Abstract
Purpose
To evaluate the clinical outcomes of patients who underwent arthroscopic rotator cuff repair augmented using subacromial bursa, concentrated bone marrow aspirate (cBMA), and platelet-rich plasma.
Methods
Sixteen patients were included in the study who underwent arthroscopic rotator cuff repair augmented using subacromial bursa, cBMA, and platelet-rich plasma from January 2018 to July 2018 and had a minimum 1-year follow-up. American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test, Constant-Murley, and Single Assessment Numerical Evaluation (SANE) scores were collected preoperatively and at terminal follow-up. To determine the clinical relevance of ASES scores, the minimal clinically important difference, substantial clinical benefit, and the patient acceptable symptomatic state thresholds were used. In vitro cellular proliferation of subacromial bursa (nucleated cells/gram) and cBMA (nucleated cells and colony-forming units/cc) samples was evaluated and correlated to clinical outcomes scores.
Results
Mean follow-up was 12.6 ± 1.8 months (range 12-19 months). Patients achieved significant improvement in ASES (45.8±22.5pre vs 88.5 ± 14.6post, Δ44.7 ± 20.7; P = .001), Simple Shoulder Test (4.3 ± 3.2pre vs 10.4 ± 1.6post, Δ5.7 ± 3.9, P = .002), Constant-Murley (44.3 ± 18.2pre vs 83.6 ± 17.5post, Δ37.2 ± 21.8; P = .001), SANE (13.3 ± 10.7pre vs 86.3 ± 17.5post, Δ71.9 ± 22.9; P = .001), and pain scores (5.0±2.8pre vs 1.1 ± 1.6post, Δ3.5±2.5, P = .001) at final follow-up. With regards to ASES score, 93.8% of patients achieved the minimal clinically important difference, 93.8% the substantial clinical benefit, and 62.5% reached or exceeded the patient acceptable symptomatic state criteria. There was a significant positive correlation of nucleated cell count of cBMA with postoperative SANE score (r = 0.707; P = .015) and delta in ASES score (r = 0.727; P = .011). All other correlations were found to be nonsignificant (P > .05, respectively).
Conclusions
Patients undergoing arthroscopic rotator cuff repair augmented using the Mega-Clot with bursa technique achieved significant improvement in functional outcomes at a minimum 1-year follow-up, with 93.8% of patients reaching substantial clinical benefit.
Level of Evidence
Level IV, therapeutic case series.
Despite advances in surgical technique and instrumentation, recurrent rotator cuff tears following primary repair remain a significant problem.1 Several studies have examined the influence of surgical techniques in an attempt to reduce mechanical failure, such as suture anchor material, configuration, and footprint preparation.2,3 However, biologic failure leading to retears is not yet fully understood. A “hypovascular zone” within the supraspinatus tendon has been hypothesized to lead to initial degenerative tears, with further implication to poor tendon healing after repair.4
Improving the healing potential following rotator cuff repair using biologic adjuvants has become increasingly popular in recent years.5, 6, 7 Platelet-rich plasma (PRP) is derived from autologous peripheral blood that is centrifuged to isolate a greater concentration of growth factors contained within alpha-granules of the platelets to promote healing.8 Clinical outcomes following PRP application have been inconsistent; however, retear rates have been found to be significantly decreased in medium-to-large tears.9 In addition, bone marrow still is the most commonly used source of mesenchymal stem cells (MSCs) for biological augmentation, and its application in patients with rotator cuff injuries has shown promising results in decreasing retear rates and improving healing outcomes.10, 11, 12
Along with these biologic adjuvants, subacromial bursal tissue, which is often discarded during arthroscopic surgery to ensure visualization of the rotator cuff tear, may also be a significant source of MSCs.13, 14, 15, 16 In vitro characterization of subacromial bursa-derived cells (SBDCs) has shown that these cells fulfill all characteristics of MSCs, including their proliferation potential, similar expression of surface antigen profiles, and multilineage differentiation.13,15,17 Besides, SBDCs have been found to have superior engraftment to host tendon along with survival as well as greater proliferation and differentiation potentials when compared with concentrated bone marrow aspirate (cBMA).18,19 Recently, Morikawa et al.20 described a novel, effective, non-enzymatic method for mechanically isolating SBDCs for clinical use. However, no studies have been published yet that report on clinical outcomes following rotator cuff repair augmented with subacromial bursa.
Delivery of these biologics during repair has been described using various techniques,21, 22, 23 with the ultimate goal to promote healing. As scaffolds are expensive and tend to loosen and small clots are difficult to deliver and may float away, a technique was developed to create a clot with great volume and easy delivery. Thus, we combined cBMA, PRP, platelet-poor plasma (PPP), subacromial bursal tissue, and bovine thrombin to create a “Mega-Clot” as an adjuvant for arthroscopic rotator cuff repair. The purpose of this study was to evaluate the clinical outcomes of patients who underwent arthroscopic rotator cuff repair augmented using subacromial bursa, cBMA, and PRP. The authors hypothesized that augmentation of arthroscopic rotator cuff repair using this “Mega-Clot” with a bursa technique would result in significant improvement of shoulder function at a minimum 1-year follow-up.
Methods
This was a retrospective review of prospectively collected data from an institutional shoulder registry. All patients included were those older than 18 years of age and undergoing primary or revision arthroscopic rotator cuff repair augmented using a clot consisting of cBMA, PRP, PPP, and subacromial bursa. Surgeries were performed by a single, shoulder fellowship-trained surgeon (A.D.M.), from January 2018 to July 2018. Institutional review board approval was obtained before initiation of the study (no. 20X-081-1). As biologic augmentation of arthroscopic rotator cuff repairs at the institution is only performed in cases in which specific patient demographics and tear characteristics prompt concern of impaired biologic healing potential, all included patients had to fulfill at least 2 of the following criteria to be eligible for inclusion: presence of comorbidities that affect healing, such as diabetes, cancer, smoking, or rheumatoid arthritis; involvement of the dominant side in manual laborers and farmers; involvement of at least 2 rotator cuff tendons; history of previously failed repairs; and presence of tissue degeneration or cuff tear arthropathy (Hamada grade ≤3). Patients who did not fulfill these criteria were not considered for biologic augmentation. Excluded from the study were also patients with less than 1 year of follow-up, rotator cuff tear arthropathy (Hamada grade ≥4), irreparable massive tears, or preoperative pseudoparalysis. All alternative treatment options were discussed with the patient, including continued conservative treatment. Basic demographic information (age, sex, and body mass index) as well as a thorough medical and surgical history were obtained for each patient.
