Dysregulation in lipid metabolism is among the most prominent metabolic alterations in cancer. This review discusses current knowledge about the advances in understanding lipid metabolism regulation in cancer cells and introduces therapies that disrupt lipid metabolism for cancer treatment.
Abstract
Dysregulation in lipid metabolism is among the most prominent metabolic alterations in cancer. Cancer cells harness lipid metabolism to obtain energy, components for biological membranes, and signaling molecules needed for proliferation, survival, invasion, metastasis, and response to the tumor microenvironment impact and cancer therapy. Here, we summarize and discuss current knowledge about the advances made in understanding the regulation of lipid metabolism in cancer cells and introduce different approaches that have been clinically used to disrupt lipid metabolism in cancer therapy.
Introduction
Lipids, together with proteins and nucleic acids, are essential components of biological membranes and building blocks that constitute cells. In addition, lipids are used in energy storage and metabolism and have important roles as signaling molecules for many cellular activities. The regulation of lipid metabolism, such as lipid uptake, synthesis, and hydrolysis, is essential for the maintenance of cellular homeostasis (Röhrig and Schulze, 2016). Cancer cells in tumor microenvironments, where nutrient availability is consistently changing during tumor progression, harness lipid metabolism to support their rapid proliferation, survival, migration, invasion, and metastasis.
Glycolipids and phospholipids (which are subcategorized into phosphoglycerides and sphingolipids), together with cholesterol, represent major components of biological membranes. Cholesterol is also a substrate for the synthesis of fat-soluble vitamins and steroid hormones (Luo et al., 2020). As major components of glycolipids and phospholipids, fatty acids (FAs) can be esterified with a glycerol moiety to form triglycerides, which are nonpolar lipids synthesized and stored in lipid droplets during high nutrient availability and hydrolyzed to generate ATP by FA oxidation (FAO, also called β-oxidation) under energy stress conditions. Aside from energy metabolism and membrane formation, lipids form second messengers derived from phospholipase-dependent hydrolyzation of membrane lipids and synthesis from essential FAs, whose availability is largely determined by lipids in the diet (Park et al., 2012). Phospholipases (PLC, PLD, and PLA) can generate many bioactive second messengers, such as diacylglycerol, phosphatidic acid, lysophosphatidic acid, and arachidonic acid. These molecules trigger the activation of the RAS, phosphoinositide 3-kinases (PI3Ks), protein kinase C, RAC, RHO, and several other signaling axes that can promote tumorigenesis, which was intensively discussed previously and is not covered in this review (Moolenaar and Perrakis, 2011; Park et al., 2012). In addition, sterols, including oxysterol and cholesterol, are critical regulators of sterol regulatory element (SRE)–binding protein (SREBP) activation for downstream gene expression, and thus their levels affect lipogenesis in cancer. Cholesterol is a component of lipid rafts for signaling and can also covalently modify Hedgehog and Smoothened proteins for Hedgehog signaling activation (Porter et al., 1996; Xiao et al., 2017).
In this review, we summarize and discuss current knowledge about the advances in the regulation of lipid (FAs and cholesterol) uptake, lipogenesis, and FAO-dependent lipolysis in cancer cells. We dedicate a separate section at the end to discuss different approaches that might be used to disrupt lipid metabolism for cancer therapy.
Lipid uptake
FA uptake
Mammals produce only certain FAs, i.e., those carrying double bonds to the Δ9 position of the hydrocarbon chain. Other FAs, particularly polyunsaturated FAs, are essential and obtained from the diet (Nakamura and Nara, 2004). The known FA protein transporters in the plasma membrane include cluster of differentiation 36 (CD36; also known as FA translocase), the family of FA transport proteins (also collectively known as SLC27), and plasma membrane FA-binding proteins (FABPs), all of which display increased gene and protein expression in tumors (Su and Abumrad, 2009).
High CD36 expression has been correlated with poor prognosis for patients with breast, ovarian, gastric, and prostate cancer (Koundouros and Poulogiannis, 2020). Deletion of Cd36 in the prostate of cancer-susceptible Pten−/− mice or mammary tissues of MMTV-neu mice attenuated increased FA uptake in cancer and mitigated tumorigenesis (Feng et al., 2019; Watt et al., 2019). In breast cancer patients, CD36 expression increases following anti-HER2 therapy and correlates with poor survival. Correspondingly, the HER2 inhibitor lapatinib-resistant breast cancer cells increase FA uptake, and CD36 inhibition suppresses the growth of lapatinib-resistant, but not that of lapatinib-sensitive, tumor cells (Feng et al., 2019). High-fat diets induced NF-κB–dependent CD36 expression and elicited O-GlcNAcylation of CD36 at S468 and T470, which enhanced FA uptake and murine gastric cancer metastasis (Jiang et al., 2019). Hydrogen sulfide, which is implicated in cancer metastasis, induces CD36 expression and reduces C333-C272 disulfide bond formation in CD36, which activates the long-chain FA-binding conformation of CD36 to promote FA uptake and accelerate gastric cancer metastasis (Wang et al., 2019). Palmitic acid induces gastric cancer cell migration and invasion through CD36-dependent activation of the protein kinase B (PKB, also known as AKT)/β-catenin signaling pathway, whereas dietary oleic acid up-regulates CD36 expression and its expression-dependent activation of the Src-ERK1/2 pathway, which promotes cervical cancer cell growth (Pan et al., 2019; Yang et al., 2018). Palmitic acid or a high-fat diet can specifically boost the metastatic potential of metastasis-initiating oral cancer cells with high CD36 expression. Blockade of CD36 with neutralizing antibodies inhibits the metastasis of oral cancer cells in mice (Pascual et al., 2017), implying a role for dietary lipids and CD36 in tumor metastasis.
The ability of cancer cells to use exogenous lipids for the provision of unsaturated FAs appears to be dependent on oxygen levels and the type of oncogene expressed, and not all lipids are exploited equally (Snaebjornsson et al., 2020). Hypoxic tumor cells exhibit increased FA import dependent on hypoxia-inducible factor–1α (HIF-1α)–induced FABP3/7 expression, which accompanies reduced de novo FA synthesis because the catabolism of glucose to acetyl–coenzyme A (CoA) is decreased (Bensaad et al., 2014). Compared with myrAKT expression, which boosts de novo FA synthesis, oncogenic H-RasV12G or K-RasG12D expression under normoxic conditions recapitulates hypoxic conditions and reestablishes cell reliance on the uptake of lipids, among which lysophospholipids containing mono- or polyunsaturated acyl chains are internalized to a far greater extent than phospholipids and saturated lysophospholipids (Kamphorst et al., 2013). In addition to being blood resources, adipocytes in the tumor microenvironment provide extracellular FAs for tumor cells. FABP4 expression was detected in ovarian cancer cells at the adipocyte–tumor cell interface. Omentum-metastatic ovarian cells activate the lipolysis in adipocytes to produce free FAs that can be subsequently secreted and taken up by cancer cells in a FABP4-dependent manner for increased FAO and rapid tumor growth (Gharpure et al., 2018; Nieman et al., 2011). In addition, omental adipocytes induce CD36 expression and associated lipid accumulation in ovarian cancer cells. Inhibition of CD36 attenuated adipocyte-induced cholesterol and lipid droplet accumulation in ovarian cancer cells, peritoneal dissemination of tumor cells, and tumor growth (Ladanyi et al., 2018). Similar to adipocytes, tissue-resident pancreatic stellate cell–derived cancer-associated fibroblasts in pancreatic ductal adenocarcinoma (PDAC) secrete lipids including lysophosphatidylcholines for cancer cell uptake and synthesis of phosphatidylcholines. In addition, PDAC cell–secreted enzyme autotaxin hydrolyzes lysophosphatidylcholines into lysophosphatidic acid as an extracellular signal to promote PDAC cell proliferation and migration (Auciello et al., 2019). Thus, hypoxia, overexpressed FA transporters in tumor cells, specific oncogene expression, and tumor cell–modulated stromal cells including adipocytes and fibroblasts can induce tumor cells to take up extracellular FAs and create mitogenic signals to sustain tumor cell proliferation.
Cholesterol uptake
Dietary cholesterol is absorbed by Niemann–Pick type C1–like 1 (NPC1L1) protein in the membrane of intestinal enterocytes (Altmann et al., 2004), where cholesterol is esterified by acylCoA:cholesterol acyltransferases (ACATs; also known as sterol O-acyltransferase) for uptake by the liver (Ko et al., 2020). The liver, the main cholesterol biosynthesis organ, delivers cholesterol as very-low-density lipoproteins to the bloodstream, where the very-low-density lipoproteins are processed into low-density lipoproteins (LDLs) for uptake by the LDL receptors (LDLRs) on peripheral cells. The cellularly absorbed cholesterol eventually reaches the ER for sensing, transport, or esterification (Goldstein and Brown, 2009).
LDLR expression was positively correlated with poor prognosis of the patients with small cell lung cancer (SCLC), breast cancers, and PDAC. LDLR depletion in HER2-overexpressing breast cancer cells and PDAC cells reduced cholesterol uptake and tumor growth in mice with hyperlipidemia (Gallagher et al., 2017; Guillaumond et al., 2015; Zhou et al., 2017). In addition, LDLR expression is transcriptionally up-regulated by epidermal growth factor receptor (EGFR) vIII mutant/PI3K-activated SREBP1 in glioblastoma (Guo et al., 2011). Loss of PTEN and subsequent PI3K/AKT activation in prostate cancer cells largely enhanced exogenous LDL uptake required for tumor growth (Yue et al., 2014). These findings underscore that the LDLR-mediated cholesterol uptake plays instrumental roles in the proliferation of some types of cancer cells.
Lipogenesis
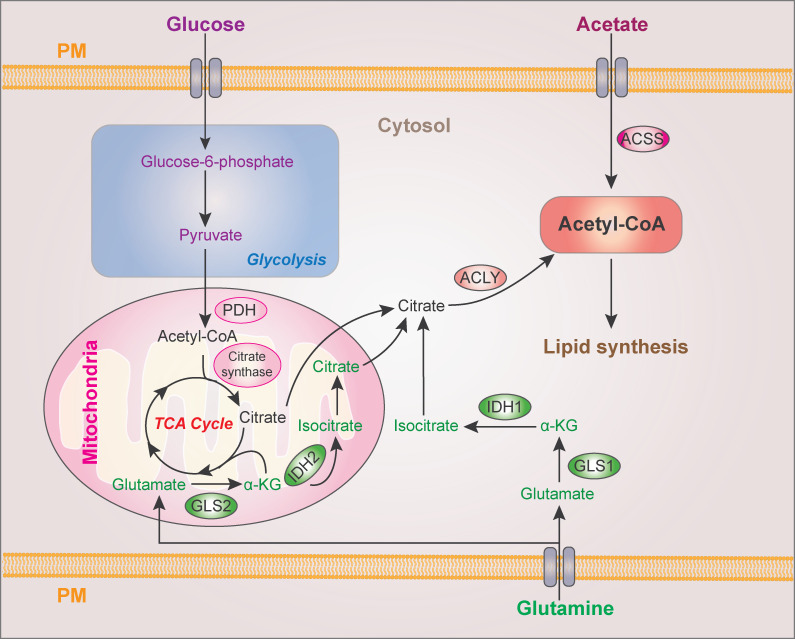
Lipogenesis in normal tissue is primarily restricted to hepatocytes and adipocytes. Nevertheless, cancer cells activate lipogenesis in response to their high metabolic demand, even in the presence of exogenous lipid sources, or to serum-derived lipid deficiency in the tumor microenvironment (Röhrig and Schulze, 2016). The main substrate for lipid synthesis is cytoplasmic acetyl-CoA, which can be derived from citrate by ATP–citrate lyase (ACLY) or acetate by acetyl-CoA synthetase (ACSS; Li et al., 2017a). Carbons from glucose and glutamine contribute to citrate production (Li et al., 2016b; Metallo et al., 2011; Fig. 1). Glucose-derived pyruvate is converted to acetyl-CoA by pyruvate dehydrogenase followed by citrate synthase–mediated production of citrate, which is then exported to cytosol from mitochondria by mitochondrial citrate transport proteins. Glutamine is converted to α-ketoglutarate mediated by cytosolic glutaminase 1 (GLS1) or mitochondrial GLS2, which is followed by cytosolic isocitrate dehydrogenase 1 (IDH1)– and mitochondrial IDH2-dependent isocitrate and subsequent citrate production. Since both FA and cholesterol are synthesized from acetyl-CoA through a series of reactions, acetyl-CoA levels are a key element for lipid production (Fig. 2).
