Abstract
Background
Impaired gastric emptying (GE) is associated with morbidity in surgical critically ill children. The relationship between inflammation, gut barrier integrity (lipopolysaccharide binding protein [LBP]; zonulin), and GE has not been described in this cohort.
Methods
Children ≥2 years of age and requiring critical care after surgery were enrolled. Preoperative and postoperative levels of serum cytokines, LBP, and zonulin, and GE by the acetaminophen absorption test, were measured, allowing patients to serve as their own controls. Postoperative delayed GE was defined as a decrease in GE by ≥20% compared with preoperative GE. The following were examined : comparison between postoperative andpreoperative values, correlations between fold change (postoperative/preoperative) in study variables, and fold change in study variables between patients with and without postoperative delayed GE.
Results
Twenty patients, median age 14 years (12.25, 18), 12 female, were included. Eight of 20 patients had postoperative delayed GE. Postoperative interleukin‐6 (IL‐6), IL‐8, IL‐10, and LBP were increased, and zonulin was decreased (P‐values < .05). Fold change in IL‐10 and zonulin were inversely correlated (ρ −0.618, P = .004). Patients with postoperative delayed GE had greater fold increase in IL‐10 (P = .0159) and fold decrease in zonulin (P = .0160). Five of 7 (71%) patients with both fold increase in IL‐10 and decrease in zonulin had delayed GE.
Conclusion
Postoperative changes in IL‐10 and zonulin were associated with delayed GE in surgical critically ill children, which might suggest a mechanism to for delayed GE in postoperative inflammation and gut barrier dysregulation after surgery.
Keywords: critical care, cytokine, enteral nutrition, epithelial barrier, gastric emptying, gut, inflammation, interleukin‐10, pediatric, zonulin
Clinical Relevancy Statement
Gastrointestinal (GI) dysfunction, which manifests as delayed gastric emptying and gut barrier dysregulation, is prevalent in critically ill patients and has been associated with increased morbidity. Zonulin is a reversible regulator of gut barrier tight junctions and an agonist of the protease‐activated receptor 2. The protease‐activated receptor 2 has been associated with changes in gastric emptying and gut barrier integrity. In a cohort of pediatric patients undergoing complex nonabdominal surgery and requiring critical care, a significant postoperative inflammatory response and gut barrier dysregulation were identified. Forty percent of patients had postoperative delayed gastric emptying, and this subcohort had a significantly greater increase in postoperative interleukin‐10 (IL‐10) and decrease in zonulin when compared with patients without postoperative delayed gastric emptying. This study sheds light onto the contributing factors for GI dysfunction in surgical critical care and will guide subsequent translational research studies. Elucidating the role of IL‐10 and zonulin in gastric emptying may support development of potential diagnostic and therapeutic alternatives for this cohort.
Introduction
Gastrointestinal (GI) dysfunction, which manifests as delayed gastric emptying and changes in gut barrier integrity, is highly prevalent in critically ill patients. 1 , 2 , 3 , 4 , 5 , 6 It has been associated with poor enteral nutrition (EN) delivery, severity of illness, length of stay, and risk of mortality in pediatric and adult critically ill patients. 1 , 2 , 3 , 4 , 7 The mechanisms underlying delayed gastric emptying and alterations in gut barrier integrity, specifically in pediatric critically ill patients, are not well understood.
Zonulin is a reversible regulator of gut barrier tight junctions that has been found to be elevated in the serum of patients with systemic inflammatory conditions such as diabetes, spondyloarthritis, and sepsis. 6 , 8 , 9 , 10 , 11 Zonulin can activate the protease‐activated receptor 2 (PAR2), a G‐couple protein present in the GI tract that has been associated with changes in gastric emptying and gut barrier integrity and is activated under conditions of inflammation. 8 , 12 , 13 , 14 Changes in zonulin, a PAR2 agonist, in the setting of postoperative acute inflammation may play a role in GI dysfunction in children requiring postoperative care in the pediatric intensive care unit (PICU).
In this prospective pilot study, associations between postoperative changes in inflammatory cytokines, markers of gut barrier integrity, gastric emptying, and zonulin were examined in a cohort of patients undergoing posterior spinal fusion. Posterior spinal fusion is an elective and complex surgery associated with postoperative inflammation. 15 , 16 We hypothesized that pediatric patients requiring critical care after major surgery would have an inflammatory response that would be associated with changes in gut barrier integrity and gastric emptying. Studies in animal models support a role for PAR2 in gastric emptying and gut barrier integrity under conditions of inflammation; therefore, an association between zonulin, a PAR2 agonist, and postoperative GI function changes was also examined. 8 , 12 , 13 , 14
Methods
Patient Population
Patients 2 years of age and older scheduled for elective posterior spinal fusion surgery and requiring postoperative care in a multidisciplinary ICU of a quaternary children's hospital between July 2017 and April 2019 were enrolled. Patients with emergent need for posterior spinal fusion, such as trauma or neurologic emergencies, were not eligible for this study, as examining markers prior to the inflammatory trigger would not have been possible. Patients with previously diagnosed medical or surgical GI pathology, including but not limited to dysmotility and chronic malabsorption, were excluded. Patients with contraindications to acetaminophen, those with suspected or confirmed liver or renal dysfunction by laboratory parameters, or those without an arterial or central venous blood‐drawing line were also excluded.
Posterior spinal fusion surgery is an elective, complex nonabdominal surgery that can be associated with postoperative inflammation and GI dysfunction. 15 , 16 Common diagnoses for elective posterior spinal fusion surgery in our institution are neuromuscular, idiopathic, and congenital scoliosis. A select team of orthopedic surgeons and anesthesiologists perform these surgeries and provide anesthesia, respectively, reducing variability among surgical and clinical practices. Table S1 provides a summary of the most common categories of medications that may have an effect on gastric emptying administered to enrolled patients as part of routine care. Per routine care, most common narcotics and benzodiazepines administered on study day 1 were fentanyl, remifentanil, sufentanil, and midazolam and, on study day 2, were morphine and diazepam. All patients received antibiotics on study day 1 and 2, per routine care. Postoperative use of vasopressors and mechanical ventilation was variable and dependent on patient clinical status and is denoted in the results.
