Summary
Sleep apnoea is associated with negative outcomes following general anaesthesia. Current recommendations suggest using short‐acting anaesthetic agents in preference to standard agents to reduce this risk, but there is currently no evidence to support this. This randomised controlled triple‐blind trial tested the hypothesis that a combination of short‐acting agents (desflurane‐remifentanil) would reduce the postoperative impact of general anaesthesia on sleep apnoea severity compared with standard agents (sevoflurane‐fentanyl). Sixty patients undergoing hip arthroplasty under general anaesthesia were randomised to anaesthesia with desflurane‐remifentanil or sevoflurane‐fentanyl. Respiratory polygraphy was performed before surgery and on the first and third postoperative nights. The primary outcome was the supine apnoea‐hypopnoea index on the first postoperative night. Secondary outcomes were the supine apnoea‐hypopnoea index on the third postoperative night, and the oxygen desaturation index on the first and third postoperative nights. Additional outcomes included intravenous morphine equivalent consumption and pain scores on postoperative days 1, 2 and 3. Pre‐operative sleep study data were similar between groups. Mean (95%CI) values for the supine apnoea‐hypopnoea index on the first postoperative night were 18.9 (12.7–25.0) and 21.4 (14.2–28.7) events.h−1, respectively, in the short‐acting and standard anaesthesia groups (p = 0.64). Corresponding values on the third postoperative night were 28.1 (15.8–40.3) and 38.0 (18.3–57.6) events.h−1 (p = 0.34). Secondary sleep‐ and pain‐related outcomes were generally similar in the two groups. In conclusion, short‐acting anaesthetic agents did not reduce the impact of general anaesthesia on sleep apnoea severity compared with standard agents. These data should prompt an update of current recommendations.
Keywords: anaesthesia, hip arthroplasty, perioperative medicine, sleep apnoea
Introduction
Obstructive sleep apnoea (OSA) is characterised by intermittent and recurrent episodes of apnoea due to partial or complete obstruction of the upper airway during sleep following a reduction in pharyngeal muscle tone [1]. The prevalence of sleep apnoea is high; 49% of men and 23% of women aged > 40 years in a Swiss population‐based cohort [2], and the condition is an important public health issue due to its association with hypertension [3]; metabolic syndrome [4]; acute coronary syndrome [5]; stroke [6]; and mortality [7, 8].
In patients with OSA, volatile anaesthetics and opioids increase the incidence of upper airway obstruction secondary to a reduction in pharyngeal muscle tone [9]. In addition, opioids aggravate the risk of prolonged apnoea by decreasing central respiratory drive [10]. Therefore, patients with OSA are at an increased risk of developing respiratory and cardiovascular complications after general anaesthesia [11, 12, 13].
Current recommendations for anaesthetic management of patients with OSA suggest that short‐acting agents such as desflurane and remifentanil should be used for general anaesthesia [14, 15]. These agents have been shown to be associated with an improved recovery profile, oxygen saturation and respiratory rate 2 h after surgery compared with sevoflurane or alfentanil in patients without OSA [16, 17]. Furthermore, morbidly obese patients who received general anaesthesia with desflurane had earlier extubation times, earlier verbal contact, and were more awake on arrival at the recovery area, compared with morbidly obese patients who received sevoflurane [18]. However, there is still uncertainty about the benefits of short‐acting anaesthetic agents in patients with OSA because they have not yet been compared with standard agents in a randomised controlled clinical trial.
This study was designed to test the hypothesis that a combination of desflurane and remifentanil (short‐acting agents) would reduce the impact of general anaesthesia on postoperative OSA severity compared with a combination of sevoflurane and fentanyl (standard agents).
Methods
This randomised, triple‐blind, parallel‐group trial was conducted at the University Hospital of Lausanne between February 2016 and May 2018. Randomisation was undertaken using a computer‐generated randomisation table in aggregates of 10. Assignments were concealed in a sealed opaque envelope. The patients, nursing staff, research team, the sleep technician and the sleep physician were not aware of treatment allocation. The trial was sponsored by the Swiss National Science Foundation. The study was approved by the hospital ethical committee and all patients provided written informed consent.
Patients were eligible for participation in the study if they were aged between 18 and 85 years and scheduled to undergo hip arthroplasty. Exclusion criteria included: treatment of sleep apnoea with continuous positive airway pressure; presence of severe respiratory or cardiovascular disease; malignant hyperthermia; pre‐operative consumption of benzodiazepines; chronic use of opioids at a dosage of 30 mg.day−1 or more morphine equivalents; and pregnancy. On the day of surgery, patients were randomised to general anaesthesia with desflurane and remifentanil (short‐acting group), or sevoflurane and fentanyl (standard group).