Figure 1.
Cytoplasmic acetyl-CoA production for lipid synthesis. Cytoplasmic acetyl-CoA is produced from ACLY-catalyzed citrate and ACSS-catalyzed acetate. Glucose and glutamine contribute to citrate production from mitochondrial pyruvate oxidation in the TCA cycle and reductive carboxylation, respectively. PDH, pyruvate dehydrogenase; α-KG, α-ketoglutarate; GLS, glutaminase; PM, plasma membrane.
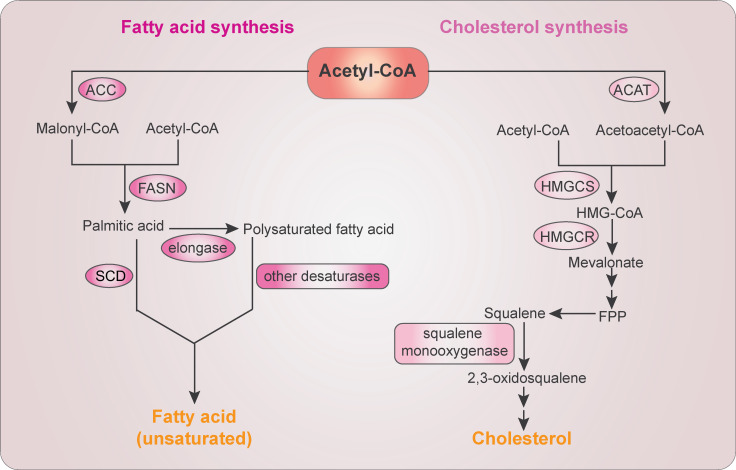
Figure 2.
The FA and cholesterol biosynthesis. FA biosynthesis starts with conversion of acetyl-CoA to malonyl-CoA by ACC. Acetyl-CoA and malonyl-CoA are then catalyzed into palmitic acid by FASN. Further elongation is mediated by elongases to form polysaturated FA. Palmitic acid and polysaturated FAs are desaturated into unsaturated FAs by SCD and other fatty acyl-CoA desaturases, respectively. Cholesterol biosynthesis starts with the condensation of two molecules of acetyl-CoA by ACAT to form acetoacetyl-CoA, which is further condensed with a third molecule of acetyl-CoA by HMG-CoA synthase (HMGCS) to form HMG-CoA. HMGCR then reduces HMG-CoA to mevalonate, which is converted to farnesyl pyrophosphate (FPP). Farnesyl pyrophosphate–converted squalene is oxidized by SM to produce 2,3-oxidosqualene, a precursor of cholesterol and sterols.
Acetyl-CoA–producing enzymes ACLY and ACSS
ACLY
ACLY catalyzes the conversion of citrate and CoA to oxaloacetate and acetyl-CoA. By inhibition of ACLY, bempedoic acid reduces patients’ LDL cholesterol levels (Giral, 2020), displaying a critical role of ACLY in lipogenesis. The overexpression or enhanced activity of ACLY is correlated with tumor progression in glioblastoma, colorectal cancer, breast cancer, lung cancer, and hepatocellular carcinoma (HCC). Decreased ACLY expression reduces tumor cell viability and suppresses tumor cell proliferation, invasion, and metastasis in various cancer types (Hatzivassiliou et al., 2005; Khwairakpam et al., 2020).
ACLY, which is transcriptionally up-regulated by SREBP1, is allosterically activated by glycolytic intermediate fructose 6-phosphate and inhibited by citrate at high concentrations via homotropic allosteric regulation of ALCY. The citrate-dependent inhibition is abolished by ACLY S455 phosphorylation by AKT or protein kinase A (PKA; Berwick et al., 2002; Potapova et al., 2000). Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation and inhibits lipid synthesis and tumor progression (Zhang et al., 2016). Under high-glucose conditions, sirtuin–2 (SIRT2) deacetylase-promoted ACLY ubiquitylation and degradation are inhibited by lysine acetyltransferase 2B (KAT2B, also known as PCAF)–mediated acetylation at K540, K546, and K554. This acetylation is increased in human lung cancers and promotes lipogenesis and tumor growth (Lin et al., 2013). In addition, IKKβ-phosphorylated ACLY binds to IKKβ-phosphorylated and stabilized ubiquitin-specific protease–30 (USP30), which deubiquitinates and stabilizes ACLY in HCC cells (Gu et al., 2020). In diethylnitrosamine (DEN)/CCl4-induced liver tumors in mice, USP30 deletion attenuated lipogenesis, inflammation, and tumor growth (Gu et al., 2020).
In addition to participating in lipid metabolism in the cytosol, nucleus-translocated ACLY generates acetyl-CoA for histone acetylation and gene transcription regulation (Wellen et al., 2009). In melanoma cells, ACLY enhances acetyltransferase p300-dependent histone acetylation, thereby promoting PPARγ coactivator (PGC) 1α–mediated mitochondrial biogenesis for cell proliferation (Guo et al., 2020). In PDAC, glioma, and prostate cancer cells, growth factors or oncogenic K-Ras expression promotes AKT–ACLY signaling, histone acetylation, cell proliferation, and tumor growth (Carrer et al., 2019; Lee et al., 2014). Following DNA damage, the ataxia telangiectasia mutated–AKT axis–induced nuclear ACLY S455 phosphorylation promotes histone acetylation at double-strand break sites, enabling BRCA1 recruitment and DNA repair by homologous recombination (Sivanand et al., 2017). Thus, ACLY-mediated acetyl-CoA production plays critical roles in multiple cellular activities in both the cytosol and nucleus.
ACSS2
ACSS produces acetyl-CoA via the ligation of acetate and CoA. ACSS1 and ACSS3 are mitochondrial proteins, and ACSS2 is localized in the cytoplasm and nucleus (Li et al., 2017b). ACSS2, which is transcriptionally up-regulated by SREBP, is expressed in a large proportion of human tumors and critical for acetate catalyzation to sustain cancer cell growth, especially under metabolic stress (Comerford et al., 2014; Luong et al., 2000). Acetate can be converted from fructose by the gut microbiota, and depletion of the microbiota or silencing of hepatic ACSS2 suppresses the conversion of bolus fructose into hepatic acetyl-CoA and FAs (Jang et al., 2018; Zhao et al., 2020). In addition, ACSS2 depletion or deficiency largely inhibits tumor cell growth and mouse tumor formation, highlighting a critical role of acetate consumption in the production of lipid biomass for tumor growth (Mashimo et al., 2014; Schug et al., 2015).
Limited amounts of oxygen, serum, or glucose increase the nuclear localization of ACSS2 (Bulusu et al., 2017; Li et al., 2017a, b). Glucose deprivation promotes 5′ AMP-activated protein kinase (AMPK)–mediated ACSS2 S659 phosphorylation, resulting in exposure of its nuclear localization signal for nuclear translocation in glioblastoma cells. In complexes with transcription factor EB, ACSS2 activates lysosome- and autophagosome-related genes by locally producing acetyl-CoA for histone H3 acetylation at the promoter regions of these genes by using the acetate released from histone deacetylation, leading to lysosomal biogenesis and autophagy, cell survival, and brain tumor growth (Li et al., 2017a, b). In addition, ACSS2 enhances the expression and activation of liver X receptor (LXR)/retinoid X receptor transcription factors to promote lipogenesis during prolonged fasting (Huang et al., 2018). Analyses of human tumor specimens showed that ACSS2 S659 phosphorylation was positively correlated with glioma malignancy and poor survival of patients with non-SCLC (NSCLC; Li et al., 2017b; Yang et al., 2019), whereas high ACSS1/2 expression led to increased histone H3 acetylation and FA synthase (FASN) expression in HCC patients (Bulusu et al., 2017). These findings reveal dual roles of ACSS2 in the direct involvement of de novo lipogenesis and the epigenetic regulation of gene expression for promoting lysosomal biogenesis, autophagy, and lipid metabolism.
FA biosynthesis enzymes
Acetyl-CoA carboxylase (ACC)
ACC, the rate-limiting enzyme for FA synthesis, catalyzes the carboxylation of acetyl-CoA to malonyl-CoA (Fig. 2; Wei and Tong, 2015). Mammalian ACC has two tissue-specific isoforms: ACC1 (also known as ACCα, encoded by ACACA) and ACC2 (also known as ACCβ, encoded by ACACB). ACC1 is a cytosolic enzyme primarily expressed in lipogenic tissues (liver and adipose tissues) and is critical for FA synthesis. In contrast, ACC2 is embedded in mitochondrial outer membrane and is mainly expressed in oxidative tissues (heart and skeletal muscles), and ACC2-produced malonyl-CoA inhibits carnitine palmitoyltransferase I (CPT1, also known as palmitoyl-CoA transferase I, CAT1, or CCAT), which governs the rate-limiting step of FA uptake and FAO by mitochondria (Luo et al., 2012).
ACC1 is highly expressed in a variety of human cancers, including breast, prostate, liver, and gastric carcinoma, and the ACACA gene is present in recurrent amplicons associated with lowered survival of breast cancer patients (Chin et al., 2006; Luo et al., 2012). ACC1 depletion decreases FA synthesis and induces the apoptosis of prostate and breast tumor cells but not nonmalignant cells (Brusselmans et al., 2005). ACC1 is regulated at the transcriptional level by SREBP and at the protein level by a complex interplay of phosphorylation, the binding of allosteric regulators, and protein–protein interactions (Koundouros and Poulogiannis, 2020). AMPK phosphorylates ACC1 S79 and ACC2 S212 and inhibits their activities, whereas the expression of ACC1/2 phosphorylation-dead mutants increases lipogenesis and lesions in mouse liver and the proliferation of human liver cancer cells (Ha et al., 1994; Lally et al., 2019). In addition, leptin and TGFβ signaling induce TGFβ-activated kinase (TAK) 1-AMPK–mediated ACC1 inhibition, resulting in increased cellular acetyl-CoA, Smad2 acetylation and activation, and breast cancer cell invasion and metastasis. These results suggest that fine-tuning ACC1 activity is critical for balanced FA synthesis and other cellular activities (Rios Garcia et al., 2017). ACC1 is allosterically activated by citrate binding and inactivated by the binding of palmitoyl-CoA and other fatty acyl-CoAs, as well as the BRCA1 C-terminal (BRCT) domains (Hunkeler et al., 2018). Mutated BRCA1, which is associated with the predisposition to inherited cancer, or insulin-like growth factor-1 (IGF-1) stimulation disrupts the interaction between BRCA1 and inactive and phosphorylated ACC. Consequently, ACC is dephosphorylated and activated for FA synthesis (Koobotse et al., 2018; Moreau et al., 2006). ACC stability can be increased by its interaction with peptidyl-prolyl cis–trans isomerase PIN1 to inhibit its lysosomal degradation in prostate cancer cells and with aldo-keto reductase family 1 B10 (AKR1B10) to inhibit its ubiquitylation-dependent degradation in breast cancer cells (Ma et al., 2008; Ueda et al., 2019). ACC1 expression and subsequent de novo FA synthesis can also be up-regulated by calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) expression in prostate cancer cells, likely in a protein translation–dependent manner (Penfold et al., 2018). ACC1 also forms a complex with CPT1 and prevents its mitochondrial distribution under nutrient-sufficient conditions. During metabolic stress, phosphorylated ACC1 dissociates from CPT1, which translocates to the mitochondria for FAO, thus maintaining HCC cell survival (Wang et al., 2016).
