting for pairwise comparison by Student’s t‐test is shown. (e) Oxygen tension measured using an oxygen microsensor probe in wild‐type plants and in pad3 plants. Data were collected at an early and late stage of infection, visually evaluated from the lesion sizes and appearance. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. To assess the statistical significance of the observed datasets (n = 11), one‐way ANOVA was performed followed by Tukey’s post‐hoc test (***, P < 0.001).
We directly measured the O2 content at the sites of infection with an oxygen microsensor, finding that it drastically dropped from a value of 18% in control leaves to < 2% in the infected area (Fig. 2b,c). The rate of respiration of infected leaves was more than three‐fold higher, when compared to mock‐treated leaves (Fig. 2d), suggesting that local hypoxia at the site of infection results from faster O2 consumption. We verified if the hypoxia was reached faster in pad3, a mutant that is more susceptible to B. cinerea. The results showed that hypoxia was reached earlier in pad3 compared to the wild‐type, correlating well with the lesion size at two different times from infection in the two genotypes (Fig. 2e).
We explored the possibility that other pathogenic fungi or bacteria also could induce hypoxia during infection. As observed post‐infection with B. cinerea (Fig. 2a), Arabidopsis leaves infected by A. brassicicola, a necrotrophic fungus, showed a very clear, localized induction of the PCO1 promoter (Fig. S1a). Infection by a hemibiotrophic bacterium, P. syringae, did not, instead, induce hypoxia‐related symptoms (Fig. S1b).
Local hypoxia at the infection site results in stabilization of RAP2.12
Next we tested if hypoxia originating at the site of B. cinerea infection was sufficient for increased abundance and relocalization of RAP2.12 to the nuclei in an otherwise fully aerobic leaf. We used a 35S:RAP2.12‐GFP reporter line to visualize RAP2.12 in epidermal cells of leaves that were treated with broth‐only (Mock) or infected with B. cinerea. Although mock‐treated leaves predominantly showed membrane localization of RAP2.12‐GFP, occasionally we also found GFP signal at the nuclei (Fig. 3a). However, B. cinerea inoculation led to a strong increase in RAP2.12‐GFP fluorescence in the nuclei at the area of infection, as well as in the area of the leaf that is close to the inoculation site (Fig. 3a,b). These results indicate successful RAP2.12 stabilization and an increase in overall RAP2.12 quantity, mostly in the nucleus‐localized component, upon B. cinerea‐induced hypoxia (Fig. 3a,b). We tested if ERF‐VII proteins are important for contrasting infection by B. cinerea, as proposed by Zhao et al. (2012) by challenging the pentuple ERF‐VII mutant (erfvii) and a line overexpressing a constitutively stable version of RAP2.12 (35S:Δ‐RAP2.12) with B. cinerea. The results confirmed that ERF‐VII proteins contribute to Arabidopsis tolerance to B. cinerea, as demonstrated by the larger lesion area observed in the erfvii mutant (Fig. S2a,b) and higher expression of the B. cinerea actin (Bc‐Actin) gene in infected plants (Fig. S2c). Overexpression of a stable version of RAP2.12, however, did not enhance tolerance to the fungus (Fig. S2a,b).
Fig. 3.
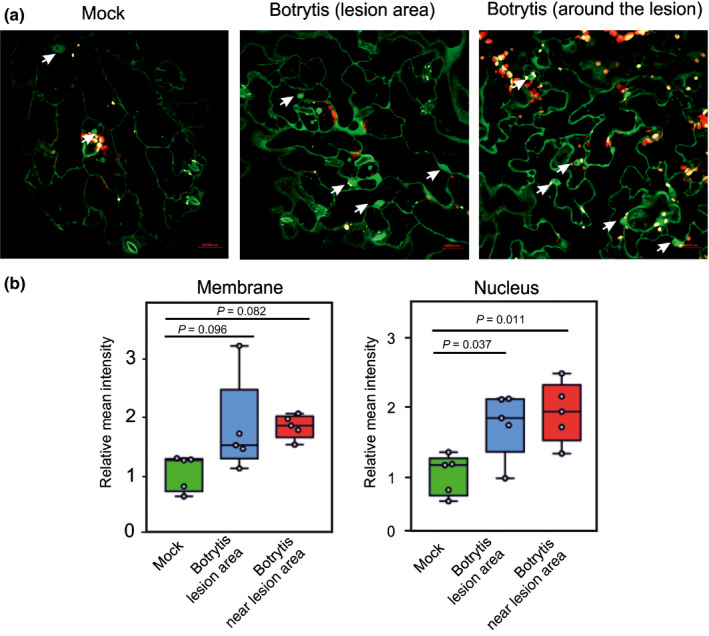
Infection by Botrytis cinerea results in increased signal and nuclear localization of RAP2.12:GFP (GFP, green fluorescent protein). (a) Representative confocal microscopy images of RAP2.12‐GFP (green) and chlorophyll fluorescence (red) in Arabidopsis epidermal leaf cells in the B. cinerea lesion area or in proximity of the infected region. The leaf area directly under a drop of culture broth (mock) was used as control. (b) Relative mean intensity (n = 5) of RAP2.12‐GFP fluorescence as shown in (a) in the membrane or nucleus following mock or B. cinerea treatments. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. To assess the statistical significance of the observed datasets, one‐way ANOVA was performed followed by Tukey post‐hoc test.
Ethylene does not influence ERF‐VII expression following B. cinerea infection
The ERF‐VII genes are ETH‐inducible (Hinz et al., 2010) and ETH is produced during the infection by B. cinerea (Qadir et al., 1997; Chague et al., 2002; Zhu & Tian 2012). We confirmed ETH production 24 h post‐infection with B. cinerea (Fig. 4a). Inhibition of ETH perception by 1‐methylcyclopropene (1‐MCP; see Fig. S3 for evidence of the efficacy of the inhibitor) reduced the expression of PDF1.2 (Fig. 4b). However, 1‐MCP did not affect the expression of pPCO1:GUS post‐infection with B. cinerea, indicating that the ETH is dispensable for the activation of the anaerobic response (Fig. 4c). As we observed an overall increase of RAP2.12‐GFP in the nuclei of B. cinerea‐infected leaves (Fig. 3), we explored the possibility that infection with B. cinerea not only might lead to hypoxia‐dependent stabilization of RAP proteins, but also to ETH‐induced expression of these ERF‐VII genes. However, we observed that most ERF‐VII genes were not upregulated in infected leaves, with the notable exception of HRE2 (Fig. 4d). The induction of HRE2 was not influenced by 1‐MCP, suggesting independence of this gene from ETH as observed previously (Hess et al., 2011).
