Abstract
Purpose
Lung adenocarcinoma is one of the common causes of cancer-related deaths worldwide. AHNAKs are giant proteins, which are correlated with cell structure and migration, cardiac calcium channel signaling, and other processes. Current studies identified AHNAK2 as a novel oncogene in some cancers; however, studies on its function in lung cancers are limited.
Materials and Methods
The expression of AHNAK2 was analyzed in normal lung tissues, lung adenocarcinoma tissues, and paracancerous tissues using the Oncomine database. It was further verified in relative cell lines by real-time quantitative polymerase chain reaction and Western blotting (WB). Adenocarcinoma cell lines were transfected with si-NC and si-AHNAK2 by lipofectamine 3000 and treated with or without TGF-β1, and cell migration and invasion were detected by wound-healing and transwell assays. The expression of epithelial-mesenchymal transition (EMT) markers was detected by WB, as well as that of phosphorylated-Smad3 (p-Smad3) and Smad3 levels. After Smad3 phosphorylation inhibitor was added to the adenocarcinoma cell lines, migration and invasion were detected by wound-healing and transwell assays, and the expression of EMT markers was detected by WB when the cells were transfected with si-NC and si-AHNAK2 and treated with or without TGF-β1.
Results
We found higher expression of AHNAK2 in lung adenocarcinoma tissues through the Oncomine database and further verified its high expression in relative cell lines. When the cells were stimulated with TGF-β1, knockdown of AHNAK2 suppressed cell migration, invasion, and EMT, and inhibited TGF-β–induced Smad3 signaling. When p-Smad3 was inhibited, knockdown of AHNAK2 had no effect on the two cell lines investigated when treated with or without TGF-β1.
Conclusion
AHNAK2 acts as an oncogenic protein and promotes migration, invasion, and EMT in lung adenocarcinoma cells via the TGF-β/Smad3 pathway. Thus, it may be a novel target for lung adenocarcinoma therapy.
Keywords: epithelial-mesenchymal transition, lung adenocarcinoma, AHNAK2, transforming growth factor-beta 1, Smad
Introduction
Lung cancer is a leading cause of death worldwide.1 Based on cancer statistics in 2020, there will be approximately 228,820 new cases of lung and bronchus cancer diagnosed in the USA alone,2 accounting for 13% of the new cancer cases in men and 12% in women.2 Meanwhile, lung cancer is still the top cause of all cancer deaths in both genders, with an estimated 23% of the cancer deaths in men and 22% in women due to lung cancer.2 A total of 85% of the lung cancer cases are diagnosed as lung adenocarcinoma, with an average five-year survival rate of 15%.3 Currently, its high mortality rate is a major public health challenge. Therefore, it is necessary to explore the potential mechanisms underlying lung adenocarcinoma metastasis.
AHNAKs are giant proteins (molecular mass >600 kDa) mainly expressed in muscular cells4 and are found in many intracellular locations, including the nucleus, cytoplasm, and plasma membrane. Two members comprise the AHNAK protein family, which are AHNAK and AHNAK2:5 AHNAK plays a protective role in vascular healing after wire injury and contributes to muscular dystrophies when it is abnormally located in muscle connective tissues,6,7 whereas AHNAK2, originally found in mouse heart tissue extract and gradually reported in recent years,5 is involved in cell migration, repair and other processes.8–11 AHNAK2 is regarded as an oncogenic protein and plays a role in the metastases of cancers, including pancreatic ductal adenocarcinoma, clear cell renal cell carcinoma, uveal melanoma, and papillary thyroid carcinoma,12–15 and some studies have revealed its possible mechanism in tumors. Kirov et al have indicated that AHNAK2 is an important element of the fibroblast growth factor1 nonclassical export pathway, which regulates carcinogenesis, angiogenesis, and inflammation,16 Li et al reported that it promotes uveal melanoma progression by regulating the PI3K signaling pathway,14 and Wang et al revealed that AHNAK2 mediates hypoxia pathway–driven epithelial-mesenchymal transition (EMT) and induction of stem cell properties by targeting hypoxia-inducible factor 1 (HIF1)-α in clear cell renal cell carcinoma.12 However, the role of AHNAK2 in lung adenocarcinoma has not been clarified.
EMT is the loss of epithelial status and maintenance of the apicobasal polarity of cell–cell adhesion molecules to obtain mesenchymal properties. This transition contributes to profound cellular reorganization by regulating different proteins to achieve enhanced migratory and invasive activities.17 It is believed that metastasis begins with this transition of some tumor cells in the primary tumor which migrate towards blood vessels.18 Therefore, EMT plays a vital role in tumor progression and metastasis.
