Abstract
Background
In early stage laryngeal squamous cell carcinoma (LSCC) radiotherapy with curative intent is a major treatment modality. TNM classification is used to define patients eligible for radiotherapy. Studies in early stage glottic LSCC identified several predictive biomarkers associated with local control. However, we recently reported that this predictive value could not be confirmed in supraglottic LSCC.
Objective
To examine whether clinical behavior and protein expression patterns of these biomarkers differ between glottic and supraglottic LSCC.
Study Design
Retrospective cohort study.
Methods
Tumor tissue sections of 196 glottic and 80 supraglottic T1‐T2 LSCC treated primarily with RT were assessed immunohistochemically for expression of pAKT, Ki‐67 and β‐Catenin. Expression data of HIF‐1α, CA‐IX, OPN, FADD, pFADD, Cyclin D1, Cortactin and EGFR in the same cohort of glottic and supraglottic LSCC, were retrieved from previously reported data. The relationship between glottic and supraglottic sublocalization and clinicopathological, follow‐up, and immunohistochemical staining characteristics were evaluated using logistic regression and Cox regression analyses.
Results
Glottic LSCC were correlated with male gender (P = .001), hoarseness as a primary symptom (P < .001), T1 tumor stage (P < .001), negative lymph node status (P < .001), and an older age at presentation (P = .004). Supraglottic LSCC patients developed more post‐treatment distant metastasis when adjusted for gender, age, and T‐status. While supraglottic LSCC was associated with higher expression of HIF‐1α (P = .001), Cortactin (P < .001), EGFR (P < .001), and Ki‐67 (P = .027), glottic LSCC demonstrated higher expression of CA‐IX (P = .005) and Cyclin D1 (P = .001).
Conclusion
Differences in clinicopathological and immunohistochemical staining characteristics suggest that T1‐T2 glottic and supraglottic LSCC should be considered as different entities.
Level of Evidence
N/A. Laryngoscope, 2020
Keywords: Biomarkers, head and neck squamous cell carcinoma, glottic laryngeal squamous cell carcinoma, supraglottic laryngeal squamous cell carcinoma
INTRODUCTION
Head and neck cancer encompasses a broad spectrum of malignancies, and is responsible for 550,000 new cases and 380,000 deaths worldwide annually. 1 Histologically, the vast majority (approximately 90%) concerns mucosal squamous cell carcinomas. Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous group of malignancies, involving multiple subsites. About 21% of all HNSCC are located in the larynx. 2 Squamous cell carcinoma of the larynx (LSCC) originates from supraglottic, glottic, and subglottic sites in 69%, 28%, and 1% respectively (2% overlapping/unknown). 3
In early stage LSCC radiotherapy is a major treatment modality. However, local control (LC) rates vary between 40% and 100% in early stage LSCC. 4 Radiotherapy failure frequently results in highly morbid salvage surgery. A major wound complication rate of 60% and a pharyngocutaneous fistula rate of 30% have been described. 5
TNM staging, apparently, is of insufficient predictive value toward radio response. In search for new predictive markers in LSCC, immunohistochemical expression of tumor‐specific proteins has been investigated frequently. The authors 4 , 6 , 7 , 8 and others 9 , 10 , 11 studied proteins such as those involved in cell cycle regulation, tumor hypoxia, and cell adhesion. However, the predictive value of these markers varied considerably. For instance, in separate homogeneous cohorts of 91 glottic and 60 supraglottic T1‐T2 LSCC treated with primarily radiotherapy, hypoxia markers HIF‐1α and CA‐IX were predictive for LC in glottic LSCC, 7 but not in supraglottic LSCC. 4 More recently we found that pFADD overexpression was associated with a significantly improved LC rate in glottic LSCC, 8 whereas in our supraglottic cohort it was not. 6 One explanation for this discrepancy is that these LSCC sublocalizations represent other entities, each with its own clinical and biological behavior. As a consequence, the protein expression patterns might differ.
In this study, we will compare the frequency of high/low expression of hypoxia markers HIF‐1α, CA‐IX, and OPN, the 11q13‐related biomarkers FADD, pFADD, Cyclin D1 and Cortactin, and EGFR in a large cohort of pretreatment biopsies of 196 glottic and 80 supraglottic T1‐T2 LSCC. The expression of most of these markers was studied previously in association with clinical outcome upon radiotherapy in this cohort of glottic and supraglottic early stage LSCC. The data on frequency of expression of these markers were retrieved from previously published studies. 4 , 6 , 7 , 8 , 12 In addition, we selected three other biomarkers from the literature because of their typical profile in LSCC (pAKT, Ki‐67, and β‐Catenin) 13 and performed expression analysis by immunohistochemistry using the same cohort of pretreatment biopsies of 196 glottic and 80 supraglottic T1‐T2 LSCC.
MATERIALS AND METHODS
Patient and Biomarker Selection
A database was constructed with 1513 patients treated in the University Medical Center Groningen for (pre‐)malignant laryngeal disease between 1990 and 2011. The following selection criteria were used, as reported previously 4 , 6 : histologically confirmed squamous cell carcinoma restricted to the glottic and supraglottic region; staged cT1 or cT2; curatively treated with primarily radiotherapy with no other previous treatment; sufficient formaldehyde‐fixed paraffin‐embedded (FFPE) pretreatment tumor material, resulting in a cohort of 650 patients. Five patients were excluded because of a malignancy of the head and neck region in their medical history, nine patients were excluded because of the existence of a double tumor in the head and neck region, and 11 patients were excluded because of chemotherapy or other systemic treatment of malignancies before or during treatment for their LSCC.
From 276 patients, sufficient FFPE tumor tissue was available, covering 196 glottic and 80 supraglottic LSCC patients (Table 1). The collection of patient data and tissue samples was approved by the Medical Ethics Committee of our hospital.
TABLE 1.
