Abstract
Most breast cancer patients in sub‐Saharan Africa are diagnosed at advanced stages after prolonged symptomatic periods. In the multicountry African Breast Cancer‐Disparities in Outcomes cohort, we dissected the diagnostic journey to inform downstaging interventions. At hospital presentation for breast cancer, women recalled their diagnostic journey, including dates of first noticing symptoms and health‐care provider (HCP) visits. Negative binomial regression models were used to identify correlates of the length of the diagnostic journey. Among 1429 women, the median (inter‐quartile range) length (months) of the diagnostic journey ranged from 11.3 (5.7‐21.2) in Ugandan, 8.2 (3.4‐16.4) in Zambian, 6.5 (2.4‐15.7) in Namibian‐black to 5.6 (2.3‐13.1) in Nigerian and 2.4 (0.6‐5.5) in Namibian‐non‐black women. Time from first HCP contact to diagnosis represented, on average, 58% to 79% of the diagnostic journey in each setting except Nigeria where most women presented directly to the diagnostic hospital with advanced disease. The median number of HCPs visited was 1 to 4 per woman, but time intervals between visits were long. Women who attributed their initial symptoms to cancer had a 4.1 months (absolute) reduced diagnostic journey than those who did not, while less‐educated (none/primary) women had a 3.6 months longer journey than more educated women. In most settings the long journey to breast cancer diagnosis was not primarily due to late first presentation but to prolonged delays after first presentation to diagnosis. Promotion of breast cancer awareness and implementation of accelerated referral pathways for women with suspicious symptoms are vital to downstaging the disease in the region.
Keywords: Africa, breast cancer, cancer diagnosis, early diagnosis
Short abstract
What's new?
In sub‐Saharan Africa, most women with breast cancer are diagnosed long after symptoms first arise. Here, the authors studied the diagnostic journey for breast cancer among the African Breast Cancer‐Disparities in Outcome cohort. This is the largest study to quantify the length of the diagnostic journey across various settings in sub‐Saharan Africa. Time to final diagnosis decreased substantially when a woman recognized her symptoms as cancer. Most delays, they found, were due to extended time between first examination and final diagnosis. Promotion of breast cancer awareness among both women and healthcare providers could help reduce these delays.
Abbreviations
- ABC‐DO
African Breast Cancer ‐ Disparities in Outcomes (study)
- AD
absolute difference
- BC
breast cancer
- CI
confidence interval
- HCP
health‐care provider
- HIC
high‐income country
- HIV
human immunodeficiency virus
- IQR
inter‐quartile range
- IRR
incidence rate ratio
- LMIC
low‐ and middle‐income county
- SEP
socioeconomic position
- SSA
sub‐Saharan Africa
1. INTRODUCTION
In high‐income countries (HICs), most breast cancer (BC) patients are diagnosed at an early stage, 1 when the disease is potentially curable. 2 In contrast, in sub‐Saharan Africa (SSA), most BC women are diagnosed at advanced stages. 3 Delays in seeking care, and in getting a definitive diagnosis, are the major drivers of advanced stage at diagnosis, with studies in HICs having shown that a time interval greater than 3 months between symptom discovery and diagnosis is associated with advanced‐stage disease and poor outcomes. 4 The Breast Health Global Initiative has emphasized the need for downstaging interventions which promote early diagnosis and timely access to appropriate treatment in low‐ and middle‐income countries (LMICs). 5 However, to implement those in SSA, it is vital to understand the array of personal, sociocultural, and economic barriers on a woman's journey from symptom onset to cancer diagnosis. During this diagnostic period, “delays” may occur either before or after the first contact with a health‐care provider (HCP), hereafter referred to as the pre‐contact and post‐contact intervals. Few SSA studies have examined the diagnostic period, and they involved small numbers of patients, no standardized collection of data on key events along the diagnostic journey and limited data on potential correlates. 6
The African Breast Cancer‐Disparities in Outcomes (ABC‐DO) study is a multicountry prospective cohort of BC patients in SSA, 7 which obtained recalled information on the navigational pathway to BC diagnosis. In a previous ABC‐DO analysis, poor BC awareness, low educational level and unskilled employment were identified as drivers of late‐stage diagnosis with their mediating pathway being mainly through prolonged time to diagnosis. 8 , 9 The main aims of the present analysis are to characterize and dissect the navigational path to BC diagnosis and to identify the main drivers of its length to inform cancer control policies in the region.
2. MATERIALS AND METHODS
2.1. Study participants and data collection
A detailed protocol of the ABC‐DO study has been published. 7 Briefly, women aged ≥18 years with histologically confirmed or suspected BC were recruited between September 2014 and September 2017, through hospitals located in five SSA countries: Namibia (Windhoek Central Hospital, Windhoek), Uganda (Mulago Hospital and the Uganda Cancer Institute, Kampala), Nigeria (Abia State University Teaching Hospital and the Maranatha private clinic, Aba, and the Federal Medical Centre, Owerri), Zambia (Cancer Diseases Hospital and University Teaching Hospital, Lusaka, and Kabwe General Hospital, Kabwe) and South Africa (Chris Hani Baragwanath Academic Hospital, Soweto). The overall response rate was 99%. The present analysis excludes data from South Africa (as this site used a different baseline questionnaire) and from Kabwe General Hospital (as recruitment was not clinic/hospital‐based). The study populations will be referred to hereafter as Nigerian, Ugandan, Zambian, Namibian‐black and Namibian‐non‐black women.
ABC‐DO study implementation, management and data collection were enabled via a specifically tailored m‐health mobile phone application. Participants completed a face‐to‐face baseline interview at, or near, the time of the first visit to the participating hospital for possible BC diagnosis. This interview captured information on sociodemographic variables as well as recalled information on the diagnostic journey (eg, nature and date of first symptom, dates of all HCP visits) as detailed in Tables 1 and 2. Information on TNM BC stage at diagnosis was extracted from clinical records.
TABLE 1.
