Abstract
Purpose
During preclinical testing, teriparatide caused a dose‐dependent increase in the incidence of osteosarcoma in rats. This study compared the incidence rate of osteosarcoma among patients aged ≥65 years treated with teriparatide vs a matched‐comparator cohort.
Methods
This population‐based comparative‐cohort study matched exposure details for each teriparatide user, identified via Medicare Part D prescription claims, and up to four comparators based on age, sex, zip code, date of claim for filled prescription, and number of unique therapeutic classes dispensed. Outcomes were identified via linkage with participating cancer registries. All US state cancer registries were invited to participate.
Results
Overall, 153 316 patients in the teriparatide cohort and 613 247 in the comparator cohort were linked to 811 osteosarcoma cases from 26 participating state cancer registries (68% of US patients aged ≥65 years diagnosed 2007‐2014). Analysis on a subset of cohorts revealed they were balanced for known osteosarcoma risk factors and Charlson comorbidity index. Mean duration of teriparatide treatment was 10 months. No osteosarcoma cases were observed in the teriparatide cohort; the incidence rate in the comparator cohort was consistent with the background incidence rate among adults aged ≥65 years. The incidence rate ratio was 0.0 (95% confidence interval, 0.0‐3.2).
Conclusions
For US patients aged ≥65 years, incidence of osteosarcoma among those treated with teriparatide ranges from 0 to 3.2 times the incidence of osteosarcoma in those treated with other medications. Given low incidence of osteosarcoma, this range of effect is inconsistent with a large absolute increase in osteosarcoma risk.
Keywords: cohort, Medicare, osteosarcoma, pharmacoepidemiology, registry, safety, teriparatide
KEY POINTS.
This population‐based comparative cohort study aimed to compare the incidence rate of osteosarcoma among patients aged ≥65 years treated with teriparatide, identified via Medicare Part D prescription claims, to a cohort of matched comparators. Outcomes were identified via linkage with participating state cancer registries. Patients were followed to date of osteosarcoma diagnosis, death, or end of study.
A total of 153 316 patients in the teriparatide cohort and 613 247 patients in the comparator cohort were linked to 811 osteosarcoma cases from 26 participating state cancer registries (covering 68% of US osteosarcoma cases aged ≥65 diagnosed from 2007 to 2014).
No cases of osteosarcoma were observed in the teriparatide cohort, and the rate in the comparator cohort was consistent with the background rate among adults aged ≥65 years. The incidence rate ratio was 0.0 (95% confidence interval, 0.0‐3.2).
The incidence of osteosarcoma among teriparatide‐treated patients aged ≥65 years in the US ranges from 0 to 3.2 times the incidence among those treated with other medications. Given the low incidence of osteosarcoma, this range of effect is inconsistent with a large absolute increase in risk for osteosarcoma.
1. INTRODUCTION
The prevalence of osteoporosis among individuals aged ≥50 years in the United States was estimated to be 10.2 million in 2014. 1 Teriparatide (Forteo; Eli Lilly and Company), a recombinant human parathyroid hormone, stimulates new bone formation on trabecular and cortical bone surfaces by preferential stimulation of osteoblastic over osteoclastic activity. In the United States, teriparatide is indicated for the treatment of postmenopausal women with osteoporosis at high risk for fracture, for the increase of bone mass in men with primary or hypogonadal osteoporosis at high risk for fracture, and for the treatment of men and women with osteoporosis associated with sustained systemic glucocorticoid therapy at high risk for fracture. In clinical studies in postmenopausal women, teriparatide significantly reduced the incidence of vertebral fractures 2 and was well tolerated.
In preclinical testing, teriparatide caused dose‐dependent increases in osteosarcoma incidence in rats. 3 Studies have shown that the rat skeleton is more sensitive to the pharmacological effects of parathyroid hormone in the formation of new bone and osteosarcoma than monkey or human skeleton. 4 No cases of osteosarcoma were reported during clinical trials of teriparatide or in a 5‐year post‐treatment follow‐up study that included seven long‐term teriparatide clinical trials, and few spontaneous cases of osteosarcoma have been reported in patients treated with teriparatide. 5 These studies were planned and agreed upon in collaboration with regulatory authorities and the manufacturer as part of postmarketing responsibilities. Those completed noninterventional studies have yielded frequencies of teriparatide‐exposed cases of osteosarcoma that are consistent with the background frequency in the similarly aged general population. Results from these noninterventional studies have wide confidence intervals (CIs) owing to relatively small study sizes, given the rare outcome frequency. 6 , 7
Osteosarcoma in humans is a primary malignant bone tumor with an incidence rate varying from 1.7 per million in those aged 25 to 59 years to 3.9 per million in those aged ≥60 years. 8 , 9 Little is known about the etiology of osteosarcoma in adults. 10 , 11 Potential risk factors include injury, infection, and metallic implants at the tumor site and metallic implants, Paget's disease of the bone, and radiation treatment to the bones. 11 , 12
This study aimed to evaluate a potential association between teriparatide and osteosarcoma. The primary objective was to compare the incidence rate of osteosarcoma among patients aged ≥65 years with a prescription claim for teriparatide in Medicare Part D data with a cohort of matched comparators using an incidence rate ratio (IRR) and 95% CI.
All authors of this paper contributed to the design of the study protocol, which was reviewed by the US Food and Drug Administration, interpretation of results; and writing or critical review of the manuscript. RTI Health Solutions was solely responsible for the study conduct and analysis and had contractual agreement that allowed for freedom to publish results, irrespective of the findings, provided the sponsor was given an opportunity to review for intellectual property.
