Abstract
Purpose
To evaluate whether allograft anterior cruciate ligament reconstruction (ACLR) is superior or inferior to autograft ACLR or conservative management in terms of effectiveness and safety.
Methods
A systematic review of the evidence for allograft ACLR was conducted. Randomized controlled trials with a minimum mean follow-up time of 5 years were included. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and the EUnetHTA-Core-Model were used as reporting standards. A meta-analysis was conducted for selected crucial outcomes using a random-effects model. The strength of the available evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results
Six randomized trials were included comparing allograft with autograft. Patients were on average between 28 and 32.8 years of age (allograft group) and 28.9 and 31.7 years of age (autograft group). Based on the crucial outcomes, the meta-analyses showed no statistically significant differences in Lysholm score, Tegner score, and Cincinnati Knee Score between groups. A small statistical difference favoring autografts was found across studies in the subjective International Knee Documentation Committee score (–2.25; 95% confidence interval –3.02 to –1.47; I2 = 0%; range of all scores: 73.7-90). Two of six studies reported on graft failure, with a statistically significant difference to the detriment of using allografts (13/49 [26.5%] vs 4/48 [8.3%] in one study, 13/43 [30.2%] vs 3/40 [7.5%] in the other study).
Conclusions
Although no substantial difference in patient-reported function, activity level, and symptoms was demonstrated, evidence from the included studies showed a greater risk for graft failure or revision that may make allograft a less safe treatment modality in ACLR. The strength of available evidence is low based on the crucial outcomes due to the lack of high-quality research and the present increased risk of bias in primary studies. Priority should be shifted toward reflecting on whether there is a subpopulation for whom allograft ACLR may still be advantageous in theory (e.g., less-active older patients) and further conduct RCTs in this population.
Level of Evidence
Level II, systematic review of Level II evidence studies.
Anterior cruciate ligaments (ACLs) are important structures for the knee, providing both support and stability.1 Ruptures can result from sports injuries and can be treated conservatively (i.e., progressive physiotherapy, rehabilitation) or surgically. The latter is usually indicated if patients are in physically demanding situations (e.g., if they are athletes, have high demands with regard to knee function in the future), or if suffering from complex capsule ligament injuries.2 Yet, the need for surgical treatment is not always that clear—as shown within a randomized controlled trial (RCT)3,4 indicating that in young, active adults with acute ACL tears, a strategy of rehabilitation plus early anterior cruciate ligament reconstruction (ACLR) was not superior to a strategy of rehabilitation plus optional delayed ACLR in terms of patient-reported knee function. Based on this RCT, a Cochrane review5 concluded that there is low-quality evidence showing no difference in patient-reported knee function outcomes when comparing surgical management and conservative treatment 2 and 5 years after the ligament was ruptured. The review authors did, however, note that many of the patients in the conservative management group in the RCT did opt for an ACLR later.
If surgical treatment is chosen, the reconstruction is usually performed using arthroscopy. However, there is an ongoing debate on graft selection: grafts can be either autogenous (i.e., ligament’s from patients’ own body) or allogenous (i.e., from a donor body).6, 7, 8 Both of these graft types have their own strengths and weaknesses: allografts eliminate donor-site morbidity, potentially reduce pain, surgery time, and eventually lead to quicker recovery,9,10 but it may be on the offset of potential disadvantages: immunologic reactions, slower remodeling, integration, disease transmission, and increased costs.7 In this context, patients undergoing ACLR using allografts and younger patients may be at increased risk of subsequent graft failure.11
As this controversy continues, the Ludwig Boltzmann Institute for Health Technology Assessment (HTA) was asked to conduct a systematic review regarding the effectiveness and safety of cruciate ligament reconstruction (CLR) using allografts as part of the annual evaluation of “extra-medical services” in the hospital benefit catalogue. The policy question was whether there is a patient-relevant added benefit of using allograft CLR instead of conventional autograft CLR or conservative treatment in several different indications to guide an evidence-based reimbursement decision (the full HTA report is available online12). In this article, results of the conducted HTA report in the context of ACLR are described and an additional meta-analysis is conducted. Hence, the purpose of this systematic review was to evaluate whether allograft ACLR is superior or inferior to autograft ACLR or conservative management in terms of effectiveness and safety. Our hypothesis was that there would a difference using allografts versus autografts for ACLR regarding clinical outcome, safety, and complication rates.
Methods
To identify eligible studies, a systematic review on the comparative effectiveness and safety of allografts in comparison to autografts in ACLR was conducted. The EUnetHTA Core Model for rapid relative effectiveness assessment13,14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement15,16 were used as reporting standards.
Search Strategy
A systematic literature search was carried out in four databases: Medline, Embase, the Cochrane Library, and the University of York Centre for Reviews and Dissemination. The search was conducted in December 2018. The full search strategy can be found online.12 Supplementary, a manual search in PubMed and websites such as UpToDate (https://www.uptodate.com/home) was conducted. The search was limited to articles in English and German. In addition, we conducted a manual search in PubMed and arthroscopy.org and could not find further eligible publications published in 2019 or through May 2020.
Selection Criteria
RCTs that elaborated on the long-term effectiveness of allograft ACLR were considered eligible to be included in this assessment. Autograft ACLR or conservative management were selected as relevant distinct active comparators. Concerning sample size, only studies with more than 50 patients were considered to be eligible for the evidence synthesis. Due to the fact that the aim of this article was to assess the long-term comparative effectiveness and safety of allograft ACLR, the minimum length of follow-up was set to be 5 years. Studies with a mean follow-up period less than 5 years were excluded. An overview of the eligibility criteria according to the PICO principle (i.e., Population, Intervention, Comparison, and Outcome) can be found in Table 1.
Table 1.
Eligibility Criteria Based on the PICOS (Population, Intervention, Control, Outcomes) Tool
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Patients who are candidates for anterior cruciate ligament reconstruction (ACLR) | All other indications |
| Intervention | Allograft cruciate ligament reconstruction | Other forms of therapy (e.g., using other graft choices)/conservative management |
| Control | Other techniques of cruciate ligament reconstruction (autograft, synthetic graft, etc.) Conservative management |
No restriction |
| Outcomes | ||
| Effectiveness | Crucial: Patient-reported function, activity level and symptoms measured using a validated instrument (e.g., Lysholm score, Tegner score, IKDC scores) Important: Clinical knee stability measured using a validated instrument (e.g., KT-1000/2000 arthrometer, the Lachman test or pivot shift test), health-related quality of life measured using a validated instrument, patient satisfaction measured using a validated instrument |
All other outcomes |
| Safety | Crucial: Graft failure, re-ruptures, re-operations and revisions, complications Important: Procedure-related mortality |
All other outcomes |
| Study design | Randomized controlled trials with mean follow up of at least 5 years and more than 50 patients. | Randomized controlled trials with a mean follow-up of less than 5 years and less or equal to 50 enrolled patients. Not-randomized trials, observational studies. Studies published as an abstract only. |
IKDC, International Knee Documentation Committee.
Study Selection
Three researchers were involved in the study selection of relevant publications. Two review authors screened the abstracts (G.G., S.G.G.), and consequently reviewed potentially relevant full-text articles (G.G., C.d.V.), based on the predefined inclusion criteria. Discrepancies were resolved by consensus, and a senior researcher (S.G.G.) was consulted in case these could not be resolved as outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.15,16
Selected Outcomes
Patient-relevant outcomes were chosen within the scoping phase. In accordance with Grading of Recommendations Assessment, Development and Evaluation (GRADE),17 these outcomes were judged as either critical or important. In addition, the critical outcomes were judged to be crucial for supporting the evidence-based recommendation whether to adopt the technology in the hospital benefit catalog of Austria. For the evaluation of the comparative effectiveness of allograft ACLR, patient-reported function, activity level and symptoms measured using validated instruments (e.g., Lysholm score, Tegner score) were judged to be crucial. Further important outcomes included clinical knee stability, health-related quality of life, and patient satisfaction. Similarly, the important outcomes also had to be measured using a validated instrument. To elaborate on how allograft ACLR compares in terms of safety, crucial outcomes covered graft failure, re-ruptures, reoperations, and revisions as well as complications. The conclusions were mainly based on the results of the outcomes that were judged to be crucial for patients.