This study was approved by the Boston Children's Hospital institutional review board. All eligible patients were approached and written consent was obtained from parents/guardians and assent was obtained from patients when applicable.
Study Procedures
All procedures were performed preoperatively and postoperatively. This study design allowed for patients to serve as their own control and to specifically examine for changes in study variables after surgery. Patients received nothing by mouth and no specialized EN for the duration of all procedures on both study days. Preoperative procedures, study day 1, were performed after anesthesia induction and vascular access was obtained but prior to surgical incision. Postoperative procedures, study day 2, were completed on the morning of the first postoperative day in the PICU. Daily blood samples (3 mL) from an existing peripheral arterial or central venous line were processed for serum by spinning at 5000 rpm in a centrifuge for 10 minutes and stored at −80 °C for subsequent measurement of cytokine, lipopolysaccharide binding protein (LBP), and zonulin levels. A baseline 250‐μL blood sample and an additional 4–5 samples were drawn and placed in EDTA microtubes, processed for plasma, and stored at −80 °C for the acetaminophen absorption test (AAT), as detailed below. Total blood volume drawn for all assays was 9 mL over a 2‐day study period.
Cytokines
Cytokines were examined using the MSD V‐Plex Proinflammatory Panel kit (Rockville, MD), a multiplex cytokine immunoassay that measures interleukin‐6 (IL‐6), IL‐8, IL‐ 1β, IL‐2, IL‐4, IL‐13, IL‐10, IL‐12p70, interferon‐γ (IFN‐γ), and tumor necrosis factor‐α (TNF‐α) levels. The assay was completed according to the manufacturer's instructions. Samples were run in batches and each sample was run in duplicate. The first batch of samples was run at 3 different dilutions, and results were used to guide subsequent testing with samples diluted 1:2, consistent with the manufacturer's recommendations. Results were analyzed using the MSD software, and all cytokine levels are reported as picograms per milliliter (pg/mL). Preoperative and postoperative levels of IL‐1β, IL‐2, IL‐4, IL‐13, and IL‐12p70 were <0.1 pg/mL or undetectable and therefore not included for further analysis. Median (range) cytokine values as reported by the manufacturer in a cohort of healthy adults are as follows: IL‐6 0.47 pg/mL (0.16–27.2); IL‐8 9.61 pg/mL (1.48–1720); IL‐10 0.20 pg/mL (0.06–3.08); TNF‐α 0.36 pg/mL (0.10–1.75); and IFN‐γ 3.77 pg/mL (0.64–14.4).
Lipopolysaccharide Binding Protein
LBP served as a proxy marker of gut barrier dysregulation. 17 , 18 , 19 LBP levels were measured using the MSD Human LBP Kit (Rockville, MD). The assay was completed per the manufacturer's instructions, and samples were run in batches and in duplicate. LBP levels are reported as nanograms per milliliter (ng/mL). Median (range) LBP values as reported by the manufacturer in healthy adults are 2900 ng/mL (1440–6500).
Gastric Emptying: Acetaminophen Absorption Test
Gastric emptying was assessed on study day 1 and 2 by the AAT, which has been previously described. 1 , 20 Fifteen mg/kg or a maximum of 650 mg of a standard formulation of liquid acetaminophen was administered on study day 1 via a nasogastric or gastrostomy tube and on study day 2 by mouth, if patient was extubated and allowed to take liquids, or alternatively through a nasogastric or gastrostomy tube. Six plasma acetaminophen levels were obtained including a baseline level prior to acetaminophen administration, and up to 5 additional blood samples following acetaminophen administration at the following time periods: 10–20 minutes, 35–45 minutes, 60 minutes, 120–150 minutes, and 220–260 minutes. Samples were analyzed in the Quantitative Clinical Pharmacology and Pharmacokinetics Laboratory at Boston Children's Hospital as previously described. 1 The pharmacokinetic parameter area under the curve at 60 minutes (AUC60, μg/mL*min) was calculated to determine gastric emptying. The AUC60 has been correlated with scintigraphy, the gold‐standard test for gastric emptying measurement, and with EN advancement. 1 , 20 Previously reported ranges of AUC60 are 30 μg/mL*min to 1320 μg/mL*min. 1 , 20 Postoperative delayed gastric emptying was defined as a 20% or greater decrease in postoperative AUC60 compared with each patient's individual preoperative AUC60. Postoperative AUC60 change in gastric emptying is presented as fold change, with negative fold change representing a postoperative decrease in AUC60.
Zonulin
Zonulin, a PAR2 agonist and reversible regulator of the tight junction, served as proxy marker for potential PAR2 activation and as a marker of gut barrier dysregulation. 8 , 9 , 10 The IDK Zonulin ELISA kit (Bensheim, Germany) was utilized for this study. The IDK Zonulin ELISA kit measures zonulin family peptides , therefore all serum zonulin results in this study represent zonulin family peptides. The assay was completed per manufacturer's instructions and samples were run in batches and in duplicate. Zonulin levels are reported as ng/mL. Median ± standard deviation zonulin levels as reported by the manufacturer in a healthy adult cohort are 34 ± 14 ng/mL.