Transforming growth factor β (TGF-β), a bifunctional regulator, can either inhibit or stimulate cell proliferation. In cancer progression, TGF-β frequently serves as a tumor suppressor during the early stages of tumor development. However, it can switch into a tumor promoter for the persistence of tumor cells due to its antimitogenic effects in advanced tumors. The expression of TGF-β is higher in tumor cells, which accelerates tumor progression by enhancing the migratory and invasive abilities during the advanced phases of tumorigenesis by stimulating extracellular matrix deposition and tissue fibrosis, interfering with immune and inflammatory functions, supporting angiogenesis, promoting EMT that enables increased migration and invasion, and maintaining cancer stem cells.19
We investigated the function of AHNAK2 in lung adenocarcinoma cells in our study. The results showed that AHNAK2 is an oncogenic protein in lung adenocarcinoma. We further studied the mechanisms of AHNAK2 and found that it may promote migration, invasion, and EMT in human lung adenocarcinoma cells through the TGF-β/Smad3 pathway.
Materials and Methods
Patients Information Source
The gene expression profiles of two different kinds of lung tissues, Bhattacharjee Lung and Landi Lung were downloaded from the Oncomine database (https://www.oncomine.org/resource/login.html) to analyze the expression of AHNAK2. Bhattacharjee Lung data were obtained to analyze the AHNAK2 expression differences between normal lung and lung adenocarcinoma tissues, and Landi Lung data were to analyze between lung adenocarcinoma and paracancerous tissues.
Cell Type and Culture Conditions
Human lung cancer cell lines (A549 and H1299) and normal lung epithelial cells (BEAS-2B) were purchased from the Chinese Academy of Science (Shanghai, China). A549 was initiated in 1972 by D.J. Giard et al through an explant culture of lung carcinomatous tissues from a 58-year-old Caucasian male, H1299 was established from a lymph node metastasis of the lung cancer from a 43-year-old Caucasian male who had received prior radiation therapy, and BEAS-2B was isolated from normal bronchial epithelium obtained from autopsy of non-cancerous individuals. BEAS-2B, A549, and H1299 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Camarillo, CA, USA) with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), and all cells were maintained at 37 °C in a 5% CO2 incubator. The cells were passaged upon reaching 90% confluence.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
All cells were seeded on 12-well plates. When they reached nearly 90% confluence, TRIzol (Invitrogen, Camarillo, CA, USA) was used for the total RNA (1 μg) extraction from the cells. Reverse transcription converted the mRNA into cDNA with a RevertAid First Strand cDNA Synthesis Kit (Thermo, Waltham, MA, USA) per the manufacturer’s instructions. The cells were incubated for 60 min at 42 °C, and synthesis was terminated for 5 min at 70 °C. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using the QuantStudio 6 system and the Power SYBR Green PCR Master Mix (Applied Biosystems and Thermo Fisher Scientific, respectively). The thermal cycling conditions were as follows: polymerase activation at 95 °C for 2 min, 40 cycles of denaturation at 95 °C for 1 s, and annealing and extension at 60 °C for 30 s. β-actin was used as a control for the variability in expression levels, and the 2–ΔΔCT method was adopted to calculate the expression of AHNAK2. With respect to the ΔΔCT of the 2–ΔΔCT method, ΔCT is the difference in threshold cycle between the target and reference genes. Thus, ΔΔCT is the difference in ΔCT between the target and reference samples. Primer sequences are listed in Table 1.
Table 1.
Primer Sequences
| AHNAK2 | 5ʹ-GTGCAGAAACGGAAGATGACC-3’ | 5ʹ-GCCTCAGTCGTGTATTCGTAGA-3’ |
| E-cadherin | 5ʹ-CGAGAGCTACACGTTCACGG-3’ | 5ʹ-GGGTGTCGAGGGAAAAATAGG-3’ |
| N-cadherin | 5ʹ-TGCGGTACAGTGTAACTGGG-3’ | 5ʹ-GAAACCGGGCTATCTGCTCG-3’ |
| Vimentin | 5ʹ-GACGCCATCAACACCGAGTT-3’ | 5ʹ-CTTTGTCGTTGGTTAGCTGGT-3’ |
| β-Actin | 5ʹ-CCTGGCACCCAGCACAATG-3’ | 5ʹ-G GGCCGGACTCGTCATACT-3’ |
Transfection
Si-RNAs (including si-NC, si-AHNAK2-1,-2,-3) were purchased from Shanghai GenePharma Co., Ltd (http://www.genepharma.com/). Once the lung adenocarcinoma cell lines seeded on 6-well plates reached ~50% confluence, they were transfected with si-RNAs, respectively (5 nM each). Briefly, 20 µM si-RNA (5 µL) and lipofectamine 3000™ (5 µL) were separately diluted into Opti-MEM (250 µL) as the dilated ratio of 1:50 for 5 min. Then si-RNAs and lipofectamine 3000™ diluent were mixed and incubated at room temperature for 15 min. The mixture (500 µL) was added the each well of 6-well plates containing 1.5 mL DMEM with 10% FBS. 24 h later, the cell culture medium was harvested. The transfection effect of si-RNAs was detected. The operation was performed using the Lipofectamine 3000 kit (Invitrogen). The expression of AHNAK2 was lower in the cells transfected with si-AHNAK2-1 and −2 than that in cells transfected with si-AHNAK2-3 on the RNA and protein levels, demonstrating better transfection effects of si-AHNAK2-1 and −2. Therefore, they were selected for subsequent experiments. Si-RNA sequences are listed in Table 2.
