Abstract
Associations between body mass index (BMI) and the cardiovascular (CV) and kidney efficacy of glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) in patients with type 2 diabetes (T2D) are uncertain; therefore, data analysed separately from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial and the Trial to Evaluate Cardiovascular and Other Long‐term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6) were examined. These international, randomized, placebo‐controlled trials investigated liraglutide and semaglutide (both subcutaneous) in patients with T2D and at high risk of CV events. In post hoc analyses, patients were categorized by baseline BMI (<25, ≥25‐<30, ≥30‐<35 and ≥35 kg/m2), and CV and kidney outcomes with GLP‐1 RA versus placebo were analysed. All baseline BMI data from LEADER (n = 9331) and SUSTAIN 6 (n = 3290) were included (91% and 92% of patients with overweight or obesity, respectively). In SUSTAIN 6, nominally significant heterogeneity of semaglutide efficacy by baseline BMI was observed for CV death/myocardial infarction/stroke (major adverse CV events, primary outcome of both; P interaction = .02); otherwise, there was no statistical heterogeneity for either GLP‐1 RA versus placebo across BMI categories for key CV and kidney outcomes. The lack of statistical heterogeneity from these cardiorenal outcomes implies that liraglutide and semaglutide may be beneficial for many patients and is probable not to depend on their baseline BMI, but further study is needed.
Keywords: body mass index, cardiovascular, liraglutide, major adverse cardiovascular events, semaglutide
1. INTRODUCTION
While some glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) have been shown to reduce major adverse cardiovascular (CV) events (MACE) in people with type 2 diabetes (T2D), they are also a recommended treatment when there is a compelling need for such patients to lose weight. 1 , 2 Several factors have been linked to these reported CV benefits, but their precise roles are unknown. 3 One such factor is baseline body mass index (BMI), whose impact has only been investigated on limited treatment outcomes with GLP‐1 RAs. 4 Although weight loss associated with GLP‐1 RA use increases with increasing BMI, 4 it is unknown if other effects vary by BMI. We investigated if the CV and kidney outcomes with GLP‐1 RAs are consistent across the spectrum of BMI, using data from the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER 5 ) trial and the Trial to Evaluate Cardiovascular and Other Long‐term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN 6 6 ) analysed separately.
2. MATERIALS AND METHODS
2.1. Study design
The LEADER and SUSTAIN 6 trial designs have been published. 5 , 6 In brief, both trials were double‐blind and placebo‐controlled. Patients with T2D and at high risk of CV events were randomly assigned to the GLP‐1 RA or placebo (once‐daily subcutaneous [s.c.] liraglutide 1.8 mg or maximum tolerated dose vs. placebo in LEADER, 1:1 ratio; once‐weekly s.c. semaglutide 0.5 or 1.0 mg vs. volume‐matched placebo in SUSTAIN 6, 1:1:1:1 ratio [pooled as semaglutide vs. placebo for analyses]), with all patients otherwise treated according to standard of care. 5 , 6 Key inclusion criteria in both trials were being aged 50 years or older with established CV disease (previous coronary, cerebrovascular or peripheral vascular disease), heart failure (New York Heart Association class II or III), or chronic kidney disease stage 3 or higher; or being aged 60 years or older with at least one CV risk factor (microalbuminuria or proteinuria, hypertension with left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle–brachial index of <0.9). 5 , 6 Major exclusion criteria included use of GLP‐1 RAs, dipeptidyl peptidase‐4 inhibitors, pramlintide or rapid‐acting insulin, and recent history of an acute coronary or cerebrovascular event. 5 , 6