Clinical Data
Baseline demographics, including age, sex, home medications, and comorbidities, were documented. Intraoperative duration of anesthesia was recorded. Postoperative data included need for invasive mechanical ventilatory and/or vasoactive support, length of ICU stay, and length of hospital stay. Nutrition data were recorded and included the time to achieve 50% of prescribed EN goal from admission for patients receiving specialized EN by an enteral feeding tube (nasogastric, nasojejunal, gastrostomy, jejunostomy) and the time of solid‐diet initiation for patients on an oral diet. Patients requiring >48 hours to achieve 50% prescribed EN volume goal were categorized as having “slow EN advancement,” whereas patients who required 48 hours or less to achieve 50% of prescribed EN goal were categorized as “fast EN advancement.” Patients on an oral diet were included and their time to initiating a solid diet was considered as achieving 50% of prescribed EN goal. An institutional EN algorithm guides the route (gastric vs postpyloric), initiation, and advancement of nutrition for patients who require specialized EN. 21 Patients who were eligible to initiate an oral diet would have advanced as tolerated per their preference.
Statistical Analysis
Data were assessed for normality by the D'Agostino and Pearson test and skewness was identified. 22 Continuous variables are presented as median and interquartile range (25th and 75th percentiles) and nonparametric statistical methods were used.
Examining preoperative and postoperative values allowed for patients to serve as their own controls. A Wilcoxon matched‐pairs signed rank test to examine the differences in each patient's preoperative and postoperative serum cytokine, LBP, zonulin, and AUC60 values was performed (Table 2). Fold change for all variables (IL‐6, IL‐8, IL‐10, IFN‐γ, TNF‐α, LBP, zonulin, and AUC60) was calculated by dividing each patient's postoperative value by the preoperative value of each respective study variable. A fold change value <1 reflected a decrease in a variable postoperatively and was converted to negative values by the following equation: −1/ x. Spearman ρ correlations were calculated between postoperative fold change in AUC60 and fold change in cytokine, LBP, and zonulin levels (Table 3). Differences in postoperative fold change for cytokines, LBP, and zonulin were compared using the nonparametric Mann‐Whitney U test for patients dichotomized by postoperative gastric emptying status (Table 4) and EN advancement (Table 5), as previously defined. Receiver operating characteristic (ROC) curve analysis was applied to evaluate the predictive accuracy of fold change in IL‐10 and zonulin in differentiating patients with and without postoperative delayed gastric emptying (Figure 1). The AUC and 95% CIs with Youden J‐index were used to determine cutoff values that maximized the sensitivity and specificity of these markers in predicting postoperative delayed gastric emptying. 23 A descriptive model of postoperative delayed gastric emptying was developed (Figure 2). Statistical analyses were completed using GraphPad Prism Version 8 (GraphPad Software, La Jolla, CA).
Table 2.
Preoperative and Postoperative Levels of Markers of Inflammation, Gut Barrier Integrity, Gastric Emptying, and Zonulin (n = 20)
| Study variables | Preoperative Median (IQR a ) | Postoperative Median (IQR a ) | Wilcoxon matched‐pairs signed rank test P‐value |
|---|---|---|---|
| Cytokines, pg/mL | |||
| IL‐6 | 0.329 (0.176, 0.851) | 59.34 (36.02, 104) | <.0001 |
| IL‐8 | 7.55 (4.47, 12.72) | 14.42 (11.25, 28.23) | <.0001 |
| TNF‐α | 1.39 (0.953, 1.78) | 1.277 (1.07, 1.56) | .6487 |
| IFN‐γ | 1.73 (0.798, 3.28) | 1.433 (0.589, 2.88) | .4091 |
| IL‐10 | 0.102 (0.034, 0.244) | 1.586 (0.655, 2.22) | .0001 |
| LBP, ng/mL | 277 (182.6, 591.4) | 2050 (1233, 4650) | <.0001 |
| Zonulin, ng/mL | 50.09 (41.40, 66.38) | 38.87 (32.11, 42.16) | .0003 |
| Gastric emptying AUC, μg/mL*min | |||
| All patients (n = 20) | 158.4 (90.78, 483.7) | 244.4 (99.48, 337.2) | .8695 |
| Patients with postoperative delayed gastric emptying (n = 8) a | 476.0 (189.8, 515.5) | 102.1 (63.70, 251.0) | .0078 |
AUC60, area under the curve at 60 minutes as determined by the acetaminophen absorption test; IFN‐γ, interferon γ; IL, interleukin; IQR, interquartile range; LBP, lipopolysaccharide binding protein; TNF‐α, tumor necrosis factor α.
Postoperative delayed gastric emptying defined as having 20% or greater decrease in the postoperative AUC60 compared with each patient's preoperative AUC60.
IQR represented as 25th percentile, 75th percentile.
Table 3.
Univariate Spearman ρ Correlation Analysis of the Postoperative Fold Change in Markers of Inflammation, Gut Barrier Integrity, Gastric Emptying, and Zonulin (n = 20)
| Zonulin | LBP | AUC60 | ||||
|---|---|---|---|---|---|---|
| Study variables | ρ | P | ρ | P | ρ | P |
| Cytokines‐ | ||||||
| IL‐6 | −0.334 | .150 | 0.391 | .088 | −0.220 | .352 |
| IL‐8 | −0.280 | .232 | 0.343 | .139 | −0.093 | .696 |
| TNF‐α | −0.406 | .076 | 0.250 | .289 | −0.344 | .137 |
| IFN‐γ | −0.223 | .336 | 0.347 | .133 | 0.128 | .591 |
| IL‐10 | −0.618 | .004 | 0.005 | .985 | −0.465 | .039 |
| LBP | −0.104 | .663 | X | X | 0.002 | .995 |
| Zonulin | X | X | X | X | 0.370 | .108 |
Fold change—Postoperative value/preoperative value; when fold change was <1, it was converted to negative value by the following equation: −1/x
AUC60, area under the curve at 60 minutes as determined by the acetaminophen absorption test; IFN‐γ, interferon γ; IL, interleukin; LBP, lipopolysaccharide binding protein; TNF‐α, tumor necrosis factor α.
