Abstract
Background and Objectives
A novel helium plasma device was evaluated for efficacy and safety for dermal resurfacing (ClinicalTrials.gov Identifier: NCT03286283). The helium plasma device delivers energy in a controlled, bimodal fashion that when compared with the nitrogen plasma predicate device in a porcine animal model demonstrated a more limited depth of thermal effect but a greater skin tissue contraction.
Study Design/Materials and Methods
Fifty‐five eligible subjects seeking improvement in facial rhytids were enrolled for study at one of three investigational sites. Most subjects underwent full‐face treatment. Power levels were limited to 20% at peri‐oral and peri‐orbital areas—a level that correlates to an energy density 40% lower than the highest setting on the predicate device. Three‐month post‐treatment Fitzpatrick Wrinkle and Elastosis Scale (FWS) scores were compared with baseline scores as determined by blinded independent photographic reviewers (IPRs) and study investigators.
Results
Blinded IPRs observed a ≥1‐point FWS improvement in 63.64% of subjects whereas study investigators noted a ≥1‐point FWS improvement in 54 of 55 subjects (98.18%) of subjects. 90.9% of subjects indicated “improvement” in appearance utilizing the modified Global Aesthetic Improvement Scale. Subgroup analysis showed 1‐point (±0.05) FWS improvement by IPRs and study investigators for Fitzpatrick Skin Types II and III, age≥62, two of three study sites, and post‐treatment oral steroid use. Eighty Non‐Serious Adverse Events in 39 subjects were reported, most of which resolved within 14 days or less. There were no Serious Adverse Events or Unanticipated Device Effects reported.
Conclusion
At the modest power level studied, a significant improvement from a single pass helium plasma dermal resurfacing treatment was observable in most subjects by IPRs and investigators, and no serious adverse events were reported. The discrepancy between IPR and study investigator FWS improvement may be explained in part by the limitations of assessing two‐dimensional photographs versus live in‐person evaluation of subjects. Studies evaluating higher energy levels and/or multiple treatment passes are ongoing. Lasers Surg. Med. © 2020 The Authors. Lasers in Surgery and Medicine published by Wiley Periodicals, Inc.
Keywords: helium plasma, dermal resurfacing, radio frequency, clinical trial, facial wrinkle score
INTRODUCTION
In appropriate skin types and skin conditions, full‐field ablative laser skin resurfacing (10600 nm CO2; 2940 nm Erbium‐YAG; and 2780 nm Erbium‐YSGG) remains the “gold standard” for long term correction of rhytids and reduction of photodamage, solar elastosis, and dyschromia [1, 2, 3]. Nevertheless, energy‐based treatment options for facial skin rejuvenation have continued to evolve in search of effective alternative treatments that may have less downtime, fewer unanticipated side effects and/or allow for treatment of a greater diversity of skin types.
Following experimental use of radiofrequency (RF) energy to create a superficial skin injury through a conductive gel plasma interface (coblation) [4], alternative skin rejuvenation treatments that have entered widespread clinical use include non‐ablative fractional resurfacing (NFR) [5, 6], ablative fractional resurfacing (AFR) [7], treatment with dual‐wavelength NFR lasers (e.g., 1550 and 1927 nm) [8] as well as dual‐modality AFR and NFR (e.g., micro‐ablative 2940 nm and non‐ablative 1470 nm) [9], variable depth RF microneedle treatments [10] and nitrogen plasma skin regeneration (PSR) [11, 12]. Known as the fourth state of matter, plasmas are gases in temporary higher energy states wherein electrons have transitioned to higher orbits and where the higher energy states quickly dissipate without additional energy input.
Skin healing after nitrogen PSR treatment involves a process of natural skin regeneration, wherein the upper/outer layers of skin that are desiccated during treatment remain intact as a natural biological dressing during the early phase of healing; as the “old” skin desquamates, the newly regenerated and smoother pink skin appears [13]. Clinical benefits of nitrogen PSR treatment include a range of treatment protocols for diverse skin types and conditions, effective reversal of photodamage and dyschromia, preservation of natural skin tone, and modest reduction of acne scarring and rhytidosis [10, 12, 13, 14, 15, 16, 17, 18].
An alternative Food and Drug Administration cleared gas (helium) plasma that was introduced in 2015 for general indications of soft tissue ablation, coagulation, and cutting (Renuvion®; Apyx Medical Corporation, Clearwater, Fl) has more recently been assessed for its potential use in dermal resurfacing. Although the helium plasma generator initially generates helium plasma in a similar fashion to the nitrogen plasma device through the use of RF energy, helium gas flow is continuous (e.g., 4 L/min) and helium plasma is also continuously generated across the distance from the treatment tip to the skin's surface [19]. With the treatment tip as the cathode (positive electrode) and the skin tissue as the anode (negative electrode) electrical coupling occurs, and RF energy travels down to the skin's surface and into the superficial skin tissue in a process known as Joule (or resistive) heating [19]. Although the RF energy ionizes only a tiny amount of the helium gas, it is sufficient to enable propagation of the RF energy to the skin tissue across a radio frequency bridge and to create a visible violet white “beam” (Lewis Rayleigh afterglow) from continuous de‐excitation (neutralization) of ionized helium plasma atoms across the length of the beam path [19].
Although both nitrogen gas and helium gas plasma treatments involve heating of the skin surface via heat transfer from flowing hot gas/plasma, helium plasma is unique in also directing RF energy into the tissue resulting in resistive or Joule heating and a more powerful bimodal energy deposition [19]. Preclinical studies evaluating helium plasma skin tissue effects compared to that of the predicate nitrogen plasma device in a porcine animal model demonstrated (i) modestly reduced depth of thermal effect (more superficial tissue injury), (ii) greater skin tissue contraction, and (iii) similarity of acute and chronic histopathological findings [19]. The preclinical study authors suggest that these findings result from differences in the nature of plasma generation and of plasma—skin tissue interaction—with helium plasma's modest depth of tissue injury/repair resulting from impedance changes in treated tissue and quickly dispersing low current RF energy and from helium plasma's bimodal energy delivery and more thorough full field treatment [19]. The more superficial tissue injury and paradoxically greater magnitude skin tissue contraction point to helium plasma's potential suitability for use in skin rejuvenation. Following this preclinical study, a prospective, multi‐center, single‐arm clinical study evaluating the use of helium plasma for dermal resurfacing was performed to evaluate its potential for wrinkle reduction—these initial clinical results are reported herein.
METHODS
Study Subjects
Eligible subjects were healthy male and female adults ≥30 years old seeking improved appearance of facial wrinkles and rhytids from among the patient population at three participating study sites.
Inclusion Criteria
To be eligible for inclusion, subjects were required to have a facial wrinkle score ≥2 on the Fitzpatrick Wrinkle and Elastosis Scale, a Fitzpatrick Skin Scale score ≤III, and express their willingness to comply with protocol requirements, including abstaining from other facial cosmetic procedures through the 6‐month follow‐up visit. These included but were not limited to, laser or chemical resurfacing, dermabrasion, neuromodulators, and dermal fillers.
