Abstract
Guidance from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency recommends using Child‐Pugh classification for pharmacokinetic evaluation in noncancer subjects with hepatic impairment (HI). Therefore, dosing recommendations for oncology compounds for patients with HI are commonly based on Child‐Pugh classification. In oncology clinical practice, National Cancer Institute classification (NCIc), is commonly used for evaluating hepatic function and dosing decisions for oncology patients. This work evaluated the discordance between the 2 systems and the impact on dosing recommendations. The classification system in HI studies was reviewed for FDA‐approved oncology compounds. Discordance between Child‐Pugh and NCIc was evaluated for sunitinib, dacomitinib, palbociclib, bosutinib, and axitinib. Pharmacokinetic (PK) analyses were conducted based on Child‐Pugh classification and NCIc. Review of 117 approved oncology compounds showed prevalent use of Child‐Pugh classification for dedicated HI studies in noncancer subjects. NCIc is commonly used in cancer patient studies. NCIc tended to classify subjects as less impaired versus Child‐Pugh (64.9%, 73.7%, and 61.5% of subjects with mild, moderate, and severe HI, respectively, via Child‐Pugh were classified as at least 1 category less impaired via NCIc). PK analyses by NCIc were consistent with Child‐Pugh for sunitinib, dacomitinib, and palbociclib. For bosutinib, NCIc showed less impact of HI than Child‐Pugh; an opposite trend was observed for axitinib. The impact of this considerable discordance between the 2 systems on dosing decisions bears consideration. When Child‐Pugh is used for HI study enrollment, exploratory PK analyses based on NCIc should be conducted. Prescribers should attempt to use the same classification system in the product label for dosing decisions.
Keywords: Child‐Pugh classification, clinical pharmacology, hepatic impairment, National Cancer Institute classification, oncology
Efficacy and safety of new anticancer agents are often established in clinical studies that exclude patients with advanced degrees of organ impairment. 1 , 2 Therefore, dedicated hepatic impairment (HI) studies are conducted to compare drug exposure in subjects with varying degrees of liver dysfunction with a control group with normal hepatic function. The results from these studies are interpreted in the context of the exposure‐safety and exposure‐efficacy relationships to guide dosing recommendation for oncology compounds in these subpopulations. Regulatory guidance recommends conducting HI studies when drug pharmacokinetics (especially metabolism and biliary excretion) are expected to be significantly altered in patients with liver dysfunction or for drugs with a relatively narrow therapeutic index. 3 , 4 , 5
In noncancer subjects, the most common causes of liver disease include hepatitis B or C, alcoholic liver disease, and nonalcoholic fatty liver disease. 6 Regardless of etiology, liver fibrosis is a generic wound‐healing response to chronic liver disease. 7 Progressive liver fibrosis can eventually lead to cirrhosis, end‐stage liver disease, hepatocellular carcinoma (HCC), and death. 8 In contrast to progressive liver deterioration because of cirrhosis, hepatic insufficiency in cancer patients, other than HCC, is generally a result of metastatic spread of the primary tumor to or near the liver, a common site of metastasis. 9 Liver damage in cancer patients could also be from toxicities associated with anticancer treatment, as well as biliary obstruction or portal vein thrombosis. 10 , 11 , 12
The Child‐Pugh classification assesses hepatic function by grouping subjects based on 2 clinical features (ascites and encephalopathy) and 3 laboratory‐based parameters (serum albumin, bilirubin, and prothrombin time or international normalized ratio) (Supplemental Table 1). 8 It was initially developed to predict operative mortality in patients undergoing portosystemic shunt surgery. 8 , 13 Guidance from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) on HI studies recommends using Child‐Pugh classification as the basis for enrollment and analysis in HI trials conducted in healthy subjects (ie, subjects with no underlying hepatic dysfunction used as a control group) and subjects with varying degrees of hepatic dysfunction but otherwise healthy. The guidance highlights that in cancer patients, hypoalbuminemia, encephalopathy, and ascites may be related to cancer cachexia or cancer metastases to the brain or peritoneal surfaces rather than impaired hepatic function and indicate that although other approaches to assess hepatic function might be appropriate, Child‐Pugh classification should still be evaluated for each subject. 3 , 4 Therefore, the product label of many oncology compounds provides dosing recommendations based on Child‐Pugh classification.
Another system to assess HI, specifically in oncology patients, was developed by the National Cancer Institute (NCI) Organ Dysfunction Working Group (ODWG) to guide chemotherapy dosing for NCI‐sponsored clinical trials. 14 , 15 The NCI classification system (NCIc) is a simpler method to implement that uses 2 laboratory‐based parameters to grade hepatic dysfunction: total bilirubin and aspartate aminotransferase (Supplemental Table 2). The use of readily available laboratory values by the NCIc makes it likely to be implemented in routine clinical practice for dosing decisions of approved oncology compounds, even though the dosing recommendations in a product label might be based on Child‐Pugh classification. 16
Given the differences in the parameters used for Child‐Pugh classification and NCIc, discordance in hepatic function classification between the 2 systems may occur as indicated in prior unpublished analyses. 17 , 18 This discrepancy may result in an incorrect prescribed dose when the dosing decision in clinical practice is based on a classification system different from that is used in the product label (Figure 1). A hypothetical example is assumed with a drug label that requires a 50% dose reduction for patients with moderate HI based on Child‐Pugh classification but no change for patients with mid HI (Figure 1). If NCIc, instead of Child‐Pugh classification, was used by the treating oncologist to assess a patient's hepatic function that will be used for dose adjustment related to HI, and the patient was classified as mild NCIc, instead of moderate Child‐Pugh classification, the full dose would be prescribed for this patient, which might result in overexposure and potentially increased adverse events (Figure 1).
Figure 1.
