Abstract
Multiple system atrophy (MSA) is a rare and fatal neurodegenerative disease with limited symptomatic treatment options. Aggregation of α‐synuclein in oligodendrocytes is believed to be a central mechanism of the neurodegenerative process. PD01A and PD03A are 2 novel therapeutic vaccine candidates containing short peptides as antigenic moieties that are designed to induce a sustained antibody response, specifically targeting pathogenic assemblies of α‐synuclein. The objectives of the current study were to evaluate primarily the safety and tolerability of PD01A and PD03A in patients with early MSA. Thirty patients (11 women) were randomized to receive 5 subcutaneous injections of either PD01A (n = 12), PD03A (n = 12), or placebo (n = 6) in this patient‐ and examiner‐blinded, placebo‐controlled, 52‐week phase 1 clinical trial (ClinicalTrial.gov identifier: NCT02270489). Immunogenicity and clinical scores were assessed as secondary objectives. Twenty‐nine patients reported a total of 595 treatment‐emergent adverse events (mild or moderate, n = 555; severe, n = 40). Treatment‐related adverse events included 190 injection‐site reactions typically observed in vaccination trials with similar per‐subject incidence in the treatment groups over time. Sustained IgG titers were observed in the PD01A‐treated group, and 89% of treated patients developed a PD01‐specific antibody response after receiving all injections. Induced antibodies displayed clear reactivity to the α‐synuclein target epitope. Titers and antibody responder rate (58%) were lower in the PD03A‐treated group. In conclusion, both PD01A and PD03A were safe and well tolerated. PD01A triggered a rapid and long‐lasting antibody response that specifically targeted the α‐synuclein epitope. © 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: MSA, α‐synuclein, active immunization, treatment
Multiple system atrophy (MSA) is a debilitating and fatal neurodegenerative disease characterized by a variable combination of autonomic failure, parkinsonism, and cerebellar and pyramidal features. The estimated prevalence of this rare disease ranges between 1.9 and 4.9 per 100,000. 1 Clinically, 2 major subtypes are distinguished, one with predominant parkinsonism (MSA‐P) and one with dominating cerebellar impairment (MSA‐C). Both have poor prognosis. Current treatment options are limited and provide only some symptomatic relief. Therefore, disease‐modifying therapies remain an urgent unmet need. 2 , 3
The neuropathological hallmark is the accumulation of α‐synuclein in oligodendrocytes, forming glial cytoplasmic inclusions, 4 , 5 , 6 qualifying MSA as a synucleinopathy together with Parkinson's disease and Lewy body dementia. The origin of α‐synuclein in glial cytoplasmic inclusions remains under debate, and the exact mechanisms underlying the pathogenesis are only incompletely understood. Nevertheless, the accumulation of α‐synuclein aggregates and their cell‐to‐cell propagation are believed to play a central role at molecular and cellular levels in the neurodegenerative process in MSA. 1 , 3 , 7 , 8 , 9 , 10
As a consequence, α‐synuclein‐targeting therapies are currently considered a promising approach to prevent disease progression in MSA. 3 , 11 One suitable approach being investigated is specific active immunotherapy. This involves immunization with short peptides (AFFITOPEs) mimicking the amino acid sequence of a segment of the target protein. 12 The respective AFFITOPE is conjugated to the carrier protein keyhole limpet hemocyanin (KLH) and is absorbed to aluminum hydroxide. The carrier protein provides the required T‐helper epitopes for the induction of a long‐lasting and boostable antibody response, whereas the antigenic component (ie, the AFFITOPE) operates solely as a B‐cell epitope and is responsible for the specificity of the humoral immune response. Specific active immunotherapy against α‐synuclein has been designed to induce a long‐lasting antibody response specific for aggregated α‐synuclein species and has been shown to interfere with α‐synuclein pathogenic mechanisms. 11 , 12 , 13
The specific active immunotherapy candidates PD01A and PD03A have proven to be highly immunogenic and to induce α‐synuclein aggregate‐specific antibodies in mice. 14 , 15 In a transgenic mouse model of MSA, active immunization with PD01A induced specific antibodies against α‐synuclein and mitigated the accumulation of α‐synuclein aggregates, which was associated with reduced neurodegeneration. 14
Here, we assessed in a first‐in‐human trial, the safety, tolerability, and immunogenicity of the 2 α‐synuclein targeting AFFITOPE vaccines PD01A and PD03A in patients with early MSA.
1. Methods
1.1. Study Design
This bicenter, randomized, patient‐ and examiner‐blinded, placebo‐controlled, parallel‐group phase 1 clinical trial assessed safety, tolerability, and immunogenicity of repeated subcutaneous injections of either PD01A, PD03A or placebo in weeks 0, 4, 8, 12, and 36 in patients with early MSA. Patients were enrolled between December 2014 and March 2016 at the 2 sites of the French Reference Center for MSA at the University Hospitals in Bordeaux and Toulouse. This study received ethical approval (CPP Sud‐Ouest et Outre‐Mer III, 2014/65) and was conducted according to the Declaration of Helsinki (EudraCT: 2014–000567‐40, ClinicalTrial.gov identifier: NCT02270489).
