Abstract
Aim
In 9‐17‐year‐old Chinese girls, the AS04‐adjuvanted HPV‐16/18 vaccine (AS04‐HPV‐16/18) given as three‐dose schedule induced high antibody levels, which were noninferior 1 month after the third dose to those observed in 18‐25‐year‐old Chinese women in a large efficacy study. We assessed the persistence of antibodies 8‐9 years after vaccination in the same subjects.
Methods
This follow‐up phase III, open‐label study (NCT03355820) included subjects who had received three doses of AS04‐HPV‐16/18 in the initial trial (NCT00996125). Serum antibody concentrations were assessed by ELISA and compared to antibody persistence observed in 18‐25‐year‐old Chinese women 6 years after first vaccination in the efficacy study (NCT00779766).
Results
Out of the 227 enrolled subjects, 223 were included in the per‐protocol immunogenicity analysis. Mean interval from first AS04‐HPV‐16/18 dose to blood sampling was 101.4 months (8.5 years). For antibodies against HPV‐16 and ‐18, 8.5 years after first vaccine dose all subjects remained seropositive and antibody. Geometric mean concentrations (GMCs) were 1236.3 (95% confidence interval [CI]: 1121.8; 1362.4) and 535.6 (95% CI: 478.6; 599.4) ELISA Units/mL, respectively. These seropositivity rates and antibody GMCs were higher than those observed 6 years after first vaccination of 18‐25‐year‐old women.
Conclusion
Sustained anti‐HPV‐16 and ‐18 immune responses were observed 8‐9 years after AS04‐HPV‐16/18 vaccination of 9‐17 year‐old Chinese girls that were higher than the ones observed 6 years after first vaccination in Chinese adult women in whom AS04‐HPV‐16/18 efficacy against cervical intraepithelial neoplasia of grade ≥2 was demonstrated.
Keywords: AS04‐adjuvanted HPV‐16/18 vaccine, cervical cancer, China, human papillomavirus, immunogenicity
1. INTRODUCTION
Persistent human papillomavirus (HPV) infection is causal for developing cervical cancer. 1 In 2018, about 570 000 new cervical cancer cases were estimated to be annually diagnosed worldwide. 2 In China, this estimated number is approximately 105 000. 3 Cervical cancer ranks as the third most frequent cancer among women aged 15‐44 years and is the second leading cause of cancer deaths in this age segment. 3 Among oncogenic HPV types, HPV‐16 and HPV‐18 are the most frequently associated with cervical cancers. In China, it was estimated that 59.5% and 9.6% of cervical cancers were attributable to HPV‐16 and HPV‐18, respectively. 3
To date, three vaccines are available for the prevention of HPV‐related lesions: the AS04‐adjuvanted HPV‐16/18 (AS04‐HPV‐16/18) vaccine (Cervarix, GSK), the quadrivalent HPV‐6/11/16/18 vaccine, and the nonavalent HPV‐6/11/16/18/31/33/45/52/58 vaccine (both MSD). These HPV vaccines contain virus‐like particles and have been demonstrated to be efficacious against infections and precancerous lesions caused by the vaccine HPV‐types. 4 , 5 , 6 The AS04‐HPV‐16/18 vaccine showed a 93.2% efficacy against cervical intraepithelial neoplasia grade three or greater (CIN3+) irrespective of HPV type in HPV naïve young women. 6 It is adjuvanted with AS04, an adjuvant system containing an agonist of the toll‐like receptor 4 adsorbed onto aluminum hydroxide. 7 , 8 This vaccine was first licensed in 2007 and more recently (2016) in China. It was the first vaccine approved for cervical cancer prevention in female population aged 9‐15 years in mainland China with the broadest age indication 9‐45‐year‐old.