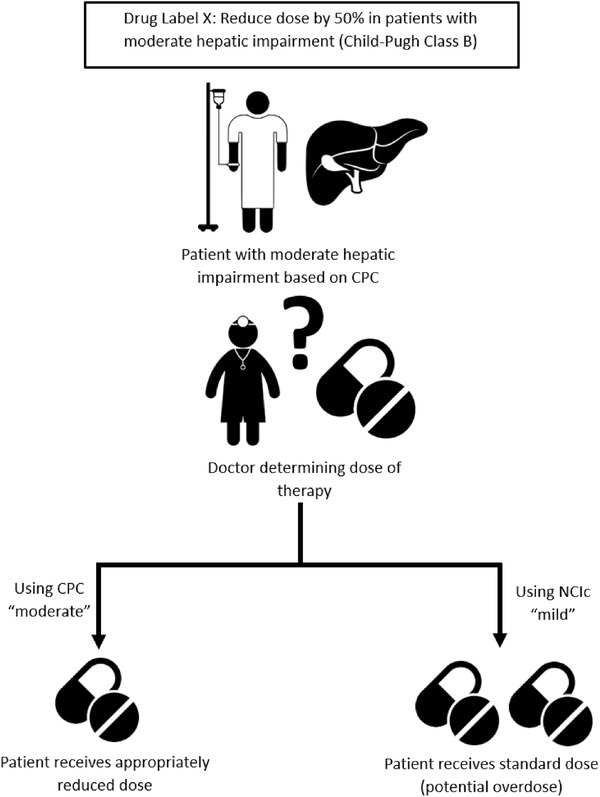
Schematic demonstrating potential for dosing errors based on the discordance between Child‐Pugh and NCI classifications.
In this study, we investigated the prevalence of using Child‐Pugh classification versus NCIc in HI studies for oncology compounds approved by the FDA (1999 to 2019), explored the discordance between the 2 classification systems for approved oncology compounds with dedicated HI studies in noncancer subjects within the Pfizer portfolio to date, and evaluated the implications of such discordance on pharmacokinetic (PK) analysis and dosing recommendations.
Methods
Survey of FDA Oncology Approvals
Clinical Pharmacology & Biopharmaceutics reviews, multidiscipline reviews, product inserts, approval letters as accessed from fda.gov, available information on clinicaltrials.gov, and available literature for oncology compounds approved by the U.S. FDA from 1999 to August 2019 were reviewed. The following parameters were assessed: (1) the study population in the HI study (ie, cancer patients or noncancer subjects; the term “noncancer subjects” is used hereafter to describe the population of healthy subjects and subjects with hepatic impairment but who are otherwise healthy), (2) the approach used for the evaluation, that is, dedicated HI study or population (PopPK) approach, and (3) the classification system used as for subject enrollment and PK analyses (ie, Child‐Pugh classification, NCIc, or liver function biomarkers). Studies conducted as part of the initial submission to the FDA and those conducted after the initial approval, were all included in this review.
Cell therapies, cancer supportive therapies (eg, treatment for chemotherapy‐associated cytopenia or bisphosphonates), oncology approvals with insufficient review documents, and studies rejected by the FDA or those that did not result in a labeling recommendation were excluded.
Evaluation of the Discordance Between Child‐Pugh Classification and NCIc in Pfizer's Oncology Portfolio
The retrospective discordance analysis was based on Pfizer's oncology compounds that were (1) granted their first approval within the period from 2006 to 2018 and (2) for which a HI trial conducted in noncancer subjects using Child‐Pugh classification was completed as of October 2019. All studies in scope were single‐dose studies. Each study was reanalyzed by classifying subjects using NCIc (ie, using aspartate aminotransferase [AST] and total bilirubin values at baseline; Supplemental Table 2) to different categories of hepatic dysfunction (ie, normal, mild, moderate, and severe). A concordance analysis between the Child‐Pugh classification and NCIc was conducted for each study and across all studies. In addition, non‐Pfizer compounds with available subject‐level listings of the discordant Child‐Pugh classification versus NCIc in FDA reviews were also included in this analysis.
Evaluation of Exposure Difference in Subjects With Hepatic Impairment Versus Normal Hepatic Function Using Child‐Pugh Classification and NCIc
AUCinf and Cmax for available studies in scope were summarized by hepatic function group as defined based on Child‐Pugh classification and NCIc. PK parameters for total drug exposure were summarized unless differences in protein binding between hepatic function groups were considered relevant, in which case free drug exposure was used for the analysis. Geometric mean ratio and 90% confidence interval were generated for each hepatic dysfunction group (test) compared with normal group (reference) using a 1‐way analysis of variance (ANOVA) model based on natural log‐transformed data. The results from the 2 classification systems were evaluated to assess the impact of using NCIc in clinical practice if labeling recommendations were based on Child‐Pugh classification. All analyses were performed using R software, version 3.5.1.
Assessment of Overall Similarity in Drug Exposure Within NCIc Categories
To determine whether NCIc results in similar exposure in subjects within the same hepatic function category, the AUCinf and Cmax for each NCIc group (eg, mild NCIc) were plotted by the corresponding Child‐Pugh classification group (ie, normal, mild, and moderate), whenever supported by the available data. For example, a box plot showing similar exposure for the mild NCIc group across different normal, mild, and moderate Child‐Pugh groups may suggest that NCIc is a better system for evaluating exposure differences in subjects with varying categories of HI and consequently for dosing recommendations. On the other hand, if exposure in the mild NCIc/normal Child‐Pugh group is lower than that in the mild NCIc/mild Child‐Pugh group, then NCIc might not be adequate for elucidating exposure differences between HI groups.
Results
Use of Child‐Pugh Classification and NCIc in Hepatic Impairment Studies for Oncology Compounds
FDA‐approved oncology drugs from 1999 to August 2019 were reviewed with respect to dedicated HI studies and the classification system used for subject enrollment, PopPK analyses of clinical trials to assess the impact of hepatic function on drug exposure and label recommendations (n = 117). Eighty‐two dedicated HI studies with available information were identified and included in the current analysis (Supplemental Table 3). Overall, hepatic dysfunction was found to be more commonly assessed using Child‐Pugh criteria (72%) for subject enrollment in dedicated HI studies compared with NCIc (27%); see Supplemental Table 3.
In the identified HI studies, 32 studies (32 of 82, 39%) and 50 studies (50 of 82, 61%) were conducted in patients with cancer and noncancer subjects, respectively (Table 1). Hepatic dysfunction was found to be assessed consistently using Child‐Pugh when the studies were conducted in noncancer subjects (100%). Of the 32 HI studies conducted in cancer subjects, 22 of the studies (69%) defined hepatic dysfunction using NCIc, whereas 9 studies (28%) used Child‐Pugh classification.