Anaesthesia was induced using intravenous propofol 1.5–2.0 mg.kg−1 and either remifentanil 0.5 µg.kg−1 (short‐acting) or fentanyl 1–2 μg.kg−1 (standard), with tracheal intubation facilitated by intravenous rocuronium 0.6 mg.kg−1. Anaesthesia was maintained using desflurane (short‐acting) or sevoflurane (standard) in an air‐oxygen mixture at a concentration of 0.8–1.2 minimum alveolar concentration (MAC) to achieve a bispectral index (Aspect Medical Systems, Norwood, MA, USA) of between 40 and 60. Analgesia to manage increases in heart rate or blood pressure of more than 20% above pre‐operative values was provided with an infusion of remifentanil 0.1 µg.kg−1.min−1 (short‐acting) or 25 µg bolus doses of fentanyl (standard) [19]. Positive pressure ventilation was initiated, and tidal volume and rate adjusted to maintain EtCO2 between 4.7 and 5.3 kPa.
After prosthesis implantation, the surgical site was infiltrated with 50 ml of ropivacaine 0.2%. As per routine institutional practice, at the end of surgery all patients received intravenous acetaminophen 1000 mg and intravenous ketorolac 30 mg for multimodal analgesia and intravenous ondansetron 4 mg for anti‐emetic prophylaxis [20, 21]. In case of residual neuromuscular blockade (defined as a train‐of‐four ratio < 0.9), muscle relaxation was antagonised with neostigmine 50 μg.kg−1 and glycopyrrolate 5–10 μg.kg−1 [22]. In Phase 1 recovery, pain was assessed on a visual analogue scale with a range of 0–10. A score of 4 or more or patient request for analgesia was managed with morphine 2 mg every 10 min as needed. After resumption of oral intake, patients received acetaminophen 1000 mg every 6 h, ibuprofen 400 mg every 6 h, and oxycodone 5 mg every 3 h as needed. Ongoing anti‐emetic medication included intravenous ondansetron 4 mg as needed. Patients received oxygen at a rate of 2–4 l.min−1 in Phase 1 recovery, but not after transfer to the ward.
Sleep‐related parameters and outcomes were measured on the night before surgery (pre‐operative baseline) and on the first and third nights after surgery using a portable respiratory polygraph recorder (Embletta®, ResMed, San Diego, CA, USA). This system, previously validated against polysomnography [23], allows non‐invasive recording of nasal airflow via nasal cannula, oxygen saturation using finger pulse oximetry, respiratory efforts using thoracic and abdominal belts, and body position. All recordings were scored by a specialised sleep technician, supervised and reviewed by a sleep specialist, and both were unaware of treatment allocation. Apnoea was defined as breathing cessation lasting for 10 s or more, and hypopnoea was defined as a fall of 30% or more in the respiratory flow signal associated with a 3% or greater drop in oxygen saturation. The apnoea‐hypopnoea index (AHI) was defined as the number of apnoeic and hypopnoeic events per hour of recording time, and the oxygen desaturation index reflected the number of oxygen desaturations of 3% or more per hour of recording time. Central apnoea was defined as the presence of > 50% of events without abdominal or thoracic movements.
The primary outcome was the AHI in the supine position on the first postoperative night. Secondary sleep‐related outcomes were: supine AHI on the third postoperative night; AHI; obstructive apnoea index; mixed apnoea index; central apnoea index; hypopnoea index; oxygen desaturation index; percentage of recording time with oxygen saturation below 90%; and proportion of time in the supine position on the first and third postoperative nights. Secondary pain‐related outcomes were: intravenous morphine AHI equivalent consumption in the recovery area and on postoperative days 1, 2 and 3; pain scores at rest on arrival and at departure and on postoperative days 1, 2 and 3; rates of postoperative nausea and vomiting or pruritus on postoperative days 1, 2 and 3; and satisfaction score (rated on a visual analogue scale from 0 to 10).
It was calculated that 22 patients were required in each treatment group to detect a difference in supine AHI of 5 events.h−1 with a standard deviation of 5, 90% power and a two‐sided alpha error of 0.05. Based on an estimated drop‐out rate of 30%, the recruitment target was set at 60 subjects (30 per group). A between‐group difference in AHI of 5 events.h−1 was chosen because this has previously been defined as clinically relevant [3, 24].
Statistical analysis was performed using SPSS Statistics for Windows Version 25.0 (IBM, Armonk, NY, USA). Categorical data were summarised as rates and continuous data were summarised as mean with 95%CI. Categorical data were compared using the Fisher's exact test or Pearson Chi‐square test with Yates' correction as appropriate. Continuous independent variables were analysed using general linear models, while categorical and continuous repeated measurements were analysed using generalised estimating equations according to time, anaesthesia group, and interaction between time and anaesthesia effects. When more than one distribution fitted the model, the best was chosen based on the lowest quasi‐likelihood under independence model criterion for generalised estimating equations and lowest Akaike information criterion for general linear models.
Briefly, generalised estimating equations are an extension of general linear models to longitudinal or clustered data, where observations are no longer independent. The aim was to extend the general linear models estimating equations to the multivariate setting by replacing the vector of responses and the vector of means by their corresponding multivariate counterparts and using a matrix of weights. Generalised estimating equations take into account the dependence of observations by specifying a working correlation matrix [25]. This increases the efficiency of the estimators of the parameters compared with those arising under the assumption that repeated observations from a subject are independent of one another, as long as this assumption is true and the resulting estimators remain consistent in the absence of missing data [26]. This method uses all the available information without excluding any individual, even if they are missing data at some time‐points.