Fig. 4.
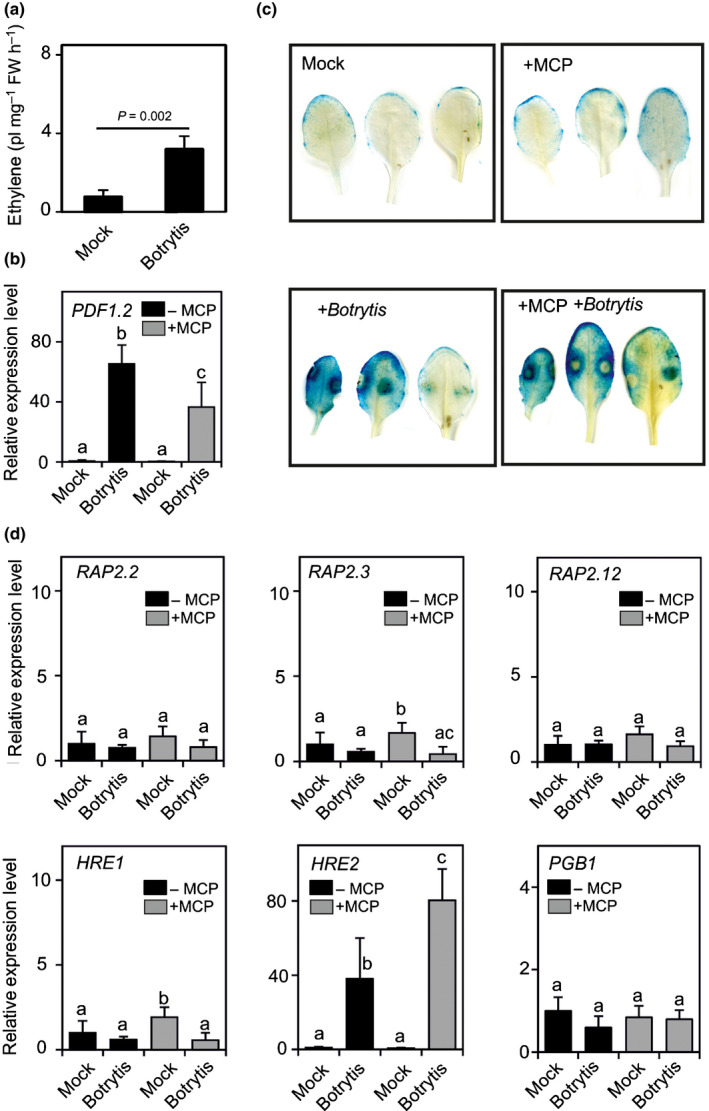
Ethylene production during Botrytis cinerea infection does not influence subgroup VII of ETHYLENE RESPONSE FACTOR (ERF‐VII) expression. (a) Ethylene (ETH) production by plants infected with B. cinerea compared to mock‐treated plants (culture broth). Data are mean ± SD (n = 5). The P‐value resulting for pairwise comparison by Student’s t‐test is shown. (b) PDF1.2 expression in leaves infected with B. cinerea compared to mock‐treated leaves (culture broth). A set of plants was treated with 1‐methylcyclopropene (1‐MCP) to inhibit ETH signalling. Data are mean ± SD (n = 5). Differences (P < 0.05; two‐way ANOVA) are indicated by different letters. (c) GUS staining revealing the expression of the transgenic line having the β‐glucuronidase (GUS) gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) reporter. A set of plants was treated with 1‐MCP to inhibit ETH signalling. (d) Expression of ERF‐VII genes in leaves infected with B. cinerea compared to mock‐treated leaves. A set of plants was treated with 1‐MCP to inhibit ETH signalling. Data are mean ± SD (n = 5). Differences (P < 0.05; two‐way ANOVA) are indicated by different letters.
Induction of anaerobic genes by B. cinerea is independent from wounding and ABA
We explored the possibility that infection by B. cinerea causes wounds in the leaf and that this could lead to induction of hypoxia‐regulated genes. Wounding induces ADH1 expression (Kato‐Noguchi, 2001) and wounding triggers resistance to B. cinerea (Chassot et al., 2008), making the link between wounding caused during B. cinerea infection and ADH1 expression possible. We confirmed that wounding triggers expression of anaerobic genes, as indicated by GUS activity along mechanically‐induced wounds in pPCO1:GUS plants (Fig. 5a). HRE2 and ADH1 expression was induced by wounding, whereas PDF1.2 was not (Fig. 5b). The expression of an ABA‐inducible gene, RD29A occurs as early as 1 h after wounding, suggesting that wounding induces the synthesis of ABA. Given that ADH1 is induced by ABA (de Bruxelles et al., 1996), it is likely that the expression of ADH1 is the consequence of wounding‐induced ABA synthesis (Fig. 5b; Kato‐Noguchi, 2001). However, although wounding induced an increased O2 consumption in leaves, this was transient (Fig. 5c) and did not lead to hypoxia in the wounded leaf areas (Fig. 5d).
Fig. 5.
