Abstract
Reported median overall survival (mOS) in metastatic colorectal cancer (mCRC) patients participating in systemic therapy trials has increased to over 30 months. It is uncertain whether trial results translate to real‐life populations. Moreover, patients prefer presentation of multiple survival scenarios. Population‐based data of all stage IV CRC patients diagnosed between 2008 and 2016 were obtained from the Netherlands Cancer Registry, which has a case ascertainment completeness surpassing 95%. We calculated the following percentiles (scenarios) of OS per year of diagnosis for the total population, and for treatment subgroups: 10th (best‐case), 25th (upper‐typical), 50th (median), 75th (lower‐typical) and 90th (worst‐case). Twenty‐five percent of patients did not receive any antitumor treatment. From 2008 to 2016, mOS of the total population (n = 27275) remained unchanged at approximately 12 months. OS improved only for the upper‐typical and best‐case patients; by 4.2 to 29.1 months (P < .001), and by 6 to 62 months (P < .001), respectively. No clinically relevant change was observed among patients who received systemic therapy, with mOS close to 15 months and best‐case scenario approximately 40 months. A clinically relevant improvement in survival over time was observed in patients who initially received metastasectomy and/or HIPEC only. In contrast to the wide belief based on trial data that mOS of mCRC patients receiving systemic therapy has improved substantially, improvement could not be demonstrated in our real‐life population. Clinicians should consider quoting multiple survival scenarios based on real‐life data instead of point estimates from clinical trials, when informing patients about their life expectancy.
Keywords: metastatic colorectal cancer, population‐based, prognosis, real‐world, scenarios for survival
Short abstract
What's new?
Clinical trials on first‐line systemic therapy in metastatic colorectal cancer (mCRC) patients suggest a marked improvement in median overall survival (mOS). However, uncertainty exists in how the survival reported in trials relates to the real‐life mCRC population. This is the first population‐based study providing multiple scenarios for patient survival. The results show that real‐life mCRC patients receiving systemic therapy have a shorter mOS compared to trial patients, likely reflecting patient selection in clinical trials. Clinicians should consider presenting multiple scenarios for survival based on real‐life data instead of point estimates from clinical trials when informing patients about their life expectancy.
Abbreviations
- ASCO
American Society of Clinical Oncology
- CI
confidence interval
- CRC
colorectal cancer
- ESMO
European Society for Medical Oncology
- HIPEC
hyperthermic intraperitoneal chemotherapy
- ICD
International Classification of Diseases for Oncology
- IKNL
Netherlands Comprehensive Cancer Organisation
- mCRC
metastatic colorectal cancer
- mOS
median overall survival
- NCR
Netherlands Cancer Registry
- OS
overall survival
- PALGA
Dutch Pathological‐Anatomical National Automated Archive
- PTR
primary tumor resection
- RFA
radiofrequency ablation
- WHO PS
World Health Organization performance status
1. INTRODUCTION
During the past two decades, much progress has been made in the treatment of patients with metastatic colorectal cancer (mCRC). More effective systemic therapy became available, including chemotherapy, targeted therapy, immunotherapy and combinations of these drugs. Additionally, advances were made in local treatment of metastases such as surgical metastasectomy, cytoreductive surgery/HIPEC, radiofrequency ablation (RFA) and radiotherapy. Guidelines recommend mono‐ or multimodal treatment strategies depending on the extent and resectability of metastases. 1 , 2 Multidisciplinary meetings (including surgeons) are essential to identify the optimal treatment strategy for each individual patient. The progress in treatment is reflected in clinical trials on first‐line systemic therapy in mCRC patients, which suggest a marked improvement in median overall survival (OS) from approximately 20 months 3 , 4 to over 30 months in recent years. 5 , 6 , 7 Supplementary Table 4 summarizes relevant clinical trials on first‐line systemic therapy. When informing patients, many clinicians quote the mOS reported in these trials.
Yet only a minority (2.5%‐20%) of cancer patients participate in clinical trials. 8 , 9 , 10 The prognosis communicated to patients in clinical practice is therefore often based upon data from a presumably highly selected population. Uncertainty exists in how the survival reported in trials relates to the real‐life mCRC population.
A realistic sense of prognosis is of vital importance for patients in pursuance of informed decision‐making and advance care planning. 11 , 12 To support physicians in depicting a realistic image of patients' prognosis, reliable data on survival of the total (“real‐life”) population of patients with mCRC are indispensable. Moreover, patients prefer presentation of best‐case, worst‐case and typical scenarios to presentation of just the median survival time. 13 These scenarios for survival have been evaluated in trial patients, 14 , 15 but not yet in real‐life patients.
The aim of our study was to determine typical, best‐case and worst‐case survival scenarios of real‐life mCRC patients. Since ideally life expectancy of an individual patient is estimated based on survival of a group of patients with comparable prognostic features receiving similar treatments, 14 we present survival scenarios for different treatment subgroups. Additionally, we describe trends in survival and treatment over time.
2. METHODS
2.1. Patients
In this nationwide population‐based study, we obtained data from the Netherlands Cancer Registry (NCR) on all stage IV (synchronous metastatic) CRC patients ≥18 years old diagnosed between January 2008 and December 2016 in the Netherlands. The NCR differs from other registries in that the overall completeness of case ascertainment of the NCR exceeds 95%. 16 The NCR thus includes both trial and nontrial patients and has nationwide coverage. Clinical data on all newly diagnosed malignancies in the Netherlands are registered in the NCR. These clinical data contain the initial treatment, consisting of the mono‐ or multimodal treatment received from diagnosis of mCRC until progression of disease. Data on metachronous metastatic disease were not available in the NCR. For inclusion in the NCR, CRC needs to be either histologically proven or strongly suspected based on clinical and radiologic grounds. Main sources of notification are the automated pathology archive (PALGA) and the National Registry of Hospital Discharge Diagnoses. Following the notification, trained data managers collect patient, tumor and treatment characteristics directly from medical records.