Diagnostic Imaging
All patients undergoing surgery had preoperative radiographs (true anterior/posterior view, axillary lateral view, scapular Y view) and magnetic resonance imaging (MRI) of the involved shoulder. On radiographs, acromiohumeral distance (AHD) was measured preoperatively by calculating the perpendicular distance between the most lateral portion of the undersurface of the acromion and a line parallel to the superior border of the greater tuberosity on the true anterior/posterior view.24 Further, rotator cuff tear arthropathy was graded on plain radiographs according to Hamada et al.25 MRI scans were used to determine rotator cuff tear characteristics including tear size, number of involved tendons, tendon retraction, fatty infiltration, and atrophy. According to Gerber et al.,26 massive tears were defined as those including complete tears of at least 2 tendons. Tendon retraction was quantified on coronal T2 fat-saturated images using the classification proposed by Patte.27 Fatty infiltration of the tendon and the associated muscle belly was evaluated on T1 sagittal oblique views based on the presence of fatty streaks within the supraspinatus muscle belly using Goutallier’s grading system.28 Muscle atrophy was graded according to Thomazeau et al.29 by determining the occupation ratio between surface of the cross-section of the muscle belly and the supraspinatus fossa on sagittal oblique images.
Surgical Technique: Mega-Clot with Bursa Technique
All surgeries were performed with the patient in the beach chair position by a single, shoulder fellowship-trained surgeon (A.D.M.). Following an interscalene block and successful induction of general anesthesia, diagnostic arthroscopy was performed to confirm the presence of the rotator cuff tear and assess mobility for repair. Loose suture material and/or anchors from previous repairs were removed and the torn rotator cuff tendons were mobilized. All rotator cuff repairs were performed arthroscopically using a double-row technique.
PRP and PPP Preparation
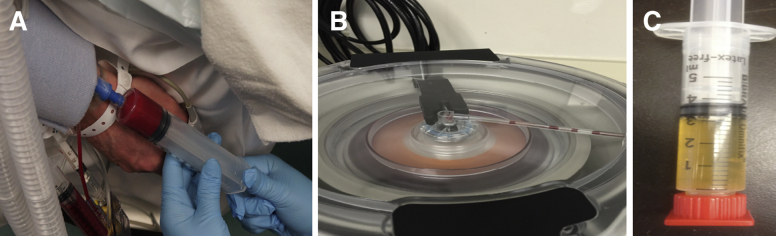
Before surgery, 60 mL of venous peripheral whole blood was drawn using a 60-mL syringe prefilled with 8 mL of Anticoagulant Citrate Dextrose Solution A (Fig 1A).30 The blood was processed using a fully automated 3-sensor technology system based on flow cytometry and light absorption (Angel System; Arthrex, Naples, FL) (Fig 1B) to obtain approximately 2 to 3 mL of PRP (Fig 1C) and 20 to 25 mL of PPP.30 The high-spinning centrifugal process with a hematocrit setting of 7% took about 17 to 20 minutes.30
Fig 1.
Harvest and processing of PRP. At the beginning of surgery, 60 mL of venous peripheral whole blood are drawn (A) and then processed using a fully automated 3-sensor technology system based on flow cytometry and light absorption (B) to obtain approximately 3 mL of PRP (C). (PRP, platelet-rich plasma.)
Bone Marrow Aspiration and Concentration
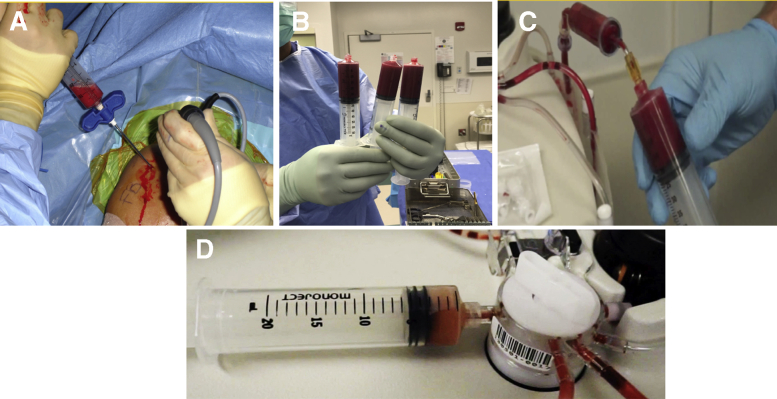
Bone marrow aspirate (BMA) was obtained from the proximal humeral head during arthroscopic rotator cuff repair according to a previously published technique (Fig 2).31 The proximal humerus is an ideal harvest site for BMSCs, as the epiphysis consists of trabecular bone and is rich in hematopoietic cells.18 A heparin-flushed (10,000 IU/mL) non-fenestrated bone marrow aspiration trocar (14 gauge), was inserted 25 to 30 mm into the medial aspect of the greater tuberosity (Bone Marrow Aspiration Kit; Arthrex).18,30,31 After a 60-mL syringe containing 2 mL of Anticoagulant Citrate Dextrose Solution A (Baxter Healthcare Corp) was connected to the trocar, the syringe was pulled back to maximize suction (Fig 2A).18,30,31 This standardized aspiration method was repeated 6 times, allowing 18 mL of BMA to flow into each of the six 60-mL syringes for a total of 120 mL of aspirate (Fig 2B).18,30,31 All syringes coming into contact with BMA were flushed with heparin (10,000 IU/mL) before use. The BMA, consisting of blood, bone marrow, and arthroscopic fluid, was transferred to the Angel System (Arthrex) and concentrated using a 15% hematocrit setting (cBMA) (Fig 2 C and D).18,30,31 The tunnel created for the aspiration was later used to insert the first suture anchor of the medial row.30
Fig 2.
Harvest and processing of BMA. BMA is obtained from the proximal humeral head during arthroscopic rotator cuff repair using a non-fenestrated trocar (A). The harvested BMA, consisting of blood, bone marrow, and arthroscopic fluid (B), is transferred to the Angel System (Arthrex, Naples, FL) (C) and concentrated using a 15% hematocrit setting (D). (BMA, bone marrow aspirate.)