Patients between 30 and 75 years with early MSA (defined as <4 years from symptom onset) fulfilling current consensus criteria for a diagnosis of possible or probable MSA 16 were randomized to receive treatment with either PD01A (n = 12), PD03A (n = 12), or placebo (n = 6); see Figure 1. Participants had no major cognitive impairment (Montreal Cognitive Assessment score at screening ≥ 21) and were on stable medications for at least 30 days at the screening visit. Treatments for concomitant illnesses had to remain stable during the entire study period. In addition, efforts were made to keep treatments for MSA symptoms (eg, dopamine replacement therapy, midodrine, fludrocortisone, laxatives, antidepressants) as stable as possible during the entire study period. Patients were randomized according to the permuted blocks method with fixed block size. The randomization sequence was computer‐generated by the trial sponsor's statistician. Patients were randomized accordingly by a responsible unblind person (designated staff member at each study site without any involvement in the study assessments).
FIG. 1.
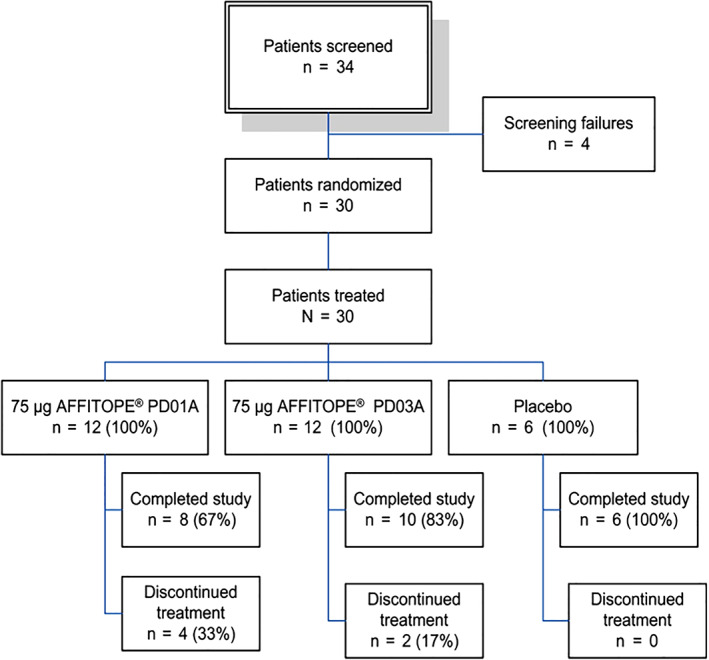
Study design and patient enrollment. [Color figure can be viewed at wileyonlinelibrary.com]
According to the request of the French regulatory authorities (Agence Nationale de Sécurité du Médicament), enrollment was stopped after the 6th and 16th patient had completed the 3 primary immunizations for interim safety assessments. If a suspected unexpected serious adverse reaction was reported, the Data Safety Monitoring Board was to be consulted to evaluate the event and to provide recommendations concerning continuation of the trial.
1.2. Study Treatments
PD01A, PD03A, and placebo were administered 5 times via subcutaneous injection in weeks 0, 4, 8, 12, and 36. Injections of PD01A and PD03A contained the AFFITOPE peptide (either PD01 or PD03)‐KLH conjugates adjuvanted with aluminum hydroxide (Alhydrogel) in phosphate‐buffered saline (PBS), whereas placebo injections consisted of aluminum hydroxide in PBS only. The administered dose of 75 μg corresponds to the amount of immunizing peptide in the conjugate. The investigational medicinal product (IMP) was prepared on site by the responsible unblind staff member (see above).
1.3. Study End Points
The primary end point was safety and tolerability as determined by withdrawal criteria, adverse events (AEs), serious adverse events (SAEs), physical and neurological examination results, vital signs, body mass, safety reads of brain magnetic resonance imaging (MRI), and laboratory assessments. Secondary end points included immunogenicity (ie, the change from baseline of antibody titers specific for the immunizing peptides PD01A and PD03A and the targeted α‐synuclein epitope) and clinical rating scales (ie, the change from baseline in Unified MSA Rating Scale [UMSARS] I–IV, Geriatric Depression Scale [GDS], and Composite Autonomic Symptom Score 31 [COMPASS 31] scores).