HPV vaccination programs were introduced in more than 90 countries but disparity in coverage rates has been reported. 9 , 10 Surveillance data evidenced the global impact of vaccination on HPV‐16/18 incidence when sufficient coverage rate is achieved. 11 Recently published results from a retrospective population study in Scotland demonstrated the high effectiveness of the AS04‐HPV‐16/18 vaccine against preinvasive cervical disease and evidenced substantial herd protection in unvaccinated women. 12 Moreover, results from two observational studies conducted in Scotland and the Netherlands reported the effectiveness of the AS04‐HPV‐16/18 vaccine against nonvaccine types HPV‐31/33/45 and HPV‐31/35/45/52, respectively. 13 , 14 This real‐world evidence of effectiveness against nonvaccine types is aligned with the cross‐protective vaccine efficacy against 6‐ and 12‐month persistent infections for the combined HPV‐31/33/45 types shown in Chinese women aged 18‐25 years vaccinated with the AS04‐HPV‐16/18 vaccine. 15 Based on the evidence available to date, the protection provided by the AS04‐HPV‐16/18 vaccine against HPV types other than 16/18 is presumably due to the adjuvant system. 16 , 17 , 18
As the risk of cervical HPV infection is related to sexual activity and considering the prophylactic nature of the HPV vaccines, 19 , 20 vaccination should occur before onset of sexual activity. 21 Long‐lasting protection is, however, crucial to protect girls and women during the early years of their sexual activity when the risk of infection is the highest. 22 , 23 , 24 The basis for long‐lasting protection may be a sustained high immune response, though no immune correlate of protection has been established so far. 25 , 26 Recent expert reviews even postulated that aside from the neutralizing antibodies, nonneutralizing antibodies as well as cellular immunity were important contributors to the mechanism of protection of HPV vaccines. 17 , 27 , 28 High levels of anti‐HPV‐16 and ‐18 antibodies have been measured up to 10 years after AS04‐HPV‐16/18 vaccination in different age groups. 25 , 29 Even though antibody kinetic profiles present a sharp increase after vaccination followed by a slow decrease to reach a plateau, its level tends to be higher with younger age at vaccination. This trend, together with the need of vaccinating women before their sexual debut, supports the World Health Organization (WHO) recommendation of vaccination programs targeting young adolescents. 30
In China, the introduction of the AS04‐HPV‐16/18 vaccine followed a broad clinical development program. The pivotal efficacy study in China (NCT00779766) showed high efficacy against CIN grade 2 or greater (CIN2+) in Chinese women aged 18‐25 years. 31 , 32 As such, clinical endpoint could not be analyzed in younger subjects due to ethical considerations, and licensure in younger age groups was supported by immunobridging results. A phase III, double blind, randomized, controlled trial (NCT00996125) demonstrated noninferiority of the immune response 1 month after completion of three‐dose vaccination in Chinese girls aged 9‐17 years when compared to the 18‐25 years old women from the pivotal efficacy study. 33
Here, we present results from the phase III, follow‐up study (NCT03355820) assessing the persistence of immune response against HPV‐16/18, up to 8.5 years after the first dose or approximately 8 years after completion of the three‐dose course in the same subjects. Figure 1 summarizes the research, clinical relevance, and impact of this study on the patient population.
FIGURE 1.
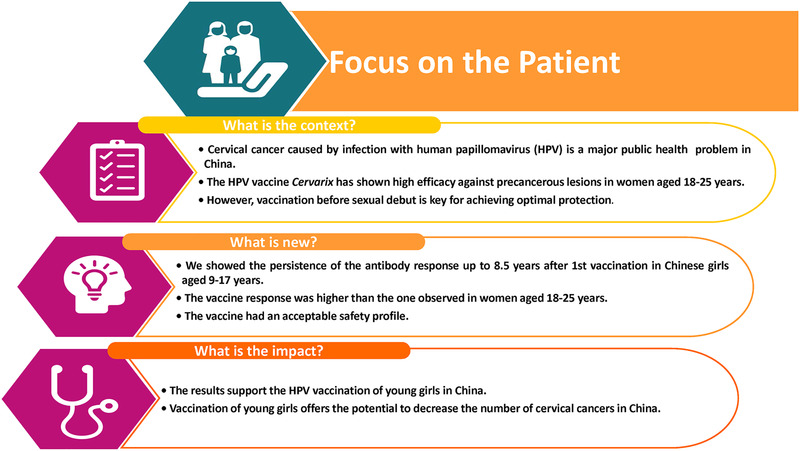
Focus on the patient [Color figure can be viewed at wileyonlinelibrary.com]
2. METHODS
2.1. Study design and participants
The initial study (NCT00996125) was a phase III, double‐blind, randomized, controlled trial, conducted in the Center for Disease Control and Prevention (CDC) in Thaizhou city (Jiangsu, China) between October 2009 and December 2010, in which girls aged 9‐17 years at the time of vaccination received three doses of the AS04‐HPV‐16/18 vaccine or an aluminum hydroxide (AlOH3) placebo at months 0, 1, and 6. 33 AS04 is a GSK proprietary Adjuvant System containing 3‐O‐desacyl‐4′‐monophosphoryl lipid A (50 μg MPL) adsorbed on aluminum salt (500 μg Al3+). Dosage, route of administration, and criteria for inclusion and exclusion of subjects have been previously described. 33
All healthy subjects who completed three‐dose vaccination with the AS04‐HPV‐16/18 vaccine in the previous study were eligible for inclusion. The present phase III, follow‐up study (NCT03355820) was an open‐label study conducted in the same CDC center between February and June 2018 (Figure 2). Subjects having received any anti‐HPV vaccination outside of the previous study, or any investigational/nonregistered product that could have had an impact on the study objectives during the 30‐day period before the study visit, were excluded. Concomitant participation in another clinical study and contraindication related to blood drawing procedure were also exclusion criteria. All subjects having completed the initial study were invited to this follow‐up study. Written informed consent was obtained from each subject before their enrollment in the study. For subjects below 18 years old, that is, the legal age of consent, written informed consent was obtained from their parents or legally acceptable representatives as well as written informed assent from the subject. The study was conducted in accordance with the country regulatory requirements and following the guidelines from the Declaration of Helsinki and the International Conference on Harmonization‐Good Clinical Practices. The study protocol, any amendments, the informed consent, and any other information that required approval were submitted to the Ethics Committee of Jiangsu Provincial CDC and to the Human Genetics Resources Administration of China for review and approval.
