Abstract
Background
There has been a concern that blood donations can increase the risk of hematological malignancies. We investigated if blood donations increase the risk of developing hematological malignancies, specifically acute lymphoblastic leukemia, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myeloid leukemia, Hodgkin lymphoma, and myeloma, as well other non‐Hodgkin lymphoma.
Study Design and Methods
In total, the study included 1,021,433 Swedish blood donors, with 19.5 million person‐years of follow‐up. Two sets of analysis were performed. In the first cohort analysis, standardized incidence ratios (SIRs) were calculated, comparing the incidence of the different types of hematological cancers in blood donors to that of the general population. In the second analysis, a nested case–control study was conducted, investigating the association between number of donations and the risk of each type of malignancy.
Results
Apart from a modestly elevated risk of CLL (SIR, 1.07; 95% confidence interval [CI], 1.01‐1.15) and a modestly decreased risk of AML (SIR, 0.85; 95% CI, 0.77‐0.83), the risk of hematological malignancies did not differ between blood donors and the general population. In the nested case–control study there were no convincing associations between number of prior whole blood donations and site‐specific malignancy risk.
Conclusions
There was no convincing evidence of an increased risk in any hematological malignancy when interpreting the results from both series of analyses.
Abbreviations
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- CML
chronic myeloid leukemia
- IQR
interquartile range
- SCANDAT3‐S
Scandinavian Donations and Transfusions database
- SIRs
standardized incidence ratios
1. INTRODUCTION
Blood donors are selected for their good health, and unsurprisingly, studies on blood donor health have shown that donors have an overall decreased risk of cancer compared to the general population. 1 In particular, associations have been found between whole blood donations with a decreased risk for iron‐dependent malignancies such as cancer of the lung, liver, colon, stomach, and esophagus, 2 , 3 , 4 , 5 , 6 which could perhaps be explained by relative iron deficiency due to frequent phlebotomy.
However, a study published in 1990 investigating the risk of cancer in 37 795 blood donors reported that while blood donors have lower risk for cancers overall, this was not observed for hematological malignancies. 7 This was reproduced in an observational cohort study with data up to 2002 in Sweden and Denmark. 1 A more recent study published in 2013 on blood donors in the United States reported an overall increased incidence of cancer in blood donors, including hematological malignancies; however, this was speculated to be the result of increased detection in the donor population. 8 Still, given the lack of recent large‐scale studies, there is persistent concern that blood donations may increase the risk of hematological malignancies.
Using data from the Swedish part of the newly updated Scandinavian Donations and Transfusions database (SCANDAT3‐S) (reference to concurrently submitted SCANDAT3‐S manuscript), we conducted a nationwide cohort study and nested case–control study to study the risk of hematological malignancies. In addition, we also characterized the patterns of hemoglobin concentration before diagnosis of a hematological malignancy.
2. METHODS
2.1. Setting and data sources
In Sweden, blood banks and blood collection centers are part of the public health care system. Donors are nonremunerated and are required to have a hemoglobin concentration of at least 125 and 135 g/L for men and women, respectively. Whole blood donations are limited to four times and three times per year for men and women, respectively. Oral iron supplementation is routinely supplied to active donors.
Analyses were based on the Swedish portion of SCANDAT3‐S (reference to concurrently submitted SCANDAT3‐S manuscript). In brief, the database contains all electronically available data on blood donors, blood donations, blood transfusions and transfused patients, with the earliest data going back to the late 1960s. Using national registration numbers, assigned to all Swedish inhabitants, the database has been linked with a range of health outcomes registers, including the Swedish Cancer Register and Registers of the Swedish Total Population, to allow identification of individuals with hematological cancers and migration status as well as date of death throughout the study period. 9
2.2. Study population, study design, and statistical analyses
From SCANDAT3‐S, we extracted information on all whole blood donations between 1980 and 2017. Donors were followed from the date of their first whole blood donation until the date of first diagnosis of any hematological malignancy, death, emigration, or December 31, 2017, whichever occurred first. Diagnosis of hematological malignancy was ascertained from the Swedish Cancer Register, which were coded using revisions 8 and 10 of the International Classification of Diseases. Hematological malignancies were divided into six groups: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), chronic myeloid leukemia (CML), Hodgkin lymphoma, multiple myeloma, and other non‐Hodgkin lymphoma. For more details on classification, see Table S1.