Characteristics of the ABC‐DO study participants at the time of cohort recruitment (not including South Africa)
| N = 1429 | Percent a | |
|---|---|---|
| ABC‐DO population group | ||
| Namibia‐black women | 371 | 25.9 |
| Namibia‐non‐black women | 96 | 6.7 |
| Nigeria | 397 | 27.8 |
| Uganda | 400 | 28.0 |
| Zambia | 165 | 11.6 |
| Sociodemographic | ||
| Age at diagnosis: mean (SD) | 50.1 | (13.9) |
| Low SEP (vs medium/high) b | 810 | 56.7 |
| Not married (vs married) c | 710 | 49.7 |
| Having any children living at home (vs none) | 1096 | 76.7 |
| Primary/no education (vs secondary/higher) | 628 | 44.0 |
| Working in unskilled employment (vs skilled) | 1007 | 70.5 |
| Health‐related | ||
| Recent birth (<3 years prior to BC diagnosis) | 176 | 12.3 |
| Having a personal or family history of BC (vs no) | 174 | 12.2 |
| Positive HIV status (vs negative) | 136 | 9.5 |
| Having ever had other chronic comorbidities (vs never) d | 740 | 51.8 |
| Knowledge and beliefs | ||
| Heard previously about BC (vs no/don't know) | 1176 | 82.3 |
| Know someone with BC (vs no/don't know) | 663 | 46.4 |
| Thinks BC is common (vs no/don't know) | 577 | 40.3 |
| Thinks BC is curable (vs no/don't know) | 754 | 52.8 |
| Attributed first symptom(s) to cancer (vs no/don't know)) | 144 | 10.1 |
| Belief in traditional medicine/healing (vs no/don't know) | 346 | 24.2 |
| Belief in spiritual/faith healing (vs no/don't know) | 1010 | 70.7 |
| Being Muslim (vs no) e | 54 | 13.5 |
| Breast symptom and final diagnosis | ||
| Self‐recognition of symptoms (vs screen/CBE detection) f | 1399 | 97.9 |
| First change noticed: breast lump (vs no) | 1230 | 86.1 |
| Final diagnosis: Benign condition | 33 | 2.3 |
| Final diagnosis: BC | 1396 | 97.7 |
| Presenting with advanced BC stage (TNM III/IV; vs TNM I/II) g | 831 | 63.2 |
Abbreviations: BC, breast cancer; CBE, clinical breast examination; HCP, health‐care provider; SEP, socioeconomic position.
Column percentages unless stated otherwise.
Calculated as setting‐specific tertiles (low, medium and high) of the distribution of a SEP score (range: 0‐9) based on the following self‐reported possessions and facilities: home ownership; indoor water; flush toilet; electricity; vehicle; refrigerator; landline; gas or electric stove; and a bed.
Marital status at enrolment defined as married or not married (ie, single, divorced or widowed).
Having ever suffered from one of the following non‐HIV chronic conditions: hypertension, heart disease, diabetes, chronic anemia, chronic obstructive pulmonary disease (COPD, eg, chronic bronchitis, emphysema), asthma, hepatitis B or C, tuberculosis, other chronic infection, other cancer, other chronic disease.
Percentage restricted to the Ugandan setting, the only with a sizeable Muslim population.
For 30 women (including 15 Namibian‐non‐black and 8 Namibian‐black women) the breast abnormality was first detected through mammographic/ultrasound screening or a routine CBE.
Percentage out of all women with a final BC diagnosis and with known stage (n = 1314; information on stage was missing for 81 women: 32 from Uganda, 23 from Zambia and 26 from Nigeria).
TABLE 2.
Navigation‐related features of the journey to breast cancer diagnosis in the ABC‐DO cohort, by study population group
| Variable | Study population group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Namibia, black (n = 371) | Namibia, non‐black (n = 96) | Nigeria (n = 397) | Uganda (n = 400) | Zambia (n = 165) | All (n = 1429) | |||||||
| n a | % a | n a | % a | n a | % a | n a | % a | n a | % a | n a | % a | |
| Urban (vs rural) residence b | 207 | 55.8 | 85 | 88.5 | 262 | 66.0 | 108 | 27 | 111 | 67.3 | 773 | 54.1 |
| First person told | ||||||||||||
| Relative/friend | 297 | 80.1 | 67 | 69.8 | 313 | 78.8 | 343 | 85.8 | 157 | 95.2 | 1177 | 82.4 |
| Formal HCP c | 60 | 16.2 | 28 | 29.2 | 76 | 19.1 | 34 | 8.5 | 5 | 3.03 | 203 | 14.2 |
| Informal HCP d | 2 | 0.5 | 0 | 0 | 6 | 1.5 | 8 | 2.0 | 1 | 0.6 | 17 | 1.2 |
| Other | 12 | 3.2 | 1 | 1.0 | 2 | 0.5 | 15 | 3.8 | 2 | 1.2 | 32 | 2.2 |
| First HCP visited e | ||||||||||||
| Type of first HCP | ||||||||||||
| Primary f | 208 | 56.1 | 82 | 85.4 | 20 | 5.0 | 97 | 24.3 | 58 | 35.2 | 465 | 32.5 |
| Secondary/tertiary g | 160 | 43.1 | 13 | 13.5 | 362 | 91.2 | 249 | 62.3 | 97 | 58.8 | 881 | 61.7 |
| Informal h | 3 | 0.8 | 1 | 1.0 | 15 | 3.8 | 54 | 13.5 | 10 | 6.1 | 83 | 5.8 |
| Barriers to first HCP visit i | ||||||||||||
| No transport available | 81 | 21.8 | 0 | 0 | 6 | 1.5 | 48 | 12 | 4 | 2.4 | 139 | 9.7 |
| Transport/treatment costs | 68 | 18.3 | 2 | 2.1 | 35 | 8.8 | 77 | 19.3 | 11 | 6.7 | 193 | 13.5 |
| Pain and/or fear | 15 | 4.04 | 2 | 2.1 | 45 | 11.3 | 37 | 9.3 | 29 | 17.6 | 128 | 9.0 |
| Other j | 39 | 10.5 | 9 | 9.4 | 46 | 11.6 | 115 | 28.8 | 17 | 10.3 | 226 | 17.6 |
| None | 250 | 67.4 | 87 | 90.1 | 274 | 69.0 | 237 | 59.3 | 117 | 70.9 | 965 | 67.5 |
| Outcome of first HCP visited | ||||||||||||
| Appropriate diagnosis/referral k | 257 | 69.4 k | 80 | 83.3 k | 95 | 55.2 k | 144 | 42.9 k | 103 | 64.0 k | 679 | 59.9 k |
| Inappropriate diagnosis/referral k , l | 112 | 30.4 k | 16 | 16.7 k | 77 | 44.8 k | 192 | 57.1 k | 58 | 36.0 k | 455 | 40.1 k |
| Not applicable m | 2 | — | 0 | — | 225 | — | 64 | — | 4 | — | 295 | — |
| No. HCPs visited e | ||||||||||||
| 1 | 5 | 1.4 | 2 | 2.1 | 238 | 60.0 | 69 | 17.3 | 6 | 3.6 | 320 | 22.4 |
| 2 | 55 | 14.8 | 11 | 11.5 | 117 | 29.5 | 5 | 1.3 | 78 | 47.3 | 266 | 18.6 |
| 3 | 122 | 32.9 | 38 | 39.6 | 40 | 10.1 | 89 | 22.3 | 65 | 39.4 | 354 | 24.8 |
| 4 | 104 | 28.0 | 24 | 25.0 | 2 | 0.5 | 83 | 20.8 | 15 | 9.09 | 228 | 16.0 |
| ≥5 | 85 | 22.9 | 21 | 21.9 | 0 | 0 | 154 | 38.5 | 1 | 0.6 | 261 | 18.3 |
| Median (IQR) | 4 | (3‐4) | 3 | (3‐4) | 1 | (1‐2) | 4 | (3‐5) | 2 | (2‐3) | 3 | (2‐4) |
| Median (IQR) time (months) between HCP contacts n | ||||||||||||
| 1‐2 | 1.4 | (0.5‐4.0) | 0.6 | (0.2‐1.2) | 5.0 | (1.7‐12.1) | 2.0 | (0.6‐5.9) | 3.0 | (1.2‐7.9) | 1.1 | (0.1‐4.3) |
| 2‐3 | 1.1 | (0.5‐2.7) | 0.7 | (0.2‐1.9) | 3.2 | (1.2‐7.9) | 1.2 | (0.3‐3.5) | 2.1 | (1.1‐5.0) | 1.2 | (0.4‐3.3) |
| 3‐4 | 0.8 | (0.3‐1.6) | 0.9 | (0.5‐1.4) | 7.1 | (6.0‐8.3) | 0.8 | (0.2‐2.0) | 0.8 | (0.2‐2.0) | 1.6 | (0.2‐3.0) |
| Length of the post‐contact interval as % of the length of the diagnostic journey | ||||||||||||
| All women, median (IQR) | 60.6% | (12.1%‐96.2%) | 77.8% | (15.7%‐96.4%) | 10.6% | (0.9%‐66.7%) | 63.7% | (24.6%‐87.0%) | 78.3% | (18.0%‐98.2%) | 51.5% | (7.8%‐89.1%) |
| Symptomatic women, median (IQR) o | 57.6% | (11.8%‐95.3%) | 69.6% | (9.5%‐90.9%) | 10.5% | (0.9%‐66.6%) | 63.6% | (24.6%‐87.0%) | 78.6% | (18.9%‐98.2%) | 50.2% | (7.3%‐88.7%) |
Abbreviations: HCP, health‐care provider; IQR, inter‐quartile range.