Data Extraction, Quality Appraisal, and Analysis
Risk of bias of the eligible studies was assessed using the Cochrane Risk of Bias tool.18 The single data extraction method with verification by another reviewer was used. The data were extracted by means of piloted forms for systematic reviews by one researcher (G.G.) and verified by another researcher (C.d.V.). The evidence was primarily qualitatively synthesized and the strength of the available evidence was assessed using the GRADE approach.17 Evidence of different comparisons (allograft vs autograft ACLR; allograft ACLR vs conservative treatment) was separated accordingly.
To strengthen the interpretation of the results, a meta-analysis was performed for selected crucial outcomes when data was reported by more than 2 studies. A random-effects model was chosen hereby and the meta-analysis was performed using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark; 2014). For continuous variables, the mean difference between groups was calculated. For dichotomous variables, the risk ratio is chosen as a statistical measure. A significance level of .05 was chosen and the confidence intervals were reported.
Results
Search Results
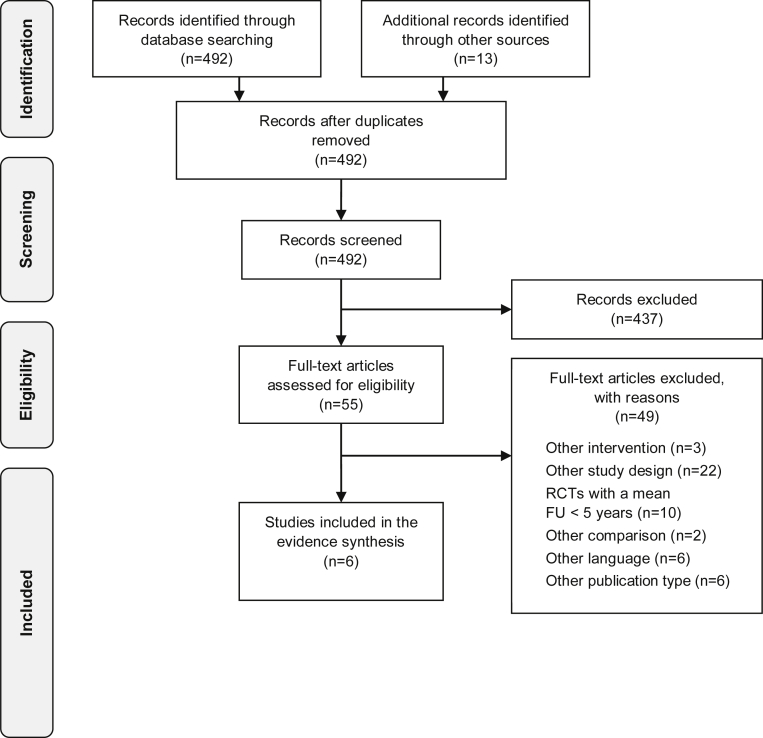
The database search resulted in 492 records after de-duplication. The abstract screening revealed that 437 publications did not meet the inclusion criteria. As a result, 55 full-text articles were further assessed for eligibility. A total of 49 records were not eligible and excluded with reason, resulting in 6 studies eligible for the evidence synthesis (see Fig 1)
Fig 1.
PRISMA flow chart. (PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.)
Literature Review
Characteristics of Included Studies
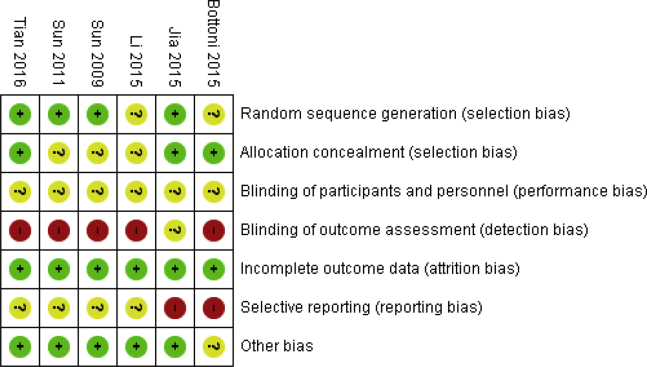
Table 2 provides an overview of the characteristics of all included studies. The evidence consists of 6 randomized controlled trials,19, 20, 21, 22, 23, 24 comparing allograft with autograft ACLR in patients with a ruptured anterior cruciate ligament. Notably, differences existed when it came to graft type and procedures used. That is to say, these differences were primarily in the context of whether the grafts were fresh-frozen, irradiated or not, or as to which tendons were used (e.g., bone–patellar bone tendon–bone, hamstring tendons, etc.). Two studies,19,22 for instance, used irradiation as a method to disinfect allografts, whereas no irradiation was used in the remaining studies.20,21,23,24 As shown in Figure 2, the risk of bias was unclear to high in all studies, mainly due to the lack of blinding and reporting bias.
Table 2.
Characteristics of Included Studies
| Author, Year | Bottoni et al., 201520 | Sun et al., 200923 | Sun et al., 201124 | Tian et al., 201619 | Li et al., 201522 | Jia and Sun, 201521 |
|---|---|---|---|---|---|---|
| Country | USA | China | China | China | China | China |
| Sponsor | Arthrex, and the Musculoskeletal Transplant Foundation | Supported by Natural Science Foundation of China Grant no. 2004GG2202034 | Research funding was provided by the Key Project of the Provincial Science Foundation of Shandong |
NR | NR | Nil |
| Interventions/products | Fresh-frozen, nonirradiated tibialis posterior tendon allograft | Fresh frozen, nonirradiated BPTB hemi-allograft |
Fresh-frozen, nonirradiated hamstring tendon allograft | Fresh-frozen, irradiated hamstring tendon allograft | 4-stranded, y-irradiated tibialis anterior tendon allograft | Bone-patellar tendon-bone allograft |
| Comparator | 4-stranded hamstring autograft | BPTB autograft | 4-stranded hamstring tendon autograft | Hamstring tendon autograft | 4-stranded gracilis and semitendinosus tendon autograft Hybrid graft (y-irradiated tibialis anterior tendon allograft and semitendinosus tendon autograft) |
Hamstring autograft |
| Surgical procedure | ACLR (not further specified) | Arthroscopic ACLR |
Arthroscopic ACLR |
Arthroscopic anatomic double-bundle ACLR | ACLR (not further specified) | Arthroscopic ACLR |
| Study design | RCT | RCT | RCT | RCT | RCT | RCT |
| Number of patients∗ | 99 patients (100 knees) 50 vs 50 (knees) |
172† 86 vs 86 |
208‡ 104 vs 104 |
107§ 53 vs 54 |
102‖ 34 vs 34 vs 34 |
106¶ 53 vs 53 |
| Inclusion criteria | Patients 18 years of age or older with symptomatic ACL deficiency, confirmed by MRI |
Only primary unilateral reconstructions of the ACL were included in the study.# Patients with minor medial collateral ligament sprains (lower than grade II), previous diagnostic arthroscopy, or meniscal tears were not excluded from the study. |
Only primary unilateral reconstructions of the ACL were included in the study.# No previous injury or surgery on the affected knee, No multiple ligamentous injuries or malalignment, and Ability to complete the study protocol. Patients with minor medial collateral sprains (<grade 2), meniscal tears and or previous diagnostic arthroscopes were not excluded. |
Patients with acute or chronic ACL ruptures Only primary unilateral reconstructions of the ACL were included in the study.# |
Unilateral ACL rupture verified clinically by positive Lachman test and positive pivot shift test findings. All patients had undergone a preoperative MRI scan to confirm the ACL rupture. All patients with normal preoperative CRP (<10 mg/L) and ESR (≤15 mm/h) values were included. |
Diagnosis with ACL tear by physical examination and MRI, normal alignment, normal contralateral knee, and willingness to join the rehabilitation program |
| Exclusion criteria | Multiligamentous injuries (concomitant grade I or II medial collateral ligament injuries were not excluded), Previous knee ligament surgery (previous knee arthroscopic surgery was not excluded), and Time remaining on the island of less than 6 months |
Patients were excluded from the study if they had had a previous injury to or surgery on the affected knee; multiple ligamentous injuries, or malalignment; or if they lacked the ability to complete the study protocol. Patients undergoing revision reconstruction and those with associated injuries of the posterior cruciate ligament or posterolateral corner or with deficiency or reconstruction of the ACL in the contralateral knee were also excluded. |
Revision reconstruction and patients with associated injuries of the posterior cruciate ligament or the posterolateral corner, with deficiency, or a reconstruction of the ACL in the contralateral knee were excluded. | Patients were excluded if they had a previous injury or surgery on the affected knee, had open physes present, had severe arthritic changes in the knee, had multiple ligamentous injuries, had malalignment, lacked the ability to complete the study protocol, a revision reconstruction, associated injuries of the posterolateral corner, and deficiency or reconstruction of the ACL in the contralateral knee. |