Description of Pretreatment Clinical Characteristics, Stratified by Supraglottic (n = 80) and Glottic LSCC (n = 196).
| Characteristic | Value | Supraglottic LSCC | Glottic LSCC | Univariate log. Regression OR (95% CI) * | Multivariate log. Regression OR (95%CI) * |
|---|---|---|---|---|---|
| Gender | Male | 59 (73.8) | 177 (90.3) | 1 | 1 |
| Female | 21 (26.3) | 19 (9.7) | 3.32 (1.67–6.59) † | 3.53 (1.37–9.13) † | |
| Age (yr) | <64 | 53 (66.3) | 92 (46.9) | 1 | 1 |
| >64 | 27 (33.7) | 104 (53.1) | 0.45 (0.26–0.78) † | 0.34 (0.15–0.77) † | |
| Median (range) | 62.0 (33–96) | 65.0 (35–89) | |||
| Primary symptom | Hoarse voice | 39 (48.8) | 191 (97.4) | 1 | 1 |
| Other | 41 (51.3) | 2 (0.5) | 100.40 (23.30–432.52) ‡ | 128.40 (26.65–618.56) ‡ | |
| Duration of complaint (wk) | <22 | 42 (53.8) | 94 (48.7) | 1 | NA |
| >22 | 36 (46.2) | 99 (51.3) | 0.81 (0.48–1.38) | ||
| Median (range) | 18.0 (0–520) | 24.0 (0–520) | |||
| T‐status | T1 | 22 (27.5) | 100 (51.0) | 1 | 1 |
| T2 | 58 (72.5) | 96 (49.0) | 2.75 (1.56–4.83) ‡ | 2.98 (1.33–6.68) † | |
| N‐status | N0 | 61 (76.3) | 192 (98.0) | 1 | 1 |
| N+ | 19 (23.8) | 3 (1.5) | 19.93 (5.70–69.67) ‡ | 8.54 (1.67–43.70) † | |
| N‐status | N1 | 9 (11.3) | 3 (1.5) | NA | NA |
| N2 | 9 (11.3) | 0 (0.0) | |||
| N3 | 1 (1.3) | 0 (0.0) | |||
| Nx | 0 (0.0) | 1 (0.5) |
Presented are N (%), unless specified otherwise. Performed were logistic regression analyses where the presence of supraglottic LSCC was considered as the outcome.
P < .05.
P < 0.001.
CI = confidence interval; LSCC = laryngeal squamous cell carcinoma; N = node; NA = not applicable; OR = odds ratio; T = tumor.
The cohort of patients treated between 1990–2008 was described in detail in previously reported studies on the expression of HIF‐1α, CA‐IX, OPN, FADD, pFADD, Cyclin D1, Cortactin, and EGFR in relation to response to radiotherapy. 4 , 6 , 7 , 8 , 12 For the expression analysis of pAKT, Ki‐67, and β‐Catenin, the cohort was expanded with early stage LSCC patients treated between 2009 and 2011.
Immunohistochemistry
First, hematoxylin and eosin staining was performed after 4‐μm serial sections were cut, to evaluate whether sufficient tumor material was available for immunohistochemistry.
Antigen retrieval was achieved by heating in a microwave in preheated citrate buffer (pH 6.0) for 15 minutes at 100°C, by incubation overnight in Tris–HCl (pH 9.0) at 80°C, and by heating in a microwave in preheated Tris/HCL buffer for 15 minutes at 100°C, respectively. Next, endogenous peroxidases were blocked for 30 minutes at room temperature (RT) with 0.3% H2O2. The slides were incubated at RT for 1 hour with antibodies against pAKT (1:50, clone 736E11, Cell Signaling Technology, Danvers, Massachusetts, USA), Ki‐67 (1:350, Clone MIB‐1, Dako, Glostrup, Denmark), and β‐Catenin (1:1,000, clone 14/B, BD Biosciences, San Jose, California, USA). Subsequently, for pAKT immunostaining Envision treatment was applied (Dako) for 30 minutes at RT. For Ki‐67 and β‐Catenin stainings, secondary antibodies were applied for 30 minutes at RT (1:100, RAMPO, Dako), followed by tertiary antibodies, as well for 30 minutes at RT (1:100, GARPO, Dako). The peroxidase reaction was performed by applying 3,3′‐diaminobenzide tetrachloride for 10 minutes, followed by counterstaining with hematoxylin, dehydration, and mounting.
Evaluation of Immunohistochemical Staining
All slides were scored by two observers separately, blinded for follow‐up data. In general, in this study, protein expression is classified as high when the percentage of positive tumor cells is higher than the predefined cut‐off.
For evaluation of cytoplasmic pAKT immunostaining, expression levels (high or low) were defined using a cut‐off of 10% of tumor cells with positive expression, as reported previously in HNSCC. 13 , 14 , 15 , 16 The evaluation of Ki‐67 expression has been performed using different cut‐offs, varying from 10% to 60%. 17 , 18 , 19 , 20 , 21 Therefore, Ki‐67 expression was evaluated using the median staining percentage (49%). Expression of β‐Catenin was scored on both membranous as well as cytoplasmic staining. For membranous staining a cut‐off percentage of 10% was used, as reported previously. 13 In case of β‐Catenin cytoplasmic staining any staining above background staining was considered positive as reported earlier. 11 , 22 Examples of pAKT, Ki‐67, β‐Catenin, and EGFR immunostainings are shown in Figure 1A–D.
Fig 1.
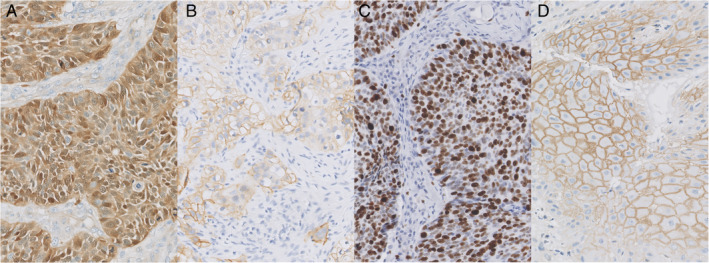
Positive staining for pAKT A, EGFR B, Ki‐67 C, and β‐Catenin D, in laryngeal squamous cell carcinoma. Original magnification, 200x. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Expression data of HIF‐1α, CA‐IX, OPN, FADD, pFADD, Cyclin D1, Cortactin, and EGFR in our two cohorts of glottic and supraglottic LSCC were retrieved from our previous studies. 4 , 6 , 7 , 8 , 12 , 23 The used antibodies, the immunohistochemical staining conditions, and the scoring criteria of the immunostainings of all used biomarkers are summarized in Supplementary data, Table S1.