Subacromial Bursal Cells Harvest
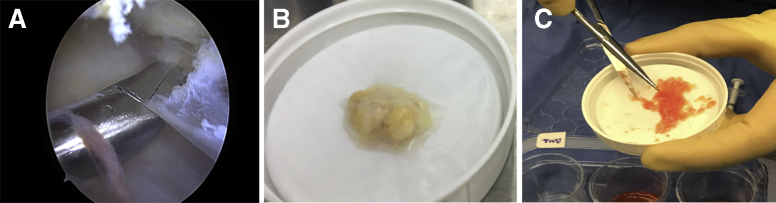
Two bursa samples were obtained from the subacromial space overlying the rotator cuff tendons using an arthroscopic grasper device.18 A syringe was used to measure the sample size to ensure exactly 1 cc of subacromial bursal tissue, respectively. One tissue sample was chopped until becoming a finely minced, gooey particulate (Fig 3) according to a previously published technique20 and added to the 30-mL syringe containing the cBMA, PRP, and PPP. The other sample of bursal tissue was placed into a sterile 3-mL syringe and immediately transported from the operating room to a laminar flow hood for processing.
Fig 3.
Harvest and processing of subacromial bursal tissue. Subacromial bursa is obtained from over the rotator cuff tendon using a laparoscopic grasper device (A). The sample (B) is then chopped using sterile tenotomy scissors until becoming a finely minced, gooey particulate (C).
Mega-Clot With Bursa Preparation and Delivery to Repair Site
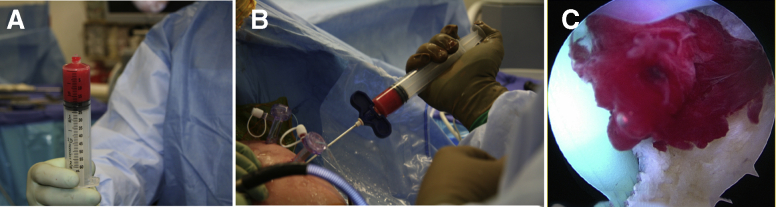
A “Mega-Clot” was used as a biologic scaffold to deliver MSCs, growth factors, and subacromial bursal cells directly to the repair site, thus enhancing the biological healing process.30 To ensure biological and mechanical stability of the clot, volumes of 0.1 cc of cBMA, 0.1 cc of PRP, 0.6 cc of PPP, 0.2 cc of bovine thrombin (5000 IU/mL), and 1 cc of subacromial bursal tissue were combined, scaled up, and added to a 30-mL syringe according to the amount of product produced. Bovine thrombin was used to activate and obtain a stable clot.30 During preparation of the clot, the medial row was placed at the articular margin using 2 double-loaded suture anchors (PEEK Corkscrew FT Suture Anchor, 5.5 mm × 14.7 mm w/two No.2 FiberWire, Arthrex) and the sutures were tied. The final clot, usually having a total size of 16 to 24 cc (Fig 4A), was then delivered into the repair site using the same non-fenestrated trocar that was used for bone marrow aspiration (Fig 4 B and C). Subsequently, the lateral row was completed and secured using two additional suture anchors in a horizontal mattress fashion (PEEK SwiveLock, 4.75 mm × 19.1 mm; Arthrex).
Fig 4.
Delivery of the prepared clot during rotator cuff surgery in a right shoulder. The final clot, usually having a total size of 16 to 24 cc (A), is delivered to the repair site using the same non-fenestrated trocar that was used for bone marrow aspiration (B). Arthroscopic view of the delivered clot via the anterior portal (C).
In Vitro Cellular Proliferation of Subacromial Bursa
A 200-mg sample of each bursa specimen was carefully weighed for plating. The sample was placed in a culture dish and mechanically digested for 60 seconds using tenotomy scissors sterilized in 100% ethanol.20 When the tissue sample resembled a finely minced, liquified particulate, it was re-suspended and placed into 100-mm Primaria culture dishes (Thermo Fisher Scientific, Waltham, MA) containing 10 mL of complete Dulbecco’s Modified Eagle’s Medium (DMEM [1X], Thermo Fisher Scientific), 10% fetal bovine serum (Thermo Fisher Scientific), and 1% penicillin/streptomycin (Pen Strep Glutamine [100X]; Thermo Fisher Scientific). The culture dishes were stored in a humidified, low oxygen tension (5% CO2) incubator at 37°C. To allow time for the cells to adhere to the culture dish, the media were not replaced during the first week but was replaced twice per week thereafter. SBDCs harvested using the described method have been shown to fulfill all required characteristics proposed by the International Society for Cellular Therapy, including their adherence to tissue culture plastic, ability to form colonies, positive fluorescence-activated cell sorting analysis of characteristic surface markers CD73, CD90, and CD105 as well as the ability of multilineage differentiation.18, 19, 20
Cellular proliferation was evaluated after 3 weeks of incubation, to allow the cells to achieve full confluence. Culture dishes were aspirated of media and incubated at 37°C with 1.5 mL of sterile 0.5% trypsin/ethylenediamine tetra-acetic acid for 20 minutes. Following incubation, 1.5 mL of complete DMEM was added to each dish to inactivate the trypsin. Two separate 100-μL samples of the cellular solution were drawn up using a micro-pipette and placed in transparent cuvettes filled with 9.9 mL of 0.9% NaCl solution. Nucleated cells were counted using a Z1 Coulter Particle Counter (Beckman Coulter Life Sciences, Indianapolis, IN) calibrated to detect particles greater than 8 μm. The cellular concentrations (cells/mL) were multiplied by the final volume of the trypsinized dishes (3 mL) and normalized by the mass of tissue originally plated (0.2 g) to obtain the cell mass density in cells per gram.
Nucleated Cell Count and Colony-Forming Units (CFUs) of cBMA
To obtain the total of nucleated cell count, 10 μL of cBMA was diluted in 9.9 mL of saline. Using the Coulter Counter, the number of nucleated cells in this 10 mL of solution was immediately calculated after surgery and multiplied by 10 to obtain the total number of nucleated cells in 1 cc of cBMA.