Table 1.
Hepatic Impairment Studies for Oncology FDA Approvals by Classification Systems and Study Population
| Study Population | ||
|---|---|---|
| Hepatic Impairment Classification System | Cancer Patients | Noncancer Subjects |
| Total number of studies | 82 a | |
| Child‐Pugh | 9 b (28%) | 50 (100%) |
| NCIc | 22 (69%) | 0 |
| WHO c | 1 (3%) | 0 |
| Total (% from total number of studies) | 32 (39%) | 50 (61%) |
Five compounds conducted postapproval studies to evaluate the effect of advanced categories of hepatic impairment on drug exposure, for example, to evaluate the effect of severe HI by Child‐Pugh classification when the initial study evaluated the effect of Child‐Pugh mild and moderate groups. The initial and postapproval studies used the same classification system and were considered as 1 study. A total of 80 compounds were included in the analysis. Two dedicated HI studies were conducted and included in the current analysis for gefitinib, 1 conducted in noncancer subjects using Child‐Pugh classification and another used liver biochemistry test (serum BILI, AP, and either AST or ALT were each scored on a 0‐4 scale according to the WHO grading system). Two HI studies were included for sorafenib, 1 conducted in patients with hepatocellular carcinoma (HCC) and another in noncancer patients. For other compounds (n = 78), 1 study was conducted/ongoing and included in the current analysis.
Sorafenib, regorafenib, osimertinib, olaparib, erlotinib, brentuximab‐vedotin, temozolomide, arsenic trioxide, eribulin mesylate. The study population for the sorafenib, regorafenib, temozolomide, and arsenic trioxide studies, that is, 4 of 9 studies (44%) conducted using Child‐Pugh classification in cancer patients, were patients with HCC. The effect of Child‐Pugh mild and moderate HI on regorafenib single‐dose exposure was evaluated in an expansion cohort in the dose‐escalation trial.
WHO, World Health Organization guidelines. 19
Among the 117 oncology drugs reviewed, 86 compounds that included PopPK analyses to assess hepatic function were identified (Supplemental Table 4). Liver function biomarker (eg, AST or alanine transaminase or total bilirubin) were included as continuous covariates without categorization of hepatic function in 25 of the 86 PopPK analyses (29%). A total of 55 of 86 compounds (64%) used hepatic dysfunction category defined using either Child‐Pugh or NCIc as a categorical covariate in the PopPK analysis. Of the 55 PopPK analyses, 54 (98%) used NCIc to define categories of hepatic dysfunction, whereas only 1 analysis for selinexor, 2%, used Child‐Pugh (Supplemental Table 4).
Evaluation of the Discordance Between Child‐Pugh Classification and NCIc in Pfizer Oncology Portfolio
Five HI studies of Pfizer oncology compounds were included in the current analysis to evaluate the discordance between Child‐Pugh and NCIc, including HI studies for axitinib, bosutinib, dacomitinib, palbociclib, and sunitinib. All 5 studies were conducted as single‐dose studies in noncancer subjects with varying degrees of hepatic function. Child‐Pugh classification was the primary basis for enrollment and PK analysis for all 5 studies. A summary of the design of the HI study, high‐level overview of the results based on Child‐Pugh, and the current label language is presented in Supplemental Table 5.
The total number of noncancer subjects included in the current analysis across all 5 studies was 128, with similar representation across the Child‐Pugh normal (healthy subjects with no underlying hepatic dysfunction, n = 40), mild (class A, n = 37), and moderate (class B, n = 38) hepatic impairment groups; Child‐Pugh severe HI group (class C) had 13 subjects. The concordance analysis between Child‐Pugh and NCIc is presented in Table 2. The NCIc was discordant with the Child‐Pugh classification for a considerable proportion of patients. Overall, the NCIc tended to classify a given subject as less impaired than with Child‐Pugh classification. For example, 64.9%, 73.7%, and 61.5% of subjects with mild, moderate, and severe HI, respectively, via Child‐Pugh were classified in at least 1 lower HI category when staged via NCIc (Table 2). An additional example was identified in FDA reviews comparing individual subject hepatic function category using both Child‐Pugh and NCIc for pexidartinib. 20 The concordance analyses for the pexidartinib study (Supplemental Table 6) and for all 6 studies (ie, 5 Pfizer compounds in addition to pexidartinib, total number of subjects, 161; Supplemental Table 7) were consistent with analysis for the 5 Pfizer compounds.
Table 2.
Discordance Between Child‐Pugh and NCIc for Axitinib, Bosutinib, Dacomitinib, Palbociclib, and Sunitinib
| Child‐Pugh Classification a | |||||
|---|---|---|---|---|---|
| Normal n = 40 | Mild n = 37 | Moderate n = 38 | Severe n = 13 | ||
| NCI classification | Normal | 38 (95%) | 24 (64.9%) | 5 (13.2%) | 0 (0%) |
| Mild | 1 (2.5%) | 12 (32.4%) | 23 (60.5%) | 1 (7.7%) | |
| Moderate | 1 (2.5%) | 1 (2.7%) | 9 (23.7%) | 7 (53.8%) | |
| Severe | 0 (0%) | 0 (0%) | 1 (2.6%) | 5 (38.5%) | |
Subjects in the Normal category included healthy subjects without underlying hepatic dysfunction and were not scored on the Child‐Pugh scale. Mild group corresponded to class A (5‐6 points), moderate group corresponded to class B (7‐9 points), and the severe group corresponded to class C (10‐15 points) on the Child‐Pugh classification scale.
Evaluation of the Difference in the Magnitude of HI Impact on Drug Exposure Using Child‐Pugh Classification and NCIc for Oncology Compounds With Available Data in Pfizer Portfolio
The PK analyses for AUCinf and Cmax for all 5 studies are presented in Table 3 and Supplemental Table 9, respectively. The design, PK results based on Child‐Pugh, and dosing recommendations in the product label for all 5 studies are presented in Supplemental Table 5.
Table 3.