Statistical Analysis
All statistical analyses were performed using the SPSS 23.0 software (IBM, Armonk, New York, USA). For analyzing relations between clinicopathological characteristics and immunohistochemical staining characteristics on the one side and glottic/supraglottic LSCC sublocalization on the other side, uni‐ and multivariate logistic regression analyses were used. Follow‐up data were analyzed using uni‐ and bivariate Cox regression analyses. P‐values of <.05 were considered to be statistically significant.
RESULTS
Differences in Clinicopathological and Follow‐up Data in T1‐T2 Glottic and Supraglottic LSCC
We compared common clinicopathological and follow‐up data observed in relation with tumor sublocalization in our cohort of pretreatment biopsies of 196 glottic and 80 supraglottic LSCC patients (Table 1). This analysis revealed that the duration of complaints was not correlated significantly with LSCC sublocalization (P = .414). However, male gender (P = .001), hoarseness as a primary symptom (P < .001), T1 tumor stage (P < .001), negative lymph node status (P < .001), and an older age of tumor presentation (P = .004) were statistically significantly correlated with glottic sublocalization. On multivariate logistic analysis these findings remained statistically significant (Table 1).
Both LSCC sublocalizations demonstrated a similar number of patients with locoregional recurrences (see Supporting Information, Table S2). Adjusted bivariate Cox regression analyses revealed no significant difference in the case of locoregional recurrence (Table 2). Patients with supraglottic LSCC developed more distant metastasis after initial diagnosis/treatment (P = .009), losing significance when adjusted for primary symptom (P = .203), and N‐status (P = .114). More supraglottic LSCC patients died, which was only significant when adjusted for age (P = .015). Significance of death of disease (DOD) was present only when additionally corrected for gender (P = .035).
TABLE 2.
The Impact of LSCC Sublocalization (Supraglottic vs. Glottis) on Outcome (Locoregional Recurrence, Distant Metastasis, Overall Death an Death of Disease. *
| Correction for | Sublocalization | Locoregional Recurrence HR (95%CI) | Distant Metastasis HR (95%CI) | Death HR (95%CI) | Death of Disease HR (95% CI) |
|---|---|---|---|---|---|
| Non‐adjusted estimate | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 1.14 (0.68–1.90) | 4.95 (1.49–16.50) † | 1.33 (0.92–1.93) | 0.54 (0.25–1.19) | |
| Gender | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 1.29 (0.77–2.17) | 5.19 (1.53–17.65) † | 1.41 (0.96–2.06) | 0.40 (0.17—0.94) † | |
| Age | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 1.17 (0.69–1.97) | 5.40 (1.56–18.64) † | 1.61 (1.10–2.35) † | 0.56 (0.25–1.26) | |
| Primary symptom | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 1.25 (0.67–2.34) | 2.78 (0.58–13.44) | 1.31 (0.83–2.06) | 0.50 (0.17–1.47) | |
| T‐status | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 1.02 (0.60–1.73) | 5.14 (1.51–17.51) † | 1.32 (0.91–1.91) | 0.50 (0.23–1.11) | |
| N‐status | Glottic LSCC | 1 | 1 | 1 | 1 |
| Supraglottic LSCC | 0.91 (0.51–1.60) | 2.93 (0.77–11.09) | 1.23 (0.83–1.81) | 0.70 (0.23–2.13) |
Cox regression analysis adjusted for gender, age, primary symptom, T‐status and N‐status.
P < .05.
CI = confidence interval; LSCC = laryngeal squamous cell carcinoma; HR = hazard ratio.
Comparison of Expression Profiles of Biomarkers Associated with Clinical Outcome Between Glottic and Supraglottic LSCC
In order to validate differences between glottic and supraglottic T1‐T2 LSCC, we compared the frequency of expression of these 11 selected biomarkers (pAKT, Ki‐67, β‐Catenin, HIF‐1α, CA‐IX, OPN, FADD, pFADD, Cyclin D1, Cortactin, and EGFR), reported as potential prognostic or predictive markers toward clinical outcome in LSCC (summarized in Table 3). The percentage of tumor cells with high expression in both the glottic and supraglottic sublocalization was similar for OPN, FADD, pFADD, pAKT, and β‐Catenin. The percentage of supraglottic LSCC with high expression of HIF‐1α, Cortactin, EGFR, and Ki‐67 was significantly higher, compared to glottic LSCC (P = .001, P < .001, P < .001, P = .027, respectively). On the other hand, expression of CA‐IX and Cyclin D1 was significantly increased in glottic LSCC compared to supraglottic LSCC (P = .005, P = .001, respectively).
TABLE 3.
Results of Immunohistochemistry, Logistic Regression Analysis.