Unless otherwise specified.
Usual place of residence defined as urban (ie, city/town) or rural (ie, village/rural).
Includes doctors, nurses and midwives.
Includes other health‐related professionals (eg, chemists/pharmacists), church pastors, elders and traditional and spiritual healers.
Including the study hospital. HCP either part of the formal health system (primary/secondary/tertiary): medical facilities including private doctors/general practitioners, hospitals, community and outreach clinics—or the informal health system: other non‐medically trained health professionals (eg, chemist/pharmacist), traditional and spiritual healers or church leaders.
Includes private doctors, community clinics, pharmacists and community health‐care workers.
Includes both public and private secondary and tertiary hospitals.
Includes traditional and spiritual healers as well as other informal HCPs.
Women could select more than one answer.
Include barriers selected by less than 5% of the participants—for example, could not get time off from job; lack of childcare; fear of being unwell or dying; felt that treatment would not help; fear of rejection by husband/family; preference for traditional or spiritual medicine; could not get an appointment.
Percentages out of those women who visited at least one formal or informal HCP prior to visiting the study hospital.
Inappropriate outcome includes women who were reassured, and told not to worry (no. across all population groups [n] = 161); women who underwent tests, but were not informed of their results (n = 65); women who were told they had something else, and treatment was offered (n = 211); and those who were told they had something else, but no treatment was offered (n = 18).
Not applicable if women did not visit any formal or informal HCP prior to visiting the study hospital.
Time interval between first visits to consecutive HCP, that is, between first and second, second and third, and third and fourth HCP visited among women who visited at least a total of two, three and four HCPs, respectively. A breakdown in the length of these intervals by total number of HCP visited is shown in Figure 2.
Excluding 30 women with screen‐detected cancers.
The term “patient delay” is often used in HICs with universal access to free health care to refer to the time interval from symptom recognition to presentation to a HCP, as its length is essentially driven by patient‐mediated factors. In contrast, the terms “provider delay,” “health‐system delay” or “diagnostic delay” are often used to refer to the time interval from presentation to definitive diagnosis, as its length is driven predominantly by health system‐mediated factors. However, these terms may not properly capture conditions in most SSA settings—that is, without free access to health care, the length of each interval is likely to be the result of a complex interplay between patient and health system drivers. For instance, a woman may delay presentation not only because of patient‐related factors (eg, lack of BC awareness) but also because of lack of a HCP in her area of residence. Similarly, a woman who first presents with a suspicious cancer may delay final diagnosis due to fear of its consequences (eg, mastectomy, death) or desire to try first an informal HCP (eg, traditional healer). Hence, to avoid any a priori judgement on the reasons underlying the length of these time intervals, the diagnostic journey of a woman with a suspicious BC was divided into a pre‐contact interval (date of symptom discovery to date of first HCP visit) and a post‐contact interval (date of first HCP visit to date of diagnosis). HCP contacts included those with either the formal or the informal health system (Table 2). The date of final diagnosis was defined according to the European Network of Cancer Registries guidelines, 10 that is, prioritizing date of biopsy/cytology or date of hospital admission. If histological confirmation was not available (12.7% [n = 182]), diagnosis was based on the clinical history or imaging examinations (eg, mammography).
Women were excluded from this analysis if: their first reported symptom occurred >5 years (n = 70) previously (likely related to a previous condition); the date of symptom discovery was missing (n = 6); or, due to errors, the recorded date of symptom discovery was later than the date of diagnosis (n = 7). A further 43 women were excluded from the post‐contact interval analyses because the date of diagnosis preceded the self‐reported date of the first HCP visit.
2.2. Statistical methods
The primary outcomes were the lengths (in months) of the diagnostic journey, and of its two components: the pre‐ and post‐contact intervals. As the distributions of these lengths were positively skewed, medians (inter‐quartile ranges [IQR]) are reported. The cumulative probability of obtaining a diagnosis by time since symptom recognition, and time since first HCP contact, were estimated using Kaplan‐Meier. Negative binomial regression models were fitted to identify woman‐level correlates of interval lengths. These models yielded incidence rate ratios (IRRs) which can be interpreted as an estimate of the ratio of interval lengths. Variables within groups of sociodemographic, health‐related, knowledge and belief factors (Table 1) were highly correlated. Hence, minimally adjusted models, which adjusted for study population and age, were fitted first to identify the variable within each group with the strongest association with interval lengths. Thereafter, fully adjusted models were fitted which further controlled for the variables identified by the minimally adjusted models, that is, educational level, ever‐suffering from a non‐human immunodeficiency virus (HIV) comorbidity, and attributing the initial symptom(s) to cancer. Odds ratio (OR) for the association between advanced‐stage (III/IV) relative to early stage (I/II) at diagnosis and length of the diagnostic journey were estimated using logistic regression models. Analyses were conducted in Stata version 14.2.