ACC2 protein is highly expressed in laryngocarcinoma, and ACC2 expression level is positively associated with clinical cancer stage and a decreased 5-yr survival rate (Li et al., 2019a). During nutrient abundance, prolyl hydroxylase domain protein 3 (PHD3) hydroxylates and activates ACC2, thereby repressing FAO. In acute myeloid leukemia (AML), PHD3 expression is dramatically decreased, contributing to a boost of FAO that drives AML cell proliferation and disease severity (German et al., 2016).
FASN
FASN condenses one molecule of acetyl-CoA and seven malonyl-CoA molecules into the 16-carbon palmitate (C16:0; Fig. 2). FASN overexpression and hyperactivity commonly occur in many human epithelial cancers and their preneoplastic lesions and are correlated with a higher risk of both cancer recurrence and death (Menendez and Lupu, 2007). FASN expression has been shown to be stimulated by PTEN loss, steroid hormones, and the activation of EGFR and ERBB2 in a manner dependent on the activation of the PI3K–AKT and ERK1/2 signaling cascades (Menendez and Lupu, 2007; Yang et al., 2002b). Tumor-associated FASN overexpression is preferentially regulated at the transcription level by SREBP1 (Rawson, 2003). Posttranslational regulation of FASN was exhibited by its interaction with androgen-regulated and overexpressed USP2a in prostate cancer and USP30 in HCC cells, both of which deubiquitinate and stabilize FASN (Graner et al., 2004; Gu et al., 2020). FASN can be acetylated by KAT8 and deacetylated by HDAC3. FASN acetylation enhances its association with the ubiquitin ligase TRIM21 for degradation and is frequently reduced in human HCC samples with up-regulated HDAC3 expression (Lin et al., 2016). The tumor suppressor speckle-type POZ protein is another ubiquitin ligase for FASN ubiquitylation. The speckle-type POZ protein mutants commonly found in prostate cancer cannot bind to FASN, thereby up-regulating FASN expression and lipid accumulation in prostate cancer cells (Gang et al., 2019). FASN stability is also regulated by its SUMOylation, which protects FASN against proteasomal degradation in breast cancer cells (Floris et al., 2020).
FASN inhibition decreases FA synthesis and induces malonyl-CoA accumulation to inhibit CPT1-mediated FAO and cause subsequent cell-cycle arrest and apoptosis of tumor cells (Bandyopadhyay et al., 2006; Menendez and Lupu, 2007). FASN inhibition alters membrane phospholipid composition, structure, and fluidity and lipid raft formation, thereby impairing the correct localization and/or functioning of tyrosine kinase receptors, such as EGFR and ERBB2 (Menendez and Lupu, 2007). FASN inhibition also dampens palmitate-dependent palmitoylation and activation of plasma membrane– and mitochondria-associated EGFR, resulting in EGFR ubiquitylation, abrogation of EGFR activation-induced mitochondrial fusion, blunted tumor growth, and increased sensitivity of cancer cells to EGFR inhibitors (Ali et al., 2018; Bollu et al., 2014). Overexpression of FASN reduces the expression of the E-twenty six (ets)-DNA–binding protein PEA3, a negative regulator of HER2 gene transcription, and consequently increases HER2 mRNA expression (Menendez et al., 2004; Xing et al., 2000). Although intrahepatic cholangiocarcinoma showed insensitivity to FASN depletion, an outcome likely attributed to the high expression of FA uptake-related proteins and robust long-chain FA uptake in intrahepatic cholangiocarcinoma cells, ablation of FASN significantly delayed mouse HCC formation. Notably, FASN deficiency promoted nuclear translocation and activation of SREBP2 for cholesterogenesis. SREBP2 inhibition in combination with FASN ablation completely prevented PTEN deficiency/c-Met–driven mouse hepatocarcinogenesis (Che et al., 2020; Li et al., 2016a), highlighting the critical role of FASN-dependent FA synthesis and SREBP2-mediated cholesterogenesis in HCC development.
Stearoyl-CoA desaturase (SCD)
SCD (SCD1 and SCD5/hSCD2 in humans) is a rate-limiting enzyme in the ER that catalyzes the formation of a double bond at position Δ9 in stearic acid (C18:0) and, to a lesser extent, palmitic acid (C16:0), to generate the monounsaturated FA (MUFA) oleic acid (C18:1) and palmitoleic acid (C16:1), respectively (Snaebjornsson et al., 2020; Fig. 2).
SCD requires NADPH and oxygen to function, further explaining why, under hypoxic conditions, cancer cells rely more on the exogenous supply of unsaturated FA-containing lipids. When cells are deprived of exogenous lipids, the subsequent SCD1 inhibition induced both ferroptosis and apoptosis. Inhibition of SCD1 decreases CoQ10, an endogenous membrane antioxidant whose depletion has been linked to ferroptosis. A concomitant decrease in unsaturated fatty acyl chains in the phospholipids of membranes, including the ER membrane, and an increase in long-chain saturated ceramides leads to the unfolded protein response, ER stress, and apoptosis (Ackerman and Simon, 2014; Tesfay et al., 2019). Inhibited desaturation impairs mitochondrial respiration, which results in oxidative stress, and decreases MUFA incorporation into cardiolipins, a specific class of lipids exclusive to the inner mitochondrial membrane that bind cytochrome c and prevent its release from the membrane. SCD inhibition coincides with increased cytochrome c release and apoptosis (Peck and Schulze, 2016; Potze et al., 2016).
Activated EGFR in lung cancer cells phosphorylates SCD1 at Y55 to stabilize SCD1 expression leading to increased MUFA levels and lung tumor growth in mice (Zhang et al., 2017a). In Burkitt’s lymphoma cells, overexpressed Myc induces the activation of ER stress sensor inositol-requiring enzyme 1 (IRE1) and transcriptional factor X-box binding protein 1 (XBP1) and subsequent SCD1 expression for maintaining ER homeostasis and cell survival (Xie et al., 2018).
SCD1 overexpression was also found in HCC and predicts the clinical response of HCC patients to sorafenib treatment and shorter disease-free survival. Suppression of SCD1 forces liver tumor–initiating cells to differentiate via the ER stress–induced unfolded protein response, resulting in enhanced sensitivity to sorafenib (Ma et al., 2017). SCD1 silencing or inhibition in xenograft mouse models also reduced formation of tumors derived from human stomach, colon, lung, and prostate cancer cells (Snaebjornsson et al., 2020), underscoring critical roles of SCD1 in tumor development.
Cholesterol biosynthesis enzymes
Mammalian 3-hydroxy-3-methylglutaryl (HMG)–CoA reductase (HMGCR)
HMGCR, the rate-limiting enzyme of the mevalonate pathway for cholesterol biosynthesis, is an ER-localized glycoprotein and converts HMG-CoA to mevalonate (Fig. 2; Liscum et al., 1985). The regulation of HMGCR is achieved at transcriptional, posttranscriptional, translational, and posttranslational levels. HMGCR gene transcription is activated by SREBP2, whereas its mRNA translation can be blocked by unknown mevalonate-derived nonsterol metabolites (Luo et al., 2020; Nakanishi et al., 1988). In addition, HNRNPA1 regulates the alternative splicing of HMGCR to increase the expression of an alternatively spliced HMGCR transcript lacking exon 13, which exhibits diminished HMGCR activity (Yu et al., 2014). HMGCR degradation can be induced by sterols, mostly oxysterols and methylated sterols, and vitamin E family members δ-tocotrienol and γ-tocotrienol. Accumulated sterols in cells stimulate insulin-induced gene (INSIG) protein binding to the sterol-sensing domain of HMGCR and subsequent HMGCR ubiquitylation and degradation involving multiple ubiquitin ligases, the GP78 complex, TRC8 (also known as RNF139), and RNF145 (Luo et al., 2020). Under hypoxic conditions, hypoxia-inducible factor–1α activates INSIG2 transcription to trigger HMGCR degradation and inhibit cholesterol biosynthesis in the liver (Hwang et al., 2017). AMPK phosphorylates HMGCR S872 and inhibits HMGCR-dependent cholesterol biosynthesis (Clarke and Hardie, 1990; Sato et al., 1993). SIRT1, which can be down-regulated by miR-34a, a microRNA increased in nonalcoholic fatty liver disease, deacetylates and activates LKB1 and LKB1-AMPK signaling for HMGCR inhibition (Min et al., 2012; Ruderman et al., 2010).
HMGCR expression is up-regulated in multiple types of cancer, including gastric cancer, glioblastoma, and prostate cancer. The overexpression of HMGCR promoted the growth and migration of these cancer cells, while HMGCR knockdown inhibited tumorigenesis. HMGCR inhibition has been targeted for treating solid and blood cancers and cancer with drug resistance (Kong et al., 2018; Lee et al., 2018; Yang et al., 2020). Intriguingly, lipophilic HMGCR inhibitor simvastatin treatment reduced the mevalonate pathway-produced geranylgeranyl diphosphate, which is required for protein prenylation, and the prenylation levels of the small guanosine triphosphatase Rab5 in antigen-presenting cells, resulting in arrested endosomal maturation, prolonged antigen retention, enhanced antigen presentation, and antigen-specific antitumor immunity. In addition, simvastatin treatment robustly enhances cancer vaccinations and synergizes with anti–PD-1 antibodies for tumor treatment (Xia et al., 2018). These results highlight the potential of the mevalonate pathway as a target for boosting cancer immune therapy.
Squalene monooxygenase (SM)
SM (encoded by SQLE), the second rate-limiting ER-associated cholesterol biosynthesis enzyme downstream of HMGCR, converts nonsterol intermediate squalene to 2,3(S)-oxidosqualene (Fig. 2). Similar to HMGCR, SM is also a target of SREBP2 (Sharpe and Brown, 2013). SM has a cholesterol-sensing domain (the first N-terminal 100 amino acids, termed SM N100), which regulates the proteasomal SM degradation in a cholesterol, but not INSIG-, 24,25-dihydrolanosterol–, or side-chain oxysterol–dependent manner (Chua et al., 2017; Gill et al., 2011; Padyana et al., 2019). Cholesterol-accelerated SM degradation is mediated by E3 ligase membrane-associated RING finger 6 (MARCH6), which is concomitantly stabilized by the cholesterol-dependent inhibition of MARCH6 autoubiquitylation (Sharpe et al., 2019; Zelcer et al., 2014). Squalene, as a feedforward factor for cholesterol synthesis, directly binds to the N100 region, thereby reducing the interaction of SM with and ubiquitination by MARCH6 (Yoshioka et al., 2020). In contrast to cholesterol, unsaturated FAs, such as oleate, can stabilize SM by inhibiting MARCH6-mediated degradation (Stevenson et al., 2014), thereby maintaining cholesterol synthesis.