| Staining Characteristics | Glottic LSCC | Supraglottic LSCC | Logistic Regression OR (95% CI) * | Previously Reported | |
|---|---|---|---|---|---|
| HIF‐1α | Low n (%) | 45 (49.5) | 13 (21.7) | 1 | Schrijvers 2008 161, Wachters 2013 2154 |
| High n (%) | 46 (50.5) | 47 (78.3) | 3.54 (1.69–7.41) † | ||
| Median (range) | 1.0 (0–60) | 6.0 (0–50) | |||
| CA‐IX | Low n (%) | 54 (59.3) | 49 (81.7) | 1 | Schrijvers 2008 161, Wachters 2013 2154 |
| High n (%) | 37 (40.7) | 11 (18.3) | 0.33 (0.15–0.71) † | ||
| Median (range) | 10.0 (0–90) | 1.0 (0–40) | |||
| OPN | Low n (%) | 69 (76.7) | 40 (66.7) | 1 | Wachters 2013 2154 |
| High n (%) | 21 (23.3) | 20 (33.3) | 1.64 (0.80–3.40) | ||
| Median (range) | 0.0 (0–50) | 0.0 (0–80) | |||
| FADD | Low n (%) | 71 (77.2) | 40 (66.7) | 1 | Schrijvers 2012 1220, Wachters 2017 0000 |
| High n (%) | 21 (22.8) | 20 (33.3) | 1.69 (0.82–3.49) | ||
| Median (range) | 2.0 (0–4) | 2.0 (0–4) | |||
| pFADD | Low n (%) | 30 (32.6) | 17 (28.3) | 1 | Schrijvers 2012 1220, Wachters 2017 0000 |
| High n (%) | 62 (67.4) | 43 (71.7) | 1.22 (0.60–2.49) | ||
| Median (range) | 80 (20–100) | 80.0 (5–100) | |||
| Cyclin D1 | Low n (%) | 43 (49.4) | 46 (78.0) | 1 | Glottic: Schrijvers 2012 122 |
| High n (%) | 44 (50.6) | 13 (22.0) | 0.28 (0.13–0.58) † | ||
| Median (range) | 10.0 (0–85) | 35.0 (0–95) | |||
| Cortactin | Low n (%) | 42 (51.9) | 12 (20.3) | 1 | Glottic: Schrijvers 2012 122 |
| High n (%) | 39 (48.1) | 47 (79.7) | 4.22 (1.95–9.10) ‡ | ||
| Median (range) | 20.0 (0–100) | 65.0 (0–100) | |||
| pAKT | Low n (%) | 48 (34.3) | 14 (22.6) | 1 | NA |
| High n (%) | 92 (65.7) | 48 (77.4) | 1.79 (0.90–3.57) | ||
| Median (range) | 50 (0–100) | 70 (0–100) | |||
| EGFR | Low n (%) | 41 (47.1) | 3 (5.9) | 1 | Supraglottic: Bruine de Bruin 2019 1 |
| High n (%) | 46 (52.9) | 48 (94.1) | 14.26 (4.13–49.28) ‡ | ||
| Median (range) | 2 (1–4) | 2 (1–4) | |||
| Ki‐67 | Low n (%) | 76 (56.7) | 25 (39.7) | 1 | NA |
| High n (%) | 58 (43.3) | 38 (60.3) | 1.99 (1.08–3.66) † | ||
| Median (range) | 48.0 (15–86) | 51 (4–89) | |||
| β‐Catenin | Low n (%) | 6 (4.4) | 4 (6.3) | 1 | NA |
| (membr.) | High n (%) | 131 (95.6) | 60 (93.8) | 0.69 (0.19–2.52) | |
| Median (range) | 75.0 (0–100) | 76.5 (0–100) | |||
| β‐Catenin | Low n (%) | 129 (94.2) | 63 (98.4) | 1 | NA |
| (cytopl.) | High n (%) | 8 (5.8) | 1 (1.6) | 0.26 (0.03–2.09) | |
| Median (range) | 0 (0–1) | 0 (0–1) |
Logistic regression analysis for the presence of supraglottic LSCC.
P < .05.
P < .001.
CI = confidence interval; LSCC = laryngeal squamous cell carcinoma; OR = odds ratio; NA not applicable.
DISCUSSION
Despite many studies on the prognostic and/or predictive value of immunohistochemical biomarkers in HNSCC cohorts, conflicting and divergent results keep these biomarkers from being incorporated in daily strategies. This might be explained by population diversity in TNM stages, immunohistochemical techniques, staining evaluation methodologies, and anatomical tumor sublocalizations. 24 , 25 , 26
Previously, we studied the association between local control and expression of several markers in separate homogeneous cohorts of T1‐T2 glottic and supraglottic LSCC, all treated with radiotherapy only. This association with some markers observed in the glottic cohort, 7 , 8 could not be confirmed in the supraglottic cohort. 4 , 6 Recently, we found that pATM expression was associated with local control in supraglottic but not in glottic LSCC. 27 These observations suggested that within these tumor sublocalizations, clinical and biological behavior, and hence protein expression patterns might differ.
In this study we analyzed both the clinicopathological characteristics and the differences in frequency of high expression of these biomarkers in two homogeneous, well‐defined cohorts of 196 glottic and 80 supraglottic LSCC, all diagnosed and treated at the same institute. To circumvent differences as a result of technical issues, all tissue samples were collected from the same cohort, immunohistochemistry of all markers was performed in the same lab using similar conditions and all immunostainings were evaluated using the same scoring criteria as previously defined (see Supporting Information, Table S1). 4 , 7 , 8 , 12 , 13 , 23
From an epidemiological perspective, male gender was significantly more dominant in the glottic than in the supraglottic LSCC patients. This finding is in concordance with other studies, 28 , 29 whereas in several studies gender was not related with tumor sublocalization. 30 , 31 Geographical differences in the use of alcohol between both sexes are known confounders. 32 Moreover, compared to the (sub)glottis, the supraglottis is exposed to a relatively higher degree to ingested agents and to a lesser degree to inhaled agents, 33 suggesting that etiological factors, like the use of alcohol and tobacco, might have distinguishable epidemiological effects. Because of the notoriously unreliable and frequently lacking data on the use of tobacco and alcohol in our cohorts, these characteristics were not analyzed in our study.
In the literature differences between the age at which early stage glottic and supraglottic LSCC was diagnosed have not been described. In our study both the median age and mean age (65.19 and 62.04, respectively, data not shown) were 3 years higher in glottic LSCC patients. Significantly more patients in our supraglottic cohort died during our follow‐up period, which is in agreement with the literature, 3 although disease‐related death rates were similar (see Supporting Information, Table S2). However, in both glottic and supraglottic LSCC only a minority died of disease‐related causes.
From a clinical perspective, differences between glottic and supraglottic LSCC can be pointed out as well. Relatively small mucous membrane disruptions on a glottic level produce vibratory disturbances of the involved vocal fold during speech, hence hoarseness in the patient, whereas comparable mucous membrane disturbances on a supraglottic level can remain unnoticed or misinterpreted by the patient for a longer period of time. 34 This is in good agreement with the observation that hoarseness was a significantly more frequently experienced primary symptom in our glottic LSCC cohort. Additionally, it can explain the significantly higher T‐stage at presentation in our supraglottic cohort.