Table 2.
siRNA Sequences
| Si-AHNAK2-1 | 5ʹ-CCAAGUGGAUGUGAAACUUTT-3’ | 5ʹ-AAGUUUCACAUCCACUUGGTT-3’ |
| Si-AHNAK2-2 | 5ʹ-GCCCUGAAAUAGACAUCAATT-3’ | 5ʹ-UUGAUGUCUAUUUCAGGGCTT-3’ |
| Si-AHNAK2-3 | 5ʹ-GCGGACAAGGAUGUGACUATT-3’ | 5ʹ-UAGUCACAUCCUUGUCCGCTT-3’ |
| Si-NC | 5ʹ-UUCUCCGAACGUGUCACGUTT-3’ | 5ʹ-ACGUGACACGUUCGGAGAATT-3’ |
TGF-β1 and SIS3 Treatment
The lung adenocarcinoma cell lines were transfected with si-NC and si-AHNAK2-1 and −2 for 24 h, and 3 μM of inhibitor of Smad3 phosphorylation, SIS3 (Selleck Chemicals, Houston, USA), was added to the wells and incubated for 6 h. Likewise, 5 ng/mL TGF-β1 (Cell Signaling Technology, Danvers, MA, USA) was added to low-serum DMEM (containing 1% FBS) for the indicated periods. The control groups were also incubated in low-serum medium.
Analysis of Cell Morphology
Once the A549 cells seeded on 6-well plates reached 40% confluence, the cells were transfected with si-NC and si-AHNAK2-1 and −2, incubated with or without TGF-β1 for 24 h, and viewed under an inverted microscope to observe the morphological changes.
Wound-Healing Assay
Once the two lung adenocarcinoma cell lines reached 95% confluence, a 200-μL tip was used to make a scratch in the cell layers to form a wound gap. Photographs were taken at 0 h and 24 h after the wound gap was formed. Image J was used to calculate the migration area.
Transwell Assay
After transfection and treatment as described above, the two lung adenocarcinoma cell lines in serum-free DMEM were inoculated in the upper chambers of transwell plates coated with Matrigel (Corning, Cambridge, MA, USA). The 10% FBS-supplemented DMEM medium was added to the lower chambers. After 24 h, the cells left on the upper surfaces of the filters were removed, and the cells invading the membranes were fixed in 4% cold paraformaldehyde and stained with 0.1% crystal violet. The adherent cells were photographed under a light microscope and calculated using Image J.
Western Blot Analysis
The two lung adenocarcinoma cell lines were treated with sodium dodecyl sulfate (SDS) lysis buffer suspension after transfection and TGF-β1 treatment. The protein concentration was detected after total cellular protein extraction using a BCA protein assay kit (Beyotime Biotechnology, Shanghai, China). The proteins were separated by 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% bovine serum albumin for 1 h, the membrane was incubated with primary antibodies for 24 h. The dilution ratios of the antibodies were as follows: anti-AHNAK2 (1:1000; rabbit polyclonal; 17,682-1-AP; ProteinTech Group, Inc., Chicago, IL, USA), anti-E-cadherin (1:1000; rabbit monoclonal; #3195; Cell Signaling Technologies), anti-N-cadherin (1:1000; mouse monoclonal; ab98952; Abcam, Cambridge, UK), anti-Vimentin (1:4000; rabbit monoclonal; ab92547; Abcam), anti-Smad3 (1:600; rabbit polyclonal; 25,494-1-AP; ProteinTech), anti-p-Smad3 (1:2000; rabbit monoclonal; ab52903; Abcam), and anti-β-actin (1:5000; mouse monoclonal; ab8224; Abcam). The membrane was then treated with secondary antibodies (1:10,000; Donkey anti-rabbit IgG; ab205718; Abcam) (1:10,000; Goat anti-mouse IgG; ab205719; Abcam) in the dark for 1 h the following day. The membrane was exposed to an ECL assay kit (Beyotime Biotechnology). The quantitative data were analyzed using Image J.
Statistical Analysis
The data were analyzed with GraphPad Prism 6.0 software. Each experiment was independently repeated three times, and the results were presented as the mean ± standard deviation (SD). A t-test was performed to determine the differences between two groups and one-way analysis of variance was performed among more than three groups. When p < 0.05, the differences were considered statistically significant.