Combined multiple-ligament injuries Previous ACL surgery Contralateral knee ligament injury, Radiographically verified osteoarthritis Patients with pre-existing metabolic pathologies such as diabetes mellitus or uremia were excluded from the study. Those patients who could not finish the minimum clinical follow-up period of 5 years were also excluded. |
NR |
| Rehabilitation (before or after ACLR) | Physical therapy∗∗ (standardized protocol) | Physical therapy∗∗ (same protocol) | Physical therapy∗∗ (same protocol) | Physical therapy∗∗ (same protocol) | Physical therapy∗∗ (standardized protocol) | Physical therapy∗∗ (same program) |
| Age of patients, y, mean ± SD (range) | 29.2 ± 5.5 (20.7-41.5) vs 28.9 ± 5.8 (20.6-42.5) | 32.8 ±7.1 (19-65) vs 31.7 ±6.3 (20-54)†† | 31.2 ± 8.3 (18-59) vs 29.6 ±6.9 (19-56)†† | 28.6 ±7.2 (18-50) vs 29.2 ± 6.9 (18-55)†† | 30.5 ±6.1 vs 29.8 ±7.9 vs 31.6 ±8.2††,‡‡ | 28 vs 31§§ |
| Sex, n female (%) | 6 (12.2) vs 7 (14.6) | 17 (21.3) vs 15 (20) | 17 (17.9) vs 20 (22) | 9 (20.9) vs 8 (20) | 17 (53.1) vs 15 (46.9) vs 13 (41.9) | 27 (50.9) vs 25 (47.2) |
| Further relevant patient characteristics at baseline and cointerventions | 95% of patients were in the military (active-duty). Concomitant meniscal and chondral pathologic abnormalities, microfracture, and meniscal repair performed at the time of reconstruction were similar in both groups. No statistically significant differences were found when comparing the respective baseline characteristics between allograft and autograft groups, except for lateral compartment: grade 0: 43 (87.8%) vs 35 (72.9%); grade 1: 3 (6.1%) vs 3 (6.3%); grade 2: 1 (2%) vs 6 (12.5%); grade 3: 2 (4.1%) vs 0 (0%); grade 4: 0 (0%) vs 4 (8.3%). Difference of LC category was s.s. with P = .034. |
No statistically significant differences between treatment groups when considering arthroscopic findings and treatments at time of ACLR No. of patients with normal meniscus (no treatment of meniscal tears at time of ACL reconstruction): 36/80 patients (45%) vs 36/76 patients (47%) |
No statistically significant differences between treatment groups when considering arthroscopic findings and treatments at time of ACLR No. of patients with normal meniscus (no treatment of meniscal tears at ACL reconstruction): 48/95 patients (50.5%) vs 45/91 patients (49.5%) |
No statistically significant differences between treatment groups when considering arthroscopic findings and treatments at time of ACLR No. of patients with normal meniscus (no treatment of meniscal tears at ACL reconstruction): 18/43 patients (41.9%) vs 16/40 patients (40.0%) |
No statistically significant differences when considering associated injuries and treatments before ACLR No. of patients with no treatment of meniscal tears: 3/32 patients (9.4%) vs 6/32 patients (18.8%) vs 7/31 patients (22.6%) |
Baseline characteristics insufficiently described |
| Mean follow-up, y | 10.5, range: 10-11 | 5.6, range: 4-8 | 7.8, range: 6-10 7.9 (SD 1.1) vs 7.6 (SD 0.9) |
6.9, range: 5.5-8 6.8 (SD 0.8) vs 7 (SD 0.7) |
5.9 (overall mean), range: 5-7 6.1 (SD 0.3) vs 5.8 (SD: 0.9) vs 5.9, SD 0.6 |
6.75, range: 2.33-7.16 |
| Loss to follow-up, n (%)‖‖ | Overall: 3 (3) 1 vs 2¶¶ |
Overall: 16 (9.3) 6 (6.9) vs 10 (11.6) |
Overall: 22 (10.6) 9 (8.7) vs 13 (12.5) |
Overall: 24 (22.4) 10 (18.9) vs 14 (25.9) |
Overall: 7 (6.8) 2 (5.9) vs 2 (5.9) vs 3 (8.8) | Overall: 0 (0) 0 (0) vs 0 (0) |
| Patients included in analysis, n | 49 vs 48 (knees)## | 80 vs 76 | 95 vs 91 | 43 vs 40 | 32 vs 32 vs 31 | 53 vs 53 |
NOTE. Source: Goetz and de Villiers.12
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; BPTB, bone–patellar tendon–bone; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LC, lateral compartment; MRI, magnetic resonance imaging; NR, not reported; RCT, randomized controlled trial; SD, standard deviation; s.s., statistically significant.
At time of randomization.
218 patients underwent ACL reconstruction. Of those, 195 patients were eligible to participate in the study. 172 patients provided written, informed consent and were randomized to different treatment groups.
256 patients were assessed for eligibility, of whom 208 were randomized to the different treatment groups.
121 patients were assessed for eligibility, of whom 107 were eligible and randomized to the different treatment groups.
281 patients were assessed for eligibility, of whom 102 patients were randomized to the different treatment groups.
122 patients were assessed for eligibility, of whom 106 patients were randomized to the different treatment groups.
All patients had an MRI scan obtained preoperatively to exclude combined, complicated ligament injuries to their knees.
Physical therapy may have included, but was not limited to, the following: preoperative therapy to restore full knee range, normal gait, and eliminate knee swelling; postoperative: Full extension range of motion, strengthening exercises, range of motion brace (for 4 weeks postsurgery), and a functional brace for sport activities (for 1-2 years after surgery). Adaptations for range of motion restriction and weightbearing status applied, with accompanying meniscal and chondral surgery. 2 studies explicitly reported that physical therapy provided outside of the institution may have varied and may have been a factor that influenced the outcomes.
The study only described information on the age and sex of the patients who were analyzed (as opposed to the number of patients who were originally randomized). Therefore, the denominator used to calculate the percentages is the number of patients analyzed in the respective treatment group.
The range of the variable age was not reported in the study.
SD and range were not reported.
Due to the fact that calculating the loss to follow-up is sometimes confused in clinical studies, the loss to FU was calculated by the review authors using consistent criteria: The follow-up rate was calculated using the number of randomised patients as the denominator and the number of patients analyzed as the numerator. The difference between randomised and analyzed patients was therefore considered to be patients lost to FU.46
The review authors judged it to be spurious that the investigators switched constantly between knees and patients when reporting characteristics of patients and results. Given that it was only reported that 50 knees were randomised in two groups, the percentage for the loss to FU in each group was not estimable. Of the patients lost to follow-up, 2 were deceased, and 1 patient was lost to follow-up for other reasons.
It was unclear to the review authors whether the presented results refer to 97 knees or patients, because the study did not clearly report it. Given that 3 patients were lost to follow-up, 97 knees must have been considered in the analysis.
Fig 2.
Risk of bias of included studies. Note that the full risk of bias assessment is available in the appendix of the HTA report (available online12). (HTA, Health Technology Assessment.)