Summary of Descriptive Statistics a and Statistical Analysis b of AUCinf by Child‐Pugh Classification and NCIc
| Compound Name | |||||
|---|---|---|---|---|---|
| N | HI Classification | Normal | Mild | Moderate | Severe |
|
Sunitinib N = 23 |
Child‐Pugh, n, GM (CV%) | 7, c 1369 (37%) | 8, 1514 (42%) | 8, 1477 (13%) | |
|
Child‐Pugh Adjusted GMR % (90%CI) |
— | 110.6 (83.5‐146.7) | 107.9 (81.4‐143.1) | ||
| NCIc, n, GM (CV%) | 14, 1342 (30%) | 7, 1690 (33%) | 2, 1527 (19%) | ||
| NCIc adjusted GMR % (90%CI) | — | 125.9 (99.2‐159.8) | 113.8 (77.1‐167.9) | ||
|
Dacomitinib N = 25 |
Child‐Pugh, n, GM (CV%) | 8, 805 (42%) | 8, 811 (32%) | 9, 682 (39%) | |
|
Child‐Pugh Adjusted GMR % (90%CI) |
— | 100.8 (73.4‐138.4) | 84.7 (62.3‐115.3) | ||
| NCIc, n, GM (CV%) | 14, 800 (38%) | 8, 753 (42%) | 2, 583 (23%) | 1, 685 (NA) | |
| NCIc adjusted GMR % (90%CI) | — | 94.2 (70.7‐125.6) | 72.9 (44.7‐119.1) | 85.6 (43.8‐167.5) | |
|
Palbociclib d N = 28 |
Child‐Pugh, n, GM (CV%) | 7, 197 (26%) | 7, 163 (32%) | 7, 264 (25%) | 7, 348 (23%) |
|
Child‐Pugh Adjusted GMR % (90%CI) |
— | 83.0 (65.4‐105.4) | 134.3 (105.7‐170.5) | 176.9 (139.3‐224.6) | |
| NCIc, n, GM (CV%) | 13, 198 (31%) | 6, 237 (37%) | 5, 260 (55%) | 4, 335 (30%) | |
| NCIc adjusted GMR % (90%CI) | — | 119.6 (88.6‐161.5) | 131.2 (95.3‐180.6) | 169.1 (119.5‐239.4) | |
|
Bosutinib N = 27 |
Child‐Pugh, n, GM (CV%) | 9, 864 (37%) | 6, 1938 (24%) | 6, 1731 (48%) | 6, 1655 (44%) |
|
Child‐Pugh Adjusted GMR % (90%CI) |
— | 224.4 (160.2‐314.1) | 200.4 (143.1‐280.5) | 191.6 (136.9‐268.3) | |
| NCIc, n, GM (CV%) | 13, 1129 (56%) | 6, 1583 (42%) | 7, 1858 (43%) | 1, 1350 (NA) | |
| NCIc adjusted GMR % (90%CI) | — | 140.3 (94.2‐209.0) | 164.7 (112.8‐240.4) | 119.6 (51.7‐276.5) | |
|
Axitinib N = 24 |
Child‐Pugh, n, GM (CV%) | 8, 156 (63%) | 8, 122 (167%) | 8, 304 (44%) | |
|
Child‐Pugh Adjusted GMR % (90%CI) |
‐ | 78.3 (39.9‐153.7) | 195.2 (99.5‐383.2) | ||
| NCIc, n, GM (CV%) | 12, 137 (120%) | 10, 210 (76%) | 2, 410 (36%) | ||
| NCIc adjusted GMR % (90%CI) b | — | 152.8 (83.7‐278.8) | 299.1 (102.3‐874.6) |
AUCinf, area under the plasma concentration‐time curve from time zero to infinity; CI, confidence interval; CV%, geometric CV%; GM, geometric mean; GMR, geometric mean ratio; NCIc, National Cancer Institute classification.
N represents the total number of subjects in the study, n is the number of subjects for each HI group/classification. Geometric mean (geometric CV%) is presented for all parameters. Unit for AUCinf for all compounds is ng·h/mL. Total N for all studies = 127.
Adjusted geometric mean ratio for the hepatic impaired group (test) compared with normal hepatic function group (reference) for each classification (ie, Child‐Pugh and NCIc) using ANOVA model based on natural log‐transformed data.
One subject in the normal Child‐Pugh group in the sunitinib HI study vomited after dosing and was excluded from the PK analysis. This subject was included in the discordance evaluation.
For palbociclib, the summary and statistical analysis of unbound AUCinf are presented. For other compounds, the summary and statistical analysis are presented for total AUCinf.
Sunitinib
The sunitinib HI trial enrolled mild and moderate hepatically impaired noncancer subjects and matching controls with normal hepatic function based on Child‐Pugh (8 subjects/group). 21 Mild and moderate hepatic impairment based on Child‐Pugh did not alter sunitinib exposure compared with subjects with normal hepatic function; therefore, no dose adjustment is needed for mild or moderate HI (Supplemental Table 5).
Based on NCIc, all normal Child‐Pugh subjects were classified as normal NCIc: 5 of 8 mild Child‐Pugh subjects (62.5%) were classified as normal NCIc; 6 of 8 subjects with moderate Child‐Pugh (75%) were categorized as less hepatically impaired, either as normal NCIc (2 of 8, 25%) or mild NCIc (4 of 8, 50%); see Supplemental Table 8.
Despite the discordance in the 2 classification systems, which led to an imbalanced number of subjects per group for the NCIc, the summary statistics and the results of the ANOVA analysis indicated similar exposure (AUCinf and Cmax) for the mild and moderate groups compared with normal hepatic function based on either classification system (Table 3 and Supplemental Table 9). A limited number of subjects with NCIc moderate HI were available (n = 2).
Dacomitinib
The dacomitinib HI study enrolled noncancer subjects with Child‐Pugh mild and moderate HI (8 to 9 subjects per group) and matching controls with normal hepatic function based on Child‐Pugh. Mild and moderate HI based on Child‐Pugh did not alter dacomitinib exposure; therefore, no dose adjustment is needed for mild or moderate HI (Supplemental Table 5). 16
Using NCIc, all normal Child‐Pugh subjects were classified as normal NCIc, 6 of 8 mild Child‐Pugh subjects (75%) were classified as normal NCIc, and 6 of 9 subjects with moderate Child‐Pugh (66.7%) were categorized as mild NCIc (Supplemental Table 10).
