Abstract
Objective
This paper aims at examining the clinical characteristics of ischemic stroke patients with different levels of prestroke functional dependency, their long‐term outcome, and determinants of five‐year mortality.
Materials and methods
We describe demographics, comorbidity, treatment, as well as long‐term mortality, and functional status of 5899 prestroke‐dependent ischemic stroke patients stratified by dependency level and compared to a concurrent cohort of 14 148 prestroke‐independent patients. The study was based on 2016 survey data from Riksstroke, the Swedish national stroke register, and patients were followed up at three months, 12 months, and either at three or five years. We used Cox regression for mortality predictor analysis and multiple imputation was performed to minimize bias from loss to follow‐up.
Results
With increasing level of prestroke dependency, comorbidity burden was higher, drug prescription lower, and prognosis less favorable. At three years, the proportion that had died or deteriorated were 82.6%, 87.5%, and 86.3% in moderate, moderately severe, and severe dependency, respectively. In moderate dependency, prognosis was relatively favorable: Three‐month mortality was half of that seen in severe dependency (25.3% versus 49.6%). Differences in overall outcome between groups of varying prestroke functional dependency level were statistically significant (P < .05) at all follow‐up time points.
Conclusions
There was great heterogeneity between groups of different level of prestroke dependency; those of moderate dependency had a relatively favorable prognosis. Patients of different prestroke level of dependency need to be addressed separately, and further research is needed characterizing this group and exploring management strategies.
Keywords: function, ischemic, long‐term outcome, mortality, prognosis, stroke
1. INTRODUCTION
Stroke is the second leading cause of disability worldwide. 1 However, many patients are already functionally dependent prior to stroke and up to 90% have comorbidity, that is, one or multiple coexisting chronic conditions. 2 Prestroke disability is associated with increased mortality, 3 , 4 , 5 and there is evidence of increasingly poor prognosis with higher comorbidity burden. 6 , 7
Management in prestroke comorbid and/or functionally dependent patients is complex and includes many important considerations. The benefit of treating these patients is weighted against the risk of potentially dangerous adverse effects such as major bleed, which may lead to hesitancy in initiating therapy. Age and disability is associated with decreased prescription of oral anticoagulants (OAC) for atrial fibrillation (AF) 8 , 9 and are cited as major reasons for excluding patients from thrombolytic therapy. 10 Management is further complicated by lack of evidence. There are few comprehensive descriptions of prestroke‐dependent patients, who are often lumped together into one single group. Moreover, these individuals are frequently excluded from randomized controlled trials.
Our objective was to present a comprehensive description of ischemic stroke (IS) patients from a large national cohort, stratified by level of prestroke functional dependency. We included data on demographics, comorbidity, treatment, as well as long‐term prognosis (mortality and functional status) on several follow‐up occasions up to five years following stroke.
2. MATERIALS AND METHODS
2.1. Study population
In 2016, the Swedish Stroke Register (Riksstroke) conducted a long‐term follow‐up survey including over 23 000 patients who had a stroke three (2013), or five years (2011) earlier. This paper describes a subgroup from this survey: 5899 prestroke functionally dependent patients with IS. We excluded patients with intracerebral hemorrhage or unspecified stroke, those < 18 years of age, and those with unknown prestroke functional status. Patients were divided into groups based on level of prestroke dependency: moderate (modified Rankin Scale [mRS] 3), moderately severe (mRS 4), and severe (mRS 5). In addition, we included 14 148 prestroke‐independent (mRS ≤ 2) IS patients from the same survey cohort to serve as a reference group. These individuals were divided into two age groups, ≤77 years (median age 68 years) and ≥ 78 years (median age 83 years), to facilitate comparison to the dependent patients who were of advanced age (median age 85 years).
2.2. Data sources
Data on demographics, treatment, and functional status were obtained from Riksstroke, the Swedish quality register for stroke care, with an estimated coverage of > 90% of stroke patients admitted to hospital. 11 , 12 There were missing data in < 2% of patients in baseline and treatment parameters. Patients were followed up at three months, 12 months, three years (2013 cohort only), and five years (2011 cohort only) via paper‐based questionnaires. Patients were considered lost to follow‐up upon failure to return the questionnaire or if there was incomplete information in any of the variables needed for estimating a mRS score. Deceased patients were classified as followed up. In prestroke‐dependent patients, there was loss to follow‐up of 13.7% at three months, 23.0% at 12 months, 16.2% at three years, and 8.6% at five years. For the prestroke‐independent patients, loss to follow‐up was 12.6% at three months, 21.0% at 12 months, 22.4% at three years, and 20.9% at five years.
Data on mortality status and date of death were obtained from the Swedish Cause of Death register.
Data on comorbidity were obtained from the Swedish National Patient Register (SNPR), which includes data from outpatient and inpatient healthcare contacts, and the Charlson Comorbidity Index (CCI) 13 was used to determine which conditions to include in the analysis. Also, data from the Swedish Prescribed Drug Register were used to identify additional cases of dementia.
We obtained data for highest level of education from Statistics Sweden.
The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statements 14 and the local ethics approval committee (Regional Ethical Review Board, Lund) approved the project in 2017 (Dnr 2017/529). The committee waived the need for patient consent.