In total, there were 794 patients enrolled in these studies at time of randomization (range 99-208). There were 69 patients lost to follow-up, and 34 patients received hybrid grafts. In addition, one study referred to knees instead of patients. As a result, there were 352 and 340 patients receiving allografts and autografts, respectively (n = 692). The reader must hereby be aware that one of these patients refer to a knee.
On average, the studies followed the patients between 5.6 and 10.5 years, with a loss to follow-up ranging from 3% to 22.4%. Across all studies, the patients were on average between 28 and 32.8 years of age (allograft group) and 28.9 and 31.7 years of age (autograft group), respectively. The rate of female patients within the included studies ranged from 12.2% to 53.1% (allograft group) and 14.6% to 47.2% (autograft group).
There was no difference in baseline characteristics detected in the included studies. That is, 5 of 6 included studies19,20,22, 23, 24 stated that differences in baseline characteristics were tested and mostly not statistically different. However, chondral pathologic abnormalities of the lateral femoral condyle were statistically significantly different in 1 study,20 with 87.8% in the allograft group as opposed to 72.9% with grade 0 lateral compartment in the autograft group, respectively. Cointerventions included, for instance, pre- and postoperative rehabilitation (including physiotherapy), but all studies reported that the same postoperative rehabilitation program was followed. A notable further cointerventions included meniscus treatment (e.g., reconstruction) in 5 of 6 studies.19,20,22, 23, 24 One of the included studies insufficiently reported on baseline characteristics or cointerventions.21 In all of the included studies,19, 20, 21, 22, 23, 24 the significance level was defined as P < .05.
Allograft Versus Autograft in ACLR
Effectiveness
The outcomes patient-reported function, activity level, and symptoms were judged to be critical and, hence, crucial for deriving a recommendation within the HTA process. Further important outcomes covered clinical knee stability, health-related quality of life (HRQOL), and patient satisfaction.
Patient-Reported Function, Activity Level, and Symptoms
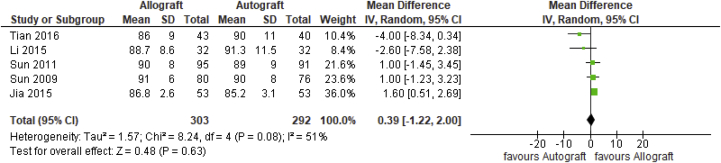
Mean difference was only statistically significantly different in 1 of 4 outcomes for which a meta-analysis could have been performed. The Lysholm score was reported in 5 included studies,19,21, 22, 23, 24 with overall 595 analyzed patients. As depicted in Figure 3, the pooled not-statistically significant mean difference between allograft and autograft groups across studies was 0.39 (95% confidence interval [CI] –1.22 to 2.00; P = .63; I2 = 51%). The mean Lysholm scores ranged from 86 (±9) to 91 (±6) in the allograft groups and 85.2 (±3.1) to 91.3 (±11.5) in the autograft groups
Fig 3.
Lysholm score outcome: forest plot of the results of individual studies. (CI, confidence interval; IV, inverse variance; SD, standard deviation.)
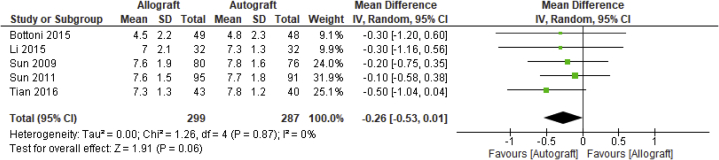
Five studies reported on the Tegner score,19,20,22, 23, 24 with 586 analyzed patients across studies. As depicted in Figure 4, the pooled nonstatistically significant mean difference between allograft and autograft groups across studies was –0.26 (95% CI –0.53 to 0.01; P = .06, I2 = 0%). The mean Tegner scores ranged from 4.5 (±2.2) to 7.6 (±1.9) in the allograft groups and 4.8 (±2.3) to 7.8 (±1.6) in the autograft groups.
Fig 4.
Tegner score outcome: forest plot of the results of individual studies. (CI, confidence interval; IV, inverse variance; SD, standard deviation.)
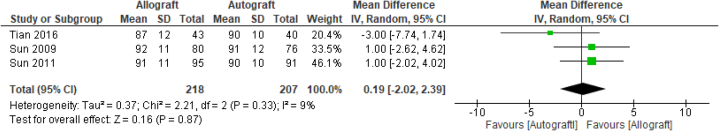
Three further studies19,23,24 reported on the Cincinnati Knee score, with 425 analyzed patients. As depicted in Figure 5, the pooled nonstatistically significant mean difference between allograft and autograft groups across studies was 0.19 (95% CI –2.02 to 2.39; P = .87; I2 = 9%). The mean Cincinnati Knee Scores ranged from 87 (±12) to 92 (±11) in the allograft groups and 90 (±10) to 91 (±12) in the autograft groups. The Single Assessment Numerical Evaluation score was only reported in 1 study,20 with a nonstatistically significant measured mean score difference of 78.8 ± 18.8 in the allograft group and 81.5 ± 16.4 in the autograft group.
Fig 5.
Cincinnati Knee Score: forest plot of the results of individual studies. (CI, confidence interval; IV, inverse variance; SD, standard deviation.)
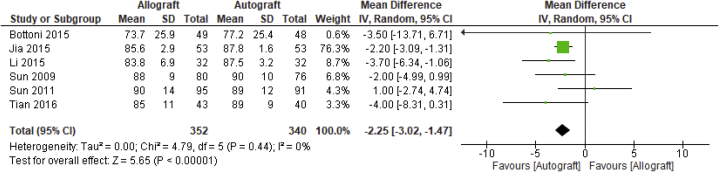
All of the 6 included studies reported on the subjective International Knee Documentation Committee (IKDC) score,19, 20, 21, 22, 23, 24 with 692 analyzed patients across studies. As depicted in Figure 6, the pooled mean difference between allograft and autograft groups across studies was –2.25 (95% CI –3.02 to –1.47; P < .00001; I2 = 0%). The mean subjective IKDC scores ranged from 73.7 (±25.9) to 90 (±14) in the allograft groups and 77.2 (±25.4) to 90 (±10) in the autograft groups.
Fig 6.
Subjective International Knee Documentation Committee: forest plot of the results of individual studies. (CI, confidence interval; IV, inverse variance; SD, standard deviation.)
The results of the patient reported function activity level and symptoms outcomes reached low (Lysholm score, Single Assessment Numerical Evaluation score) to moderate (Tegner score, Cincinnati Knee score, subjective IKDC score) certainty according to GRADE (Table 3).25 The other selected patient-reported outcomes (Knee injury and Osteoarthritis Outcome Score and Marx activity scale) were not reported in any of the included studies.
Table 3.