To obtain the total number of CFUs, 1 cc of cBMA was added to a 100-mm tissue culture plate with 9 cc of complete alpha minimum essential medium containing 10% fetal bovine serum (Thermo Fisher Scientific) and 0.1% Penicillin/Streptomycin (Thermo Fisher Scientific). After 48 hours, the medium was changed to remove the non-adherent cells. CFUs were counted with their first appearance, usually between 7 and 10 days, under the microscope (Eclipse TS100; Nikon Instruments Inc., Melville, NY) by the same experienced investigator each time. A colony was defined as a cluster of 8 or more cells.17 The CFUs were counted for one quarter of the plate and multiplied by 4 to obtain the total number of CFUs per 1 cc of cBMA. Connective tissue progenitor prevalence also was calculated (no. of CFUs/no. of nucleated cells).32
Live/Dead Assay and Scanning Electron Microscopy of Reimplanted Clot
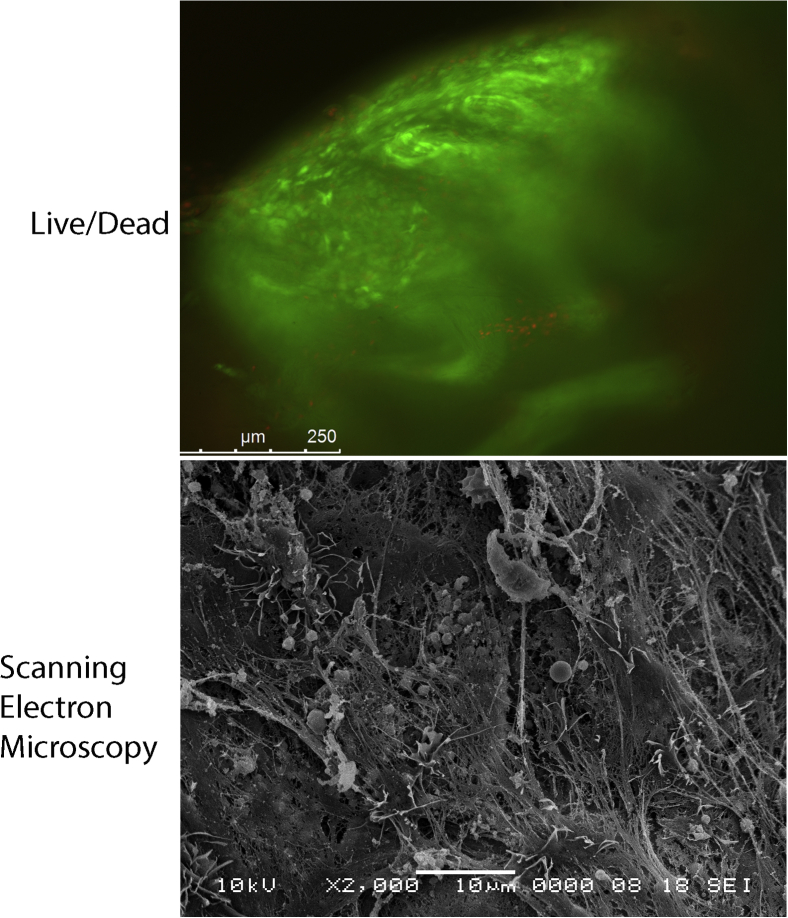
The viability of reimplanted MSCs in a fibrin clot was assessed for each patient using a live/dead assay. The clot was cultured in 10 mL of complete DMEM in a humidified, low oxygen tension (5% CO2) incubator at 37°C for 3 weeks before analysis. For the assay, the clot was incubated in 1X phosphate-buffered saline containing 5 μM calcein and 10 μg/mL propidium iodide (Thermo Fisher Scientific) for 30 minutes to stain for live and dead cells within the clot. After washing 2X with phosphate-buffered saline, the green or red fluorescence was visualized using a Leica DMI 6000B fluorescent microscope (Leica Microsystems, Buffalo Grove, IL) (Fig 5).
Fig 5.
The viability of reimplanted MSCs within the clot is assessed using a live/dead assay, with 90% of the cells within the clot being viable (green fluorescence) while only 10% are dead (red fluorescence). The morphology of the clot using scanning electron microscopy shows a scaffold rich in fibrous strands, which are formed from fibrinogen found in the ACP and can be seen entangling platelets, red blood cells, erythrocytes, and MSCs within the clot. (ACP, autologous conditioned plasma; MSCs, mesenchymal stem cells)
Scanning electron microscopy was employed to further confirm viability of the cells within the clot. Samples were fixed in 2% glutaraldehyde in 0.1 M cacodylic buffer, pH 7.4, for 24 hours. Clots were sputter coated with gold/palladium for 20 seconds with a Polaron E5100 SEM coating unit. Images were obtained using a JeOL JSM-633F field emission scanning microscope by use of an accelerating voltage of 10 to 15 kV, at various magnifications (Fig 5).
Clinical Outcome Measures
The American Shoulder and Elbow Surgeons (ASES) Score,33 Constant-Murley Score,34 Single Alpha-numeric Evaluation (SANE) Score,35 Simple Shoulder Test (SST),36 and range of motion in terms of active flexion, abduction, and external rotation, were collected preoperatively and at terminal follow-up. Patients were assessed by the senior surgeon (A.D.M.). Pain scores were obtained from the ASES survey. Previous studies have confirmed these scores in terms of reliability, validity, and responsiveness.35,37, 38, 39 To determine the clinical significance of ASES scores, the minimal clinically important difference (MCID), the substantial clinical benefit (SCB), and the patient acceptable symptomatic state (PASS) thresholds were calculated.40 To assess the clinical relevance of a change in score with respect to an outcome measure, the SCB and MCID were used.40 The PASS was employed as a tool to assess the minimum score associated with patient satisfaction.40 In rotator cuff tear populations, these metrics included an 11-point change for the MCID, 17.5-point change for SCB, and a final ASES score of 86.7 for the PASS.41
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) as well as median and interquartile range (IQR) for continuous variables and frequency and proportion for categorical variables. Given the small sample size, the Wilcoxon sign rank test (the nonparametric analog to the paired t test) was used to compare pre- to postoperative clinical outcome and range of motion values. Pearson correlation coefficients were calculated to explore the relationship between cellular data and patient metrics. A P value of less than .05 was considered statistically significant. All statistical analyses were performed using Stata (StataCorp 2017. Stata Statistical Software: Release 15; StataCorp LLC, College Station, TX).