Table 4.
Fold Change in Markers of Inflammation, Gut Barrier Integrity, and Zonulin in Patients With and Without Postoperative Gastric Emptying Delay (n = 20)
| Postoperative gastric emptying delay a (n = 8) | No postoperative gastric emptying delay (n = 12) | Mann‐Whitney U | |||
|---|---|---|---|---|---|
| Fold change of study variables c | Median | IQR b | Median | IQR b | P |
| Cytokines | |||||
| IL‐6 | 270.5 | 44.94, 876.8 | 169.4 | 50.31, 334.2 | .5714 |
| IL‐8 | 3.64 | 1.90, 7.77 | 2.70 | 1.35, 5.32 | .5713 |
| TNF‐α | 1.18 | −1.32, 1.29 | −1.11 | −1.55, 1.22 | .2703 |
| IFN‐γ | −1.71 | −6.77, 0.652 | −0.141 | −2.57, 4.54 | .2704 |
| IL‐10 | 42.8 | 14.93, 104.5 | 8.49 | 4.84, 17.55 | .0159 |
| LBP | 10.07 | 3.07, 16.73 | 8.13 | 2.71, 17.33 | .9101 |
| Zonulin | −1.68 | −2.30, −1.43 | −1.12 | −1.32, −1.05 | .0160 |
IFN‐γ, interferon γ; IL, interleukin; IQR, interquartile range; LBP, lipopolysaccharide binding protein; TNF‐α, tumor necrosis factor α.
Postoperative delayed gastric emptying defined as having 20% or greater decrease in the postoperative area under the curve at 60 minutes (AUC60) compared with each patient's preoperative AUC60
IQR represented as 25th percentile, 75th percentile.
Fold change—Postoperative value/preoperative value; when fold change was <1, it was converted to negative value by the following equation: −1/x.
Table 5.
Fold Change in Markers of Inflammation, Gut Barrier Integrity, Zonulin, and Gastric Emptying in Patients With Slow vs Fast EN Advancement (N = 20)
| Slow EN advancement a (n = 6) | Fast EN advancement (n = 14) | Mann‐Whitney U | |||
|---|---|---|---|---|---|
| Fold change of study variables c | Median | IQR b | Median | IQR b | P |
| Cytokines | |||||
| IL‐6 | 204.9 | 63.71, 521.4 | 207.2 | 39.29, 336.7 | .9044 |
| IL‐8 | 2.65 | 0.605, 9.42 | 3.35 | 1.83, 4.59 | .8411 |
| TNF‐α | 0.017 | −1.22, 1.32 | −0.003 | −1.69, 1.24 | .6015 |
| IFN‐γ | −2.20 | −3.65, −0.742 | 0.021 | −3.62, 3.89 | .2740 |
| IL‐10 | 38.06 | 8.94, 101.5 | 15.07 | 4.18, 20.75 | .2391 |
| LBP | 2.91 | 2.27, 9.72 | 11.66 | 4.26, 18.18 | .0622 |
| Zonulin | −1.81 | −2.58, −1.29 | −1.211 | −1.43, −0.530 | .0408 |
| Gastric emptying, AUC60 | −2.06 | −7.77, 0.258 | 1.628 | −1.41, 2.83 | .1297 |
AUC60, area under the curve at 60 minutes as determined by the acetaminophen absorption test; EN, enteral nutrition; IFN‐γ, interferon γ; IL, interleukin; IQR, interquartile range; LBP, lipopolysaccharide binding protein; TNF‐α, tumor necrosis factor α.
Slow EN advancement defined as requiring >48 hours to achieve EN goal.
IQR represented as 25th percentile, 75th percentile.
Fold change—Postoperative value/preoperative value; when fold change was <1, it was converted to a negative value by the following equation: −1/x.
Figure 1.
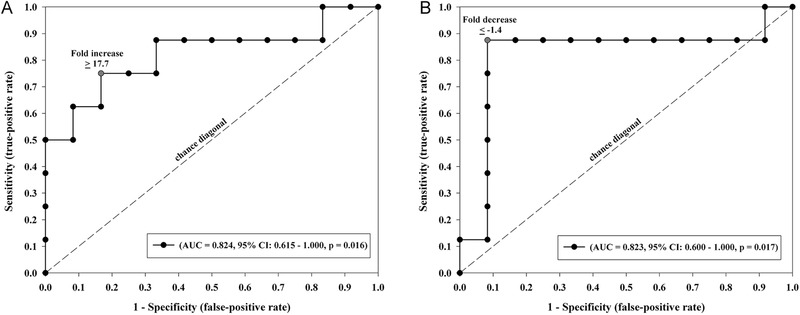
Receiver operating characteristic (ROC) curves demonstrating the predictive ability of fold increase in interleukin‐10 (IL‐10) and fold decrease in zonulin for postoperative delayed gastric emptying. (A) Depiction of the ROC curve for fold change in IL‐10 as a predictor of postoperative delayed gastric emptying, defined as 20% or greater decrease in postoperative compared with preoperative area under the curve at 60 minutes (AUC60) by the acetaminophen absorption test. A fold increase of ≥17.7 in IL‐10 had an AUC of 0.824; 95% CI, 0.615–1.00; P = 0.016. (B) Depiction of the ROC curve for fold change in zonulin as a predictor of postoperative delayed gastric emptying, defined as in Figure1A. A fold decrease of ≤−1.4 in zonulin had an AUC of 0.823; 95% CI, 0.600–1.00; P = 0.017, respectively.
Figure 2.