FIGURE 2.
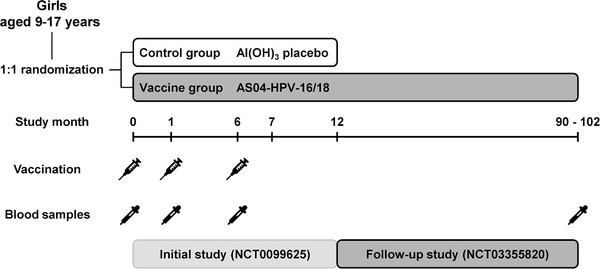
Study design AS04‐HPV‐16/18: AS04‐adjuvanted HPV‐16/18 vaccine
2.2. Immunogenicity assessment
One blood sample of approximately 5 mL was collected to assess the immune response against HPV‐16 and HPV‐18 (primary objective) by measuring antibody concentrations by Enzyme Linked Immunosorbent Assays (ELISA). The methodology was described previously and transferred to the National Institute for Food and Drug Control, China. 34 Seropositivity was defined as a concentration above assay cutoff, that is, 19 ELISA Units (EU)/mL (3.1 International Units [IU]/mL) for HPV‐16 and 18 EU/mL (3.2 IU/mL) for HPV‐18. The conversion factors from EU to IU are 1/6.1 for HPV‐16 and 1/5.7 for HPV‐18.
A secondary objective of the study was to compare the persistence of immune response against HPV‐16/18, 8.5 years after the first vaccine dose in Chinese girls aged 9‐17 years, with the antibody persistence observed 6 years after first vaccination of Chinese women aged 18‐25 years from the efficacy study (NCT00779766). 32
2.3. Safety assessment
Only serious adverse events related to the study procedures or to concomitant GSK product and events with a fatal outcome were to be reported.
2.4. Statistical methods
The statistical analyses were performed using the Statistical Analysis Systems Drug Development. Immunogenicity analyses were performed on the per‐protocol set consisting of subjects who were included in the according‐to‐protocol cohort for immunogenicity analysis in the initial study and had serology results available for the follow‐up study. 33 Seroconversion and seropositivity rates with exact 95% confidence intervals (CI), as well as geometric mean concentrations (GMCs) with 95% CI, were calculated.
2.5. Data availabilities
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
3. RESULTS
3.1. Study participants
All 369 girls who completed the initial study were invited to participate in this follow‐up; 228 were enrolled, of which 227 were included in the exposed set, meaning that they had received the three doses of AS04‐HPV‐16/18 vaccine during the initial study. After elimination of four subjects who were eliminated from the according‐to‐protocol cohort in the initial vaccination study, a total of 223 women constituted the per‐protocol set (Figure 3).
FIGURE 3.