We performed two separate analyses to investigate the risk of hematological cancer after blood donation. First, we computed standardized incidence ratios (SIRs) comparing the incidence of hematological malignancies in the donor cohort to that of the background population. SIRs were computed as the ratio of the observed to expected number of cancers, where the latter was calculated by multiplying the age‐, sex‐, and calendar year–specific cancer incidence in the general population with the corresponding person‐time at risk in the donor cohort. Confidence limits for the SIRs were constructed with use of an exact method. 10
Second, to investigate the association between number of whole blood donations and risk of hematological malignancy, we performed a nested case–control study. We used the same case definitions as for the SIR calculations and selected up to 10 controls for each case. Controls were selected using incidence‐density sampling, matching on sex, geographical region of first donation, age at first donation (± 2.5 years), as well as date of first donation (± 1 year). 11 Time since first donation was used as the time scale.
Relative risks of each hematological malignancy, in relation to total number of prior whole blood donations, expressed as odds ratios, were computed with conditional logistic regression accounting for the matched sets. In addition to number of prior whole blood donations (categorized as 1‐5, 6‐10, 11‐25, 26‐50, or >50 donations), the models incorporated an interaction term between sex and age at first donation, as well as a categorical term for country of birth (Sweden, northern Europe, rest of Europe, or outside of Europe).
Recognizing that early signs of a hematological malignancy—including a reduced hemoglobin concentration—might influence donors' propensity to donate blood, we first characterized the patterns of changing hemoglobin concentration in both cases and controls before diagnosis of the case. This was done by modeling the hemoglobin concentration, recorded for each blood donation as a function of time to diagnosis of the case, using a mixed‐effects model with time until diagnosis modeled as a restricted cubic spline (with knots at −3000, −1000, −730, −365, −180 days before diagnosis of the case), indicator variable for cases, interaction between the indicator and time until diagnosis, with random intercepts for the individual as well as the for each matched set of cases and controls.
This analysis demonstrated considerable heterogeneity between the different types of malignancies (Figure 1), where the hemoglobin began to decline as early as 3 to 4 years before the malignancy was finally diagnosed. We therefore ran all analyses both overall and with a 3‐year latency.
FIGURE 1.
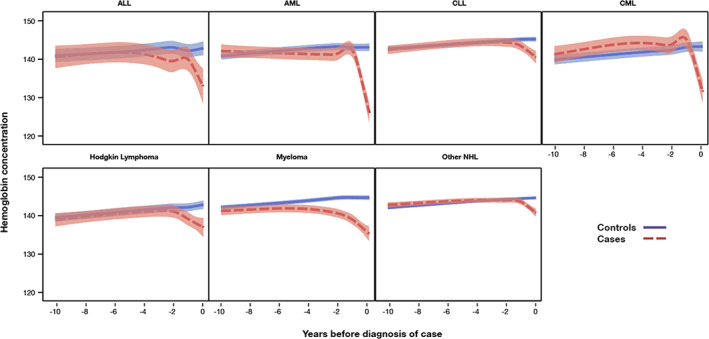
Depicts the average hemoglobin concentration (g/L) in cases and controls for the different types of investigated hematological malignancies, as a function of time before diagnosis of case [Color figure can be viewed at wileyonlinelibrary.com]
All statistical analyses and data handling were conducted with computer software (SAS version 9.4, (SAS Institute). The study was approved by the regional ethics committee in Stockholm, Sweden (2018/167‐31, 2019‐02636).
3. RESULTS
We included a total of 1 021 433 blood donors. Baseline characteristics are presented in Table 1. A total of 480 043 (47.0%) donors were female. The median age at first donation was 29.9 years (interquartile range [IQR], 23.0‐40.1) and the median length of follow‐up was 19.4 years (IQR, 9.7‐27.6).
TABLE 1.
Characteristics of donor cohort
| Number of donors | 1 021 433 | |
|---|---|---|
| Female sex, n (%) | 480 043 | (47.0) |
| Age at first donation, n (%) | ||
| 18‐30 y | 513 660 | (50.3) |
| 31‐40 y | 249 731 | (24.4) |
| 41‐50 y | 171 773 | (16.8) |
| 51‐65 y | 84 141 | (8.2) |
| >65 y | 2128 | (0.2) |
| Median (IQR) | 29.9 | (23.0‐40.1) |
| Duration of follow‐up, n (%) | ||
| <5 years | 126 485 | (12.4) |
| 5‐9 years | 137 999 | (13.5) |
| 10‐19 years | 269 754 | (26.4) |
| ≥20 years | 487 195 | (47.7) |
| Median (IQR) | 19.4 | (9.7‐27.6) |
| Year of first donation, n (%) | ||
| 1980‐1989 | 277 593 | (27.2) |
| 1990‐1999 | 320 501 | (31.4) |
| 2000‐2009 | 242 469 | (23.7) |
| 2010‐2017 | 180 870 | (17.7) |
Abbreviation: IQR, interquartile range.