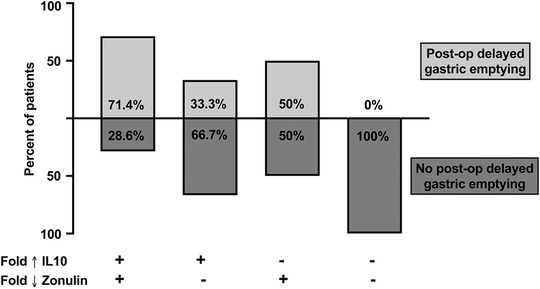
Descriptive model of the association between interleukin‐10 (IL‐10), zonulin, and postoperative (post‐op) delayed gastric emptying. Patients were categorized by a fold change increase (Fold ↑) in IL‐10 and decrease (Fold ↓) in zonulin. (Fold change is the result of dividing the postoperative value of IL‐10 or zonulin by its respective preoperative value.) A plus sign (+) represented the presence of a postoperative IL‐10 fold increase greater than the cohort's median and the presence of a zonulin postoperative fold decrease less than the cohort's median. Percentage of patients within these categories with postoperative delayed gastric emptying (as previously defined) were represented by light gray bars, whereas patients without postoperative delayed gastric emptying were represented by dark gray bars. No bar represents 0% of patients with postoperative delayed gastric emptying.
Results
Baseline Clinical Characteristics
Twenty‐four eligible patients were enrolled, 3 of which were excluded from the analysis because of incomplete postoperative gastric emptying testing and 1 because of error in sample processing (Figure S1). Final statistical analysis included 20 patients who completed all preoperative and postoperative study procedures with a median age 14 years (12.25, 18), and 60% were female (12/20). Table 1 depicts the cohort's baseline demographics and clinical data. Medications administered preoperatively and postoperatively were not significantly different (Table S1).
Table 1.
Study Cohort Demographics and Clinical Characteristics (n = 20)
| Demographic and clinical variables | Median (IQR d ) or frequency (%) |
|---|---|
| Age, y | 14 (12.25, 18) |
| Sex, female | 12/20 (60%) |
|
Comorbidities a Respiratory Neurologic Cardiac disease Other |
7/20 (35%) 14/20 (70%) 4/20 (20%) 3/20 (15%) |
| Anesthesia duration (minutes) | 661 (570, 759) |
| Number of patients who required postoperative vasoactive agents | 7/20 (35%) |
| Number of patients who required postoperative mechanical ventilator support | 10/20 (50%) |
| Length of ICU stay (days) | 2 (1, 3.75) |
| Length of hospital stay (days) | 6.5 (5, 11.25) |
|
Route of nutrition Specialized EN b Oral feeding |
10 (50%) 10 (50%) |
| Time to 50% EN goal (hours) c | 34.54 (15.88, 62.42) |
EN, enteral nutrition; ICU, intensive care unit; IQR, interquartile range.
Comorbidities are not mutually exclusive.
EN administered via a feeding tube.
Patients receiving oral feeding were counted as reaching 50% of EN goal when solid diet was initiated.
IQR represented as 25th percentile, 75th percentile.
Inflammation, Gut Barrier Integrity, and Gastric Emptying Markers
Table 2 describes the differences between preoperative and postoperative cytokine, LBP, zonulin, and AUC60 absolute values. Matched to preoperative values, IL‐10, IL‐6, and IL‐8 were significantly increased postoperatively, P ≤ .0001 (Wilcoxon). There were no significant differences between preoperative and postoperative levels of IFN‐γ and TNF‐α. Postoperative LBP values were increased and zonulin values were decreased compared with matched preoperative values (P < .0001 and P = .0003, respectively). Eight of 20 (40%) patients had postoperative delayed gastric emptying, with median preoperative and postoperative AUC60 values of 476.0 μg/mL*min (189.8–515.5) and 102.1 μg/mL*min (63.70–251.0), respectively, P = .0078.
Correlation Analysis Between Inflammation, Gut Barrier Integrity, and Gastric Emptying
A univariate Spearman ρ correlation analysis between the fold change in cytokine values, LBP, zonulin, and gastric emptying was performed (Table 3). A fold decrease in AUC60 correlated with a fold increase in postoperative IL‐10 (ρ = −0.465, P = .039). Postoperative fold increase in IL‐10 correlated with a fold decrease in zonulin (ρ = −0.618, P = .004). Postoperative fold decrease in AUC60 was inversely related to the time to reach 50% EN goal, though statistical significance was not reached (ρ = −0.420, P = .066, data not shown).
Dichotomous Analysis of Gastric Emptying and EN Advancement
Differences in the median fold change of cytokine, LBP, and zonulin levels in patients dichotomized into postoperative delayed gastric emptying (n = 8) vs no postoperative delayed gastric emptying (n = 12) were examined by Mann‐Whitney U test (Table 4). Patients with postoperative delayed gastric emptying had greater postoperative fold increase in IL‐10 (P = .0159) and fold decrease in zonulin (P = .0160) when compared with patients without postoperative delayed gastric emptying (Table 4).
EN delivery has been previously correlated with gastric emptying; therefore, differences in the median fold change of cytokine, LBP, and zonulin levels in patients dichotomized into slow EN advancement (n = 6) vs fast EN advancement (n = 14) was performed (Table 5). 1 Patients with slow EN advancement had greater postoperative fold decrease in zonulin (P = .0408) when compared with patients with fast EN advancement (Table 5).
ROC Curve Analysis and Descriptive Model of Association Between IL‐10, Zonulin, and Gastric Emptying
IL‐10 and zonulin were found to be significantly associated with postoperative delayed gastric emptying as reported in the previous analyses. An ROC analysis to examine the predictive accuracy of fold increase in IL‐10 and fold decrease in zonulin for delayed gastric emptying was performed. A fold increase of ≥17.7 in IL‐10 had a predictive accuracy of AUC 0.824; 95% CI, 0.615–1.00; P = .016 (Figure 1A). A fold decrease of ≤−1.4 in zonulin had a predictive accuracy of AUC 0.823; 95% CI, 0.600–1.00; P = .017 (Figure 1B).