PK analysis via either classification indicated similar dacomitinib exposure for the mild and moderate groups compared with normal hepatic function based on either classification system (Table 3 and Supplemental Table 9). A limited number of subjects with NCIc moderate or severe HI were available (n = 2 and n = 1, respectively).
Palbociclib
An HI trial for palbociclib enrolled noncancer subjects based on Child‐Pugh, including 7 subjects in each HI group (mild, moderate, and severe) and matching controls with normal hepatic function based on Child‐Pugh. The mean fraction unbound of palbociclib in plasma increased with worsening hepatic function. Similar unbound palbociclib exposure was achieved in subjects with Child‐Pugh mild HI compared with subjects with normal hepatic function. In subjects with Child‐Pugh moderate and severe HI, there were a 34% and 77% increase in unbound palbociclib AUCinf, respectively, compared with normal. As a result, the palbociclib label recommendation indicates no dose adjustment is needed for mild or moderate HI (ie, 125 mg palbociclib), whereas a patient with severe HI should receive a reduced dose of 75 mg palbociclib (Supplemental Table 5). 22
When staging subjects using NCIc, 1 of the 7 subjects in the normal Child‐Pugh group was reclassified as moderate on NCIc. Six of 7 subjects in mild Child‐Pugh (85.7%) were normal on NCIc, 5 of 7 subjects in moderate Child‐Pugh (71.4%) were normal on NCIc, and 3 of 7 subjects in the severe Child‐Pugh group (42.9%) were moderate on NCIc (Supplemental Table 11).
PK analysis via either classification indicated overall similar unbound palbociclib AUCinf for the mild group compared with subjects with normal hepatic function (Table 3). A similar increase in effect of HI on unbound palbociclib AUCinf was observed via either classification for the moderate group (34% and 31%, respectively) and the severe group (77% and 69%, respectively); see Table 3.
Bosutinib
The bosutinib HI study enrolled noncancer subjects with Child‐Pugh mild, moderate, and severe HI (6 to 9 subjects/group) and matching controls with normal hepatic function based on Child‐Pugh. Based on Child‐Pugh, subjects with any degree of HI had ∼2‐fold higher bosutinib exposure and longer plasma elimination half‐life compared with subjects with normal hepatic function. Therefore, bosutinib label recommendations indicate that a dose reduction to 200 mg once daily is recommended for patients with any degree of HI (Supplemental Table 5). 23
Based on NCIc, all normal Child‐Pugh subjects were classified as normal NCIc, 4 of 6 mild Child‐Pugh subjects (66.7%) were classified as normal NCIc, and 4 of 6 subjects with moderate Child‐Pugh (66.7%) were categorized as mild NCIc. Similarly, 5 of 6 subjects with severe Child‐Pugh (83.4%) were categorized as less hepatically impaired either as mild NCIc (1 of 6, 16.7%) or moderate NCIc (4 of 6, 66.7%); see Supplemental Table 12.
In contrast to the results using Child‐Pugh, PK analysis based on NCIc indicated less impact of mild and moderate NCIc HI with these groups demonstrating 40% and 65% higher AUCinf compared with normal NCIc hepatic function (Table 3).
Axitinib
The axitinib HI trial enrolled noncancer subjects with Child‐Pugh mild and moderate HI and matching controls with normal hepatic function based on Child‐Pugh; severe Child‐Pugh group was not included. Each category enrolled 8 subjects to assess axitinib PK. Mild Child‐Pugh HI did not affect axitinib exposures (Cmax and AUCinf) compared with subjects with normal hepatic function; therefore, no dose adjustment was needed for patients in the mild Child‐Pugh group. In contrast, patients with moderate HI based on Child‐Pugh had 2‐fold higher AUCinf. As a result, a dose reduction of 50% is recommended for patients with moderate HI based on Child‐Pugh (Supplemental Table 5). 24
Based on NCIc, 7 of 8 normal Child‐Pugh subjects (87.5%) were classified as normal NCIc, with 1 of 8 subjects (12.5%) classified as mild NCIc. Four of 8 mild Child‐Pugh subjects (50%) were classified as normal NCIc, 6 of 8 subjects with moderate Child‐Pugh (75%) were categorized as less hepatically impaired either as normal NCIc (1 of 8, 12.5%) or mild NCIc (5 of 8, 62.5%); see Supplemental Table 13.
PK analysis via NCIc indicated a higher impact of mild and moderate NCIc compared with Child‐Pugh, with these groups demonstrating 53% and 195% higher AUCinf compared with normal NCIc hepatic function (Table 3), although the number of subjects with NCIc moderate HI was limited (n = 2).
Similarity in Drug Exposure Within NCIc Categories
To determine whether NCIc results in similar exposure in subjects within the same hepatic function category, PK parameters for subjects with NCIc normal hepatic function were plotted versus the respective Child‐Pugh classification for axitinib (Figure 2) and bosutinib (Figure 3). For axitinib, with the exception of the AUCinf of 1 subject with moderate Child‐Pugh, the distribution of AUCinf and Cmax appeared similar for axitinib across all subjects with normal NCIc irrespective of the Child‐Pugh classifications (Figure 2A). A similar finding was observed for axitinib AUCinf and Cmax for subjects with mild NCIc HI (Figure 2B). Overall, NCIc appears to provide similar axitinib exposure within a certain HI group.
Figure 2.