Results
AHNAK2 is Upregulated in Human Lung Adenocarcinoma and Relative Cell Lines
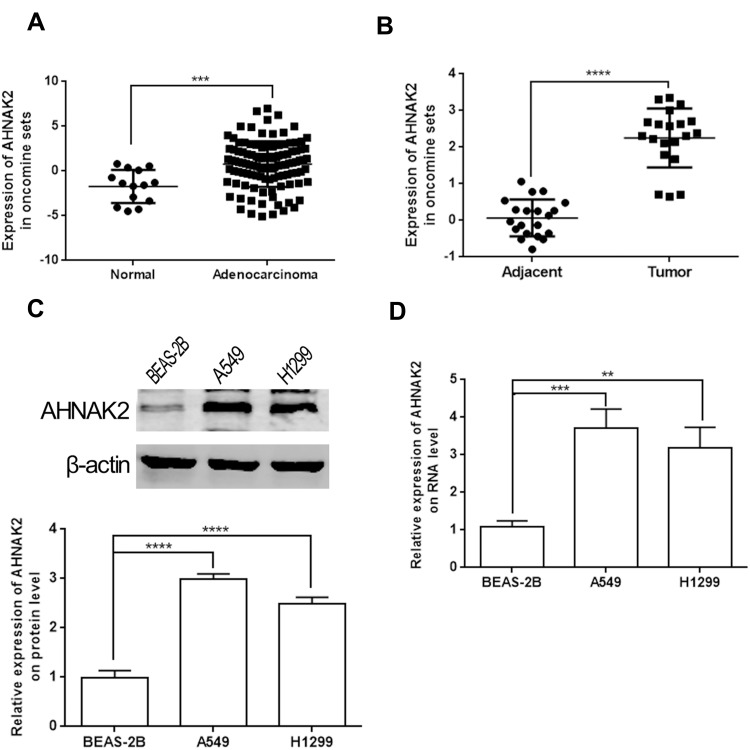
Analysis of the expression of AHNAK2 from lung tissue samples (Bhattacharjee Lung) in the Oncomine database revealed higher expression in human lung adenocarcinoma tissues than that in the normal lung tissues (Figure 1A). Furthermore, we compared 20 pairs of lung adenocarcinoma and paracancerous tissues (Landi Lung), which also showed higher expression of AHNAK2 in lung adenocarcinoma tissues (Figure 1B). WB and RT-qPCR results showed that AHNAK2 expression in human lung adenocarcinoma cell lines A549 and H1299 obviously increased, compared with the normal lung epithelial cell line BEAS-2B (Figure 1C and D).
Figure 1.
AHNAK2 is highly expressed in lung adenocarcinoma. (A) The expression of AHNAK2 between normal tissues (14 samples) and lung adenocarcinoma tissues (114 samples) from Bhattacharjee Lung of Oncomine database. (B) The expression of AHNAK2 between lung adenocarcinoma and paracancerous tissues (20 pairs) from Landi Lung of Oncomine database. (C) The relative expression of AHNAK2 in normal lung epithelial cells (BEAS-2B), lung adenocarcinoma cell lines (A549, H1299) on protein level. (D) The relative expression of AHNAK2 in normal lung epithelial cells (BEAS-2B), lung adenocarcinoma cell lines (A549, H1299) on RNA level (**p < 0.01, ***p < 0.001, ****p < 0.0001).
Downregulation of AHNAK2 Suppresses TGF-β1–Induced Lung Adenocarcinoma Cell Migration and Invasion
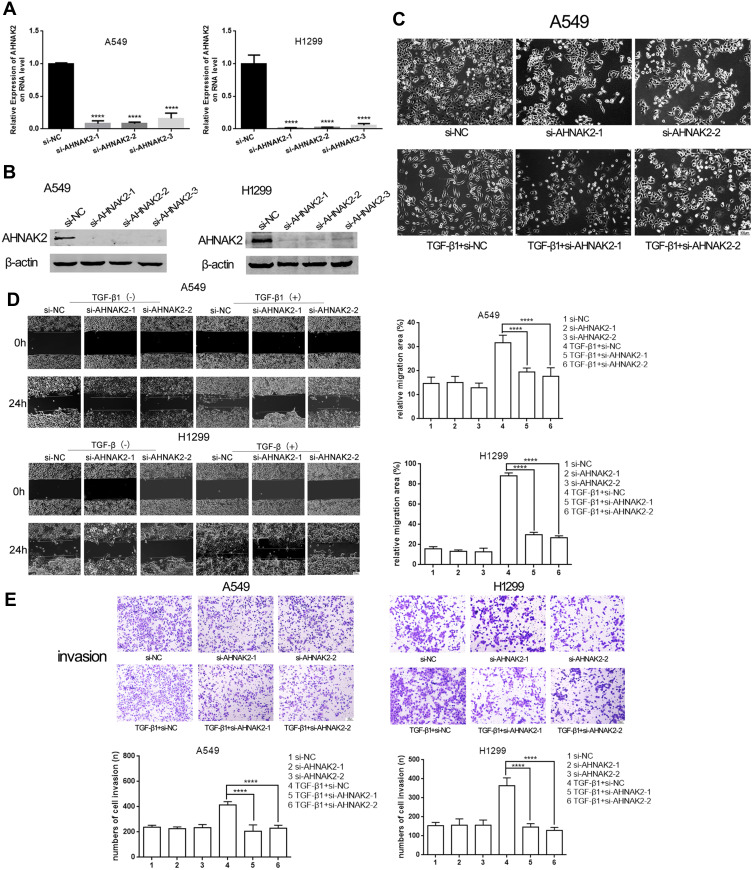
To observe the function of AHNAK2 in lung adenocarcinoma, the two lung adenocarcinoma cells were transfected with si-NC and si-AHNAK2-1, −2, and −3 for the knockdown of AHNAK2, and the transfection efficiency was detected at the RNA and protein levels. AHNAK2 expression obviously decreased when transfected with si-AHNAK2 (Figure 2A and B). Finally, si-AHNAK2-1 and −2 were selected in the following experiments owing to their better efficiency.