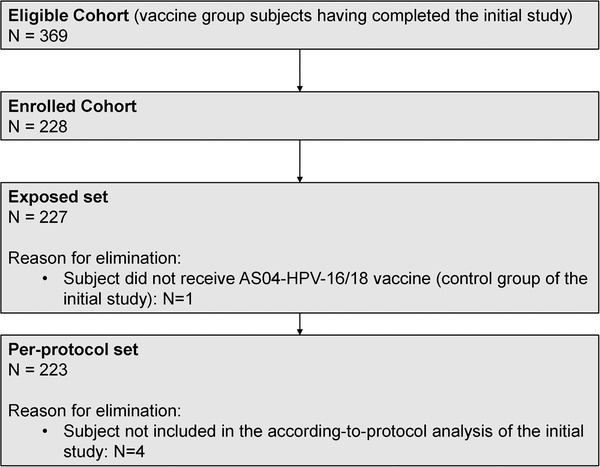
CONSORT diagram. AS04‐HPV‐16/18 vaccine: AS04‐adjuvanted HPV‐16/18 vaccine. Abbreviation: N, number of subjects
Demographic characteristics of the subjects included in the per‐protocol set are presented in Table 1. The mean age of the 223 subjects at the time of the follow‐up visit was 21.6 years (standard deviation [SD]: 2.3) and seven (3.1%) of them were 17‐year‐old adolescents. The mean time between the first vaccine dose received during the initial study and the follow‐up visit of the present study was 101.4 months (SD: 1.2), approximately 8.5 years. All subjects were of Asian ethnicity.
TABLE 1.
Demographic characteristics of the subjects included in the per‐protocol set (N = 223)
| Age (years) | |
|---|---|
| Mean (SD) | 21.6 (2.3) |
| Median (Min – Max) | 22.0 (17‐26) |
| Age group, n | |
|---|---|
| <18 years old (%) | 7 (3.1) |
| ≥18 years old (%) | 216 (96.9) |
| Time between first vaccine dose and blood sampling (months) | |
|---|---|
| Mean (SD) | 101.4 (1.2) |
| Median (Min – Max) | 101.2 (100‐104) |
| Ethnicity, n | |
|---|---|
| Asian‐East Asian Heritage (%) | 223 (100) |
Abbreviations: Max, maximum; Min, minimum; N, total number of subjects; n, number of subjects in a category; SD, standard deviation.
3.2. Immunogenicity
Approximately 8.5 years after having received their first vaccine dose at age of 9‐17 years, all subjects were still seropositive for antibodies against HPV‐16 and HPV‐18 (Table 2). These seropositivity rates were higher compared to the persistence rates reported by Zhu and colleagues 6 years after first vaccine dose in women from the pivotal efficacy study. 32 They measured that 95.2% (95% CI: 91.6; 97.6) and 96.1% (95% CI: 92.7; 98.2) of women aged 18‐25 years at vaccination were still seropositive for antibodies against HPV‐16 and HPV‐18 at month 72, respectively (reported in Table 2 for comparison).
TABLE 2.
Year 8.5 anti‐HPV‐16/18 antibody seropositivity rates and GMCs in subjects aged 9‐17 years at vaccination compared with year 6 results in women aged 18‐25 years at vaccination from the pivotal efficacy study (per‐protocol set)
| Study | Seropositivity rate | GMC | |||
|---|---|---|---|---|---|
| Age group (years) | Timing a (years) | N | N | % (95% CI) | EU/mL (95% CI) |
| HPV‐16 | |||||
| Follow‐up | 223 | 223 | 100 (98.4‐100) | 1236.3 (1121.8‐1362.4) | |
| 9‐17 | 8.5 | ||||
| Pivotal efficacy | 229 | 218 | 95.2 (91.6‐97.6) | 691.7 (578.6‐826.8) | |
| 18‐25 | 6 | ||||
| HPV‐18 | |||||
| Follow‐up | 223 | 223 | 100 (98.4‐100) | 535.6 (478.6‐599.4) | |
| 9‐17 | 8.5 | ||||
| Pivotal efficacy | 229 | 220 | 96.1 (92.7‐98.2) | 355.3 (305.4‐413.4) | |
| 18‐25 | 6 | ||||
Abbreviations: CI, confidence interval; EU, ELISA unit; Follow‐up study, NCT03355820; GMC, geometric mean concentration; N, total number of subjects; n, number of seropositive subjects; Pivotal efficacy study, NCT00779766.
Years between first vaccine dose administration and blood sampling.
In subjects aged 9‐17 years, antibody GMCs measured by ELISA were 1236.3 EU/mL (95% CI: 1121.8; 1362.4) and 535.6 EU/mL (95% CI: 478.6; 599.4) for anti‐HPV‐16 and anti‐HPV‐18, respectively (Table 2). These values were, respectively, 2‐ and 1.5‐fold higher than compared to antibody GMCs measured 6 years after vaccination of women aged 18‐25 years in the pivotal efficacy study 32 (presented in Table 2 for comparison).