3. RESULTS
3.1. Study participants
In all, 1429 women were included in the analysis (Table 1). Mean age at diagnosis was 50.1 years, 44% reported low education, 71% believed in spiritual or faith healing (range: 50% in Uganda to 96% in Zambia) and 24% believed in traditional medicine (12% in Namibia to 38% in Uganda). A breast lump was the first symptom noticed by 86% of the participants. Only 10% attributed their symptoms to cancer (3% in Nigeria to 14% in Namibian‐black and 33% in Namibian‐non‐black women). In all, 98% of BCs were symptomatic detections.
3.2. Navigational nodes to BC diagnosis
Most women reported first approaching a close relative/friend before visiting a HCP. Few women first visited an informal HCP except in Uganda (14%) (Table 2). Half of the participants reported having experienced barriers to first visiting a HCP (multiple answers possible); the most common for Namibian‐black and Ugandan women were lack of transport (22% and 12%, respectively) and treatment/transport costs (18% and 19%, respectively), while in Zambia and Nigeria the most common were pain and/or fear (18% and 11%, respectively). Of the 1134 participants who had visited other HCPs prior to the study hospital, 40% reported inappropriate outcomes (eg, told not to worry, wrong diagnosis) of their first visit (range: 57% in Uganda to 17% in Namibian‐non‐black women). The median number of HCP visited per woman ranged from 1 in Nigeria to 4 in Uganda and Namibian‐black women.
3.3. Length of the diagnostic journey
Lengths of the diagnostic journey (Figure 1, left panel) were shortest for Namibia‐non‐blacks (median [months]: 2.4) and longest in Uganda (11.3). Consequently, the percent of women diagnosed within 3 months of symptom recognition ranged from 59% in Namibia‐non‐black women to 11% in Uganda (Figure 1, right panel). The diagnostic journey was much shorter for women whose breast abnormality was first detected during a routine hospital visit (screen‐detected/CBE, 30 women including 15 Namibian‐non‐black and 8 Namibian‐black) than for symptomatic women, with medians (IQR) of 1.6 (0.3‐5.9) and 7.3 (3.1‐16.7) months, respectively.
FIGURE 1.
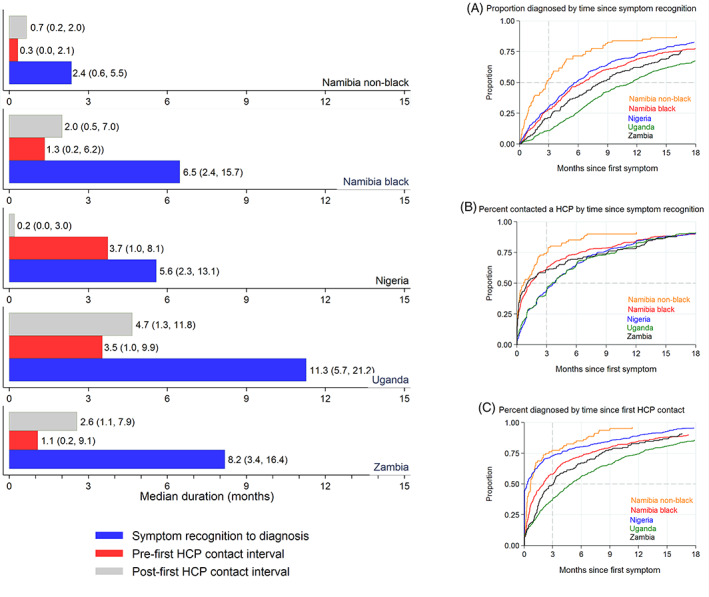
Left panel: Median (IQR) length (in months) of the diagnostic journey from symptom discovery to diagnosis (breast cancer or other), and of its pre‐ and post‐contact components, in the ABC‐DO study, by population group. Right Panel: Cumulative probabilities of: A, a definitive diagnosis by time since self‐recognition of a suspicious symptom (diagnostic interval); B, a first visit to a HCP by time since discovery of a suspicious symptom (pre‐contact interval); and C, a definitive diagnosis by time since first visit to a HCP (post‐contact interval) [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Length of the diagnostic journey and BC stage at diagnosis
Among women with malignant BC, 65% had advanced disease (Stages III/IV), with this percent being highest in Nigerian women (76%) and lowest in Namibian‐non‐black women (26%). Excluding screen‐detected women, the odds of being diagnosed with Stage III/IV BC increased with increasing length of the diagnostic journey (age‐population‐adjusted OR (95% confidence interval [CI]): 1 (reference), 1.32 (0.93‐1.86), 1.75 (1.25‐2.45) and 1.98 (1.45‐2.70) for <3, 3 to <6, 6 to <12 and ≥12 months, respectively; P‐for‐trend <.001), with no clear evidence of between‐population group heterogeneity.
3.5. Partitioning the diagnostic journey into pre‐ and post‐first HCP contact intervals
The median post‐contact interval represented at least 60% of the diagnostic journey in all settings except Nigeria, where the pre‐contact interval dominated (Table 2). Although the number of HCP visited was not excessive, the time intervals between visits to consecutive HCPs were long (Figure 2). For instance, the median length (in months) between visits to the first and second HCP ranged from 0.6 in Namibian‐non‐black and 1.4 in Namibian‐black women to 3 in Zambia and 5 in Nigeria.
FIGURE 2.
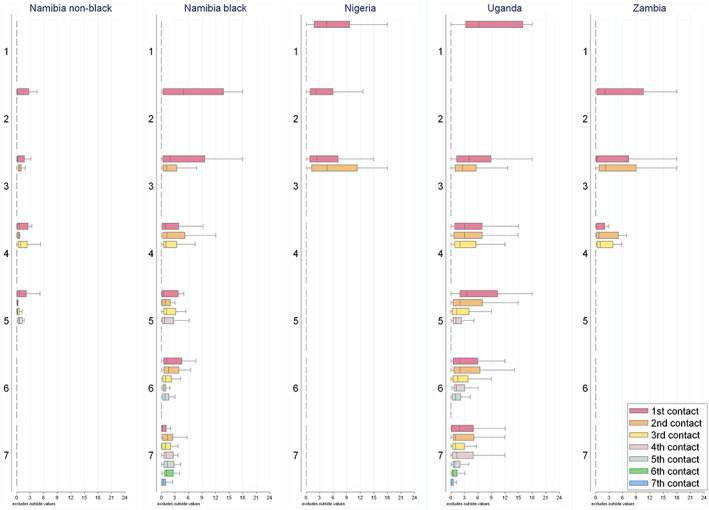
Median (IQR) time intervals between first visits to consecutive HCP, by total number of HCPs visited, among ABC‐DO symptomatic women. Total number of HCP visited includes the study hospital. Outlier values were excluded and estimates for categories with <10 women were omitted [Color figure can be viewed at wileyonlinelibrary.com]
3.6. Woman‐level correlates of the diagnostic journey length
Women‐level correlates of the diagnostic journey length were analyzed excluding screen‐detected/CBE women. Minimally adjusted and fully adjusted analyses yielded similar IRR estimates and hence only findings from the latter are presented (Table 3). The length of the diagnostic journey increased by 32% per every 10‐year increase in age at diagnosis (IRR: 1.32, 95% CI: 1.02‐1.69). The diagnostic journey was 12% longer (IRR: 1.12, 95% CI: 1.00‐1.25) among women of low relative to those of medium/high socioeconomic position (SEP) and 24% longer (1.24, 1.10‐1.40) among those with primary/none relative to those with higher education, both driven mainly by longer post‐contact intervals. The association with low education was particularly marked in Namibian‐black (1.47, 1.16‐1.86) and Zambian (1.37, 1.00‐1.87) women. Being unmarried was associated with a longer diagnostic journey in Namibian‐black and non‐black women, but with a shorter journey in Nigeria.