Results
Demographics
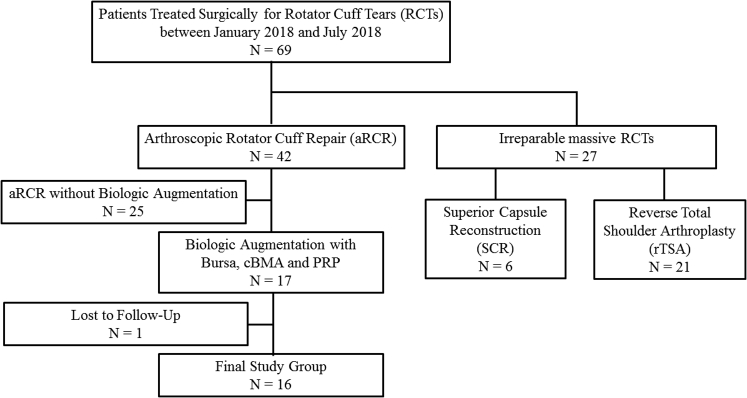
Sixty-nine patients underwent surgical treatment of rotator cuff tears between January 2018 and July 2018. Of these patients, 27 patients were excluded because they had irreparable massive rotator cuff tears, of whom 6 underwent superior capsule reconstruction and 21 underwent reverse total shoulder arthroplasty. Further, 25 patients were excluded for undergoing arthroscopic rotator cuff repair without biologic augmentation. In addition, 1 patient was excluded for having been lost to follow-up. This resulted in a final study group of 16 patients who underwent rotator cuff repairs augmented using Mega-Clot with bursa technique and had a minimum 1-year follow-up (Fig 6). The mean age of patients was 57.4 ± 5.4 years (range 47 – 64 years) with an average follow-up of 12.6 ± 1.8 months (range 12-19 months). Four patients (25%) had a previously failed rotator cuff repair and most of the patients were male (62.5%). Patient demographics are demonstrated in Table 1.
Fig 6.
Flowchart of patients included in the study. (cBMA, concentrated bone marrow aspirate; PRP, platelet-rich plasma.)
Table 1.
Patient Demographics (N = 16)
| n | % | |
|---|---|---|
| Mean age, y, ± SD | 57.4 ± 5.4 | |
| Mean BMI ± SD | 29.4 ± 4.2 | |
| Sex | ||
| Male | 10 | 62.5 |
| Female | 6 | 37.5 |
| Dominant side involved | 11 | 68.8 |
| Smoker | 3 | 18.6 |
| Diabetes | 2 | 12.5 |
| Revision | 4 | 25.0 |
BMI, body mass index; SD, standard deviation.
Radiographic Analysis
All patients had preoperative standard radiographs and MRI scans. Most patients (87.5%) had rotator cuff tears with at least 2 tendons involved. Tissue degeneration was moderate with most of the patients having grade 2 fatty infiltration according to Goutallier (43.8%) and grade 2 muscle atrophy according to Thomazeau (56.3%). The average preoperative AHD was 7.2 ± 3.3 mm. Preoperative tear characteristics are demonstrated in Table 2.
Table 2.
Preoperative Tear Characteristics (n = 16)
| n | % | |
|---|---|---|
| Number of tendons involved | ||
| 1 | 2 | 12.5 |
| 2 | 11 | 68.8 |
| 3 | 3 | 18.8 |
| Tendon retraction | ||
| Patte 1 | 7 | 43.8 |
| Patte 2 | 5 | 31.3 |
| Patte 3 | 4 | 25.0 |
| Fatty infiltration | ||
| Goutallier 0 | 1 | 6.3 |
| Goutallier 1 | 6 | 37.5 |
| Goutallier 2 | 7 | 43.8 |
| Goutallier 3 | 1 | 6.3 |
| Goutallier 4 | 1 | 6.3 |
| Atrophy (supraspinatus) | ||
| Thomazeau 0 | 1 | 6.3 |
| Thomazeau 1 | 3 | 18.8 |
| Thomazeau 2 | 9 | 56.3 |
| Thomazeau 3 | 3 | 18.8 |
| Cuff tear arthropathy | ||
| Hamada 1 | 10 | 62.5 |
| Hamada 2 | 5 | 31.3 |
| Hamada 3 | 1 | 6.3 |
| Acromiohumeral distance, mm, mean ± SD | 7.2 ± 3.3 | |
SD, standard deviation.
Clinical Outcomes
With regards to active range of motion, patients achieved significant improvement in forward elevation (130.3 ± 46.0°pre vs 174.0 ± 9.9°post, Δ 45.0 ± 45.4°; P = .001) and abduction (117.5 ± 51.6°pre vs 164.3 ± 30.5°post, Δ 48.3 ± 46.9°; P = .001). There was no significant improvement in external rotation at terminal follow-up (40.9 ± 19.9°pre vs 47.3 ± 10.3°post, Δ 5.7 ± 21.5°; P = .162).
Overall, there was significant improvement in ASES (45.8 ± 22.5pre vs 88.5 ± 14.6post, Δ 44.7 ± 20.7; P = .001), SST (4.3 ± 3.2pre vs 10.4 ± 1.6post, Δ 5.7 ± 3.9, P = .002), Constant-Murley (44.3 ± 18.2pre vs 83.6 ± 17.5post, Δ 37.2 ± 21.8; P = .001), SANE (13.3 ± 10.7pre vs 86.3 ± 17.5post, Δ 71.9 ± 22.9; P = .001), and pain scores (5.0 ± 2.8pre vs 1.1 ± 1.6post, Δ 3.5 ± 2.5, P = .001) (Fig 7). A greater preoperative AHD showed a significant positive correlation to postoperative ASES (r = 0.672; P = .009) and SST (r = 0.588; P = .027) scores and a significant negative correlation to pain scores (r = –0.728; P = .003). All other correlations of patient demographics to clinical outcomes were found to be nonsignificant (P > .05, respectively). When we evaluated the clinical relevance of improvement in ASES scores by using patient outcome thresholds, 93.8% of all patients achieved the MCID, 93.8% crossed the SCB, and 62.5% reached or exceeded the PASS criteria (Fig 8).
Fig 7.
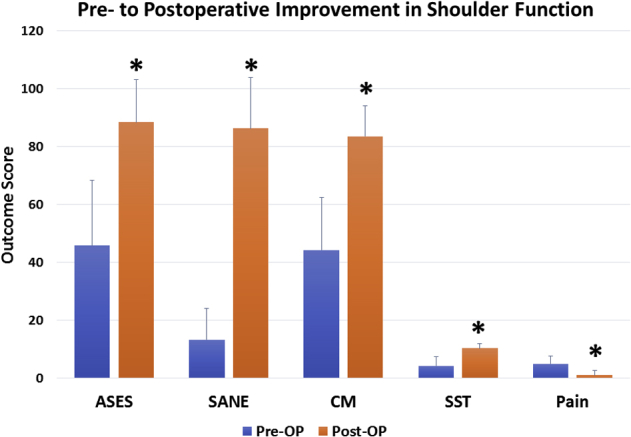
Preoperative to postoperative improvement in shoulder function following biologically augmented rotator cuff repair. ∗Indicates statistical significance. (ASES, American Shoulder and Elbow Surgeons; CM, Constant-Murley; SANE, Single Assessment Numerical Evaluation; SST, Simple Shoulder Test.)