Moreover, already on an early embryological basis, the glottic and supraglottic larynx have distinguishable origins. The glottic and subglottic larynx are derived from the sixth branchial arch and the supraglottis from the third and fourth branchial arches. 34 , 35 The supraglottic compartment ultimately develops a rich lymphatic network and, with relatively few anatomical barriers, forms a rather easy entrance for locoregional tumor spread. Tumor spread in the glottic compartment, however, is hampered by elastic layers and a more limited network of lymphatics. 36 The observed higher lymph node status at diagnosis in supraglottic T1‐T2 LSCC as compared to glottic LSCC is in agreement with this organic perspective and with the literature as well. 37
On a molecular level, the literature demonstrates some clues that protein expression profiles between glottic and supraglottic LSCC might differ. One study, using a relatively small series of 35 glottic and 25 supraglottic T1‐T4 N0‐N+ LSCC, revealed a significantly higher EGFR and RXRa expression in glottic LSCC, whereas expression of NF‐kB and Cox2 were similar in both supraglottic and glottic LSCC. 38 Another study showed a decreased expression of β‐Catenin in supraglottic LSCC, but no difference regarding ILK, pAKT, E‐Cadherin, Vimentin, AR, and Er‐b). 13 These findings suggested the existence of distinguishable protein expression profiles in glottic and supraglottic LSCC. On the other hand, α‐Catenin, CD44, hyaluronan, p53, and Bcl‐2 was not correlated with tumor sublocalization within 136 glottic and 62 supraglottic Tis‐T2 LSCC cohort. 36
In the present paper, we selected 11 biomarkers, frequently reported in LSCC/HNSCC and often reported to be associated with clinical outcome. 4 , 6 , 7 , 8 , 23 , 39 Differential expression between glottic and supraglottic LSCC was observed for six markers (HIF‐1α, CA‐IX, Cyclin D1, Cortactin, EGFR, and Ki‐67) (Table 3). Tumor hypoxia is an extensively studied process in HNSCC and has been shown to induce genetic instability, tumor cell aggression, and treatment failure. 40 , 41 Predominantly, endogenous hypoxia markers HIF‐1α and its down‐stream upregulated intracellular pH regulating CA‐IX have been under investigation concerning their relation with survival and locoregional control. 40 We observed that the number of cases with expression of both HIF‐1α and CA‐IX was significantly different between glottic and supraglottic LSCC (Table 3). In the literature, expression of CA‐IX was not investigated in glottic and supraglottic LSCC separately, whereas two reports on expression of HIF‐1α did not find a difference between these sublocalizations. 42 , 43 This discordance with our data might be explained by the fact that these two studies are composed of T1‐T4 LSCC with a relatively low number of T1‐T2 cases (n = 41 and n = 63, respectively). Both studies used a cut‐off value of 10%. Our predetermined cut‐off value was 0.5%, as in our earlier studies, based on its relation with treatment response. Yet, when a cut‐off value of 10% was applied, results remained similar (HR 2.88 CI, 1.24–6.69, P = .014, data not shown). The distribution of the expression of hypoxia markers is a good explanation that the hypoxia markers HIF‐1α and CA‐IX were predictive for local control in glottic LSCC, 7 but not in supraglottic LSCC. 4 Because tumor hypoxia can be counteracted by breathing carbogen (98% O2 and 2% CO2) and nicotinamide (a vasoactive drug) during radiotherapy 44 to improve treatment response, the localization of squamous cell carcinomas in the larynx should be taken into account in treatment decision making.
In HNSCC, amplification of chromosome region 11q13.3 is a frequently observed event 45 , 46 and is associated with poor prognosis. 47 Genes for FADD, Cyclin D1, and Cortactin are located within this region, resulting in frequent overexpression in almost all HNSCC with this amplification. 48 Although FADD and pFADD expression did not show significant differences in our glottic and supraglottic cohort, Cyclin D1 and Cortactin did. Comparative studies on FADD, pFADD, and Cortactin expression in glottic and supraglottic LSCC separately have not been published before, other than our previous publications. 6 , 8 Several studies on Cyclin D1 expression do report on glottic and supraglottic LSCC separately. 49 , 50 , 51 , 52 , 53 None of these studies revealed expression differences between tumor sublocalization, but the number of T1‐T2 LSCC cases in most of these study populations was rather small (n = 46, n = 75, n = 38, n = 21, and n = 8, respectively). Evaluation methods and cut‐off percentages in the literature referred to above, differed (three‐point scale, immunoreactive score, 5% cut‐off, 5‐point scale, 10% cut‐off, respectively). As in previous studies in LSCC, we utilized a cut‐off of 32.5%. 8
The AKT‐pathway is a complex signal transduction pathway that promotes cell cycle regulation in response to extracellular signals. Both pAKT and EGFR are key proteins in this pathway and the corresponding protein expression has been demonstrated to be of clinical significance in HNSCC. 54 , 55 For EGFR staining we utilized the methods of Pattje et al. 23 Complete circular membranous staining was considered high expression and no or incomplete circular membranous staining was considered low expression. Four reports analyzed the expression of EGFR in the LSCC sublocalizations separately, all using different staining evaluation methodologies. 38 , 52 , 56 , 57 Only one publication described a significantly increased frequency of tumors with high expression of EGFR in glottic LSCC. 38 In our cohorts, a significantly increased frequency of cases with high expression of EGFR was found in supraglottic LSCC. The population of Kourelis et al. was, however, too small to draw firm conclusions, containing only 18 glottic and 12 supraglottic early stage LSCC. Regarding the expression of pAKT, only Goulioumis et al. evaluated glottic and supraglottic LSCC separately and described no significantly differing expression pattern, 13 which is in agreement with our results. Cetuximab, a monoclonal antibody targeting the extracellular domain of EGFR, has been in use in clinical praxis in advanced stage HNSCC. 58 As high expression of EGFR was found particularly in supraglottic LSCC, anti‐EGFR treatment might be more effective in LSCC originating from the supraglottic larynx.