Safety monitoring performed at every study visit included assessments of local site reactions, vital signs, physical and neurological examinations, and blood laboratory testing. Adverse events were coded using MedDRA dictionary version 18.1. Grading of injection‐site reactions was performed according to the US Food and Drug Administration Guidance for Industry: “Toxicity Scale for Healthy Adult and Adolescents Patients Enrolled in Preventive Vaccine Clinical Trials” from 2007. Investigator‐blinded brain MRI (high‐field 3.0 T with fast 3‐plane localizer, T1‐3‐D‐weighted, T2‐3‐D‐weighted, and FLAIR‐3D sequences) and clinical assessments (UMSARS I–IV, GDS, and COMPASS 31) were performed at baseline, week 26, and the end of study. Lumbar puncture was performed at baseline and week 40. The total study duration was 52 weeks.
1.4. Specific Active Immunotherapy IgG Response
For immunogenicity testing, serum samples were serially diluted (1:3 dilution steps) and evaluated by an external provider (eBioscience, Vienna) using an ELISA validated to specifically detect IgG antibodies. Titers were determined for reactivity with either PD01 or PD03, with the native epitope on the target α‐synuclein protein, an irrelevant control peptide, and the carrier protein KLH (to confirm patients' immune competence). A serial dilution of a human IgG pool coated to the ELISA plate was used as a calibration curve, and results are presented as geometric mean end‐titers. End‐titers were defined as last serum dilution that gave a signal that was higher than the signal of the calibration curve at penultimate dilution. Immune responders (seroconversion) were defined as patients with a PD01 or PD03 peptide titer ratio ≥ 4‐fold relative to baseline. 17
1.5. Statistical Analysis
The safety population was defined as patients enrolled into the study and receiving at least 1 immunization (identical to the intention‐to‐treat population [ITT]).
Statistical analyses were performed based on the 3 treatment groups (frequency table for categorical data and descriptive statistics for continuous data). All statistical tests in this study were regarded as hypothesis‐generating and P values as descriptive. The sample size of this exploratory study was based on a clinical rationale; no formal sample size calculation was performed.
Immune parameters were statistically compared between treatment groups at the different visits using analysis of covariance with baseline as covariate based on log‐transformed data (geometric mean and 95% CI). For the comparison of baseline UMSARS II (motor examination) scores and their change over time, a mixed model for repeated measurements was used to obtain the average treatment effect over the whole study period with the fixed‐effects parameter baseline, treatment, and time as well as treatment‐by‐time interaction. Depending on normality of data, a paired t test or a Wilcoxon signed‐rank test was performed to compare the changes of UMSARS I/IV, GDS, and COMPASS 31 scores from baseline to study end for each treatment group.
2. Results
2.1. Demographics
Thirty patients were enrolled (11 women) with a median age of 61 years and a mean interval of 2.75 years since symptom onset (0.75 years since MSA diagnosis). Baseline characteristics with corresponding clinical scores are presented in Table 1. Twenty‐five patients received at least 1 concomitant symptomatic medication, mostly dopamine replacement therapy with 5 subjects receiving a combination of several drugs.
TABLE 1.
Demographics and clinical baseline characteristics
| Parameter | PD01A | PD03A | Placebo | Total | |
|---|---|---|---|---|---|
| (n = 12) | (n = 12) | (n = 6) | (n = 30) | ||
| Age (years) | Median (range) | 62 | 60 | 66 | 61 |
| (42–74) | (47–71) | (48–70) | (42–74) | ||
| Body weight (kg) | Median (range) | 69.5 | 76.5 | 73.0 | 73.0 |
| (45–97) | (56–102) | (57–94) | (45–102) | ||
| Sex, n | Female | 6 | 4 | 1 | 11 |
| Male | 6 | 8 | 5 | 19 | |
| Time since symptom onset (years) | Mean | 2.93 | 2.50 | 2.88 | 2.75 |
| Time since MSA diagnosis (years) | Mean | 0.68 | 0.79 | 0.82 | 0.75 |
| MSA‐P/MSA‐C | Number | 7/5 | 6/6 | 1/5 | 14/16 |
| UMSARS I score | Median (range) | 16.0 | 16.5 | 15.5 | — |
| (4–25) | (8–21) | (10–27) | |||
| UMSARS II score | Median (range) | 14.5 | 18.5 | 14.5 | — |
| (7–32) | (11–27) | (12–29) | |||
| UMSARS III: systolic blood pressure in supine position (mm Hg) | Median (range) | 128 | 142 | 135 | — |
| (103–223) | (104–170) | (116–203) | |||
| UMSARS III: diastolic blood pressure in supine position (mm Hg) | Median (range) | 79 | 83 | 85 | — |
| (63–125) | (67–100) | (70–96) | |||
| Maximal drop in systolic blood pressure (mm Hg) | Mean | 30 | 28 | 31 | — |
| Maximal drop in diastolic blood pressure (mm Hg) | Mean | 13 | 13 | 14 | — |
| UMSARS III: orthostatic symptoms | No/yes | 8/4 | 7/5 | 4/2 | — |
| UMSARS IV | Median (range) | 2 | 2 | 2 | — |
| (1–4) | (1–2) | (1–3) | |||
| GDS | Median (range) | 4.0 | 5.5 | 7.5 | — |
| (0–9) | (0–11) | (2–10) | |||
| COMPASS 31 score | Median (range) | 41.0 | 37.1 | 53.1 | — |
| (3.2–56.6) | (11.1–57.1) | (1.9–59.8) |
UMSARS, Unified Multiple System Atrophy Rating Scale I–IV; GDS, Geriatric Depression Scale; COMPASS 31, Composite Autonomic Symptom Score; data from ITT population.