Exclusion Criteria
Reasons for exclusion from the study included use of isotretinoin or other medication that can cause dermal hypersensitivity prior to treatment, active herpes simplex virus‐1, diabetes mellitus, autoimmune disease, bleeding disorders or blood‐thinning medications, connective tissue disease or active skin disease in the planned treatment area, known susceptibility to keloid formation or hypertrophic scarring, a facelift procedure or facial injections within the past year, hypersensitivity to anesthetics, a concurrent therapy that might place the subject at risk or jeopardize the study objectives, enrollment in another investigational trial and pregnancy or lactation.
Study Design
Subject eligibility, physical examination, and wrinkle and rhytid assessments were completed at one of three investigational sites within 21 days prior to the study procedure. One or two urine pregnancy tests were obtained if the pre‐procedure screening and helium plasma procedure were not performed on the same day. Digital images of the planned treatment area were obtained to document pretreatment facial appearance (Visia‐CR 2.3 System; Canfield Scientific, Inc., Parsippany, NJ). The same standardized imaging was obtained throughout the study at subsequent follow‐up visits. Subjects received medication for prophylactic treatment of bacterial and viral infections at the discretion of the investigator. Subjects also completed a visual analog scale (VAS) pain assessment [20] pre‐ and immediately post‐procedure.
The face of each subject was divided into five zones: Zone 1 (perioral), Zone 2 (periorbital), Zone 3 (forehead), Zone 4 (nose), and Zone 5 (cheeks). Topical anesthesia is not indicated for helium plasma dermal resurfacing (interferes with device to tissue RF coupling) and was not used. Patient comfort was facilitated with trigeminal nerve blocks, peripheral ring blocks, and labial blocks followed by sequential infiltration of tumescent anesthesia for each treatment zone. The volume used in each Zone was at the discretion of the investigator. Investigators were instructed to use a steady movement of the plasma beam to ablate the tissue in each Zone and to treat all Zones with only a single non‐overlapping pass of the plasma beam.
Treatment of Zone 1 and Zone 2 (perioral and periorbital zones) was limited to a maximum of 20% power and helium flow of 4 L/min. Zones 3, 4, and 5 (forehead, nose, and cheeks, respectively) could be treated with a maximum of 40% power and helium flow of 4 L/min. Energy delivery was performed in continuous (no pulsing) mode. Investigators were instructed not to wipe away treated tissue.
Subjects underwent assessments immediately following the procedure and then at 10 days and 1‐, 3‐ and 6‐month post‐procedure.
Post‐Treatment Care
Subjects were instructed to keep their skin moist at all times during the first 10 days. The skin was to be covered with a generous, occlusive layer of petroleum jelly at all times. Cool water and vinegar soaks (1 tablespoon white vinegar per cup of water) were to be performed up to every 1–2 hours as tolerated on days 0‐2, then up to every 2–4 hours on days 3–10. Appropriately moistened 4 × 4 gauze pads were applied over the face for approximately 15–30 minutes, then removing them before they dried. At the end of each soak, petroleum jelly was reapplied. The skincare regimen was ideally adjusted at day 10, wherein the occlusive petrolatum was discontinued, and a light moisturizer and sun protection were started as directed by the investigator.
Study Assessments
Following the study procedure, subjects returned to the study site at 10 days (9–14 days), 1 month (23–37 days), 3 months (80–100 days), and 6 months (166–194 days) for VAS pain assessment, post‐procedure assessments and to complete questionnaires. Digital images were obtained at each visit. Using daily diaries, subjects reported post‐procedure complications and adverse events, daily VAS 0‐10 scale pain scores, and the date when they first felt comfortable and willing to go out in public following treatment.
Assessment of subject wrinkle severity was made at baseline and each follow‐up visit by the investigator and by three sourced, blinded, board‐certified dermatologists, or plastic surgeons (Independent Photographic Reviewers [IPRs]) using the Fitzpatrick Wrinkle and Elastosis Scale (FWS) [21]. The FWS is a clinically validated assessment tool used to assess skin wrinkle severity and elastosis on a scale from 1 through 9 (Table 1). Assessment of randomized baseline and 3‐month follow‐up images was performed by the blinded IPRs and included right, front, and left views.
Table 1.
Fitzpatrick Winkle and Elastosis Scale. The FWS is a Clinically Validated Assessment Tool Used to Assess Skin Wrinkle Severity and Elastosis on a Scale From 1 Through 9. Study Participants Were Required to Have a Wrinkle and Elastosis Score of 2 or Above
| Class | Description | Score | Description |
|---|---|---|---|
| I | Fine wrinkles | 1‐3 | Mild: Fine texture changes with subtly accentuated skin lines. |
| II | Fine to moderate depth wrinkles, moderate number of lines | 4‐6 | Moderate: Distinct papular elastosis (individual papules with yellow translucency under direct lighting) and dyschromia |
| III | Fine to deep wrinkles, numerous lines, with or without redundant skin folds | 7‐9 | Severe: Multipapular and confluent elastosis (thickened, yellow and pallid) approaching or consistent with cutis rhomboidalis |
Modified Global Aesthetic Improvement Scale (GAIS)
The modified GAIS is a subjective rating of improvement in baseline appearance [22]. Subjects and Investigators each rated subject appearance ranging from Very Much Improved to Very Much Worse (Table 2).
Table 2.
Modified Global Aesthetic Improvement Scale Evaluation (GAIS) for Investigators and Subjects. The Modified GAIS is a Subjective Rating of Improvement in Baseline Appearance. Subjects and Investigators Each Rated Subject Appearance Ranging From Very Much Improved to Very Much Worse
| Investigators/subjects rating | Description |
|---|---|
| Very Much Improved | Optimal cosmetic result from this procedure in this subject |
| Much Improved | Marked improvement in appearance from the initial condition, but not completely optimal for this subject |
| Improved | Obvious improvement in appearance from the initial condition |
| No Change | The appearance is essentially the same as the original condition |
| Worse | The appearance is worse than the original condition |
| Much Worse | The appearance is worse than the original condition |
| Very Much Worse | The appearance is worse than the original condition |
Study Endpoints
The primary efficacy endpoint was the proportion of subjects achieving individual treatment success, defined as a ≥1‐point improvement on the FWS at the 3‐month visit by at least two out of the three blinded IPRs. The secondary efficacy endpoint was a ≥1‐point improvement in the baseline FWS scores and at least an “Improved” rating on the modified GAIS at the 3‐month visit.
Additional efficacy endpoints were ≥1‐point improvement in FWS and ≥75% agreement with at least an “Improved” rating by the subject on the modified GAIS; mean change in baseline FWS at the 3‐month visit; subject satisfaction with the treatment procedure at the 3‐month visit; achievement of re‐epithelialization by facial zone and across all facial zones at the 10 day, 1‐, and 3‐month follow‐up visits as assessed by the investigator; mean duration until subject felt comfortable going out in public; and the proportion of subjects with correctly identified 3‐month images by at least two out of three blinded IPRs.
The primary safety endpoint included all reports of adverse events up to the 3‐month post‐treatment visit. The secondary safety endpoint was VAS pain and discomfort assessment after treatment and change in daily VAS pain assessment scores through the 10‐day follow‐up visit.