To assess the generalisability of our findings, sensitivity analyses were performed on sub‐groups of patients with OSA (AHI ≥ 5 events.h−1 or ≥ 15 event.h−1) or at high risk of OSA (NoSAS score ≥ 8, STOP‐BANG score ≥ 3, or Berlin score ≥ 2). Multiple comparisons (for time or interaction effects) were performed using Bonferroni's post‐hoc test.
Results
Sixty patients were recruited and 49 completed the study for the primary outcome (Fig. 1). Twelve and 15 patients did not perform the last polygraphy in the standard and short‐acting groups respectively (p = 0.48). Patient characteristics were similar in the two treatment groups (Table 1) and between those who did vs. did not complete the last polygraphy (online Supporting Information, Table S1). Only one patient in the standard agents group had central apnoea (58% of all apnoea events were not accompanied by thoracic or abdominal effort).
Figure 1.
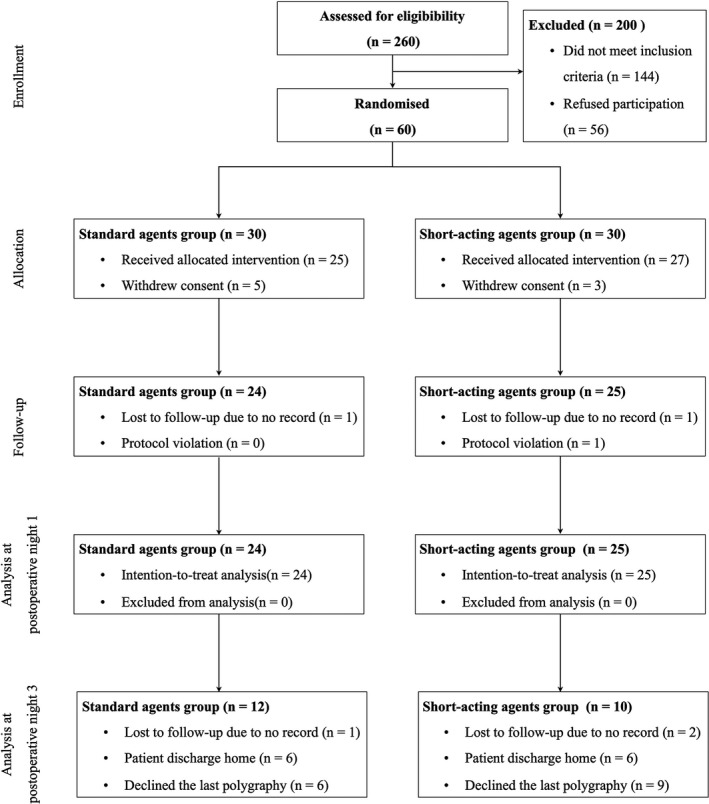
Study flow diagram.
Table 1.
Baseline and clinical characteristics of patients. Values are mean (SD), median (IQR [range]) or number (proportion) as appropriate.
| Characteristic |
Standard agents n = 24 |
Short‐acting agents n = 25 |
|---|---|---|
| Age; y | 64 (15) | 66 (10) |
| Men; n | 17 (71%) | 16 (64%) |
| Weight; kg | 81 (71–93 [58–130]) | 82 (69–90 [62–135]) |
| Height; cm | 174 (9) | 170 (9) |
| BMI; kg.m−2 | 27.1 (24.3–29.6 [19.5–41.0]) | 27.0 (24.6–31.0 [22.1–43.9]) |
| ESS score ≥ 11 | 4 (17%) | 4 (17%) |
| ASA physical status | ||
| 1 | 6 (25%)* | 0 |
| 2 | 12 (50%) | 22 (88%)* |
| 3 | 6 (25%) | 3 (12%) |
| Duration of surgery; min | 132 (106–162 [80–360]) | 136 (112–165 [76–252]) |
| Hip arthroplasty | ||
| Primary | 12 (50%) | 11 (44%) |
| Secondary | 12 (50%) | 14 (56%) |
| Comorbidities | ||
| Coronary artery disease | 0 | 2 (8%) |
| Hypertension | 10 (42%) | 10 (40%) |
| Renal failure | 0 | 2 (8%) |
| Diabetes | 1 (4%) | 1 (4%) |
| Hyperlipidaemia | 2 (8%) | 3 (12%) |
| Sleep apnoea scores | ||
| NoSAS score ≥ 8 | 16 (67%) | 19 (79%) |
| STOP‐BANG score ≥ 3 | 22 (92%) | 20 (83%) |
| Berlin score ≥ 2 | 13 (54%) | 10 (42%) |
| Pre‐operative AHI | ||
| < 5 events.h−1 | 4 (17%) | 3 (12%) |
| 5–14.9 events.h−1 | 10 (42%) | 9 (36%) |
| 15–29.9 events.h−1 | 6 (25%) | 9 (36%) |
| ≥ 30 events.h−1 | 4 (17%) | 4 (16%) |
AHI, apnoea‐hypopnoea index; ESS, Epworth Sleepiness Scale.
Observed frequency significantly different from overall frequency (adjusted residual > |2|).