The primary composite outcome in both trials was first occurrence of MACE (CV death, non‐fatal myocardial infarction or non‐fatal stroke). The key secondary expanded outcome (expanded MACE) also included hospitalization for unstable angina or heart failure, or revascularization. The secondary composite renal outcome (termed nephropathy) was comprised of new‐onset or persistent macroalbuminuria, persistent doubling of serum creatinine level and creatinine clearance of less than 45 mL/min/1.73m2, the need for continuous renal‐replacement therapy or death from kidney disease. Both trials were approved by institutional review boards or ethics committees for each centre; all patients provided written informed consent. 5 , 6
Weight and height were measured by investigators at baseline and BMI was calculated. BMI was also assessed at designated visits throughout both trials. 5 , 6
2.2. Statistical methods
Details of the primary statistical analyses conducted in these trials have been described. 5 , 6 For the present post hoc analyses, the effects of liraglutide and semaglutide on the time‐to‐first primary MACE, expanded MACE, CV death and nephropathy were evaluated by baseline BMI category, separately for the two trials. BMI was categorized based on cut‐off values described by the World Health Organization (<25, ≥5 to <30, ≥30 to <35 and ≥35 kg/m2, defining overweight as BMI ≥25 kg/m2 and obesity as BMI ≥30 kg/m2). 7 The significance of the differences between the baseline characteristics across these BMI categories was assessed using a Kruskal–Wallis test for continuous variables and a chi‐square test for categorical variables regardless of treatment group. The Cochran–Armitage trend test was used to analyse event rates across BMI groups in the placebo groups of both trials. The hazard ratios (HRs) and 95% confidence intervals (CIs) for treatment versus placebo were calculated using Cox proportional hazard regression models with treatment and BMI category as fixed factors and included a treatment‐by‐BMI term to test for quantitative interaction between both. The models were adjusted for baseline characteristics related to cardiorenal risk (sex, smoking status, antihyperglycaemic treatments, prior CV events, geographic region, age, diabetes duration, estimated glomerular filtration rate), with a P‐interaction of less than .05 considered significant. No adjustments for multiple testing were performed.
Quadratic spline regression was applied using Cox proportional hazard regression to analyse treatment differences in time‐to‐first MACE by continuous baseline BMI. The percentage weight loss by BMI category was calculated over 3 years for LEADER and 104 weeks for SUSTAIN 6, including P‐interaction for both. All analyses were performed using the software package SAS (version 9.4).
3. RESULTS
The disposition and baseline characteristics of trial participants have been published. 5 , 6 In LEADER, a total of 9340 patients were randomized (4668 to liraglutide; 4672 to placebo), with a median follow‐up of 3.8 years. 5 In SUSTAIN 6, 3297 patients were randomized (1648 to semaglutide; 1649 to placebo), with a median follow‐up of 2.1 years. 6
The proportions of patients in LEADER with a baseline BMI of less than 25 kg/m2, of 25 to less than 30 kg/m2, of 30 to less than 35 kg/m2, and of 35 kg/m2 or higher, were 9%, 29%, 32%, and 30%, respectively, and in SUSTAIN 6 these were 8%, 28%, 33%, and 31%, respectively ( Table S1). Baseline characteristics varied across the BMI categories within each trial ( Table S1). Notably, in LEADER, the mean diabetes duration was longest in the BMI less than 25 kg/m2 category versus the other BMI categories, with a similar trend in SUSTAIN 6. As expected, a greater percentage of patients were treated with insulin at baseline with increasing baseline BMI in both trials ( Table S1). The percentage of patients with established CV disease was similar across the BMI categories in LEADER (P = .30; range: 80.3%‐82.2%; Table S1), while, in SUSTAIN 6, it differed (P = .02; range: 78.7%‐84.7%; Table S1). Within both trials, the mean estimated glomerular filtration rates were similar across the BMI categories (P = .27 for LEADER; P = .14 for SUSTAIN 6; Table S1).
The placebo event rates for MACE, expanded MACE and CV death were similar across BMI categories within each trial ( Table S2). In SUSTAIN 6, the risk of nephropathy declined with increasing BMI category (P trend = .0002) and, although the nephropathy event rate declined in LEADER, it did not reach significance (P trend = .18) ( Table S2).