Because of the sensitive nature of the data collected for this study, requests to access the data set may be sent to Riksstroke after obtaining the appropriate ethics approval.
2.3. Measures and definitions
Comorbidity was defined as chronic conditions registered in the SNPR at any time five years prior to index stroke.
Comorbidity burden was estimated based on the sum of individual conditions as none (0), low (1), moderate (2‐3), and high (≥4) from a specified list of 17 chronic conditions (Appendix 2).
Prestroke and post‐stroke functional status was described using the mRS, a well established, valid, and reliable instrument for assessing function in stroke. 15 Scores were estimated from information on dependency in specified ADL domains (toileting, dressing, mobility), living conditions, and help from next of kin (post‐stroke only), using a validated and previously specified translation algorithm. 16 Prestroke status was reported by hospital personnel in conjunction with index stroke, and post‐stroke status was reported by the stroke patient or caregiver via the Riksstroke follow‐up questionnaires.
In 2011/2013, the Riksstroke register did not include routine registration of parameters on stroke severity such as NIHSS or stroke size. Instead, we used level of consciousness at admission as a proxy marker of stroke severity.
Also, highest level of education was used as a proxy marker for socioeconomic status.
2.4. Statistical methods
Categorical variables were summarized as proportions (percentages) and quantitative variables as means or medians. Group differences were assessed using Chi‐squared test for categorical variables (including outcome variables such as mRS score), and Student's t test for continuous variables (age only). Significance level was set to 5% and tests were two‐sided.
Multiple imputation was performed to estimate likely mRS scores for survivors lost to follow‐up. 17 The imputation model included a large number of selected baseline characteristics and outcome variables as predictors. Five imputations were conducted, each based on ten iterations of the underlying Markov chain, and an average was calculated. The methodology was described in detail in Appendix 1, Appendix 3, and Appendix 4.
Five‐year cumulative survival was visualized using Kaplan‐Meier curves. For the 2013 cohort, survival data were only available for up to three years after stroke and these individuals were censored at this time point. The log rank test was used to analyze differences between groups.
A Cox regression model with time since baseline examination as time scale was used to estimate the effect of selected prognostic factors on five‐year‐mortality in prestroke dependents (including a reference group of prestroke independent patients of comparable age [≥78]). The significance level was set to 5%. Covariates were chosen based on clinical relevance, and we included common potential confounders such as age, sex, and socioeconomic status. The model included several, potentially correlated, explanatory variables. To ensure that this did not cause unreliability of parameter estimates and corresponding standard errors, collinearity diagnostics were carried using standard techniques as implemented in SAS PROC REG. Also, we tested the proportional hazards assumption (in a rather experimental fashion) by simply adding different time‐dependent covariates (corresponding to interactions between prognostic factors and certain transformations of time) to the regression model. In our large data, the global test of no effect of these covariates was highly significant for almost all choices of time transformation. However, when inspecting plots of smoothed sums of parameter estimates and suitably scaled Schoenfeld residuals, we can see that there is almost no variation over time in the effects on mortality hazard of the prognostic factors during the five years following the baseline examination. In other words, the statistically significant tests do not seem to correspond to variations in effects that would change the conclusion of the analysis. In the light of this, we decide to present, interpret, and discuss the outcome of the Cox regression analysis despite violations of model assumptions.
Statistical analyses were conducted using IBM SPSS Statistics version 24 and SAS 9.4, CARY NC
3. RESULTS
3.1. Patient characteristics
Prestroke dependent patients differed significantly from independents in several key parameters. They were on average ten years older (mean age 83.7 compared to 73.3 years), the proportion of females was higher (61.3% versus 42.3%), and comorbidity burden was greater, for example, dementia was present in 12.9% versus 1.5%, and 35.4% versus 16.4% had previous stroke, respectively (Table 1). When only comparing to independent patients of comparable age (≥78), differences were somewhat attenuated but remained significant.
Table 1.