3.3. Safety
There were no serious adverse events nor deaths reported during the study.
4. DISCUSSION
Due to their prophylactic nature, HPV vaccines have the highest impact when administered to HPV‐naive subjects. Young adolescent girls are, therefore, the primary target population of most immunization programs. 30 Here, we present the first data on long‐term persistence of the immune response elicited in Chinese girls aged 9‐17 years at first vaccination with the AS04‐HPV‐16/18 vaccine.
In all subjects, seropositivity for HPV‐16 and HPV‐18 antibodies was sustained up to 8.5 years after first vaccination. Seropositivity rates were higher compared to the persistence rates measured 6 years after first vaccine dose in women from the pivotal efficacy study.
The same trend was observed for the GMCs, even though sampling was done after 2 additional years in the current study (Table 2). This difference was already observed 1 month after the third vaccine dose as anti‐HPV‐16 GMCs were 18 682.4 EU/mL (95% CI: 17 162.7; 20 336.6) versus 6996.2 EU/mL (95% CI: 6211.7; 7879.7) for women aged 9‐17 and 18‐25 years, respectively. 33 The same trend was observed for anti‐HPV‐18 GMCs, that is, 7882.4 EU/mL (95% CI: 7079.0; 8777.1) versus 3309.4 EU/mL (95% CI: 2941.9; 3722.8) in women aged 9‐17 and 18‐25 years at vaccination, respectively. 33 From the comparison of the values measured 1 month after administration of the third dose with the results from the 8.5‐years follow‐up, we observe antibody GMC decline over the 8 years period for both HPV‐16 and HPV‐18 in girls aged 9‐17 years at vaccination. 33
As expected from previous studies, 25 , 29 the immune response to HPV‐16 and HPV‐18 detected in vaccinated subjects remains, respectively, 41.5‐ and 23.6‐fold above the antibody concentrations observed in unvaccinated women with evidence of having cleared an HPV‐16 or HPV‐18 infection (ie, seropositive and DNA negative; 29.8 and 22.7 EU/mL, respectively). 26 , 35 Although not unequivocal, the immune response elicited after natural infection with HPV can reduce the risk of reinfection with the same HPV type. 36 , 37 While no immune correlate of protection for HPV‐16/18 vaccination has been identified, antibody GMC plateau levels measured in vaccinated women in whom vaccine efficacy has been demonstrated up to 9.4 years (418.3 EU/mL [95% CI: 344.0; 508.6] for HPV‐16 and 242.6 EU/mL [95% CI: 199.3; 295.2] for HPV‐18 at month 107‐113 timepoint) and GMCs levels in women who were able to clear a natural infection, are valuable benchmarks above which serological protection is thought to be effective. 25 , 38 Thus, despite the lack of immune correlate of protection, the high immune response sustained in the 9‐17 years age group should be indicative of protective effect conferred by the AS04‐HPV‐16/18 vaccine, as demonstrated in Chinese women aged 18‐25 years at vaccination. 31 , 32 , 33
The exact mechanism of protection conferred by HPV vaccination is still to be fully elucidated. Strong and persistent neutralizing antibody levels together with cellular immune response triggered by the vaccine are thought to be involved in its ability to protect the host from HPV infections, even though they may not be the only mechanism of action. 17 , 27 , 28 , 39 , 40 , 41 Correlation between serum and mucosal antibody concentrations in cervicovaginal samples suggests transudation of immunoglobulin G in the female genital tract. 29 , 42 To date, follow‐up studies have been conducted for up to 12 years. 25 , 29 , 43 , 44 The current results add Chinese‐specific data to the body of evidence describing the long‐term kinetic of immune response in women vaccinated with AS04‐HPV‐16/18. Previous fitting of experimental kinetic profiles of anti‐HPV‐16/18 concentrations with mathematical models predicted that girls vaccinated at 15‐25 years of age would have anti‐HPV‐16 and ‐HPV‐18 antibody levels above natural infection levels for 38 and 28 years after vaccination, respectively. 29 , 45 Such long‐lasting immune response is key for achieving optimal protection of vaccinated women, being at risk of HPV infection throughout their period of sexual activity.