When analysing data from the treatment groups, only the interaction for MACE in SUSTAIN 6 showed significance; for all others, there was no statistically significant heterogeneity of the treatment effects of liraglutide or semaglutide versus placebo across baseline BMI groups (Figure 1). Correspondingly, P‐interaction values for treatment‐by‐BMI for MACE, expanded MACE and CV death in LEADER were .34, .22 and .79, respectively; and in SUSTAIN 6 these were .02, .27 and .82, respectively.
FIGURE 1.
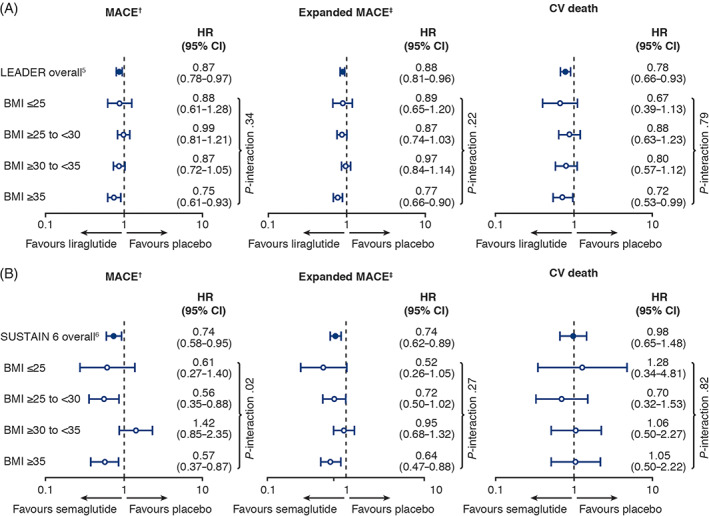
Cardiovascular outcomes by baseline body mass index category in A, LEADER and B, SUSTAIN 6. Primary and expanded MACE analyses adjusted for sex, smoking status, antihyperglycaemic treatments, prior cardiovascular (CV) events, geographic region, age, diabetes duration, estimated glomerular filtration rate. Smoking status was not adjusted for in the SUSTAIN 6 analysis for CV death because of low event numbers. †Primary major adverse cardiovascular events (MACE): composite of CV death, non‐fatal myocardial infarction (MI) and non‐fatal stroke. ‡Expanded MACE: components of primary MACE plus revascularization (coronary only in LEADER; coronary or peripheral in SUSTAIN 6) or hospitalization for unstable angina pectoris or heart failure. BMI, body mass index (in kg/m2); CI, confidence interval; HR, hazard ratio
For new‐onset or worsening nephropathy, there was no heterogeneity of treatment efficacy across the BMI categories, with P‐interaction values of .92 for LEADER and .21 for SUSTAIN 6 (Figure 2).
FIGURE 2.
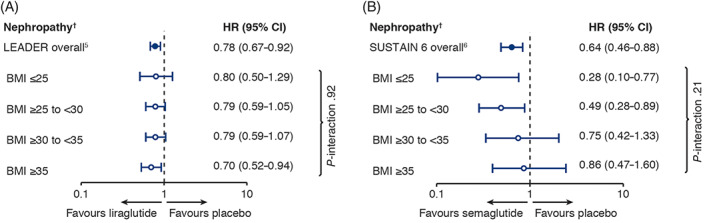
Renal outcomes by baseline body mass index category in A, LEADER and B, SUSTAIN 6. LEADER analysis adjusted for sex, smoking status, antihyperglycaemic treatments, prior cardiovascular events, geographic region, age, diabetes duration, estimated glomerular filtration rate. SUSTAIN 6 analysis adjusted for sex, antihyperglycaemic treatments, prior cardiovascular events, geographic region, age, diabetes duration, estimated glomerular filtration rate (smoking status was omitted because of low event numbers). †Nephropathy: new or persistent macroalbuminuria, doubling of serum creatinine, creatinine clearance of less than 45 mL/min/1.73m2, end‐stage kidney disease or death from kidney disease. BMI, body mass index (in kg/m2); CI, confidence interval; HR, hazard ratio
In the regression analysis of baseline BMI as a continuous variable, liraglutide showed consistent benefits across BMI categories in analysis of time‐to‐first MACE, within the quartile boundaries, where 50% of the events occurred. Semaglutide also showed similar results across baseline BMI values for MACE (Figure S1).