Statistical Analysis
The sample size was chosen to provide sufficient power for a statistical comparison based on a power calculation. Categorical data was provided as proportions and counts while continuous data were presented with the mean, median, minimum, maximum, or standard deviation. Statistics were produced using statistical software (SAS Version 9.3 or later; SAS Institute, Cary, NC; Kaleidagraph 4.0, Synergy Software, Reading, PA).
Ethics
Each subject provided signed informed consent prior to participating in any study‐related activities and a required release of subject images including possible use in publications. This protocol was approved by a commercial Institutional Review Board (Western Institutional Review Board, Puyallup, WA). ClinicalTrials.gov Identifier: NCT03286283.
RESULTS
Fifty‐five eligible subjects underwent the study procedure with the helium plasma device and completed the 6‐month follow‐up visit study requirements. The study cohort included 51 females (92.7%) and 4 males (7.3%) with an overall group average age of 61.5 years (±9.2 standard deviation [SD]) and a range of 31–82. Fitzpatrick Skin Scale Type I–III were enrolled; 4 (7.3%) Type I (white skin that never tans and always burns easily), 25 (45.5%) Type II (white skin that tans slightly and always burns easily), and 26 (47.3%) Type III (light brown skin that tans gradually and can burn moderately). All subjects enrolled in the study who had baseline FWS values were included in the full analysis set. The demographics and clinical characteristics of treated subjects are summarized in Table 3. Prior cosmetic treatments included filler injections (n = 21; 38.2%), neuromodulator injections (n = 22; 40.0%), facelift procedures (n = 15; 27.3%), laser resurfacing (n = 12; 21.8%), chemical resurfacing (n = 7; 12.7%), and fat transplant (n = 5; 9.1%).
Table 3.
Demographics and Baseline Characteristics. Aggregate Data From the Full Study Cohort of 55 Subjects Were Used to Develop Overall Demographics and Baseline Characteristics
| Mean age (SD), years | 61.5 (9.2), range 31–82 |
|---|---|
| Gender, n (%) | |
| Female | 51 (92.7) |
| Male | 4 (7.3) |
| Race/ethnicity, n (%) a | |
| White | 48 (87.3) |
| Hispanic or Latino | 10 (18.2) |
| American Indian/Alaska Native | 1 (1.8) |
| Mean weight (SD), kg | 69.0 (13.1), range 41–110 |
| Mean height (SD), cm | 165.0 (6.9), range 150–178 |
| Fitzpatrick skin type | |
| Type I | 4 (7.3) |
| Type II | 25 (45.5) |
| Type III | 26 (47.3) |
| Sun exposure, n (%) | |
| Extensive | 16 (29.1) |
| Natural | 32 (58.2) |
| None | 7 (12.7) |
| Tobacco use, n (%) | |
| None | 36 (65.5) |
| Past history only, n (%) | 17 (30.9) |
| Current smoker | 2 (3.6) |
| Packs per day | 0.9 (0.4) |
| Alcohol use, n (%) | |
| None | 12 (21.8) |
| 1–2 drinks weekly | 32 (58.2) |
| 3–4 drinks a weekly | 11 (20.0) |
SD, standard deviation.
Race and ethnicity were not mutually exclusive.
All 55 subjects were treated with a single, non‐overlapping pass of helium plasma with a helium flow rate of 4 L/min and power level of 4 to 40% (Table 4). Fifty‐four subjects received treatment in the perioral area (Zone 1) and 47 subjects received treatment in the periorbital area (Zone 2). Most subjects underwent full‐face treatment with the forehead (Zone 3) treated in 51, the nose (Zone 4) treated in 49, and the cheeks (Zone 5) treated in 51 (Table 4). Mean total volume of injected tumescent was 52.7 ml; mean volumes of tumescent per treatment area were also recorded (Table 4). At the investigators’ discretion, some subjects were given anxiolytic/sedative and/or pain medication prior to treatment. Mean (SD) procedure time (start of helium plasma treatment) was 44.0 (16.9) minutes and ranged from 14 to 109 minutes.
Table 4.
Study Treatment Parameters. Aggregate Data for Each Facial Zone for cc Tumescent Used and % Power Used for Subjects That Underwent Helium Plasma Single Pass Treatment in Each of the Five Different Facial Zones
| Tumescent (cc) | Power (%) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| n | Range | Range | |
| Zone 1 (PORL) | 54 | 9.5 ± 7.4 | 19.6 ± 2.7 |
| 1–40 | 10–30 | ||
| Zone 2 (PORB) | 47 | 5.9 ± 4.5 | 18.1 ± 4.4 |
| 0–16 | 5–20 | ||
| Zone 3 (Forehead) | 51 | 9.4 ± 11.5 | 24.1 ± 8.8 |
| 0–50 | 10–40 | ||
| Zone 4 (Nose) | 49 | 2.9 ± 2.4 | 22.7 ± 8.9 |
| 0–10 | 4–40 | ||
| Zone 5 (Cheeks) | 51 | 25.0 ± 18.8 | 23.7 ± 8.9 |
| 1–80 | 10–40 |
SD, standard deviation.
None of the subjects were lost to follow‐up (i.e., all subjects completed all required study visits and underwent photographic images acquisition at all study visits). A total of 42 protocol deviations occurred in the study. There were 17 cases where the informed consent was not appropriately obtained (subject name and date were pre‐filled by study staff on ICF form), 7 cases where follow‐up visits occurred outside of the pre‐defined visit window, 12 cases with procedure deviation (Zones 1 or 2 treated above or below 20% power: Zone 1 with 3 subjects at 10% power and 1 subject with 30% power; Zone 2 with 2 subjects at 5% power and 6 subjects at 10% power), 3 cases where post‐procedural care were not done per protocol instructions, 2 cases where subjects were enrolled with one of the Exclusion Criteria (history of Diabetes Mellitus), and 1 case where the daily diary was not completed. In addition, 17 subjects were given medication (methylprednisolone mini burst and taper) to alleviate significant facial swelling following treatment.
Efficacy Endpoints
The primary efficacy endpoint of ≥1‐point improvement in baseline FWS scores as assessed by blinded IPRs at the 3‐month visit was achieved by 35 subjects (63.64%) in the full analysis population (N = 55), whereas study investigators noted a >1‐point improvement in baseline FWS at the 3‐month visit in 54 of 55 subjects (98.18%). Interrater reliability amongst the three IPRs in their determination of baseline and 3‐month FWS scores was assessed via determination of the Intraclass Correlation Coefficient (ICC, an assessment of the consistency, or conformity, of measurements made by multiple observers measuring the same quantity). IPR ICCs for baseline and 3‐month FWSs were 0.82 and 0.72 indicating good and moderate interrater reliability, respectively.
The secondary efficacy endpoint was the proportion of subjects with a ≥1‐point improvement in FWS scores and at least an “Improved” rating on the modified GAIS at the 3‐month visit: 61.82% as assessed by IPRs versus 96.36% for study investigators. Among these subjects, the modified GAIS ratings were Very Much Improved (n = 7; 12.96%), Much Improved (n = 32; 59.26%), Improved (n = 14; 25.93%) or No Improvement (n = 1; 1.85%); the subject with <1‐point change in FWS score was rated as No Improvement on the modified GAIS scale.