The supine AHI did not differ significantly between the short‐acting and standard groups at baseline, or on the first or third night after surgery (Fig. 2). The other secondary sleep‐related outcomes were comparable between groups (Table 2). Changes from pre‐operative baseline to the first postoperative night were similar in those who did vs. did not complete the final polygraphy (online Supporting Information, Table S2).
Figure 2.
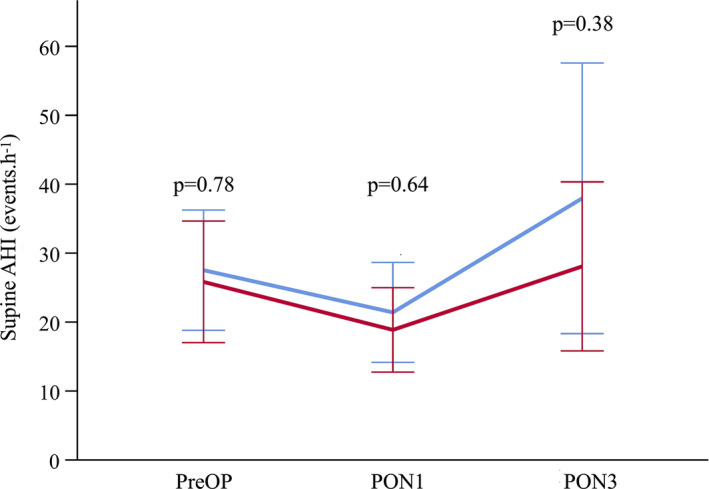
Change in the apnoea‐hypopnoea index (AHI) in the supine position over time (values are shown as mean with 95%CI). PreOP, pre‐operative; PON1, postoperative night 1; PON3, postoperative night 3. Blue line, standard agents; red line, short‐acting agents.
Table 2.
Sleep study data. Values are mean (95%CI).
| Standard agents | Short‐acting agents | p value | |
|---|---|---|---|
| Pre‐operative baseline | (n = 24) | (n = 25) | |
| AHI; events.h−1 | 17.6 (11.0–24.1) | 19.4 (11.7–27.1) | 0.68 |
| OAI; events.h−1 | 4.1 (1.2–7.0) | 4.7 (0–9.4) | 0.68 |
| CAI; events.h−1 | 1.5 (0.1–2.9) | 2.6 (1.3–3.9) | 0.12 |
| MAI; events.h−1 | 8.3 (0.5–16.0) | 10.8 (1.2–20.5) | 0.42 |
| HI; events.h−1 | 10.8 (7.0–14.7) | 10.5 (7.3–13.7) | 0.86 |
| ODI; events.h−1 | 21.0 (13.6–28.3) | 22.9 (15.7–30.1) | 0.67 |
| Mean SpO2; % | 92.2 (91.0–93.3) | 92.8 (92.1–93.5) | 0.35 |
| Time with SpO2 < 90%; % | 16.1 (4.5–27.6) | 8.7 (2.1–15.3) | 0.09 |
| Supine time; % | 52.6 (39.8–65.4) | 53.5 (41.7–65.4) | 0.92 |
| Postoperative night 1 | (n = 24) | (n = 25) | |
| AHI; events.h−1 | 20.7 (13.4–28.0) | 18.8 (12.7–25.0) | 0.73 |
| OAI; events.h−1 | 4.4 (−1.1–9.9) | 1.9 (−0.3–4.1) | 0.06 |
| CAI; events.h−1 | 2.1 (−0.2–4.4) | 1.7 (0.6–2.8) | 0.56 |
| MAI; events.h−1 | 5.4 (1.7–9.0) | 2.9 (−0.8–6.7) | 0.37 |
| HI; events.h−1 | 13.4 (8.7–18.1) | 14.7 (10.2–19.1) | 0.67 |
| ODI; events.h−1 | 29.0 (19.8–381) | 28.0 (20.3–35.7) | 0.89 |
| Mean SpO2; % | 91.5 (90.4–92.7) | 91.1 (89.6–92.5) | 0.60 |
| Time with SpO2 < 90%; % | 24.6 (11.6–37.5) | 25.3 (10.1–40.5) | 0.93 |
| Supine time; % | 93.7 (88.0–99.3) | 98.4 (95.2–101.7) | 0.16 |
| Postoperative night 3 | (n = 12) | (n = 10) | |
| AHI; events.h−1 | 37.2 (17.8–56.6) | 28.1 (15.8–40.3) | 0.41 |
| OAI; events.h−1 | 15.2 (−0.1–30.5) | 7.9 (0.3–15.6) | 0.16 |
| CAI; events.h−1 | 4.5 (0.7–8.4) | 2.7 (0.2–5.2) | 0.22 |
| MAI; events.h−1 | 28.3 (4.5–52.2) | 7.8 (−1.3–16.9) | 0.05 |
| HI; events.h−1 | 13.6 (6.0–21.2) | 16.2 (8.5–23.9) | 0.58 |
| ODI; events.h−1 | 41.3 (20.0–62.6) | 34.5 (20.9–48.1) | 0.59 |
| Mean SpO2; % | 91.5 (89.7–93.3) | 93.1 (91.3–95.0) | 0.14 |
| Time with SpO2 < 90%; % | 19.7 (2.1–37.3) | 14.1 (−3.4–31.5) | 0.51 |
| Supine time; % | 97.7 (94.8–100.6) | 100.0 (100.0–100.0) | 0.10 |
AHI, apnoea‐hypopnoea index; CAI, central apnoea index; HI, hypopnoea index; MAI, mixed apnoea index; OAI, obstructive apnoea index; ODI, oxygen desaturation index.