Fig 8.
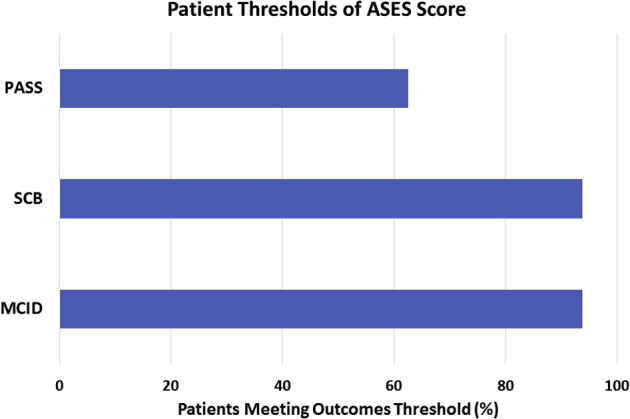
Percentage of patients meeting outcomes thresholds. (ASES, American Shoulder and Elbow Surgeons; MCID, minimal clinically-important difference; PASS, patient acceptable symptomatic state; SCB, substantial clinical benefit.)
Biologic Findings and Correlation to Clinical Outcomes
Overall, 1 cc of the processed cBMA contained 25.4 × 106 nucleated cells (SD: 4.5 × 106; median: 27.6 × 106; IQR: 6.0 × 106) and 1283.6 CFUs (SD: 304.2; median: 1256; IQR: 239). This resulted in a mean connective tissue progenitor prevalence of 49.1 CFUs per 106 nucleated cells (SD: 13.1; median: 45; IQR: 20.6). When we looked at the cellular proliferation potential of the harvested and mechanically processed subacromial bursal tissue, there was an average cell density of 471,769 nucleated cells per gram of bursa (SD: 401,353; median: 312,000; IQR: 404,000). Age, body mass index, and AHD had no significant influence on cellular measures of cBMA and subacromial bursa (P > .05, respectively).
When evaluating the relationship between biologic findings and clinical outcomes, there was a significant positive correlation of nucleated cell count of cBMA with postoperative SANE score (r = 0.707; P = .015) as well as pre- to postoperative delta in ASES score (r = 0.727; P = .011). All other correlations were found to be nonsignificant (P > .05, respectively).
Complications and Revisions
None of the 16 patients who underwent arthroscopic rotator cuff repair augmented using the technique had postoperative complications. However,1 patient (6.3%) failed due to trauma to the shoulder and subsequently underwent superior capsule reconstruction 3 months after the previous surgery. This patient (54-year old male) had a massive tear with grade 3 tendon retraction, grade 2 fatty infiltration, and grade 2 atrophy.
Live/Dead Assay and Scanning Electron Microscopy of Reimplanted Clot
The live/dead assay was performed on the clots after 3 weeks in culture. When the cellular viability in each clot was evaluated, 90% of the cells within the clot were viable (green fluorescence) whereas only 10% were dead (red fluorescence) (Fig 5).
The morphology of the clot using scanning electron microscopy showed a scaffold rich in fibrous strands (Fig 5). These strands are formed from fibrinogen found in the autologous conditioned plasmaand can be seen entangling platelets, red blood cells, erythrocytes, and MSCs within the clot.
Discussion
The most important finding of the study was that patients undergoing arthroscopic rotator cuff repair augmented using the Mega-Clot with bursa technique achieved significant improvement in functional outcomes at a minimum 1-year follow-up. Further, 93.8% of patients reached substantial clinical benefit following surgery, highlighting the promising preliminary results of a novel, potent biologic repair augmentation combining subacromial bursa, cBMA, and PRP.
As the endogenous healing potential of the rotator cuff tendon seems to be limited, biologic augmentation options have garnered recent interest, including the clinical application of growth factors, platelet concentrates, or MSCs.9,10,12,42,43 Even recent meta-analyses of randomized controlled trials have reported mixed results, with some showing decreased failure-to-heal rate for small- to medium-sized tears as well as decreased re-tear rates for large tears treated with PRP,9,44 and others finding no difference in outcome scores and structural healing rates.42,45 A study by Malavolta et al.46 found that PRP application did not significantly improve clinical outcomes, pain, and structural healing in 51 prospectively randomized patients undergoing rotator cuff repair at 5-year follow-up. Similar to our results, Randelli et al.47 reported short-term benefits following repair augmentation using PRP, including significantly lower pain scores 1 month after surgery and greater functional improvement at 3-month follow-up.
Only a few studies have investigated the effectiveness of BMA for augmenting rotator cuff repairs, with most reporting on bone marrow stimulation techniques, rather than direct application of cBMA.11,12,48,49 Hernigou et al.12 reported long-term results of primary rotator cuff repairs augmented using cBMA showing improved healing rates on MRI compared with a nonaugmented control group. In 14 patients with a minimum follow-up of 1 year, Ellera Gomes et al.11 described improved clinical outcomes along with tendon integrity in 100% of patients following augmentation of mini-open transosseous suture repair for full-thickness rotator cuff tears. However, current literature does not allow for drawing definite conclusions regarding the clinical efficacy of BMA and PRP applications, which is mainly due to inconsistent relationships between successful rotator cuff healing and clinical outcomes scores as well as disparities in underlying pathologies, repair techniques, and patient demographics.43
Along with these biologic adjuvants, subacromial bursal tissue may also be a significant, easily accessible source of MSCs.13, 14, 15,17,19 Although SBDCs have been shown to demonstrate superior engraftment to host tendon along with survival when compared with cBMA, there remains a lack of clinical data supporting these promising in vitro findings.18,19 Morikawa et al.20 recently described an effective, clinically feasible method for mechanically isolating SBDCs. The authors found that mechanically processing subacromial bursa resulted in a significantly higher release of SBDCs compared to no manipulation.20 Using this previously published isolation technique, the present study contributes to the current knowledge reporting on clinical outcomes following repair augmentation using subacromial bursa.