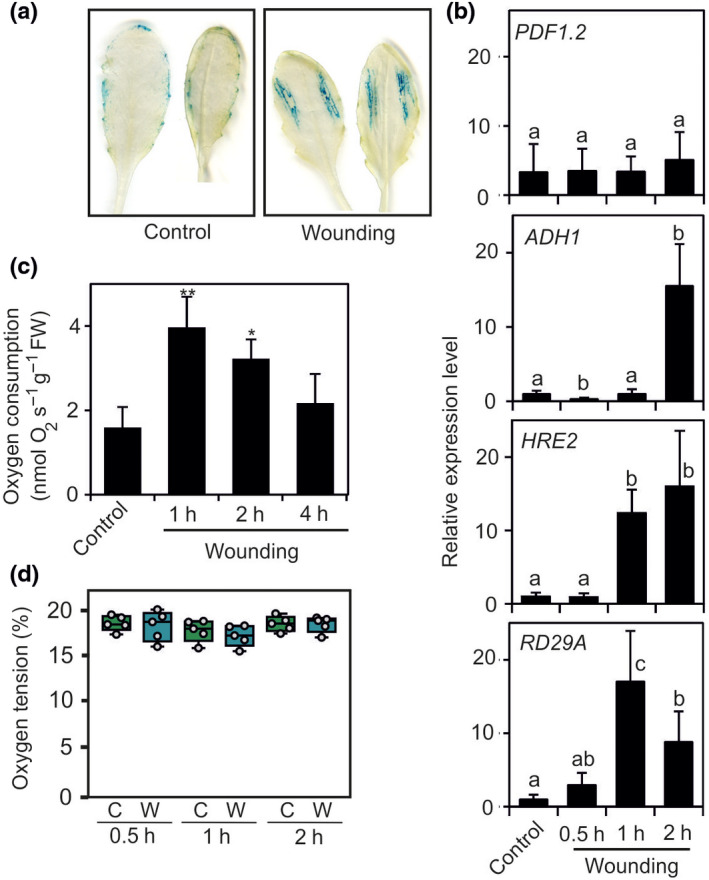
Wounding‐dependent induction of anaerobic genes is hypoxia‐independent. (a) β‐glucuronidase (GUS) staining revealing the expression of the transgenic line having the GUS gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) reporter in wounded leaves. (b) Expression of PDF1.2, ADH1, RD29A in Arabidopsis leaves that were wounded. Sampling was as shown in the figure. Data are mean ± SD (n = 5). Differences (P < 0.05; one‐way ANOVA) are indicated by different letters. (c) Oxygen consumption of infected leaves compared to mock‐treated leaves (culture broth). Data are mean ± SD (n = 5). Pairwise comparison by Student’s t‐test: *, P < 0.05; **, P < 0.01. (d) Oxygen tension measured using an oxygen microsensor probe. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. Differences were not significant for P < 0.05.
Botrytis cinerea is known to produce ABA (Marumo et al., 1982; Izquierdo‐Buono et al., 2018). We checked if this hormone contributes to the induction of anaerobic genes during B. cinerea infection. pPCO1:GUS leaves infected with B. cinerea showed GUS staining in the areas where the inoculum was placed (Fig. 6a). The effect of droplets of ABA applied to the leaves on the expression of GUS in pPCO1:GUS leaves was limited, but present (Fig. 6a). Furthermore, ADH1 expression was clearly induced by ABA, whereas it had no effect of HRE2 expression (Fig. 6b). The expression of RD29A, a gene responding strongly to ABA, showed very high levels in the ABA‐treated leaves but its expression was negligible in leaves infected with B. cinerea (Fig. 6b), indicating that B. cinerea infection does not lead to markedly increased ABA levels in the infected tissue. The O2 concentration at the sites where ABA was applied on the leaves was unaffected, as evident from the oxygen microsensor measurements (Fig. 6c). Overall, these results showed that, although ABA can induce ADH1, the contribution of ABA produced by the B. cinerea pathogen to the induction of anaerobic genes is negligible.
Fig. 6.
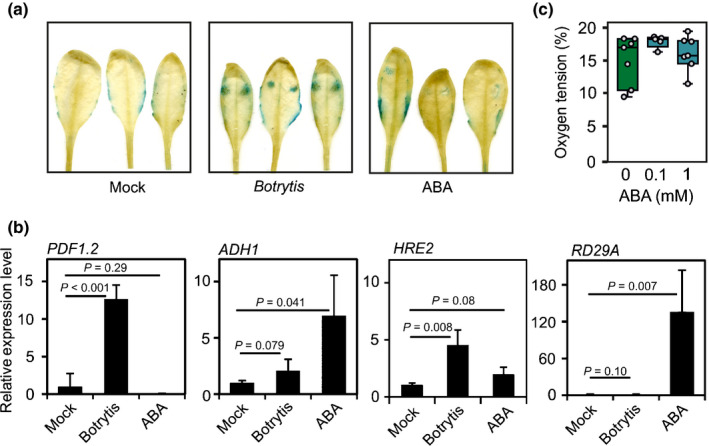
Abscisic acid (ABA) does not induce hypoxia. (a) β‐glucuronidase (GUS) staining revealing the expression of the transgenic line having the GUS gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) reporter in Botrytis cinerea‐infected leaves and, to a minor extent, in ABA‐treated leaves. (b) Expression of PDF1.2, ADH1, RD29A in Arabidopsis leaves that were infected with B. cinerea or treated with ABA. Data are mean ± SD (n = 5). Differences (P < 0.05; one‐way ANOVA) are indicated by different letters. (c) Oxygen tension measured using an oxygen microsensor probe. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. Differences were not significant for P < 0.05.
Elicitors of plant defence moderately decrease O2 availability but cannot induce anaerobic genes
The induction of hypoxia at the site of B. cinerea infection raises the question about the trigger that leads to a marked induction of respiration and a concomitant reduction in oxygen availability. We verified if elicitation of plant defence is enough to induce the low O2 condition associated with B. cinerea infection. The bacterial flagellin peptide elicitor flg22 enhances resistance to B. cinerea (Ferrari et al., 2007) and induces several pathogenesis‐related genes (Denoux et al., 2008). We treated Arabidopsis plants with flg22 and observed induction of PDF1.2 but not of ADH1 and HRE2 (Fig. 7a). GUS staining was absent in pPCO1:GUS leaves treated with flg22, whereas B. cinerea was able to induce GUS expression as also shown Fig. 2a, but also Fig. 4cand 6a (Fig. 7b). Only when used at 10 µM, flg22 was able to trigger a moderate decrease in O2 availability, from c. 15% in control leaves to < 10% in flg22‐treated leaves, an O2 concentration still too high to induce the expression of anaerobic genes (Fig. 7c). A time‐course with 10 µM flg22 confirmed that this elicitor is unable to trigger hypoxia even with shorter or longer treatment times (Fig. S4). We next explored the possibility that signalling originating from fragments of pectin (OGs) produced by the degradation of the host cell wall may induce hypoxia in the leaves of B. cinerea‐infected plants. OGs activate plant innate immunity by functioning as damage‐associated molecular patterns and induce several pathogen responses genes, such as PAD3 (Ferrari et al., 2007). We failed to observe induction of PAD3 when the length of the treatment was > 24 h (not shown) and decided to check the plant's response to OGs in a time‐course experiment. The results showed that PAD3 is induced after 10 h from the start of OG treatment (Fig. 7d). However, ADH1 and HRE2 were not induced at any of the analyzed time points (Fig. 7d). Analysis of the O2 content in the leaves indicated that leaves responding to OGs are fully aerobic (Fig. 7e) and the pPCO1:GUS leaves did not show any sign of GUS activity (Fig. 7f). Finally, we used heat‐killed mycelium as an elicitor, confirming also in this case the lack of hypoxia following the treatment, which was able to induce expression of defence‐related genes but not the hypoxia‐related ones (Fig. S5). Overall, these results indicated that elicitation of the defence pathway in the plant cells is not sufficient for induction of local hypoxia at the site of infection, suggesting that the metabolic/eliciting activity of the living fungus is responsible for the increase in O2 consumption.