Patient demographics and comorbidity
| Prestroke‐independent patients (mRS 0‐2) | Prestroke‐dependent patients | ||||||
|---|---|---|---|---|---|---|---|
|
Total n = 14 148 |
Age ≤ 77 n = 8408 |
Age ≥ 78 n = 5740 |
Total n = 5899 |
Moderate (mRS 3) n = 3300 |
Moderately severe (mRS 4) n = 1889 |
Severe (mRS 5) n = 710 |
|
| Age, mean (SD), years | 73.3 (11.7) | 66.0 (9.2) | 84.1 (4.4) | 83.7 (8.8) | 83.7 (8.8) | 83.8 (8.7) | 83.6 (9.3) |
| Age, median (IQR), years | 75 (16) | 68 (11) | 83 (7) | 85 (10) | 86 (10) | 85 (11) | 85 (11) |
| Male sex | 8167 (57.7) | 5323 (63.3) | 2844 (49.5) | 2283 (38.7) | 1228 (37.2) | 803 (42.5) | 252 (35.5) |
| Total comorbidity burden | |||||||
| None | 209 (20.6) | 2201 (26.2) | 708 (12.3) | 412 (7.0) | 254 (7.7) | 122 (6.5) | 36 (5.1) |
| Low | 4014 (28.4) | 2510 (29.9) | 1504 (26.2) | 953 (16.2) | 581 (17.6) | 261 (13.8) | 111 (15.6) |
| Moderate | 5241 (37.0) | 2817 (33.5) | 2424 (42.2) | 2439 (41.3) | 1385 (42.0) | 790 (41.8) | 264 (37.2) |
| High | 1984 (14.0) | 880 (10.5) | 1104 (19.2) | 2095 (35.5) | 1080 (32.7) | 790 (37.9) | 299 (42.1) |
| Selected comorbidities | |||||||
| Atrial fibrillation | 3564 (25.2) | 1492 (17.7) | 2072 (36.1) | 2509 (42.5) | 1410 (42.7) | 781 (41.3) | 318 (44.8) |
| Chronic kidney failure | 336 (2.4) | 177 (2.1) | 159 (2.8) | 285 (4.8) | 150 (4.5) | 100 (5.3) | 35 (4.9) |
| COPD | 514 (3.6) | 306 (3.6) | 208 (3.6) | 364 (6.2) | 221 (6.7) | 103 (5.5) | 40 (5.6) |
| Congestive heart failure | 1059 (7.5) | 450 (5.4) | 609 (10.6) | 1256 (21.3) | 648 (19.6) | 448 (23.7) | 160 (22.5) |
| Dementia | 206 (1.5) | 74 (0.9) | 132 (2.3) | 760 (12.9) | 256 (7.8) | 316 (16.7) | 188 (26.5) |
| Diabetes | 2925 (20.7) | 1843 (21.9) | 1082 (18.9) | 1454 (24.6) | 739 (22.4) | 512 (27.1) | 203 (28.6) |
| Hypertension | 8680 (61.4) | 4718 (56.1) | 3962 (69.0) | 4122 (69.9) | 2340 (70.9) | 1329 (70.4) | 453 (63.8) |
| Myocardial infarction | 709 (5.0) | 360 (4.3) | 349 (6.1) | 494 (8.4) | 269 (8.2) | 172 (9.1) | 53 (7.5) |
| Previous stroke | 2310 (16.4) | 1267 (15.1) | 1043 (18.3) | 2071 (35.4) | 984 (30.0) | 764 (40.8) | 322 (45.7) |
Data presented as no. (percent) if not indicated otherwise. Differences between groups were statistically significant for all variables (P < .05).
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; mRS, modified Rankin scale; SD, standard deviation.
Dependent patients were split into three groups based on prestroke level of dependency: moderate (50.9%), moderately severe (32.0%), and severe (12.2%). Age distribution was similar, but comorbidity burden increased with dependency level. There was high comorbidity (≥4 conditions) in 32.7%, 37.9%, and 42.1% in moderate, moderately severe, and severe dependency, respectively. This was partly explained by the increasing proportion of previous stroke (30.0% to 45.7%) and dementia (7.8% to 26.5%).
3.2. Medication and care parameters
Overall, prestroke‐dependent patients alive at discharge had a longer length of hospital stay (median 10 days) compared to independent patients of comparable age (≥78 years) (median 8 days) (Table 2). However, in patients of severe dependency, hospital stay was shorter (median 6 days). These differences were statistically significant.
Table 2.
Patient care parameters and medication
| Prestroke‐independent patients (mRS 0‐2) | Prestroke‐dependent patients | ||||||
|---|---|---|---|---|---|---|---|
|
Total n = 14 148 |
Age ≤ 77 n = 8408 |
Age ≥ 78 n = 5740 |
Total n = 5899 |
Moderate (mRS 3) n = 3300 |
Moderately severe (mRS 4) n = 1889 |
Severe (mRS 5) n = 710 |
|
| First admitted to stroke unit a | 11 891 (84.4) | 7166 (85.6) | 4725 (82.6) | 4647 (78.9) | 2616 (79.4) | 1478 (78.4) | 553 (78.1) |
| Thrombolytic therapy | 1395 (9.9) | 1014 (12.1) | 381 (6.6) | 246 (4.2) | 160 (4.9) | 65 (3.4) | 21 (3.0) |
| Length of hospital stay, median (IQR), days b | 6 (10) | 5 (8) | 8 (12) | 10 (11) | 10 (12) | 10 (11) | 6 (9) |
| Medication at discharge | |||||||
| Antihypertensive drugs c | 4678 (79.2) | 2758 (76.1) | 1920 (84.0) | 1679 (79.1) | 986 (82.8) | 532 (76.3) | 161 (68.5) |
| Statins | 9718 (73.4) | 6532 (81.1) | 3186 (61.5) | 1898 (39.6) | 1219 (43.9) | 537 (36.3) | 142 (26.2) |
| Antiplatelet drugs in non‐cardioembolic stroke d | 9122 (91.2) | 6044 (91.1) | 3078 (91.5) | 2446 (85.6) | 1442 (87.8) | 771 (85.6) | 233 (74.4) |
| Anticoagulants in cardioembolic stroke | |||||||
| Total | 1999 (62.9) | 1010 (73.0) | 989 (55.1) | 580 (30.3) | 396 (35.4) | 149 (26.0) | 35 (15.6) |
| Warfarin | 1803 (56.7) | 897 (64.9) | 906 (50.5) | 505 (26.3) | 345 (30.8) | 134 (23.4) | 26 (11.6) |
| Other Anticoagulants e | 210 (6.6) | 120 (8.7) | 90 (5.0) | 85 (4.4) | 59 (5.3) | 17 (3.0) | 9 (4.0) |
Data presented as no. (percent) if not indicated otherwise. Differences between groups were statistically significant for all variables (P < .05).