In an attempt to combine the benefits of these various biologic adjuvants, thus maximizing the healing potential of the repaired tendon, the technique reported on in this study was developed. This may be especially of importance in the treatment of massive tears, in which the usually occurring postoperative healing rate of 71% to 89% of cases drops to 47% to 50%.50,51 With 56.3% of our patient population undergoing repair for massive tears, the preliminary clinical results using the Mega-Clot with bursa technique seem promising, with only one surgical failure requiring revision. Besides, Cvetanovich et al.41 showed that only 52.1% of patients who underwent primary rotator cuff repair reached or exceeded the PASS criteria for ASES, compared with 62.5% of our subjects. However, it remains to be seen whether this significant improvement in shoulder function will be maintained in the longer term.
Delivery of biologic adjuvants during repair has been described using various techniques,21, 22, 23 with the ultimate goal to promote healing. The clot used in this study presents with great volume to reduce the risk of floating away and to provide an easy delivery. In addition to its unknown cost-effectiveness, it has to be acknowledged that it is a complex technique, as various biologic adjuvants have to be mixed in the correct ratio. As maintenance of a sufficient rotator cuff function has been shown to be vital in delaying the development of glenohumeral arthritis, the clot was stabilized using bovine thrombin, to ensure maximum stability and retainment of the applied biologic augments at the repair site.52 However, there is a lack of confirmation that the delivered clot containing the potent adjuvants remains at the repair site during the postoperative period.
Limitations
There were several limitations to the study. Although the data were collected prospectively, the chart review was performed retrospectively and may create a selection bias, which may also be amplified by the large number of excluded patients. Even though the sample size was small, the results were statistically significant, which helps in minimizing the concern over a type 2 error. In addition, with this study only reporting on outcomes of a single surgeon’s practice, external validity may be limited in terms of both patient population and surgical technique. The large patient-individual variability in harvested biologic adjuvants, including cBMA, subacromial bursa, and PRP, also may have influenced the results. More importantly, due to the combination of various biologic adjuvants, it remains unknown which of the factors is most important for sufficient healing. The reliability of the Goutallier classification also has been shown to be limited in predicting rotator cuff tear characteristics on MRI scans, including repairability or outcome after surgery.53 Further, this study only reports on preliminary outcomes after a minimum 1-year follow-up; thus, it does not allow for drawing definite conclusions and limits comparability to previously published studies, also due to the lack of a control group. It remains to be seen if patients will maintain significant improvement in shoulder function over a longer term. Lastly, repeat MRI or ultrasound examinations were not available to evaluate for the integrity of the repaired tendon.
Conclusions
Patients undergoing arthroscopic rotator cuff repair augmented using the Mega-Clot with bursa technique achieved significant improvement in functional outcomes at a minimum 1-year follow-up, with 93.8% of patients reaching substantial clinical benefit.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: The University of Connecticut Health Center/Musculoskeletal Institute receives funding from Arthrex. A.D.M. receives consulting fees as well as research support from Arthrex, outside the submitted work. K.B. is a consultant for Arthrex, outside the submitted work. The company was not involved in the study design, data collection, or final manuscript. R.A.A. receives grants from Arthrex. In addition, he is a consultant for Biorez, outside the submitted work. M.P.C. receives personal fees from the Arthroscopy Association of North America (AANA) for the Arthroscopy Journal, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Investigation performed at the Department of Orthopaedic Surgery, University of Connecticut, Farmington, Connecticut, U.S.A.
Supplementary Data
References
- 1.Galatz L., Ball C., Teefey S. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cummins C.A., Appleyard R.C., Strickland S., Haen P.S., Chen S., Murrell G.A. Rotator cuff repair: an ex vivo analysis of suture anchor repair techniques on initial load to failure. Arthroscopy. 2005;21:1236–1241. doi: 10.1016/j.arthro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Cole B.J., ElAttrache N.S., Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23:662–669. doi: 10.1016/j.arthro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Gamradt S.C., Gallo R.A., Adler R.S., et al. Vascularity of the supraspinatus tendon three months after repair: Characterization using contrast-enhanced ultrasound. J Shoulder Elbow Surg. 2010;19:73–80. doi: 10.1016/j.jse.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Zumstein M.A., Rumian A., Thelu C.E., et al. SECEC Research Grant 2008 II: Use of platelet- and leucocyte-rich fibrin (L-PRF) does not affect late rotator cuff tendon healing: A prospective randomized controlled study. J Shoulder Elbow Surg. 2016;25:2–11. doi: 10.1016/j.jse.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Barber F.A., Hrnack S.A., Snyder S.J., Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27:1029–1035. doi: 10.1016/j.arthro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Zumstein M.A., Ladermann A., Raniga S., Schar M.O. The biology of rotator cuff healing. Orthop Traumatol Surg Res. 2017;103:S1–S10. doi: 10.1016/j.otsr.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Lubkowska A., Dolegowska B., Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26:3S–22S. [PubMed] [Google Scholar]
- 9.Warth R.J., Dornan G.J., James E.W., Horan M.P., Millett P.J. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: A meta-analysis and meta-regression. Arthroscopy. 2015;31:306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Imam M.A., Holton J., Horriat S., et al. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J. 2017;3:58. doi: 10.1051/sicotj/2017039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellera Gomes J.L., da Silva R.C., Silla L.M., Abreu M.R., Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20:373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernigou P., Flouzat Lachaniette C.H., Delambre J., et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 13.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 14.Song N., Armstrong A.D., Li F., Ouyang H., Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinert A.F., Kunz M., Prager P., et al. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther. 2015;6:114. doi: 10.1186/s13287-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldino J.B., Muench L.N., Kia C., et al. Intraoperative and in vitro classification of subacromial bursal tissue. Arthroscopy. 2020;36:2057–2068. doi: 10.1016/j.arthro.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Dyrna F., Zakko P., Pauzenberger L., McCarthy M.B., Mazzocca A.D., Dyment N.A. Human subacromial bursal cells display superior engraftment versus bone marrow stromal cells in murine tendon repair. Am J Sports Med. 2018;46:3511–3520. doi: 10.1177/0363546518802842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa D., Johnson J.D., Kia C., et al. Examining the potency of subacromial bursal cells as a potential augmentation for rotator cuff healing: An in vitro study. Arthroscopy. 