The generalised estimating equations model did not show any significant interaction (p = 0.63) or group effect (p = 0.43), whereas a time effect was present (p < 0.0001) when analysing the three nights in all patients (Table 3). The supine AHI was significantly lower on postoperative night 1 compared with the pre‐operative baseline (p = 0.03) and postoperative night 3 (p = 0.003). There was no significant difference between pre‐operative baseline and the third postoperative night (p = 0.41) (Table 3).
Table 3.
Data from the generalised estimating equations model for secondary sleep‐related outcomes. Values are mean (95%CI).
|
Pre‐operative night n = 49 |
Postoperative night 1 n = 49 |
Postoperative night 3 n = 22 |
p value | |
|---|---|---|---|---|
| Supine AHI; events.h−1 | 26.7 (21.5–33.1) | 20.1 (16.2–25.0) ‡ | 32.6 (24.6–43.4) ¶ | < 0.01 |
| AHI; events.h−1 | 18.5 (14.4–23.8) | 19.7 (15.8–24.7) | 32.3 (24.3–43.0) ‡¶ | < 0.01 |
| OAI; events.h−1 | 4.4 (2.5–7.7) | 2.9 (1.3–6.4) | 11.0 (6.1–19.7) ¶ | < 0.01 |
| CAI; events.h−1 | 2.0 (1.2–3.2) | 1.9 (1.1–3.4) | 3.5 (2.1–5.9) | < 0.01 |
| MAI; events.h−1 | 1.4 (0.4–0.8) | 0.7 (0.3–1.4) | 2.2 (1.2–4.1) ¶ | < 0.01 |
| HI; events.h−1 | 10.6 (8.6–13.2) | 14.0 (11.3–17.4) | 14.9 (10.9–20.2) | 0.09 |
| ODI; events.h−1 | 21.9 (17.6–27.2) | 28.5 (23.5–34.6) ‡ | 37.7 (28.7–49.6) ‡ | < 0.01 |
| Mean SpO2; % | 92.5 (91.8–93.1) | 91.3 (90.4–92.1) ‡ | 92.3 (91.3–93.4) | 0.01 |
| Time with SpO2 < 90%; % | 11.8 (7.3–19.2) | 24.9 (17.2–36.2) ‡ | 16.7 (8.8–31.5) | < 0.01 |
| Supine time; % | 53.1 (96.0–93.0) | 96.0 (93.0–99.1) ‡ | 98.8 (97.6–100.1) ‡ | < 0.01 |
AHI, apnoea‐hypopnoea index; CAI, central apnoea index; HI, hypopnoea index; MAI, mixed apnoea index; OAI, obstructive apnoea index; ODI, oxygen desaturation index.
Indicating a time effect.
p < 0.05 compared to pre‐operative night.
p < 0.05 compared to postoperative night 1.
Overall, the prevalence of severe OSA (defined as an AHI > 30 events.h−1) on the third postoperative night was significantly higher than on the pre‐operative night (OR 7.00, 95%CI 2.07–23.60; p < 0.0001). The prevalence of severe OSA on the first postoperative night did not differ significantly from the pre‐operative baseline (OR 2.50, 95%CI 0.86–7.31); p = 0.09). Sensitivity analyses showed that the study findings were consistent across patient sub‐groups based on OSA definition and in patients at high risk of OSA.
Pain scores were similar between groups during the course of the study (online Supporting Information, Figure S1). Other pain‐related outcomes did not differ significantly between treatment groups (Table 4), apart from intravenous morphine equivalent consumption in the recovery area, which was significantly higher in the short‐acting group (Table 4).
Table 4.
Acute pain‐related outcomes. Data are mean (95%CI) or number (proportion).
| Standard agents | Short‐acting agents | p value | |
|---|---|---|---|
| Recovery area | |||
| i.v. morphine equivalent consumption; mg | 7 (4–10) | 13 (10–16) | 0.02 |
| Postoperative day 1 | |||
| i.v. morphine equivalent consumption; mg | 6 (0–11) | 4 (2–6) | 0.51 |
| PONV | 2/23 (9%) | 1/23 (4%) | 1.00 |
| Pruritus | 0/23 | 0/23 | – |
| Postoperative day 2 | |||
| i.v. morphine equivalent consumption; mg | 12 (2–23) | 8 (4–12) | 0.30 |
| PONV | 4/24 (17%) | 2/25 (8%) | 0.42 |
| Pruritus | 1/24 (4%) | 0/25 | 0.49 |
| Postoperative day 3 | |||
| i.v. morphine equivalent consumption; mg | 10 (3–18) | 6 (3–9) | 0.19 |
| PONV | 6/24 (25%) | 2/23 (9%) | 0.25 |
| Pruritus | 0/24 | 2/23 (9%) | 0.23 |
| VAS satisfaction score | 9.0 (8.6–9.4) | 9.4 (9.0–9.7) | 0.82 |
i.v., intravenous; PONV, postoperative nausea and vomiting; VAS, visual analogue scale score (from 0–10).