Ki‐67 is a good marker to quantify the degree of proliferative activity in neoplastic cells, since nuclear Ki‐67 is present in all phases of the cell cycle, but not in the G0 phase (resting cells). 59 The nuclear expression of Ki‐67 was significantly higher in our supraglottic cohort compared to our glottic cohort, confirming the findings of Tamas et al. 60 There are publications without significant association between Ki‐67 expression and LSCC sublocalization as well. 61 , 62 Study populations existed of T1‐T4 or unknown stage LSCC and differing evaluation methods were used, complicating a firm comparison.
In LSCC, loss of expression of the membrane protein β‐Catenin, has been associated with tumor dedifferentiation and a decrease in disease‐specific survival. 22 , 63 In a group of 46 glottic and 12 early stage supraglottic LSCC loss of membranous β‐Catenin expression was associated with supraglottic sublocalization. 13 Moreover, a combined cytoplasmic and membranous β‐Catenin expression was associated with supraglottic sublocalization in a population of 32 LSCC of unknown TNM stage. 11 In our LSCC cohorts β‐Catenin did not demonstrate a sublocalizational difference, following the results of Greco et al. 22
CONCLUSION
Our data on protein expression patterns and clinicopathological features in a well‐defined cohort of 276 early stage LSCC suggest that supraglottic and glottic LSCC should be considered as different entities. This is in agreement with differences observed in tumor response upon radiotherapy and clinical outcome. Our findings imply that results of reported studies that have included both glottic and supraglottic LSCC should be interpreted with caution. In future studies, we strongly recommend to evaluate supraglottic and glottic LSCC separately. Ultimately, these plausible biological differences between glottic and supraglottic LSCC may lead to more differential treatment schedules in (subpopulations of) glottic and supraglottic LSCC.
Supporting information
Table S1 Details on immunohistochemical staining methods.
Table S2. Description of follow‐up data, stratified by supraglottic (n = 80) and glottic LSCC (n = 196).
Editor's Note: This Manuscript was accepted for publication on December 31, 2020.
The authors have no conflicts of interest to declare.
Funding: A grant from the Jan Kornelis de Cock Foundation (Groningen, The Netherlands); epidemiological data by Dr. B. van Dijk (Integraal Kankercentrum Noord Oost, Groningen, The Netherlands).
BIBLIOGRAPHY
- 1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. van Dijk BA, Karim‐Kos HE, Coebergh JW, Marres HA, de Vries E. Progress against laryngeal cancer in the Netherlands between 1989 and 2010. Int J Cancer 2014;134:674‐681. [DOI] [PubMed] [Google Scholar]
- 4. Wachters JE, Schrijvers ML, Slagter‐Menkema L, et al. Prognostic significance of HIF‐1a, CA‐IX, and OPN in T1‐T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope 2013;123:2154‐2160. [DOI] [PubMed] [Google Scholar]
- 5. Yeh DH, Sahovaler A, Fung K. Reconstruction after salvage laryngectomy. Oral Oncol 2017;75:22‐27. [DOI] [PubMed] [Google Scholar]
- 6. Wachters JE, Schrijvers ML, Slagter‐Menkema L, et al. Phosphorylated FADD is not prognostic for local control in T1‐T2 supraglottic laryngeal carcinoma treated with radiotherapy. Laryngoscope 2017;127:E301‐E307. [DOI] [PubMed] [Google Scholar]
- 7. Schrijvers ML, van der Laan BF, de Bock GH, et al. Overexpression of intrinsic hypoxia markers HIF1alpha and CA‐IX predict for local recurrence in stage T1‐T2 glottic laryngeal carcinoma treated with radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:161‐169. [DOI] [PubMed] [Google Scholar]
- 8. Schrijvers ML, Pattje WJ, Slagter‐Menkema L, et al. FADD expression as a prognosticator in early‐stage glottic squamous cell carcinoma of the larynx treated primarily with radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 9. Bredell MG, Ernst J, El‐Kochairi I, Dahlem Y, Ikenberg K, Schumann DM. Current relevance of hypoxia in head and neck cancer. Oncotarget 2016;7:50781‐50804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Miguel‐Luken MJ, Chaves‐Conde M, Carnero A. A genetic view of laryngeal cancer heterogeneity. Cell Cycle 2016;15:1202‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galera‐Ruiz H, Ríos‐Moreno MJ, González‐Cámpora R, et al. The cadherin‐catenin complex in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 2012;269:1183‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruine DB, Wachters JE, Schrijvers ML, et al. PTEN is associated with worse local control in early stage supraglottic laryngeal cancer treated with radiotherapy. Laryngoscope Investig Otolaryngol 2019;4:399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goulioumis AK, Varakis J, Goumas P, Papadaki H. Differential beta‐catenin expression between glottic and supraglottic laryngeal carcinoma. Eur Arch Otorhinolaryngol 2010;267:1573‐1578. [DOI] [PubMed] [Google Scholar]
- 14. Ongkeko WM, Altuna X, Weisman RA, Wang‐Rodriguez J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am J Clin Pathol 2005;124:71‐76. [DOI] [PubMed] [Google Scholar]
- 15. Moreno‐Galindo C, Hermsen M, García‐Pedrero JM, Fresno MF, Suárez C, Rodrigo JP. p27 and BCL2 expression predicts response to chemotherapy in head and neck squamous cell carcinomas. Oral Oncol 2014;50:128‐134. [DOI] [PubMed] [Google Scholar]