The SQLE locus has increased copy numbers in multiple cancers, and its overexpression was detected in nonalcoholic fatty liver disease–induced HCC and linked to radioresistance in pancreatic cancer and progression or poor prognosis of breast cancer, prostate cancer, colorectal cancer, and squamous lung cancer (Brown et al., 2016; Cirmena et al., 2018; Liu et al., 2018). In addition, the proliferation of SCLC cells is sensitive to SM inhibition, which results not from cholesterol biosynthesis inhibition but from the toxic accumulation of the SM substrate squalene (Mahoney et al., 2019). The loss of SM expression, which has been found in anaplastic lymphoma kinase–positive anaplastic large cell lymphoma cells, contributes to cholesterol auxotrophy and renders these cells dependent on LDLR for cholesterol uptake and tumor growth (Garcia-Bermudez et al., 2019). These findings reveal distinct features of cholesterol metabolism in different types of tumor.
Transcriptional regulation of lipogenesis
Lipogenesis is transcriptionally governed by SREBPs (Fig. 3), a family of helix-loop-helix leucine zipper transcription factors consisting of three isoforms: SREBP1a and SREBP1c encoded by the SREBF1 gene, and SREBP2 encoded by the SREBF2 gene. SREBP-1c is ubiquitously expressed, whereas SREBP-1a is expressed in intestinal epithelium, the heart, and macrophages, and SREBP2 is expressed in the liver and adipose tissue. SREBP1 mainly regulates the expression of FA synthesis genes and LDLR, whereas SREBP2 preferentially controls cholesterol biosynthesis gene expression (Horton et al., 2003; Im et al., 2011).
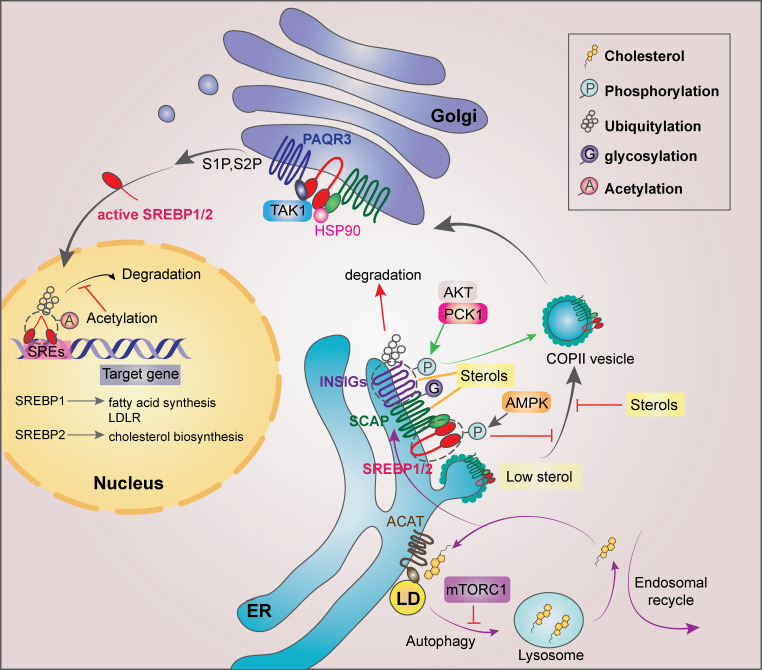
Figure 3.
Regulation of SREBP1/2 in cancer cells. SREBP activity can be regulated at multiple levels and at different subcellular localizations. In the ER, sterols bind to SCAP and disrupt the direct interaction between SCAP and COPII for the SREBP ER exit. When sterol level decreases, SCAP dissociates from INSIGs and facilitates the incorporation of SCAP/SREBP into COPII-coated vesicles. mTORC1 suppresses autophagy and subsequent cholesterol trafficking from the lysosome to the ER, leading to SREBP2 activation. Long-chain unsaturated FAs inhibit SREBP activation through inhibition of ubiquitylation of INSIG1. AKT-phosphorylated PCK1 phosphorylates INSIG1/2 and disrupts the bindings of oxysterols to INSIG1/2 for SREBP1/2 activation. In addition, activated AMPK can phosphorylate SREBP1/2 for their retention in the ER. EGFR activation enhances N-glycosylation of SCAP, triggering its dissociation from INSIG1. In the Golgi, SREBP1/2 are cleaved by S1P and S2P, releasing the transcriptionally active SREBP1/2. HSP90 facilitates the SREBP–SCAP complex transit from the ER to the Golgi. PAQR3 potentiates SREBP processing in the Golgi, whereas TAK1-mediated phosphorylation of SREBP1/2 inhibits SREBP. In the nucleus, truncated SREBP1/2 bind to SREs within the promoters of their target genes. GSK3-phosphorylated SREBP1/2 undergoes ubiquitylation and degradation, which can be counteracted by acetylation of the ubiquitylated Lys residues of SREBP1/2. LD, lipid droplet.
Inactive SREBPs reside in the ER membrane, where their C-terminal domains interact with the WD-repeat domain in the C terminus of SREBP cleavage-activating protein (SCAP; Gong et al., 2015). The N-terminal domain of SCAP binds to INSIG1 and INSIG2, forming an INSIG/SCAP/SREBP complex that retains SREBPs in the ER (Yabe et al., 2002; Yang et al., 2002a). The binding of coatomer II (COPII) to SCAP leads to the translocation of the SCAP–SREBP complex from the ER to the Golgi, where SREBPs are sequentially cleaved by membrane-bound site-1 protease (S1P) and site-2 protease (S2P), releasing the transcriptionally active N-terminal domains. Mature SREBPs then translocate to the nucleus and bind as homodimers to SREs and E-boxes within their target gene promoters (Nohturfft and Zhang, 2009). SREBP activity can be regulated at multiple levels and in different subcellular localizations, such as the ER, Golgi, and nucleus.
Regulation of SREBPs in the ER
SREBP activation is regulated by sterol fluctuations in the ER. Cholesterol binds to SCAP, thereby disrupting the interaction between SCAP and COPII and retaining SREBP in the ER (Shimano and Sato, 2017). Oxysterols, such as 25-hydroxycholesterol, are much more potent than cholesterol in the SREBP’s ER retention by binding to INSIGs and promoting INSIG binding to SCAP (Eberlé et al., 2004). When the sterol level decreases, SCAP dissociates from INSIGs, which facilitates the incorporation of SCAP/SREBP into COPII-coated vesicles (Menendez and Lupu, 2007). p53 transcriptionally induces the ATP-binding cassette transporter (ABCA1), a cholesterol transporter gene. p53 loss or ABCA1 ablation decreases the retrograde transportation of cholesterol from the plasma membrane to the ER, thereby promoting SREBP2 maturation and mouse liver tumorigenesis (Moon et al., 2019). ER-residing ACAT esterifies ER cholesterol with FAs to form cholesteryl esters for the storage in lipid droplets, thereby controlling ER cholesterol levels. Inhibition of ACAT accumulates ER cholesterol, reduces lipid droplet formation, and suppresses glioblastoma growth and the aggressiveness of prostate cancer cells by blocking SREBP1-regulated gene expression (Geng et al., 2016; Yue et al., 2014).
mTORC1 suppresses autophagy and maintains endosomal recycling to the plasma membrane, thereby preventing membrane-derived cholesterol from reaching lysosomes and subsequent ER localization, leading to SREBP2 activation (Eid et al., 2017). Long-chain unsaturated FAs inhibit SREBP activation through interaction with ubiquitin regulatory X domain–containing protein 8 (UBXD8). UBXD8 binds to polyubiquitylated INSIG1, in turn recruiting the valosin-containing protein complex, which facilitates INSIG1 degradation. Long-chain unsaturated FAs induce the detachment of UBXD8 from INSIG1, thereby stabilizing INSIG1 (Lee et al., 2008; Lee et al., 2010).
The INSIG/SCAP/SREBP complex can also be regulated in lipid-independent manners. Receptor tyrosine kinase activation and active K-RAS mutations in cancer cells induce AKT-mediated phosphorylation of S90 of cytosolic phosphoenolpyruvate carboxykinase 1 (PCK1), resulting in inhibition of the gluconeogenic activity of PCK1 and its translocation to the ER, where it acts as a protein kinase phosphorylating INSIG1 S207 and INSIG2 S151. Phosphorylated INSIGs reduce their binding to oxysterols, thereby activating SREBP-dependent lipogenesis for liver tumor formation. In addition, PCK1-mediated INSIG phosphorylation is associated with poor HCC prognoses (Xu et al., 2020). EGFR activation also leads to enhanced SCAP N-glycosylation to trigger its dissociation from INSIG1 to induce lipogenesis and glioblastoma growth (Cheng et al., 2015). In addition to regulation by the AKT–PCK1 axis, SREBP1 activation by AKT- and mTORC1-dependent or mTORC1-independent mechanisms was revealed (Düvel et al., 2010; Guo et al., 2009; Yecies et al., 2011). CREB-regulated transcription coactivator 2 (CRTC2) competes with the COPII complex subunit Sec23A to interact with Sec31A, thereby disrupting SREBP1 transport, and this inhibitory effect was attenuated by mTOR-mediated CRTC2 phosphorylation (Han et al., 2015). It was shown that insulin signaling and AKT activation suppress INSIG2 expression by promoting its mRNA decay (Yecies et al., 2011). In addition, INSIG1, but not INSIG2, is ubiquitinated by GP78 and degraded upon sterol depletion (Lee et al., 2006). INSIG1 degradation, which is also induced in HCC cells in an AKT-mediated INSIG1 S207 phosphorylation–dependent manner, results in SREBP activation–increased transcription of downstream genes, including INSIG1 itself (Shao and Espenshade, 2012; Xu et al., 2020). The newly synthesized INSIG1 continues to be degraded until a sufficient amount of cholesterol is produced to induce a conformational change of SCAP that enables INSIG1 binding in normal cells (Gong et al., 2006). However, switching off SREBP activation by the feedback-produced cholesterol and INSIG1 protein is disrupted by INSIG1 S207 phosphorylation in tumor cells, and thus a high level of lipogenesis is maintained (Xu et al., 2020). In contrast to AKT-mediated INSIG phosphorylation, activated AMPK phosphorylates SREBPs for their potential retention in the ER, thereby suppressing lipogenesis in HepG2 liver cancer cells (Li et al., 2011). In addition, the SCAP–SREBP2–INSIG complex can be also retained in the ER upon its binding to the LATS2 tumor suppressor, interaction with ERLINs and RNF139 independent of ubiquitylation, or the RNF145-dependent SCAP ubiquitylation that prevents COPII binding (Aylon et al., 2016; Huber et al., 2013; Irisawa et al., 2009; Zhang et al., 2017b). Mice harboring liver-specific Lats2 knockout spontaneously developed fatty liver disease in association with impaired p53 activation (Aylon et al., 2016),
Thus, the INSIG/SCAP/SREBP complex in the ER can be regulated by sterol and FA fluctuations; posttranslational modifications of INSIG, SCAP, and SREBP; mRNA and protein stability of INSIG; and the protein interaction that affects the binding of SCAP to COPII.