Fig. 7.
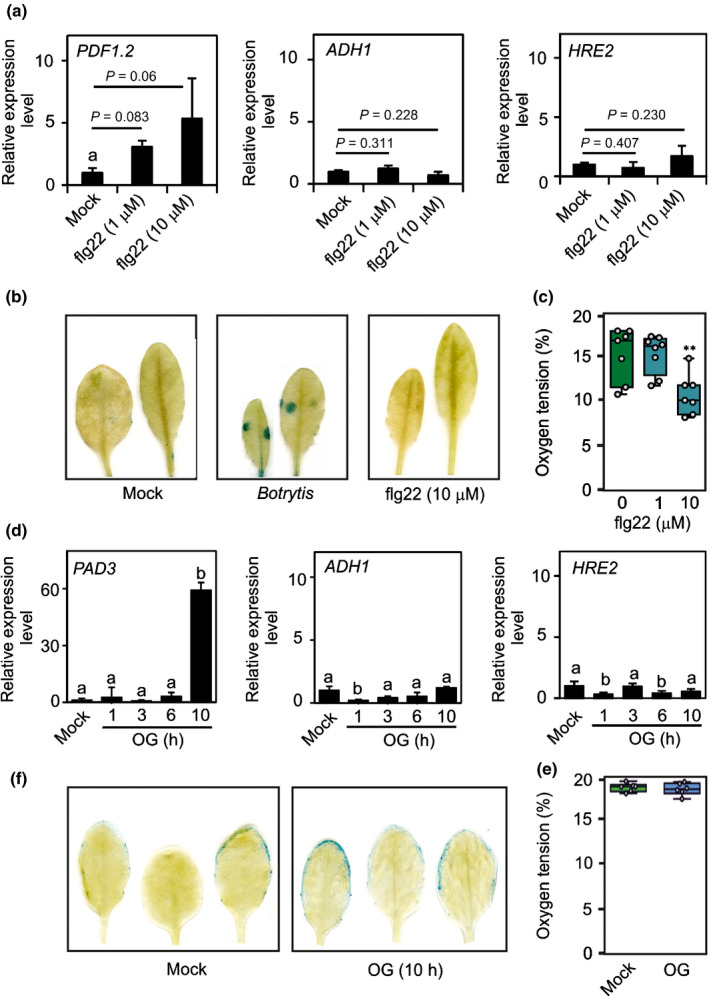
Elicitors of plant defence responses do not induce hypoxia. (a) Expression of PDF1.2 and ADH1 in Arabidopsis leaves that were infected with Botrytis cinerea or treated with flagellin peptide elicitor flg22. Data are mean ± SD (n = 5). The elicitor was applied as two droplets/leaf. The P‐value resulting for pairwise comparison by Student’s t‐test is shown. (b) β‐glucuronidase (GUS) staining revealing the expression of the transgenic line having the GUS gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) reporter in B. cinerea‐infected leaves but not in flg22‐treated leaves. The elicitor was applied as two droplets/leaf. (c) Oxygen tension measured using an oxygen microsensor probe. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. Significant differences: **, P < 0.01. (d) Expression of PAD3 and ADH1 in Arabidopsis leaves that were infected with B. cinerea or treated with oligogalacturonides (OGs) for the time indicated in figure. The elicitor was applied as two droplets per leaf. Data are mean ± SD (n = 5). Differences (P < 0.05; one‐way ANOVA) are indicated by different letters. (e) Oxygen tension measured using an oxygen microsensor probe. Lines in the boxes indicate the median, the bottom and top of each box denotes the first and third quartile, respectively, the dots represent the single data points and whiskers denote the min/max values. Differences were not significant for P < 0.05. (f) GUS staining revealing the absence of expression of the pPCO1:GUS reporter in in OG‐treated leaves. The elicitor was applied as two droplets per leaf.
Discussion
Subgroup VII of the ethylene transcription factors (ERF‐VII) proteins are key components of the oxygen (O2) sensing mechanism in plants (Bailey‐Serres et al., 2012). Under hypoxia the low concentration of O2 permits the stabilization of these proteins, which accumulate in the nucleus to activate the hypoxia‐responsive gene network. Besides their well‐established role in hypoxia signalling (Loreti et al., 2016), ERF‐VII proteins also contribute to pathogen resistance (Zhao et al., 2012; Gravot et al., 2016; Kim et al., 2018; Vicente et al., 2019). Given the instability of ERF‐VII proteins under aerobic conditions, their involvement in pathogen resistance implies that they can get stabilized in the plant's infected tissues. This may occur if hypoxia is established during pathogen infection or by modulation of nitric oxide (NO), the other important determinant for ERF‐VII stability (Gibbs et al., 2014; Gravot et al., 2016), possibly by the NO‐scavenging activity of the ethylene (ETH)‐induced PHYTOGLOBIN1 (PGB1; Hartman et al., 2019).
Induction of hypoxia‐responsive genes was indeed reported in roots infected by Plasmodiophora brassicae (Gravot et al., 2016) or upon Agrobacterium tumefaciens infection in stems (Kerpen et al., 2019). Hypoxia in clubroot and in crown galls may result from slower O2 diffusion rate in tumorigenic tissues (Jubault et al., 2013) or from an intracellular competition for O2 between the pathogen and the host (Gravot et al., 2016). It also has been postulated that increased respiration rates to fuel rapid cell proliferation rates may cause hypoxia in the case of crown galls (Kerpen et al., 2019), although O2 consumption rate did not increase in clubroot tissue.