Subgroup analysis of 3‐month primary endpoint FWS as assessed by study investigators and blinded IPRs included stratification by Fitzpatrick Skin Score, Age, Study Site and use of oral steroid medication following treatment (Table 5). A 3‐month net FWS change (with negative values indicating aesthetic improvement) ≥1 was observed in all subgroups as assessed by study investigators but in only 4 of the 10 subgroups as assessed by blinded IPRs with two additional subgroups narrowly missing inclusion as assessed by blinded IPRs (Fitzpatrick Skin Scale II subgroup, −0.99 FWS and Study Site 01 subgroup, FWS −0.95) (Table 5). The highest percentage of responders (≥1 FWS score change based on IPR assessed FWS) of 76.92% (n = 20/26) was observed in subjects with Fitzpatrick Skin Scale Type III, with subjects of skin Type II and Type I exhibiting similar but lower rates of 52% (n = 13/25) and 50% (n = 2/4), respectively. Hispanic/Latino and White subjects were determined to be a “treatment responders” based on a ≥1 FWS score change as assessed by the IPRs in 71.43% (n = 5/7) and 68.18% (n = 30/44) subjects, respectively. The majority of subjects age 62 or above (70.97%; n = 22/31) showed a ≥1 FWS score change based on IPR assessment. Thirty‐three out of 51 females (64.71%) and 2 out of 4 of male (50%) subjects demonstrated a ≥1 FWS score change based on IPR assessment.
Table 5.
Baseline and 3‐Month FWS Data, Subgroup Analysis. Stratification of the Full Study Cohort by Fitzpatrick Skin Scale, Age, Study Site, and Post‐Treatment Oral Steroid Use With Baseline and 3‐Month FWS Values (SD) and 3‐Month Net FWS Change (Delta, Δ) Shown for Investigators Versus IPRs
| Baseline FWS | 3‐Month FWS | 3‐Month Net FWS Δ | |||||
|---|---|---|---|---|---|---|---|
| Subgroup | Full cohort N = 55 | Investigator | IPR | Investigator | IPR | Investigator | IPR |
| Fitzpatrick Type I | n = 4 | 4.25 (±1.5) | 4.83 (±2.2) | 2.5 (±1.0) | 4.08 (±2.2) | −1.75 | −0.75 |
| Fitzpatrick Type II | n = 25 | 5.24 (±1.2) | 5.92 (±2.4) | 2.96 (±1.0) | 4.93 (±2.3) | −2.28 | −0.99 |
| Fitapatrick Type III | n = 26 | 5.04 (±1.5) | 6.72 (±2.2) | 3.00 (±0.9) | 5.58 (±2.2) | −2.04 | −1.14 |
| Age ≥ 62 | n = 31 | 5.32 (±1.4) | 7.00 (±1.9) | 3.00 (±1.1) | 5.72 (±2.1) | −2.32 | −1.28 |
| Age ≤ 61 | n = 24 | 4.75 (±1.3) | 5.21 (±2.6) | 2.88 (±0.7) | 4.47 (±2.3) | −1.87 | −0.74 |
| Study Site 01 | n = 22 | 4.4 (±1.4) | 5.54 (±0.77) | 2.9 (±1.1) | 4.59 (±0.84) | −1.5 | −0.95 |
| Study Site 02 | n = 11 | 6.2 (±1.1) | 6.34 (±1.09) | 3.6 (±0.7) | 5.53 (±1.19) | −2.6 | −0.81 |
| Study Site 03 | n = 22 | 5.2 (±1.1) | 7.83 (±0.78) | 2.6 (±0.7) | 6.05 (±0.83) | −2.6 | −1.78 |
| Post‐tx oral steroid | n = 17 | 5.2 (±1.1) | 7.37 (±1.3) | 2.7 (±0.7) | 5.71 (±1.9) | −0.5 | −1.66 |
| No post‐tx oral steroid | n = 38 | 5.0 (±1.5) | 5.70 (±2.5) | 3.1 (±1.0) | 4.94 (±2.4) | −1.9 | −0.76 |
FWS, Fitzpatrick Wrinkle and Elastosis Scale; IPR, independent photographic reviewer; SD, standard deviation.
Representative before and 3‐ and 6‐month post‐treatment digital photographs are shown in Figures 1, 2 and 3.
Figure 1.
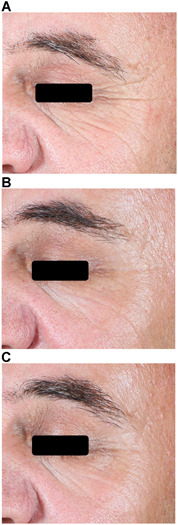
Helium plasma dermal resurfacing in 51‐year‐old male, Fitzpatrick Skin Scale (FWS) III. Before (A), 3‐month (B) and 6‐month (C) VISIA‐CR photographs. Zones 2, 3, 4, and 5 treated at 20% power (except 30% power Zone 4), single pass, 4 L/min helium gas flow—significant improvement of Zone 2 (peri‐orbital) lines evident by month 3 and maintained at month 6. Baseline FWS Investigator and IPR 7 and 8, respectively. Three‐month FWS Investigator and IPR 5 and 7, respectively. Three‐month FWS net change Investigator and IPR‐2 and ‐1, respectively. IPRs, independent photographic reviewers.
Figure 2.
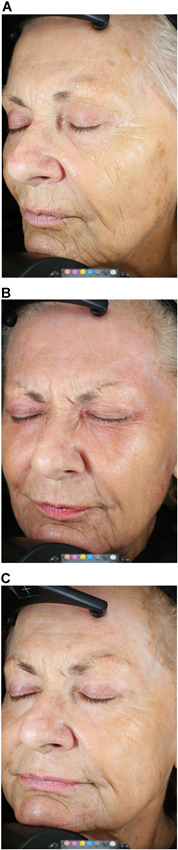
Helium plasma dermal resurfacing in 82‐year‐old female, Fitzpatrick Skin Scale III. Before (A), 3‐month (B), and 6‐month (C) VISIA‐CR photographs. Zones 1 through 5 treated at 20% power, single pass, 4 L/min helium gas flow—significant improvement of Zones 1 (peri‐oral), 2 (peri‐orbital), and 3 (cheeks) with reduction of dynamic and static facial lines evident by month 3 and maintained at month 6. Baseline FWS Investigator and IPR 7 and 9, respectively. Three‐month FWS Investigator and IPR 3 and 6, respectively. Three‐month FWS net change Investigator and IPR‐4 and ‐3, respectively. IPRs, independent photographic reviewers.
Figure 3.