Regulation of SREBPs in the Golgi apparatus
Heat shock protein 90 (HSP90) binds to and stabilizes the SREBP–SCAP complex in both the ER and the Golgi and facilitates its transit to the Golgi, whereas HSP90 inhibition leads to proteasomal degradation of the complex (Kuan et al., 2017). The Golgi-anchoring protein progestin and adipoQ receptor 3 (PAQR3), which is transcriptionally induced under cholesterol-depleting conditions, interacts with SCAP and SREBP and tethers them to the Golgi to potentiate SREBP processing and enhance lipid synthesis (Xu et al., 2015). ER- or Golgi-localized SREBPs can be phosphorylated and inhibited by TAK1 without blocking their nuclear localization, and hepatic Tak1 deficiency causes steatosis (Morioka et al., 2016).
Regulation of nuclear SREBPs
mTORC1 promotes the nuclear localization of mature SREBP1 through the phosphorylation and cytoplasmic retention of the phosphatidate phosphatase LPIN1, which, in its unphosphorylated state, inhibits SREBP1 by sequestering it at the nuclear periphery (Peterson et al., 2011). Nuclear SREBP can be degraded by the SCFFBXW7 ubiquitin ligase dependent on GSK3-mediated SREBP phosphorylation (Sundqvist et al., 2005). This phosphorylation is prevented by SREBP1a R321 dimethylation by protein arginine methyltransferase 5 (PRMT5), resulting in increased lipogenesis, accelerated tumor cell proliferation, and poor prognosis for HCC patients (Liu et al., 2016). The SREBP degradation can be also inhibited by the acetylation of the ubiquitylated Lys residue by p300 and its related protein CBP (Giandomenico et al., 2003), and this inhibition can be abrogated by SIRT1 deacetylatase (Walker et al., 2010). In addition, ERK-mediated phosphorylation of nuclear SREBP2 at S432 and S455 enhances SREBP2 activity, whereas SUMO-1–conjugating enzyme Ubc9-dependent SREBP SUMOylation decreases its transcriptional activity (Hirano et al., 2003; Kotzka et al., 2004).
The vicinity of the SRE motif of the promoter regions of lipogenic genes, including SREBF2, contains binding sites for transcription factors SP1 and/or NFY (Shimano and Sato, 2017). SP1 or NFY interacts with SREBPs and coordinates the expression of a subset of SREBP target genes. Hepatocyte nuclear factor 4 (HNF4) and PGC1β up-regulate SREBP activity also via direct binding (Lin et al., 2005; Misawa et al., 2003). Upon ER stress or glucose deprivation, ATF6 interacts with SREBP2, resulting in the recruitment of HDAC1 to the ATF6–SREBP2 complex and the inhibition of SREBP2 (Zeng et al., 2004). Thus, the activity of nuclear SREBPs can be regulated by phosphorylation, acetylation, ubiquitylation, and protein–protein interactions.
Transcriptional regulation of SREBPs and the roles of LXRs in lipid homeostasis
The SREBF1 and SREBF2 promoters each contain an SRE for their own autoloop activation, which generates positive feedback self-regulation upon SREBP activation (Shimano and Sato, 2017). In addition, LXRα and LXRβ form heterodimers with retinoid X receptor and bind to conserved LXR response elements in the promoters of SREBF1 in the presence of different nuclear oxysterols, which function as LXR ligands, to promote lipid synthesis (Lin and Gustafsson, 2015; Repa et al., 2000). The expression of SREBP2 can be inhibited by the binding of FOXO3 to the SREBF2 promoter, leading to the recruitment of SIRT6 to deacetylate histone H3 (Tao et al., 2013). In addition to transcriptional regulation, the negative regulation of SREBPs at the mRNA level by miR-29, miR-185, and miR-342 has also been revealed (Cheng et al., 2018).
The role of LXRs in lipid metabolism in cancer cells is complicated by the expression of their multiple downstream genes, and LXR has been shown to exert positive or negative regulation on the growth and survival of different tumor cells (Flaveny et al., 2015; Guo et al., 2011). LXRs can reduce intracellular cholesterol levels by enhancing cholesterol efflux mediated by up-regulated ABCA1 expression, and decreasing cholesterol uptake mediated by increased expression of inducible degrader of the LDLR (Idol), a ubiquitin ligase for LDLR degradation (Lin and Gustafsson, 2015; Repa et al., 2000; Zelcer et al., 2009). In contrast, in addition to activating SREBF1 expression, LXRs can increase lipogenesis by inducing FASN and SCD1 expression and enhances glycolysis by inducing phosphofructokinase-2 (PFK2) and glucokinase (GCK1) expression (Kim et al., 2009; Tao et al., 2013; Zhao et al., 2012). Given that LXR activation also induces the expression of cell cycle regulators and genes involved in immune cell functions, studying LXR in cancer in intact tumor immune microenvironments may elucidate more insightful information.
Lipolysis and FAO
FAO allows the mitochondrial conversion of long-chain FAs into acetyl-CoA, which enters the TCA cycle, and NADH and FADH2, which are coenzymes used in the electron transport chain (Fig. 4). FAO occurs in peroxisomes when the FA chains are too long to be oxidized in mitochondria. On the outer mitochondrial membrane, fatty acyl-CoA is converted to fatty acylcarnitine by CPT1, for which the isoforms CPT1A, CPT1B, and CPT1C are predominantly expressed in the liver, muscle, and brain, respectively. Carnitine/acylcarnitine translocase located on the inner mitochondrial membrane shuttles acylcarnitine into the mitochondrial matrix. CPT2 on the matrix side of the inner membrane reconverts acylcarnitine to acyl-CoA for being cleaved into acetyl-CoA, which enters the TCA cycle to generate ATP and malic enzyme-dependent NADPH (Fig. 4). In addition, FAO-generated citrate can be exported to the cytoplasm for NADPH production by IDH-mediated isocitrate oxidation. NADPH is a reducing agent that supports biosynthesis and redox homeostasis (Carracedo et al., 2013).
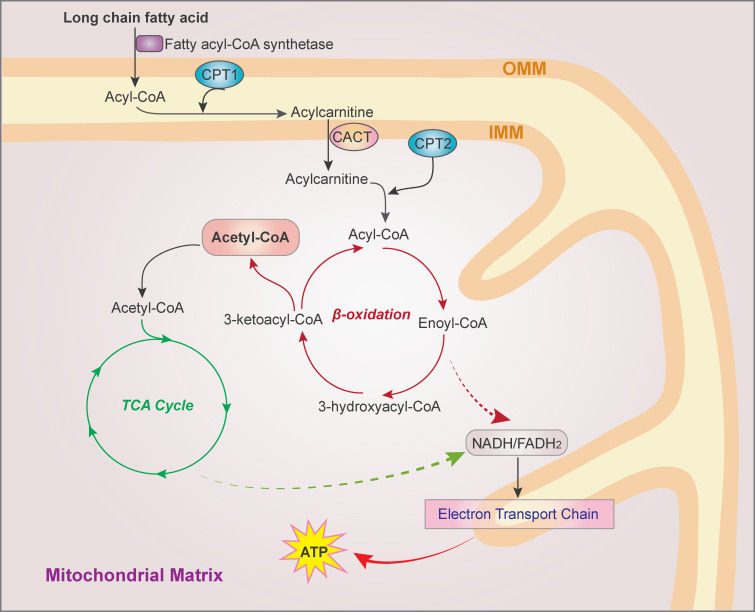
Figure 4.
The FAO pathway. FAs are activated to fatty acyl-CoA by fatty acyl-CoA synthetase. On the outer mitochondrial membrane, fatty acyl-CoA is converted to fatty acylcarnitine by CPT1 and shuttled into the mitochondrial matrix. CPT2 on the matrix side of the inner membrane then reconverts acylcarnitine to acyl-CoA, which is cleaved into acetyl-CoA by a repeated four-step cycle catalyzed sequentially by the activity of acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl CoA dehydrogenase, and 3-ketoacyl-CoA thiolase, resulting in the shortening of FAs by two carbons in each cycle. The breakdown product acetyl-CoA enters the TCA cycle, and the produced NADH and FADH2 are coenzymes used in the electron transport chain to generate ATP. CACT, carnitine/acylcarnitine translocase; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane.
Many types of cancer, such as lung cancer with K-Ras mutation, triple-negative breast cancer, and glioma, exhibit high FAO activity (Carracedo et al., 2013; Padanad et al., 2016). Consistently, the overexpression of various FAO pathway proteins, including CPT1A, CPT1B, CPT1C, CPT-2, carnitine transporter CT2, and acyl-CoA synthetase long-chain family member 3 (ACSL3), is found in multiple types of cancers, while some FAO enzymes, such as CPT1A, are highly correlated with poor outcomes patients with AML or ovarian cancer (Carracedo et al., 2013). CPT1C, expressed normally in the brain, is up-regulated in human lung tumors, depending on AMPK, and promotes FAO, ATP production, and the resistance of tumor cells to energy stress (Zaugg et al., 2011).
The most prominent transcriptional regulators of FAO are PPARs (Poulsen et al., 2012). Promyelocytic leukemia protein, which is overexpressed in triple-negative breast cancer, activates PPAR and FAO by reducing PGC-1α acetylation (Carracedo et al., 2012). Jumonji D3 (JMJD3) histone demethylase, interacting with SIRT1 and PPARα, decreases H3K27me3 at FAO genes and thereby epigenetically activates the expression of these genes (Seok et al., 2018). Acting as a transcription factor, HNF4 directly activates FAO genes and is required for the renewal of intestinal stem cells (Chen et al., 2020). In breast cancer cells, c-Myc/PGC-1β/ERRα signaling induces FAO enzyme expression (Yan et al., 2017), and FAO inhibition blocked MYC-driven breast tumor growth in mice (Camarda et al., 2016). In addition, mammary adipocyte–derived leptin up-regulates STAT3-induced CPT1B expression and FAO activity in breast cancer stem cells, which promotes cancer cell stemness and chemoresistance (Wang et al., 2018). Similar to CPT1 overexpression, acyl-CoA-binding protein (also known as diazepam binding inhibitor [DBI]) is highly expressed in glioblastoma and binds to acyl-CoAs to promote mitochondrial long-chain fatty acyl-CoA accumulation, FAO, and tumor growth (Duman et al., 2019). As the first and rate-limiting enzyme in FAO and a major producer of H2O2 in peroxisomes, acyl-CoA oxidase 1 (ACOX1) exhibits increased succinylation and activity due to the down-regulated expression of SIRT5 desuccinylase, which enhances DNA damage response to oxidative stress in HCC cells (Chen et al., 2018). FAO can also be stimulated by the AMPK activation, which reduces ACC-produced malonyl CoA, an allosteric CPT1 inhibitor (Jeon et al., 2012). CircACC1, an ACC1-derived circular RNA with elevated expression in human colon cancer, regulates the assembly and activation of the AMPK complex under metabolic stress, thereby facilitating FAO and xenograft tumor growth (Li et al., 2019b).
In addition to its role in ATP production and cell proliferation, FAO is critical for NADPH homeostasis, mitochondrial function, and cell survival. FAO inhibition decreases NADPH levels with correspondingly increased ROS levels and apoptosis rates of glioma cells (Pike et al., 2011). During energy stress, AMPK activation increases NADPH production through FAO and inhibits NADPH consumption in FA synthesis, thereby inhibiting lung cancer cell death (Jeon et al., 2012).
MCL-1, which is a BCL-2 family protein linked to a FAO signature in AML, interacts with very-long-chain acyl-CoA dehydrogenase and promotes long-chain FA β-oxidation (Escudero et al., 2018). Truncated BH3 interacting domain death agonist, a proapoptotic mitochondrial protein, decreases CPT-1 activity and inhibits FAO, while overexpressed CPT-1 interacts with Bcl-2 and counteracts the effects exerted by BH3 interacting domain death agonist on FAO. Inhibition of FAO promotes Bak and Bax oligomerization, mitochondrial permeability transition, cytotoxic accumulation of long-chain FAs, ER stress, and subsequent apoptosis (Giordano et al., 2005; Ma et al., 2018; Samudio et al., 2010).