To date, there is no evidence of hypoxic condition in leaves infected by nontumorigenic pathogens like Botryitis cinerea apart from a transient induction of ALCOHOL DEHYDROGENASE 1 (ADH1) in leaves infected by the hemibiotroph pathogen Pseudomonas syringae (Vicente et al., 2019). However, this could have resulted from synthesis of abscisic acid (ABA) occurring in Pseudomonas‐infected leaves (de Torres‐Zabala et al., 2007), which induces ADH1 expression (de Bruxelles et al., 1996).
Although there is evidence linking ERF‐VII proteins to B. cinerea resistance (Zhao et al., 2012), the potential role of ERF‐VIIs in aerobic tissues such as leaves is thus actually challenged by their instability if O2 and/or NO are present. An environment characterized by high O2 and NO concentrations is not compatible with RAP2.3 stability, making its interaction with OCTADECANOID‐RESPONSIVE ARABIDOPSIS 59 (ORA59) impossible (Kim et al., 2018). Likewise, it was unclear how an unstable RAP2.2 can contribute to resistance to B. cinerea (Zhao et al., 2012). Vicente et al. (2019) demonstrated a role for ERF‐VII in stomatal defence against Pseudomonas – therefore in an anaerobic tissue. Also in this case the instability of ERF‐VII would compromise their action.
Here, we demonstrated that infection by B. cinerea and Alternaria brassicicola, but not P. syringae, leads to the rapid establishment of localized hypoxia in an otherwise fully aerobic leaf. In the case of B. cinerea, this occurs though enhanced respiration at the site of infection (Fig. 2d), which locally depletes O2 availability (Fig. 2b) at a level compatible with ERF‐VII (RAP2.12) stabilization and accumulation in the plant's nuclei (Fig. 3a,b). It remains to be elucidated if the absence of hypoxia at the site of P. syringae infection is related to the plant's response to the pathogen in terms of respiratory metabolism. Hypoxia following infection by necrotrophic fungi also may arise from the tissue waterlogging in the necrotic area, although the increased respiration observed in Botrytis‐infected leaves suggests that hypoxia is generated by a sequence of intense respiratory mechanisms at the site of infection. The establishment of hypoxia correlates with the plant's susceptibility to B. cinerea infection, as demonstrated by the faster hypoxia establishment in the pad3 mutant (Fig. 2e). Although flagellin peptide (flg)22 and oligogalacturonides (OGs) do not induce hypoxia (Fig. 7b,e), it is very likely that other signalling molecules released by B. cinerea enhance the plant's respiration (Fig. 2d). This is supported by the evidence of enhanced respiration in Arabidopsis cells that were cultured in a millicell culture insert that enables molecular communication between the fungus and the plant cells without physical contact (Veillet et al., 2017). Water‐soaking of the leaf tissues infected by B. cinerea also may contribute to generating hypoxia.
Given the drastic decline in O2 concentration at the sites where the infection occurs we discounted the possibility of an indirect effect of B. cinerea triggering a low‐pH environment on components of the O2 sensing machinery such as the PCO enzymes, which display reduced activity at pH < 7 (White et al., 2018).
Wounding induced by penetration of B. cinerea in the plant’s cells could potentially contribute to establishing local hypoxia in the leaves, but the strong induction of the GUS gene under the control of the PLANT CYSTEINE OXIDASE 1 (PCO1) promoter (pPCO1:GUS) in mechanically wounded leaves (Fig. 5a) is likely ABA‐dependent and uncoupled from the B. cinerea‐dependent effect (Fig. 5b). ABA can indeed induce ADH1 (Fig. 6b; de Bruxelles et al., 1996) and wounding causes an ABA‐dependent response that we observed in terms of RD29A expression (Fig. 5b). But these symptoms of ABA signalling were absent in leaves infected with B. cinerea (Fig. 6b) and ABA did not induce Hypoxia Responsive ERF 2 (HRE2) (Fig. 6b), indicating that, although B. cinerea may cause an increase in the leaf ABA content (Marumo et al., 1982), this is insufficient to trigger RD29A expression and an ABA‐dependent response on hypoxic genes such as ADH1 (Fig. 6b).
Ethylene production during B. cinerea infection (Chaqué et al., 2002) could have two consequences. On the one hand, it may contribute to induce the expression of ERF‐VII at the transcriptional level (Hinz et al., 2010; Hartman et al., 2019), and on the other, it could enhance ERF‐VII stability before hypoxia by increasing expression of the NO‐scavenger PGB1 (Hartman et al., 2019). We observed production of ETH in the plants infected with B. cinerea (Fig. 4a), but we could not observe induction of ERF‐VII genes, with the exception of a strong induction of HRE2 (Fig. 4d). However, although HRE1 is induced by ETH, HRE2 is not (Hess et al., 2011). This suggests that upregulation of HRE2 was triggered upon B. cinerea‐induced hypoxia and was independent of ETH. This is supported by the fact that the ETH signalling inhibitor 1‐MPC (Mitochondrial Pyruvate Carrier) did not affect the induction of HRE2 (Fig. 4d). Overall, given that the ETH‐responsive ERF‐VIIs (Hinz et al., 2010; Hess et al., 2011) are not induced by B. cinerea infection and that HRE2 induction is ETH‐independent, we conclude that the amount of ETH produced during B. cinerea infection is not sufficient to affect the induction or stabilization of ERF‐VII. In line with the absence of a marked ETH signalling pathway connecting with hypoxia sensing, PGB1 was not induced by B. cinerea infection (Fig. 4d).
Oligogalacturonides are released during interaction with B. cinerea, which secretes polygalacturonase to degrade pectin. Those OGs with a degree of polymerization between 10 and 25 act as elicitors that contribute to trigger immunity against the fungal pathogen. Interestingly, OGs were found to induce NO production (Rasul et al., 2012), which is known to negatively affect ERF‐VII stability (Gibbs et al., 2014). In several cases, we measured O2 concentrations close to anoxia at the site of B. cinerea infection (Fig. 2b), indicating that the amount of O2 available for PCOs activity is very low. Under these conditions the contribution of NO to ERF‐VII stability is presumably marginal; however, we did observe a decrease in the aerobic expression of ADH1 and HRE2 after the addition of OGs (Fig. 7d), suggesting that the release of OGs during B. cinerea infection may indeed negatively influence ERF‐VII stability.