2. METHODS
2.1. Study design
This study was initiated in 2014 using a population‐based cohort of patients aged ≥65 years from secondary data to compare the incidence of osteosarcoma among teriparatide users with incidence among nonusers. Exposure was ascertained from prescription drug claims, and the primary outcome (osteosarcoma) was ascertained through linkage with state cancer registries. The study period to identify exposure was January 2007 to December 2014 (Figure 1). Follow‐up began on the index date (ie, the first dispensing of teriparatide in the exposed cohort or the corresponding index date for the comparator cohort) and ended on the date of diagnosis with osteosarcoma, date of death, or end of the study period, whichever occurred first.
FIGURE 1.
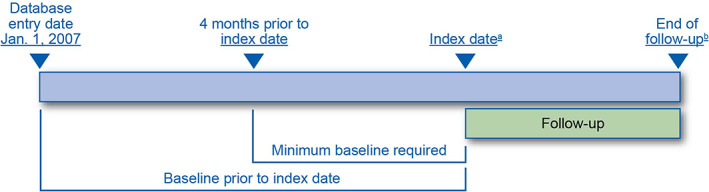
Teriparatide Medicare linkage study design. (A) Date of teriparatide prescription for the exposed and comparator cohort. (B) Osteosarcoma diagnosis, death, or end of study period [Colour figure can be viewed at wileyonlinelibrary.com]
2.2. Study population
The study cohorts were selected from all people enrolled in Medicare Part D in the United States. Medicare was chosen because general eligibility begins at age 65 years and an estimated 98% of the US population aged 65 years and older are covered. 13 Moreover, the vast majority of teriparatide users are over 65. The teriparatide (exposed) cohort comprised patients with a Medicare claim for an outpatient medication dispensing of teriparatide beginning in 2007, the first full year in which Medicare Part D was available. Exposed patients were individually matched with up to four patients from the general population of Medicare Part D patients with a prescription for a medication other than teriparatide in the same calendar month (comparator cohort). Patients were matched for age, sex, three‐digit zip code, date of claim for filled prescription, and number of unique therapeutic classes of medications dispensed in the previous 4 months. A general population comparator was chosen over a comparator treated with other osteoporosis medications because of concern regarding potential selection bias: patients receiving teriparatide might have a more intensive clinical and treatment history and higher cancer risk than those treated with other osteoporosis medications. Patients in both cohorts were required to have 4 months or more of continuous Medicare enrollment before the index date (ie, look‐back period). Both new users and prevalent uses of teriparatide were included to maximize the study size.
2.3. Variables
Exposure to teriparatide was identified by its National Drug Codes in Medicare Part D claims data and was classified as incident or prevalent based on whether the patient had a teriparatide prescription during the look‐back period. Time at risk started at the index date and ended at the diagnosis of osteosarcoma, death, or end of the study period. Minimum look‐back period was 4 months; however, all available look‐back time was used to characterize the cohorts at baseline. Incident osteosarcoma was defined by International Classification of Diseases for Oncology, Third Edition (ICD‐O‐3) codes (Table 1) that were pathologically confirmed and newly reported any time after the index date and was ascertained through linkage with cancer registries. Cancer registries in all 50 US states and the District of Columbia were invited to participate in the study. Cancer registries collect detailed clinical information including tumor site, type, and stage of cancer (extent of disease) at the time of diagnosis and cancer treatment received during the first 6 months following diagnosis (ie, the first course of therapy); data are coded using ICD‐O‐3 codes. Clinics are required by law to report all cancers to their central statewide cancer registry. Data are reported by year of the cancer diagnosis. Because of the expected lag time between diagnosis and reporting, the study included outcomes from years in which case reporting was considered complete or nearly complete (95% complete for the last year).
TABLE 1.
International Classification of Diseases for Oncology, Third Edition Codes used to identify osteosarcoma
| Code | Description |
|---|---|
| 9180/3 | Osteosarcoma NOS (not otherwise specified) |
| 9181/3 | Chondroblastic osteosarcoma |
| 9182/3 | Fibroblastic osteosarcoma |
| 9183/3 | Telangiectatic osteosarcoma |
| 9184/3 | Osteosarcoma in Paget's disease of bone |
| 9185/3 | Small cell osteosarcoma |
| 9186/3 | Central osteosarcoma |
| 9187/3 | Intraosseous well differentiated osteosarcoma |
| 9192/3 | Parosteal osteosarcoma |
| 9193/3 | Periosteal osteosarcoma |
| 9194/3 | High‐grade surface osteosarcoma |
| 9195/3 | Intracortical osteosarcoma |
The use of other osteoporosis drugs and glucocorticoids, medications indicating potentially confounding underlying conditions, in both the teriparatide and comparator cohorts during all available look‐back times was described. For the teriparatide cohort, characteristics of teriparatide treatment were described. For both the teriparatide and comparator cohorts, number of deaths during the follow‐up period and identified risk factors for osteosarcoma were characterized. Cause of death was not available in the Medicare data set; therefore, only total mortality could be characterized during follow‐up. Detailed operational definitions can be found in Supplementary Appendix A.
For all Medicare data, to protect patient privacy, non‐zero cells of <11, or any percentages or other data that allow a non‐zero cell of <11 to be derived from other information, could not be disclosed. Also, the reporting of minima, maxima, medians, modes, and percentiles is not permitted.
2.4. Data linkage
The study cohort identified in Medicare Part D was linked to participating state cancer registry data by an independent third‐party organization (General Dynamics Information Technology [GDIT]). A deterministic data linkage (ie, exact match) was conducted between the Medicare Part D beneficiaries selected for the study cohorts and patients diagnosed with osteosarcoma in the cancer registry data. The study investigators did not have access to personally identifying information for the study cohort and submitted encrypted beneficiary IDs for individuals in the study cohort to GDIT for linkage. GDIT linked using either Social Security number (SSN) or at least three of the following variables: the last four digits of the SSN, last name, date of birth, and sex (zip code and state were used to clarify possible matches). When matches were found during the linkage, the encrypted beneficiary ID was returned to the coordinating study center, which then requested tumor‐related variables from the cancer registry. Registries were blinded to the exposure status of patients who matched, and GDIT was not provided with cancer diagnosis information for patients with osteosarcoma submitted by the registries. The tumor‐related information was used to establish the date of diagnosis to confirm that a linked patient was exposed to teriparatide before the cancer diagnosis. Information on cancer site and morphology was also used to ensure that the patient met the study case definition for osteosarcoma.