TABLE 3.
Associations between woman‐level factors and length of the diagnostic journey to breast cancer, and its pre‐contact and post‐contact intervals, among symptomatic women in the ABC‐DO cohort
| Variable | Study population group | Pre‐diagnostic interval | Pre‐contact interval | Post‐contact interval | ||||
|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) a | IRR (95% CI) a | IRR (95% CI) a | ||||||
| Overall | Group‐specific | P het b | Overall | Group‐specific | Overall | Group‐specific | ||
| (n = 1399) c | (n = 1399) c | (n = 1356) c , d | ||||||
| Sociodemographic | ||||||||
| Age (10 years increase) | 1.32 (1.02, 1.69) | .148 | 1.26 (0.89, 1.79) | 1.35 (0.94, 1.93) | ||||
| Low SEP (vs medium/high) | 1.12 (1.00, 1.25) | .306 | 1.10 (0.93, 1.30) | 1.22 (1.03, 1.43) | ||||
| Not married (vs married) | 1.07 (0.96, 1.20) | <.001 | 1.06 (0.91, 1.24) | 1.11 (0.95, 1.31) | ||||
| Namibia‐non‐blacks | 2.21 (1.23, 4.00) | 2.63 (1.22, 5.64) | 1.96 (0.85, 4.52) | |||||
| Namibia‐blacks | 1.39 (1.10, 1.74) | 1.28 (0.90, 1.80) | 1.50 (1.10, 2.05) | |||||
| Nigeria | 0.75 (0.60, 0.94) | 0.70 (0.54, 0.91) | 0.91 (0.60, 1.36) | |||||
| Uganda | 1.04 (0.88, 1.23) | 1.15 (0.91, 1.47) | 0.96 (0.75, 1.21) | |||||
| Zambia | 1.07 (0.79, 1.44) | 1.09 (0.63, 1.87) | 1.04 (0.70, 1.54) | |||||
| Any children living at home (yes vs no) | 0.97 (0.86, 1.10) | .86 | 1.01 (0.85, 1.19) | 0.92 (0.77, 1.11) | ||||
| Primary/no education (vs secondary/higher) | 1.24 (1.10, 1.40) | .037 | 1.16 (0.98, 1.37) | 1.35 (1.13, 1.61) | ||||
| Namibia‐non‐blacks | 0.89 (0.40, 1.98) | 0.75 (0.27, 2.07) | 0.90 (0.31, 2.65) | |||||
| Namibia‐blacks | 1.47 (1.16, 1.86) | 1.83 (1.30, 2.57) | 1.21 (0.86, 1.69) | |||||
| Nigeria | 1.16 (0.89, 1.53) | 1.12 (0.81, 1.55) | 1.33 (0.80, 2.20) | |||||
| Uganda | 1.20 (1.00, 1.45) | 0.91 (0.70, 1.18) | 1.45 (1.13, 1.88) | |||||
| Zambia | 1.37 (1.00, 1.87) | 1.00 (0.56, 1.80) | 1.71 (1.13, 2.57) | |||||
| Working in unskilled employment (yes vs no) | 1.10 (0.96, 1.26) | .302 | 1.22 (1.01, 1.47) | 1.06 (0.86, 1.29) | ||||
| Knowledge and beliefs | ||||||||
| Ever heard about BC (yes vs no) | 1.05 (0.92, 1.21) | .015 | 0.89 (0.74, 1.08) | 1.28 (1.04, 1.57) | ||||
| Namibia‐non‐blacks | — | — | — | |||||
| Namibia‐blacks | 1.06 (0.79, 1.43) | 1.01 (0.64, 1.60) | 1.20 (0.81, 1.79) | |||||
| Nigeria | 1.08 (0.83, 1.40) | 0.93 (0.68, 1.26) | 1.30 (0.82, 2.06) | |||||
| Uganda | 1.19 (0.97, 1.46) | 0.99 (0.74, 1.33) | 1.40 (1.04, 1.89) | |||||
| Zambia | 0.85 (0.54, 1.33) | 0.92 (0.40, 2.10) | 0.95 (0.51, 1.76) | |||||
| Know someone with BC | 1.03 (0.92, 1.14) | .916 | 0.93 (0.81, 1.08) | 1.12 (0.96, 1.32) | ||||
| Thinks BC is common | 0.98 (0.87, 1.10) | <.001 | 0.78 (0.67, 0.92) | 1.14 (0.97, 1.36) | ||||
| Namibia‐non‐blacks | 0.31 (0.14, 0.65) | 0.35 (0.13, 0.95) | 0.19 (0.06, 0.58) | |||||
| Namibia‐blacks | 1.10 (0.88, 1.39) | 0.91 (0.64, 1.27) | 1.32 (0.97, 1.80) | |||||
| Nigeria | 0.88 (0.67, 1.16) | 0.89 (0.65, 1.22) | 0.75 (0.45, 1.26) | |||||
| Uganda | 1.15 (0.96, 1.37) | 0.82 (0.64, 1.05) | 1.50 (1.17, 1.93) | |||||
| Zambia | 0.97 (0.71, 1.33) | 0.78 (0.44, 1.39) | 1.04 (0.70, 1.57) | |||||
| Thinks BC is curable | 0.96 (0.86, 1.08) | .174 | 0.93 (0.80, 1.09) | 0.96 (0.81, 1.14) | ||||
| Attributed symptom(s) to cancer | 0.56 (0.47, 0.67) | .115 | 0.50 (0.39, 0.64) | 0.62 (0.47, 0.80) | ||||
| Belief in spiritual medicine | 1.06 (0.94, 1.19) | .346 | 0.97 (0.82, 1.15) | 1.11 (0.93, 1.32) | ||||
| Belief in traditional medicine | 1.10 (0.98, 1.25) | .007 | 1.03 (0.87, 1.22) | 1.24 (1.03, 1.48) | ||||
| Namibia‐non‐blacks | 0.24 (0.06, 0.91) | 0.09 (0.01, 0.75) | 0.40 (0.08, 2.12) | |||||
| Namibia‐blacks | 0.89 (0.65, 1.20) | 1.03 (0.65, 1.64) | 0.70 (0.46, 1.07) | |||||
| Nigeria | 1.42 (1.11, 1.81) | 1.20 (0.89, 1.62) | 1.92 (1.23, 3.00) | |||||
| Uganda | 0.89 (0.65, 1.20) | 0.94 (0.74, 1.21) | 1.12 (0.88, 1.42) | |||||
| Zambia | 1.08 (0.78, 1.49) | 0.95 (0.51, 1.75) | 1.21 (0.79, 1.85) | |||||
| Muslim (vs other religions) e | 0.76 (0.60, 0.97) | n.a. | 0.94 (0.67, 1.32) | 0.69 (0.48, 0.97) | ||||
| Health related | ||||||||
| Recent birth (<3 years) | 1.02 (0.85, 1.21) | .104 | 1.08 (0.84, 1.38) | 0.95 (0.73, 1.22) | ||||
| Other chronic comorbidities (ever vs never) | 0.91 (0.81, 1.02) | .297 | 0.83 (0.71, 0.98) | 0.99 (0.84, 1.17) | ||||
| Personal or known family history of BC | 1.09 (0.92, 1.28) | .261 | 0.88 (0.70, 1.11) | 1.33 (1.05, 1.70) | ||||
| HIV positive | 1.04 (0.87, 1.25) | .022 | 1.18 (0.92, 1.51) | 0.96 (0.74, 1.25) | ||||
| Namibia‐non‐blacks | — | — | — | |||||
| Namibia‐blacks | 0.93 (0.68, 1.26) | 1.11 (0.70, 1.76) | 0.77 (0.50, 1.19) | |||||
| Nigeria | 1.14 (0.61, 2.13) | 0.64 (0.31, 1.35) | 2.16 (0.72, 6.47) | |||||
| Uganda | 0.77 (0.60, 1.00) | 0.77 (0.53, 1.13) | 0.71 (0.49, 1.03) | |||||
| Zambia | 1.77 (1.17, 2.68) | 2.12 (0.97, 4.62) | 1.60 (0.93, 2.75) | |||||
| Symptom related | ||||||||