Discussion
The results of this randomised controlled trial suggest that short‐acting anaesthetic agents do not reduce the impact of general anaesthesia on OSA severity on the first and third postoperative nights, compared with standard agents. In that context, given that both desflurane and remifentanil are expensive agents [27], desflurane releases more carbon dioxide into the atmosphere than other inhaled anaesthetics [28] and that remifentanil is associated with secondary hyperalgesia [29], we suggest that current recommendations for the use of short‐acting anaesthetics in patients with OSA should probably be revised. Furthermore, our results do not support existing recommendations that encourage monitoring of patients on the first postoperative night only [14, 15]. Our data suggest that monitoring should be continued up to at least the third postoperative night. However, this may not be feasible given the expansion of ambulatory surgery and overall reductions in length of hospital stay. Therefore, a temporary prescription for continuous positive airway pressure therapy or a mandibular advancement device might represent satisfactory and cost‐effective alternative measures for postoperative management of patients with OSA [30].
There are a number of potential explanations for the lack of difference seen between short‐acting and standard anaesthetic agents on OSA severity during the first postoperative night in our study. Firstly, although desflurane has lower blood: gas and oil: gas partition coefficients than sevoflurane, reducing absorption from the fat compartment and resulting in quicker induction and emergence times, the improved recovery profile does not appear to extend beyond a period of 15–30 min [18]. Therefore, differences in pharmacodynamics between these agents may be too small to have an impact on postoperative OSA severity. Secondly, administration of intravenous morphine postoperatively could have neutralised any potential beneficial effects of the short‐acting agents. Indeed, patients in the short‐acting group actually received more morphine in the recovery area (mean difference of 6 mg). This phenomenon, known as secondary hyperalgesia, is well known after remifentanil administration and primarily occurs during the first two postoperative hours [29, 31]. However, intravenous administration of long‐acting opioids after a painful surgical procedure is consistent with standard postoperative pain management [32, 33] and mirrors the daily practice of the recovery area.
The decrease in supine AHI on the first postoperative night compared with the pre‐operative baseline seen in our study might be due to greater time awake (which artificially decreases the AHI on polygraphy recordings) or a decreased proportion of rapid eye movement sleep [34] (because respiratory events occur predominantly during this phase). Subsequently, apnoeic and hypopnoeic events, and the oxygen desaturation index all increased on the third postoperative night compared with previous recordings, potentially due to a rebound in the amount of rapid eye movement sleep, as reported previously [35]. Unfortunately, we cannot confirm this because sleep stage data are not available from polygraphy. However, polygraphy data from 38 sleep apnoea patients showed that time in rapid eye movement sleep was lowest on the first postoperative night and increased up to the fifth postoperative night [34]. Therefore, it is possible that the negative impact of general anaesthesia on OSA severity could increase further after the third postoperative night.
The pre‐operative AHI values of 17.6 and 19.4 events.h−1 in the two groups in our study might seem high for unselected patients, but are in line with the results of a recent meta‐analysis showing that the mean (95%CI) AHI was 15.5 (12.9–18.2) events.h−1 in a general population aged 65–79 years [36]. Moreover, rates of sleep apnoea in our study (85% with an AHI of 5 events.h−1 or more) and those reported previously [12, 13] suggest that OSA is highly prevalent in pre‐operative populations. Therefore, implementation of protocols to manage OSA patients undergoing surgery would place a significant burden on anaesthetic departments. Thus, recommendations based on evidence rather than expert opinion are essential. Furthermore, current inclusive respiratory event definitions and highly sensitive nasal pressure sensors may artificially elevate the AHI, as previously suggested by our group [37]. On that basis and given that some older studies were performed between 2006 and 2010 [12], we also wonder whether the current AHI threshold should be revised, as previously suggested [38].
Further research is needed to determine which patients are at increased postoperative risk based on different definitions of OSA. For example, data from a recent prospective study showed that only severe OSA (AHI > 30 events.h−1) was significantly associated with a composite cardiovascular event outcome [13]. Therefore, we believe that not only the recommendations for OSA management during anaesthesia, but also the AHI threshold above which they are implemented should be revised.
Although rigorously designed to minimise bias, our study has some limitations. Polygraphy does not record total sleep time, meaning that differences in sleep quality between recording nights may have contributed to the AHI variations observed. We were aware of this limitation but elected not to perform standard polysomnography with an electroencephalogram because this would create more distress for patients already under stress in a peri‐operative setting. Nevertheless, the polygraphy system we used has been validated against polysomnography with a high sensitivity and specificity [23], and therefore we do not believe this represents a major limitation. In addition, early discharge or withdrawal of consent reduced the number of patients participating in the final assessment, although total numbers were still sufficient based on sample size calculation. Nevertheless, secondary sleep outcomes should be considered as exploratory, requiring further investigation. Finally, registration of the study was performed after the inclusion of the first two patients, but we do not believe that this short delay impacted the validity of our results.