The advantages of establishing hypoxia at the site of B. cinerea infection can be multiple. The fate of OGs as elicitors might depend on the establishment of local hypoxia at the site of B. cinerea infection. It was indeed recently discovered that OGs can get oxidized by a group of four Arabidopsis berberine bridge enzyme‐like proteins, named OGOX1‐4 (Benedetti et al., 2018). Oxidized OGs display a reduced capability of activating the immune responses and are less hydrolysable by fungal polygalacturonases (Benedetti et al., 2018). In the context of the dialog between the pathogen and the host, the O2‐dependent activity of OGOXs is potentially unfavourable for the plant because of the consequent reduced activity of OGs as elicitors. From another perspective, the fungus might induce hypoxia to maintain the OGs in a nonoxidized status and therefore metabolizable by the fungus itself. This latter possibility is more likely, given that overexpression of OXOGs results in enhanced resistance to B. cinerea (Benedetti et al., 2018). Therefore, the B. cinerea‐induced localized hypoxia at the site of infection may provide a means to avoid oxidation of OGs and thus allow their metabolism by the fungus (Fig. 8).
Fig. 8.
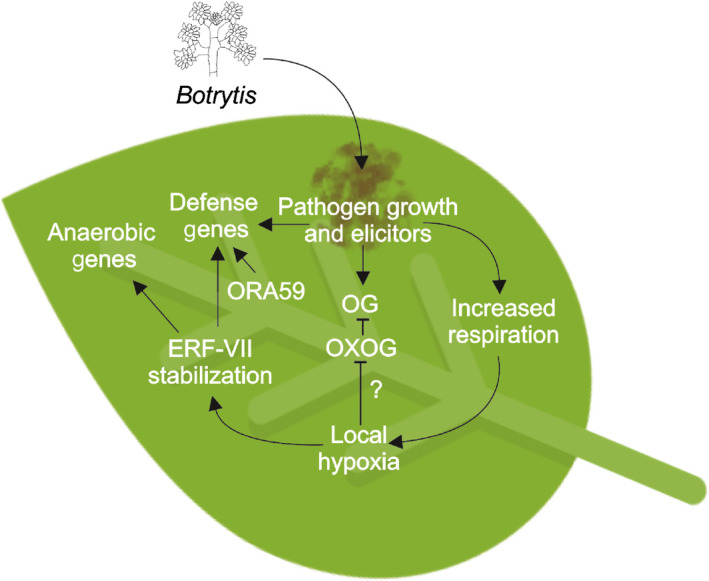
Model explaining the establishment of local hypoxia at the sites of Botrytis cinerea infection. Botrytis cinerea infection results in increased respiration, inducing hypoxic conditions that lead to subgroup VII of ETHYLENE RESPONSE FACTOR (ERF‐VII) stabilization and translocation to the nucleus. Here, these transcription factors induce the expression of genes required for hypoxia adaptation as well as, together with OCTADECANOID‐RESPONSIVE ARABIDOPSIS 59 (ORA59) (Kim et al., 2018) and other still nonidentified partners, genes involved in plant defence. Hypoxia may also influence the fate of oligogalacturonides (OGs), that are oxidized by OXOG in a reaction requiring molecular oxygen (Benedetti et al., 2018).
The establishment of hypoxia at the site of B. cinerea infection also may promote pathogen resistance because it leads to the stabilization of ERF‐VII proteins (Fig. 3). This will allow RAP2.3 to form a nucleus‐localized complex with ORA59 to regulate plant defence genes (Kim et al., 2018). RAP2.2 would be stabilized as well, contributing to the plant's resistance to B. cinerea (Zhao et al., 2012). We indeed observed increased susceptibility to B. cinerea infection in a pentuple mutant (erfvii) defective in all five ERF‐VII proteins (Fig. S2). This suggests that the establishment of hypoxia is part of plant defence response rather than a mere consequence of pathogen infection. Furthermore, all three RAP‐type ERF‐VIIs (RAP2.2, RAP2.3, RAP2.12) will activate the hypoxia‐responsive genes to protect the plant tissue from the negative consequences of hypoxia. Induction of HRE2 by B. cinerea infection, and an ERF‐VII gene which is strongly and stably induced under low‐ O2 conditions, ensures that the transcription of hypoxic genes is steadily maintained (Licausi et al., 2010). Enhanced stabilization of ERF‐VII, as in 35S:Δ‐RAP2.12 plants, however, does not enhance tolerance to B. cinerea (Fig. S2), suggesting also that a hypoxic pre‐treatment would be ineffective for enhancing B. cinerea tolerance. A model explaining the mechanism of hypoxia induction and its relations with the plant's response to B. cinerea is reported in Fig. 8.
Hypoxia occurs in plants as a consequence of abiotic constraints such as soil waterlogging or flooding, but also in plant tissues and organs whose compactness limits O2 diffusion (van Dongen & Licausi, 2015). Local hypoxic niches influence shoot apical meristem development (Weits et al., 2019) as well as lateral root growth (Eysholdt‐Derzsó & Sauter, 2017; Eysholdt‐Derzsó & Sauter, 2019; Shukla et al., 2019). We demonstrated here that not only tumorigenic root tissues (Jubault et al., 2013) but also leaves develop hypoxia as a consequence of pathogen infection. This indicates that pathogen infection by itself can be sufficient to induce hypoxia and may be a common feature of biotic stresses. These sources of evidence expand the influence of hypoxia in plant growth and development well beyond plant's tolerance to flooding (Bailey‐Serres & Voesenek, 2008). Besides ERF‐VII proteins, the hypoxia‐dependent stabilization of other proteins that are target of the Cys‐branch of the N‐degron pathway (Millar et al., 2019) may influence pathogen resistance pathways, an area of study certainly deserving further work.
Author contributions
PP and EL conceived the work and devised the experimental design; MCV, EL and GN carried out the experimental work and data analysis; DAW performed the confocal microscopy and oxygen measurement experiments; AM performed the ETH assays; PP and EL wrote the manuscript and all authors reviewed and commented on the manuscript.