2.5. Statistical analyses
The IRR and 95% CI for osteosarcoma occurrence in teriparatide users and nonusers were estimated using exact conditional Poisson regression. The incidence rate of osteosarcoma among teriparatide users and comparators was estimated by the number of cases of osteosarcoma captured by the participating state cancer registries during the observation period divided by the total person‐time of observation among individuals at risk. The IRR of osteosarcoma was calculated as the ratio of the incidence rate of osteosarcoma in teriparatide users to the incidence rate in the comparator cohort. It was assumed that there was no induction or latency period between teriparatide exposure and development of clinically detectable osteosarcoma.
Although all US state cancer registries were invited to participate, not all registries participated owing resource constraints or other local restrictions relating to use of state cancer registry data. Incomplete capture of osteosarcoma cases in the US population resulting from registries not participating in the study was addressed in two ways: (a) by applying a coverage fraction that represents the percentage of osteosarcoma cases captured in this study (based on cancer registry participation) to the total person‐time observed; and (b) by recalculating the person‐time at risk using the exposure information for only patients from states with participating registries and comparing it with the proposed person‐time calculation using the coverage fraction to see if patients in these states differed in a meaningful way from patients in states with nonparticipating registries. We also calculated the results using only patients from participating states.
Demographic and baseline characteristics were summarized descriptively for the teriparatide and comparator cohorts. Sensitivity analyses included (a) excluding a 6‐month lag time to account for potential latency and (b) counting only teriparatide patients with two or more prescriptions to minimize possible exposure misclassification. Because Medicare Part D data did not contain clinical information other than prescription records, sensitivity analyses were conducted to compare the similarity of the teriparatide cohort and the matched comparison cohort among a subset of patients also represented in Medicare databases for Parts A and B, which contained more detailed clinical information. Factors suspected of increasing the risk of osteosarcoma 14 or that were a proxy for overall health status were assessed for each cohort, as were demographics, baseline characteristics, and total follow‐up time.
All analyses were performed using SAS software version 9.3 or higher (SAS Institute).
3. RESULTS
3.1. Baseline characteristics
The study cohort included 153 316 patients with a teriparatide prescription with whom 613 247 comparators were matched. The study cohort was predominantly female (91%); 59% of patients were aged ≥75 years on the index date (Table 2). More than 65% of patients in the study cohort were from states with a participating cancer registry. Nearly 70% of patients were on six or more unique therapeutic classes of medications within the 4 months before the index date. Before the index date, a higher percentage of teriparatide users had a dispensing of a corticosteroid drug (39%) than did the comparators (31%). Osteoporosis drugs other than teriparatide were more frequently dispensed in the teriparatide cohort (60%) than the comparator cohort (27%). Cardiovascular drugs were more frequently dispensed in the comparator cohort (83%) than the teriparatide cohort (73%). The mean duration of follow‐up was 3.8 years for the teriparatide cohort and 3.6 years for the comparator cohort.
TABLE 2.
Population characteristics
| Characteristic | Teriparatide cohort (n = 153 316) | Comparator cohort (n = 613 247) |
|---|---|---|
| Sex, n (%) | ||
| Male | 13 426 (8.8) | 53 699 (8.8) |
| Female | 139 890 (91.2) | 559 548 (91.2) |
| Age (years) on index date, mean (SD) | 76.9 (7.64) | 77.0 (7.85) |
| Length of look‐back period (months), mean (SD) | 38.2 (28.69) | 38.0 (27.65) |
| Patients from states with participating cancer registries, n (%) | 100 033 (65.2) | 400 119 (65.2) |
| Use of corticosteroid drugs before the index date, n (%) | 58 953 (38.5) | 186 924 (30.5) |
| Use of other osteoporosis drugs before the index date, n (%) | 92 632 (60.4) | 162 905 (26.6) |
| Medications by AHFS therapeutic class within the 4 months before the index date, n (%) | ||
| Antihistamine drugs | 6758 (4.4) | 26 353 (4.3) |
| Anti‐infective agents | 69 434 (45.3) | 280 925 (45.8) |
| Antineoplastic agents | 10 732 (7.0) | 29 034 (4.7) |
| Autonomic drugs | 47 648 (31.1) | 167 263 (27.3) |
| Blood derivatives | 0 (0.0) | n < 11 |
| Blood formation, coagulation, and thrombosis agents | 27 958 (18.2) | 122 156 (19.9) |
| Cardiovascular drugs | 112 092 (73.1) | 508 567 (82.9) |
| Central nervous system agents | 109 903 (71.7) | 392 259 (64.0) |
| Diagnostic agents | 139 (0.1) | 2210 (0.4) |
| Electrolytic, caloric, and water balance | 56 889 (37.1) | 303 711 (49.5) |
| Enzymes | 409 (0.3) | 2274 (0.4) |
| Respiratory tract agents | 8567 (5.6) | 28 263 (4.6) |
| Eye, ear, nose, and throat preparations | 38 452 (25.1) | 162 231 (26.5) |
| Gastrointestinal drugs | 74 206 (48.4) | 265 962 (43.4) |
| Gold compounds | 18 (0.0) | n < 11 |
| Heavy‐metal antagonists | 45 (0.0) | 59 (0.0) |
| Hormones and synthetic substitutes | 82 052 (53.5) | 336 900 (54.9) |
| Local anesthetics | 1302 (0.8) | 6094 (1.0) |
| Oxytocics | 0 (0.0) | 0 (0.0) |
| Serums, toxoids, and vaccines | 2209 (1.4) | 7113 (1.2) |
| Skin and mucous membrane agents | 40 572 (26.5) | 148 909 (24.3) |
| Smooth muscle relaxants | 12 621 (8.2) | 45 692 (7.5) |
| Vitamins | 3735 (2.4) | 9982 (1.6) |
| Miscellaneous therapeutic agents | 53 507 (34.9) | 132 440 (21.6) |
| Unclassified | 0 (0.0) | 0 (0.0) |
| Number of unique AHFS therapeutic classes within the 4 months before the index date, n (%) | ||
| 0‐2 | 14 442 (9.4) | 57 767 (9.4) |
| 3‐5 | 32 311 (21.1) | 129 244 (21.1) |
| 6‐8 | 37 512 (24.5) | 150 047 (24.5) |
| 9‐11 | 30 431 (19.8) | 121 722 (19.8) |
| 12‐15 | 24 141 (15.7) | 96 563 (15.7) |
| >15 | 14 479 (9.4) | 57 904 (9.4) |
Abbreviations: AHFS = American Hospital Formulary Services.