Evidence Profile: Comparative Effectiveness and Safety of Allograft Versus Autograft in ACLR
| Certainty Assessment |
No. Analyzed Patients∗,† |
Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | LoE | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Allograft | Autograft | |||
| Effectiveness | ||||||||||||
| Patient-reported function, activity level, and symptoms (follow-up: mean ≥5 years; assessed with: Lysholm score) | ||||||||||||
| 5 19,21, 22, 23, 24 |
RCT | II | Serious‡ | Serious§ | Not serious‖ | Not serious | None | 303 | 292 | MD 0.39 (95% CI –1.22 to 2.00; P = .63; I2 = 51%) | ⨁⨁⊕⊕◯◯ Low |
Critical |
| Patient-reported function, activity level and symptoms (follow-up: mean ≥5 years; assessed with: Tegner score) | ||||||||||||
| 5 19,20,22, 23, 24 | RCT | II | Serious¶ | Not serious# | Not serious‖ | Not serious | None | 299 | 287 | MD –0.26 (95% CI –0.53 to 0.01; P = 0.06 I2 = 0%) | ⨁⨁⨁⊕⊕⊕◯ Moderate |
Critical |
| Patient-reported function, activity level and symptoms (follow-up: mean ≥5 years; assessed with: Cincinnati Knee score) | ||||||||||||
| 3 19,23,24 | RCT | II | Serious∗∗ | Not serious†† | Not serious‖ | Not serious | None | 218 | 207 | MD 0.19 (95% CI –2.02 to 2.39; P = 0.87; I2 = 9%) | ⨁⨁⨁⊕⊕⊕◯ Moderate |
Critical |
| Patient-reported function, activity level and symptoms (follow-up: mean 10.5 years; assessed with: SANE score) | ||||||||||||
| 120 | RCT | II | Serious‡‡ | Not serious | Serious§§ | Not serious | None | 49 | 48 | The study did not find a statistically significant difference: The postoperative mean score was 2.7 points lower in the allograft group when compared to the autograft group. Postoperative mean SANE score: 78.8 ± 18.8 vs 81.5 ± 16.4 |
⨁⨁⊕⊕◯◯ Low |
Critical |
| Patient-reported function, activity level and symptoms (follow-up: mean ≥5 years; assessed with: subjective IKDC score) | ||||||||||||
| 6 19, 20, 21, 22, 23, 24 |
RCT | II | Serious‡‡ | Not serious | Not serious‖ | Not serious | None | 352 | 340 | MD –2.25 (95% CI –3.02 to –1.47; P < .00001; I2 = 0%). | ⨁⨁⨁⊕⊕⊕◯ Moderate |
Critical |
| Clinical knee stability (follow-up ≥5 years; assessed with: Lachman test) | ||||||||||||
| 4 19,22, 23, 24 |
RCT | II | Very serious‖‖ | Serious¶¶ | Not serious | Not serious | None | 250 | 239 | s.s. difference in Lachman scores (grade 0-1) in 1 study19: 31/43 (72%) vs 37/40 (93%). No statistically significant differences in Lachman scores in 3 studies22, 23, 24 Lachman test (grade 0-1; ranges across studies): 31/43 (72%) to 74/80 (92.5%) vs 84/91 (92.3%) to 30/32 (93.8%) |
⨁⊕◯◯◯ very low |
Important |
| Clinical knee stability (follow-up: mean ≥5 years; assessed with: pivot shift test) | ||||||||||||
| 4 19,22, 23, 24 |
RCT | II | Very serious‖‖ | Serious¶¶ | Not serious | Not serious | None | 250 | 239 | s.s. difference in Pivot shift test (Grade 0-1) in 1 study19: 38/43 (88.4%) vs 40/40 (100%) No statistically significant differences in pivot shift test in 3 studies22, 23, 24 Pivot shift (grade 0-1; ranges across studies): 38/43 (88.4%) to 95/95 (100%) vs 32/32 (100%) to 91/91 (100%) |
⨁⊕◯◯◯ Very low |
Important |
| Clinical knee stability (follow-up: mean ≥5 years; assessed with: KT arthrometer; better indicated by lower values) | ||||||||||||
| 4 19,22, 23, 24 |
RCT | II | Serious‡‡ | Serious¶¶ | Not serious | Not serious | None | 250 | 239 | 2/4 studies19,22 found a statistically significant difference in instrumented knee laxity favoring autografts, while the other 2/4 studies23,24 did not find any statistically significant difference in side-to-side differences measured with the KT arthrometer between treatment groups. Mean side-to-side differences (in mm; ranges across studies): 2.5 ± 0.9 to 5.5 ± 1 vs 2.1 ± 1.6 to 2.5 ± 0.7 |
⨁⨁⊕⊕◯◯ Low |
Important |
| Clinical knee stability (follow-up: mean ≥5 years; assessed with: objective IKDC score) | ||||||||||||
| 4 19,22, 23, 24 |
RCT | II | Serious‡‡ | Not serious | Not serious | Not serious | None | 250 | 239 | None of the studies found a statistically significant difference in the objective IKDC score between treatment groups. Objective IKDC score (normal or nearly normal scores; ranges across studies): 38/43 (88.4%) to 75/80 (93.8%) vs 29/32 patients (90.6%) to 38/40 (95%) |
⨁⨁⨁⊕⊕⊕◯ Moderate |
Important |
| Patient satisfaction (assessed with: NR) | ||||||||||||
| 1 21 | RCT | II | Very serious## | Not serious | Not serious | Not serious | None | 53 | 53 | Patient satisfaction was analyzed in 106 patients from 1 study. The study found no statistically significant difference between patients undergoing allograft ACLR (n = 53) or autograft ACLR (n = 53). The instrument used to measure patient satisfaction was not reported. Satisfied: 46/53 (86.8%) vs 47/53 (88.7%) Nearly satisfied: 7 (13.2%) vs 5 (9.4%) Diff. n.s.; P > .05 |
⨁⨁⊕⊕◯◯ Low |
Important |
| Safety | ||||||||||||
| Graft failure (follow-up: mean ≥5 years) | ||||||||||||
| 2 19,20 |
RCT | II | Not serious∗∗∗ | Not serious | Serious‖,§§ | Not serious | None | 92 | 88 | Bottoni et al.20: 13/49 (26.5%) vs 4/48 (8.3%), diff. s.s. with P < .05 Tian et al.19: 13/43 (30.2%) vs 3/40 (7.5%), diff. s.s. with P < .001††† |
⨁⨁⨁⊕⊕⊕◯ Moderate |
Critical |
| Revisions (follow-up: mean ≥5 years) | ||||||||||||
| 220,22 | RCT | II | Not serious∗∗∗ | Serious‡‡‡ | Serious‖,§§ | Not serious | None | 81 | 80 | Bottoni et al.20: 13/49 (26.5%) vs 4/48 (8.3%), diff. s.s. with P < .05 Li et al.22: no patient needed additional surgery because of recurrent or residual symptoms (0/32 vs 0/32) |
⨁⨁⊕⊕◯◯ Low |
Critical |
| Complications (follow-up: mean ≥5 years) | ||||||||||||
| 6 19, 20, 21, 22, 23, 24 |
RCT | II | Serious§§§ | Not serious | Not serious‖ | Serious‖‖‖ | None | 352 | 340 | Overall complication rate: NR Arthrofibrosis (reported in 2/6 studies19,24; 269 patients): 0/138 (0%) vs 0/131 (0%) Effusion (0/6 studies): NR Tenderness (reported in 1 study24; 186 patients): 0/95 (0%) vs 2/91 (2.1%) Infections (reported in 4 studies19,22, 23, 24; 489 patients): 5/250 (2%) vs 0/239 (0%), range: 0-4.6% vs 0% Hypoesthesia (reported in 2 studies19,24; 269 patients): 0/138 (0%) vs 6/131 (4.6%), range: 0% vs 3.3-7.5%. Synovitis was not reported in any of the included studies. Deep venous thrombosis (reported in 3 studies19,23, 24; 425 patients): 2/218 (0.9%) vs 1/207 (0.5%), range: 0%-2.5% vs 0%-1.3% Further reported complications: Postoperative mean fever time in days (reported in 1 study23; 156 patients): 6.8 vs 4.4 diff. s.s., with P < .05) Arthritic progression (reported in 1 study19; 83 patients): 14/43 (32.6%) vs 4/40 (10%), diff. s.s. with P < .05. Tibial and femoral tunnel widening in millimeters (reported in 2 studies; 203 patients): Jia et al.21: Tibial (in mm), mean ± SD: 7.8 ± 0.4 vs 7.61 ± 0.22, diff. s.s. with P < .05 Femoral (in mm), mean ± SD: 7.64 ± 0.35 vs 7.51 ± 0.42, diff. s.s. with P < .05 Bottoni et al.20: Tibial (in mm), mean (range): 9.2 (7-10) vs 8.9 (7-10); diff. n.s. with P = .651 Femoral (in mm), mean (range): 8.8 (7-10) vs 8.3 (7-10), diff n. s. with P = .453 Furthermore, some of the included studies specifically stated that there were no cases of pain when kneeling, anterior knee pain, etc. |
⨁⨁⊕⊕◯◯ Low |
Critical |
NOTE. Source: Goetz and de Villiers12; results of crucial outcomes (for which at least 3 studies reported on) are depicted quantitatively using MDs between allograft groups and autograft groups across studies. Results of the remaining outcomes for which no meta-analysis was conducted are depicted qualitatively. Furthermore, the LoE of individual studies was further added based on a guidance document.25
ACLR, anterior cruciate ligament reconstruction; CI, confidence interval; Diff., difference; IKDC, International Knee Documentation Committee; LoE, Level of Evidence, MD, mean difference; n.s., not statistically significant; RCT, randomized controlled trial; SANE, Single Assessment Numerical Evaluation; s.s., statistically significant.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence17:
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The reader is reminded that in Bottoni et al. the number of patients actually refers to the number of knees.