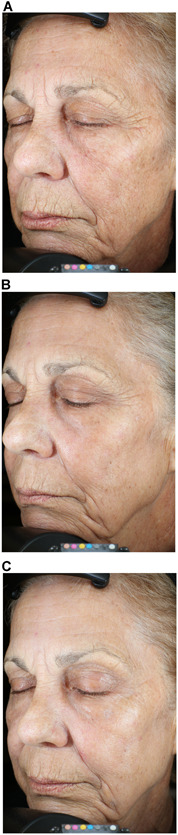
Helium plasma dermal resurfacing in 63‐year‐old female, Fitzpatrick Skin Scale III. Before (A), 3‐month (B), and 6‐month (C) VISIA‐CR photographs. Zones 1 through 5 treated at 20% power, single pass, 4 L/min helium gas flow−significant improvement of Zones 1 (peri‐oral), 2 (peri‐orbital), and 3 (cheeks) with reduction of dynamic and static facial lines evident by month 3 and further improved at month 6. Baseline FWS Investigator and IPR 6 and 9, respectively. Three‐month FWS Investigator and IPR 4 and 8, respectively. Three‐month FWS net change Investigator and IPR‐2 and ‐1, respectively. IPRs, independent photographic reviewers.
Additional Endpoints
Using the modified GAIS, most subjects (n = 50; 90.9%) self‐reported improvement in appearance at 3 months post‐procedure, specifically, Very Much Improved (n = 11; 20.4%), Much Improved (n = 17; 31.5%) and Improved (n = 22; 40.0%); five subjects (9.1%) rated themselves as No Improvement (Fig. 4). At 3‐month post‐treatment, the investigators reported a mean (SD) change in baseline FWS of −2.13 (1.02) points, which is indicative of clinically significant improvement.
Figure 4.
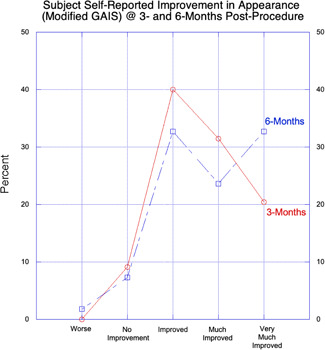
Subject self‐reported improvement in appearance (modified GAIS) at 3‐ and 6‐month post‐procedure with percent improvement at 3‐ and 6‐month on y‐axis and five step grading system on x‐axis. Number of respondents at 3‐month (n = 50) slightly higher than at 6‐month (n = 48). A greater percentage of subjects reported “Very Much Improved” at 6‐month (32.7%) versus 3‐month (20.4%). Although 1 subject (1.8%) self‐reported “Worse” at 6‐month GAIS the 6‐month VAS satisfaction for this subject was “1” on 0–10 scale with “0” = best. GAIS, Global Aesthetic Improvement Scale.
Among subjects judged by investigators to have achieved a ≥1‐point improvement in FWS scores (n = 54), the mean (SD) improvement in subjects VAS satisfaction scores was 2.6 (3.0) points at 3 months.
Facial re‐epithelialization was assessed at the 10‐day, 1‐, and 3‐month follow‐up visits. An overall mean of 96.8% re‐epithelization was achieved for all treated zones at the 10‐day follow‐up visit and 100% was achieved at the 1‐month follow‐up visit.
At the 10‐day follow‐up visit, more than half of subjects (n = 31; 56.4%) said they felt comfortable going out in public. Subjects felt comfortable going out in public after a mean (SD) of 8.5 (2.5) days following facial helium plasma skin regeneration treatment.
An unbiased IPR assessment of treatment responders was performed in which baseline and 3‐month images were presented simultaneously in random order to blinded IPRs who were asked to identify the 3‐month images. Treatment success was achieved when at least two of three IPRs correctly identified the 3‐month images. Using this analysis, 54 subjects (98.18%) achieved treatment success.
Safety Endpoints
Overall, 80 adverse events (AEs) were reported by 39 subjects (70.9%) (Table 6). AEs reported in >1 subject included hypersensitivity to treatment (resulting in erythema, swelling, induration and/or urticaria) (n = 26; 47.3%), post‐inflammatory hyperpigmentation (n = 8; 14.5%), acne (n = 5; 9.1%), itching (n = 4; 7.3%), prolonged wound healing (n = 4; 7.3%), pain (n = 3, 5.5%), sensitivity to topical care (n = 3; 5.5%), hypertrophic scarring (n = 2; 3.6%), bleeding (n = 2; 3.6%) and systemic events (flu‐like symptoms) (n = 2; 3.6%). Two severe AEs were bronchitis and folliculitis associated with MRSA; however, neither were treatment related. The remaining AEs were mild to moderate in severity. For many subjects (n = 23; 41.8%), AEs resolved within 7 days while the majority of those remaining (n = 41; 55.3%) resolved within 14 days. Focal hypertrophic scarring in two subjects (lower chin area in both subjects) required serial triamcinolone injections (10 mg/ml) and several months to resolve.
Table 6.
Adverse Events, Full Cohort. Anticipated and Non‐Anticipated Adverse Events by Type With Number and PercentNon‐Treatment Related AEs Included MRSA Folliculitis, New Onset Hypothyroidism, Injury, Diarrhea, Rash, Contact Dermatitis, Pain, Worsening Acne, and Bronchitis
| n | Percent | |
|---|---|---|
| Anticipated | ||
| Hypersensitivity with one or more of: edema, erythema, induration, urticaria | 30 | 37.50 |
| Post‐inflammatory hyperpigmentation (temporary) | 8 | 10.00 |
| Acne | 5 | 6.25 |
| Pruritis | 5 | 6.25 |
| Pain | 2 | 2.50 |
| Transient bleeding | 2 | 2.50 |
| Subtotal | 52 | 65.00 |
| Non‐anticipated | ||
| Other treatment (device or procedure) related^ | 11 | 13.80 |
| Non‐treatment related^^ | 9 | 11.20 |
| Prolonged healing | 4 | 5.00 |
| Hypertrophic scarring | 2 | 2.50 |
| Systemic effects | 2 | 2.50 |
| Subtotal | 28 | 35.00 |
Other Treatment related aes included milia, dry eyes, eye irritation, focal skin congestion with inflammation, sensitivity to topical care (3 subjects), weeping wound, blurred vision (2 subjects), conjunctivitis.
Non‐treatment related AEs included MRSA folliculitis, new onset hypothyroidism, injury, diarrhea, rash, contact dermatitis, pain, worsening acne, and bronchitis.
The mean VAS pain scores decreased from a high of 4.3 (2.6) (N = 55) immediately following the procedure decreasing to 1.8 (3.6) on post‐procedure day 10 (n = 42).
Additional 6‐month data
At 6‐month post‐treatment, the investigators reported a mean (SD) change in baseline FWS of −3.0 (0.92) points which is indicative of clinically significant improvement. Using the modified GAIS, most subjects (n = 49; 89.1%) self‐reported improvement in appearance at 6 months post‐procedure, specifically, Very Much Improved (n = 18; 32.7%), Much Improved (n = 13; 23.6%) and Improved (n = 18; 32.7%); four subjects (7.3%) rated themselves as No Improvement, one subject stated that appearance had worsened and one subject not included due to incorrectly completing the questionnaire (Fig. 4). For the one subject that stated “worse”, the 6‐month VAS satisfaction was “1” on 0–10 scale with “0” = best and this subject indicated “Perhaps” would recommend to a friend. Mean VAS (SD) pain score was 0.33 (1.3) (N = 55). Mean (SD) VAS satisfaction score was 3.2 (3.3) (N = 55) wherein 91% of subjects surveyed would or perhaps would recommend the procedure to a friend.