Patients were classified as stage IV when the first metastasis was detected prior to the start of the initial treatment or during surgical exploration. As possible stage migration due to better staging at initial cancer diagnosis could invalidly contribute to an increased survival, this was investigated by calculating the proportion of CRC patients with stage IV disease per incidence year. Stage at diagnosis is available in the NCR for approximately 94% to 97% of CRC patients. 17
Topography and morphology were coded according to the International Classification of Diseases for Oncology (ICD‐O‐3). Tumor location was categorized as right‐sided colon (C18.0, C18.1, C18.2, C18.3 or C18.4), left‐sided colon (C18.5, 18.6, 18.7), rectum (C19.9, C20.9) or unknown (C18.8 or C18.9). Follow‐up on vital status occurs through annual linkage between the NCR and the National Municipal Personal Records Database, which contains information on vital status of all Dutch inhabitants.
Survival scenarios were analyzed for (a) the total patient population, and separately for the following different treatment subgroups based on the initial treatment received: (b) no antitumor therapy, (c) any form of local and/or systemic antitumor therapy, (d) systemic therapy with or without primary tumor resection (PTR) (but no metastasectomy, hyperthermic intraperitoneal chemotherapy [HIPEC], nonsurgical treatment of liver metastasis such as RFA, or radiotherapy of metastasis), (e) exclusively systemic antitumor therapy, (f) PTR and systemic antitumor therapy (but no metastasectomy, HIPEC, nonsurgical treatment of liver metastasis, or radiotherapy of metastasis), (g) metastasectomy and/or HIPEC, plus systemic therapy, with or without PTR/nonsurgical local treatment of liver metastasis/radiotherapy of metastasis and (h) metastasectomy and/or HIPEC only (ie, without systemic therapy) with or without PTR/nonsurgical local treatment of liver metastasis/radiotherapy of metastasis.
2.2. Statistical analyses
Categorical data were presented as proportions with percentages. Numerical data were presented as mean (±SD) or median (interquartile range). OS was defined as the number of months between date of diagnosis and death or censoring. In case of multiple primary CRC tumors, survival was calculated from date of diagnosis of the first primary tumor. Surviving patients were censored at 31 January 2019. Crude OS stratified by year of diagnosis was calculated using the Kaplan‐Meier method for the total patient population, and for treatment subgroups. Point estimates of OS per calendar year of diagnosis was determined for various percentiles with 95% confidence intervals (CIs) using the R functions survfit of the R package survival 18 and the generic function quantile. The following percentiles (representing scenarios) were calculated (Figure 1): 10th (best‐case), 25th (upper‐typical), 50th (median), 75th (lower‐typical) and 90th (worst‐case). The typical scenario is defined as the 75th to 25th percentile (interquartile range), representative of the middle 50% of patients. These percentiles (scenarios) and terminology were chosen in line with previous studies. 13 , 14 , 15 The point estimates represent the number of months survived by 10%, 25%, 50%, 75% or 90% of patients, that is, p10, p25, p50, p75 and p90. No point estimates were reported if a 95% CI could not be calculated due to limited follow‐up time or limited events after a certain time point. Additionally, the 5‐year OS percentages of the total Stage IV CRC population were calculated.
FIGURE 1.
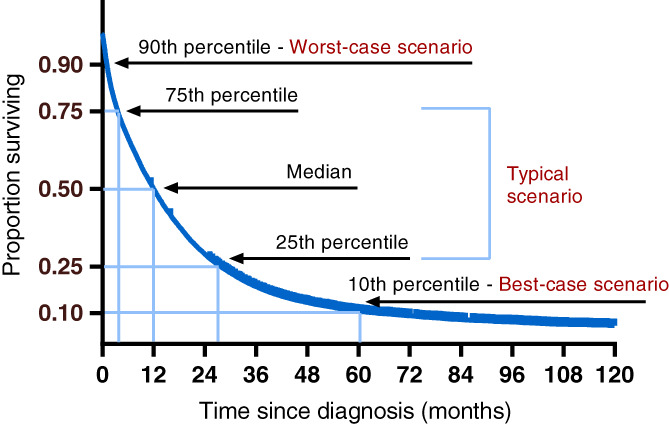
Survival curve percentiles and their corresponding scenarios, adapted from Kiely [14], for illustrative purposes only [Color figure can be viewed at wileyonlinelibrary.com]
We analyzed trends over time in survival and treatment. We estimated survival percentiles for each incidence year. A change in OS ≥3 months was defined as clinically relevant and statistical significance was determined by quantile regression (R package ctqr 19 ). P values <0.05 were considered statistically significant and all tests were two‐sided. To facilitate comparison of our survival results to population‐based data from other countries, we also calculated relative survival estimates (both age standardized and not age‐standardized). For the relative survival analysis (Pohar Perme method 20 , 21 ) we compared the observed deaths with those expected from the general population life table. 22 For the age‐standardization, we used the cluster 1 standard cancer patient population as proposed by Corzziari et al. 23 All analyses were carried out using SPSS version 25 and R version 3.5.1. 24
3. RESULTS
Between January 2008 and December 2016, 27 275 patients were diagnosed with stage IV CRC in the Netherlands (Table 1). Since the start of population screening in 2014, an increase in diagnosis of early‐stage CRC and a slight decrease in the proportion of stage IV disease was observed (Table 2). Approximately 25% of stage IV CRC patients did not receive any antitumor treatment either because they were ineligible or refused antitumor treatment. This percentage remained stable over time. The proportion of patients receiving both systemic therapy and PTR has halved since 2008. In contrast, the proportion of patients who initially underwent metastasectomy and/or HIPEC without systemic treatment has tripled.
TABLE 1.