2.2. Safety
The safety population included all 30 enrolled subjects. A total of 595 treatment‐emergent adverse events (TEAEs) were reported by 29 patients (Table 2). Fifteen serious adverse events (SAE) were reported in this study in eleven patients (10 SAEs in 7 PD01A patients, 2 SAEs in 2 PD03A patients, and 3 SAEs in 2 placebo‐treated patients; Table S1).
TABLE 2.
Overview of TEAE by severity and relation to study medication
| Parameter | PD01A (n = 12) | PD03A (n = 12) | Placebo (n = 6) | Total (n = 30) | |
|---|---|---|---|---|---|
| All TEAE | Subjects (events) | 11 (231) | 12 (217) | 6 (147) | 29 (595) |
| Mild TEAE | 11 (116) | 12 (108) | 6 (101) | 29 (325) | |
| Moderate TEAE | 11 (94) | 11 (92) | 6 (41) | 28 (227) | |
| Severe TEAE | 8 (19) | 7 (16) | 2 (5) | 17 (40) | |
| Fatal events | 2 (2) | 1 (1) | 0 | 3 (3) | |
| SAE | 7 (10) | 2 (2) | 2 (3) | 11 (15) | |
| Treatment‐related AE | Subjects (events) | 11 (109) | 10 (97) | 6 (71) | 27 (277) |
| Unrelated AE | 11 (122) | 12 (120) | 6 (76) | 29 (318) |
AE, adverse event; treatment‐related AE, AE classified as possibly, probably, or certainly related to the IMP; SAE, serious adverse event; TEAE, treatment‐emergent AE; data from ITT population.
Twenty‐four patients completed the trial, and 6 discontinued the study treatment. Reasons for study discontinuation were death (2 patients), withdrawal shortly followed by death (1 patient), fatigue (2 patients), and concomitant disease (1 patient). The reported cause of death was worsening of MSA‐related conditions for 2 patients and pulmonary embolism for 1 (pulmonary embolism and MSA diagnosis were confirmed in this patient by autopsy). All 3 deaths were considered unrelated to the active treatments after a detailed investigation involving the French Competent Authority (Agence Nationale de Sécurité du Médicament) and the Data Safety Monitoring Board.
The vast majority of reported TEAE were of mild or moderate intensity, leaving 40 adverse events classified as severe (Table 2). The number of TEAEs and treatment‐related AEs per subject was very similar in all treatment groups. Overall, there were 190 local reactions and 200 systemic reactions when including only events with an incidence >10% of subjects (Table S2). There were 17 injection‐site reactions after the first immunization and 52 after the second immunization, followed by a plateau in both absolute number and affected subjects. Severe local reactions were sporadically observed.
There were 277 adverse events classified as related to study medication (possibly, probably, or certainly related), of which 190 were injection‐site reactions, typically observed in vaccination trials, and systemic events such as fatigue, headache, and nausea. The number of injection‐site reactions per sex was similar for PD01A, but higher for women in the PD03A and placebo treatment groups (PD01A, 8.6 ISR/woman vs 8.8 ISR/man; PD03A, 9.3 ISR/woman vs 5.4 ISR/man; placebo, 11.0 ISR/woman vs 6.8 ISR/man); see Table S3.
Compatible with the underlying disease, vital signs, physical and neurological examinations, and some hematology and clinical chemistry laboratory results were abnormal and considered clinically significant at baseline and during the study. Brain MRI and cerebrospinal fluid (CSF) assessments did not reveal any sign of an adverse neuroinflammatory response induced by the IMP.
2.3. Antibody Titers
Both active treatments, PD01A and PD03A, induced a sustained IgG antibody response against the immunizing peptides PD01 and PD03, respectively (Fig. 2A,B). Titers peaked in week 12 (4 weeks after the third immunization) and subsequently declined, with a half‐life of approximately 12 weeks. The geometric group mean titer against the immunizing peptide increased significantly in the PD01A‐treated group compared with placebo by a factor of 114, from 1:41 at baseline to 1:4673 in week 12 (P = 0.0078 change from baseline; Fig. 2A) and in the PD03A‐treated group compared with placebo by a factor of 14.6, from 1:94 to 1:1379 (P = 0.0179 change from baseline; Fig. 2B). After 3 priming injections, 9 of 11 patients in the PD01A group (82%) and 7 of 12 patients in the PD03A group (58%) met the predefined cutoff for seroconversion. The boost immunization in week 36 reactivated the antibody response, with peak titers achieved 4 weeks after the administration, resulting in geometric group mean titers of 1:6740 in the PD01A group (P = 0.0066 compared with placebo, with 8 of 9 patients showing seroconversion — 89%) and 1:1080 in the PD03A group (P = 0.0437 compared with placebo, with 7 of 12 patients classified as serological responders — 58%). In both groups, the booster immunization triggered a long‐lasting antibody response, which could be observed until the end of the study.