Abbreviations: IQR, interquartile range; mRS, modified Rankin scale.
Including intensive care unit and observational unit.
Only patients alive at discharge.
Only the 2013 cohort.
Acetylsalicylic acid, dipyramidole, clopidogrel alone or in combination.
Primarily non‐vitamin K antagonist oral anticoagulants.
A small proportion (4.2%) of prestroke‐dependent patients received thrombolytic therapy. This proportion was seen to significantly decrease with increasing dependency level. Also, non‐treatment was associated with old age, severe stroke, and high comorbidity (data not shown).
There was a significantly lower prescription of stroke‐specific drugs such as statins, antihypertensive, antiplatelet, and anticoagulant drugs with increasing level of prestroke dependency. In those without AF (non‐cardioembolic stroke), 74.4% of severe dependency patients were prescribed antiplatelet drugs at discharge compared to 91.5% in independent patients ≥ 78 years. Similarly, in patients with AF (cardioembolic stroke), OAC prescription at discharge was 15.6% in severe dependency compared to 55.1% in independent patients ≥ 78 years.
3.3. Long‐term mortality and functional status
At three‐month follow‐up, the majority (58.6%) of all prestroke‐dependent patients were either dead or had deteriorated to a lower functional level than they had prior to stroke (Figure 1A). Prognosis varied substantially between patients of differing prestroke dependency level. Three‐month mortality was 49.6% in severe dependency (mRS 5), 37.3% in moderately severe dependency (mRS 4), 25.3% in moderate dependency (mRS 3), and 14.0% in independent (mRS 0‐2) patients of comparable age (≥78).
Figure 1.
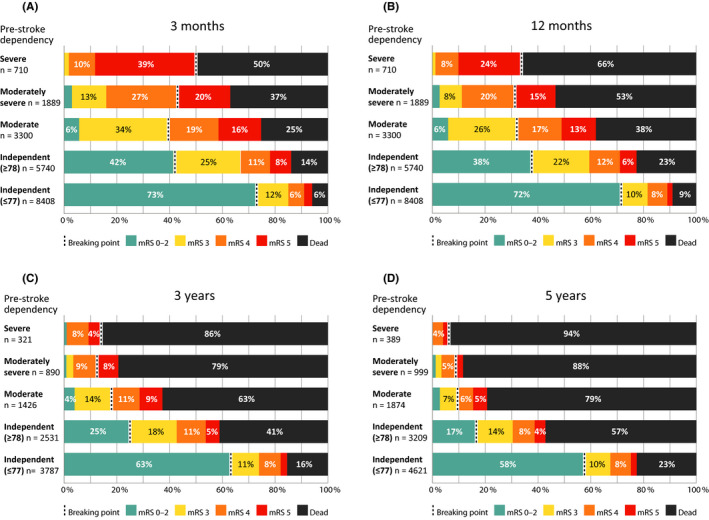
(A‐D) Functional status on several follow‐up occasions after stroke. Groups of different prestroke dependency levels are shown as separate bars. The breaking point in each bar separates those who maintained prestroke functional status (to the left) and those who had died or deteriorated (to the right). Differences in overall outcome between groups were statistically significant (P < .05) at all follow‐up time points. Moderate prestroke dependency = mRS 3, moderately severe prestroke dependency = mRS 4, severe prestroke dependency = mRS 5. Percentages ≤ 3 were not labeled
Between three and 12 months, patients experiencing functional improvement outnumbered those that died or deteriorated in prestroke‐independents, while the inverse relationship was seen in prestroke‐dependents: 10.9% improvement versus 31.7% death or deterioration in the moderate dependency group and 9.4% versus 38.1% in the moderately severe group (Figure 2A). Between 12 months and three years, death or deterioration dominated in all groups (Figure 2B).
Figure 2.
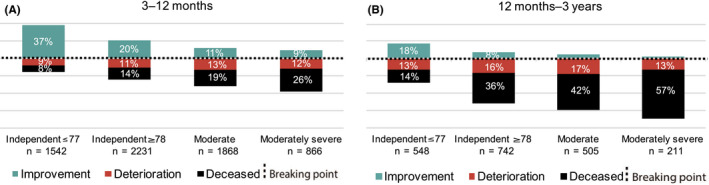
(A, B) Change in functional status over time. From 3 months to 12 months (A), and from 12 months to 3 years (B). The breaking point in each column separates those who improved and those who deteriorated or died. Only those alive and functionally dependent (mRS 3‐5) at 3 (A) and 12 months (B), respectively, were included. Differences in overall outcome between groups were statistically significant (P < .05) at all follow‐up time points. Moderate prestroke dependency = mRS 3, moderately severe prestroke dependency = mRS 4. Severe dependency patients (mRS 5) were not shown since no further deterioration was possible within the mRS classification. Percentages ≤ 5 were not labeled
At three‐year follow‐up, the proportion that had died or deteriorated in functional status was 86.3% in severe dependency, 87.5% in moderately severe dependency, 82.6% in moderate dependency, and 75.1% in independent patients ≥ 78 years (Figure 1C). These proportions were further increased at five‐year follow‐up (Figure 1D).