Supporting information
Fig. S1 Infection by Alternaria brassiciola, but not Pseudomonas syringae (Pst DC3000), results in localized hypoxia and expression of the pPCO1:GUS reporter.
Fig. S2 Effects of B. cinerea infection on wild‐type plants (Col‐0), the pentuple ERFVII mutant (erfvii) and a line overexpressing a constitutively stable version of RAP2.12 (35S:Δ‐RAP2.12).
Fig. S3 Effects of MCP on Arabidopsis plants.
Fig. S4 Effects of flg22 in a time‐course experiment.
Fig. S5 Effects of heat‐killed mycelium on Arabidopsis plants.
Table S1 Primers used for gene expression analysis using real‐time quantitative RT‐PCR.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The B. cinerea strain was kindly provided by Simone Ferrari (La Sapienza, University of Rome). We thank Benedetta Mattei (L’Aquila University) for useful discussion and Sabrina Sarrocco (University of Pisa) for advice on B. cinerea cultures management and for providing us with the Alternaria brassicicola strain.
Contributor Information
Pierdomenico Perata, Email: p.perata@santannapisa.it.
Elena Loreti, Email: loreti@ibba.cnr.it.
References
- Bailey‐Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT. 2012. Making sense of low oxygen sensing. Trends in Plant Science 17: 129–138. [DOI] [PubMed] [Google Scholar]
- Bailey‐Serres J, Voesenek LACJ. 2008. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59: 313–339. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Verrascina I, Pontiggia D, Locci F, Mattei B, De Lorenzo G, Cervone F. 2018. Four Arabidopsis berberine bridge enzyme‐like proteins are specific oxidases that inactivate the elicitor‐active oligogalacturonides. The Plant Journal 94: 260–273. [DOI] [PubMed] [Google Scholar]
- Broekgaarden C, Caarls L, Vos IA, Pieterse CM, Van Wees SC. 2015. Ethylene: traffic controller on hormonal crossroads to defense. Plant Physiology 169: 2371–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. 1996. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiology 111: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagué V, Elad Y, Barakat R, Tudzynski P, Sharon A. 2002. Ethylene biosynthesis in Botrytis cinerea . FEMS Microbiology Ecology 40: 143–149. [DOI] [PubMed] [Google Scholar]
- Chassot C, Buchala A, Schoonbeek HJ, Métraux JP, Lamotte O. 2008. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. The Plant Journal 55: 555–567. [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. 2008. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Molecular Plant 1: 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Licausi F. 2015. Oxygen sensing and signaling. Annual Review of Plant Biology 66: 345–367. [DOI] [PubMed] [Google Scholar]
- Eysholdt‐Derzsó E, Sauter M. 2017. Root bending is antagonistically affected by hypoxia and ERF‐mediated transcription via auxin signaling. Plant Physiology 175: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysholdt‐Derzsó E, Sauter M. 2019. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biology 21: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. 2007. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3 . Plant Physiology 144: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. 2003. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4 . The Plant Journal 35: 193–205. [DOI] [PubMed] [Google Scholar]
- Gasch P, Fundinger M, Müller JT, Lee T, Bailey‐Serres J, Mustroph A. 2016. Redundant ERF‐VII transcription factors bind to an evolutionarily conserved cis‐motif to regulate hypoxia‐responsive gene expression in Arabidopsis. Plant Cell 28: 160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Isa NM, Movahedi M, Lozano‐Juste J, Mendiondo GM, Berckhan S, Marin de la Rosa N, Vincente Conde J, Sousa Correia C, Pearce SP et al 2014. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Molecular Cell 53: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey‐Serres J et al 2011. Homeostatic response to hypoxia is regulated by the N‐end rule pathway in plants. Nature 479: 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Lee SC, Licausi F, Kosmacz M, Oosumi T, van Dongen JT, Bailey‐Serres J, Perata P. 2014. A trihelix DNA binding protein counterbalances hypoxia‐responsive transcriptional activation in Arabidopsis. PLoS biology 12: e1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Perata P. 2018. Group VII ethylene response factors in Arabidopsis: regulation and physiological roles. Plant Physiology. 176: 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Shukla V, Maggiorelli F, Giorgi FM, Lombardi L, Perata P, Licausi F. 2017. Age‐dependent regulation of ERF‐VII transcription factor activity in Arabidopsis thaliana . Plant, Cell & Environment 40: 2333–2346. [DOI] [PubMed] [Google Scholar]
- Gravot A, Richard G, Lime T, Lemarié S, Jubault M, Lariagon C, Jocelyne J, Vicente J, Seilaniantz AR, Holdsworth MJ et al 2016. Hypoxia response in Arabidopsis roots infected by Plasmodiophora brassicae supports the development of clubroot. BMC Plant Biology 16: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman S, Liu Z, Van Veen H, Vicente J, Reinen E, Martopawiro S, Zhang H, van Dongen N, Bosman F, Visser EJ et al 2019. Ethylene‐mediated nitric oxide depletion pre‐adapts plants to hypoxia stress. Nature Communications 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess N, Klode M, Anders M, Sauter M. 2011. The hypoxia responsive transcription factor genes ERF71/HRE2 and ERF73/HRE1 of Arabidopsis are differentially regulated by ethylene. Physiologia Plantarum 143: 41–49. [DOI] [PubMed] [Google Scholar]
- Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R. 2010. Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiology 153: 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PY, Catinot J, Zimmerli L. 2015. Ethylene response factors in Arabidopsis immunity. Journal of Experimental Botany 67: 1231–1241. [DOI] [PubMed] [Google Scholar]
- Izquierdo‐Bueno I, González‐Rodríguez VE, Simon A, Dalmais B, Pradier JM, Le Pêcheur P, Mercier A, Walker AS, Garrido C, González Collado I et al 2018. Biosynthesis of abscisic acid in fungi: identification of a sesquiterpene cyclase as the key enzyme in Botrytis cinerea . Environmental Microbiology 20: 2469–2482. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubault M, Lariagon C, Taconnat L, Renou JP, Gravot A, Delourme R, Manzanares‐Dauleux MJ. 2013. Partial resistance to clubroot in Arabidopsis is based on changes in the host primary metabolism and targeted cell division and expansion capacity. Functional & Integrative Genomics 13: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato‐Noguchi H. 2001. Wounding stress induces alcohol dehydrogenase in maize and lettuce seedlings. Plant Growth Regulation 35: 285–288. [Google Scholar]