Baseline table: patient and tumor characteristics
| n | % of total study population | |
|---|---|---|
| Total study population | 27 275 | 100% |
| Male sex | 15 270 | 56% |
| Age in years at diagnosis | ||
| Mean (±SD) | 68.6 (±11.7) | |
| Median (IQR) | 69.0 (61.0‐77.0) | |
| Categorical: | ||
| 0‐49 | 1723 | 6% |
| 50‐59 | 4087 | 15% |
| 60‐69 | 8097 | 30% |
| 70‐79 | 8198 | 30% |
| 80+ | 5170 | 19% |
| WHO performance status before start of treatment a | ||
| 0‐1 | 2643 | 43.4% |
| 2 | 369 | 6.1% |
| 3‐4 | 166 | 2.7% |
| Missing | 2912 | 47.8% |
| Primary tumor site | ||
| Right‐sided colon | 9649 | 35% |
| Left‐sided colon | 9021 | 33% |
| Rectal | 7540 | 28% |
| Location not specified or overlapping parts of colon | 1065 | 4% |
| >1 Primary tumor | 232 | 0.9% |
| Morphology | ||
| No pathologic diagnosis | 1578 | 6% |
| Adenocarcinoma | 22 518 | 83% |
| Mucinous adenocarcinoma | 2299 | 8% |
| Signet ring cell carcinoma | 610 | 2% |
| Other | 270 | 1% |
| Molecular pathology a | ||
| BRAF mutation | 197 | 3.2% |
| BRAF wildtype | 1121 | 18.4% |
| BRAF status unavailable | 4772 | 78.4% |
| RAS mutation | 794 | 13.0% |
| RAS wildtype | 846 | 13.9% |
| RAS status unavailable | 4450 | 73.1% |
| MSI | 89 | 1.5% |
| MSS | 1382 | 22.7% |
| MS status unavailable | 4619 | 75.8% |
| Number of metastatic sites at diagnosis | ||
| 1 organ | 16 800 | 61.6% |
| 2 organs | 7379 | 27.1% |
| 3 organs | 2441 | 8.9% |
| >3 organs | 655 | 2.4% |
| Localization metastases at diagnosis | ||
| Liver | 20 390 | 74.8% |
| Liver‐only | 11 657 | 42.7% |
| Lung | 6470 | 23.7% |
| Lung‐only | 1271 | 4.7% |
| Peritoneal | 6101 | 22.4% |
| Peritoneal‐only | 2422 | 8.9% |
| Bone | 934 | 3.4% |
| Brain | 190 | 0.7% |
Abbreviations: IQR, interquartile range; MS, microsatellite, MSI, microsatellite instable; MSS, microsatellite stable.
Available as of incidence year 2015. Calculated percentages are the proportion of the total population diagnosed in 2015 and 2016, see Table 2 for these numbers.
TABLE 2.
Trends in number of patients diagnosed and initial treatment over time
| Total population | Year of diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | ||
| No. of patients diagnosed with CRC (any stage) 25 | 123 190 | 12 150 | 12 311 | 12 773 | 13 210 | 13 256 | 13 057 | 15 214 | 15 913 | 15 306 |
| No. of patients diagnosed with stage IV CRC (% of all CRC patients) | 27 275 (22%) | 2807 (23%) | 2844 (23%) | 3030 (24%) | 3086 (23%) | 3044 (23%) | 3198 (25%) | 3176 (21%) | 3097 (20%) | 2993 (20%) |
| Percentage of stage IV patients deceased at end of FU a | 24 342 (89%) | 2665 (95%) | 2691 (95%) | 2850 (94%) | 2848 (92%) | 2798 (92%) | 2889 (90%) | 2756 (87%) | 2564 (83%) | 2281 (76%) |
|
Treatment modalities (% of stage IV patients) b | ||||||||||
| No antitumor therapyB | 6081 (22%) | 630 (22%) | 643 (23%) | 705 (23%) | 649 (21%) | 706 (23%) | 720 (23%) | 646 (20%) | 677 (22%) | 705 (24%) |
| Systemic therapy ± PTRD | 12 240 (45%) | 1299 (46%) | 1329 (47%) | 1383 (46%) | 1422 (46%) | 1354 (45%) | 1465 (46%) | 1443 (45%) | 1318 (43%) | 1227 (41%) |
| Systemic therapy onlyE | 7245 (27%) | 665 (24%) | 786 (28%) | 786 (26%) | 809 (26%) | 799 (26%) | 853 (27%) | 901 (28%) | 822 (27%) | 824 (28%) |
| Systemic therapy + PTRF | 3768 (14%) | 523 (19%) | 436 (15%) | 455 (15%) | 471 (15%) | 435 (14%) | 449 (14%) | 396 (13%) | 351 (11%) | 252 (8%) |
| Metastasectomy/HIPEC + systemic therapyG | 2956 (11%) | 213 (8%) | 260 (9%) | 294 (10%) | 371 (12%) | 375 (12%) | 389 (12%) | 371 (12%) | 383 (12%) | 300 (10%) |
| Metastasectomy/HIPEC onlyH | 1564 (6%) | 109 (4%) | 95 (3%) | 124 (4%) | 118 (4%) | 119 (4%) | 153 (5%) | 238 (8%) | 270 (9%) | 338 (11%) |
Note: B, D, E, F, G, and H correspond to the graphs in Figure 2.
Abbreviations: CRC, colorectal cancer; FU, follow‐up; HIPEC, hyperthermic intraperitoneal chemotherapy; PTR, primary tumor resection.
31 January 2019.
The percentages for the treatment modalities do not add up to 100% because the categories are not all mutually exclusive.
Median OS (p50) of the total stage IV CRC population remained unchanged in the period 2008 to 2016 at approximately 12 months (Figure 2 and Table 3). OS improved only for the “upper‐typical” and “best” patients of the total population; p25 by 4.2 months from 24.9 to 29.1 months (P < .001), and p10 by 6.0 months from 56.0 to 62.0 months (P < .001), respectively. OS of the lower‐typical (p75) and worst (p90) patients remained stable at around 3.5 and 1 months, respectively. Five‐year OS rate of the total stage IV CRC population improved from 9·1% to 12·4% between 2008 and 2014 (Table 3). Survival trends over time of patients who received any form of antitumor therapy are similar to survival trends of the total population. No clinically relevant change in any of the percentiles was observed among patients who received systemic therapy, with median OS (p50) close to 15 months and best‐case scenario (p10) approximately 40 months. Median OS (p50) and worst‐case scenario for survival (p90) were highest in patients who underwent both metastasectomy and/or HIPEC, and systemic therapy: that is, around 48 and 15 months, respectively. A large, clinically relevant improvement in survival over time was observed only in patients who initially received metastasectomy and/or HIPEC without systemic treatment. Median OS (p50) in this treatment group improved by 13.3 months from 28.0 to 41.3 months (P = .0351), lower‐typical survival scenario (p75) improved by 11.0 months from 10.4 to 21.4 months (P < .001) and worst‐case survival scenario (p90) by 7.2 months from 3.2 to 10.4 months (P < .001). Survival for the upper‐typical (p25) and best‐case (p10) patients in the metastasectomy and/or HIPEC (± systemic therapy) treatment groups cannot yet be displayed due to insufficient follow‐up time.