We examined the relative effect of several key variables on long‐term outcome (five‐year mortality). Figure 3 shows significantly diverging survival in groups of different prestroke dependency level (P < .05). This finding was also demonstrated in a Cox regression model adjusted for several potential confounding factors (Table 3). The model also showed a significant effect of comorbidity burden as well as stroke severity.
Figure 3.
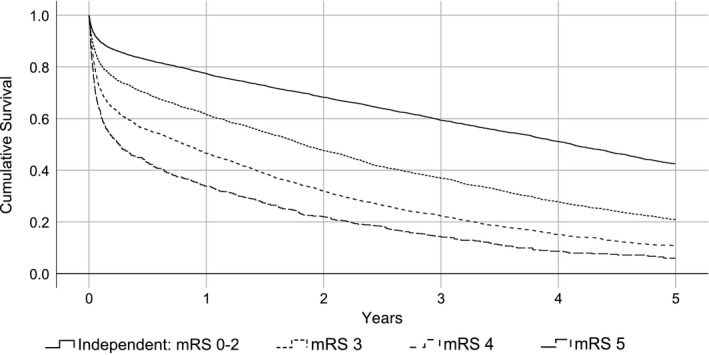
Cumulative 5‐year survival in patients of different prestroke dependency level. Group differences were statistically significant (P < .05). Total n = 11 639. A group of prestroke‐independent patients of comparable age (≥78) (n = 5740) was included for reference
Table 3.
Prognostic impact on mortality within 5 years in prestroke‐dependent patients
| HR (95% CI) | |
|---|---|
| Level of consciousness at admission | |
| Alert (reference) | ‐ |
| Drowsy | 2.16 (2.03‐2.29) |
| Comatose | 5.02 (4.56‐5.52) |
| Total comorbidity burden | |
| None (reference) | ‐ |
| Low | 1.14 (1.03‐1.26) |
| Moderate | 1.43 (1.30‐1.57) |
| High | 1.87 (1.70‐2.06) |
| Prestroke level of dependency | |
| Independent (mRS ≤ 2) of comparable age (≥78) (reference) | ‐ |
| Moderate (mRS 3) | 1.72 (1.63‐1.82) |
| Moderately severe (mRS 4) | 2.45 (2.30‐2.62) |
| Severe (mRS 5) | 2.77 (2.53‐3.04) |
Cox regression proportional hazards model adjusted for age, sex, and socioeconomic status. Including prestroke‐dependent patients plus a reference group of prestroke independents of comparable age(≥78). Total n = 11 263.
Abbreviations: CI, confidence interval; HR, Hazard ratio; mRS, modified Rankin scale.
4. DISCUSSION
Prestroke functionally dependent patients differed substantially from prestroke independents in key characteristics, most notably age: dependents were on average 10 years older. Also, compared to independents of comparable age, they had a considerably higher prevalence of dementia, previous stroke, AF, and other comorbidities. At three‐year follow‐up, only a small proportion (16.8%) of prestroke dependents had maintained prestroke functional status. However, there was substantial heterogeneity within this group: There was greater comorbidity burden as well as increasingly poor prognosis with higher level of dependency. Two distinct groups could be discerned from our results: those of moderate dependency and those of moderately severe to severe dependency. For instance, at three‐month follow‐up, mortality in moderate dependency patients was half of that seen in severe dependency patients (25.3% versus 49.6%, respectively).
The association between physical disability and poor prognosis after stroke has been demonstrated by others as well. For instance, Kwok et al (n = 14 437) have described a linear relationship between prestroke mRS score and probability of death in the acute phase after stroke, 4 and Ganesh et al (n = 380) have reported a strong relationship between early functional deterioration (within three months) and death or institutionalization at five years in prestroke dependent patients. 3 However, compared to our study, most previous studies use small and/or selected cohorts, only include mortality follow‐up data, and do not stratify analyses based on prestroke degree of dependency. We have provided an in‐depth characterization of a group of patients which has previously received little attention in relation to their considerable consumption of healthcare resources. The needs of functionally dependent patients, many of which are suffering from multiple comorbidities, are highly variable and complex, which warrants a structured and comprehensive approach: a major challenge for health professionals and healthcare systems. We would like to mention two interventions that we believe have the potential to substantially improve management of these individuals: community multidisciplinary teams and formal frailty assessment. Multidisciplinary teams include health professionals with different competences working together to address complex needs. Such teams are now considered standard of care in the inpatient setting, but are not well implemented in community‐based care. 18 Also, many functionally dependent patients can be considered frail, a result of cumulative decline in many physiological systems during a lifetime, leading to increased vulnerability to physiological stressors, such as a stroke. 19 Frailty is a (loosely) defined clinical concept and frailty assessment in selected patients has proven useful in identifying high‐risk individuals. 20 It is frequently used in the geriatric setting and it has been suggested that frailty assessment should be integrated into the care of all patients over 70. 20 It would likely benefit functionally dependent patients, particularly those with a history of stroke.