The observation of such high and sustainable immune response in Chinese girls vaccinated with three doses of AS04‐HPV‐16/18 vaccine also suggests that reduction of schedule to two doses in adolescent may be considered to be sufficient for protection in this population. 46 , 47 , 48 , 49 As recommended by the WHO, 30 two‐dose schedule in adolescent has been adopted by many countries, such as the United Kingdom, the United States, Germany, and Canada. By 2017, 48 countries worldwide had adopted a two‐dose vaccination schedule in their national programs as it improves compliance and cost‐effectiveness. 50
The present study has several limitations. Follow‐up was conducted for the sole vaccine group, using results of the pivotal study as historical comparator. The comparison is, however, relevant as both studies were conducted in the same geographical area.
Although the immune response detected 8.5 years after vaccination was in line with data generated in other studies, the intermediate timepoints, that is,, between 7 months and 8.5 years after first vaccination, could have been helpful for determining the kinetics and experimental plateau level in this population. The study was limited to serological endpoints, while assessment of cellular‐mediated immunity could also have been of interest for corroborating the assumed mechanisms of protection. Finally, the initial study was an immunobridging study in subjects too young for the assessment of gynecological endpoints. Direct demonstration of long‐term protection could support the impact of HPV‐vaccination in this population.
In conclusion, vaccination of Chinese girls aged 9‐17 years with three doses of AS04‐HPV‐16/18 vaccine induced high and sustained immune response up to 8.5 years after first dose administration. Antibody levels were higher than those observed in vaccinated Chinese adult women although they were assessed after 2 years longer period in vaccinated girls than in women. They were also several folds higher than after natural infection in adult women (global data). These findings are consistent with global AS04‐HPV‐16/18 vaccine data and suggest that subjects vaccinated with three‐dose schedule at 9‐17 years of age are likely to be protected for decades. The vaccine showed an acceptable safety profile in this population.
FUNDING
GlaxoSmithKline Biologicals SA funded this study (NCT03355820; ClinicalTrials.gov) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of the present manuscript.
CONFLICTS OF INTEREST
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare the following potential conflicts of interest: Frank Struyf, Nicolas Folschweiller, Johny Jiang, Sylviane Poncelet, Naveen Karkada, and Dorota Borys are employees of the GSK group of companies. Frank Struyf, Nicolas Folschweiller, Sylviane Poncelet, and Dorota Borys hold shares in the GSK group of companies. Archana Jastorff was consultant of XPE Pharma & Science for the GSK group of companies at the time the study was conducted. Yuemei Hu, Xiang Zhang, Yilin He, Zhilong MA, Yan Xie, Xiangbin Lu, Yabin Xu, Yanqiu Zhang, Yunyu Jiang, and Hui Xiao have nothing to disclose.
AUTHORS CONTRIBUTIONS
All authors participated in the design or implementation or analysis, and interpretation of the study; and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
TRADEMARK
Cervarix is a trademark owned by or licensed to the GSK group of companies.
ACKNOWLEDGMENTS
The authors thank all study participants, investigators, nurses, and other staff members for contributing to this study and especially Bénédicte Brasseur, Yu He, Jenny Jiang, Ivy Luan, Jazelle Torres, Yuan Xu, and Helen Zhang. The authors thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Bruno Baudoux coordinated the manuscript development and editorial support. Jonathan Ghesquière provided medical writing support.
Hu Y, Zhang X, He Y, et al. Long‐term persistence of immune response to the AS04‐adjuvanted HPV‐16/18 vaccine in Chinese girls aged 9‐17 years: Results from an 8‐9‐year follow‐up phase III open‐label study. Asia-Pac J Clin Oncol. 2020;16:392–399. 10.1111/ajco.13398
REFERENCES
- 1. de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2‐13. [DOI] [PubMed] [Google Scholar]
- 2. Bruni L, Barrionuevo‐Rosas L, Albero G, et al. Human Papillomavirus and Related Diseases in the World . 2017. Available from: http://hpvcentre.net/statistics/reports/XWX.pdf. Summary Report 27 July. Accessed April 29, 2019.
- 3. ICO/IARC Information Centre on HPV and Cancer . Human Papillomavirus and Related Diseases Report — China . 2018. Available from: https://hpvcentre.net/statistics/reports/CHN.pdf. Accessed May 23, 2019.