Patients were categorized as having “postoperative IL‐10 fold increase” if their IL‐10 was above the cohort's median fold change vs “no postoperative fold increase” if their IL‐10 was equal to or less than the cohort's median fold change. Patients were also categorized as having “postoperative zonulin fold decrease” if their zonulin was below the cohort's median fold change vs “no postoperative zonulin fold decrease” if their zonulin was equal to or greater than the cohort's median fold change. The proportion of patients with postoperative delayed gastric emptying within these categories were examined (Figure 2). Five of 7 (71.4%) patients with postoperative IL‐10 increase and zonulin decrease had postoperative delayed gastric emptying, whereas patients without this combination did not have postoperative delayed gastric emptying.
Discussion
In this prospective pilot study, we have demonstrated a feasible model to study and describe the complex interactions between inflammation and GI dysfunction in pediatric patients undergoing complex surgery and requiring critical care. In this cohort, a significant postoperative proinflammatory and antiinflammatory response was identified. Postoperative GI function was altered, with a majority of patients having gut barrier dysregulation and 40% had delayed gastric emptying. Postoperative increase in IL‐10 and decrease in zonulin were associated with delayed gastric emptying and predicted postoperative delayed gastric emptying with >80% accuracy. A postoperative response with an increase in IL‐10 and decrease in zonulin may represent a compensatory mechanism for the significant proinflammatory trigger of surgery and associated gut barrier dysregulation. This potential mechanism was most prominent in patients with postoperative delayed gastric emptying, and their relationship may be direct or divergent. These results add to the limited literature on mechanisms of postoperative GI function changes in critically ill children and provide new insights that will need further examination.
Major surgery in this study cohort resulted in a robust inflammatory response with increases in IL‐6, IL‐8, and IL‐10, which is consistent with the most common cytokines reported to be increased in similar study cohorts. 15 , 16 , 24 , 25 In addition to an inflammatory response, a majority of patients in this study had an increase in LBP on postoperative day 1 compared with preoperative levels. LBP is an acute phase reactant. It is released primarily when lipopolysaccharide, a microbial product of intestinal bacteria, translocates across the gut barrier, thereby serving as a proxy marker for altered gut permeability. 17 , 18 , 19 The postoperative increase in LBP in this study cohort is suggestive of transient gut barrier dysregulation, as the absolute LBP levels were lower than previously reported in other inflammatory conditions such as sepsis. The increase in LBP was also positively correlated with postoperative cytokine levels, though statistical significance was not reached. A direct relationship between inflammatory response and loss of gut barrier integrity has been previously reported in pediatric and adult patients undergoing major surgery, including congenital heart diseases and multiple trauma. 4 , 26 , 27
Zonulin is a PAR2 agonist, and PAR2 has been associated with modulating GI epithelial barrier integrity and motility in animal models. 8 , 12 , 13 , 14 Zonulin is also known as a reversible regulator of tight junctions and has been shown to be elevated in patients with systemic inflammation and disrupted gut barrier integrity. 6 , 8 , 9 , 10 , 11 Two studies have examined zonulin in critically ill adults. One study reported higher zonulin levels in adults admitted to the ICU with sepsis when compared with adults admitted to the ICU without sepsis, including surgical patients, and healthy controls. 11 Another study reported elevated zonulin levels in adults requiring mechanical ventilation with sepsis, respiratory failure, or cardiac failure when compared with healthy controls, and specifically excluded surgical patients. 6 Both studies examined zonulin only at 1 time point. Pediatric studies examining zonulin levels have not included critically ill patients and have focused on patients with chronic inflammatory conditions. 28 , 29 , 30 In addition to differences in the study population, our study examined zonulin at 2 time points, allowing for an assessment of zonulin under 2 conditions for the same patient, and found a decrease in zonulin postoperatively. Downregulation of zonulin has been reported and found to be associated with beneficial physiologic changes to the epithelial barrier. 31 , 32 Therefore, as a reversible effector in the epithelial barrier structure, among many tight junction proteins, it is physiologically plausible that zonulin is downregulated in an effort to counterbalance postoperative loss of gut barrier integrity.
Delayed gastric emptying affected 40% of the study cohort, consistent with previous reports. 1 , 5 , 7 Patients with both postoperative increase in IL‐10 and decrease in zonulin had a greater prevalence of delayed gastric emptying, and IL‐10 and zonulin were inversely correlated. The correlation between delayed gastric emptying and elevated levels of IL‐10 is consistent with previous literature on IL‐10 and GI homeostasis, including gut barrier integrity and GI motility. 33 , 34 , 35 , 36 An inverse relationship between IL‐10 and zonulin has also been previously reported, though not in critically ill patients. 30 , 37 Few studies have examined zonulin and gastric emptying or the relationship between IL‐10, zonulin, and gastric emptying, and results vary among existing studies. 6 , 32 , 38 , 39 In nonsurgical critically ill adults, delayed gastric emptying was associated with markers of loss of gut barrier integrity and elevated levels of zonulin, but there was no correlation with IL‐10. 6 In healthy patients on an inulin‐enriched diet, a decrease in zonulin was associated with improved epithelial barrier function and a delay in gastric emptying, but inflammatory markers were not examined. 32 , 38 The observations from our current study suggest an inverse and synergistic relationship for IL‐10 and zonulin in pediatric patients with delayed gastric emptying during postoperative inflammatory conditions. Furthermore, these findings support a potential role for IL‐10 and zonulin as predictive biomarkers of delayed gastric emptying in this cohort.