FIG. 2.
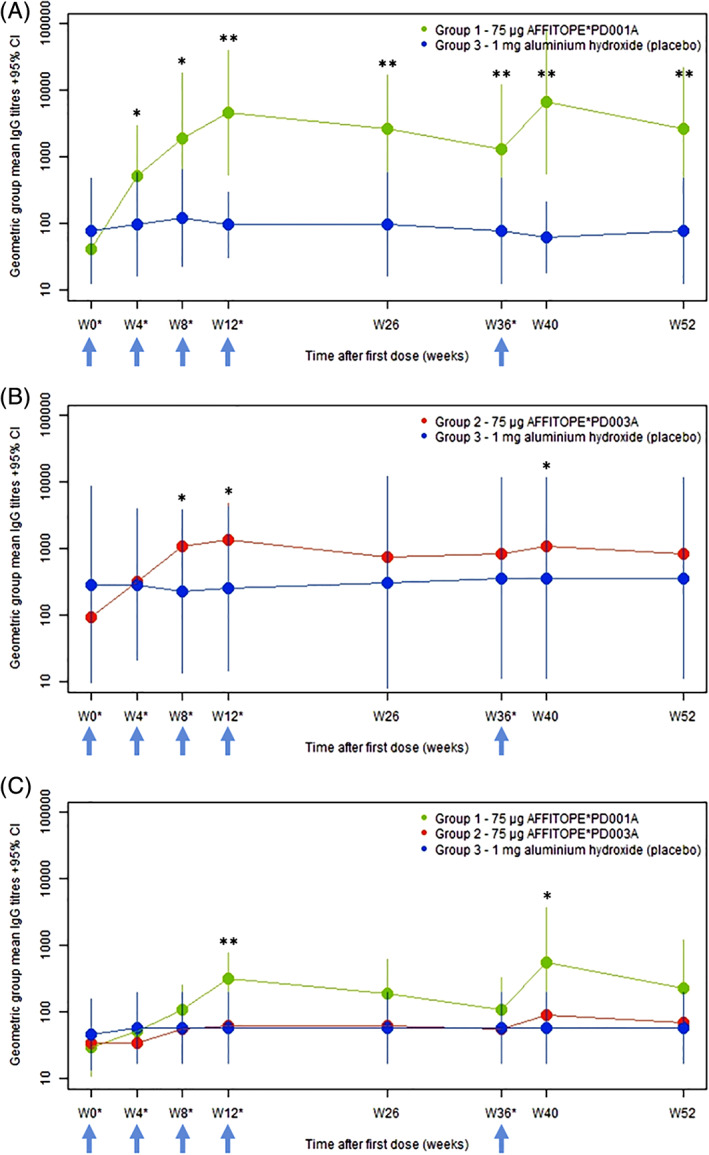
PD01, PD03, and α‐synuclein target epitope‐specific titers over time. Primary immune response to the immunizing peptides PD01 (A), PD03 (B), and the α‐synuclein target epitope (C). Bars represent the 95% confidence intervals. Arrows indicate times of injection. *P < 0.05 compared with placebo (change from baseline); **P < 0.01 compared with placebo (change from baseline). [Color figure can be viewed at wileyonlinelibrary.com]
Antibody titers against the α‐synuclein target epitope were lower than those measured against both immunizing peptides, but the time profile of antibody development was very similar for all. Following immunization with PD01A peptide, antibody titers against the α‐synuclein target epitope increased from 1:30 at baseline to 1:324 in week 12 (P = 0.0097 compared with placebo; Fig. 2C) and 1:562 in week 40 (P = 0.0273 compared with placebo; Fig. 2C). Antibody levels against the α‐synuclein target epitope did not increase significantly in the PD03A group, reaching geometric group mean titers of 1:62 and 1:90 in weeks 12 and 40, respectively (Fig. 2C). As expected, antibody reactivity to the immunizing PD01A and PD03A peptides and to the α‐synuclein target epitope did not change over time in the placebo group (Fig. 2A–C).