Excluding patients with hybrid grafts.
In 4/5 studies, the risk of bias for blinding the outcome assessors was judged to be high. Therefore, we judged that this may have seriously affected the certainty.
Heterogeneity: I2 = 51%.
Differences in interventions were present across studies (e.g., irradiated vs nonirradiated grafts, single-bundle vs double-bundle, etc.).
In 5/5 studies, the risk of bias for blinding the outcome assessors was judged to be high. Therefore, we judged that this may have seriously affected the certainty.
Heterogeneity: I2 = 0%.
In 3/3 studies, the lack of blinding significantly increases the risk of bias. Therefore, we judged that this may have seriously affected the certainty.
None of the studies showed any statistically significant differences in Cincinnati Knee scores between treatment groups. The nonstatistical findings showed slightly greater scores in allograft patients in 2/3 studies and lower scores in allograft patients in 1/3 study/studies when compared with the autograft groups, respectively. The difference of the mean scores ranged from 1 to –3.
The lack of blinding in the study/studies may seriously affect the certainty to believe in the evidence of this outcome measure.
The overall applicability for the broad population selected in these assessment results may suffer due to the fact that numerous different graft types were used and that some studies used a subpopulation of the population of interest. Bottoni et al., for instance, only included highly active military (mostly) men, and Tian et al. used irradiated allografts. It is unclear in how far the generalizability suffers due to the aforementioned factors.
It was judged that the lack of blinding may have very seriously affected the certainty to believe this specific outcome.
Heterogeneity was suspected within the included studies. It appears that the studies do not consistently show any difference/difference favoring a treatment group.
There were 2 substantial factors that increased the risk of bias: lack of blinding and selective outcome reporting; the latter was present insofar as it was insufficiently described how patient satisfaction was measured. In addition, no scores were reported, but it was stated that no statistically significant differences between treatment groups was found.
Lack of blinding for outcome assessors was judged to be less likely to affect this outcome.
Graft failure, however, was defined differently in the studies. Tian et al. defined it as knee laxity >5 mm measured with a KT-2000, and Bottoni et al. did not clearly mention how graft failure was defined.
Bottoni et al. found a considerably large difference in the revision rate, whereas Li et al. stated that no additional surgeries were needed in either of the treatment groups.
The risk of bias for selective outcome reporting was judged high in 2/6 studies, and unclear in the remaining 4/6 studies. Most of the studies, however, did not report on an overall complication rate. Instead, they were presented narratively in the studies.
The optimal information size may have not been reached for most of the specific complications.
Clinical Knee Stability
The important outcome clinical knee stability was reported by 4 studies using different instruments. Four studies with overall 489 enrolled patients measured the Lachman test.19,22, 23, 24 A statistically significant difference to the detriment of allografts was found in one of these studies19 (Table 3). No statistically significant difference was found in the remaining studies.22, 23, 24 The pivot shift test was reported in 4 of 6 studies.19,22, 23, 24 Although 1 study19 found a statistically significant difference to the detriment of allografts, none of the remaining 3 studies22, 23, 24 found a statistically significant difference in pivot shift test results (Table 3). Side-to-side difference (in millimeters) was measured using the KT-1000 arthrometer and reported in 4 studies.19,22, 23, 24 Of these, 2 studies19,22 found a statistically significant difference favoring autografts, and the other 3 studies23,24 did not find a statistically significant difference hereby. Objective IKDC was reported in 4 trials,19,22, 23, 24 but none of these studies found a statistically significant difference in objective IKDC score between treatment groups. For a more nuanced description on results of the important end point clinical knee stability, the reader is referred to the evidence table (Table 3) and the original HTA report.12
The results of the clinical knee stability outcomes reached very low (Lachman test, pivot shift test), low (side-to-side difference) to moderate (objective IKDC) certainty according to GRADE (Table 3).
HRQOL and Patient Satisfaction
HRQOL was not reported in any of the included studies. Yet, patient satisfaction was reported by 1 of 6 included studies.21 The study found no statistically significant difference in patient satisfaction between patients receiving allografts (n = 53) and autografts (n = 53). The evidence must be interpreted with caution, because the study authors did not mention which instrument was used for the measurement of patient satisfaction. The results of the patient satisfaction reached low certainty according to GRADE (Table 3).
Safety
Outcomes of interest that were judged to be critical, and hence crucial to derive a recommendation, covered graft failure, re-ruptures, reoperations, revisions, and complications. For graft failure, 2 studies19,20 reported on this outcome. In the study by Bottoni et al.,20 the reported graft failure rate was 13 of 49 knees (26.5%) in the allograft group as opposed to 4 of 48 knees (8.3%) in the autograft group (P < .05). Similarly, the other study19 reported on a graft failure rate of 13 of 43 patients (30.2%) in the allograft group as compared with 3 of 40 patients (7.5%) in the autograft group (P < .001).
The outcome revision rate was reported in 2 of 6 studies.20,22 Bottoni et al.20 reported on a revision rate of 13 of 49 knees (26.5%) in the allograft group as compared with 4 of 48 knees (8.3%) in the autograft group (P < .05). Yet, it is noteworthy to state that the sample included in the study by Bottoni et al.20 included highly active, young patients and that those patients with revisions may be identical to the ones with graft failure (see above). The other study, by Li et al.,22 did not sufficiently report on the outcome, and it appeared that no statistical testing was undertaken. The study stated narratively that none of the analyzed patients received/needed additional surgery because of recurrent or residual symptoms (32 patients receiving y-irradiated allografts, 32 analyzed patients receiving autografts, and an additional 31 patients receiving hybrid grafts). The results of the safety outcomes reached low (revision rate) to moderate (graft failure) certainty according to GRADE (Table 3).
The re-rupture rate or reoperation rate was not reported by any of the included studies. In addition, none of the studies reported on the overall complication rate. Studies reported on some complications but did not statistically test differences between treatment groups. The reader is referred to Table 3 for a narrative description on some of the reported complications.
Allograft ACLR Versus Conservative Management
No studies were identified comparing the effectiveness and safety of allograft ACLR with conservative treatment.
Discussion
The evidence found in this systematic review consisted of 6 RCTs comparing allograft with autograft ACLR. For the outcome patient-reported function, activity level, and symptoms, mean difference was only statistically significantly different in 1 of 4 outcomes for which a meta-analysis could have been performed. Yet, this statistically significant difference was not judged to be clinically relevant. Although most studies either insufficiently, or failed to, report on safety outcomes, risk of graft failure was found to be substantially greater in patients receiving allograft ACLR compared with patients receiving autograft ACLR by 2 studies.19,20 The risk for revisions was subsequently also found to be greater in patients having undergone allograft ACLR in comparison with patients having undergone autograft ACLR in one study20 and one further study insufficiently reported on this outcome22 but stated that none of the patients needed additional surgery because of recurrent or residual symptoms. In addition, no evidence was found, comparing allograft ACLR with conservative treatment.
Most of the studies included in this systematic review suffer from substantial (high) risk of bias, e.g., due to the lack of blinding. In this context, poor reporting on complications must especially be highlighted as a limitation of the available evidence hereby. More broadly and as evident in most studies that evaluate the effectiveness and safety of surgical procedures is the lack of objective outcome measurements, inter alia, with regard to measuring knee stability. Further, there are 10 publications26, 27, 28, 29, 30, 31, 32, 33, 34, 35 available to evaluate the short-term difference between allograft and autografts with a follow-up time of less than 5 years. It is questionable as to whether these publications have overlapping samples because re-publishing the results of trials with different follow up may have been present. While these studies cannot answer our research question on the long-term comparative effectiveness and safety of allograft ACLR, it appears that safety reporting may be seen as a general problem within some of the arthroscopic studies more broadly, that is, numerous studies also do not report on complications in a standardized and rigorous manner.