FIGURE 2.
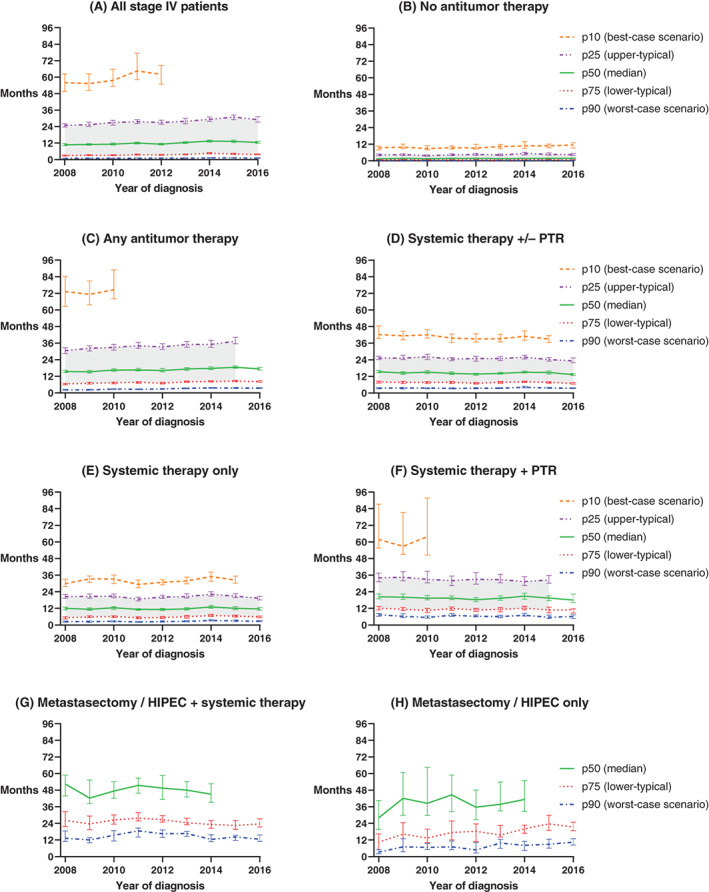
Change in OS of stage IV CRC patients over time for different scenarios. The patterned lines represent five survival percentiles, and three scenarios for survival. For example, the p10 line represents the tenth percentile, or the time at which 10 percent of all patients were still alive (best‐case scenario). The p50 line represents the fiftieth percentile (the median), or the time at which 50 percent of patients were still alive. The grey shading represents the typical scenario, i.e. the 75th to 25th percentile (interquartile range), representative of survival times for the middle 50% of patients. Error bars are 95% CI. (A) All stage IV patients, (B) Patients who did not receive any antitumour therapy (only best supportive care), (C) Patients who received any form of local and/or systemic antitumour therapy, (D) Patients who received systemic antitumour therapy +/‐ primary tumour resection, without metastasectomy / HIPEC / non‐surgical local treatment of liver metastasis / radiotherapy of metastasis, (E) Patients who received systemic antitumour therapy only, (F) Patients who received systemic therapy and primary tumour resection, without metastasectomy / HIPEC / non‐surgical local treatment of liver metastasis / radiotherapy of metastasis, (G) Patients who received both metastasectomy and/or HIPEC plus systemic therapy, with or without primary tumour resection / non‐surgical local treatment of liver metastasis / radiotherapy of metastasis, (H) Patients who received metastasectomy and/or HIPEC only (i.e. without systemic therapy), with or without primary tumour resection / non‐surgical local treatment of liver metastasis / radiotherapy of metastasis. Treatment subgroups are based on the initial treatment received. For some treatment groups and incidence years, the highest percentiles cannot yet be displayed due to insufficient follow‐up time to calculate point estimates or complete the 95% CIs. For the same reason, the typical scenario cannot yet be displayed for groups (G) and (H) [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
(1) Median OS in months (95% CI) per treatment strategy, and (2) 5‐year OS of stage IV CRC patients diagnosed between 2008 and 2016
| Treatment strategy | Year of diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | ||
| Median OS in months | Total Stage IV populationA |
10·8 (10·2‐11·6) |
11·0 (10·4‐11·6) |
11·2 (10·6‐12·1) |
12·0 (11·4‐12·7) |
11·2 (10·6‐11·9) |
12·4 (11·6‐13·1) |
13·5 (12·7‐14·2) |
13·2 (12·4‐14·2) |
12·5 (11·8‐13·2) |
| No antitumor treatmentB |
1·5 (1·3‐1·7) |
1·8 (1·7‐2·0) |
1·6 (1·4‐1·8) |
1·7 (1·5‐2·0) |
1·8 (1·6‐2·0) |
1·6 (1·4‐1·9) |
1·7 (1·5‐1·9) |
1·8 (1·5‐2·1) |
1·8 (1·6‐2·1) |
|
| Any antitumor therapyC |
15·5 (14·7‐16·4) |
15·3 (14·3‐16·4) |
16·5 (15·6‐17·5) |
16·6 (15·9‐17·6) |
16·2 (15·2‐17·6) |
17·3 (16·3‐18·3) |
17·7 (16·9‐18·8) |
18·6 (17·9‐19·5) |
17·3 (16·5‐18·5) |
|
| Systemic therapy ± PTRD |
15·4 (14·7‐16·3) |
14·4 (13·5‐15·4) |
15·1 (14·1‐16·1) |
14·2 (13·4‐15·3) |
13·7 (12·8‐14·3) |
14·2 (13·6‐15·0) |
15·0 (14·4‐15·9) |
14·8 (13·7‐15·9) |
13·3 (12·7‐14·1) |
|
| Systemic therapy onlyE |
12·0 (10·9‐13·0) |
11·4 (10·7‐12·4) |
12·4 (11·3‐13·2) |
11·3 (10·7‐11·9) |
11·2 (10·4‐12·0) |
11·6 (10·8‐12·6) |
13·1 (12·1‐14·1) |
12·0 (11·1‐13·2) |
11·6 10·6‐12·6) |
|
| Systemic therapy + PTRF |
20·4 (18·9‐22·4) |
20·1 (18·5‐22·4) |
19·4 (18·2‐21·4) |
19·4 (18·4‐21·1) |
18·3 (16·8‐19·8) |
19·3 (17·9‐21·0) |
20·8 (18·6‐23·0) |
19·5 (17·6‐21·0) |
18·0 (16·2‐22·4) |
|
| Metastasectomy/HIPEC + systemic therapyG |
52·4 (43·8‐58·8) |
42·3 (38·2‐55·3) |
47·4 (42·1‐54·1) |
51·5 (45·9‐56·7) |
49·5 (41·3‐58·6) |
48·0 (43·1‐54·1) |
45·1 (39·2‐52·7) |
a | a | |
| Metastasectomy/HIPEC onlyH |
28·0 (19·6‐40·6) |
42·1 (29·8‐60·8) |
38·5 (29·7‐64·4) |
44·6 (32·5‐59·0) |
35·7 (26·6‐48·0) |
37·8 (33·0‐53·9) |
41·3 (32·6‐54·9) |
a | a | |
| Percentage 5‐year OS |
Total Stage IV population |
9·1 (8·1–10·2) |
9·1 (8·1‐10·2) |
9·8 (8·8‐10·9) |
10·8 (9·8‐12·0) |