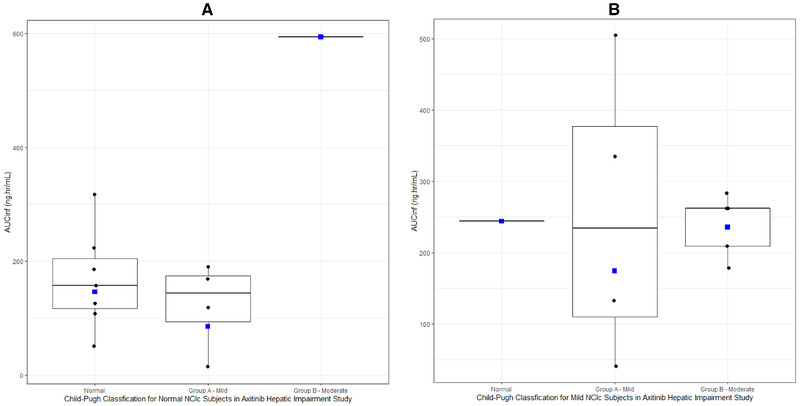
(A) Box plot of axitinib AUCinf in normal NCIc subjects by Child‐Pugh classification. Box plot provides median and 25% and 75% quartiles with whiskers to the last point within 1.5 × the interquartile range. Geometric mean is shown as blue squares. After recategorization using Child‐Pugh classification, 1 subject was classified as moderate (n = 1). (B) Box plot of axitinib AUCinf in mild NCIc subjects by Child‐Pugh classification. Box plot provides median and 25% and 75% quartiles with whiskers to the last point within 1.5 × the interquartile range. Geometric mean is shown as blue squares. After recategorization using Child‐Pugh classification, 1 subject was classified as normal (n = 1).
Figure 3.
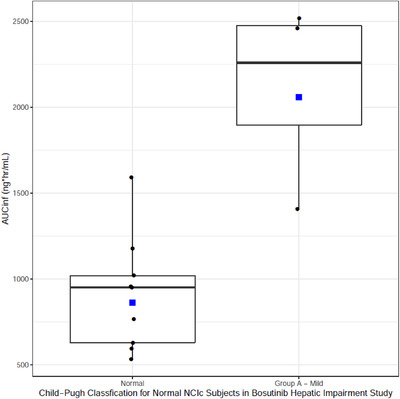
Box plot of bosutinib AUCinf in normal NCIc subjects by Child‐Pugh classification. Box plot provides median and 25% and 75% quartiles with whiskers to the last point within 1.5 × the interquartile range. Geometric mean is shown as blue squares.
For bosutinib, subjects who were classified as normal NCIc showed higher exposure in the mild Child‐Pugh group versus normal Child‐Pugh (Figure 3). Although NCI appears to be a more specific delineator of exposure compared with Child‐Pugh classification for axitinib (Figure 2), this finding did not apply for bosutinib. Therefore, for bosutinib, NCIc was not a good differentiator of exposure and Child‐Pugh appears to be a better classification system for correlation with changes in drug exposure.
Discussion
This analysis assessed the discordance between Child‐Pugh and NCIc and evaluated the potential impact of such discordance on PK exposure using Pfizer oncology compounds as examples. Considerable discordance was observed between Child‐Pugh and NCIc across all assessed compounds, with the latter tending to classify patients as less hepatically impaired than Child‐Pugh. This suggests that these 2 systems are not interchangeable, as they are based on different laboratory parameters/clinical evaluations and were developed for different purposes/patient populations. Given that dosing recommendations for many approved oncology compounds are using Child‐Pugh, whereas the prescribing oncologists are more commonly using NCIc (as illustrated in Figure 1), an integrated approach to inform dosing recommendation for oncology compounds based on hepatic function is needed.
Our review based on data from about 20 years of FDA oncology approvals indicated that all HI studies conducted in noncancer subjects used Child‐Pugh classification for enrollment, PK analyses, and dosing recommendations in the product label. The predominant use of Child‐Pugh classification in HI studies in noncancer subjects is consistent with FDA and EMA guidance on the topic. 3 , 4 Although the EMA guidance on HI studies recommends classification by Child‐Pugh classification, the guidance acknowledged that such a classification system was not developed for the purpose of predicting drug elimination capacity. 4
In contrast, most studies conducted in cancer patients used NCIc. This is consistent with the FDA's guidance on the potential for using alternative approaches for HI classification when the underlying diseases, such as cancer metastasis or cachexia, are the cause of alterations in Child‐Pugh classification components (eg, albumin level, encephalopathy, and ascites). 3 , 25 NCIc uses readily available laboratory values and does not require evaluation of subjective assessments of clinical features such as ascites or hepatic encephalopathy, which renders the implementation of the NCIc system simpler and more consistent across physicians/hospital systems compared with Child‐Pugh classification, which led the ODWG to propose the NCIc for grading hepatic function in HI studies. 15 As such, NCIc is more commonly used in real‐world oncology setting for classifying hepatic function and making dosing decisions even if dosing recommendations in the product label are provided based on Child‐Pugh classification. 16 Hence, there is a need for an assessment of the discordance between the 2 systems and the impact of this discordance on PK analyses to rule out a potential impact on dosing decisions. Although previous reports have evaluated the discordance between the 2 systems, 14 , 17 to our knowledge, the current analysis is the first systematic simultaneous evaluation of the concordance of the 2 classification systems and the impact on PK analyses and dose recommendations.
In our analysis, ≥60% of patients were classified in at least 1 lower HI category via NCI than Child‐Pugh classification. A similar discordance rate has been noted based on an unpublished analysis that included 65 HI studies involving 1841 subjects. 17
Classification by Child‐Pugh classification or NCIc resulted in a similar magnitude of exposure changes for sunitinib, dacomitinib, and palbociclib. This indicates no impact of the noted discordance on these compounds. For bosutinib, the increase in exposure in the NCIc mild and moderate HI groups versus normal NCIc hepatic function was less than that with Child‐Pugh classification. This finding should be interpreted in the context of bosutinib dosing recommendations, which require a dose adjustment for patients with Child‐Pugh classification mild, moderate, or severe HI (200 mg once daily) compared with the approved dose of 400 mg once daily in newly diagnosed chronic myeloid leukemia (CML) patients or 500 mg once daily in CML patients with resistance or intolerance to prior therapy. 16 , 21 , 22 The exposure difference between NCIc mild or moderate versus normal NCIc suggests that a dose reduction to 200 mg may result in underexposure in this subpopulation. However, the impact on efficacy is expected to be minimal in this case, given that bosutinib dose up‐titration is recommended if no clinical response is achieved and if no grade 3 or higher adverse events occur, which is expected to compensate for potential underexposure in this subpopulation. For axitinib, the increase in axitinib exposure in NCIc mild and moderate HI versus normal NCIc was more than that based on Child‐Pugh classification, a direction opposite the bosutinib findings. Similarly, this finding should be interpreted in the context of the axitinib dosing recommendations, which do not require a dose adjustment for patients with Child‐Pugh classification mild HI, whereas a 50% dose reduction is needed for patients with Child‐Pugh classification moderate HI. 23 This may suggest that axitinib dose adjustment for patients with mild HI is needed. However, the magnitude of increase in axitinib plasma exposure, its established safety profile, and the labeled dose titration algorithm may subvert the need for an axitinib starting dose reduction in this patient population. In summary, the current dosing recommendations for evaluated programs appear adequate for either classification system, with special considerations for bosutinib and axitinib. However, these case studies indicate that the impact of this discordance on PK exposure and dosing recommendations does not follow a common trend (eg, bosutinib and axitinib showed opposite trends). Therefore, PK analysis from the dedicated HI study via NCIc needs to be conducted to assess the impact of this discordance for each compound.