| First symptom recognized was breast lump (yes vs no) f | 1.16 (0.99, 1.36) | .574 | 1.42 (1.14, 1.76) | 1.04 (0.82, 1.31) | ||||
| Navigation‐related | ||||||||
| Urban residence | 0.99 (0.88, 1.11) | .957 | 1.09 (0.93, 1.28) | 0.95 (0.81, 1.13) | ||||
| First contact | ||||||||
| Primary HCP | 1 (ref.) | .409 | 1 (ref.) | 1 (ref.) | ||||
| Secondary HCP | 1.04 (0.92, 1.18) | 1.29 (1.08, 1.53) | 0.87 (0.73, 1.05) | |||||
| Informal HCP | 1.27 (1.00, 1.62) | 1.07 (0.77, 1.50) | 1.49 (1.05, 2.11) | |||||
Abbreviations: BC, breast cancer; HCP, health care provider; n.a., not applicable; ref., reference category; SEP, socioeconomic position; —, estimates based on <10 women omitted.
Incidence rate ratios (IRR), with 95% confidence intervals (CI), adjusted for study population group, age (in four categories: 18‐39; 40‐49; 50‐59; 60+), educational level (primary or none vs secondary or higher), having suffered from a non‐HIV chronic comorbidities (ever vs never) and attributing first breast symptom(s) to cancer (yes vs no).
P‐value for interaction with study population group.
Excludes 30 women whose symptoms were first discovered by a routine clinical breast examination or through screening mammography/ultrasound (see Results section).
Excludes a further 43 women whose date of final diagnosis preceded the self‐reported date of their first HCP visit (see Methods section).
IRR (95% CI) estimates for the Ugandan population group only as this is the only one with a sizeable Muslim population (n = 395 for the pre‐diagnostic and the pre‐contact intervals; n = 386 for the post‐contact interval).
Excludes an additional eight women with missing data for this variable.
The length of the diagnostic journey decreased by about half (0.56, 0.47‐0.67) if a woman attributed her initial symptom(s) to cancer, reflecting both shorter pre‐ and post‐contact intervals. Belief in traditional medicine was associated with a longer (1.42, 1.11‐1.81) diagnostic journey in Nigeria, driven mainly by a longer post‐contact interval, but with a shorter (0.24, 0.06‐0.91) journey in Namibian‐non‐black women, reflecting both shorter pre‐ and post‐contact intervals. In Uganda, the only setting with a sizeable Muslim population, Muslim women had a shorter diagnostic journey (0.76, 0.60‐0.97), reflecting a shorter post‐contact interval. Being HIV‐positive was associated with a longer diagnostic journey in Zambia (1.77, 1.17‐2.68; HIV prevalence: 15.1%), driven by longer pre‐ and post‐contact intervals, but no association was found for Namibian‐black or Ugandan women, who had similar HIV‐prevalence (13.2% and 11.8%, respectively). Noticing a breast lump as the first symptom was associated with a longer pre‐contact interval. Visiting first an informal HCP was associated with a longer diagnostic journey, reflecting a longer post‐contact interval.
The absolute difference (AD) in the median length of the diagnostic journey between women with low vs high education was 3.6 months, and between those who attributed their symptoms to cancer vs those did not was 4.1 months, translating into AD in the proportion diagnosed ≤3 months of 20% and 22%, respectively (Figure 3). Hence, the AD in median lengths of the diagnostic journey between higher‐educated women who attributed their symptoms to cancer and lower‐educated women who did not was 7.2 months, corresponding to a 34% AD in the proportion diagnosed ≤3 months.
FIGURE 3.
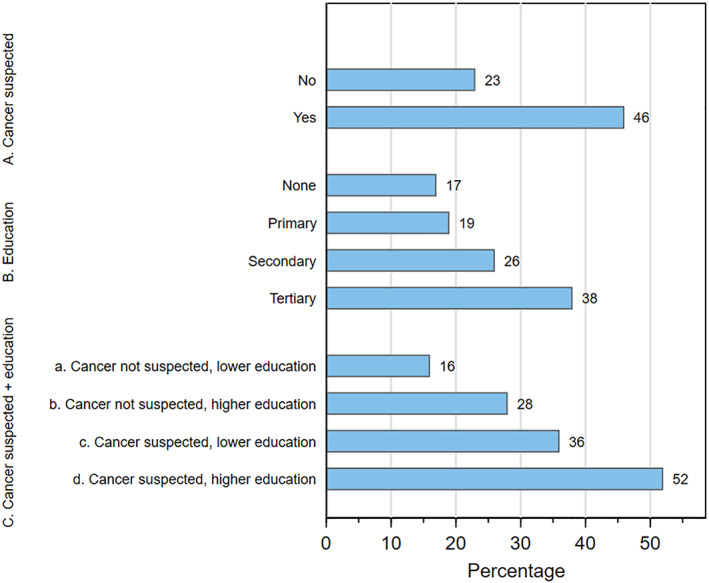
Percentage of ABC‐DO symptomatic women diagnosed within 3 months from the time of their symptom recognition by: A, whether the woman suspected first symptom might be cancer; B, the woman's highest level of formal education; and c, combined education and suspicion of cancer, where lower education is none/primary and higher education is secondary or more [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The diagnostic journey of ABC‐DO women was much longer than reported for white and black women in North America, 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 stretching back to the 1940 to 1960s, but consistent with those reported for black women in SSA 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 (Supplemental Material). The post‐contact interval accounted for over 60% of the diagnostic journey in all settings except Nigeria, the sole sites with regional rather than national catchment population, consistent with considerable delays after a woman's first HCP contact and in line with the high proportion of women reporting an inappropriate outcome of their first HCP visit. The prolonged post‐contact interval mainly reflected long intervals between visits to a few HCP, rather than visits to multiple HCPs. These findings highlight the importance of educating primary and secondary health‐care professionals about BC, and the need for health‐system implementation of clear referral pathways to fast‐track patients with suspicious breast abnormalities to specialized centers for early diagnosis.