Note: To protect patient privacy, non‐zero cell counts <11 cannot be disclosed for Medicare data.
3.2. Teriparatide exposure, medication use, and deaths during follow‐up
Among the teriparatide cohort, 120 302 patients (79%) had an incident exposure to teriparatide during the study period (Table 3). During the follow‐up period, the average duration of teriparatide exposure was approximately 10 months. Use of corticosteroids was higher among the teriparatide cohort (45%) than the comparator cohort (36%) during the follow‐up, as was the use of osteoporosis medications excluding teriparatide (41% in the teriparatide cohort vs 23% in the comparator cohort) (Table 3). Use of medications during follow‐up in most unique therapeutic classes was higher in the teriparatide cohort than the comparator cohort, except for cardiovascular drugs and electrolytic, caloric, and water balance treatments. The mean person‐years of observation did not vary appreciably by whether patients were from a state with a participating cancer registry (data not shown). Women consistently had higher mean person‐years of observation in every age category than men (data not shown). Among both cohorts combined, 227 296 deaths were recorded after the index date: 28% of the teriparatide cohort (42 180 of 153 316) and 30% of the comparator cohort (185 116 of 613 247).
TABLE 3.
Medication use during follow‐up
| Category | Teriparatide cohort(n = 153 316) | Comparator cohort(n = 613 247) |
|---|---|---|
| Use of teriparatide | ||
| Type of exposure a , n (%) | ||
| Incident | 120 302 (78.5) | — |
| Prevalent | 33 014 (21.5) | — |
| Number of dispensings, mean (SD) | 9.4 (8.43) | — |
| Average days' supply per dispensing episode b , mean (SD) | 33.3 (14.91) | — |
| Duration of exposure (months) c , mean (SD) | 9.5 (8.21) | — |
| Use of other medication | ||
| Use of corticosteroid drugs, n (%) | 68 348 (44.6) | 220 591 (36.0) |
| Among those with at least 1 corticosteroid dispensing, mean (SD): | ||
| Number of dispensings per patient | 7.7 (12.91) | 4.9 (8.90) |
| Duration of exposure (months) d | 7.0 (14.37) | 3.6 (9.57) |
| Use of other osteoporosis drugs, n (%) | 62 616 (40.8) | 139 984 (22.8) |
| Among those with at least 1 other osteoporosis drug dispensing, mean (SD): | ||
| Number of dispensings per patient | 14.4 (17.16) | 15.6 (17.60) |
| Duration of exposure (months) d | 18.6 (19.41) | 20.2 (20.10) |
| Medications by AHFS therapeutic class, n (%) | ||
| Antihistamine drugs | 16 404 (10.7) | 54 167 (8.8) |
| Anti‐infective agents | 133 581 (87.1) | 502 006 (81.9) |
| Antineoplastic agents | 24 973 (16.3) | 66 401 (10.8) |
| Autonomic drugs | 90 945 (59.3) | 317 113 (51.7) |
| Blood derivatives | n < 11 | n < 11 |
| Blood formation, coagulation, and thrombosis agents | 50 750 (33.1) | 200 044 (32.6) |
| Cardiovascular drugs | 131 191 (85.6) | 548 074 (89.4) |
| Central nervous system agents | 138 267 (90.2) | 524 750 (85.6) |
| Diagnostic agents | 444 (0.3) | 5043 (0.8) |
| Electrolytic, caloric, and water balance | 91 338 (59.6) | 400 776 (65.4) |
| Enzymes | 4608 (3.0) | 15 716 (2.6) |
| Respiratory tract agents | 18 954 (12.4) | 55 747 (9.1) |
| Eye, ear, nose, and throat preparations | 97 026 (63.3) | 348 122 (56.8) |
| Gastrointestinal drugs | 120 292 (78.5) | 434 154 (70.8) |
| Gold compounds | 20 (0.0) | 15 (0.0) |
| Heavy‐metal antagonists | 75 (0.0) | 153 (0.0) |
| Hormones and synthetic substitutes | 114 393 (74.6) | 441 357 (72.0) |
| Local anesthetics | 10 937 (7.1) | 33 686 (5.5) |
| Oxytocics | n < 11 | n < 11 |
| Serums, toxoids, and vaccines | 22 386 (14.6) | 70 473 (11.5) |
| Skin and mucous membrane agents | 103 756 (67.7) | 358 596 (58.5) |
| Smooth muscle relaxants | 27 786 (18.1) | 88 290 (14.4) |
| Vitamins | 7733 (5.0) | 22 988 (3.7) |
| Miscellaneous therapeutic agents | 76 531 (49.9) | 213 086 (34.7) |
| Unclassified | 220 (0.1) | 513 (0.1) |
Abbreviations: AHFS = American Hospital Formulary Services.