An interesting example elucidating the importance of categorization of HI with Child‐Pugh classification versus NCIc is the case of pexidartinib, which is approved for the treatment of tenosynovial giant‐cell tumor. 20 The pexidartinib HI study was conducted in noncancer subjects using Child‐Pugh classification mild and moderate groups and matched normal subjects. The FDA requested PK analysis based on NCIc. Similar to Pfizer compounds analyzed in this article, recategorization resulted in a limited number of moderate NCI patients (only 2 of the original 8 categorized as moderate Child‐Pugh classification); see Supplemental Table 6. Because of the limited data for subjects with NCIc moderate HI, the FDA issued a postmarketing requirement to evaluate the effect of moderate hepatic impairment using NCIc on pexidartinib plasma exposure. 20 Although the pexidartinib case study could be impacted by the hepatotoxic potential of this drug, this example demonstrates that the regulatory authorities are increasingly examining this issue, which might have real implications on the recommended dosing of oncology compounds.
Given the discordance between the 2 systems and the potential implications on dosing recommendations, evaluating which of the 2 classifications is “better” could be useful. It is important to highlight that neither classification system has been validated for the evaluation of hepatic dysfunction in cancer patients nor that they have been evaluated for predicting drug elimination capacity. Child‐Pugh classification is often used for staging patients with HCC 26 and appears to be a comprehensive assessment of the functional capacity of the liver. 27 However, Child‐Pugh classification might also have some limitations including (1) not validated for the assessment of liver function in patients with cancers other than HCC; (2) albumin, bilirubin, and INR are not specific for liver disease and could be affected by other factors not related to liver dysfunction such as inflammation, nutritional status, hemolysis, or vitamin K deficiency 28 , 29 ; (3) evaluation of ascites and hepatic encephalopathy could be subjective 28 , 29 ; and (4) treatment‐induced changes, that is, the use of diuretics for ascites or antibiotics and lactulose for encephalopathy, might not be incorporated into Child‐Pugh classification staging. NCIc is simpler and only uses AST and total bilirubin, with the latter being the more important parameter in classifying severity of hepatic dysfunction. NCIc, however, is subject to similar limitations to Child‐Pugh classification regarding the use of total bilirubin as an important parameter for classification. 4 Therefore, each system has some limitations, and it remains unclear which is superior.
Another important aspect for either classification system is whether it can elucidate exposure differences in different groups of HI. Our analysis could not conclusively address this question given the contradicting trends observed in the axitinib and bosutinib examples. Axitinib exposure appeared similar in subjects categorized as NCIc normal or mild hepatic function regardless of their Child‐Pugh classification, which suggests that NCIc could result in homogenous exposure within each HI group. However, in subjects with normal NCI hepatic function, bosutinib AUCinf was ∼2‐fold higher in subjects with Child‐Pugh classification mild HI compared with Child‐Pugh classification normal hepatic function. Therefore, it remains unclear which classification system is a better differentiator of exposure, and further exploration using a larger data set is warranted.
Although NCIc is not necessarily ideal in all cases (eg, bosutinib), it could be argued that given its common use among oncologists, ideally NCIc should be the primary classification system in HI trials so that the classification system commonly used by the oncologist for dose adjustment is consistent with that from which dose adjustment recommendations are derived. The challenge of such an approach is the limited number of subjects who will satisfy the criteria for moderate or severe NCIc; therefore, using NCIc as the primary classification system in HI trials may result in difficulties in enrolling an adequate number of subjects in the moderate and severe NCIc groups, which may limit the practical application of NCIc, especially for studies conducted in noncancer subjects.
Given the challenges associated with using NCIc as the primary classification system in HI trials for oncology compounds conducted in noncancer subjects and given that the current regulatory guidances recommend using Child‐Pugh classification for HI trials, Child‐Pugh classification remains the main classification system for HI trials conducted in noncancer subjects. To mitigate the potential impact of the discordance between the 2 systems, an integrated approach to characterize the impact of hepatic impairment on dosing recommendations is proposed as follows: (1) when Child‐Pugh classification is the primary classification for enrollment and PK analysis of HI trials, PK analysis for investigational agents based on NCIc should be conducted as an exploratory objective (these additional analyses should be interpreted in light of the numbers of subjects in each NCIc HI group and alongside the primary analysis using Child‐Pugh classification); although this recategorization could result in unbalanced matching based on demographics between NCIc HI groups compared with the NCIc normal group and limited number of subjects in some groups, it provides key information on the potential for dosing errors in the clinic; (2) evaluate the consistency of PK findings between the HI study (typically using Child‐Pugh classification) and PK data collected from cancer patients in clinical trials (typically using NCIc) while acknowledging that clinical trials typically exclude patients in the moderate and severe NCIc groups; and (3) the prescribing oncologist should attempt to use the same classification system as outlined in the product label for dosing decisions. In addition, for approved oncology drugs with a completed HI trial using Child‐Pugh classification as the primary classification, a similar retrospective exercise should be conducted to evaluate the potential impact of the discordance on dosing recommendations.