Figure 2.
Silencing AHNAK2 inhibits lung cancer cells migratory and invasive abilities in TGF-β1 inducement. (A and B) The relative expression of AHNAK2 when the two lung adenocarcinoma cells were transfected with si-NC, si-AHNAK2-1,-2,-3 on RNA level and protein level. (C) The morphological changes of A549 after the different transfection and TGF-β1 induction. Original magnification ×100. (D) The effect of si-AHNAK2 on the migration of the two lung adenocarcinoma cells by wound-healing with or without TGF-β1 induction. Original magnification ×100. (E) The effect of si-AHNAK2 on the invasion of the two lung adenocarcinoma cells by transwell with or without TGF-β1 induction. Original magnification ×200 (****p < 0.0001).
It is known that cells can take on a spindle shape with TGF-β1 stimulation.20 Thus, when A549 cells were transfected with si-NC and stimulated with TGF-β1, they showed an obvious spindle shape. However, when transfected with si-AHNAK2 and stimulated with TGF-β1, the phenomenon was reversed (Figure 2C). The above results suggest that downregulation of AHNAK2 reverses the spindle shape induced by TGF-β1. As H1299 are mesenchymal cells, it is difficult to morphologically observe them.21
When the cells were stimulated with TGF-β1, the migratory and invasive abilities of AHNAK2 were detected. The wound-healing experiment result suggested that low expression of AHNAK2 dramatically slowed down the speed of healing when the cells were stimulated with TGF-β1 (Figure 2D), and the transwell experiment result suggested that knockdown of AHNAK2 expression inhibited the invasive ability of lung cancer cells after treatment with TGF-β1, which is similar to the wound-healing result (Figure 2E).
Downregulation of AHNAK2 Suppresses TGF-β1–Induced Lung Adenocarcinoma Cell EMT Process and TGF-β1/Smad3 Signaling
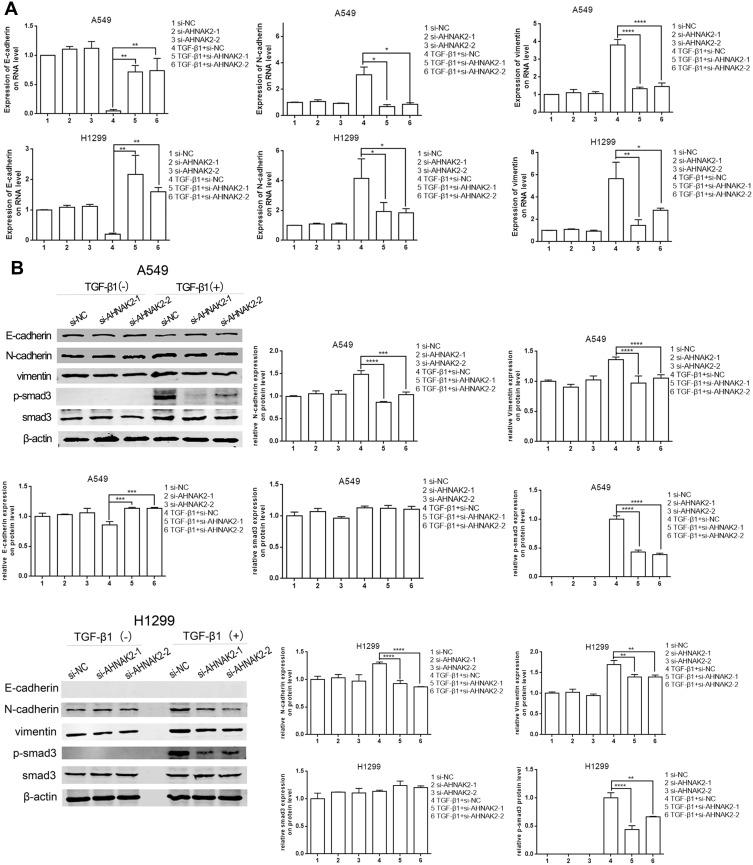
The expression of EMT markers was detected by RT-qPCR and WB. Downregulation of AHNAK2 increased the expression of E-cadherin and repressed that of N-cadherin and vimentin in TGF-β1–induced cells (Figure 3A and B). In fact, the expression of E-cadherin was almost absent in H1299 cells, which indicates that H1299 cells are mesenchymal cells.20
Figure 3.
Silencing AHNAK2 reverses TGF-β1-induced EMT and its transcriptional response in lung adenocarcinoma cell. (A) Downregulation of AHNAK2 suppresses EMT process of the two lung adenocarcinoma cells in the TGF-β1 inducement by RT-qPCR. (B) Downregulation of AHNAK2 suppresses EMT process and its transcriptional response of the two lung adenocarcinoma cells TGF-β1 inducement by WB (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Moreover, as the current model of TGF-β ligand–induced reaction is a typical signal pathway from the type II to the type I receptor for Smad activation and target gene transcription,22 the phosphorylated Smad3 (p-Smad3) level was detected to determine whether AHNAK2 affects the TGF-β/Smad3 pathway in EMT. The results suggested that p-Smad3 expression was downregulated when A549 and H1299 cells were transfected with si-AHNAK2-1 and −2 and stimulated with TGF-β1, compared with those transfected with si-NC and stimulated with TGF-β1 (Figure 3B).