However, overly active FAO can be cytotoxic. In glioblastoma cells, highly expressed diacylglycerol-acyltransferase 1 (DGAT1) converts diacylglycerol and excessive fatty acyl CoA to triglycerides, which are stored in lipid droplets. Inhibiting DGAT1 resulted in excessive FAO and ROS production and apoptosis (Cheng et al., 2020). DGAT1/2 depletion also increased saturated toxic FAs in clear cell renal cell carcinoma cells and impaired mouse tumor growth (Ackerman et al., 2018; Chitraju et al., 2017). In obesity- and nonalcoholic steatohepatitis–driven HCC, CPT2 down-regulation prevents lipotoxicity in HCC cells and promotes liver tumorigenesis (Fujiwara et al., 2018). Thus, dynamic and balanced control of FAO supports tumor cell proliferation and survival by providing the needed ATP and NADPH, eliminating potentially toxic lipids, inhibiting proapoptotic pathways, and producing the metabolic intermediates required for anabolism.
Targeting lipid metabolism for cancer treatment
The integrated and mutual regulation between oncogenic signaling and lipid metabolism promotes cancer cell growth, survival, proliferation, migration, invasion, and metastasis. Lipid metabolism in cancer cells and other cells in the tumor microenvironment, including immune cells, adipocytes, endothelial cells, and fibroblasts, is dynamically regulated and interconnected, and great efforts have been made to develop therapeutic drugs to intervene in lipid metabolism at different levels. Numerous preclinical studies on many inhibitors of a variety of lipid metabolism enzymes have been reported (Koundouros and Poulogiannis, 2020; Ma et al., 2018; Röhrig and Schulze, 2016) and will not be covered in detail here. Instead, advanced studies, especially clinical trials, are discussed.
To target cholesterol synthesis, statin family drugs, such as HMGCR inhibitors, are currently being tested as anticancer agents in multiple clinical trials (Mullen et al., 2016). Some inconsistent results were reported. Retrospective studies showed that statin treatment prolonged the survival of patients with multiple myeloma, colorectal cancer, and metastatic pancreatic cancer cotreated with first-line chemotherapy (Abdel-Rahman, 2019; Brånvall et al., 2020; Cardwell et al., 2014). In contrast, randomized phase III trials in patients with SCLC, metastatic colorectal cancer, HCC, or gastric cancer showed that addition of pravastatin or simvastatin to standard chemotherapy offers no additional benefit (Jouve et al., 2019; Kim et al., 2014; Lim et al., 2015; Seckl et al., 2017). Notably, a phase II study revealed that simvastatin in combination with the EGFR inhibitor gefitinib, but not afatinib, resulted in higher tumor response rates and longer progression-free survival than did EGFR inhibitor alone in NSCLC patients (Han et al., 2011; Lee et al., 2017). It was suggested that the lipophilic statin drugs more readily enter extrahepatic cells, whereas hydrophilic statins are more hepatoselective (Duncan et al., 2006). In addition, clinical data indicate that the anticancer effects of statins are both dose and time dependent (Kim et al., 2014). Thus, identification of predictive biomarkers for patient stratification and selection of appropriate statin type, dosage, and treatment durations likely provide more definitive evaluations of statins as adjuvant treatments.
Nelfinavir, an S2P inhibitor that has been used in HIV treatment, was administered concurrently with chemoradiotherapy during early-phase clinal trials, and the results showed encouraging antitumor activity and acceptable safety in patients with NSCLC, inoperable pancreatic cancer, or multiple myeloma, although the exact contribution of lipid synthesis inhibition by nelfinavir to the anticancer effect warrants further study (Das, 2019; Driessen et al., 2016; Rengan et al., 2012; Wilson et al., 2016).
To target FA synthesis, the FASN inhibitor TVB-2640 is currently being evaluated in phase II clinical trials as a single agent in NSCLC with KRAS multination (NCT03808558), in combination with paclitaxel and trastuzumab in triple-negative breast cancer (NCT03179904), or with the anti-angiogenic drug bevacizumab in high-grade astrocytoma (NCT03032484). Preclinical animal studies showed that ACC inhibitors ND-646 and ND-654 markedly suppressed growth of mouse lung tumor and rat HCC, respectively (Lally et al., 2019; Svensson et al., 2016). In addition, the ACC inhibitor ND-630, originally developed for the treatment of nonalcoholic steatohepatitis, is currently undergoing phase I testing as a cancer treatment (NCT02876796; Snaebjornsson et al., 2020).
Given that lipid uptake and synthesis both contribute to lipid resources in cancer cells, simple treatment strategies inhibiting FA or cholesterol biosynthesis may be less effective due to the compensation from dietary lipids. In addition, the dynamic FAO regulation exerts critical roles in cancer progression. Thus, more specific and combinational approaches for the intervention of lipid uptake, synthesis, and lipolysis, such as FAO, are needed. Tumor lipid metabolism in each patient can manifest unique features and render profiles showing specific regulation with distinct genetic fingerprints determined by genetic mutations and epigenetic gene regulation in each type and subtype of cancer. A rational combination of conventional chemotherapy and/or radiation treatment with immunotherapy and targeted therapies, including lipid metabolism–targeted approaches, should be investigated for developing more efficient cancer treatments that do not elicit drug resistance.
Conclusion and perspectives
Our current understanding of the aberrant regulation of lipid metabolism in cancer indicates increased comprehension of the metabolic wiring of cancer cells. Lipid metabolism in cancer cells can be regulated not only through intracellular oncogenic signaling but also by the input from the tumor microenvironment composed of various types of cells, cytokines, growth factors, DNA, RNA, and nutrients, including lipids. In turn, aberrant lipid metabolism reroutes oncogenic signaling pathways in cancer cells and affects neighboring normal cell populations through secretory components, including lipids. This complexity highlights the need for studying not only the lipid metabolism network in cancer cells but also the interconnected pathways in the tumor microenvironment and the effects of interfering with lipid metabolism in the cells in the tumor microenvironment on tumor progression and treatment responses, especially the antitumor immune and anti-angiogenesis responses. In addition, structural elucidation of oncogenic signaling–induced and posttranslationally modified lipid enzymes, as well as lipid metabolism–regulating enzymes, will facilitate the identification of specific interventions that target aberrantly regulated, but not normal, lipid metabolism. Insight into the tumor-specific regulation of lipid metabolism will reveal new and exciting therapeutic opportunities for cancer elimination with minimal side effects.
Acknowledgments
This study was supported by Ministry of Science and Technology of the People's Republic of China grants (2020YFA0803300 to Z. Lu), the Zhejiang University Research Fund (188020*194221901/029 to Z. Lu), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01001 to Z. Lu), the National Natural Science Foundation of China (81902880 to X. Bian), the Qingdao Postdoctoral Application Research Project (to X. Bian), and the China Postdoctoral Science Foundation (2019M660160 to X. Bian). Z. Lu is the Kuancheng Wang Distinguished Chair.
Author contributions: All authors researched data for the article, contributed to the discussion of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
References
- Abdel-Rahman O. 2019. Statin treatment and outcomes of metastatic pancreatic cancer: a pooled analysis of two phase III studies. Clin. Transl. Oncol. 21:810–816. 10.1007/s12094-018-1992-3 [DOI] [PubMed] [Google Scholar]
- Ackerman D., and Simon M.C.. 2014. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 24:472–478. 10.1016/j.tcb.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman D., Tumanov S., Qiu B., Michalopoulou E., Spata M., Azzam A., Xie H., Simon M.C., and Kamphorst J.J.. 2018. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 24:2596–2605.e5. 10.1016/j.celrep.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Levantini E., Teo J.T., Goggi J., Clohessy J.G., Wu C.S., Chen L., Yang H., Krishnan I., Kocher O., et al. 2018. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 10:e8313 10.15252/emmm.201708313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S.W., Davis H.R. Jr., Zhu L.J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303:1201–1204. 10.1126/science.1093131 [DOI] [PubMed] [Google Scholar]
- Auciello F.R., Bulusu V., Oon C., Tait-Mulder J., Berry M., Bhattacharyya S., Tumanov S., Allen-Petersen B.L., Link J., Kendsersky N.D., et al. 2019. A Stromal Lysolipid-Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer Discov. 9:617–627. 10.1158/2159-8290.CD-18-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y., Gershoni A., Rotkopf R., Biton I.E., Porat Z., Koh A.P., Sun X., Lee Y., Fiel M.I., Hoshida Y., et al. 2016. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30:786–797. 10.1101/gad.274167.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Zhan R., Wang Y., Pai S.K., Hirota S., Hosobe S., Takano Y., Saito K., Furuta E., Iiizumi M., et al. 2006. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 66:5934–5940. 10.1158/0008-5472.CAN-05-3197 [DOI] [PubMed] [Google Scholar]
- Bensaad K., Favaro E., Lewis C.A., Peck B., Lord S., Collins J.M., Pinnick K.E., Wigfield S., Buffa F.M., Li J.L., et al. 2014. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 9:349–365. 10.1016/j.celrep.2014.08.056 [DOI] [PubMed] [Google Scholar]
- Berwick D.C., Hers I., Heesom K.J., Moule S.K., and Tavare J.M.. 2002. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J. Biol. Chem. 277:33895–33900. 10.1074/jbc.M204681200 [DOI] [PubMed] [Google Scholar]
- Bollu L.R., Ren J., Blessing A.M., Katreddy R.R., Gao G., Xu L., Wang J., Su F., and Weihua Z.. 2014. Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells. Cell Cycle. 13:2415–2430. 10.4161/cc.29338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brånvall E., Ekberg S., Eloranta S., Wästerlid T., Birmann B.M., and Smedby K.E.. 2020. Statin use is associated with improved survival in multiple myeloma: A Swedish population-based study of 4315 patients. Am. J. Hematol. 95:652–661. 10.1002/ajh.25778 [DOI] [PubMed] [Google Scholar]
- Brown D.N., Caffa I., Cirmena G., Piras D., Garuti A., Gallo M., Alberti S., Nencioni A., Ballestrero A., and Zoppoli G.. 2016. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci. Rep. 6:19435 10.1038/srep19435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselmans K., De Schrijver E., Verhoeven G., and Swinnen J.V.. 2005. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 65:6719–6725. 10.1158/0008-5472.CAN-05-0571 [DOI] [PubMed] [Google Scholar]