An additional important finding in our study was the difference in treatment. Stroke‐specific drug prescription (including antihypertensive drugs, statins, antiplatelet drugs, and OAC) decreased with increasing level of dependency. Specifically, only 15.6% of severe dependency patients with AF were prescribed OAC at discharge compared to 35.4% in moderate dependency patients and 55.1% in independent patients of comparable age. Our results suggest that OAC may be under‐prescribed in functionally dependent patients (who have a high baseline risk for stroke). However, since prescription of OAC is affected by many factors (eg, risk versus benefit considerations), we cannot draw any such conclusions based on our data. Moreover, in the most recent Riksstroke data, the prescription of OAC has increased substantially, particularly for non‐vitamin K antagonist oral anticoagulants (NOAC), 21 which in our study comprised a very modest proportion of total OAC prescriptions. These drugs have a more favorable risk profile as compared to warfarin and continuous monitoring is generally not required. Also, a relatively small proportion (4.2%) of prestroke‐dependent patients received thrombolytic therapy. However, acute interventional therapy in stroke is in rapid development and the study cohort had their index stroke before major revisions of the Swedish national guidelines in 2014. Thrombolysis rates have now increased substantially in the elderly, reaching 12% in those > 80 of age in 2018 21 compared to 4.6% in the study cohort. Several studies support the effectiveness of thrombolytic therapy in individuals with prestroke disability. 22 , 23 , 24 However, evidence is limited regarding effectiveness and safety in particular subgroups (eg, those with dementia or severe disability).
Further research describing functionally dependent and multimorbid stroke patients is warranted. Important areas include clinical characteristics and prognosis, the implementation of comprehensive management strategies (eg, multidisciplinary teams and frailty assessment), and comorbidity (literature is currently sparse and methodologically heterogeneous 25 ). As is highlighted by our results, this research needs to be stratified because of the heterogeneity within this group of patients.
4.1. Strengths
This comprehensive description of prestroke‐dependent patients is, to our knowledge, the most extensive of its kind to date. Most previous studies include smaller and/or selected cohorts, only report mortality follow‐up data, and/or do not use a stratified approach.
4.2. Limitations
Several important potential sources of bias are discussed below.
First, attrition bias. Overall, loss to follow‐up was relatively low. However, to minimize the impact of potential bias, we used multiple imputation.
Secondly, selection bias. The Riksstroke register only includes stroke patients admitted to hospital (estimated to around 84%‐92% 26 , 27 ). Non‐admittance might have been greater in certain groups, for example, those of severe prestroke dependency where the effects of a new stroke may be less apparent.
Thirdly, classification bias. Multiple types of evidence attest to the validity and reliability of the mRS as a measure of function after stroke. 15 As for prestroke mRS, the reliability has been shown to be comparable with standard mRS. However, there may be a poor correlation with certain markers of function. 28 No specific clinical assessment was made to determine mRS scores, which makes the estimation less reliable. Since the estimation was primarily made from information reported via paper‐based follow‐up surveys, this might have introduced reporter bias. Specifically, information on post‐stroke functional status was provided by the patient or next of kin, whereas information on prestroke status was reported by hospital personnel. The use of different sources may explain why a small proportion of patients seemed to improve in functional status relative to their prestroke status. Further, within the mRS scale, certain scores have greater intra‐class variability and the mRS is unable to capture subtle changes that may still be clinically relevant and for severe dependency patients (mRS 5), further deterioration is not accounted for. This makes comparative analysis of functional outcome problematic which is why we only used mortality as measure of outcome in our multivariable analyses.
5. CONCLUSION
Stroke had a large impact on prognosis in prestroke‐dependent patients. Only four out of ten patients maintained their prestroke functional level at three‐month follow‐up, and less than two out of ten at three years. However, there was great heterogeneity within this group. In severe prestroke‐dependent patients, the comorbidity burden was higher, drug prescription was lower, and prognosis less favorable. In those with moderate prestroke dependency, a relatively large proportion survived and maintained prestroke functional level. Our results highlight the need for research efforts and treatment management strategies addressing patients of different prestroke level of dependency separately.
CONFLICT OF INTEREST
BN received honoraria for serving on data monitoring committees from Astra Zeneca (SOCRATES and THALES trials) and Bayer AG (NAVIGATE‐ESUS trial).
ACKNOWLEDGEMENTS
We would like to thank the staff at Riksstroke, in particular statistician Fredrik Jonsson for preparing the data from Riksstroke used in the study. Also, we thank the external proofreader Lee Nolan.
Appendix 1.
Supplemental methods
This section expands upon our use of multiple imputation on the whole Riksstroke long‐term follow‐up data set of 22 905 patients which also included those with intracerebral hemorrhage. We present key information as suggested by Sterne et al. 29
Total loss to follow‐up in the four surveys (three months, 12 months, three years, and five years) ranged from 12.8%‐21.2% (Appendix 3). We used multiple imputation to estimate likely values of missing data in the variables needed to compute mRS scores. These variables were:
-
Functional ability in:
Toileting
Dressing
Mobility
Living conditions
Need of support from next of kin
We used a large number of predictor variables, both from baseline and follow‐up, likely associated with functional outcome. Therefore, it seemed reasonable to assume that data were missing at random (MAR). Under the assumption of MAR, we may obtain valid inferences by applying a MI technique. However, it is important to stress that missingness likely contained some element of non‐missing at random (NMAR) and that the assumption of MAR is reasonable since we included a large number of predictors.