2019;35:2978–2988. doi: 10.1016/j.arthro.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Morikawa D., Muench L.N., Baldino J.B., et al. Comparison of preparation techniques for isolating subacromial bursa-derived cells as a potential augment for rotator cuff repair. Arthroscopy. 2020;26:80–85. doi: 10.1016/j.arthro.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Pandey V., Bandi A., Madi S., et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25:1312–1322. doi: 10.1016/j.jse.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Castricini R., Longo U.G., De Benedetto M., et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: A randomized controlled trial. Am J Sports Med. 2011;39:258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 23.Rodeo S.A., Delos D., Williams R.J., Adler R.S., Pearle A., Warren R.F. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: A prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 24.Jeon Y.S., Rhee Y.G. Factors associated with poor active anterior elevation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:786–793. doi: 10.1016/j.jse.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Hamada K., Yamanaka K., Uchiyama Y., Mikasa T., Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469:2452–2460. doi: 10.1007/s11999-011-1896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber C., Fuchs B., Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990;254:81–86. [PubMed] [Google Scholar]
- 28.Goutallier D., Postel J.M., Bernageau J., Lavau L., Voisin M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 29.Thomazeau H., Rolland Y., Lucas C., Duval J.M., Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand. 1996;67:264–268. doi: 10.3109/17453679608994685. [DOI] [PubMed] [Google Scholar]
- 30.Voss A., McCarthy M.B., Allen D., et al. Fibrin scaffold as a carrier for mesenchymal stem cells and growth factors in shoulder rotator cuff repair. Arthrosc Tech. 2016;5:e447–e451. doi: 10.1016/j.eats.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzocca A.D., McCarthy M.B., Chowaniec D.M., Cote M.P., Arciero R.A., Drissi H. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med. 2010;38:1438–1447. doi: 10.1177/0363546509360924. [DOI] [PubMed] [Google Scholar]
- 32.Voss A., McCarthy M.B., Singh H., et al. The influence of trocar fenestration and volume on connective tissue progenitor cells (stem cells) in arthroscopic bone marrow aspiration from the proximal humerus. Arthroscopy. 2017;33:1167–1174 e1161. doi: 10.1016/j.arthro.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Richards R., An K.N., LU Bigliani, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 34.Constant C.R., Murley A.H. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 35.Williams G.N., Gangel T.J., Arciero R.A., Uhorchak J.M., Taylor D.C. Comparison of the single assessment numeric evaluation method and two shoulder rating scales. Am J Sports Med. 1999;27:214–221. doi: 10.1177/03635465990270021701. [DOI] [PubMed] [Google Scholar]
- 36.Lippitt S.B., Harryman D.T., Matsen F.A. In: The shoulder: A balance of mobility and stability. Matsen F.A., Fu F.H., Hawkins R.J., editors. American Academy of Orthopaedic Surgeons; Rosemont, IL: 1993. A practical tool for evaluating function: The simple shoulder test; pp. 501–518. [Google Scholar]
- 37.Beaton D., Richards R. Assessing the reliability and responsiveness of 5 shoulder questionnaires. J Shoulder Elbow Surg. 1998;7:565–572. doi: 10.1016/s1058-2746(98)90002-7. [DOI] [PubMed] [Google Scholar]
- 38.Godfrey J., Hamman R., Lowenstein S., Briggs K., Kocher M. Reliability, validity, and responsiveness of the simple shoulder test: Psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16:260–267. doi: 10.1016/j.jse.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Thigpen C.A., Shanley E., Momaya A.M., et al. Validity and responsiveness of the single alpha-numeric evaluation for shoulder patients. Am J Sports Med. 2018;46:3480–3485. doi: 10.1177/0363546518807924. [DOI] [PubMed] [Google Scholar]
- 40.Harris J.D., Brand J.C., Cote M.P., Faucett S.C., Dhawan A. Research pearls: The significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy. 2017;33:1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 41.Cvetanovich G.L., Gowd A.K., Liu J.N., et al. Establishing clinically significant outcome after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2019;28:939–948. doi: 10.1016/j.jse.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Saltzman B.M., Jain A., Campbell K.A., et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32:906–918. doi: 10.1016/j.arthro.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Carr J.B., 2nd, Rodeo S.A. The role of biologic agents in the management of common shoulder pathologies: Current state and future directions. J Shoulder Elbow Surg. 2019;28:2041–2052. doi: 10.1016/j.jse.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 44.Hurley E.T., Lim Fat D., Moran C.J., Mullett H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: A meta-analysis of randomized controlled trials. Am J Sports Med. 2019;47:753–761. doi: 10.1177/0363546517751397. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J.G., Zhao L., Jiang Y.X., Wang Z.L., Wang J., Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: A meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:125–135. doi: 10.1016/j.arthro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Malavolta E.A., Gracitelli M.E.C., Assuncao J.H., Ferreira Neto A.A., Bordalo-Rodrigues M., de Camargo O.P. Clinical and Structural evaluations of rotator cuff repair with and without added platelet-rich plasma at 5-year follow-up: A prospective randomized study. Am J Sports Med. 2018;46:3134–3141. doi: 10.1177/0363546518795895. [DOI] [PubMed] [Google Scholar]
- 47.Randelli P., Arrigoni P., Ragone V., Aliprandi A., Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: A prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Milano G., Saccomanno M.F., Careri S., Taccardo G., De Vitis R., Fabbriciani C. Efficacy of marrow-stimulating technique in arthroscopic rotator cuff repair: A prospective randomized study. Arthroscopy. 2013;29:802–810. doi: 10.1016/j.arthro.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi N., Suenaga N., Oizumi N., et al. Bone marrow stimulation at the footprint of arthroscopic surface-holding repair advances cuff repair integrity. J Shoulder Elbow Surg. 2015;24:860–866. doi: 10.1016/j.jse.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Bigliani L.U., Cordasco F.A., McIlveen S.J., Musso E.S. Operative treatment of failed repairs of the rotator cuff. J Bone Joint Surg Am. 1992;74:1505–1515. [PubMed] [Google Scholar]
- 51.Nho S.J., Delos D., Yadav H., et al. Biomechanical and biologic augmentation for the treatment of massive rotator cuff tears. Am J Sports Med. 2010;38:619–629. doi: 10.1177/0363546509343199. [DOI] [PubMed] [Google Scholar]
- 52.Ecklund K.J., Lee T.Q., Tibone J., Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007;15:340–349. doi: 10.5435/00124635-200706000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lippe J., Spang J.T., Leger R.R., Arciero R.A., Mazzocca A.D., Shea K.P. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy. 2012;28:154–159. doi: 10.1016/j.arthro.2011.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.