Note: To protect patient privacy, non‐zero cell counts <11 cannot be disclosed for Medicare data.
If the patient did not have a previous prescription for teriparatide before the index date, the exposure was classified as incident; if the patient had a previous prescription for teriparatide before the index date, the exposure was classified as prevalent.
For each teriparatide user, the per‐episode average was calculated using all dispensings of teriparatide during follow‐up.
For each teriparatide user, the duration of exposure was calculated as the sum of the days' supply of all teriparatide dispensings during follow‐up, without regard to overlaps or gaps.
For each patient, the duration of exposure was calculated as the sum of the days' supply of all dispensings of the medication of interest during follow‐up, without regard to overlaps or gaps.
3.3. Data linkage and adjustment to person‐time of observation
None of the 153 316 patients in the teriparatide cohort was identified as a match among the cases of osteosarcoma submitted for linkage by participating state cancer registries (Table 4). The exact number cannot be reported, but there were fewer than 11 and more than zero patients in the comparator cohort of 613 247 patients that were identified as matched cases among the osteosarcoma cases submitted by the cancer registries (Table 4). Of the 27 cancer registries that agreed to participate, 26 registries were included in the analysis. From these registries, 811 cases of osteosarcoma diagnosed from 2007 through 2014 were submitted to GDIT for linkage against the teriparatide and comparator cohorts. The overall match rate, a proxy measure for match success, was the percentage of cancer registry cases that matched with any patients in Medicare Part D data, including subjects outside of the two study cohorts. The match rate was 92%; 95% (461 of 485) using the primary linkage method (SSN) and 87% (219 of 252) using alternative methods. Registry data from Michigan and Oklahoma were excluded from the match rate calculation because those registries provided additional non‐study cases for linkage to mask the identity of patients submitted who were aged ≥65 years.
TABLE 4.
Incidence rates of osteosarcoma, incidence rate ratio, and incidence rate difference
| Statistic | Teriparatide cohort(n = 153 316) | Comparator cohort(n = 613 247) |
|---|---|---|
| Number of matched osteosarcoma cases by linkage to the participating cancer registries, n | 0 | n < 11 |
| Total person‐time of observation (years), PT | 585 955 | 2 212 036 |
| Adjusted for the coverage fraction (PT × 0.68) | 397 000 | 1 498 715 |
| Among patients from only the states with participating cancer registries a | 378 631 | 1 426 199 |
| Incidence rates per million person‐years (n/PT × 1 000 000) | ||
| Adjusted for the coverage fraction (95% CI) | 0.00 (0.00‐9.29) | Suppressed (1.47‐8.71) |
| Among patients from only the states with participating cancer registriesa (95% CI) | 0.00 (0.00‐9.74) | Suppressed (1.54‐9.16) |
| Incidence rate ratio | ||
| Adjusted for the coverage fraction (95% CI) | 0.00 (0.00‐3.21) | — |
| Primary study population with a 6‐month latency period | ||
| Incidence rate ratio | ||
| Adjusted for the coverage fraction (95% CI) | 0.00 (0.00‐3.19) | — |
| Primary study population requiring two teriparatide prescriptions | ||
| Incidence rate ratio | ||
| Adjusted for the coverage fraction (95% CI) | 0.00 (0.00‐3.54) | — |
Abbreviations: CI = confidence interval.
Note: To protect patient privacy, non‐zero cell counts <11 cannot be disclosed for Medicare data.
100 033 patients in the teriparatide cohort and 400 119 patients in the comparator cohort were from states with participating cancer registries.
The primary adjustment to the person‐time of observation was made by applying the coverage fraction. During the study period, 1197 US cases of osteosarcoma were diagnosed. 15 Therefore, the percentage of incident cases covered by participating state cancer registries was 68% (811 of 1197). Applying this adjustment resulted in 397 000 person‐years of observation in the teriparatide cohort and 1 498 715 person‐years of observation in the comparator cohort (Table 4).
3.4. Incidence rates of osteosarcoma, IRR, and incidence rate difference
No cases of osteosarcoma were observed in the teriparatide cohort (incidence rate per million person‐years, 0.0; 95% CI, 0.0‐9.3) (Table 4). The incidence rate in the comparator cohort is not reportable because of small numbers, but the 95% CI (1.5‐8.7 per million person‐years) indicates that the rate is similar to what would be expected in the general US population aged ≥65 years, given the estimated background incidence rate of osteosarcoma and the person‐years observed in this cohort. The IRR was 0.0 (95% CI, 0.0‐3.2), and the incidence rate difference per million person‐years was −4.5 (95% CI, −8.2 to −0.8). Rates were similar when restricting results to patients from participating states.
3.5. Sensitivity analyses
In sensitivity analyses examining balance between cohorts for believed risk factors for osteosarcoma and proxies for health status, the teriparatide and comparator subcohorts were similar regarding radiation treatment and history of Paget's disease of the bone (Table 5). The proportion of patients with a history of vertebral, hip, or pelvic fracture in the teriparatide subcohort (23%) was nearly triple that among the comparator subcohort (8%); the teriparatide cohort also had more inpatient and outpatient visits. However, the mean Charlson comorbidity index was nearly the same between groups.
TABLE 5.