- 16. Rodrigo JP, Martínez P, Allonca E, et al. Immunohistochemical markers of distant metastasis in laryngeal and hypopharyngeal squamous cell carcinomas. Clin Exp Metastasis 2014;31:317‐325. [DOI] [PubMed] [Google Scholar]
- 17. Re M, Zizzi A, Ferrante L, et al. p63 and Ki‐67 immunostainings in laryngeal squamous cell carcinoma are related to survival. Eur Arch Otorhinolaryngol 2014;271:1641‐1651. [DOI] [PubMed] [Google Scholar]
- 18. Rademakers SE, Hoogsteen IJ, Rijken PF, et al. Prognostic value of the proliferation marker Ki‐67 in laryngeal carcinoma: results of the accelerated radiotherapy with carbogen breathing and nicotinamide phase III randomized trial. Head Neck 2015;37:171‐176. [DOI] [PubMed] [Google Scholar]
- 19. Cordes C, Münzel A, Rudolph P, Hoffmann M, Leuschner I, Gottschlich S. Immunohistochemical staining of Ki‐67 using the monoclonal antibody Ki‐s11 is a prognostic indicator for laryngeal squamous cell carcinoma. Anticancer Res 2009;29:1459‐1465. [PubMed] [Google Scholar]
- 20. Fischer CA, Jung M, Zlobec I, et al. Co‐overexpression of p21 and Ki‐67 in head and neck squamous cell carcinoma relative to a significantly poor prognosis. Head Neck 2011;33:267‐273. [DOI] [PubMed] [Google Scholar]
- 21. Nikkuni O, Kaira K, Toyoda M, et al. Expression of amino acid transporters (LAT1 and ASCT2) in patients with stage III/IV laryngeal squamous cell carcinoma. Pathol Oncol Res 2015;21:1175‐1181. [DOI] [PubMed] [Google Scholar]
- 22. Greco A, De Virgilio A, Rizzo MI, Pandolfi F, Rosati D, de Vincentiis M. The prognostic role of E‐cadherin and β‐catenin overexpression in laryngeal squamous cell carcinoma. Laryngoscope 2016;126:148. [DOI] [PubMed] [Google Scholar]
- 23. Pattje WJ, Schuuring E, Mastik MF, et al. The phosphatase and tensin homologue deleted on chromosome 10 mediates radiosensitivity in head and neck cancer. Br J Cancer 2010;102:1778‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gold KA, Kim ES. Role of molecular markers and gene profiling in head and neck cancers. Curr Opin Oncol 2009;21:206‐211. [DOI] [PubMed] [Google Scholar]
- 25. Ow TJ, Pitts CE, Kabarriti R, Garg MK. Effective biomarkers and radiation treatment in head and neck cancer. Arch Pathol Lab Med 2015;139:1379‐1388. [DOI] [PubMed] [Google Scholar]
- 26. Lampri ES, Chondrogiannis G, Ioachim E, et al. Biomarkers of head and neck cancer, tools or a Gordian knot? Int J Clin Exp Med 2015;8:10340‐10357. [PMC free article] [PubMed] [Google Scholar]
- 27. Bruine de Bruin L, Schuuring E, de Bock GH, et al. High expression of DNA damage response marker pATM is associated with poor response to radiotherapy in early stage laryngeal squamous cell carcinoma. submitted for publication.
- 28. Silvestri F, Bussani R, Stanta G, Cosatti C, Ferlito A. Supraglottic versus glottic laryngeal cancer: epidemiological and pathological aspects. ORL J Otorhinolaryngol Relat Spec 1992;54:43‐48. [DOI] [PubMed] [Google Scholar]
- 29. Grenman R, Pekkola‐Heino K, Kinnala P. The incidence of laryngeal cancer by anatomical site in south‐western Finland. Eur Arch Otorhinolaryngol 1996;253:377. [DOI] [PubMed] [Google Scholar]
- 30. Li X, Izumaru S, Sakamoto K, Miyajima Y, Nakashima T. The immunohistochemical expression of p21WAF1/Cip1 and proliferating cell nuclear antigen in laryngeal squamous cell carcinomas. J Laryngol Otol 2006;120:1042‐1048. [DOI] [PubMed] [Google Scholar]
- 31. Hashibe M, Boffetta P, Zaridze D, et al. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol 2007;165:814‐820. [DOI] [PubMed] [Google Scholar]
- 32. De Stefani E, Boffetta P, Deneo‐Pellegrini H, et al. Supraglottic and glottic carcinomas: epidemiologically distinct entities? Int J Cancer 2004;112:1065‐1071. [DOI] [PubMed] [Google Scholar]
- 33. La Vecchia C, Zhang ZF, Altieri A. Alcohol and laryngeal cancer: an update. Eur J Cancer Prev 2008;17:116‐124. [DOI] [PubMed] [Google Scholar]
- 34. Fried MP, Ferlito A. The Larynx. 3rd ed. II: Plural Publishing; Inc. San Diego CA: 2009. [Google Scholar]
- 35. Lesperance MM, Lund VJ. Cummings Otolaryngology ‐ Head and Neck Surgery. 5th Rev ed.: Amsterdam: The Netherlands, Elsevier ‐ Health Sciences Division; 2010. [Google Scholar]
- 36. Hirvikoski P, Virtaniemi J, Kumpulainen E, Johansson R, Kosma VM. Supraglottic and glottic carcinomas. Clinically and biologically distinct entities? Eur J Cancer 2002;38:1717‐1723. [DOI] [PubMed] [Google Scholar]
- 37. Ferlito A, Haigentz M, Bradley PJ, et al. Causes of death of patients with laryngeal cancer. Eur Arch Otorhinolaryngol 2014;271:425‐434. [DOI] [PubMed] [Google Scholar]
- 38. Kourelis K, Papadas T, Vandoros G, Goumas P, Sotiropoulou‐Bonikou G. Glottic versus supraglottic tumors: differential molecular profile. Eur Arch Otorhinolaryngol 2008;265:79‐84. [DOI] [PubMed] [Google Scholar]
- 39. Gioacchini FM, Alicandri‐Ciufelli M, Kaleci S, Magliulo G, Presutti L, Re M. The prognostic value of cyclin D1 expression in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 2016;273:801‐809. [DOI] [PubMed] [Google Scholar]
- 40. Swartz JE, Pothen AJ, Stegeman I, Willems SM, Grolman W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: a systematic review. Cancer Med 2015;4:1101‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoogsteen IJ, Marres HA, Bussink J, van der Kogel AJ, Kaanders JH. Tumor microenvironment in head and neck squamous cell carcinomas: predictive value and clinical relevance of hypoxic markers. A review. Head Neck 2007;29:591‐604. [DOI] [PubMed] [Google Scholar]