Silencing AHNAK2 Inhibits Migration, Invasion, and EMT in Lung Adenocarcinoma Cells by Repressing the TGF-β/Smad3 Pathway
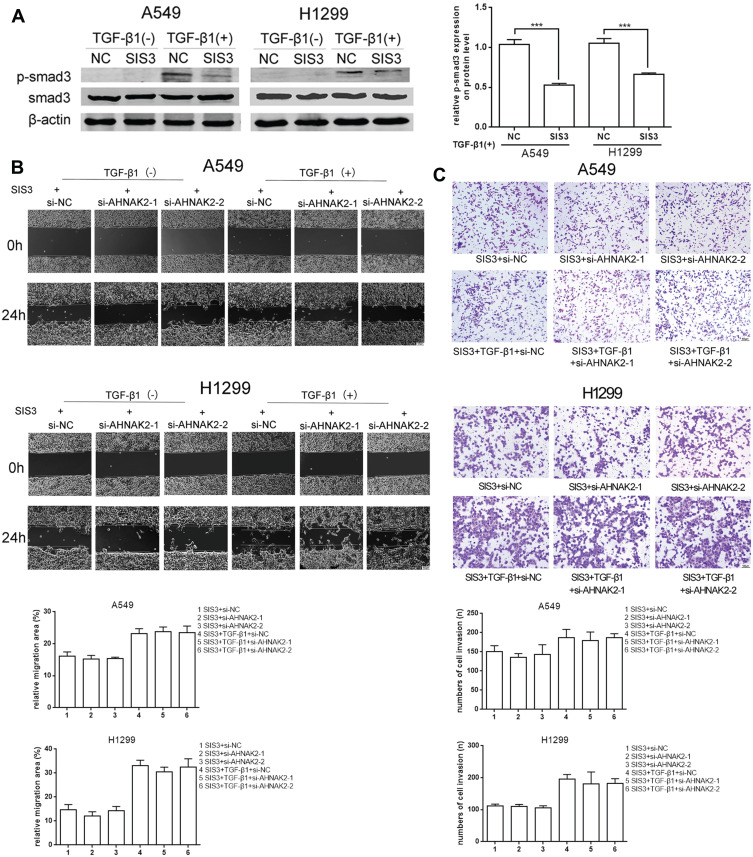
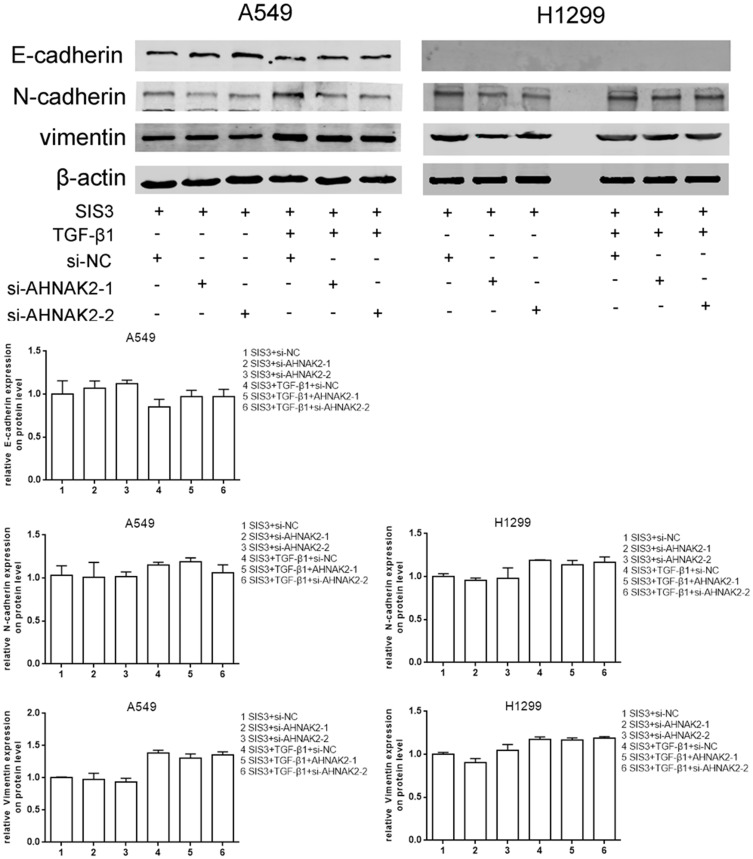
As si-AHNAK2 suppressed the expression of p-Smad3 in TGF-β1–induced cells, we added SIS3, an inhibitor of Smad3 phosphorylation, to the two cell lines when they were stimulated with or without TGF-β1. The results showed that SIS3 inhibited the expression of p-Smad3 in TGF-β–induced cells (Figure 4A). Moreover, SIS3 was added to A549 and H1299 cells stimulated with or without TGF-β1 when they were transfected with si-NC and si-AHNAK2-1 and −2. The wound-healing experiment result suggested that no statistical difference was observed among si-NC, si-AHNAK2-1, and −2–transfected cells regardless of TGF-β1 stimulation (Figure 4B). Similar results were observed in the transwell assay (Figure 4C). E-cadherin, N-cadherin, and vimentin expression showed no statistical difference after transfection and TGF-β1 induction (Figure 5). These results suggest that the inhibition of AHNAK2 suppresses migration, invasion, and EMT in lung adenocarcinoma cells by repressing the TGF-β/Smad3 pathway.