There was no significant interaction between treatment and BMI category for percentage weight loss with either liraglutide (P interaction = .07; Figure S2A) or semaglutide (P interaction = .51; Figure S2B).
4. DISCUSSION
The present results of post hoc analyses from LEADER and SUSTAIN 6 show that there was no heterogeneity in the CV and renal benefits of liraglutide and semaglutide versus placebo across the spectrum of baseline BMI evaluated either categorically or continuously, excepting a nominally significant interaction observed by baseline BMI category for the effect of semaglutide on MACE. These data should be considered by prescribers when choosing these agents for CV risk reduction in appropriate patients.
The exact nature of the relationship between any baseline characteristic, including BMI, and CV benefit of liraglutide and semaglutide (via glycaemic control and/or weight loss and/or other mechanisms) remains difficult to establish, 3 , 8 with published meta‐analysis results showing that baseline BMI was not associated with achieved glycaemic control across seven different antihyperglycaemic treatments. 9 Thus, the dose–response curves for any treatment may differ for MACE, glucose levels and weight, and our analyses have shown that there appeared to be generally no effect of baseline BMI on MACE.
Also, prior data evaluating the associations of weight loss on CV outcomes are varied. The Look AHEAD trial randomized patients with overweight/obesity and T2D to intensive lifestyle (diet and exercise) intervention versus control. 10 Despite significantly greater weight loss achieved in the intervention group, there was no significant difference in CV disease‐related morbidity and mortality. 10 Conversely, in the Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes) trial that randomized patients with T2D at high CV risk to the GLP‐1 RA albiglutide or placebo, a statistically significant 22% reduction in first occurrence of CV death, myocardial infarction or stroke (HR, 0.78 [95% CI 0.68; 0.90]) was observed with albiglutide versus placebo. While weight loss was marginally greater in the albiglutide group versus placebo at 8 and 16 months, the differences were less than 1 kg (−0.66 and − 0.83 kg, respectively), and at 28 months, weight in both the placebo and albiglutide groups was similar to their baseline values. 11 Yet another type of association was evident in the Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial, in which treatment of patients with T2D with dulaglutide resulted in a significant decrease in CV events (HR, 0.88 [95% CI 0.79; 0.99]) and a significant decrease in body weight (−1.46 kg [95% CI 1.25; 1.67]) versus placebo. 12 Mediation analyses utilizing data from such trials may provide evidence as to how weight loss impacts upon CV risk, but to date, it appears that the size of any such mediation of body weight on CV outcomes may be small with liraglutide. 13
The many mechanisms that have been proposed to underlie the cardioprotective effects of GLP‐1 RAs are complex. They include anti‐inflammatory effects, attenuation of cardiac ischaemic injury through a variety of direct and indirect actions on the myocardium and coronary arteries, modification of lipid synthesis and secretion, and improvement in endothelial dysfunction, among others. 3 For example, in one study, liraglutide and semaglutide reduced plaque lesion development through altering inflammatory pathways in mouse models of atherosclerosis. 14 These pathways could be involved in the significant improvements in the carotid intima‐media thickness of patients who were treated with liraglutide for 8 months versus baseline. 15 Such cardioprotective mechanisms of GLP‐1 RAs appear to be independent of the lipid levels of patients. 15
The renal protective effects of GLP‐1 RAs have been less well studied than the cardioprotective effects, and may be linked to renal tubular effects, oxidative stress and haemodynamic effects. 16 For liraglutide and semaglutide, renal benefits were found in LEADER and SUSTAIN 6, where they were investigated as secondary, composite endpoints. 5 , 6 Analysis of the nephropathy components revealed that the renal benefits were driven by new or persistent macroalbuminuria. 6 , 17 Within our post hoc analyses, the renal benefit with semaglutide appeared to decrease with increasing BMI, but this effect modification by BMI status was not statistically significant. However, in the placebo‐treated population of SUSTAIN 6, it was evident that nephropathy decreased with increasing BMI, which may seem counterintuitive, but fits with some studies of patients with chronic kidney disease and end‐stage renal disease. 18 The reason for the discrepancy between the LEADER and SUSTAIN 6 data in this particular regard remains unknown, but could be related to any of the baseline characteristics that varied by BMI category in SUSTAIN 6, but not in LEADER (e.g. established CV disease).