Identified woman‐level correlates of prolonged time to diagnosis were consistent with recent literature, 6 , 39 with higher SEP being associated with shorter diagnostic journeys. Attributing the initial symptom(s) to cancer was associated with shorter time to diagnosis independently of educational level, indicating that improvement of a woman's BC awareness should be a priority, particularly among socioeconomic disadvantaged populations. Contrary to studies in HICs, 40 , 41 women whose initial symptom was a lump experienced a longer pre‐contact interval. Being married was associated with shorter diagnostic journeys in Namibia and Uganda. In contrast, being married and, consistent with the low level of BC awareness, believing in traditional medicine were associated with longer diagnostic journeys in Nigeria. 9 Being HIV+ was associated with a longer diagnostic journey in Zambia, but not in the other two populations with high HIV‐prevalence (Namibian‐blacks and Uganda), reflecting perhaps between‐setting differences in health‐care access for HIV+ patients. Treatment and transport costs were the main self‐reported barriers to first visiting a HCP in Namibia and Uganda, where women often had to travel long‐distances to access health care.
ABC‐DO is the largest study yet to quantify the length of the diagnostic journey, and its components, across a range of different SSA settings, and the first to examine their relationship with stage at diagnosis. 8 A major strength was the use of a specifically tailored m‐health application to collect time‐annotated events through a woman's diagnostic journey and information on potential correlates of its length. Weaknesses include the fact that participants were recruited in public tertiary referral centers and thus might be unrepresentative as not all BC patients are referred to these hospitals or can reach them (eg, lack of resources, early death). Furthermore, the self‐reported length of the diagnostic journey might have been affected by between‐woman variation in the ability to recognize symptoms (eg, among women with a similar diagnosis journey length mean tumor size was smaller among the more educated than among less‐educated women 8 ) and across settings (eg, the percent of cancers diagnosed at stages III/IV among women who reported a pre‐contact interval of ≤3 months ranged from 21% in Namibian‐non‐black women to 54% in Nigerian and Namibian‐black women). In particular, the relatively short diagnostic journey in Nigeria seems at odds with the very high percent of women diagnosed with advanced disease (76%), suggesting later recognition (or admission) of symptoms, perhaps due to poorer BC awareness, greater fear and stigma or faster tumor growth associated with more aggressive tumor subtypes—the lack of immunohistochemistry testing in the Nigerian settings precluded examination of this. Reassuringly, overall, the self‐reported length of the diagnostic journey was associated with tumor stage at diagnosis.
In summary, the diagnostic journeys of women with symptoms suspicious of BC, a disease that is a potentially curable if diagnosed and treated early, are unacceptably long in SSA. Priority should be given to promotion of BC awareness among both women and front‐line health‐care workers and implementation of accelerated mechanisms for referral of women with suspicious abnormalities to specialized centers for early diagnosis and treatment.
CONFLICT OF INTEREST
All authors declared no potential conflicts of interest. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
ETHICS STATEMENT
The study was approved by all local and institutional ethics committees. 7 Participants provided written informed consent or, if illiterate, a fingerprint.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGMENTS
We would like to thank all ABC‐DO participants for their time and all ABC‐DO study interviewers for their dedicated work. The ABC‐DO study was funded by Susan G Komen (IIR 13264158 to Valerie McCormack and Isabel dos‐Santos‐Silva, GSP18IARC001 and GSP19IARC001, and as part of “Implementing breast cancer care efficiency in Zambia through specialized health provider training and m‐health evaluation of patient outcomes” for the Zambian site to Groesbeck Parham) and by the International Agency for Research on Cancer. Moses Galukande is a THRiVE‐2 fellow (supported by DELTA African initiative DEL‐15‐011). Leeya Pinder is supported by the University of Washington T32 Fellowship (5T32CA009515‐34).
Foerster M, McKenzie F, Zietsman A, et al. Dissecting the journey to breast cancer diagnosis in sub‐Saharan Africa: Findings from the multicountry ABC‐DO cohort study. Int. J. Cancer. 2021;148:340–351. 10.1002/ijc.33209
Milena Foerster and Fiona McKenzie contributed equally to this study.