2.4. Clinical Rating Scores
Results for UMSARS I–IV, GDS, and COMPASS 31 over time are summarized in Table 3. UMSARS I and II scores increased significantly during the trial period in all 3 treatment groups (PD01A: change UMSARS I [V0–V8], P = 0.0261; change UMSARS II [V0–V8], P = 0.0322; PD03A: change UMSARS I [V0–V8], P = 0.0031; change UMSARS II [V0–V8], P = 0.0043; placebo: change UMSARS I [V0–V8], P = 0.0028, change UMSARS II [V0–V8], P = 0.0033). Differences between groups were not significant, and observed changes in activities of daily living and motor symptoms were within the expected ranges because of disease progression in all groups.
TABLE 3.
Clinical scores over time by treatment group
| Parameter | PD01A | PD03A | Placebo | |
|---|---|---|---|---|
| (n = 12/10/8) a | (n = 12/12/11) a | (n = 6/6/6) a | ||
| UMSARS I score, median (range) | Baseline | 16.0 (4–25) | 16.5 (8–21) | 15.5 (10–27) |
| Week 26 (V5) | 18.0 (9–33) | 20.5 (7–29) | 19.5 (12–37) | |
| Week 52 (V8) | 19.5 (10–34) | 20.0 (7–26) | 23.5 (18–40) | |
| UMSARS II score, median (range) | Baseline. | 14.5 (7–32) | 18.5 (11–27) | 14.5 (12–29) |
| Week 26 (V5) | 20.5 (5–32) | 21.5 (15–35) | 23.0 (11–35) | |
| Week 52 (V8) | 22.5 (5–41) | 26.0 (11–41) | 24.5 (20–43) | |
| UMSARS III: orthostatic hypotension (yes/no) | Baseline | 8/4 | 9/3 | 4/2 |
| Week 26 (V5) | 7/3 | 7/5 | 5/1 | |
| Week 52 (V8) | 6/2 | 7/4 | 5/1 | |
| UMSARS IV score median (range) | Baseline | 2.0 (1–4) | 2.0 (1–2) | 2.0 (1–3) |
| Week 26 (V5) | 2.5 (1–4) | 2.0 (1–4) | 2.0 (2–4) | |
| Week 52 (V8) | 2.0 (1–4) | 3.0 (1–3) | 2.0 (2–4) | |
| GDS score, median (range) | Baseline | 4.0 (0–9) | 5.5 (0–11) | 7.5 (2–10) |
| Week 26 (V5) | 4.5 (0–9) | 5.5 (0–12) | 9.5 (2–13) | |
| Week 52 (V8) | 4.0 (1–11) | 5.0 (1–12) | 9.0 (2–12) | |
| COMPASS 31 score, median (range) | Baseline | 41.0 (3.2–56.6) | 37.1 (11.1–57.1) | 53.1 (1.9–59.8) |
| Week 26 (V5) | 35.3 (13.5–54.3) | 22.7 (3.1–52.3) | 47.4 (5.0–52.3) | |
| Week 52 (V8) | 39.6 (13.0–53.1) | 40.2 (1.6–52.4) | 52.8 (12.2–71.8) |
Number of evaluated subjects at baseline/V5/V8; data from ITT population.
UMSARS IV scores increased within the PD03A treatment group (change V0–V8, P = 0.0261), whereas no significant changes were observed for GDS and COMPASS 31 for all treatment groups.
3. Discussion
The primary objective of this first‐in‐human randomized phase 1 trial was to evaluate the safety and tolerability of repeated administrations of 2 specific active immunotherapies, PD01A and PD03A, in patients diagnosed with early MSA compared with placebo. The immunization schedule had been designed to both evaluate the primary antibody response after the first immunizations administered within 12 weeks and the long‐lasting antibody response after the boost immunization in week 36.
The overall safety profile showed no substantial difference between the 3 treatment groups. The 3 deaths reported in this study were all classified as unrelated to the IMP after external review, similar to all SAEs, although the total number of SAEs was higher in the PD01A group. There was a higher number of injection‐site reactions and a lower number of systemic adverse events in the active treatment groups compared with placebo. The high number of reported adverse events was expected because of the severity of the underlying disease itself, possible adverse events of the concomitant symptomatic medications, and the TEAEs caused by the active immunizations. The TEAEs classified as related to PD01A and PD03A were expected and generally well tolerated including fatigue, headache, nausea, and injection‐site reactions. The incidence of local reactions increased after the first immunization, but remained relatively constant after the second immunization with no evidence for cumulative severity.
The detected antibody response triggered by PD01A was higher than that of PD03A. Immunization with PD01A resulted in a significant increase in titers against the immunizing AFFITOPE PD01 peptide in week 12 (4 weeks after the third immunization), which translated to a humoral immune response against the α‐synuclein target epitope of approximately 1 order of magnitude lower. The antibody response increased rapidly following a booster injection in week 40 (4 weeks after the booster application), suggesting that the priming immunizations produced a significant memory effect, and antibody levels persisted until the end of the study. The observation of lower titers for the α‐synuclein target epitope compared with the immunizing AFFITOPE peptide may at least partially be explained by the binding of vaccine‐induced antibodies to the target structure (ie, masking the number of detected antibodies). IgG titers monitored after PD03A application were lower than those observed for PD01A. The reasons for this observation are currently unclear, although impaired capacity of the immune system in the PD03A treatment group could be ruled out, as the immune response against the carrier protein KLH was almost identical in both active treatment groups.