- 4. Joura EA, Giuliano AR, Iversen O‐E, et al. A 9‐valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711‐723. [DOI] [PubMed] [Google Scholar]
- 5. Kjaer SK, Sigurdsson K, Iversen O‐E, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high‐grade cervical and external genital lesions. Cancer Prev Res. 2009;2(10):868‐878. [DOI] [PubMed] [Google Scholar]
- 6. Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV‐16/18 AS04‐adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4‐year end‐of‐study analysis of the randomised, double‐blind PATRICIA trial. Lancet Oncol. 2012;13(1):89‐99. [DOI] [PubMed] [Google Scholar]
- 7. Garçon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther. 2011;11(5):667‐677. [DOI] [PubMed] [Google Scholar]
- 8. Baldridge JR, McGowan P, Evans JT, et al. Taking a Toll on human disease: toll‐like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4(7):1129‐1138. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization . Vaccine in National Immunization Programme — Update . 2019. Available from: http://www.who.int/immunization/monitoring_surveillance/VaccineIntroStatus.pptx. Accessed April 24, 2019.
- 10. Bruni L, Diaz M, Barrionuevo‐Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453‐e463. [DOI] [PubMed] [Google Scholar]
- 11. Harper DM, DeMars LR. HPV vaccines — a review of the first decade. Gynecol Oncol. 2017;146(1):196‐204. [DOI] [PubMed] [Google Scholar]
- 12. Palmer T, Wallace L, Pollock KG, et al. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: retrospective population study. BMJ. 2019;365:l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7‐year cross‐sectional study. Lancet Infect Dis. 2017;17(12):1293‐1302. [DOI] [PubMed] [Google Scholar]
- 14. Woestenberg PJ, King AJ, van Benthem BHB, et al. Bivalent vaccine effectiveness against type‐specific HPV positivity: evidence for cross‐protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis. 2018;217(2):213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu F‐C, Hu S‐Y, Hong Y, et al. Efficacy, immunogenicity, and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in Chinese women aged 18–25 years: event‐triggered analysis of a randomized controlled trial. Cancer Med. 2016;6(1):12‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Einstein MH, Levin MJ, Chatterjee A, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine and HPV‐6/11/16/18 vaccine in healthy women aged 18–45 years: follow‐up through month 48 in a phase III randomized study. Hum Vaccin Immunother. 2014;10(12):3455‐3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryser M, Berlaimont V, Karkada N, Mihalyi A, Rappuoli R, van der Most R. Post‐hoc analysis from phase III trials of human papillomavirus vaccines: considerations on impact on non‐vaccine types. Expert Rev Vaccines. 2019;18(3):309‐322. [DOI] [PubMed] [Google Scholar]
- 18. van der Weele P, Breeuwsma M, Donken R, et al. Effect of the bivalent HPV vaccine on viral load of vaccine and non‐vaccine HPV types in incident clearing and persistent infections in young Dutch females. PLoS One. 2019;14(3):e0212927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins S, Mazloomzadeh S, Winter H, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. Br J Obstet Gynaecol. 2002;109(1):96‐98. [DOI] [PubMed] [Google Scholar]
- 20. Moscicki A‐B. HPV infections in adolescents. Dis Markers. 2007;23(4):229‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mariani L, Venuti A. HPV vaccine: an overview of immune response, clinical protection, and new approaches for the future. J Transl Med. 2010;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arbyn M, Benoy I, Simoens C, Bogers J, Beutels P, Depuydt C. Prevaccination distribution of human papillomavirus types in women attending at cervical cancer screening in Belgium. Cancer Epidemiol Biomarkers Prev. 2009;18(1):321‐330. [DOI] [PubMed] [Google Scholar]
- 23. Sabol I, Milutin Gašperov N, Matovina M, et al. Cervical HPV type‐specific pre‐vaccination prevalence and age distribution in Croatia. PLoS One. 2017;12(7):e0180480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tachezy R, Smahelova J, Kaspirkova J, Salakova M. Human papillomavirus type‐specific prevalence in the cervical cancer screening population of Czech women. PLoS One. 2013;8(11):e79156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naud PS, Roteli‐Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV‐16/18 AS04‐adjuvanted vaccine: final analysis of a long‐term follow‐up study up to 9.4 years post‐vaccination. Hum Vaccin Immunother. 2014;10(8):2147‐2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet. 2007;369(9580):2161‐2170. [DOI] [PubMed] [Google Scholar]
- 27. Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18(4):240‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32, Part A):4768‐4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwarz TF, Galaj A, Spaczynski M, et al. Ten‐year immune persistence and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in females vaccinated at 15–55 years of age. Cancer Med. 2017;6(11):2723‐2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Human papillomavirus vaccines: WHO position paper, May 2017—recommendations. Vaccine. 2017;35(43):5753‐5755. [DOI] [PubMed] [Google Scholar]