DISCUSSION
Following treatment with a single, non‐overlapping pass of helium plasma, almost all subjects achieved treatment success based on IPR assessment with the appropriate selection of the post‐treatment photograph in a comparison review of pre‐ and post‐treatment photographs in randomized side‐by‐side order and many achieved a ≥1‐point improvement in FWS scores. Almost all demonstrated substantial improvements in subject GAIS scores at both 3‐ and 6‐month with an increased percentage reporting “Very Much Improved” at the 6‐month endpoint (Fig. 4). The mean decrease in FWS scores as assessed by study investigators was 2.1 points at 3‐month and further decreased to 3.0 points at 6‐month, indicating significant clinical improvement in facial appearance and interval improvement between 3‐ and 6‐month post‐procedure. The mean decrease in investigator FWS scores at both 3‐ and 6‐month post‐procedure are statistically significant (unpaired t test) with P ≤ 0.0001 at 3‐month (N = 55) and P ≤ 0.0001 at 6‐month (N = 55).
Overall subject satisfaction with treatment results was high (90.9% self‐reported improvement in appearance at 3‐month post‐procedure using modified GAIS). These improvements occurred both in subjects who were aesthetic treatment naive and who had undergone previous facial rejuvenation procedures including filler injections, neuromodulator injections, face‐lift procedures, and laser skin resurfacing. Facial skin re‐epithelialization was typically complete within 10 days at which time most subjects reported being comfortable going out in public.
When presented with both baseline and 3‐month post‐procedure images two of three IPRs correctly identified the 3‐month images in 54 of 55 subjects (98.18%). However, while the primary efficacy endpoint of ≥1‐point improvement in baseline FWS as assessed by blinded IPRs at the 3‐month visit was achieved in 64.64% in the full analysis population (N = 55), study investigators noted a >1‐point improvement in baseline FWS at the 3‐month visit in 54 of 55 subjects (98.18%). Although interrater agreement amongst IPRs was good for baseline FWS assessment and only moderate for 3‐month efficacy endpoint FWS assessment (see Results section), we do not believe that the interrater reliability data undermine our conclusions as to potential reasons for the disparities in the IPR versus Investigator FWS baseline and 3‐month primary endpoint assessment data (see following).
These positive results are in keeping with preclinical studies that indicate that substantial skin tissue contraction may be achieved with the new helium plasma technology [19]. The results are also significant given that only a single pass with a power setting of 20% was used in the majority of subjects; for perspective, 20% power is on the lower end of useful (above minimum tissue coupling threshold) power for the device and correlates to an energy density approximately 40% lower than that of nitrogen plasma at 4.0 J and 2.5 Hz [19]. Anecdotally, off‐label treatments that have included higher power and two or more passes have achieved very significant skin tissue remodeling in patients with severe rhytidosis and skin laxity.
This initial clinical study was designed for use of the helium plasma device in a similar energy density range to that commonly employed with the predicate nitrogen plasma device while also keeping in mind that helium PDR is a full field treatment with continuous energy delivery versus static energy pulses (Guassian) delivered with the predicate device. Energy density data have been previously reported: 14.1 J/cm2 for nitrogen plasma at 4.0 J with offset appropriate for a 6 mm spot and with a treatment speed of 2.5 Hz versus 8.6 J/cm2 for helium plasma at 20% power with a 3 mm continuous beam and optimal treatment speed of 1 cm per second [19]. As helium plasma treatment was performed with a free hand painting technique during this study and as the energy density for helium plasma treatment varies inversely with treatment tip velocity visual cues (superficial skin tissue coagulation with frosting and darkening) were used during treatment to assess adequacy of treatment.
Investigator FWS improvement score changes from baseline to 3‐month primary endpoint exceeded those of the IPRs for each study site and all other measures were similarly positive amongst the three study sites. Although IPR ratings were based on review of stored photographic images, investigators evaluated subjects in person face to face when determining baseline and 3‐month primary endpoint FWSs, thereby benefiting from a greater three‐dimensional assessment, that is, depth perception by the human eye observing a live subject, that cannot be matched by two‐dimensional photography.
Although confirmation bias should be considered to have influenced the unblinded investigators’ FWS assessments, investigator versus IPR baseline FWSs stratified by study site reveal higher baseline grading by IPRs for two of three study sites, that is, the unblinded investigators demonstrated greater conservatism (lower wrinkle severity assessments) in baseline FWS assessments compared to the IPRs. At 3‐month post‐treatment, FWSs stratified by study site reveal a higher grading by IPRs for all three study sites, that is, unblinded investigators also demonstrated greater conservatism (lower wrinkle severity assessments) in 3‐month FWS assessments compared with the IPRs. IPRs evaluated single images presented in random order where, in contrast to unblinded investigator assessments, no baseline image was available for comparison to 3‐month post‐treatment images.
Although one might suggest that non‐treatment of or treatment with reduced power in Zone 1 and Zone 2 treatment areas may have negatively impacted IPR 3‐month versus baseline FWS assessments, IPRs were not asked to give FWSs by zone but as a global assessment of the entire face. Nevertheless, we analyzed Study Site 01 IPR FWS data (Study Site 01 accounted for the majority of subjects that did not undergo full face treatment) further and found no statistically significant difference (unpaired t test, data not shown) when comparing 3‐month FWS versus baseline FWS for entire subgroup (n = 22), per protocol subgroup with full face treatment (n = 16) or group with non‐treatment or treatment with reduced power in zones 1 and 2 (n = 8). In addition, we found that IPRs graded 5 of 9 subjects that did not undergo treatment in Zone 1 and/or Zone 2 with a 3‐month versus baseline FWS improvement of 1—this presumably reflects lack of need for treatment in Zones 1 and/or 2 and modest improvement in adjacent treatment areas. These findings are supportive of the integrity of the study protocol related to IPR FWS grading.
It is difficult to reconcile the possibility of confirmation bias among the unblinded investigators with their more conservative baseline FWS grading and with clear advantages of three‐dimensional depth perception and availability of baseline images for comparison when performing 3‐month FWS assessments. Despite greater conservatism in baseline FWS grading, the net change (higher negative score indicating greater observed improvement) in FWS from baseline to the 3‐month primary endpoint was consistently greater for the unblinded investigators’ versus IPR assessments. In addition, it is apparent that in some cases (e.g., Fig. 3), significant additional improvement of facial rhytids occurred between 3 and 6 months post‐treatment; this suggests that the peak for maximum improvement of FWS was not captured in the 3‐month primary endpoint data for all subjects. Examples of FWS grading disparities between unblinded investigators and IPRs at baseline and 3‐month post‐treatment are detailed in Figures 1, 2, and 3.