Variables used only as predictors
Sex, age, diagnosis, living conditions before stroke, previous stroke, smoking, previous TIA/amaurosis fugax, atrial fibrillation, diabetes, hypertension, level of consciousness at admission, and functional status before stroke.
Variables both used as predictors and imputed
Functional ability in toileting, dressing, mobility as well as living conditions and need of support from next of kin.
Missing data for variables only used as predictors was less than 1%, except for previous TIA/amaurosis fugax (1.8%), level of consciousness at admission (1.2%), and smoking (6.9%). The distribution of missing values in each of the imputed variables at each time point is shown in Appendix 3.
Since data displayed a non‐monotone pattern of missing values and the missing variables were categorical, we imputed using the fully conditional specification method based on a logistic regression model. Five imputations were conducted, each based on ten iterations of the underlying Markov chain. The spread in imputed values is presented in Appendix 4.
Appendix 2.
List of the 17 included chronic conditions and ICD‐10 codes
| ICD‐10 code | |
|---|---|
| CCI conditions included | |
| Solid tumor, non‐metastatic | C00‐76 |
| Solid tumor, metastatic | C77‐79 |
| Leukemia/myeloma | C88‐96 |
| Lymphoma | C81‐86 |
| Chronic liver disease | B18, K70, K72, K73, K74 |
| Chronic kidney failure | N18 |
| COPD | J44 |
| Rheumatoid arthritis | M04, M05 |
| Peripheral vascular disease | I73 |
| Congestive heart failure | I50 |
| Myocardial infarction | I21, I22 |
| Diabetes | Data obtained from Riksstroke |
| Dementia | F00‐03 |
| Cerebrovascular disease | Data obtained from Riksstroke |
| CCI conditions not included | |
| Ulcer disease | ‐ |
| Hemiplegia | ‐ |
| AIDS | ‐ |
| Non‐CCI conditions included | |
| Atrial fibrillation/flutter | Data obtained from Riksstroke |
| Angina pectoris | I20 |
| Hypertension | Data obtained from Riksstroke |
| CCI, Charlson Comorbidity Index. | |
Appendix 3.
Proportion of missing values in imputed variables at different time points
| 3 months | 12 months | 3 years | 5 years | |
|---|---|---|---|---|
| Functional ability in | ||||
| Toileting | 11.5% | 20% | 18.8% | 15.7% |
| Dressing | 11.6% | 19.9% | 18.9% | 15.8% |
| Mobility | 10.4% | 20.2% | 19.4% | 16% |
| Living conditions | 9.9% | 20.3% | 19.7% | 16.3% |
| Need of support from next of kin | 12.9% | 20.5% | 20.1% | 16.7% |
Appendix 4.
Proportion of mRS scores in survivors at different time points: comparison between original and imputed data sets. In the original data set, cases lost to follow‐up were omitted. In the imputed data sets, missing data were replaced with imputed values. Five imputations were performed and a mean was calculated. The mean as well as lowest and highest score in the five individual imputation is presented
| Original data | Imputed data | ||||
|---|---|---|---|---|---|
| Mean | Lowest | Highest | |||
| 3 months | Independent (mRS 0‐2) | 51.7% | 51% | 50.8% | 51.2% |
| mRS 3 | 22.2% | 22.7% | 22.6% | 22.8% | |
| mRS 4 | 14.3% | 14.2% | 14.1% | 14.4% | |
| mRS 5 | 11.8% | 12% | 11.9% | 12.1% | |
| 12 months | Independent (mRS 0‐2) | 58.7% | 54.7% | 54.6% | 54.9% |
| mRS 3 | 18.7% | 20.4% | 20.3% | 20.5% | |
| mRS 4 | 14.9% | 15.6% | 15.4% | 15.8% | |
| mRS 5 | 7.6% | 9.2% | 9.1% | 9.4% | |
| 3 years | Independent (mRS 0‐2) | 61% | 56.1% | 55.1% | 57.1% |
| mRS 3 | 18.8% | 19.8% | 19.2% | 20.6% | |
| mRS 4 | 14% | 16% | 15.3% | 16.7% | |
| mRS 5 | 6.2% | 8.1% | 7.9% | 8.3% | |
| 5 years | Independent (mRS 0‐2) | 62.2% | 59.1% | 58% | 60.5% |
| mRS 3 | 17.6% | 18.7% | 18.3% | 19.1% | |
| mRS 4 | 14.4% | 15.1% | 14.4% | 15.7% | |
| mRS 5 | 5.8% | 7% | 6.7% | 7.3% | |
Sennfält S, Pihlsgård M, Norrving B, Ullberg T, Petersson J. Ischemic stroke patients with prestroke dependency: Characteristics and long‐term prognosis. Acta Neurol Scand. 2020;143:78–88. 10.1111/ane.13328
Funding information
This work was supported by the Swedish Stroke Association, Neuro Sweden, Sparbanken Färs och Frosta and received ALF funding from Region Skåne. Financial sponsors played no role in the design, execution, analysis and interpretation of data, or writing of the study.