No data are yet available about the level of α‐synuclein‐specific antibodies in the brain required to achieve a therapeutic clinical effect. However, antibody levels in CSF and presumably also in the brain seem to be dependent on the antibody concentration in the circulation. 18 , 19 Furthermore, different antibody concentration dynamics induced either by active or passive immunotherapy might further influence the concentration of α‐synuclein‐specific antibodies in the brain.
Our study has limitations. First, the small sample size allowed us to obtain first insights into the safety profile of PD01A/PD03A and potential efficacy measures in MSA patients, which will help design future clinical studies. However, the sample size was not sufficient to detect statistically significant differences, and the study was not designed to perform such tests. Second, blinding was limited to patients and examiners. However, the involvement of an unblind person for randomization and IMP preparation without any involvement in the study assessments, a similar number of TEAEs in all 3 treatment groups and the assessment of antibody titers after completion of the last study follow‐up visit ensured successful blinding of patients and examiners throughout the trial.
In conclusion, disease‐modifying or neuroprotective treatments remain an urgent unmet treatment need for MSA, and targeting α‐synuclein with specific active immunotherapy seems a promising strategy in this regard. The results of this trial in terms of safety, tolerability, and immune reaction of repeated administrations of 2 active immunotherapies, PD01A and PD03A, warrant further investigation of specific active immunotherapy in MSA patients.
AFF009 Study Investigators
Mathieu Anheim, CHU Strasbourg, Strasbourg, France.
Anna Castrioto, CHU Grenoble, Grenoble, France.
Pascal Derkinderen, CHU Nantes, Nantes, France.
Sophie Drapier, CHU Rennes, Rennes, France.
Alexandra Eusebio, CHU de la Timone, Marseille, France.
David Grabli, Hôpital de la Salpêtrière, Paris, France.
Ana Marques, CHU Clermont‐Ferrand, Clermont‐Ferrand, France.
Caroline Moreau, CHRU Lille, Lille, France.
Elena Moro, CHU Grenoble, Grenoble, France.
Christine Tranchant, CHU Strasbourg, Strasbourg, France.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution;
2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3) Manuscript: A. Writing of the first draft, B. Review and Critique.
W.G.M.: 1A‐C, 2C, 3A.
A.P.L.T.: 1B, 1C, 3B.
A.F.S.: 1B, 1C, 3B.
G.G.: 1C, 3B.
M.G.: 1B, 1C, 3B.
A.K.: 1B, 1C, 3A.
B.L.: 1C, 3B.
P.L.: 2C, 3A.
R.M.: 2C, 3A.
P.P.: 1B, 1C, 3B.
U.S.: 1C, 3B.
S.V.: 1C, 3B.
D.V.: 1A, 3B.
W.P.: 1A, 3B.
A.S.: 1A‐C, 3B.
G.S.: 1B, 1C, 2C, 3A.
O.R.: 1A‐C, 2C, 3B.
Financial Disclosures of all authors for the preceding 12 months
W.G.M. has received fees for editorial activities with Springer Nature and Elsevier, has served as adviser for Lundbeck and Biohaven, has received teaching honoraria from UCB and Boehringer Ingelheim, as well as research support from the Michael J. Fox Foundation, the University Hospital Bordeaux, the French Health Ministry, the European Community, ANR, ARAMISE, PSP‐France, MSA Coalition, LABEX Excellence Initiative.
A.P.L.T. has received research support from the French Health Ministry.
A.F.S. has received personal fees from LVL medical.
G.G. is an employee of Origenis GmbH.
M.G., B.L., U.S., and S.V. have nothing to declare.
A.K. was an employee of Affiris AG.
P.L. is employed by Affiris AG.
R.M. serves as consultant for Affiris AG.
P.P. has served as consultant for Roche.
D.V. has contracts with Roche (PASSPORT‐study) and Biohaven (M‐STAR study).
W.P. reports personal fees from AbbVie, Affiris, AstraZeneca, BIAL, Boston Scientific, Britannia, Intec, Ipsen, Lundbeck, Neuroderm, Neurocrine, Denali Pharmaceuticals, Novartis, Orion Pharma, Prexton,Teva, UCB, and Zambon (consultancy and lecture fees in relation to clinical drug development programmes for PD). He has also received royalties from Thieme, Wiley Blackwell, Oxford University Press and Cambridge University Press.
A.S. is employed by Acanis GmbH.
G.S. is employed by Affiris AG.