Table 2 presents the total number of observed and expected events, together with corresponding SIRs. Over a total of 19.5 million person‐years, we observed 150 ALL cases, 438 AML cases, 909 CLL cases, 232 CML cases, 397 Hodgkin lymphoma cases, 1051 myeloma cases, and 2772 other non‐Hodgkin lymphoma cases. Compared to the general population, this corresponded to SIRs of 1.06 (95% confidence interval [CI], 0.90‐1.24) for ALL, 0.85 (95% CI, 0.77‐0.93) for AML, 1.07 (95% CI, 1.01‐1.15) for CLL, 1.02 (95% CI, 0.89‐1.16) for CML, 0.92 (95% CI, 0.83‐1.01) for Hodgkin lymphoma, 1.03 (95% CI, 0.97‐1.10) for myeloma, and 1.03 (95% CI, 0.99‐1.07) for other non‐Hodgkin lymphoma. Estimates were largely unchanged by implementing a 3‐year latency.
TABLE 2.
Standardized incidence ratios for hematological malignancies, comparing the donor cohort to the general population
| Outcome | Observed number of events | Expected number of events | Standardized incidence ratio (95% CI) |
|---|---|---|---|
| ALL | |||
| Overall | 150 | 141.6 | 1.06 (0.90‐1.24) |
| With 3‐year latency | 134 | 124.3 | 1.08 (0.90‐1.28) |
| AML | |||
| Overall | 438 | 514.6 | 0.85 (0.77‐0.93) |
| With 3‐year latency | 412 | 480.2 | 0.86 (0.78‐0.95) |
| CLL | |||
| Overall | 909 | 846.1 | 1.07 (1.01‐1.15) |
| With 3‐year latency | 891 | 821.2 | 1.08 (1.01‐1.16) |
| CML | |||
| Overall | 232 | 227.7 | 1.02 (0.89‐1.16) |
| With 3‐year latency | 204 | 206.0 | 0.99 (0.86‐1.14) |
| Hodgkin lymphoma | |||
| Overall | 397 | 432.6 | 0.92 (0.83‐1.01) |
| With 3‐year latency | 324 | 356.8 | 0.91 (0.81‐1.01) |
| Myeloma | |||
| Overall | 1051 | 1015.9 | 1.03 (0.97‐1.10) |
| With 3‐year latency | 1015 | 980.3 | 1.04 (0.97‐1.10) |
| Other non‐Hodgkin lymphoma | |||
| Overall | 2772 | 2688.3 | 1.03 (0.99‐1.07) |
| With 3‐year latency | 2647 | 2550.1 | 1.04 (1.00‐1.08) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia. [Correction added on Sept 29, 2020, after first online publication: Alternative Table 2 with forest plot was removed.]
Results from the nested case–control study are presented in Table 3. With the exception of a decreased risk of myeloma in donors who had performed >50 whole blood donations (odds ratio, 0.7; 95% CI, 0.5‐0.9), we did not find any evidence of any association between number of whole blood donations and risks of hematological malignancies. Patterns were largely unaffected by the introduction of a 3‐year latency.
TABLE 3.