This study has several limitations. The convenience sample in this pilot study may not be representative of all age groups or surgical populations admitted to the PICU, and this sample size does not allow for multivariable analysis to assess, for example, additional clinical differences among patients. However, studying a population undergoing the same elective surgery contributes to uniform surgical and clinical practices. Delayed gastric emptying has been correlated with difficulties in advancing EN, and therefore, EN delivery serves as a practical bedside outcome. 1 Although the same relationship between zonulin and EN advancement as between zonulin and gastric emptying was identified, examining EN delivery as an outcome was limited because 50% of patients took an oral diet, for which initiation and advancement is not uniform, reducing the validity of any comparisons and results. Serum levels of LBP and zonulin were within the detectable range for each respective assay per the manufacturer. However, as serum biomarkers, they may not be specific to GI changes only, and whether reported adults levels are the same in this younger study cohort is unknown, thereby limiting their clinical applicability at this time. 17 , 40 , 41 There is growing evidence to support zonulin to be a family of proteins, and zonulin as previously studied in animal and cell models may not be identical to that tested in human serum samples with available assays. 42 In this study, an association is reported and not a causality between markers of inflammation, gut barrier, and gastric emptying. Furthermore, GI dysfunction in critical illness is likely multifactorial, and this study was not powered to address additional potential contributing factors such as medications. 43 Future clinical studies in a larger patient population and in translational models are needed to further elucidate the relationship between zonulin, IL‐10, and gastric emptying. Elucidating the role of IL‐10 and zonulin in gastric emptying may support the development of potential diagnostic and therapeutic alternatives for this cohort.
Conclusion
Surgical critically ill children have postoperative inflammatory changes, gut barrier dysregulation, and delayed gastric emptying. Postoperative delayed gastric emptying is associated with an increase in IL‐10 and decrease in zonulin. IL‐10 and zonulin changes may have a compensatory role in the setting of the proinflammatory response and gut barrier dysregulation of surgery. Further studies to examine the role of IL‐10 and zonulin on delayed gastric emptying in pediatric critical illness, including translational models, are needed.
Statement of Authorship
E. E. Martinez, D. Zurakowski, L. Pereira, J. B. Emans, S. Nurko, C. P. Duggan, A. Fasano, and N. M. Mehta contributed to conception and design of the research; E. E. Martinez, D. Zurakowski, L. Pereira, R. Freire, A. Fasano, and N. M. Mehta contributed to acquisition, analysis, or interpretation of the data; E. E. Martinez, A. Fasano, and N. M. Mehta drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Figure 1S. Consort Diagram. GI, gastrointestinal.
Supplementary information
Acknowledgments
We acknowledge and thank the following for their contribution to this research work: the Boston Children's Hospital Department of Anesthesiology, Critical Care and Pain Medicine Pediatric Anesthesia Research Center for their research, nursing, and coordination services and the core anesthesiologists who collaborated in this work, and the Boston Children's Hospital Orthopedic Center's Spine Division surgeons for their collaboration in this research.
Financial disclosure: E. E. Martinez was supported in part by the National Institutes of Health, National Institute of Child Health and Human Development, K12HD047349, and the ASPEN Rhoads Research Foundation. Christopher P. Duggan was supported in part by NIH grants K24 DK104676 and P30 DK040561.
Conflicts of interest: Alessio Fasano is cofounder and stockholder at Alba Therapeutics.
This study was scheduled to be presented during the ASPEN 2020 Nutrition Science & Practice Conference (Tampa, FL) and was a candidate for the Harry M Vars Award and the Promising Investigator Award. The conference was cancelled due to the coronavirus disease 2019 and neither of these annual Early Career Investigator awards were determined.
This and other JPEN podcasts are available at https://nutritioncare.org/podcasts
References
- 1. Martinez EE, Pereira LM, Gura K, et al. Gastric emptying in critically ill children. J Parenter Enteral Nutr. 2017;41(7):1100‐1109. [DOI] [PubMed] [Google Scholar]
- 2. Piton G, Belon F, Cypriani B, et al. Enterocyte damage in critically ill patients is associated with shock condition and 28‐day mortality. Crit Care Med. 2013;41(9):2169‐2176. [DOI] [PubMed] [Google Scholar]
- 3. Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Resp Crit Care Med. 1998;158(2):444‐451. [DOI] [PubMed] [Google Scholar]
- 4. Typpo KV, Larmonier CB, Deschenes J, Redford D, Kiela PR, Ghishan FK. Clinical characteristics associated with postoperative intestinal epithelial barrier dysfunction in children with congenital heart disease. Pediatr Crit Care Med. 2015;16(1):37‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayer AP, Durward A, Turner C, et al. Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med. 2002;28(3):336‐340. [DOI] [PubMed] [Google Scholar]
- 6. Greis C, Rasuly Z, Janosi RA, Kordelas L, Beelen DW, Liebregts T. Intestinal T lymphocyte homing is associated with gastric emptying and epithelial barrier function in critically ill: a prospective observational study. Crit Care. 2017;21(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen T, Frenette AJ, Johanson C, et al. Impaired gastrointestinal transit and its associated morbidity in the intensive care unit. J Crit Care. 2013;28(4):537 e11‐e17. [DOI] [PubMed] [Google Scholar]
- 8. Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin‐2. Proc Natl Acad Sci U S A. 2009;106(39):16799‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sapone A, de Magistris L, Pietzak M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443‐1449. [DOI] [PubMed] [Google Scholar]