- 31. Zhu F‐C, Chen W, Hu Y‐M, et al. Efficacy, immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer. 2014;135(11):2612‐2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu F‐C, Hu S‐Y, Hong Y, et al. Efficacy, immunogenicity and safety of the AS04‐HPV‐16/18 vaccine in Chinese women aged 18–25 years: end‐of‐study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019. 8:6195‐6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu F, Li J, Hu Y, et al. Immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years. Hum Vaccin Immunother. 2014;10(7):1795‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wheeler CM, Skinner SR, Del Rosario‐Raymundo MR, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 7‐year follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16(10):1154‐1168. [DOI] [PubMed] [Google Scholar]
- 35. Schwarz TF, Spaczynski M, Schneider A, et al. Immunogenicity and tolerability of an HPV‐16/18 AS04‐adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27(4):581‐587. [DOI] [PubMed] [Google Scholar]
- 36. Castellsagué X, Naud P, Chow S‐N, et al. Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA. J Infect Dis. 2014;210(4):517‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Safaeian M, Castellsagué X, Hildesheim A, et al. Risk of HPV‐16/18 infections and associated cervical abnormalities in women seropositive for naturally acquired antibodies: pooled analysis based on control arms of two large clinical trials. J Infect Dis. 2018;218(1):84‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The GlaxoSmithKlineVaccine HPV‐007 Study Group . Sustained efficacy and immunogenicity of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine: analysis of a randomised placebo‐controlled trial up to 6·4 years. Lancet. 2009;374(9706):1975‐1985. [DOI] [PubMed] [Google Scholar]
- 39. Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04‐adjuvanted HPV‐16/18 vaccine: improving upon nature. Gynecol Oncol. 2008;110(3, Supplement 1):S1‐S10. [DOI] [PubMed] [Google Scholar]
- 40. Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2, Supplement):S15‐S21. [DOI] [PubMed] [Google Scholar]
- 41. Stanley M, Lowy DR, Frazer I. Chapter 12: prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24:S106‐S13. [DOI] [PubMed] [Google Scholar]
- 42. Schwarz TF, Kocken M, Petäjä T, et al. Correlation between levels of human papillomavirus (HPV)‐16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV‐16/18 AS04‐adjuvanted vaccine. Hum Vaccines. 2010;6(12):1054‐1061. [DOI] [PubMed] [Google Scholar]
- 43. Artemchuk H, Eriksson T, Poljak M, et al. Long‐term antibody response to human papillomavirus vaccines: up to 12 years of follow‐up in the Finnish maternity cohort. J Infect Dis. 2018;219(4):582‐589. [DOI] [PubMed] [Google Scholar]
- 44. Lehtinen M, Lagheden C, Luostarinen T, et al. Ten‐year follow‐up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end‐point‐registry‐based follow‐up of three cohorts from randomized trials. BMJ Open. 2017;7(8):e015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwarz T, Spaczynski M, Kaufmann A, et al. Persistence of immune responses to the HPV‐16/18 AS04‐adjuvanted vaccine in women aged 15–55 years and first‐time modelling of antibody responses in mature women: results from an open‐label 6–year follow‐up study. 2015;122(1):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang L‐M, Puthanakit T, Cheng‐Hsun C, et al. Sustained immunogenicity of 2‐dose human papillomavirus 16/18 AS04‐adjuvanted vaccine schedules in girls aged 9–14 years: a randomized trial. J Infect Dis. 2017;215(11):1711‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kreimer AR, Struyf F, Del Rosario‐Raymundo MR, et al. Efficacy of fewer than three doses of an HPV‐16/18 AS04‐adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16(7):775‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leung TF, Liu AP‐Y, Lim FS, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine and 4vHPV vaccine administered according to two‐ or three‐dose schedules in girls aged 9–14 years: results to month 36 from a randomized trial. Vaccine. 2018;36(1):98‐106. [DOI] [PubMed] [Google Scholar]
- 49. Romanowski B, Schwarz TF, Ferguson L, et al. Sustained immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine administered as a two‐dose schedule in adolescent girls: five‐year clinical data and modeling predictions from a randomized study. Hum Vaccin Immunother. 2016;12(1):20‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. D'Addario M, Redmond S, Scott P, et al. Two‐dose schedules for human papillomavirus vaccine: systematic review and meta‐analysis. Vaccine. 2017;35(22):2892‐2901. [DOI] [PubMed] [Google Scholar]