- Bulusu V., Tumanov S., Michalopoulou E., van den Broek N.J., MacKay G., Nixon C., Dhayade S., Schug Z.T., Vande Voorde J., Blyth K., et al. 2017. Acetate Recapturing by Nuclear Acetyl-CoA Synthetase 2 Prevents Loss of Histone Acetylation during Oxygen and Serum Limitation. Cell Rep. 18:647–658. 10.1016/j.celrep.2016.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda R., Zhou A.Y., Kohnz R.A., Balakrishnan S., Mahieu C., Anderton B., Eyob H., Kajimura S., Tward A., Krings G., et al. 2016. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 22:427–432. 10.1038/nm.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell C.R., Hicks B.M., Hughes C., and Murray L.J.. 2014. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J. Clin. Oncol. 32:3177–3183. 10.1200/JCO.2013.54.4569 [DOI] [PubMed] [Google Scholar]
- Carracedo A., Weiss D., Leliaert A.K., Bhasin M., de Boer V.C., Laurent G., Adams A.C., Sundvall M., Song S.J., Ito K., et al. 2012. A metabolic prosurvival role for PML in breast cancer. J. Clin. Invest. 122:3088–3100. 10.1172/JCI62129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A., Cantley L.C., and Pandolfi P.P.. 2013. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 13:227–232. 10.1038/nrc3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer A., Trefely S., Zhao S., Campbell S.L., Norgard R.J., Schultz K.C., Sidoli S., Parris J.L.D., Affronti H.C., Sivanand S., et al. 2019. Acetyl-CoA Metabolism Supports Multistep Pancreatic Tumorigenesis. Cancer Discov. 9:416–435. 10.1158/2159-8290.CD-18-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L., Chi W., Qiao Y., Zhang J., Song X., Liu Y., Li L., Jia J., Pilo M.G., Wang J., et al. 2020. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 69:177–186. 10.1136/gutjnl-2018-317581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.F., Tian M.X., Sun R.Q., Zhang M.L., Zhou L.S., Jin L., Chen L.L., Zhou W.J., Duan K.L., Chen Y.J., et al. 2018. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 19:e45124 10.15252/embr.201745124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Vasoya R.P., Toke N.H., Parthasarathy A., Luo S., Chiles E., Flores J., Gao N., Bonder E.M., Su X., and Verzi M.P.. 2020. HNF4 Regulates Fatty Acid Oxidation and Is Required for Renewal of Intestinal Stem Cells in Mice. Gastroenterology. 158:985–999.e9. 10.1053/j.gastro.2019.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Ru P., Geng F., Liu J., Yoo J.Y., Wu X., Cheng X., Euthine V., Hu P., Guo J.Y., et al. 2015. Glucose-Mediated N-glycosylation of SCAP Is Essential for SREBP-1 Activation and Tumor Growth. Cancer Cell. 28:569–581. 10.1016/j.ccell.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Li J., and Guo D.. 2018. SCAP/SREBPs are Central Players in Lipid Metabolism and Novel Metabolic Targets in Cancer Therapy. Curr. Top. Med. Chem. 18:484–493. 10.2174/1568026618666180523104541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Geng F., Pan M., Wu X., Zhong Y., Wang C., Tian Z., Cheng C., Zhang R., Puduvalli V., et al. 2020. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 32:229–242.e8. 10.1016/j.cmet.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K., DeVries S., Fridlyand J., Spellman P.T., Roydasgupta R., Kuo W.L., Lapuk A., Neve R.M., Qian Z., Ryder T., et al. 2006. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 10:529–541. 10.1016/j.ccr.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Chitraju C., Mejhert N., Haas J.T., Diaz-Ramirez L.G., Grueter C.A., Imbriglio J.E., Pinto S., Koliwad S.K., Walther T.C., and Farese R.V. Jr. 2017. Triglyceride Synthesis by DGAT1 Protects Adipocytes from Lipid-Induced ER Stress during Lipolysis. Cell Metab. 26:407–418.e3. 10.1016/j.cmet.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N.K., Howe V., Jatana N., Thukral L., and Brown A.J.. 2017. A conserved degron containing an amphipathic helix regulates the cholesterol-mediated turnover of human squalene monooxygenase, a rate-limiting enzyme in cholesterol synthesis. J. Biol. Chem. 292:19959–19973. 10.1074/jbc.M117.794230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirmena G., Franceschelli P., Isnaldi E., Ferrando L., De Mariano M., Ballestrero A., and Zoppoli G.. 2018. Squalene epoxidase as a promising metabolic target in cancer treatment. Cancer Lett. 425:13–20. 10.1016/j.canlet.2018.03.034 [DOI] [PubMed] [Google Scholar]
- Clarke P.R., and Hardie D.G.. 1990. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 9:2439–2446. 10.1002/j.1460-2075.1990.tb07420.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford S.A., Huang Z., Du X., Wang Y., Cai L., Witkiewicz A.K., Walters H., Tantawy M.N., Fu A., Manning H.C., et al. 2014. Acetate dependence of tumors. Cell. 159:1591–1602. 10.1016/j.cell.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M. 2019. Nelfinavir with concurrent chemoradiotherapy in NSCLC. Lancet Oncol. 20:e561 10.1016/S1470-2045(19)30567-4 [DOI] [PubMed] [Google Scholar]
- Driessen C., Kraus M., Joerger M., Rosing H., Bader J., Hitz F., Berset C., Xyrafas A., Hawle H., Berthod G., et al. 2016. Treatment with the HIV protease inhibitor nelfinavir triggers the unfolded protein response and may overcome proteasome inhibitor resistance of multiple myeloma in combination with bortezomib: a phase I trial (SAKK 65/08). Haematologica. 101:346–355. 10.3324/haematol.2015.135780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman C., Yaqubi K., Hoffmann A., Acikgöz A.A., Korshunov A., Bendszus M., Herold-Mende C., Liu H.K., and Alfonso J.. 2019. Acyl-CoA-Binding Protein Drives Glioblastoma Tumorigenesis by Sustaining Fatty Acid Oxidation. Cell Metab. 30:274–289.e5. 10.1016/j.cmet.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Duncan R.E., El-Sohemy A., and Archer M.C.. 2006. Statins and the risk of cancer. JAMA. 295:2720–, author reply:2721–2722.. 10.1001/jama.295.23.2720-a [DOI] [PubMed] [Google Scholar]
- Düvel K., Yecies J.L., Menon S., Raman P., Lipovsky A.I., Souza A.L., Triantafellow E., Ma Q., Gorski R., Cleaver S., et al. 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 39:171–183. 10.1016/j.molcel.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlé D., Hegarty B., Bossard P., Ferré P., and Foufelle F.. 2004. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 86:839–848. 10.1016/j.biochi.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Eid W., Dauner K., Courtney K.C., Gagnon A., Parks R.J., Sorisky A., and Zha X.. 2017. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc. Natl. Acad. Sci. USA. 114:7999–8004. 10.1073/pnas.1705304114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero S., Zaganjor E., Lee S., Mill C.P., Morgan A.M., Crawford E.B., Chen J., Wales T.E., Mourtada R., Luccarelli J., et al. 2018. Dynamic Regulation of Long-Chain Fatty Acid Oxidation by a Noncanonical Interaction between the MCL-1 BH3 Helix and VLCAD. Mol. Cell. 69:729–743.e7. 10.1016/j.molcel.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.W., Wilkins O., Bang S., Ung M., Li J., An J., Del Genio C., Canfield K., DiRenzo J., Wells W., et al. 2019. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 29:3405–3420.e5. 10.1016/j.celrep.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny C.A., Griffett K., El-Gendy B.-D., Kazantzis M., Sengupta M., Amelio A.L., Chatterjee A., Walker J., Solt L.A., Kamenecka T.M., and Burris T.P.. 2015. Broad Anti-tumor Activity of a Small Molecule that Selectively Targets the Warburg Effect and Lipogenesis. Cancer Cell. 28:42–56. 10.1016/j.ccell.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris A., Mazarei M., Yang X., Robinson A.E., Zhou J., Barberis A., D’hallewin G., Azara E., Spissu Y., Iglesias-Ara A., et al. 2020. SUMOylation Protects FASN Against Proteasomal Degradation in Breast Cancer Cells Treated with Grape Leaf Extract. Biomolecules. 10:529 10.3390/biom10040529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N., Nakagawa H., Enooku K., Kudo Y., Hayata Y., Nakatsuka T., Tanaka Y., Tateishi R., Hikiba Y., Misumi K., et al. 2018. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 67:1493–1504. 10.1136/gutjnl-2017-315193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher E.J., Zelenko Z., Neel B.A., Antoniou I.M., Rajan L., Kase N., and LeRoith D.. 2017. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 36:6462–6471. 10.1038/onc.2017.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang X., Xuan L., Zhao X., Lv Y., Li F., Wang Y., and Wang G.. 2019. Speckle-type POZ protein suppresses lipid accumulation and prostate cancer growth by stabilizing fatty acid synthase. Prostate. 79:864–871. 10.1002/pros.23793 [DOI] [PubMed] [Google Scholar]
- Garcia-Bermudez J., Baudrier L., Bayraktar E.C., Shen Y., La K., Guarecuco R., Yucel B., Fiore D., Tavora B., Freinkman E., et al. 2019. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 567:118–122. 10.1038/s41586-019-0945-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F., Cheng X., Wu X., Yoo J.Y., Cheng C., Guo J.Y., Mo X., Ru P., Hurwitz B., Kim S.H., et al. 2016. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin. Cancer Res. 22:5337–5348. 10.1158/1078-0432.CCR-15-2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German N.J., Yoon H., Yusuf R.Z., Murphy J.P., Finley L.W., Laurent G., Haas W., Satterstrom F.K., Guarnerio J., Zaganjor E., et al. 2016. PHD3 Loss in Cancer Enables Metabolic Reliance on Fatty Acid Oxidation via Deactivation of ACC2. Mol. Cell. 63:1006–1020. 10.1016/j.molcel.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharpure K.M., Pradeep S., Sans M., Rupaimoole R., Ivan C., Wu S.Y., Bayraktar E., Nagaraja A.S., Mangala L.S., Zhang X., et al. 2018. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 9:2923 10.1038/s41467-018-04987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giandomenico V., Simonsson M., Grönroos E., and Ericsson J.. 2003. Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol. Cell. Biol. 23:2587–2599. 10.1128/MCB.23.7.2587-2599.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Stevenson J., Kristiana I., and Brown A.J.. 2011. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 13:260–273. 10.1016/j.cmet.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Giordano A., Calvani M., Petillo O., Grippo P., Tuccillo F., Melone M.A., Bonelli P., Calarco A., and Peluso G.. 2005. tBid induces alterations of mitochondrial fatty acid oxidation flux by malonyl-CoA-independent inhibition of carnitine palmitoyltransferase-1. Cell Death Differ. 12:603–613. 10.1038/sj.cdd.4401636 [DOI] [PubMed] [Google Scholar]
- Giral P. 2020. Bempedoic Acid to Lower LDL Cholesterol - Safety and Efficacy. N. Engl. J. Med. 383:e49 10.1056/NEJMc1908495 [DOI] [PubMed] [Google Scholar]
- Goldstein J.L., and Brown M.S.. 2009. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29:431–438. 10.1161/ATVBAHA.108.179564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Lee J.N., Lee P.C., Goldstein J.L., Brown M.S., and Ye J.. 2006. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 3:15–24. 10.1016/j.cmet.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Gong X., Li J., Shao W., Wu J., Qian H., Ren R., Espenshade P., and Yan N.. 2015. Structure of the WD40 domain of SCAP from fission yeast reveals the molecular basis for SREBP recognition. Cell Res. 25:401–411. 10.1038/cr.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graner E., Tang D., Rossi S., Baron A., Migita T., Weinstein L.J., Lechpammer M., Huesken D., Zimmermann J., Signoretti S., and Loda M.. 2004. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 5:253–261. 10.1016/S1535-6108(04)00055-8 [DOI] [PubMed] [Google Scholar]
- Gu L., Zhu Y., Lin X., Lu B., Zhou X., Zhou F., Zhao Q., Prochownik E.V., and Li Y.. 2020. The IKKβ-USP30-ACLY Axis Controls Lipogenesis and Tumorigenesis. Hepatology.:hep.31249 10.1002/hep.31249 [DOI] [PubMed] [Google Scholar]