Figure 4.
Silencing AHNAK2 suppresses lung adenocarcinoma cells migration and invasion induced by TGF-β1 through repressing smad-dependent signaling. (A) Repression effect of SIS3 on p-smad3 of A549 and H1299 by WB. (B) The effect of si-AHNAK2 on migration of the two lung adenocarcinoma cells by wound-healing when added with SIS3 and treated with or without TGF-β1. (C) The effect of si-AHNAK2 on invasion of the two lung adenocarcinoma cells by transwell when added with SIS3 and treated with or without TGF-β1 (***p < 0.001).
Figure 5.
Silencing AHNAK2 suppresses lung adenocarcinoma cells EMT induced by TGF-β1 through repressing smad-dependent signaling. The effect of si-AHNAK2 on EMT of the two lung adenocarcinoma cells by WB when added with SIS3 and treated with or without TGF-β1.
Discussion
In the Oncomine database, we found that the expression of AHNAK2 in lung adenocarcinoma tissues was higher than that in normal or paracancerous tissues. In the current study, we obtained similar results in relative cell lines. Furthermore, we evaluated the function of AHNAK2 in lung adenocarcinoma cells and found that downregulation of AHNAK2 suppressed migration, invasion, and EMT in lung adenocarcinoma cells during TGF-β1 induction. As TGF-β/Smad3 is a canonical signaling pathway, we explored the possible mechanism of AHNAK2 in lung adenocarcinoma cells and found that inhibition of AHNAK2 suppressed TGF-β–induced Smad3 signaling. When p-Smad3 was inhibited, knockdown of AHNAK2 had no effect on the adenocarcinoma cells regardless of TGF-β1 stimulation.
Previous studies have suggested that AHNAK2 plays the role of a tumor promoter in some tumors. For example, overexpression of AHNAK2 is an independent prognostic factor in pancreatic ductal adenocarcinoma.13 In clear cell renal cell carcinoma, AHNAK2 can promote tumorigenesis and tumor progression by inducing EMT and cancer cell stemness through HIF1-α.12 Based on previous studies, we also observed high expression of AHNAK2 in lung adenocarcinoma.
TGF-β was initially discovered and hence named for its ability to induce fibroblast growth in soft agar, which is a characteristic connected with oncogenes.22 It can drive tumor cell metastasis by promoting EMT, which guides cancer cells to proliferate continually and maintain an undifferentiated state.23 In the present study, when A549 cells were induced by TGF-β1 and transfection, the cell morphology changed, and downregulation of AHNAK2 reversed the mesenchymal cell phenotype. Furthermore, inhibition of AHNAK2 suppressed the migration, invasion, and EMT of lung adenocarcinoma cells during TGF-β1 induction.
The TGF-β/Smad pathway is well known in many tumors.24,25 In lung cancers, this canonical pathway is activated when platelet-derived TGF-β comes in contact with platelet–tumor cells directly. This results in a transition to a more aggressive mesenchymal-like phenotype and accelerates metastasis.26 In this study, we found that after TGF-β1 stimulation, downregulation of AHNAK2 obviously inhibited the phosphorylation of Smad3 and had no effect on lung cancer metastasis after inhibition of p-Smad3. In other words, repression of p-Smad3 abrogated the inhibitory effect of si-AHNAK2 on lung cancer progression. However, Lee et al27 reported that AHNAK appears to exert a tumor-suppressive effect. However, they also found that the EMT phenotype and marker genes were significantly reduced in human keratinocyte cell line (HaCaT cells), with depleted AHNAK protein in response to TGF-β. In the present study, AHNAK2 functioned as an oncogenic protein. TGF-β itself has a dual role in tumor progression and acts as a tumor promoter in later phases. We anticipate that AHNAK and AHNAK2 both interact with Smad3 in response to TGF-β, but the downstream pathway might be different in different stages of tumor progression, which requires extensive studies.
Conclusion
In conclusion, we demonstrated that AHNAK2 acts as an oncogenic protein and promotes migration, invasion, and EMT of TGF-β1–induced lung adenocarcinoma cells. Further studies are needed on the mechanism of AHNAK2, showing that it mediates the TGF-β/Smad3 pathway. Thus, AHNAK2 may be a potential target for further preclinical studies.