- Kerpen L, Van Dongen JT, Weits DA. 2019. Crown galls become hypoxic which induces plant anaerobic responses that support tumor proliferation. Frontiers in Plant Science 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NY, Jang YJ, Park OK. 2018. AP2/ERF family transcription factors ORA59 and RAP2.3 interact in the nucleus and function together in ethylene responses. Frontiers in Plant Science 9: 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT. 2011. Oxygen sensing in plants is mediated by an N‐end rule pathway for protein destabilization. Nature 479: 419–422. [DOI] [PubMed] [Google Scholar]
- Licausi F, Van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P. 2010. HRE1 and HRE2, two hypoxia‐inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana . The Plant Journal 62: 302–315. [DOI] [PubMed] [Google Scholar]
- Loreti E, van Veen H, Perata P. 2016. Plant responses to flooding stress. Current Opinion in Plant Biology 33: 64–71. [DOI] [PubMed] [Google Scholar]
- de Marchi R, Sorel M, Mooney B, Fudal I, Goslin K, Kwaśniewska K, Ryan PT, Pfalz M, Kroymann J, Pollmann S et al 2016. The N‐end rule pathway regulates pathogen responses in plants. Scientific Reports 6: 26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐de la Rosa N, Sotillo B, Miskolczi P, Gibbs DJ, Vicente J, Carbonero P, Oñate‐Sánchez L, Holdsworth MJ, Bhalerao R, Alabadí D et al 2014. Large‐scale identification of gibberellin‐related transcription factors defines group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiology 166: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo S, Katayama M, Komori E, Ozaki Y, Natsume M, Kondo S. 1982. Microbial production of abscisic acid by Botrytis cinerea . Agricultural and Biological Chemistry 46: 1967–1968. [Google Scholar]
- Mensuali Sodi A, Panizza M, Tognoni F. 1992. Quantification of ethylene losses in different container‐seal systems and comparison of biotic and abiotic contributions to ethylene accumulation in cultured tissues. Physiologia Plantarum 84: 472–476. [Google Scholar]
- Millar AH, Heazlewood JL, Giglione C, Holdsworth MJ, Bachmair A, Schulze WX. 2019. The scope, functions, and dynamics of posttranslational protein modifications. Annual Review of Plant Biology 70: 119–151. [DOI] [PubMed] [Google Scholar]
- Perata P, Matsukura C, Vernieri P, Yamaguchi J. 1997. Sugar repression of a gibberellin‐dependent signaling pathway in barley embryos. Plant Cell 9: 2197–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pré M, Atallah M, Champion A, De Vos M. 2008. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiology 147: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadir A, Hewett EW, Long PG. 1997. Ethylene production by Botrytis cinerea . Postharvest Biology and Technology 11: 85–91. [Google Scholar]
- Rasul S, Dubreuil‐Maurizi C, Lamotte O, Koen E, Poinssot B, Alcaraz G, Wendehenne D, Jeandroz S. 2012. Nitric oxide production mediates oligogalacturonide‐triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana. Plant, Cell & Environment 35: 1483–1499. [DOI] [PubMed] [Google Scholar]
- Shukla V, Lombardi L, Iacopino S, Pencik A, Novak O, Perata P, Giuntoli B, Licausi F. 2019. Endogenous hypoxia in lateral root primordia controls root architecture by antagonizing auxin signaling in Arabidopsis. Molecular Plant 12: 538–551. [DOI] [PubMed] [Google Scholar]
- Thaler JS, Owen B, Higgins VJ. 2004. The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiology 135: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres‐Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Egea PR, Bögre L, Grant M. 2007. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO Journal 26: 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. 2011. The N‐end rule pathway and regulation by proteolysis. Protein Science 20: 1298–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillet F, Gaillard C, Lemonnier P, Coutos‐Thévenot P, La Camera S. 2017. The molecular dialogue between Arabidopsis thaliana and the necrotrophic fungus Botrytis cinerea leads to major changes in host carbon metabolism. Scientific Reports 7: 17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J, Mendiondo GM, Pauwels J, Pastor V, Izquierdo Y, Naumann C, Movahedi M, Rooney D, Gibbs DJ, Smart K et al 2019. Distinct branches of the N‐end rule pathway modulate the plant immune response. New Phytologist 221: 988–1000. [DOI] [PubMed] [Google Scholar]
- Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, Perata P, van Dongen Joost T, Licausi F. 2014. Plant cysteine oxidases control the oxygen‐dependent branch of the N‐end‐rule pathway. Nature Communications 5: 3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weits DA, Kunkowska AB, Kamps NC, Portz KM, Packbier NK, Venza ZN, Gaillochet C, Lohmann JU, Pedersen O, van Dongen JT et al 2019. An apical hypoxic niche sets the pace of shoot meristem activity. Nature 569: 714. [DOI] [PubMed] [Google Scholar]
- White MD, Kamps JJ, East S, Kearney LJT, Flashman E. 2018. The plant cysteine oxidases from Arabidopsis thaliana are kinetically tailored to act as oxygen sensors. Journal of Biological Chemistry 293: 11786–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- Williamson B, Tudzynski B, Tudzynski P, van Kan JA. 2007. Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology 8: 561–580. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wei T, Yin KQ, Chen Z, Gu H, Qu LJ, Qin G. 2012. Arabidopsis RAP2.2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses. New Phytologist 195: 450–460. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Tian S. 2012. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Scientia Horticulturae 142: 38–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Infection by Alternaria brassiciola, but not Pseudomonas syringae (Pst DC3000), results in localized hypoxia and expression of the pPCO1:GUS reporter.
Fig. S2 Effects of B. cinerea infection on wild‐type plants (Col‐0), the pentuple ERFVII mutant (erfvii) and a line overexpressing a constitutively stable version of RAP2.12 (35S:Δ‐RAP2.12).
Fig. S3 Effects of MCP on Arabidopsis plants.
Fig. S4 Effects of flg22 in a time‐course experiment.
Fig. S5 Effects of heat‐killed mycelium on Arabidopsis plants.
Table S1 Primers used for gene expression analysis using real‐time quantitative RT‐PCR.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.