REFERENCES
- 1. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallacher KI, Batty GD, McLean G, et al. Stroke, multimorbidity and polypharmacy in a nationally representative sample of 1,424,378 patients in Scotland: implications for treatment burden. BMC Med. 2014;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganesh A, Luengo‐Fernandez R, Pendlebury ST, et al. Long‐Term Consequences of Worsened Poststroke Status in Patients With Premorbid Disability. Stroke. 2018;49:2430‐2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwok CS, Clark A, Ford GA, et al. Association between prestroke disability and inpatient mortality and length of acute hospital stay after acute stroke. J Am Geriatr Soc. 2012;60:726‐732. [DOI] [PubMed] [Google Scholar]
- 5. Sennfalt S, Norrving B, Petersson J, et al. Long‐term survival and function after stroke. Stroke. 2019;50(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 6. Corraini P, Szépligeti SK, Henderson VW, et al. Comorbidity and the increased mortality after hospitalization for stroke: a population‐based cohort study. J Thromb Haemost. 2018;16:242‐252. [DOI] [PubMed] [Google Scholar]
- 7. Sennfält S, Pihlsgård M, Petersson J, et al. Long‐term outcome after ischemic stroke in relation to comorbidity – An observational study from the Swedish Stroke Register (Riksstroke). Eur Stroke J. 2020;5(1):36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchwald F, Norrving B, Petersson J. Atrial fibrillation in transient ischemic attack versus ischemic stroke: a Swedish stroke register (riksstroke) study. Stroke. 2016;47:2456‐2461. [DOI] [PubMed] [Google Scholar]
- 9. Induruwa I, Evans NR, Aziz A, et al. Clinical frailty is independently associated with non‐prescription of anticoagulants in older patients with atrial fibrillation. Geriatr Gerontol Int. 2017;17:2178‐2183. [DOI] [PubMed] [Google Scholar]
- 10. Cappellari M, Bosco M, Forlivesi S, et al. Reasons for exclusion from intravenous thrombolysis in stroke patients admitted to the Stroke Unit. J Thromb Thrombolysis. 2016;42:593‐599. [DOI] [PubMed] [Google Scholar]
- 11. Riksstroke . The Riksstroke annual report of 2013 . Umeå, Sweden: Västerbottens läns landsting, 2014.
- 12. Riksstroke . The Riksstroke annual report of 2011 . Umeå, Sweden: Västerbottens läns landsting, 2012.
- 13. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453‐1457. [DOI] [PubMed] [Google Scholar]
- 15. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091‐1096. [DOI] [PubMed] [Google Scholar]
- 16. Eriksson M, Appelros P, Norrving BO, et al. Assessment of functional outcome in a national quality register for acute stroke: can simple self‐reported items be transformed into the modified Rankin Scale? Stroke. 2007;38:1384‐1386. [DOI] [PubMed] [Google Scholar]
- 17. Shrive FM, Stuart H, Quan H, et al. Dealing with missing data in a multi‐question depression scale: a comparison of imputation methods. BMC Med Res Methodol. 2006;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Socialstyrelsen (eng. Swedish National Board of Health and Welfare). Utvärdering 2018, Vård vid stroke (eng. Evaluation of stroke care, 2018) 2018. www.socialstyrelsen.se
- 19. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381:752‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riksstroke . The Riksstroke annual report of 2018 . Umeå, Sweden: Västerbottens läns landsting, 2019.
- 22. Karlinski M, Kobayashi A, Czlonkowska A, et al. Role of Preexisting Disability in Patients Treated With Intravenous Thrombolysis for Ischemic Stroke. Stroke. 2014;45(3):770‐775. [DOI] [PubMed] [Google Scholar]
- 23. Merlino G, Corazza E, Lorenzut S, et al. Efficacy and Safety of Intravenous Thrombolysis in Patients with Acute Ischemic Stroke and Pre–Existing Disability. J Clin Med. 2019;8(3):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gensicke H, Strbian D, Zinkstok SM, et al. Intravenous Thrombolysis in Patients Dependent on the Daily Help of Others Before Stroke. Stroke. 2016;47(2):450‐456. [DOI] [PubMed] [Google Scholar]
- 25. Violan C, Foguet‐Boreu Q, Flores‐Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9:e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Appelros P, Hogeras N, Terent A. Case ascertainment in stroke studies: the risk of selection bias. Acta Neurol Scand. 2003;107:145‐149. [DOI] [PubMed] [Google Scholar]
- 27. Hallstrom B, Jonsson AC, Nerbrand C, et al. Lund Stroke Register: hospitalization pattern and yield of different screening methods for first‐ever stroke. Acta Neurol Scand. 2007;115(1):49‐54. [DOI] [PubMed] [Google Scholar]
- 28. Fearon P, McArthur KS, Garrity K, et al. Prestroke modified Rankin stroke scale has moderate interobserver reliability and validity in an acute stroke setting. Stroke. 2012;43:3184‐3188. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]