O.R. has served on advisory boards or as consultant for AbbVie, Adamas, Acorda, Addex, AlzProtect, Apopharma, Astrazeneca, Bial, Biogen, Britannia, Buckwang, Clevexel, INC Reasearch, Lundbeck, Lupin, Merck, MundiPharma, Neuratris, Neuroderm, Novartis, ONO Pharma, Orion Pharma, Osmotica, Oxford Biomedica, Parexel, Pfizer, Prexton Therapeutics, Quintiles, Sanofi, Servier, Sunovion, Théranexus, Takeda, Teva, UCB, Watermark Research, XenoPort, XO, Zambon. He has received research funding from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France‐Parkinson, INSERM‐DHOS Recherche Clinique Translationnelle, MJFox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020), as well as a grant to participate in a symposium and contribute to the review of an article IPMDS.
Supporting information
Table S1. Frequency of Serious Adverse Events (SAE)
Table S2. Local and systemic reactions over time with incidence >10%*
Table S3. Local reactions by sex
Acknowledgments
The study was part of an EU‐funded program (FP7, SYMPATH grant agreement 602999).
Relevant conflicts of interest/financial disclosures: W.G.M., R.M., and W.P. have received consultancy fees from Affiris. G.G., A.K., G.S., and A.S. are currently or were in the past employed by Affiris AG.
Funding agencies: The study was part of an EU‐funded program (FP7, SYMPATH grant agreement 602999).
Contributor Information
Wassilios G. Meissner, Email: wassilios.meissner@chu-bordeaux.fr.
the AFF009 Study Investigators:
Mathieu Anheim, Anna Castrioto, Pascal Derkinderen, Sophie Drapier, Alexandra Eusebio, David Grabli, Ana Marques, Caroline Moreau, Elena Moro, and Christine Tranchant
References
- 1. Fanciulli A, Wenning GK. Multiple‐system atrophy. N Engl J Med 2015;372(3):249–263. [DOI] [PubMed] [Google Scholar]
- 2. Lopez‐Cuina M, Foubert‐Samier A, Tison F, Meissner WG. Present and future of disease‐modifying therapies in multiple system atrophy. Auton Neurosci 2018;211:31–38. [DOI] [PubMed] [Google Scholar]
- 3. Meissner WG, Fernagut PO, Dehay B, et al. Multiple system atrophy: recent developments and future perspectives. Mov Disord 2019;34(11):1629–1642. [DOI] [PubMed] [Google Scholar]
- 4. Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy‐Drager syndrome). J Neurol Sci 1989;94(1–3):79–100. [DOI] [PubMed] [Google Scholar]
- 5. Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 1994;117(Pt 2):235–243. [DOI] [PubMed] [Google Scholar]
- 6. Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha‐synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 1998;251(3):205–208. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed Z, Asi YT, Sailer A, et al. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 2012;38(1):4–24. [DOI] [PubMed] [Google Scholar]
- 8. Fernagut PO, Dehay B, Maillard A, et al. Multiple system atrophy: a prototypical synucleinopathy for disease‐modifying therapeutic strategies. Neurobiol Dis 2014;67:133–139. [DOI] [PubMed] [Google Scholar]
- 9. Monzio Compagnoni G, Di Fonzo A. Understanding the pathogenesis of multiple system atrophy: state of the art and future perspectives. Acta Neuropathol Commun 2019;7(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valdinocci D, Radford RAW, Goulding M, Hayashi J, Chung RS, Pountney DL. Extracellular interactions of alpha‐synuclein in multiple system atrophy. Int J Mol Sci 2018;19(12):4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dehay B, Bourdenx M, Gorry P, et al. Targeting alpha‐synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol 2015;14(8):855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneeberger A, Tierney L, Mandler M. Active immunization therapies for Parkinson's disease and multiple system atrophy. Mov Disord 2016;31(2):214–224. [DOI] [PubMed] [Google Scholar]
- 13. Dehay B, Decressac M, Bourdenx M, et al. Targeting alpha‐synuclein: therapeutic options. Mov Disord 2016;31(6):882–888. [DOI] [PubMed] [Google Scholar]
- 14. Mandler M, Valera E, Rockenstein E, et al. Active immunization against alpha‐synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mandler M, Valera E, Rockenstein E, et al. Next‐generation active immunization approach for synucleinopathies: implications for Parkinson's disease clinical trials. Acta Neuropathol 2014;127(6):861–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71(9):670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siegrist C. Vaccine immunology In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2018:16–34. [Google Scholar]
- 18. Brys M, Fanning L, Hung S, et al. Randomized phase I clinical trial of anti‐alpha‐synuclein antibody BIIB054. Mov Disord 2019;34(8):1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jankovic J, Goodman I, Safirstein B, et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti‐alpha‐synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol 2018;75(10):1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Frequency of Serious Adverse Events (SAE)
Table S2. Local and systemic reactions over time with incidence >10%*
Table S3. Local reactions by sex