In summary, this analysis demonstrated the prevalence of using Child‐Pugh classification and NCIc systems in HI studies for approved oncology compounds, highlighted the considerable discordance between these classification systems, and illustrated the importance of analyzing exposure differences using both systems to ensure adequate dosing recommendations for oncology compounds.
Conflicts of Interest
M.E., K.P., and D.W. are employees of Pfizer and receive stock and stock options as part of their employment. D.Z.Y. (student assistant) and E.S. (postdoctoral fellow) were paid contractors to Pfizer in the development of this article.
Funding
This study was sponsored by Pfizer Inc.
Data Sharing
On request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinicaltrials/trial‐data‐and‐results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions and for which an exception does not apply via a secure portal. To gain access, data requesters must enter into a data‐access agreement with Pfizer.
Supporting information
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of web‐based version of this article.
Acknowledgments
We acknowledge the thoughtful comments and discussion from Dr. Chandrasekar Durairaj, Dr. Donghua Yin, Dr. Erjian Wang, Dr. Huiping Xu, Dr. Justin Hoffman, Dr. Reza Khosravan, Dr. Weiwei Tan, and Dr. Yazdi K. Pithavala.
References
- 1. Hamaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist. 2014;19(10):1069‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masters JC, Wiernik PH. Are we ready to include organ‐impaired patients in oncology trials? A clinical pharmacology perspective on recent recommendations. J Clin Pharmacol. 2018;58(6):701‐703. [DOI] [PubMed] [Google Scholar]
- 3. FDA . Guidance for industry—pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling; 2003; https://www.fda.gov/media/71311/download. Accessed June 10, 2020.
- 4. EMA . Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function; 2005; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf. Accessed June 10, 2020.
- 5. FDA . Cancer clinical trial eligibility criteria: patients with organ dysfunction or prior or concurrent malignancies; 2020; https://www.fda.gov/media/123745/download. Accessed July 13, 2020.
- 6. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524‐530.e1; quiz e560. [DOI] [PubMed] [Google Scholar]
- 7. Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181‐194. [DOI] [PubMed] [Google Scholar]
- 8. Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1‐85. [PubMed] [Google Scholar]
- 9. D'Cunha R, Lin S. A review of regulatory guidance for conducting hepatic impairment studies: a case study in oncology. J Oncol Cancr Res. 2018;2:14‐22. [Google Scholar]
- 10. Grigorian A, O'Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2(2):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Macgregor CA. Nature of liver failure due to complete biliary obstruction. AMA Arch Surg. 1953;67(6):878‐901. [DOI] [PubMed] [Google Scholar]
- 12. Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21(5):1238‐1247. [PubMed] [Google Scholar]
- 13. Turcotte JG, Lambert MJ 3rd. Variceal hemorrhage, hepatic cirrhosis, and portacaval shunts. Surgery. 1973;73(6):810‐817. [PubMed] [Google Scholar]
- 14. Patel H, Egorin M, Remick S, et al. Comparison of Child‐Pugh (CP) criteria and NCI organ dysfunction working group (NCI‐ODWG) criteria for hepatic dysfunction (HD): implications for chemotherapy dosing. J. Clin. Oncol. 2004;22(14_suppl):6051‐6051. [Google Scholar]
- 15. NCI . Protocol Template for Organ Dysfunction Studies. 2019. https://ctep.cancer.gov/protocolDevelopment/docs/CTEP_Organ_Dysfunction_Protocol_Template.docx. Accessed June 10, 2020.
- 16. Giri N, Masters JC, Plotka A, et al. Investigation of the impact of hepatic impairment on the pharmacokinetics of dacomitinib. Invest New Drugs. 2015;33(4):931‐941. [DOI] [PubMed] [Google Scholar]
- 17. Younis I. Sensitivity of Liver Function Classification Systems for Exposure Changes. 2015. http://regist2.virology-education.com/2015/16HIVHEP/03_Younis.pdf. Accessed July 14, 2020.
- 18. Pearl T ZD, Moorthy G, Masson E, Vishwanathan K. Comparison of Child‐Pugh classification and NCI classification of hepatic impairment. ASCPT 2018. 2018;PI‐023. [Google Scholar]
- 19. WHO . World Health Organization. Guidelines for the screening care and treatment of persons with hepatitis C infection. 2014. http://apps.who.int/iris/bitstream/10665/111747/1/9789241548755_eng.pdf?ua=1&ua=1. Accessed June 10, 2020. [PubMed]
- 20. FDA . FDA multi‐discipline review for pexidartinib (TURALIO(R)) for the treatment of adult patients with symptomatic tenosynovial giant cell tumor (TGCT). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211810Orig1s000TOC.cfm. Accessed June 10, 2020.
- 21. Bello CL, Garrett M, Sherman L, Smeraglia J, Ryan B, Toh M. Pharmacokinetics of sunitinib malate in subjects with hepatic impairment. Cancer Chemother Pharmacol. 2010;66(4):699‐707. [DOI] [PubMed] [Google Scholar]
- 22. FDA . IBRANCE (palbociclib) [package insert]. New York, NY: Pfizer Inc.; 2019. [Google Scholar]
- 23. Abbas R, Chalon S, Leister C, El Gaaloul M, Sonnichsen D. Evaluation of the pharmacokinetics and safety of bosutinib in patients with chronic hepatic impairment and matched healthy subjects. Cancer Chemother Pharmacol. 2013;71(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 24. Tortorici MA, Toh M, Rahavendran SV, et al. Influence of mild and moderate hepatic impairment on axitinib pharmacokinetics. Invest New Drugs. 2011;29(6):1370‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L. Studying pharmacokinetics of drugs in patients with hepatic impairment—a regulatory perspective. Paper presented at: American Society of Clinical Pharmacology and Therapeutics Annual Meeting 2011; Dallas, Texas.
- 26. Levy I, Sherman M, Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child‐Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50(6):881‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shouval D. HCC: what's the score. Gut. 2002;50(6):749‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28(1):110‐122. [DOI] [PubMed] [Google Scholar]
- 29. Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child‐Pugh versus MELD. J Hepatol. 2005;42(1):S100‐S107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supplemental information can be found by clicking the Supplements link in the PDF toolbar or the Supplemental Information section at the end of web‐based version of this article.