10·2 (9·2‐11·4) |
10·3 (9·3‐11·4) |
12·4 (11·2‐13·7) |
a | a |
Note: A, B, C, D, E, F, G, and H correspond to the graphs in Figure 2.
Abbreviations: HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival; PTR, primary tumor resection.
Results not yet available because of limited follow‐up time.
Survival estimates also differ according to primary tumor location. Survival is superior for patients with left‐sided colon or rectal tumors, compared to patients with right‐sided colon tumors (Supplementary Figure 2). In the patients with a right‐sided colon tumor, a clinically relevant but statistically nonsignificant increase in survival over time was seen only in the best‐case scenario group (from 48.0 to 53.3 months, P = .17). In patients with a left‐sided colon tumor, a clinically relevant improvement over time was seen in median OS (from 11.5 to 14.9 months, P < .001), in the upper‐typical scenario (from 26.1 to 36.5 months, P < .001) and in the best‐case scenario (from 54.0 to 64.9 months, P < .001). In patients with a rectal tumor, a clinically relevant improvement over time was seen in the upper‐typical scenario (from 29.5 to 34.8 months, P = .0193). OS of the best (p10) patients with a rectal tumor seemed to decrease over time, but this change was statistically not significant (from 71.0 to 63.0 months, P = .3079). Patients in which the location of the primary tumor was unknown, represent a group with a poor prognosis. Only the best (p10) patients seem to show an improvement in OS over time, but this was statistically not significant (from 18.6 to 33.9 months, P = .0531).
For information on change in OS over time stratified by age at diagnosis, see Supplementary Table 3and Supplementary Figure 3.
The (age‐standardized) relative survival estimates are summarized in Supplementary Figure 1, Supplementary Table 1 and Supplementary Table 2.
4. DISCUSSION
The main goal of our study was to provide clinicians with reliable data on typical, best‐ and worst‐case survival of the real‐life mCRC patient population. This is the first population‐based analysis providing multiple scenarios for survival; previous population‐based research has focused on point estimates such as median survival and 5‐year survival. 17 , 26 , 27 , 28 , 29 , 30 , 31 We found a clinically relevant increase in OS over the last decade only in the upper‐typical (p25) and best patients (p10) of the total stage IV CRC population. Median OS remained unchanged, which is remarkable since a marked increase in median OS has been observed in mCRC trial populations during this period. 3 , 4 , 5 , 6 , 7
Our study has important clinical implications. Patients often ask questions about life expectancy, which clinicians struggle to answer. 32 As a result, many patients have a poor understanding of their prognosis. 33 Our results can help clinicians to better estimate and explain life expectancy to patients with mCRC. We agree with Kiely et al. 14 that questions such as “How long have I got?” are best answered by providing best‐case, typical and worst‐case scenarios. This way, we may enable patients to hope for the best and prepare for the worst. 13 , 32 , 34 Not only are multiple scenarios preferable over merely quoting the median—quantifying the best‐case scenario representing the best 10% of patients is preferable to describing an individual long‐term survivor. 35 One study previously assessed multiple scenarios for survival of mCRC trial patients. 15 However, patients in clinical trials are not representative of those seen in routine clinical practice. Therefore, to provide truly realistic information about prognosis to patients, we suggest using multiple scenarios derived from real‐life data rather than scenarios derived from trial data. As reliable individualized prediction models are not yet available, life expectancy of individual patients is ideally estimated based on survival of a group of patients receiving similar treatments. Our survival data presented per treatment group are suitable for this purpose.