Relative risk of hematological malignancy in relation to number of whole blood donations
| Outcome | Number of cases/number of controls | Number of whole blood donations | ||||
|---|---|---|---|---|---|---|
| 1‐5 | 6‐10 | 11‐25 | 26‐50 | >50 | ||
| Odds ratio (95% CI) | ||||||
| ALL | 150/1499 | |||||
| Overall | 1.00 (ref) | 0.8 (0.5‐1.3) | 1.1 (0.7‐1.8) | 1.2 (0.6‐2.1) | 0.7 (0.3‐1.9) | |
| With 3‐year latency | 1.00 (ref) | 0.8 (0.4‐1.3) | 1.4 (0.9‐2.2) | 1.3 (0.7‐2.5) | 0.6 (0.2‐2.1) | |
| AML | 438/4380 | |||||
| Overall | 1.00 (ref) | 1.3 (1.0‐1.7) | 1.1 (0.8‐1.4) | 0.9 (0.6‐1.3) | 1.3 (0.8‐2.2) | |
| With 3‐year latency | 1.00 (ref) | 1.3 (1.0‐1.8) | 1.1 (0.9‐1.5) | 0.9 (0.6‐1.3) | 1.4 (0.8‐2.5) | |
| CLL | 909/9090 | |||||
| Overall | 1.00 (ref) | 1.1 (0.9‐1.3) | 1.0 (0.8‐1.2) | 1.0 (0.8‐1.3) | 1.2 (0.9‐1.7) | |
| With 3‐year latency | 1.00 (ref) | 1.1 (0.9‐1.4) | 1.0 (0.8‐1.2) | 1.1 (0.8‐1.3) | 1.4 (1.0‐1.9) | |
| CML | 232/2320 | |||||
| Overall | 1.00 (ref) | 0.9 (0.6‐1.4) | 1.0 (0.7‐1.5) | 1.4 (0.9‐2.2) | 1.3 (0.6‐2.7) | |
| With 3‐year latency | 1.00 (ref) | 0.9 (0.6‐1.5) | 1.1 (0.7‐1.7) | 1.5 (0.9‐2.4) | 1.1 (0.4‐2.9) | |
| Hodgkin lymphoma | 397/3970 | |||||
| Overall | 1.00 (ref) | 1.1 (0.8‐1.5) | 0.9 (0.7‐1.2) | 1.0 (0.7‐1.6) | 1.8 (0.9‐3.6) | |
| With 3‐year latency | 1.00 (ref) | 1.1 (0.8‐1.5) | 1.1 (0.8‐1.5) | 1.2 (0.7‐2.0) | 2.2 (0.9‐5.0) | |
| Myeloma | 1051/10510 | |||||
| Overall | 1.00 (ref) | 0.9 (0.7‐1.1) | 0.9 (0.8‐1.1) | 0.9 (0.7‐1.1) | 0.7 (0.5‐0.9) | |
| With 3‐year latency | 1.00 (ref) | 0.9 (0.7‐1.1) | 1.0 (0.8‐1.2) | 0.9 (0.7‐1.1) | 0.7 (0.5‐1.0) | |
| Other non‐Hodgkin lymphoma | 2772/27715 | |||||
| Overall | 1.00 (ref) | 1.0 (0.8‐1.1) | 1.0 (0.9‐1.1) | 0.9 (0.8‐1.1) | 1.0 (0.8‐1.2) | |
| With 3‐year latency | 1.00 (ref) | 0.9 (0.8‐1.1) | 1.0 (0.9‐1.2) | 1.0 (0.9‐1.1) | 0.9 (0.7‐1.1) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia.
Figure 1 depicts the average hemoglobin concentrations for cases and controls as a function of time before diagnosis of the cases. For most hematological malignancies, hemoglobin concentrations began to decrease at least 1 year before the case was diagnosed, even in diseases with an acute presentation. Patients with AML exhibited the most dramatic pattern, where hemoglobin concentration decreased almost 20 g/L in the final 2 years before diagnosis.
4. DISCUSSION
In this large, population‐based cohort study, supplemented by a nested case–control study, we use robust methods to demonstrate that there is no increased risk of developing a hematological malignancy among whole blood donors compared to the general population and that frequent donations do not increase risks. Moreover, we also demonstrate that hemoglobin concentration begins to decrease already in the presymptomatic period before a diagnosis of hematological malignancy, even in malignancies with acute presentations. This may represent the long latency between developing a premalignant/symptomatic clonal hematopoiesis to the development of overt disease even in aggressive diseases. 12
The only departures, from the otherwise inconspicuous results, were a slightly decreased risk of AML and a slightly increased risk of CLL, comparing the donor cohort to the general population. Given that we found no evidence of a dose–response association between the number of donations and the risk of these malignancies, we interpret these findings to be unlikely to represent causal effects. Indeed, it seems more likely that the former is driven by residual confounding from comorbidities (eg, prior chemotherapy or prior malignant disease, which would preclude from blood donation) and that the latter is driven by screening effects from repeated blood testing and/or from comparatively higher health consciousness in the donor population.