Sensitivity analysis: cohort characteristics at baseline, subset study population with Medicare Parts A, B, and D
| Category or statistic | Teriparatide cohort(n = 105 794) | Comparator cohort(n = 297 509) |
|---|---|---|
| Sex, n (%) | ||
| Male | 9129 (8.6) | 24 662 (8.3) |
| Female | 96 665 (91.4) | 272 847 (91.7) |
| Age (years) on index date, mean (SD) | 77.3 (7.72) | 77.7 (8.03) |
| Length of look‐back period (months), mean (SD) | 36.9 (28.25) | 35.4 (26.90) |
| Patients from states with participating cancer registries, n (%) | 68 134 (64.4) | 191 002 (64.2) |
| Use of corticosteroid drugs before the index date, n (%) | 40 841 (38.6) | 91 026 (30.6) |
| Use of other osteoporosis drugs before the index date, n (%) | 62 549 (59.1) | 78 652 (26.4) |
| Medications by AHFS therapeutic class within the 4 months before the index date, n (%) | ||
| Antihistamine drugs | 5198 (4.9) | 14 970 (5.0) |
| Anti‐infective agents | 49 827 (47.1) | 145 844 (49.0) |
| Antineoplastic agents | 7683 (7.3) | 15 330 (5.2) |
| Autonomic drugs | 34 267 (32.4) | 88 182 (29.6) |
| Blood derivatives | 0 (0.0) | n < 11 |
| Blood formation, coagulation, and thrombosis agents | 20 481 (19.4) | 65 649 (22.1) |
| Cardiovascular drugs | 78 657 (74.3) | 249 518 (83.9) |
| Central nervous system agents | 77 473 (73.2) | 198 733 (66.8) |
| Diagnostic agents | 114 (0.1) | 1290 (0.4) |
| Electrolytic, caloric, and water balance | 41 015 (38.8) | 155 028 (52.1) |
| Enzymes | 309 (0.3) | 1311 (0.4) |
| Respiratory tract agents | 6302 (6.0) | 14 931 (5.0) |
| Eye, ear, nose, and throat preparations | 27 687 (26.2) | 81 886 (27.5) |
| Gastrointestinal drugs | 52 906 (50.0) | 136 276 (45.8) |
| Gold compounds | n < 11 | n < 11 |
| Heavy‐metal antagonists | 33 (0.0) | 30 (0.0) |
| Hormones and synthetic substitutes | 57 809 (54.6) | 168 155 (56.5) |
| Local anesthetics | 988 (0.9) | 3489 (1.2) |
| Oxytocics | 0 (0.0) | 0 (0.0) |
| Serums, toxoids, and vaccines | 1268 (1.2) | 2576 (0.9) |
| Skin and mucous membrane agents | 29 268 (27.7) | 77 145 (25.9) |
| Smooth muscle relaxants | 9176 (8.7) | 24 579 (8.3) |
| Vitamins | 2335 (2.2) | 4237 (1.4) |
| Miscellaneous therapeutic agents | 36 503 (34.5) | 65 436 (22.0) |
| Unclassified | 0 (0.0) | 0 (0.0) |
| Number of unique AHFS therapeutic classes within the 4 months before the index date, n (%) | ||
| 0‐2 | 8812 (8.3) | 23 209 (7.8) |
| 3‐5 | 20 824 (19.7) | 56 619 (19.0) |
| 6‐8 | 25 592 (24.2) | 71 175 (23.9) |
| 9‐11 | 21 531 (20.4) | 60 925 (20.5) |
| 12‐15 | 17 757 (16.8) | 51 679 (17.4) |
| >15 | 11 278 (10.7) | 33 902 (11.4) |
| Risk factors, n (%) | ||
| Radiation use | 3061 (2.9) | 12 891 (4.3) |
| History of Paget's disease of the bone | 630 (0.6) | 1241 (0.4) |
| Health status proxies | ||
| History of vertebral or hip/pelvic fracture, n (%) | 24 683 (23.3) | 24 162 (8.1) |
| History of cancer, n (%) | 37 356 (35.3) | 101 575 (34.1) |
| Number of inpatient and outpatient visits in the 4 months before the index date | ||
| 0 | 27 467 (26.0) | 106 033 (35.6) |
| 1 | 19 078 (18.0) | 59 619 (20.0) |
| 2 | 14 631 (13.8) | 38 383 (12.9) |
| ≥3 | 44 618 (42.2) | 93 474 (31.4) |
| Charlson comorbidity index | ||
| Mean (SD) | 3.8 (3.25) | 3.8 (3.31) |
Abbreviations: AHFS = American Hospital Formulary Services.
Note: To protect patient privacy, non‐zero cell counts <11 cannot be disclosed for Medicare data.
The subcohorts were well balanced in terms of demographic characteristics, whereas medication‐use patterns varied between cohorts. Similar to the primary cohorts, use of corticosteroids was higher in the teriparatide subcohort and the percentage of patients in the teriparatide cohort that used other osteoporosis medications (59%) was more than double that of the comparator subcohort (26%). Cardiovascular drug use and electrolytic, caloric, and water balance treatments were higher among the comparator subcohort than the teriparatide subcohort.
Other sensitivity analyses (eg, 6‐month lag time and requirement for at least two teriparatide prescriptions) resulted in similar IRR to the primary analysis given the lack of exposed cases in the teriparatide group (Table 4). Other potential analyses, such as including only new users of teriparatide or stratifying results by other variables (eg, use of other osteoporosis medicines and glucocorticoids), were similarly not indicated given lack of exposed cases.