The results of the conducted systematic review are in line with other published systematic reviews and meta-analyses. A recent review and meta-analysis36 specifically summarized the evidence comparing soft-tissue allograft ACLR with autograft ACLR. The review and meta-analysis included 8 randomized controlled trials with no filter on length of follow-up time. Similarly, the meta-analysis found statistically significant difference in subjective IKDC, with a mean difference of 2.43 to the detriment of allografts (95% CI 0.69-4.18; P = .006) and a marginal mean difference in Tegner score (mean difference 0.24; 95% CI 0.03-0.45; P = .03) as well as in side-to-side differences (mean difference −1.37; 95% CI −2.44 to 0.30; P = .01). Wang et al.36 did not find significant differences between groups when comparing the Lysholm score, complications, pivot shift test, anterior drawer test, Lachman test, overall IKDC score, or range of motion. Based on their findings, the authors concluded that soft-tissue allografts are inferior to hamstring tendon autografts with respect to subjective patient evaluation and knee stability but superior in the complication of hypoesthesia for patients undergoing ACLR.36 Yet, some shortcomings must be noted hereby. First, it is questionable as to whether these differences are actually outside the noninferiority margin, that is, as to whether the aforementioned differences within effectiveness outcomes are clinically relevant. Second, it appears that the authors did not extract data on graft failure/revision rates—a patient-relevant outcome without question.
One further meta-analysis37 found comparative evidence consisting of 13 RCTs (n = 1636). While the review was less strict and also included studies with a shorter follow-up, the review concluded that autografts are superior when compared with irradiated allografts in ACLR based on knee function as well as laxity. The authors critically highlight, however, that these conclusions must be interpreted with caution because the primary studies lacked adequate blinding.
In addition, one less-recent review included 12 studies (1167 patients) and found that autografts exhibit little clinical advantage over nonirradiated allografts on the basis of knee stability, function, and side effects.38 Another less-recent review39 identified 11 studies comparing nonchemically processed, nonirradiated allografts with autografts: no statistically significant differences were found in the Lysholm score, IKDC score, Lachman and pivot shift test results, KT-1000 arthrometer, or failure rates. However, the review was conducted in 2013, used less-strict inclusion criteria, used a specific allogeneic graft type, and also included observational studies (prospective and retrospective comparative studies). A shorter follow-up was also present in the included studies in this review.
Further, there may be covariables influencing patient relevant outcomes of cruciate ligament reconstructions substantially. For instance, one cohort study11 analyzed 281 ACLRs using multivariable regression analysis and found that patient age as well as graft type were predictors for graft failure. Subsequently, younger patients aged between 10 and 19 years had the greatest risk of graft failures. The study further found that patients having undergone allograft ACLR had 4 times greater odds of graft failure when compared with patients having undergone autograft ACLR. These results suggest that patient age and, hence, eventually also the activity level are important to consider. None of the studies found in this review included a specific age group: age of patients ranged from 18 to 65 years across studies and participants. One RCT20 with high graft failures in allograft ACLR included highly active military men in their sample. The activity level of patients in the other study19 with high graft failures in allograft ACLR is unknown. In the other studies included in this review, the reporting on activity level of patients was sparse.
Different types of allografts (and types of reconstruction) and autografts are currently used. A systematic review and meta-analysis40 focusing on the comparison between irradiated allografts and autografts identified 4 randomized trials and 2 prospective studies concluded hereby that irradiated allografts are inferior to autografts when considering subjective evaluation and knee stability, but function and complication were not found to be statistically significantly different. However, the study authors noted that the robustness of the findings is limited and needs further validation by RCTs with long-term follow-up. In our review, 2 RCTs19,22 used irradiated allografts with one of these19 showing substantially greater failure rates in the allograft group. A recent network meta-analysis41 investigated the short-term (≤2 years follow-up) knee outcomes of 17 different tendon grafts. The number of different types of evaluated grafts show that there may not yet be a gold standard in clinical practice (also within autograft ACLR). Based on few available studies comparing nonirradiated allografts with autografts finding no difference in clinical outcomes, the authors concluded that, among others, nonirradiated allografts may be used as alternatives to autografts. As a result, each specific graft type is described to be with unique advantages and disadvantages.42
As a result and once a clear gold standard is established, it would make sense to compare a specific allogeneic graft type with a specific autograft type to be used for ACLR. In such an RCT, the patient population may be further narrowed (e.g., older, less active) to test directly whether certain types of allografts yield a clinical benefit in certain scenarios, where allografts are still hypothesized to be advantageous.
Based on the conducted meta-analysis, there was only one statistically significant difference in subjective IKDC score that is likely to be too small to be actually clinically relevant,43 with a mean difference favoring autografts of –2.25 (95% CI –3.02 to –1.47). In addition, based on data of the other instruments measuring the crucial outcome patient-reported function activity level and symptoms, no statistically significant difference within the meta-analysis was found. On the contrary, for safety outcomes, the detected differences are certainly clinically relevant. Graft failure, for instance, showed clear inferiority of allografts when compared to autografts in 2 studies that reported on this outcome. Yet, it must be noted that the other studies did not report on this outcome leading to uncertainty as to whether inferiority with regard to safety applies more broadly or whether it applies only in certain subgroups of patients. Further, it must be noted that although common outcomes are evident in the medical literature, there is no defined core outcomes set available on the COMET database.44,45
Limitations
Most of the included studies failed to report rerupture and revision rates, which is important data. Also, the research question was broad and the study included many variables. Further, excluding observational studies (especially register data) could have led to not capturing studies with a considerably larger sample size than the studies we have identified in this review. Yet, observational studies are more prone to internal validity concerns and including these studies would have come on the offset of an increased risk of including spurious correlation (with questionable causation) in our findings as well.
Conclusions
While no substantial difference in patient-reported function, activity level, and symptoms was demonstrated, evidence from the included studies showed a greater risk for graft failure or revision that may make allograft a less-safe treatment modality in ACLR. The strength of available evidence is low based on the crucial outcomes due to the lack of high-quality research and the present increased risk of bias in primary studies. Priority should be shifted toward reflecting on whether there is a subpopulation for whom allograft ACLR may still be advantageous in theory (e.g., less active older patients) and further conduct RCTs in this population.
Acknowledgments
Project support by Information specialist: Tarquin Mittermayr, B.A. (Hons), M.A.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Beutler A., Alexander A. Physical examination of the knee. 2017. https://www.uptodate.com/contents/physical-examination-of-the-knee?search=cruciate%20ligament&source=search_result&selectedTitle=4∼150&usage_type=default&display_rank=4 [updated Nov 2018; cited 06.12.2018] Accessed December 6, 2018.
- 2.Lenz M. Kreuzbandverletzung, vorderes Kreuzband. 2017. https://deximed.de/home/b/orthopaedie/krankheiten/knie/kreuzbandverletzung-vorderes-kreuzband/ Accessed December 5, 2018.
- 3.Frobell R.B., Roos E.M., Roos H.P., Ranstam J., Lohmander L.S. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331–342. doi: 10.1056/NEJMoa0907797. [DOI] [PubMed] [Google Scholar]
- 4.Frobell R.B., Roos H.P., Roos E.M., Roemer F.W., Ranstam J., Lohmander L.S. Treatment for acute anterior cruciate ligament tear: Five year outcome of randomised trial. BMJ. 2013;346:f232. doi: 10.1136/bmj.f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk A.P., Davies L.J., Hopewell S., Harris K., Beard D.J., Price A.J. Surgical versus conservative interventions for treating anterior cruciate ligament injuries. Cochrane Database Syst Rev. 2016;4:CD011166. doi: 10.1002/14651858.CD011166.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West R.V., Harner C.D. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13:197–207. doi: 10.5435/00124635-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg R. Anterior cruciate ligament injury. 2018. A https://www.uptodate.com/contents/anterior-cruciate-ligament-injury?search=rupture%20cruciate%20ligament&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1. Accessed November 27, 2018.