In addition to optimally informing patients, analyzing multiple survival scenarios also helps to identify for which proportion (percentile) of patients OS improved over time. Remarkably, we observed no clinically relevant changes in median OS since 2008, neither in the total population nor in any of the treatment groups (with the exception of patients who initially received metastasectomy and/or HIPEC without systemic treatment). The observed median OS of approximately 12 months for the total stage IV CRC population concurs with previous European and US population‐based research. 26 , 27 , 28 , 29 , 30 The relative survival estimates are in line with population‐based studies from France and the United States. 31 , 36 , 37 We noted a clinically relevant increase in OS over the last decade only in the upper‐typical (p25) and best (p10) patients of the total stage IV CRC population. This indicates that only a minority of patients benefits from the availability of more effective treatment strategies, and emphasizes the importance of real‐life data in determining the impact of treatments on the outcome of the total patient population. 38 In other tumor types such as lung cancer, novel treatments have also resulted in long‐term survival for small subgroups while survival remained unchanged for a large majority. 39
Williams et al. previously assessed multiple scenarios for survival of mCRC patients who were included in 46 different randomized clinical trials of first‐line chemotherapy published before 2012. 15 The results of their study are similar to our population‐based systemic treatment group, which includes patients diagnosed between 2008 and 2016. Since 2012, however, clinical trial results of mCRC patients receiving first‐line systemic therapy suggest a strong improvement in median OS to over 30 months. 3 , 4 , 5 , 6 , 7 This substantial improvement in median OS does not correspond to our findings in real‐life patients who received systemic therapy as their initial treatment. The divergent outcomes may stem from various causes. First, trial patients represent a selection of patients with favorable prognosis regardless of treatment (eg, younger age, better WHO PS). Second, there is a delay between the demonstration of efficacy of new drugs in trials, implementation of these drugs in national guidelines, and subsequent use in daily clinical practice. Consequently, during this delay, real‐life patients may not yet receive novel effective drugs, and their impact on OS is delayed. Third, adherence to guidelines may be suboptimal because clinicians are unaware of, or disagree with guidelines or face financial restrictions. 40 , 41 , 42 , 43 Lastly, clinicians sometimes prescribe treatments to patients who would have been ineligible for participation in the pivotal trial that demonstrated their efficacy. In general, these patients have a worse outcome compared to trial patients. 9 , 44
Given the observational nature of our study, our results are not meant to guide treatment decisions but rather to estimate patients' life expectancy given prevailing clinical practice and treatment choices. 45
Median OS (p50) and worst‐case scenario for survival (p90) were highest in patients who underwent metastasectomy and/or HIPEC, in combination with systemic therapy: i.e. around 48 and 15 months, respectively. The fluctuating median OS of patients who underwent metastasectomy and/or HIPEC during our study period may reflect the lack of consensus on resectability criteria for metastases. 46 , 47 , 48
In the Netherlands, perioperative chemotherapy is not standard of care for resectable metastases since it does not increase OS compared to surgery alone. 49 In our study, the only treatment group that showed a remarkable improvement in median OS over the last decade are patients who initially received metastasectomy and/or HIPEC without systemic treatment. This treatment group consists primarily of patients with limited metastatic disease. The improvement over time is likely due to improved surgical techniques and perioperative care. 50 , 51 The difference in OS between the different treatment groups in our study suggests that the prognosis of stage IV patients is more diverse than clinicians generally realize.
The superior survival for patients with left‐sided primary tumors compared to patients with right‐sided primary tumors is consistent with previous population based studies and clinical trials. 52 , 53 , 54
The strength of our study is the use of a registry containing high‐quality, recent, reliable and complete nationwide population‐based data. The NCR differs from other registries in that the overall completeness of case ascertainment exceeds 95%, which practically eliminates selection bias. The large sample size enables reliable reporting of multiple scenarios for survival. Our data are generalizable to other Western countries given that the healthcare system in the Netherlands is accessible and of high quality. Dutch diagnostic and treatment guidelines are written with consensus of all medical oncologists and are based on available American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) guidelines. All inhabitants of the Netherlands have obligatory health insurance covering oncological care. Patients do not experience a financial threshold as all treatments recommended in the national guideline are fully reimbursed.
We provide both observed and (age‐standardized) relative survival estimates. OS is the most meaningful outcome measure given the goal of our research: to optimally inform patients about their prognosis and to compare survival of real‐life patients to survival of trial patients. The supplementary relative survival estimates enable comparison of population‐based data between countries.
There are some limitations to our study. First, only data from patients with synchronous metastatic disease were available for our study. However, prognosis of patients with metachronous mCRC is likely similar to prognosis of patients with synchronous mCRC who underwent PTR. 55 Second, no information on salvage treatments was available in the NCR for our study period. The treatment groups we described are thus based on the initial treatment plan. Third, insufficient information on comorbidities or socioeconomic status was available in the NCR, which made it impossible to explore differences in survival according to these determinants. Also, tumor mutation status is missing for a high proportion of our patient population because mutational testing at start of initial treatment was not yet fully incorporated into standard care during our study period.
In conclusion, clinicians should consider presenting multiple scenarios for survival based on real‐life data instead of point estimates from clinical trials, when informing patients about life expectancy. This approach and recommendation is applicable to all cancer types. Real‐life mCRC patients from an unselected population‐based registry receiving systemic therapy have a significantly shorter median OS compared to trial patients. The marked increase in median OS that has been observed in mCRC trials is not reflected in our real‐life population. This finding illustrates the different outcomes between trial patients and the total patient population. As patients prefer information on multiple scenarios for survival, these percentiles from OS curves of a real‐life stage IV CRC patient population presented per treatment group can help doctors to estimate and explain life expectancy to their patients.
DISCLOSURE OF INTERESTS
C. J. A. P. reports his advisory role for Nordic Pharma. A. M. M. reports advisory fees from Novartis paid to her institution. M. K. reports personal travel/accommodation fees from Congress Care—Dutch oncology society (NVMO). M. K. reports research grants/funding paid to her institution by Amgen, Bayer, BMS, Merck‐Serono, Nordic Pharma, Roche, Servier, Sirtex and Sanofi‐Aventis. M. K. reports honoraria paid to her institution by BMS, Nordic Pharma and Servier. M. K. reports the following nonfinancial interests: an advisory role for ZON‐MW, membership of the scientific board of the Dutch Cancer Society (KWF), chairmanship of the Dutch Colorectal Cancer Group (DCCG), principal investigator (PI) of the Prospective Dutch CRC cohort (PLCRC), involvement in several clinical trials as PI or co‐investigator in colorectal cancer. G. V. reports research grants/funding paid to her institution by Servier, BMS, Bayer, Merck, PGDx and Sirtex. G. R. V. reports travel/accommodation fees from Servier. All remaining authors have declared no conflicts of interest.