- 10. Ciccia F GG, Rizzo A, Alessandro R, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Disease. 2017;76(6):1123‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klaus DA, Motal MC, Burger‐Klepp U, et al. Increased plasma zonulin in patients with sepsis. Biochem Med. 2013;23(1):107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawabata A, Kuroda R, Nagata N, et al. In vivo evidence that protease‐activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br J Pharmacol. 2001;133(8):1213‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cocks TM, Sozzi V, Moffatt JD, Selemidis S. Protease‐activated receptors mediate apamin‐sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology. 1999;116(3):586‐592. [DOI] [PubMed] [Google Scholar]
- 14. Nystedt S, Ramakrishnan V, Sundelin J. The proteinase‐activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. 1996;271(25):14910‐14915. [DOI] [PubMed] [Google Scholar]
- 15. Lee JH, Lee JH, Kim JB, Lee HS, Lee DY, Lee DO. Normal range of the inflammation related laboratory findings and predictors of the postoperative infection in spinal posterior fusion surgery. Clin Orthop Surg. 2012;4(4):269‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Memtsoudis SG, Bombardieri AM, Ma Y, Girardi FP. The effect of low versus high tidal volume ventilation on inflammatory markers in healthy individuals undergoing posterior spine fusion in the prone position: a randomized controlled trial. J Clin Anesth. 2012;24(4):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fukui H. Endotoxin and other microbial translocation markers in the blood‐a clue to understand leaky gut syndrome. Cell Mol Med. 2016;2(03):3. [Google Scholar]
- 18. Farras M, Chandwe K, Mayneris‐Perxachs J, et al. Characterizing the metabolic phenotype of intestinal villus blunting in Zambian children with severe acute malnutrition and persistent diarrhea. PLoS One. 2018;13(3):e0192092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reiberger T, Ferlitsch A, Payer BA, et al. Non‐selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL‐6 in patients with cirrhosis. J Hepatol. 2013;58(5):911‐921. [DOI] [PubMed] [Google Scholar]
- 20. Nimmo WS, Heading RC, Wilson J, Tothill P, Prescott LF. Inhibition of gastric emptying and drug absorption by narcotic analgesics. Br J Clin Pharmacol. 1975;2(6):509‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamilton S, McAleer DM, Ariagno K, et al. A stepwise enteral nutrition algorithm for critically ill children helps achieve nutrient delivery goals. Pediatr Crit Care Med. 2014;15(7):583‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Agostino RB, Belanger A, D'Agostino RB Jr. A suggestion for using powerful and informative tests of normality. Am Stat. 1990;44(4):316‐321. [Google Scholar]
- 23. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York: Oxford University Press; 2003:66‐95. [Google Scholar]
- 24. Gutierrez T, Hornigold R, Pearce A. The systemic response to surgery. Surgery. 2011;29(2):93‐96. [Google Scholar]
- 25. Easton R, Balogh ZJ. Peri‐operative changes in serum immune markers after trauma: a systematic review. Injury. 2014;45(6):934‐941. [DOI] [PubMed] [Google Scholar]
- 26. Spindler‐Vesel A, Wraber B, Vovk I, Kompan L. Intestinal permeability and cytokine inflammatory response in multiply injured patients. J Interferon Cytokine Res. 2006;26(10):771‐776. [DOI] [PubMed] [Google Scholar]
- 27. Habes QLM, Linssen V, Nooijen S, et al. Markers of intestinal damage and their relation to cytokine levels in cardiac surgery patients. Shock. 2017;47(6):709‐714. [DOI] [PubMed] [Google Scholar]
- 28. Trachtman H, Gipson DS, Lemley KV, et al. Plasma zonulin levels in childhood nephrotic syndrome. Front Pediatr. 2019;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kume T, Acar S, Tuhan H, et al. The relationship between serum zonulin level and clinical and laboratory parameters of childhood obesity. J Clin Res Pediatr Endocrinol. 2017;9(1):31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koay WLA, Lindsey JC, Uprety P, et al. Intestinal integrity biomarkers in early antiretroviral‐treated perinatally HIV‐1‐infected infants. J Infect Dis. 2018;218(7):1085‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sturgeon C, Lan J, Fasano A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann N Y Acad Sci. 2017;1397(1):130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russo F, Clemente C, Linsalata M, et al. Effects of a diet with inulin‐enriched pasta on gut peptides and gastric emptying rates in healthy young volunteers. Eur J Nutr. 2011;50(4):271‐277. [DOI] [PubMed] [Google Scholar]
- 33. Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol. 2016;2(2):120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsukamoto T, Antonic V, El H II, et al. Novel model of peripheral tissue trauma‐induced inflammation and gastrointestinal dysmotility. Neurogastroenterol Motil. 2011;23(4):379‐386, e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein K, Lysson M, Schumak B, et al. Leukocyte‐derived interleukin‐10 aggravates postoperative ileus. Front Immunol. 2018;9:2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, Bauer AJ. Role of interleukin 10 in murine postoperative ileus. Gut. 2009;58(5):648‐660. [DOI] [PubMed] [Google Scholar]
- 37. Visser JT, Lammers K, Hoogendijk A, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes‐prone BioBreeding rat. Diabetologia. 2010;53(12):2621‐2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Russo F, Linsalata M, Clemente C, et al. Inulin‐enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon‐like peptide 2 in healthy young volunteers. Nutr Res. 2012;32(12):940‐946. [DOI] [PubMed] [Google Scholar]
- 39. Singh P, Silvester J, Chen X, et al. Serum zonulin is elevated in IBS and correlates with stool frequency in IBS‐D. United European Gastroenterol J. 2019;7(5):709‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151‐175. [DOI] [PubMed] [Google Scholar]
- 41. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258(1):25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martinez EE, Douglas K, Nurko S, Mehta NM. Gastric dysmotility in critically ill children: pathophysiology, diagnosis, and management. Pediatr Crit Care Med. 2015;16(9):828‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheffler L, Crane A, Heyne H, et al. Widely used ELISA does not detect precursor of haptoglobin 2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol. 2018;5(9):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. Consort Diagram. GI, gastrointestinal.
Supplementary information