There were limitations to this study. These were post hoc analyses with numerous potential confounding factors (including not being powered to assess efficacy for CV and renal outcomes across baseline BMI strata and being of comparatively short follow‐up), and the analyses were not adjusted for differences in insulin, sodium‐glucose co‐transporter‐2 inhibitor and CV medication use. Baseline BMI categories were not corrected for application to Asian patients, who comprised 9.6% of the study population, and the BMI categories were not protected by the trial randomization, resulting in heterogeneous subgroups. Only baseline BMI was analysed, and results were more consistent with the larger, postapproval LEADER trial compared with the smaller, preapproval SUSTAIN 6 trial. With just one of the many interaction tests yielding a nominally significant P‐value, the validity of this finding is uncertain and may be a spurious finding as these analyses were post hoc and did not include correction for multiplicity of testing. Given limited power in the present analyses for interaction testing, we are not able to exclude the possibility of effect modification by BMI. These analyses used data pertaining to liraglutide and semaglutide only; further analyses with datasets utilizing other GLP‐1 RA data will help clinicians to understand if a class effect underpins these results. Although pooling data from the two trials may have increased the power of this analysis, because of the larger size of LEADER versus SUSTAIN 6, we chose to analyse the data separately, to provide a clear indication of what happened with each treatment.
In conclusion, these results from post hoc analyses of the LEADER and SUSTAIN 6 trials suggest that there are consistent CV and renal benefits of liraglutide and semaglutide across baseline BMI categories in patients with T2D and high CV risk, but they need to be confirmed in future studies.
CONFLICT OF INTEREST
SV reports personal fees and other from Boehringer Ingelheim, Eli Lilly, AstraZeneca, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Valeant, Amgen, Sun Pharma and HLS Therapeutics. DKM reports consultancy fees for clinical trial leadership from AstraZeneca, Sanofi Aventis, Janssen, Boehringer Ingelheim, Merck & Co, Pfizer, Lilly US, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline and Esperion; consultancy fees from AstraZeneca, Sanofi Aventis, Lilly US, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Afimmune and Metavant. SCB reports personal fees and other from Abbott, AstraZeneca, Boehringer Ingelheim, BMS, Cellnovo, Diartis, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi‐Aventis, Schering‐Plough, Servier and Takeda; other from Cardiff University, Doctors.net, Elsevier, Onmedica, Omnia‐Med, Medscape, All‐Wales Medicines Strategy Group, National Institute for Health and Care Excellence (NICE) UK and Glycosmedia. DLB discloses the following relationships: advisory board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Level Ex, MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); site co‐investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; trustee: American College of Cardiology; unfunded research: FlowCo, Merck, Novo Nordisk, Takeda. LAL reports consultant and speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier; research grant or support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk and Sanofi. CDM reports research grants to institution and/or consulting honoraria from Amgen, Boehringer Ingelheim, CSL Behring, OctaPharma and Quark Pharmaceuticals. TMF and SR are employees of and shareholders in Novo Nordisk. HV was an employee of Novo Nordisk during the development of this manuscript. REP reports research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Sanofi‐Aventis US LLC and Takeda; speaker for AstraZeneca, Novo Nordisk and Takeda; consultant for AstraZeneca, Boehringer Ingelheim, Eisai, Inc., GlaxoSmithKline, Janssen Scientific Affairs LLC, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Pfizer and Takeda. All payments made directly to his employer (Florida Hospital/AdventHealth). BZ reports personal fees from Merck, Sanofi‐Aventis, Eli Lilly, AstraZeneca and Janssen; personal fees and other from Novo Nordisk and Boehringer Ingelheim. JBB's contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics and Zafgen; he reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Novo Nordisk, Sanofi, Theracos, Tolerion and vTv Therapeutics; he is a consultant to Cirius Therapeutics Inc, CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics and Stability Health; he holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio and Stability Health; and he is supported by grants from the National Institutes of Health (UL1TR002489, U01DK098246, UC4DK108612, U54DK118612), PCORI and ADA.