ETHICS STATEMENT
According to the Central Committee on Research involving Human Subjects, this type of registry‐based study does not require approval from an ethics committee in the Netherlands. The study was approved by the Privacy Review Board and the scientific council of the Netherlands Comprehensive Cancer Organisation (IKNL) which collects and guards the data for the NCR. All data were pseudonymized prior to the transfer from IKNL to the researchers. The NCR uses an opt‐out approach to consent.
Supporting information
Table S1 Median relative survival per treatment strategy and 5‐year relative survival
Table S2 Median age‐standardized relative survival per treatment strategy and 5‐year age standardized relative survival
Table S3 Median OS and 5‐year OS stratified by age at diagnosis
Table S4 Summary of median OS as reported in mCRC patients participating in clinical trials on first‐line systemic therapy
Figure S1 KM observed OS/relative survival/age‐standardized relative survival
Figure S2 Change in OS over time stratified by primary tumor location
Figure S3 Change in OS over time stratified by age at diagnosis
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice. We gratefully acknowledge Ronald Damhuis MD PhD for his valuable suggestions and discussions. We are indebted to Matteo Cellamare PhD for his substantial help in calculating the age‐standardized relative survival in R.
Hamers PAH, Elferink MAG, Stellato RK, et al. Informing metastatic colorectal cancer patients by quantifying multiple scenarios for survival time based on real‐life data. Int. J. Cancer. 2021;148:296–306. 10.1002/ijc.33200
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the NCR. Restrictions apply to the availability of these data, which were used under license for our study.
REFERENCES
- 1. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386‐1422. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, The ESMO Guidelines Working Group . Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2014;25:iii1‐iii9. 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 3. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335‐2342. [DOI] [PubMed] [Google Scholar]
- 4. Van Cutsem E, Köhne C‐H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408‐1417. 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 5. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first‐line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild‐type advanced or metastatic colorectal cancer a randomized clinical trial. JAMA. 2017;317:2392‐2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306‐1315. 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 7. Heinemann V, Von WLF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2014;15:1065‐1075. [DOI] [PubMed] [Google Scholar]
- 8. Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9:e40‐e47. 10.1200/JOP.2012.000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mol L, Koopman M, Van Gils CWM, Ottevanger PB, Punt CJA. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in the Netherlands. Acta Oncol (Madr). 2013;52:950‐955. [DOI] [PubMed] [Google Scholar]
- 10. Morris PG, Kelly R, Horgan A, et al. Patterns of participation of patients in cancer clinical trials in Ireland. Ir J Med Sci. 2007;176:153‐156. [DOI] [PubMed] [Google Scholar]
- 11. Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709‐1714. [DOI] [PubMed] [Google Scholar]
- 12. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33:3809‐3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiely BE, Mccaughan G, Christodoulou S, et al. Using scenarios to explain life expectancy in advanced cancer: attitudes of people with a cancer experience. Support Care Cancer. 2013;21:369‐376. [DOI] [PubMed] [Google Scholar]
- 14. Kiely BE, Soon YY, Tattersall MHN, Stockler MR. How long have I got? Estimating typical, best‐case, and worst‐case scenarios for patients starting first‐line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29:456‐463. [DOI] [PubMed] [Google Scholar]
- 15. Williams M, Singer RA, Lerner A. A simple technique to estimate best‐ and worst‐case survival in patients with metastatic colorectal cancer treated with chemotherapy. Ann Oncol. 2014;25:2014‐2019. [DOI] [PubMed] [Google Scholar]
- 16. Schouten LJ, Höppener P, Van Den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, the Netherlands. Int J Epidemiol. 1993;22:369‐376. [DOI] [PubMed] [Google Scholar]
- 17. Brouwer NPM, Bos ACRK, Lemmens VEPP, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758‐2766. 10.1002/ijc.31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Therneau T. A Package for Survival Analysis in S. R package version 2.37‐7; 2014; http://cran.r-project.org/package=survival. Accessed 9 October 2019.
- 19. Frumento P, Bottai M. An estimating equation for censored and truncated quantile regression. Comput Stat Data Anal. 2017;113:53‐63. 10.1016/j.csda.2016.08.015. [DOI] [Google Scholar]
- 20. Perme MP, Pavlic K. Nonparametric Relative Survival Analysis with the R Package relsurv. Journal of Statistical Software. 2018;87:8 10.18637/jss.v087.i08. [DOI] [Google Scholar]
- 21. Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics. 2012;68:113‐120. [DOI] [PubMed] [Google Scholar]
- 22. EUROSTAT and Statistics Netherlands . Annual Death Counts by Sex and Single Year of Age. Data Obtained Through the Human Mortality Database. www.mortality.org. Accessed 3 April 2020.
- 23. Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307‐2316. [DOI] [PubMed] [Google Scholar]
- 24.R: A Language and Environment for Statistical Computing; 2018. https://www.r-project.org/. Accessed 9 October 2019.
- 25.Netherlands Cancer Registry (NCR), IKNL. https://www.cijfersoverkanker.nl. Accessed 10 July 2019.