Acknowledgments
The present study was funded by the Clinical Plateau Discipline Project in Shanghai Pudong New Area (PWYgy2018-06).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Carrot-Zhang J, Zhao Y, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun. 2019;10(1):5472. doi: 10.1038/s41467-019-13460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shtivelman E, Cohen FE, Bishop JM. A human gene (AHNAK) encoding an unusually large protein with a 1.2-microns polyionic rod structure. Proc Natl Acad Sci U S A. 1992;89(12):5472–5476. doi: 10.1073/pnas.89.12.5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komuro A, Masuda Y, Kobayashi K, et al. The AHNAKs are a class of giant propeller-like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proc Natl Acad Sci U S A. 2004;101(12):4053–4058. doi: 10.1073/pnas.0308619101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haase N, Rüder C, Haase H, et al. Protective function of Ahnak1 in vascular healing after wire injury. J Vasc Res. 2017;54(3):131–142. doi: 10.1159/000464287 [DOI] [PubMed] [Google Scholar]
- 7.Zacharias U, Purfürst B, Schöwel V, Morano I, Spuler S, Haase H. Ahnak1 abnormally localizes in muscular dystrophies and contributes to muscle vesicle release. J Muscle Res Cell Motil. 2011;32(4–5):271–280. doi: 10.1007/s10974-011-9271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentil BJ, Benaud C, Delphin C, et al. Specific AHNAK expression in brain endothelial cells with barrier properties. J Cell Physiol. 2005;203(2):362–371. [DOI] [PubMed] [Google Scholar]
- 9.Benaud C, Gentil BJ, Assard N, et al. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J Cell Biol. 2004;164(1):133–144. doi: 10.1083/jcb.200307098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase H, Podzuweit T, Lutsch G, et al. Signaling from beta-adrenoceptor to L-type calcium channel: identification of a novel cardiac protein kinase A target possessing similarities to AHNAK. FASEB J. 1999;13(15):2161–2172. doi: 10.1096/fasebj.13.15.2161 [DOI] [PubMed] [Google Scholar]
- 11.Sussman J, Stokoe D, Ossina N, Shtivelman E. Protein kinase B phosphorylates AHNAK and regulates its subcellular localization. J Cell Biol. 2001;154(5):1019–1030. doi: 10.1083/jcb.200105121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Li X, Zhang J, et al. AHNAK2 is a novel prognostic marker and oncogenic protein for clear cell renal cell carcinoma. Theranostics. 2017;7(5):1100–1113. doi: 10.7150/thno.18198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu D, Wang J, Shi X, Yue B, Hao J. AHNAK2 is a potential prognostic biomarker in patients with PDAC. Oncotarget. 2017;8(19):31775–31784. doi: 10.18632/oncotarget.15990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Liu Y, Meng Y, Zhu Y. Nucleoprotein AHNAK2 performs a promoting role in the proliferation and migration of uveal melanoma cells. Cancer Biother Radiopharm. 2019;34(10):626–633. doi: 10.1089/cbr.2019.2778 [DOI] [PubMed] [Google Scholar]
- 15.Xie Z, Lun Y, Li X, et al. Bioinformatics analysis of the clinical value and potential mechanisms of AHNAK2 in papillary thyroid carcinoma. Aging (Albany NY). 2020;12(18):18163–18180. doi: 10.18632/aging.103645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirov A, Kacer D, Conley BA, Vary CP, Prudovsky I. AHNAK2 participates in the stress-induced nonclassical fgf1 secretion pathway. J Cell Biochem. 2015;116(8):1522–1531. doi: 10.1002/jcb.25047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–92. doi: 10.1093/annonc/mdq292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolly MK, Jia D, Boareto M, et al. Coupling the modules of EMT and stemness: a tunable ‘stemness window’ model. Oncotarget. 2015;6(28):25161–25174. doi: 10.18632/oncotarget.4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-beta-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286(29):25992–26002. doi: 10.1074/jbc.M111.229401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu G, Yu H, Shi X, et al. Cisplatin sensitivity is enhanced in non-small cell lung cancer cells by regulating epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2. BMC Pulm Med. 2014;14(1):174. doi: 10.1186/1471-2466-14-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twardzik DR, Brown JP, Ranchalis JE, Todaro GJ, Moss B. Vaccinia virus-infected cells release a novel polypeptide functionally related to transforming and epidermal growth factors. Proc Natl Acad Sci U S A. 1985;82(16):5300–5304. doi: 10.1073/pnas.82.16.5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84. doi: 10.1097/CCO.0b013e32835b6371 [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Yang X, Fu Y, et al. Mediator MED15 modulates transforming growth factor beta (TGFbeta)/Smad signaling and breast cancer cell metastasis. J Mol Cell Biol. 2013;5(1):57–60. doi: 10.1093/jmcb/mjs054 [DOI] [PubMed] [Google Scholar]
- 25.Jingushi K, Ueda Y, Kitae K, et al. miR-629 targets TRIM33 to promote TGFbeta/Smad signaling and metastatic phenotypes in ccRCC. Mol Cancer Res. 2015;13(3):565–574. doi: 10.1158/1541-7786.MCR-14-0300 [DOI] [PubMed] [Google Scholar]
- 26.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee IH, Sohn M, Lim HJ, et al. Ahnak functions as a tumor suppressor via modulation of TGFbeta/Smad signaling pathway. Oncogene. 2014;33(38):4675–4684. doi: 10.1038/onc.2014.69 [DOI] [PMC free article] [PubMed] [Google Scholar]