AUTHOR CONTRIBUTIONS
Design (of the post hoc analysis, not the trial): all authors. Conduct/data collection (all investigators on LEADER or SUSTAIN 6): LAL, SCB, JBB, REP and BZ. Analysis: SR. All the authors had access to the final study results. SV and DKM contributed equally to writing this manuscript. SV wrote the first draft of the paper, which was edited significantly by DKM and subsequently reviewed and approved by all authors, who also assume responsibility for its content.
DATA‐SHARING STATEMENT
Data supporting these analyses are available from the corresponding author on reasonable request.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
The authors thank Emre Yildirim (Novo Nordisk) for reviewing this manuscript. Editorial and submission support were provided by Gillian Groeger, PhD, and Izabel James, MBBS, of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. The LEADER and SUSTAIN 6 trials were sponsored by Novo Nordisk and are registered with ClinicalTrials.gov (NCT01179048 and NCT01720446).
Verma S, McGuire DK, Bain SC, et al. Effects of glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: Results of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:2487–2492. 10.1111/dom.14160
Funding information The LEADER and SUSTAIN 6 trials were sponsored by Novo Nordisk and are registered with ClinicalTrials.gov (NCT01179048 and NCT01720446).
REFERENCES
- 1. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of medical Care in Diabetes‐2020. Diabetes Care. 2020;43:S98‐S110. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. 10. Cardiovascular Disease and Risk Management . Standards of medical Care in Diabetes‐2020. Diabetes Care. 2020;43:S111‐S134. [DOI] [PubMed] [Google Scholar]
- 3. Sharma A, Verma S. Mechanisms by which glucagon‐like‐peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44:93‐102. [DOI] [PubMed] [Google Scholar]
- 4. Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States: a retrospective cohort study. Adv Ther. 2014;31:986‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. WHO Technical Report Series. Vol 894; 2000. https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed] [Google Scholar]
- 8. Vansteelandt S, Linder M, Vandenberghe S, Steen J, Madsen J. Mediation analysis of time‐to‐event endpoints accounting for repeatedly measured mediators subject to time‐varying confounding. Stat Med. 2019;38:4828‐4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai X, Yang W, Gao X, Zhou L, Han X, Ji L. Baseline body mass index and the efficacy of hypoglycemic treatment in type 2 diabetes: a meta‐analysis. PLoS One. 2016;11:e0166625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutton GR, Lewis CE. The look AHEAD trial: implications for lifestyle intervention in type 2 diabetes mellitus. Prog Cardiovasc Dis. 2015;58:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 12. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 13. Buse JB, Bain SC, Mann JFE, Nauck MA, et al. Cardiovascular risk reduction with Liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43:1546‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rakipovski G, Rolin B, Nohr J, et al. The GLP‐1 analogs Liraglutide and Semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizzo M, Chandalia M, Patti AM, et al. Liraglutide decreases carotid intima‐media thickness in patients with type 2 diabetes: 8‐month prospective pilot study. Cardiovasc Diabetol. 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yin WL, Bain SC, Min T. The effect of glucagon‐like peptide‐1 receptor agonists on renal outcomes in type 2 diabetes. Diabetes Ther. 2020;11:835‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 18. Naderi N, Kleine CE, Park C, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61:168‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