Treatment‐related adverse events were mild‐to‐moderate in severity and most were anticipated following a skin resurfacing procedure. Treatment‐related discomfort was moderate and largely resolved by day 10. Observed temporary side effects that are common among energy‐based resurfacing treatments (and tabulated as anticipated AEs in this study) included erythema, swelling, induration, pruritis, exacerbation of acne, sensitivity to topical care and post‐inflammatory hyperpigmentation. Among non‐anticipated AEs prolonged wound healing (time to re‐epithelialization >10 days) in discrete focal areas was observed in four subjects (7.3%); a similar phenomenon has been observed previously with nitrogen plasma skin regeneration treatment and it has been suggested that the likelihood of delayed healing events is higher in areas where the skin may be thinner and/or less vascular (e.g., peripheral areas of the forehead, temples, cheeks, jawline, and chin). Hypertrophic scarring after skin resurfacing treatments, including in rare cases with the predicate nitrogen plasma technology, has been associated with treatment of the skin in permissive areas where the skin may be thinner and/or less vascular (e.g., neck) as well as with over‐treatment, wherein the energy density introduced into the tissue exceeds the threshold for normal repair without scarring [23]. In this study, two patients had focal hypertrophic scarring in the chin area that responded favorably to intralesional triamcinolone injections. Stratification of AEs versus FSS does not show any trend toward increased AEs versus FSS (Table 7).
Table 7.
Adverse Events (Percent) by Fitzpatrick Skin Scale. Anticipated and Non‐Anticipated Adverse Events Stratified by Fitzpatrick Skin Scale
| FSS I (n = 4) | FSS II (n = 25) | FSS III (n = 26) | |
|---|---|---|---|
| Anticipated | |||
| Hypersensitivity with one or more of: edema, erythema, induration, urticaria | 2 (50) | 14 (56) | 14 (54) |
| Post‐inflammatory hyperpigmentation (temporary) | 0 (0) | 4 (16) | 4 (150 |
| Acne | 0 (0) | 2 (8) | 3 (12) |
| Pruritis | 0 (0) | 1 (4) | 0 (0) |
| Pain | 1 (25) | 0 (0) | 1 (4) |
| Transient bleeding | 0 (0) | 1 (4) | 1 (4) |
| Non‐Anticipated | |||
| Other treatment (device or procedure) related | 1 (25) | 5 (20) | 5 (19) |
| Non‐treatment related | 0 (0) | 5 (20) | 4 (15) |
| Prolonged healing | 1 (25) | 2 (8) | 1 (4) |
| Hypertrophic scarring | 0 (0) | 1 (4) | 1 (4) |
| Systemic effects | 1 (25) | 0 (0) | 1 (4) |
Further evaluation of 3‐month primary endpoint FWSs involved stratification by Fitzpatrick Skin Score, Age, Study Site and oral steroid use after treatment as well as Gender. Study subjects were predominantly Caucasian and Female, therefore no significant trends could be determined for Race/Ethnicity or Gender. Interesting trends were observed, however, for Age, Fitzpatrick Skin Score and post‐treatment oral steroid use.
Dividing the study cohort by age at 61 and below versus 62 and over revealed greater FWS improvement for the subgroup age 62 and over. Decreased dermal collagen content and more widespread and deeper rhytidosis correlates with advancing age [24]. A finding of greater improvement with slightly thinner and therefore more compliant skin in the older subgroup is an expected finding that is consistent with observed skin response with other dermal resurfacing technologies.
Interestingly an inverse trend toward lower FWS (greater improvement) was observed with increasing Fitzpatrick Skin Score where Fitzpatrick Skin Score III subjects exhibited the greatest FWS improvement. Historically, higher Fitzpatrick Skin Scores are correlated with less aggressive laser skin resurfacing treatments and generally less improvement than could be achieved with lighter skin subjected to more aggressive treatments [25]. These study results suggest that helium plasma dermal resurfacing may enable improved skin resurfacing outcomes with preservation of natural skin tone in intermediate skin types.
The greater FWS improvement in the subgroup that received oral steroids post‐treatment suggests that a greater inflammatory response was observed that may correlate with higher energy densities delivered during treatment despite protocol disallowance of energy level variance in the peri‐orbital and peri‐oral treatment areas. Variation in handpiece (treatment tip) velocity during treatment amongst the study sites and subjects could not be controlled; inadvertent increases or decreases in velocity of tip movement from the desired speed of 1 cm per second would have inversely impacted energy density delivered to the tissue.
Compared with the Gaussian nature of energy delivery with the predicate nitrogen plasma device, the more complete full field delivery of RF energy to the skin tissue with the helium plasma device likely increases its potential for effective wrinkle reduction [19]. Within the treatment parameters of this study, the potential for delayed healing and hypertrophic scarring, however, does not appear to be increased compared to resurfacing devices of similar effectiveness. Nonetheless, careful attention to target tissue concerns, treatment tip speed and device settings remains important in the mitigation of unanticipated side effects and complications.
Users new to the helium plasma dermal resurfacing must be aware of the need for electrical coupling (grounding pad required for treatment) and the related absolute contraindications for treatment (implanted electrical devices). Helium plasma radiofrequency tissue coupling occurs when sufficient energy is applied in sufficient proximity to grounded tissue. The maximum electrical tissue coupling distance is approximately 6 mm—moving the treatment tip beyond this distance eliminates all tissue effects. Although a 3 mm offset distance from treatment tip to targeted tissue is considered optimal, negligible variance occurs in energy delivered to the tissue when the treatment tip is maintained within the tissue coupling range (just over 0 mm to approximately 6 mm). Touching the skin should be avoided because of disruption of the helium plasma beam and the potential for mechanical trauma and deeper tissue injury.
The Lewis Rayleigh afterglow phenomenon creates a visible violet white “beam” during treatment that obviates the need for a separate aiming beam. Although the helium plasma beam is approximately 3 mm in diameter, the beam will tend to widen somewhat as the impedance of treated tissue increases and the radiofrequency energy is passively redirected to adjacent untreated tissue with lower impedance values. During the initial pass, the treated tissue “frosts” and often darkens making it quite simple for the treater to distinguish treated from untreated tissue. Although precise edge‐to‐edge coverage is desirable, wherein all tissue is evenly coagulated in sequential linear fashion with no skip areas, narrow gaps that may occur do not appear to negatively affect outcomes. If present, larger gaps should be treated to ensure homogenous energy delivery.
As the helium plasma dermal resurfacing technology emerges, it is now apparent that three treatment approaches are currently available: single pass treatment (as used in this study), double (or more) pass treatment and a blend of single and double pass treatment as appropriate in different facial regions. The single pass helium dermal resurfacing treatment wherein the treated (desiccated) outer skin layer is left intact during initial healing is similar to the protocol for the predicate nitrogen plasma technology. Although not relevant to the study detailed herein, as impedance dramatically increases in desiccated tissue, the coagulated tissue must be wiped away before a second pass is performed to ensure optimum RF coupling and absorption of the helium plasma RF energy [26].
CONCLUSION
The results of this initial low energy, single pass study indicate that the helium plasma device has the potential for effective, safe treatment of facial rhytidosis: treated subjects achieved significant improvements in facial appearance with rapid recovery, relatively few unanticipated adverse events following treatment and overall subject satisfaction with aesthetic improvements was high. Greater FWS improvements were correlated with age 62 and above and with higher Fitzpatrick Skin Scale scores (Type III > Type II and Type I). Additional studies to evaluate the safety and effectiveness of higher energy levels, multiple treatment passes, and fractional treatment using helium plasma are ongoing.