In conclusion, short‐acting agents do not appear to reduce the impact of general anaesthesia on OSA severity compared with standard agents, suggesting that current recommendations for use of these agents in patients with OSA should be revised.
Supporting information
Figure S1. Change in pain score over time (values are shown as mean with 95%CI). The generalised estimating equations model showed that there was no significant interaction (p = 0.15), group effect (p = 0.43) or time effect (p = 0.37). RA, Recovery are; POD, postoperative day.
Table S1. Baseline and clinical characteristics for patients who completed the study vs. those who did not have the final polygraphy. Data are mean (SD), median (IQR [range]) or number (proportion) as appropriate.
Table S2. Differences in sleep study data for postoperative night one vs. baseline in patients who completed the study vs. those who did not have the final polygraphy. Data are mean (95%CI).
Acknowledgements
This work was supported by the Swiss National Science Foundation (353,408 CHF, grant number 32003B_169974/1). English language editing assistance was provided by N. Ryan, an independent medical writer. EA has received grants from the Swiss Academy for Anaesthesia Research, Lausanne, Switzerland, and from B. Braun Medical AG. EA has also received honoraria from B. Braun Medical AG and Sintetica Ltd UK. No other external funding or competing interests declared.
References
- 1. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383: 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinzer R, Vat S, Marques‐Vidal P, et al. Prevalence of sleep‐disordered breathing in the general population: the HypnoLaus study. Lancet Respiratory Medicine 2015; 3: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. New England Journal of Medicine 2000; 342: 1378–84. [DOI] [PubMed] [Google Scholar]
- 4. Hirotsu C, Haba‐Rubio J, Togeiro SM, et al. Obstructive sleep apnoea as a risk factor for incident metabolic syndrome: a joined Episono and HypnoLaus prospective cohorts study. European Respiratory Journal 2018; 52: 1801150. [DOI] [PubMed] [Google Scholar]
- 5. Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep 2015; 38: 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New England Journal of Medicine 2005; 353: 2034–41. [DOI] [PubMed] [Google Scholar]
- 7. Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long‐term adverse outcomes: a systematic review. Sleep Medicine Reviews 2014; 18: 49–59. [DOI] [PubMed] [Google Scholar]
- 8. Lee JE, Lee CH, Lee SJ, et al. Mortality of patients with obstructive sleep apnea in Korea. Journal of Clinical Sleep Medicine 2013; 9: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. Journal of Clinical Sleep Medicine 2012; 8: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Loadsman JA, Hillman DR. Anaesthesia and sleep apnoea. British Journal of Anaesthesia 2001; 86: 254–66. [DOI] [PubMed] [Google Scholar]
- 11. Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta‐analysis of the association between obstructive sleep apnoea and postoperative outcome. British Journal of Anaesthesia 2012; 109: 897–906. [DOI] [PubMed] [Google Scholar]
- 12. Memtsoudis SG, Stundner O, Rasul R, et al. The impact of sleep apnea on postoperative utilization of resources and adverse outcomes. Anesthesia and Analgesia 2014; 118: 407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan MTV, Wang CY, Seet E, et al. Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery. Journal of the American Medical Association 2019; 321: 1788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seet E, Chung F. Management of sleep apnea in adults – functional algorithms for the perioperative period: continuing professional development. Canadian Journal of Anesthesia 2010; 57: 849–64. [DOI] [PubMed] [Google Scholar]
- 15. Dawson D, Singh M, Chung F. The importance of obstructive sleep apnoea management in peri‐operative medicine. Anaesthesia 2016; 71: 251–6. [DOI] [PubMed] [Google Scholar]
- 16. Strum EM, Szenohradszki J, Kaufman WA, Anthone GJ, Manz IL, Lumb PD. Emergence and recovery characteristics of desflurane versus sevoflurane in morbidly obese adult surgical patients: a prospective, randomized study. Anesthesia and Analgesia 2004; 99:1848–53. [DOI] [PubMed] [Google Scholar]
- 17. Wuesten R, Van Aken H, Glass PS, Buerkle H. Assessment of depth of anesthesia and postoperative respiratory recovery after remifentanil‐ versus alfentanil‐based total intravenous anesthesia in patients undergoing ear‐nose‐throat surgery. Anesthesiology 2001; 94: 211–7. [DOI] [PubMed] [Google Scholar]
- 18. Singh PM, Borle A, McGavin J, Trikha A, Sinha A. Comparison of the recovery profile between desflurane and sevoflurane in patients undergoing bariatric surgery‐a meta‐analysis of randomized controlled trials. Obesity Surgery 2017; 27: 3031–9. [DOI] [PubMed] [Google Scholar]