The strength of this study is its size and the generally high quality of Swedish register data, resulting in high statistical precision, negligible loss to follow‐up, and high accuracy in recorded diagnosis of hematological malignancies. The SCANDAT3‐S database contains all available computerized data on blood donations, with more than 5 decades of follow‐up (of which we used data from a period of nearly 40 years), making this study the most long‐standing of its kind.
The study has several limitations. Foremost, frequent blood donations essentially screen for a hematological malignancy, as donor hemoglobin is controlled at the blood collection center. Subclinical disease, presented as a modest but persistent drop in hemoglobin, might result in an expedited diagnosis of hematological disease. Furthermore, frequent blood donors with undiagnosed hematological disease might donate less frequently due to prodromal symptoms. Both of these phenomena may weaken any dose–response relationship between blood donations and subsequent hematological disease. Also, since there is a limit on the frequency of whole blood donations per year (a maximum of four times and three times per year for men and women, respectively), we were not able to assess potential effects of more frequent whole blood donations.
In conclusion, we found no convincing evidence of a relationship between hematological malignancy and blood donations. Given the consistency between the two types of analyses, and the overall strengths of the data, this should serve as comfort to both blood donors and health care professionals involved in blood collection.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1 International Classification of Diseases coding for hematological malignancies
Zhao J, Dahlén T, Brynolf A, Edgren G. Risk of hematological malignancy in blood donors: A nationwide cohort study. Transfusion. 2020;60:2591–2596. 10.1111/trf.16020
Funding information Karolinska Institutet; Region Stockholm; Vetenskapsrådet, Grant/Award Number: 2017‐01954; The creation of the SCANDAT database and the conduct of this study was made possible by a grant to Dr Edgren from Swedish Research Council (2017‐01954). Dr Edgren is supported by Region Stockholm (clinical research appointment). Dr Zhao is supported by the Clinical Scientist Training Program and the Research Internship Program, both at Karolinska Institutet.
REFERENCES
- 1. Edgren G, Tran TN, Hjalgrim H, et al. Improving health profile of blood donors as a consequence of transfusion safety efforts. Transfusion. 2007;47:2017–2024. [DOI] [PubMed] [Google Scholar]
- 2. Knekt P, Reunanen A, Takkunen H, Aromaa A, Heliovaara M, Hakulinen T. Body iron stores and risk of cancer. Int J Cancer. 1994;56:379–382. [DOI] [PubMed] [Google Scholar]
- 3. Nelson RL, Davis FG, Sutter E, Sobin LH, Kikendall JW, Bowen P. Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst. 1994;86:455–460. [DOI] [PubMed] [Google Scholar]
- 4. Selby JV, Friedman GD. Epidemiologic evidence of an association between body iron stores and risk of cancer. Int J Cancer. 1988;41:677–682. [DOI] [PubMed] [Google Scholar]
- 5. Tiniakos G, Williams R. Cirrhotic process, liver cell carcinoma and extrahepatic malignant tumors in idiopathic haemochromatosis. Study of 71 patients treated with venesection therapy. Appl Pathol. 1988;6:128–138. [PubMed] [Google Scholar]
- 6. Nelson RL. Iron and colorectal cancer risk: human studies. Nutr Rev. 2001;59:140–148. [DOI] [PubMed] [Google Scholar]
- 7. Merk K, Mattsson B, Mattsson A, Holm G, Gullbring B, Bjorkholm M. The incidence of cancer among blood donors. Int J Epidemiol. 1990;19:505–509. [DOI] [PubMed] [Google Scholar]
- 8. Vahidnia F, Hirschler NV, Agapova M, Chinn A, Busch MP, Custer B. Cancer incidence and mortality in a cohort of US blood donors: a 20‐year study. J Cancer Epidemiol. 2013;2013(814842):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulm KA. simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990;131:373–375. [DOI] [PubMed] [Google Scholar]
- 11. Greenland S, Thomas DC. On the need for the rare disease assumption in case‐control studies. Am J Epidemiol. 1982;116:547–553. [DOI] [PubMed] [Google Scholar]
- 12. Dickson MA, Papadopoulos EB, Hedvat CV, Jhanwar SC, Brentjens RJ. Acute myeloid leukemia arising from a donor derived premalignant hematopoietic clone: a possible mechanism for the origin of leukemia in donor cells. Leuk Res Rep. 2014;3:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 International Classification of Diseases coding for hematological malignancies