- 42. Li DW, Zhou L, Jin B, Xie J, Dong P. Expression and significance of hypoxia‐inducible factor‐1alpha and survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head Neck Surg 2013;148:75‐81. [DOI] [PubMed] [Google Scholar]
- 43. Wu XH, Chen SP, Mao JY, Ji XX, Yao HT, Zhou SH. Expression and significance of hypoxia‐inducible factor‐1alpha and glucose transporter‐1 in laryngeal carcinoma. Oncol Lett 2013;5:261‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Janssens GO, Rademakers SE, Terhaard CH, et al. Improved recurrence‐free survival with ARCON for anemic patients with laryngeal cancer. Clin Cancer Res 2014;20:1345‐1354. [DOI] [PubMed] [Google Scholar]
- 45. Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes‐a review. Gene 1995;159:83‐96. [DOI] [PubMed] [Google Scholar]
- 46. Freier K, Sticht C, Hofele C, et al. Recurrent coamplification of cytoskeleton‐associated genes EMS1 and SHANK2 with CCND1 in oral squamous cell carcinoma. Genes Chromosomes Cancer 2006;45:118‐125. [DOI] [PubMed] [Google Scholar]
- 47. Gollin SM. Cytogenetic alterations and their molecular genetic correlates in head and neck squamous cell carcinoma: a next generation window to the biology of disease. Genes Chromosomes Cancer 2014;53:972‐990. [DOI] [PubMed] [Google Scholar]
- 48. Gibcus JH, Menkema L, Mastik MF, et al. Amplicon mapping and expression profiling identify the Fas‐associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin Cancer Res 2007;13:6257‐6266. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed RA, Shawky A, Hamed RH. Prognostic significance of cyclin D1 and E‐cadherin expression in laryngeal squamous cell carcinoma. Pathol Oncol Res 2014;20:625‐633. [DOI] [PubMed] [Google Scholar]
- 50. Liu YF, Zhang JG, Ni HS, et al. Expression and significance of angiopoietin‐2 and cyclin D1 in laryngeal squamous cell carcinoma and the correlation with prognosis. Exp Ther Med 2013;6:1137‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morshed K, Skomra D, Korobowicz E, Szymanski M, Polz‐Dacewicz M, Golabek W. An immunohistochemical study of cyclin D1 protein expression in laryngeal squamous cell carcinoma. Acta Otolaryngol 2007;127:760‐769. [DOI] [PubMed] [Google Scholar]
- 52. Wildeman MA, Gibcus JH, Hauptmann M, et al. Radiotherapy in laryngeal carcinoma: can a panel of 13 markers predict response? Laryngoscope 2009;119:316‐322. [DOI] [PubMed] [Google Scholar]
- 53. Fu ZJ, Ma ZY, Wang QR, et al. Overexpression of CyclinD1 and underexpression of p16 correlate with lymph node metastases in laryngeal squamous cell carcinoma in Chinese patients. Clin Exp Metastasis 2008;25:887‐892. [DOI] [PubMed] [Google Scholar]
- 54. Keren S, Shoude Z, Lu Z, Beibei Y. Role of EGFR as a prognostic factor for survival in head and neck cancer: a meta‐analysis. Tumour Biol 2014;35:2285‐2295. [DOI] [PubMed] [Google Scholar]
- 55. Freudlsperger C, Horn D, Weissfuss S, et al. Phosphorylation of AKT(Ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int J Cancer 2015;136:2775‐2785. [DOI] [PubMed] [Google Scholar]
- 56. Nijkamp MM, Span PN, Terhaard CH, et al. Epidermal growth factor receptor expression in laryngeal cancer predicts the effect of hypoxia modification as an additive to accelerated radiotherapy in a randomised controlled trial. Eur J Cancer 2013;49:3202‐3209. [DOI] [PubMed] [Google Scholar]
- 57. Bussu F, Ranelletti FO, Gessi M, et al. Immunohistochemical expression patterns of the HER4 receptors in normal mucosa and in laryngeal squamous cell carcinomas: antioncogenic significance of the HER4 protein in laryngeal squamous cell carcinoma. Laryngoscope 2012;122:1724‐1733. [DOI] [PubMed] [Google Scholar]
- 58. Saada‐Bouzid E, Le Tourneau C. Beyond EGFR targeting in SCCHN: angiogenesis, PI3K, and other molecular targets. Front Oncol 2019;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kop E, de Bock GH, Noordhuis MG, et al. Standardised Ki‐67 proliferation index assessment in early‐stage laryngeal squamous cell carcinoma in relation to local control and survival after primary radiotherapy. Clin Otolaryngol 2020;45:12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tamas L, Szentkuti G, Eros M, et al. Differential biomarker expression in head and neck cancer correlates with anatomical localization. Pathol Oncol Res 2011;17:721‐727. [DOI] [PubMed] [Google Scholar]
- 61. de Oliveira DE, Bacchi MM, Macarenco RS, Tagliarini JV, Cordeiro RC, Bacchi CE. Human papillomavirus and Epstein‐Barr virus infection, p53 expression, and cellular proliferation in laryngeal carcinoma. Am J Clin Pathol 2006;126:284‐293. [DOI] [PubMed] [Google Scholar]
- 62. Acikalin MF, Oner U, Tel N, Pasaoglu O, Cakli H, Colak E. Prognostic significance of Ki‐67 expression for patients with laryngeal squamous cell carcinoma primarily treated by total laryngectomy. Eur Arch Otorhinolaryngol 2004;261:376‐380. [DOI] [PubMed] [Google Scholar]
- 63. Lopez‐Gonzalez JS, Cristerna‐Sanchez L, Vazquez‐Manriquez ME, Jimenez‐Orci G, Aguilar‐Cazares D. Localization and level of expression of beta‐catenin in human laryngeal squamous cell carcinoma. Otolaryngol Head Neck Surg 2004;130:89‐93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Details on immunohistochemical staining methods.
Table S2. Description of follow‐up data, stratified by supraglottic (n = 80) and glottic LSCC (n = 196).