4. DISCUSSION
This study identified no cases of osteosarcoma among teriparatide‐treated patients in the study cohort. Fewer than 11 and more than zero patients in the matched comparator group developed osteosarcoma during the study period; 95% CI (1.5‐8.7 per million person‐years) was consistent with the background rate of 3.9 cases per million per year in adults aged ≥65 years. Among the teriparatide and comparator cohorts combined, 1 895 715 person‐years were observed, adjusted for the coverage fraction, and the IRR was 0.0 (95% CI, 0.0‐3.2). Findings from sensitivity analyses to evaluate any differences between cohorts supported the study findings. Overall, these results are consistent with other studies in the osteosarcoma surveillance program designed to determine the extent, if any, of an increased risk of osteosarcoma associated with teriparatide treatment. 6 , 16 , 17
This study is characterized by several strengths. The cohort design allowed for direct estimation of osteosarcoma incidence in patients with a teriparatide dispensing and allowed for comparison with patients without a teriparatide dispensing. Use of Medicare Part D prescription claims data linked with cancer registry data and use of a matched comparator group were important advantages over prior noninterventional studies included in the surveillance program. 6 , 7 , 17 The ability to characterize exposure more thoroughly using prescription data for a large cohort was also an advantage over prior studies reliant on self‐report or medical record review. Given the unique nature of teriparatide, it is unlikely that patients would pay out‐of‐pocket; therefore most, if not all, exposures would have been captured in the Medicare data. Ascertaining outcome through cancer registries reduced the possibility of misclassification of cancer diagnosis, given that ICD‐O‐3 codes used by cancer registries are more specific than International Classification of Diseases, Ninth or Tenth Revision codes in claims data. Although osteosarcoma is included as mandatory reporting for cancer, it is possible that some cases were not reported to cancer registries and it is possible that such cancers would more likely be reported, and perhaps be reported more quickly, among the teriparatide users, given the awareness of the product warnings. This would create a bias toward seeing an increased risk in teriparatide users, which we did not observe.
Some limitations of this study must be considered. The possibility that residual confounding affected the findings of this study cannot be ruled out given the nature of the prescription data source and the rarity of osteosarcoma. Misclassification bias could have resulted if patients were not categorized correctly regarding exposure or outcome. Because not all registries participated in the study, there was a potential for bias related to missing data, which we attempted to account for by using a coverage fraction to adjust patient‐years of observation and by conducting a second analysis restricted to patients from the participating states. Studies of infrequent exposures and rare outcomes are inherently challenging because they require large sample sizes and may still result in imprecise effect estimates. The entire Medicare Part D file was used to maximize the size of the cohorts, and this choice restricted information available for comparing cohorts to demographic and medication information. Furthermore, this study matched for number of unique therapeutic drug classes as a crude indicator of health status. Medication use during follow‐up and mortality experience reflected a relatively well‐balanced cohort. Sensitivity analyses using a subcohort of patients with additional clinical information from other Medicare files confirmed a reasonable balance regarding known risk factors for osteosarcoma. In general, there are few established risk factors for osteosarcoma. Most were evaluated in sensitivity analyses using additional Medicare inpatient and outpatient data. Age and sex could not be evaluated as confounders because they were balanced between groups with matching. In addition, Paget's disease of the bone, a potential confounder, was not markedly different between groups in the subcohort sensitivity analysis. Moreover, Paget's disease of the bone is a contraindication for teriparatide and was expected only in the comparator population. Because this rare condition is estimated to have prevalence <4%, 18 it should not result in appreciable confounding. Finally, history of radiation therapy may have differed between study cohorts; however, the look‐back period was insufficient to capture all prior radiation therapy.
Approximately 79% of teriparatide users were new users. Because of our desire to maximize the number of teriparatide users, we chose to include both incident and prevalent users. A sensitivity analysis restricting to new users could only have been implemented; however, given there were no cases in the teriparatide cohort, this additional analysis would not have been informative.
Another potential limitation is the duration of follow‐up time: the teriparatide patients had a mean follow‐up time of 3.8 years, while the comparators had a mean follow‐up time of 3.6 years. In the absence of a biological model for the exposure‐cancer relationship, it is recommended that epidemiologists employ sensitivity analyses to explore different risk windows. The lack of cases in the exposed group and the relatively short follow‐up period precluded such analyses.
The results of this comparative study suggest that for US patients aged ≥65 years, primarily women, the incidence of osteosarcoma among teriparatide‐treated patients ranges from 0 to 3.2 times the incidence of osteosarcoma in those treated with other medications. Given the low baseline incidence of osteosarcoma, this rate translates to a range of zero to an additional 8 or 9 cases of osteosarcoma per million person‐years, which is not consistent with a large increase in risk.
Clinical relevance of these results should be evaluated in the context of the potential benefits of treatment in this population of osteoporosis patients >65 years at high risk for fractures.
ETHICS STATEMENT
The study was approved by the RTI International institutional review board, participating cancer registry data‐use committees, and their affiliated institutional review boards, as necessary. The Centers for Medicare and Medicaid Services Privacy Board approved access to the Medicare data for analysis.
CONFLICT OF INTEREST
This study was performed under a research contract between RTI Health Solutions and Eli Lilly and Company and was funded by Eli Lilly and Company. Elizabeth Andrews, Alicia Gilsenan, David Harris, Shannon Hunter, Lisa McQuay, and Kirk Midkiff are or were salaried employees of RTI Health Solutions. Nicole Kellier‐Steele is a salaried employee of Eli Lilly and Company. The contract between RTI Health Solutions and Eli Lilly and Company assures independent publication rights for RTI Health Solutions. The authors have no other conflict of interest to declare.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
The authors acknowledge and thank the GHBX Advisory Board members and guests of the board who have provided thoughtful input throughout the conduct of this study. Advisory board members include Dr. Bruce Chabner from Harvard Medical School; Prof. Henrik Bauer from Karolinska Institute; and Dr. A. Kevin Raymond, formerly from MD Anderson. Guests of the board, Dr. Maria Schymura, Dr. Kenneth Rothman, and Dr. Thor Alvegard, also provided valuable insight and advice. The authors thank David McSorley of RTI Health Solutions who provided statistical oversight during the design aspects of the study. Kate Lothman of RTI Health Solutions provided medical writing services, which were funded by Eli Lilly and Company.