- 8.MacDonald J., Rodenberg R. Posterior cruciate ligament injury. 2018. https://www.uptodate.com/contents/posterior-cruciate-ligament-injury?search=anterior%20cruciate%20ligament%20injury&source=search_result&selectedTitle=3∼36&usage_type=default&display_rank=3 Accessed December 5, 2018.
- 9.Trabuco E. Reconstructive materials used in surgery: classification and host response. 2017. https://www.uptodate.com/contents/reconstructive-materials-used-in-surgery-classification-and-host-response?search=allograft&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H8 Accessed November 27, 2018.
- 10.Strickland S.M., MacGillivray J.D., Warren R.F. Anterior cruciate ligament reconstruction with allograft tendons. Orthop Clin North Am. 2003;34:41–47. doi: 10.1016/s0030-5898(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 11.Kaeding C.C., Aros B., Pedroza A., et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON Prospective longitudinal cohort. Sports Health. 2011;3:73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz G, de Villiers C. Allograft for anterior and posterior cruciate ligament reconstruction. Decision Support Document 116. LBI-HTA, 2019. http://eprints.hta.lbg.ac.at/1205/. Accessed September 1, 2020.
- 13.EUnetHTA Methodology Guidelines. 2015. https://www.eunethta.eu/methodology-guidelines/ Accessed February 1, 2019.
- 14.European Network for Health Technology Assessment (EUnetHTA) Joint Action on HTA 2012-2015. HTA Core Model for Rapid Relative Effectiveness. 2015. https://www.eunethta.eu/wp-content/uploads/2018/06/HTACoreModel_ForRapidREAs4.2-3.pdf Accessed July 31, 2019.
- 15.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian S., Wang B., Liu L., et al. Irradiated hamstring tendon allograft versus autograft for anatomic double-bundle anterior cruciate ligament reconstruction: Midterm clinical outcomes. Am J Sports Med. 2016;44:2579–2588. doi: 10.1177/0363546516655333. [DOI] [PubMed] [Google Scholar]
- 20.Bottoni C.R., Smith E.L., Shaha J., et al. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43:2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 21.Jia Y.H., Sun P.F. Comparison of clinical outcome of autograft and allograft reconstruction for anterior cruciate ligament tears. Chin Med J (Engl) 2015;128:3163–3166. doi: 10.4103/0366-6999.170265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Wang J., Li Y., Shao D., You X., Shen Y. A prospective randomized study of anterior cruciate ligament reconstruction with autograft, gamma-irradiated allograft, and hybrid graft. Arthroscopy. 2015;31:1296–1302. doi: 10.1016/j.arthro.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Sun K., Tian S.Q., Zhang J.H., Xia C.S., Zhang C.L., Yu T.B. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft. Arthroscopy. 2009;25:750–759. doi: 10.1016/j.arthro.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Sun K., Zhang J., Wang Y., et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: A prospective, randomized controlled study. Am J Sports Med. 2011;39:1430–1438. doi: 10.1177/0363546511400384. [DOI] [PubMed] [Google Scholar]
- 25.Hohmann E., Feldman M., Hunt T.J., Cote M.P., Brand J.C. Research pearls: How Do we establish the level of evidence? Arthroscopy. 2018;34:3271–3277. doi: 10.1016/j.arthro.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Edgar C.M., Zimmer S., Kakar S., Jones H., Schepsis A.A. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Rel Res. 2008;466:2238–2246. doi: 10.1007/s11999-008-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorschewsky O., Browa A., Vogel U., Stauffer E. Clinico-histologic comparison of allogenic and autologous bone-tendon-bone using one-third of the patellar tendon in reconstruction of the anterior cruciate ligament. Unfallchirurg. 2002;105:703–714. doi: 10.1007/s00113-001-0405-0. [in German] [DOI] [PubMed] [Google Scholar]
- 28.Lawhorn K.W., Howell S.M., Traina S.M., Gottlieb J.E., Meade T.D., Freedberg H.I. The effect of graft tissue on anterior cruciate ligament outcomes: A multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28:1079–1086. doi: 10.1016/j.arthro.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Noh J.H., Yi S.R., Song S.J., Kim S.W., Kim W. Comparison between hamstring autograft and free tendon Achilles allograft: Minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19:816–822. doi: 10.1007/s00167-010-1388-6. [DOI] [PubMed] [Google Scholar]
- 30.Sun K., Tian S., Zhang J., Xia C., Zhang C., Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: A prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464–474. doi: 10.1007/s00167-008-0714-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun K., Tian S.Q., Zhang J.H., Xia C.S., Zhang C.L., Yu T.B. ACL reconstruction with BPTB autograft and irradiated fresh frozen allograft. J Zhejiang Univ Sci B. 2009;10:306–316. doi: 10.1631/jzus.B0820335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun K., Zhang J., Wang Y., et al. Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years' follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy. 2011;27:1195–1202. doi: 10.1016/j.arthro.2011.03.083. [DOI] [PubMed] [Google Scholar]
- 33.Sun R., Chen B.C., Wang F., Wang X.F., Chen J.Q. Prospective randomized comparison of knee stability and joint degeneration for double- and single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23:1171–1178. doi: 10.1007/s00167-014-2934-4. [DOI] [PubMed] [Google Scholar]
- 34.Tian S., Wang Y., Wang B., et al. Anatomic double-bundle anterior cruciate ligament reconstruction with a hamstring tendon autograft and fresh-frozen allograft: A prospective, randomized, and controlled study. Arthroscopy. 2016;32:2521–2531. doi: 10.1016/j.arthro.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Yoo S.H., Song E.K., Shin Y.R., Kim S.K., Seon J.K. Comparison of clinical outcomes and second-look arthroscopic findings after ACL reconstruction using a hamstring autograft or a tibialis allograft. Knee Surg Sports Traumatol Arthrosc. 2017;25:1290–1297. doi: 10.1007/s00167-015-3955-3. [DOI] [PubMed] [Google Scholar]
- 36.Wang H.D., Zhang H., Wang T.R., Zhang W.F., Wang F.S., Zhang Y.Z. Comparison of clinical outcomes after anterior cruciate ligament reconstruction with hamstring tendon autograft versus soft-tissue allograft: A meta-analysis of randomised controlled trials. Int J Surg. 2018;56:174–183. doi: 10.1016/j.ijsu.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Kan S.L., Yuan Z.F., Ning G.Z., et al. Autograft versus allograft in anterior cruciate ligament reconstruction: A meta-analysis with trial sequential analysis. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J., Yang H.B., Qin J.B., Yang T.B. A meta-analysis of anterior cruciate ligament reconstruction with autograft compared with nonirradiated allograft. Knee. 2015;22:372–379. doi: 10.1016/j.knee.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Lamblin C.J., Waterman B.R., Lubowitz J.H. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29:1113–1122. doi: 10.1016/j.arthro.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Wang H.D., Zhu Y.B., Wang T.R., Zhang W.F., Zhang Y.Z. Irradiated allograft versus autograft for anterior cruciate ligament reconstruction: A meta-analysis and systematic review of prospective studies. Int J Surg. 2018;49:45–55. doi: 10.1016/j.ijsu.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Yang X.G., Wang F., He X., et al. Network meta-analysis of knee outcomes following anterior cruciate ligament reconstruction with various types of tendon grafts. Int Orthop. 2020;44:365–380. doi: 10.1007/s00264-019-04417-8. [DOI] [PubMed] [Google Scholar]
- 42.Paschos N.K., Howell S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016;1:398–408. doi: 10.1302/2058-5241.1.160032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris J.D., Brand J.C., Cote M.P., Faucett S.C., Dhawan A. Research pearls: The significance of statistics and perils of pooling. Part 1: Clinical versus statistical significance. Arthroscopy. 2017;33:1102–1112. doi: 10.1016/j.arthro.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 44.Collins N.J., Misra D., Felson D.T., Crossley K.M., Roos E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care Res. 2011;63(suppl 11):S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.COMET Initiative Core Outcome Measures in Effectiveness Trials. http://www.comet-initiative.org/ Accessed April 30, 2020.
- 46.Dettori J.R. Loss to follow-up. Evid Based Spine Care J. 2011;2:7–10. doi: 10.1055/s-0030-1267080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.