ACKNOWLEDGEMENTS
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript. This study was sponsored by the Apyx Medical Corporation, Clearwater, FL.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Study participants’ disclosed holdings in Apyx Medical Corporation include privately purchased stock (DeLozier) and stock options (DeLozier).
REFERENCES
- 1. Manuskiatti W, Fitzpatrick RE, Goldman MP. Long‐term effectiveness and side effects of carbon dioxide laser resurfacing for photoaged facial skin. J Am Acad Dermatol 1999;40(3):401–411. [DOI] [PubMed] [Google Scholar]
- 2. Ward PD, Baker SR. Long‐term results of carbon dioxide laser resurfacing of the face. Arch Facial Plast Surg 2008;10(4):238–243. 10.1001/archfaci.10.4.238 [DOI] [PubMed] [Google Scholar]
- 3. Holcomb JD. Erbium YAG laser skin resurfacing In: Truswell William H., editor. Chapter 6: Lasers and Light, Peels and Abrasions: For Health, Beauty and Disease. New York, NY: Thieme Medical Publishers; 2016. [Google Scholar]
- 4. Mancini PF. Coblation: A new technology and technique for skin resurfacing and other aesthetic surgical procedures. Aesthetic Plast Surg 2001;25(5):372–377. [DOI] [PubMed] [Google Scholar]
- 5. Beasley K, Dai JM, Brown P, Lenz B, Hivnor CM. Ablative fractional versus nonablative fractional lasers—Where are we and how do we compare differing products. Curr Derm Rep 2013;2:135–143. 10.1007/s13671-013-0043-0 [DOI] [Google Scholar]
- 6. Lee HM, Haw S, Kim JK, Chang SE, Lee MW. Split‐face study using a 1,927‐nm thulium fiber fractional laser to treat photoaging and melasma in Asian skin. Dermatol Surg 2013;39(6):879–888. 10.1111/dsu.12176 [DOI] [PubMed] [Google Scholar]
- 7. Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: Traditional versus fractional. Dermatol Surg 2014;40(Suppl 12):S147–S151. 10.1097/DSS.0000000000000230 [DOI] [PubMed] [Google Scholar]
- 8. Brauer JA, McDaniel DH, Bloom BS, Reddy KK, Bernstein LJ, Geronemus RG. Nonablative 1927 nm fractional resurfacing for the treatment of facial photopigmentation. J Drugs Dermatol 2014;13(11):1317–1322. [PubMed] [Google Scholar]
- 9. Waibel S, Pozner J, Robb C, Tanzi E. Hybrid fractional laser: A multi‐center trial on the safety and efficacy for photorejuvenation. J Drugs Dermatol 2018;17(11):1164–1168. [PubMed] [Google Scholar]
- 10. Gold M, Taylor M, Rothaus K, Tanaka Y. Non‐insulated smooth motion, micro‐needles RF fractional treatment for wrinkle reduction and lifting of the lower face: International study. Lasers Surg Med 2016;48(8):727–733. 10.1002/lsm.22546 [DOI] [PubMed] [Google Scholar]
- 11. Kilmer S, Semchyshyn N, Shah G, Fitzpatrick R. A pilot study on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med Sci 2007;22(2):101–109. [DOI] [PubMed] [Google Scholar]
- 12. Foster KW, Moy RL, Fincher FF. Advances in plasma skin regeneration. J Cosmet Dermatol 2008;7(3):169–179. [DOI] [PubMed] [Google Scholar]
- 13. Holcomb JD, Kent KJ, Rousso DE. Nitrogen plasma skin regeneration and aesthetic facial surgery: Multicenter evaluation of concurrent treatment. Arch Facial Plast Surg 2009;11(3):184–193. [DOI] [PubMed] [Google Scholar]
- 14. Fitzpatrik R, Bernstein E, Iyer S, Brown D, Andrews P, Penny K. A histopathologic evaluation of the Plasma Skin Regeneration System (PSR) versus a standard carbon dioxide resurfacing laser in an animal model. Lasers Surg Med 2008;40(2):93–99. [DOI] [PubMed] [Google Scholar]
- 15. Potter MJ, Harrison R, Ramsden A, Bryan B, Andrews P, Gault D. Facial acne and fine lines: transforming patient outcomes with plasma skin regeneration. An Plast Surg 2007;58(6):608–613. [DOI] [PubMed] [Google Scholar]
- 16. Elsaie ML, Kammer JN. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J Cosmet Dermatol 2008;7(4):309–311. [DOI] [PubMed] [Google Scholar]
- 17. Bentkover SH. Plasma skin resurfacing: Personal experience and long‐term results. Facial Plast Surg Clin North Am 2012;20(2):145–162. [DOI] [PubMed] [Google Scholar]
- 18. Theppornpitak N, Udompataikul M, Chalermchai T, Ophaswongse S, Limtanyakul P. Nitrogen plasma skin regeneration for the treatment of mild‐to‐moderate periorbital wrinkles: A prospective, randomized, controlled evaluator‐blinded trial. J Cos Dermatol 2019;18(1):163–168. [DOI] [PubMed] [Google Scholar]
- 19. Holcomb JD, Schucker A. Helium plasma skin regeneration—Evaluation of skin tissue effects in a porcine model and comparison to nitrogen plasma skin regeneration. Lasers Surg Med 2019;52:23–32. 10.1002/lsm.23167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scorring with a traditional paper‐based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev 2018;2:e088 10.5435/JAAOSGlobal-D-17-00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fitzpatrick RE, Goldman MP, Satur NM, MPhil WDT. Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch Dermatol 1996;132:395–402. [PubMed] [Google Scholar]
- 22. Vandeputte J. Real‐world experience with volume augmentation using cohesive polydensified matrix hyaluronic acid gel: A retrospective single‐center analysis of 110 consecutive patients with medium‐ to long‐term follow‐up. J Clin Aesthet Dermatol 2018;11(12):30–39. [PMC free article] [PubMed] [Google Scholar]
- 23. Avram MM, Tope WD, Yu T, Szachowicz E, Neslon JS. Hypertrophic scarring of the neck following ablative fractional carbon dioxide laser resurfacing. Lasers Surg Med 2009;41(3):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khavkin J, Ellis DA. Aging skin: Histology, physiology, and pathology. Facial Plast Surg Clin North Am 2011;19:229–234. [DOI] [PubMed] [Google Scholar]
- 25. Alster TS, Tanzi EL. Laser surgery in dark skin. SKINmed Dermatol Clin 2007;2(2):80–85. 10.1111/j.1540-9740.2003.01664.x [DOI] [PubMed] [Google Scholar]
- 26. Holcomb JD. Plasma energy skin rejuvenation. Facial Plast Surg Clin N Am 2019;28(1):67–74. 10.1016/j.fsc.2019.09.006 [DOI] [PubMed] [Google Scholar]