Funding information DELTA African Foundation, Grant/Award Number: DEL‐15‐011; Susan G. Komen, Grant/Award Numbers: GSP18IARC001, GSP19IARC001, IIR 13264158; University of Washington, Grant/Award Number: 5T32CA009515‐34; International Agency for Research on Cancer
DATA AVAILABILITY STATEMENT
Collaborations with ABC‐DO at IARC are welcome. Please email mccormackv@iarc.fr
REFERENCES
- 1. Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 2. dos‐Santos‐Silva I, De Stavola BL, Junior NLR, et al. Ethnoracial and social trends in breast cancer staging at diagnosis in Brazil, 2001–14: a case only analysis. Lancet Glob Health. 2019;7(6):e784‐e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jedy‐Agba E, McCormack V, Adebamowo C, Dos‐Santos‐Silva I. Stage at diagnosis of breast cancer in sub‐Saharan Africa: a systematic review and meta‐analysis. Lancet Glob Health. 2016;4(12):e923‐e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119‐1126. [DOI] [PubMed] [Google Scholar]
- 5. Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low‐resource and middle‐resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12(4):387‐398. [DOI] [PubMed] [Google Scholar]
- 6. Espina C, McKenzie F, Dos‐Santos‐Silva I. Delayed presentation and diagnosis of breast cancer in African women: a systematic review. Ann Epidemiol. 2017;27(10):659‐671.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKenzie F, Zietsman A, Galukande M, et al. African Breast Cancer—Disparities in Outcomes (ABC‐DO): protocol of a multicountry mobile health prospective study of breast cancer survival in sub‐Saharan Africa. BMJ Open. 2016;6(8):e011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKenzie F, Zietsman A, Galukande M, et al. Drivers of advanced stage at breast cancer diagnosis in the multicountry African breast cancer ‐ disparities in outcomes (ABC‐DO) study. Int J Cancer. 2018;142(8):1568‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKenzie F, Zietsman A, Galukande M, et al. Breast cancer awareness in the sub‐Saharan African ABC‐DO cohort: African Breast Cancer—Disparities in Outcomes study. Cancer Causes Control. 2018;29(8):721‐730. [DOI] [PubMed] [Google Scholar]
- 10. European Network of Cancer Registries . Recommendations for Coding Incidence Date; 1997. Retrieved from http://www.encr.eu/images/docs/recommendations/incideng.pdf
- 11. Potts WJ. Results of delay in treatment of breast cancer. Ann Surg. 1928;88(5):842‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higginson J. Patient delay with reference to stage of cancer. Cancer. 1962;15:50‐56. [DOI] [PubMed] [Google Scholar]
- 13. Wilkinson GS, Edgerton F, Wallace HJ Jr, Reese P, Patterson J, Priore R. Delay, stage of disease and survival from breast cancer. J Chronic Dis. 1979;32(5):365‐373. [DOI] [PubMed] [Google Scholar]
- 14. Elwood JM, Moorehead WP. Delay in diagnosis and long‐term survival in breast cancer. Br Med J. 1980;280(6227):1291‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dennis CR, Gardner B, Lim B. Analysis of survival and recurrence vs. patient and doctor delay in treatment of breast cancer. Cancer. 1975;35(3):714‐720. [DOI] [PubMed] [Google Scholar]
- 16. Buttlar CA, Templeton AC. The size of breast masses at presentation. The impact of prior medical training. Cancer. 1983;51(9):1750‐1753. [DOI] [PubMed] [Google Scholar]
- 17. Feldman JG, Saunders M, Carter AC, Gardner B. The effects of patient delay and symptoms other than a lump on survival in breast cancer. Cancer. 1983;51(7):1226‐1229. [DOI] [PubMed] [Google Scholar]
- 18. Huguley CM Jr, Brown RL, Greenberg RS, Clark WS. Breast self‐examination and survival from breast cancer. Cancer. 1988;62(7):1389‐1396. [DOI] [PubMed] [Google Scholar]
- 19. Vernon SW, Tilley BC, Neale AV, Steinfeldt L. Ethnicity, survival, and delay in seeking treatment for symptoms of breast cancer. Cancer. 1985;55(7):1563‐1571. [DOI] [PubMed] [Google Scholar]
- 20. Freeman HP, Wasfie TJ. Cancer of the breast in poor black women. Cancer. 1989;63(12):2562‐2569. [DOI] [PubMed] [Google Scholar]
- 21. Coates RJ, Bransfield DD, Wesley M, et al. Differences between black and white women with breast cancer in time from symptom recognition to medical consultation. J Natl Cancer Inst. 1992;84(12):938‐950. [DOI] [PubMed] [Google Scholar]
- 22. Hunter CP, Redmond CK, Chen VW, et al. Breast cancer: factors associated with stage at diagnosis in black and white women. J Natl Cancer Inst. 1993;85(14):1129‐1137. [DOI] [PubMed] [Google Scholar]
- 23. Caplan LS, Helzlsouer KJ, Shapiro S, Freedman LS, Coates RJ, Edwards BK. System delay in breast cancer in whites and blacks. Am J Epidemiol. 1995;142(8):804‐812. [DOI] [PubMed] [Google Scholar]
- 24. Rayson D, Chiasson D, Dewar R. Elapsed time from breast cancer detection to first adjuvant therapy in a Canadian province, 1999‐2000. CMAJ. 2004;170(6):957‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244‐2252. [DOI] [PubMed] [Google Scholar]
- 26. Partridge AH, Hughes ME, Ottesen RA, et al. The effect of age on delay in diagnosis and stage of breast cancer. Oncologist. 2012;17(6):775‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gregorio DI, Cummings KM, Michalek A. Delay, stage of disease, and survival among White and Black women with breast cancer. Am J Public Health. 1983;73(5):590‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robbins GF, Bross I. The significance of delay in relation to prognosis of patients with primary operable breast cancer. Cancer. 1957;10(2):338‐344. [DOI] [PubMed] [Google Scholar]
- 29. Ly M, Diop S, Sacko M, Baby M, Diop CT, Diallo DA. Breast cancer: factors influencing the therapeutic itinerary of patients in a medical oncology unit in Bamako (Mali). Bull Cancer. 2002;89(3):323‐326. [PubMed] [Google Scholar]
- 30. Clegg‐Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in Ghana? A pilot study. Ghana Med J. 2009;43(3):127‐131. [PMC free article] [PubMed] [Google Scholar]
- 31. Ezeome ER. Delays in presentation and treatment of breast cancer in Enugu, Nigeria. Niger J Clin Pract. 2010;13(3):311‐316. [PubMed] [Google Scholar]
- 32. Ibrahim NA, Oludara MA. Socio‐demographic factors and reasons associated with delay in breast cancer presentation: a study in Nigerian women. Breast. 2012;21(3):416‐418. [DOI] [PubMed] [Google Scholar]
- 33. Price AJ, Ndom P, Atenguena E, Mambou Nouemssi JP, Ryder RW. Cancer care challenges in developing countries. Cancer. 2012;118(14):3627‐3635. [DOI] [PubMed] [Google Scholar]
- 34. Toure M, Nguessan E, Bambara AT, Kouassi YK, Dia JM, Adoubi I. Factors linked to late diagnosis in breast cancer in sub‐Saharan Africa: case of Cote d'Ivoire. Gynecol Obstet Fertil. 2013;41(12):696‐700. [DOI] [PubMed] [Google Scholar]
- 35. Marcus TS, Lunda S, Fernandez L. Delayed breast cancer presentation: hospital data should inform proactive primary care. Afr J Prim Health Care Fam Plan. 2013;5:503. [Google Scholar]
- 36. Pace LE, Mpunga T, Hategekimana V, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist. 2015;20(7):780‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brinton L, Figueroa J, Adjei E, et al. Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa. Breast Cancer Res Treat. 2017;162(1):105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joffe M, Ayeni O, Norris SA, et al. Barriers to early presentation of breast cancer among women in Soweto, South Africa. PLoS One. 2018;13(2):e0192071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unger‐Saldana K, Infante‐Castaneda C. Delay of medical care for symptomatic breast cancer: a literature review. Salud Publica Mex. 2009;51(Suppl 2):s270‐s285. [DOI] [PubMed] [Google Scholar]
- 40. Burgess CC, Ramirez AJ, Richards MA, Love SB. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77(8):1343‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramirez AJ, Westcombe AM, Burgess CC, Sutton S, Littlejohns P, Richards MA. Factors predicting delayed presentation of symptomatic breast cancer: a systematic review. Lancet. 1999;353(9159):1127‐1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Collaborations with ABC‐DO at IARC are welcome. Please email mccormackv@iarc.fr