We owe special thanks to the 27 cancer registries and departments of health that contributed data to this study: Alaska Department of Health and Social Services, Alaska Cancer Registry; Arizona Department of Health Services, Arizona Cancer Registry; California Department of Public Health, California Cancer Registry; Delaware Health and Social Services Division of Public Health, Delaware Cancer Registry; Florida Department of Health, Florida Cancer Data System; University of Hawaii Cancer Center, Hawaii Tumor Registry; Idaho Hospital Association, Cancer Data Registry of Idaho; Indiana State Department of Health, Indiana Cancer Registry; University of Iowa, State Health Registry of Iowa; Kansas Department of Health and Environment, Kansas Cancer Registry; University of Kentucky, Markey Cancer Control Program, Kentucky Cancer Registry, Michigan Department of Community Health, Michigan Cancer Surveillance Program; Minnesota Department of Health, Minnesota Cancer Surveillance System; University of Missouri, Missouri Cancer Registry; Montana Department of Public Health and Human Services, Montana Central Tumor Registry; Nebraska Department of Health and Human Services, Nebraska Cancer Registry; The Rutgers Cancer Institute of New Jersey, Rutgers University, New Jersey State Cancer Registry; New York State Department of Health, New York State Cancer Registry; North Carolina Department of Health and Human Services, Division of Public Health, North Carolina Central Cancer Registry; Ohio Department of Health, Ohio Cancer Incidence Surveillance System; Oklahoma State Department of Health, Oklahoma Central Cancer Registry; Tennessee Department of Health, Tennessee Cancer Registry; Texas Department of State Health Services, Texas Cancer Registry; University of Utah, Utah Cancer Registry; Virginia Department of Health, Virginia Cancer Registry; West Virginia Department of Health and Human Services, West Virginia Cancer Registry; Wisconsin Department of Health Services, University of Washington, Wisconsin Cancer Reporting System; the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI); and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC). Use of these data does not imply that these registries, their departments of health, the CDC, or NCI either agrees or disagrees with any representations, analyses, interpretations, or conclusions.
Gilsenan A, Midkiff K, Harris D, et al. Assessing the incidence of osteosarcoma among teriparatide users based on Medicare Part D and US State Cancer Registry Data. Pharmacoepidemiol Drug Saf. 2020;29:1616–1626. 10.1002/pds.5103
Funding information This study was performed under a research contract between RTI Health Solutions and Eli Lilly and Company and was funded by Eli Lilly and Company.
REFERENCES
- 1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520‐2526. 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1‐34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434‐1441. 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 3. Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1‐34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312‐321. [DOI] [PubMed] [Google Scholar]
- 4. Miller PD. Safety of parathyroid hormone for the treatment of osteoporosis. Curr Osteoporos Rep. 2008;6(1):12‐16. [DOI] [PubMed] [Google Scholar]
- 5. Eli Lilly and Company . Forteo [package insert]. Revised 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021318s012lbl.pdf (Accessed May 16, 2016).
- 6. Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27(12):2429‐2437. 10.1002/jbmr.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kellier N, Krohn K, Masica D, Gilsenan AW, Harding AW, Andrews EB. Teriparatide Voluntary Patient Registry: 4‐Year Progress on a Prospective Osteosarcoma Surveillance Study. Presented at the International Osteoporosis Foundation (IOF) ISCD Skeletal Health Annual Meeting; February. Orlando, Florida; 2014. [Google Scholar]
- 8. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531‐1543. 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. SEER . SEER*Stat software version 8.1.5. Data: SEER*Stat Database: Incidence ‐ SEER 18 Regs. Limited‐Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000–2010) <Katrina/Rita Population Adjustment> − Linked To County Attributes ‐ Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics. Released April 2013, based on the November 2012 submission. Surveillance, Epidemiology, and End Results Program, 2013. https://seer.cancer.gov/seerstat/
- 10. Fletcher CDM, Unni K, Mertens F, editors. Pathology and Genetics: Tumours of Soft Tissue and Bone. Lyon: IARC Press, 2002. http://apps.who.int/bookorders/anglais/detart1.jsp?sesslan=1&codlan=1&codcol=70&codcch=5 (Accessed March 27, 2014). [Google Scholar]
- 11. Unni KK, Dahlin DC. Dahlin's Bone Tumor: General Aspects and Data on 11,087 Cases. 5th ed Philadelphia: Lippincott‐Raven, 1996. [Google Scholar]
- 12. Grimer RJ, Cannon SR, Taminiau AM, et al. Osteosarcoma over the age of forty. Eur J Cancer. 2003;39(2):157‐163. DOI: S0959804902004781 [pii. [DOI] [PubMed] [Google Scholar]
- 13. Research Data Assistance Center . Strengths and limitations of CMS administrative data in research. 2013. http://www.resdac.org/resconnect/articles/156 (Accessed May 16, 2016).
- 14. Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011;2011:548151 DOI: 10.1155/2011/548151, 1, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. SEER . SEER*Stat software version 8.3.4. Data: SEER*Stat Database: NPCR and SEER Incidence ‐ Public Use Data ‐ 2001‐2014 ‐ jbk 062817. Surveillance, Epidemiology, and End Results Program and National Program of Cancer Registries, 2017. https://seer.cancer.gov/seerstat/
- 16. Midkiff K. Forteo/Forsteo Post‐Approval Osteosarcoma Surveillance Study (Study B3D‐MC‐GHBX). 2014. http://www.encepp.eu/encepp/openAttachment/studyResult/8539 (Accessed September 20, 2017). [Google Scholar]
- 17. Gilsenan A, Harding A, Kellier‐Steele N, Harris D, Midkiff K, Andrews E. The Forteo patient registry linkage to multiple state cancer registries: study design and results from the first 8 years. Osteoporos Int. 2018;29(10):2335‐2343. 10.1007/s00198-018-4604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooper C, Harvey NC, Dennison EM, van Staa TP. Update on the epidemiology of Paget's disease of bone. J Bone Miner Res. 2006;21(Suppl 2):P3‐P8. 10.1359/jbmr.06s201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