- 19. Chan SM, Lee MS, Lu CH, et al. Confounding factors to predict the awakening effect‐site concentration of propofol in target‐controlled infusion based on propofol and fentanyl anesthesia. PLoS One 2015; 10: e0124343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al Ja'bari A, Robertson M, El‐Boghdadly K, Albrecht E. A randomised controlled trial of the pectoral nerves‐2 (PECS‐2) block for radical mastectomy. Anaesthesia 2019; 74: 1277–81. [DOI] [PubMed] [Google Scholar]
- 21. Palhais N, Brull R, Kern C, et al. Extrafascial injection for interscalene brachial plexus block reduces respiratory complications compared with a conventional intrafascial injection: a randomized, controlled, double‐blind trial. British Journal of Anaesthesia 2016; 116: 531–7. [DOI] [PubMed] [Google Scholar]
- 22. Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesthesia and Analgesia 2010; 111: 120–8. [DOI] [PubMed] [Google Scholar]
- 23. Ng SS, Chan TO, To KW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS). Respirology 2010; 15: 336–42. [DOI] [PubMed] [Google Scholar]
- 24. Kim J, Tran K, Seal K, et al. Interventions for the treatment of obstructive sleep apnea in adults: a health technology assessment. Canadian Agency for Drugs and Technologies Health. 2017. https://www.cadth.ca/dv/interventions‐treatment‐obstructive‐sleep‐apnea‐adults‐health‐technology‐assessment (accessed 20/07/2020) [PubMed]
- 25. Verbeke G, Fieuws S, Molenberghs G, Davidian M. The analysis of multivariate longitudinal data: a review. Statistical Methods in Medical Research 2014; 23: 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44: 1049–60. [PubMed] [Google Scholar]
- 27. Tabing AK, Ehrenfeld JM, Wanderer JP. Limiting the accessibility of cost‐prohibitive drugs reduces overall anesthetic drug costs: a retrospective before and after analysis. Canadian Journal of Anesthesia 2015; 62: 1045–54. [DOI] [PubMed] [Google Scholar]
- 28. Vollmer MK, Rhee TS, Rigby M, et al. Modern inhalation anesthetics: potent greenhouse gases in the global atmosphere. Geophysical Research Letter 2015; 42: 1606–11. [Google Scholar]
- 29. Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra‐operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta‐analysis with trial sequential analysis. Anaesthesia 2019; 74: 793–800. [DOI] [PubMed] [Google Scholar]
- 30. Mutter TC, Chateau D, Moffatt M, Ramsey C, Roos LL, Kryger M. A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology 2014; 121: 707–18. [DOI] [PubMed] [Google Scholar]
- 31. Frauenknecht J, Kirkham KR, Jacot‐Guillarmod A, Albrecht E. Analgesic impact of intra‐operative opioids vs. opioid‐free anaesthesia: a systematic review and meta‐analysis. Anaesthesia 2019; 74: 651–62. [DOI] [PubMed] [Google Scholar]
- 32. Stebler K, Martin R, Kirkham KR, Lambert J, De Sede A, Albrecht E. Adductor canal block versus local infiltration analgesia for postoperative pain after anterior cruciate ligament reconstruction: a single centre randomised controlled triple‐blinded trial. British Journal of Anaesthesia 2019; 123: e343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albrecht E, Bathory I, Fournier N, Jacot‐Guillarmod A, Farron A, Brull R. Reduced hemidiaphragmatic paresis with extrafascial compared with conventional intrafascial tip placement for continuous interscalene brachial plexus block: a randomized, controlled, double‐blind trial. British Journal of Anaesthesia 2017; 118: 586–92. [DOI] [PubMed] [Google Scholar]
- 34. Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W. Postoperative changes in sleep‐disordered breathing and sleep architecture in patients with obstructive sleep apnea. Anesthesiology 2014; 120: 287–98. [DOI] [PubMed] [Google Scholar]
- 35. Chung F, Liao P, Elsaid H, Shapiro CM, Kang W. Factors associated with postoperative exacerbation of sleep‐disordered breathing. Anesthesiology 2014; 120: 299–311. [DOI] [PubMed] [Google Scholar]
- 36. Boulos MI, Jairam T, Kendzerska T, Im J, Mekhael A, Murray BJ. Normal polysomnography parameters in healthy adults: a systematic review and meta‐analysis. Lancet Respiratory Medicine 2019; 7: 533–43. [DOI] [PubMed] [Google Scholar]
- 37. Heinzer R, Marti‐Soler H, Haba‐Rubio J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respiratory Medicine 2016; 4: e5–6. [DOI] [PubMed] [Google Scholar]
- 38. Memtsoudis SG, Besculides MC, Mazumdar M. A rude awakening—the perioperative sleep apnea epidemic. New England Journal of Medicine 2013; 368: 2352–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Change in pain score over time (values are shown as mean with 95%CI). The generalised estimating equations model showed that there was no significant interaction (p = 0.15), group effect (p = 0.43) or time effect (p = 0.37). RA, Recovery are; POD, postoperative day.
Table S1. Baseline and clinical characteristics for patients who completed the study vs. those who did not have the final polygraphy. Data are mean (SD), median (IQR [range]) or number (proportion) as appropriate.
Table S2. Differences in sleep study data for postoperative night one vs. baseline in patients who completed the study vs. those who did not have the final polygraphy. Data are mean (95%CI).