- 26. Sorbye H, Cvancarova M, Qvortrup C, Pfeiffer P, Glimelius B. Age‐dependent improvement in median and long‐term survival in unselected population‐based nordic registries of patients with synchronous metastatic colorectal cancer. Ann Oncol. 2013;24:2354‐2360. [DOI] [PubMed] [Google Scholar]
- 27. van der Geest LGM, Lam‐Boer J, Koopman M, Verhoef C, Elferink MAG, de Wilt JHW. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457‐465. [DOI] [PubMed] [Google Scholar]
- 28. Castleberry AW, Güller U, Tarantino I, et al. Discrete improvement in racial disparity in survival among patients with stage IV colorectal cancer: a 21‐year population‐based analysis. J Gastroinest Surg. 2014;18:1194‐1204. [DOI] [PubMed] [Google Scholar]
- 29. Golan T, Urban D, Berger R, Lawrence YR. Changing prognosis of metastatic colorectal adenocarcinoma: differential improvement by age and tumor location. Cancer. 2013;119:3084‐3091. [DOI] [PubMed] [Google Scholar]
- 30. Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677‐3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitry E, Rollot F, Jooste V, et al. Improvement in survival of metastatic colorectal cancer: are the benefits of clinical trials reproduced in population‐based studies? Eur J Cancer. 2013;49:2919‐2925. 10.1016/j.ejca.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 32. Henselmans I, Smets EMA, Han PKJ, De HHCJC, Van LHWM. How long do I have? Observational study on communication about life expectancy with advanced cancer patients. Patient Educ Couns. 2017;100:1820‐1827. 10.1016/j.pec.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 33. Chen CH, Kuo SC, Tang ST. Current status of accurate prognostic awareness in advanced/terminally ill cancer patients: systematic review and meta‐regression analysis. Palliat Med. 2017;31:406‐418. [DOI] [PubMed] [Google Scholar]
- 34. Thientosapol ES, Tran TT, Adams DH, Chantrill L, Stockler MR, Kiely BE. Survival times of women with metastatic breast cancer starting first‐line chemotherapy in routine clinical practice versus contemporary randomised trials. Intern Med J. 2013;43:883‐888. [DOI] [PubMed] [Google Scholar]
- 35. Kiely BE, Tattersall MHN, Stockler MR. Certain death in uncertain time: informing hope by quantifying a best case scenario. J Clin Oncol. 2010;28:2802‐2804. [DOI] [PubMed] [Google Scholar]
- 36. Ghiringhelli F, Hennequin A, Drouillard A, Cô L, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous and metachronous colon cancer metastases: a French population‐based study. Dig Liver Dis. 2014;46:854‐858. 10.1016/j.dld.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 37. Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104‐117. [DOI] [PubMed] [Google Scholar]
- 38. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence—what is it and what can it tell us? N Engl J Med. 2016;375:2293‐2297. [DOI] [PubMed] [Google Scholar]
- 39. Davis JS, Prophet E, Peng H, et al. Potential influence on clinical trials of long‐term survivors of stage IV non‐small cell lung cancer. JNCI Cancer Spectr. 2019;3:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keikes L, van Oijen MGH, Lemmens VEPP, Koopman M, Punt CJA. Evaluation of guideline adherence in colorectal cancer treatment in the Netherlands: a survey among medical oncologists by the Dutch colorectal cancer group. Clin Colorectal Cancer. 2018;17:58‐64. 10.1016/j.clcc.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 41. Keikes L, Koopman M, Stuiver MM, Lemmens VEPP, van Oijen MGH, Punt CJA. Practice variation on hospital level in the systemic treatment of metastatic colorectal cancer in the Netherlands: a population‐based study. Acta Oncol (Madr). 2020;0:1‐9. 10.1080/0284186X.2020.1722320. [DOI] [PubMed] [Google Scholar]
- 42. Abrams TA, Meyer G, Schrag D, Meyerhardt JA, Moloney J, Fuchs CS. Chemotherapy usage patterns in a US‐wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2014;106:1‐10. [DOI] [PubMed] [Google Scholar]
- 43. Zhao Z, Pelletier E, Barber B, et al. Patterns of treatment with chemotherapy and monoclonal antibodies for metastatic colorectal cancer in Western Europe. Curr Med Res Opin. 2012;28:221‐229. [DOI] [PubMed] [Google Scholar]
- 44. Kwakman JJM, Vink G, Vestjens JH, et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: real‐life data from the Netherlands. Int J Clin Oncol. 2018;23:482‐489. 10.1007/s10147-017-1220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harrell F, Lazzeroni L. EHRs and RCTs: Outcome Prediction vs. Optimal Treatment Selection; 2018. https://www.fharrell.com/post/ehrs-rcts/. Accessed August 23 2019.
- 46. Ruers T, Van Coevoerden F, Punt CJA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. JNCI J Natl Cancer Inst. 2017;109:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chun YS, Vauthey JN. Local therapy for colorectal liver metastases: establishing today's level of evidence and defining tomorrow's roadmap. JNCI J Natl Cancer Inst. 2017;109:17‐19. [DOI] [PubMed] [Google Scholar]
- 48. Macbeth F, Farewell V, Treasure T. RE: local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. JNCI J Natl Cancer Inst. 2017;109:1‐2. [DOI] [PubMed] [Google Scholar]
- 49. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long‐term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208‐1215. [DOI] [PubMed] [Google Scholar]
- 50. Sultana A, Meng R, Piantadosi C, et al. Liver resection for colorectal cancer metastases: a comparison of outcomes over time in South Australia. Hpb. 2018;20:340‐346. 10.1016/j.hpb.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 51. Cloyd JM, Mizuno T, Kawaguchi Y, et al. Comprehensive complication index validates improved outcomes over time despite increased complexity in 3707 consecutive hepatectomies. Ann Surg. 2020;271(4):724‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brouwer NPM, van der Kruijssen DEW, Hugen N, et al. The impact of primary tumor location in synchronous metastatic colorectal cancer: differences in metastatic sites and survival. Ann Surg Oncol. 2020;27(5):1580‐1588. 10.1245/s10434-019-08100-5. Epub 2019 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta‐analysis of first‐line clinical trials. Eur J Cancer. 2017;70:87‐98. [DOI] [PubMed] [Google Scholar]
- 54. Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Rooijen KL, Shi Q, Goey KKH, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: individual patient data analysis of first‐line randomised trials from the ARCAD database. Eur J Cancer. 2018;91:99‐106. 10.1016/j.ejca.2017.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Median relative survival per treatment strategy and 5‐year relative survival
Table S2 Median age‐standardized relative survival per treatment strategy and 5‐year age standardized relative survival
Table S3 Median OS and 5‐year OS stratified by age at diagnosis
Table S4 Summary of median OS as reported in mCRC patients participating in clinical trials on first‐line systemic therapy
Figure S1 KM observed OS/relative survival/age‐standardized relative survival
Figure S2 Change in OS over time stratified by primary tumor location
Figure S3 Change in OS over time stratified by age at diagnosis
Data Availability Statement
The data that support the findings of our study are available from the NCR. Restrictions apply to the availability of these data, which were used under license for our study.