- Guillaumond F., Bidaut G., Ouaissi M., Servais S., Gouirand V., Olivares O., Lac S., Borge L., Roques J., Gayet O., et al. 2015. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA. 112:2473–2478. 10.1073/pnas.1421601112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Prins R.M., Dang J., Kuga D., Iwanami A., Soto H., Lin K.Y., Huang T.T., Akhavan D., Hock M.B., et al. 2009. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci. Signal. 2:ra82 10.1126/scisignal.2000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D., Reinitz F., Youssef M., Hong C., Nathanson D., Akhavan D., Kuga D., Amzajerdi A.N., Soto H., Zhu S., et al. 2011. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 1:442–456. 10.1158/2159-8290.CD-11-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Ma J., Yang Y., Guo S., Zhang W., Zhao T., Yi X., Wang H., Wang S., Liu Y., et al. 2020. ATP-Citrate Lyase Epigenetically Potentiates Oxidative Phosphorylation to Promote Melanoma Growth and Adaptive Resistance to MAPK Inhibition. Clin. Cancer Res. 26:2725–2739. 10.1158/1078-0432.CCR-19-1359 [DOI] [PubMed] [Google Scholar]
- Ha J., Daniel S., Broyles S.S., and Kim K.H.. 1994. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J. Biol. Chem. 269:22162–22168. [PubMed] [Google Scholar]
- Han J.Y., Lee S.H., Yoo N.J., Hyung L.S., Moon Y.J., Yun T., Kim H.T., and Lee J.S.. 2011. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 17:1553–1560. 10.1158/1078-0432.CCR-10-2525 [DOI] [PubMed] [Google Scholar]
- Han J., Li E., Chen L., Zhang Y., Wei F., Liu J., Deng H., and Wang Y.. 2015. The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature. 524:243–246. 10.1038/nature14557 [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G., Zhao F., Bauer D.E., Andreadis C., Shaw A.N., Dhanak D., Hingorani S.R., Tuveson D.A., and Thompson C.B.. 2005. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 8:311–321. 10.1016/j.ccr.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Hirano Y., Murata S., Tanaka K., Shimizu M., and Sato R.. 2003. Sterol regulatory element-binding proteins are negatively regulated through SUMO-1 modification independent of the ubiquitin/26 S proteasome pathway. J. Biol. Chem. 278:16809–16819. 10.1074/jbc.M212448200 [DOI] [PubMed] [Google Scholar]
- Horton J.D., Shah N.A., Warrington J.A., Anderson N.N., Park S.W., Brown M.S., and Goldstein J.L.. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100:12027–12032. 10.1073/pnas.1534923100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhang M., Plec A.A., Estill S.J., Cai L., Repa J.J., McKnight S.L., and Tu B.P.. 2018. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc. Natl. Acad. Sci. USA. 115:E9499–E9506. 10.1073/pnas.1806635115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M.D., Vesely P.W., Datta K., and Gerace L.. 2013. Erlins restrict SREBP activation in the ER and regulate cellular cholesterol homeostasis. J. Cell Biol. 203:427–436. 10.1083/jcb.201305076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkeler M., Hagmann A., Stuttfeld E., Chami M., Guri Y., Stahlberg H., and Maier T.. 2018. Structural basis for regulation of human acetyl-CoA carboxylase. Nature. 558:470–474. 10.1038/s41586-018-0201-4 [DOI] [PubMed] [Google Scholar]
- Hwang S., Nguyen A.D., Jo Y., Engelking L.J., Brugarolas J., and DeBose-Boyd R.A.. 2017. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J. Biol. Chem. 292:9382–9393. 10.1074/jbc.M117.788562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S.S., Yousef L., Blaschitz C., Liu J.Z., Edwards R.A., Young S.G., Raffatellu M., and Osborne T.F.. 2011. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 13:540–549. 10.1016/j.cmet.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa M., Inoue J., Ozawa N., Mori K., and Sato R.. 2009. The sterol-sensing endoplasmic reticulum (ER) membrane protein TRC8 hampers ER to Golgi transport of sterol regulatory element-binding protein-2 (SREBP-2)/SREBP cleavage-activated protein and reduces SREBP-2 cleavage. J. Biol. Chem. 284:28995–29004. 10.1074/jbc.M109.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G., Liu W., Tesz G.J., Birnbaum M.J., and Rabinowitz J.D.. 2018. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 27:351–361.e3. 10.1016/j.cmet.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S.M., Chandel N.S., and Hay N.. 2012. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 485:661–665. 10.1038/nature11066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Wu N., Xu B., Chu Y., Li X., Su S., Chen D., Li W., Shi Y., Gao X., et al. 2019. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics. 9:5359–5373. 10.7150/thno.34024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouve J.L., Lecomte T., Bouché O., Barbier E., Khemissa Akouz F., Riachi G., Nguyen Khac E., Ollivier-Hourmand I., Debette-Gratien M., Faroux R., et al. PRODIGE-11 investigators/collaborators . 2019. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J. Hepatol. 71:516–522. 10.1016/j.jhep.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Kamphorst J.J., Cross J.R., Fan J., de Stanchina E., Mathew R., White E.P., Thompson C.B., and Rabinowitz J.D.. 2013. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA. 110:8882–8887. 10.1073/pnas.1307237110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwairakpam A.D., Banik K., Girisa S., Shabnam B., Shakibaei M., Fan L., Arfuso F., Monisha J., Wang H., Mao X., et al. 2020. The vital role of ATP citrate lyase in chronic diseases. J. Mol. Med. (Berl.). 98:71–95. 10.1007/s00109-019-01863-0 [DOI] [PubMed] [Google Scholar]
- Kim T.H., Kim H., Park J.M., Im S.S., Bae J.S., Kim M.Y., Yoon H.G., Cha J.Y., Kim K.S., and Ahn Y.H.. 2009. Interrelationship between liver X receptor alpha, sterol regulatory element-binding protein-1c, peroxisome proliferator-activated receptor gamma, and small heterodimer partner in the transcriptional regulation of glucokinase gene expression in liver. J. Biol. Chem. 284:15071–15083. 10.1074/jbc.M109.006742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.T., Kang J.H., Lee J., Park S.H., Park J.O., Park Y.S., Lim H.Y., Hwang I.G., Lee S.C., Park K.W., et al. 2014. Simvastatin plus capecitabine-cisplatin versus placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. Eur. J. Cancer. 50:2822–2830. 10.1016/j.ejca.2014.08.005 [DOI] [PubMed] [Google Scholar]
- Ko C.W., Qu J., Black D.D., and Tso P.. 2020. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 17:169–183. 10.1038/s41575-019-0250-7 [DOI] [PubMed] [Google Scholar]
- Kong Y., Cheng L., Mao F., Zhang Z., Zhang Y., Farah E., Bosler J., Bai Y., Ahmad N., Kuang S., et al. 2018. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J. Biol. Chem. 293:14328–14341. 10.1074/jbc.RA118.004442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koobotse M., Holly J., and Perks C.. 2018. Elucidating the novel BRCA1 function as a non-genomic metabolic restraint in ER-positive breast cancer cell lines. Oncotarget. 9:33562–33576. 10.18632/oncotarget.26093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzka J., Lehr S., Roth G., Avci H., Knebel B., and Muller-Wieland D.. 2004. Insulin-activated Erk-mitogen-activated protein kinases phosphorylate sterol regulatory element-binding Protein-2 at serine residues 432 and 455 in vivo. J. Biol. Chem. 279:22404–22411. 10.1074/jbc.M401198200 [DOI] [PubMed] [Google Scholar]
- Koundouros N., and Poulogiannis G.. 2020. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer. 122:4–22. 10.1038/s41416-019-0650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan Y.C., Hashidume T., Shibata T., Uchida K., Shimizu M., Inoue J., and Sato R.. 2017. Heat Shock Protein 90 Modulates Lipid Homeostasis by Regulating the Stability and Function of Sterol Regulatory Element-binding Protein (SREBP) and SREBP Cleavage-activating Protein. J. Biol. Chem. 292:3016–3028. 10.1074/jbc.M116.767277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladanyi A., Mukherjee A., Kenny H.A., Johnson A., Mitra A.K., Sundaresan S., Nieman K.M., Pascual G., Benitah S.A., Montag A., et al. 2018. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene. 37:2285–2301. 10.1038/s41388-017-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally J.S.V., Ghoshal S., DePeralta D.K., Moaven O., Wei L., Masia R., Erstad D.J., Fujiwara N., Leong V., Houde V.P., et al. 2019. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab. 29:174–182.e5. 10.1016/j.cmet.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.N., Song B., DeBose-Boyd R.A., and Ye J.. 2006. Sterol-regulated degradation of Insig-1 mediated by the membrane-bound ubiquitin ligase gp78. J. Biol. Chem. 281:39308–39315. 10.1074/jbc.M608999200 [DOI] [PubMed] [Google Scholar]
- Lee J.N., Zhang X., Feramisco J.D., Gong Y., and Ye J.. 2008. Unsaturated fatty acids inhibit proteasomal degradation of Insig-1 at a postubiquitination step. J. Biol. Chem. 283:33772–33783. 10.1074/jbc.M806108200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.N., Kim H., Yao H., Chen Y., Weng K., and Ye J.. 2010. Identification of Ubxd8 protein as a sensor for unsaturated fatty acids and regulator of triglyceride synthesis. Proc. Natl. Acad. Sci. USA. 107:21424–21429. 10.1073/pnas.1011859107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.V., Carrer A., Shah S., Snyder N.W., Wei S., Venneti S., Worth A.J., Yuan Z.F., Lim H.W., Liu S., et al. 2014. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20:306–319. 10.1016/j.cmet.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee K.H., Lee G.K., Lee S.H., Lim K.Y., Joo J., Go Y.J., Lee J.S., and Han J.Y.. 2017. Randomized Phase II Study of Afatinib Plus Simvastatin Versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-small Cell Lung Cancer. Cancer Res. Treat. 49:1001–1011. 10.4143/crt.2016.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Roberts A., Juarez D., Vo T.T., Bhatt S., Herzog L.O., Mallya S., Bellin R.J., Agarwal S.K., Salem A.H., et al. 2018. Statins enhance efficacy of venetoclax in blood cancers. Sci. Transl. Med. 10:eaaq1240 10.1126/scitranslmed.aaq1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., et al. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13:376–388. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Che L., Tharp K.M., Park H.M., Pilo M.G., Cao D., Cigliano A., Latte G., Xu Z., Ribback S., et al. 2016a Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology. 63:1900–1913. 10.1002/hep.28508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jiang Y., Meisenhelder J., Yang W., Hawke D.H., Zheng Y., Xia Y., Aldape K., He J., Hunter T., et al. 2016b Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Mol. Cell. 61:705–719. 10.1016/j.molcel.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Qian X., and Lu Z.. 2017a Local histone acetylation by ACSS2 promotes gene transcription for lysosomal biogenesis and autophagy. Autophagy. 13:1790–1791. 10.1080/15548627.2017.1349581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yu W., Qian X., Xia Y., Zheng Y., Lee J.H., Li W., Lyu J., Rao G., Zhang X., et al. 2017b Nucleus-Translocated ACSS2 Promotes Gene Transcription for Lysosomal Biogenesis and Autophagy. Mol. Cell. 66:684–697.e9. 10.1016/j.molcel.2017.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Zhang C., Chen L., Wang P., Fang Y., Zhu J., Chen S., Du J., Shen B., Wu K., and Liu Y.. 2019a The role of acetyl-coA carboxylase2 in head and neck squamous cell carcinoma. PeerJ. 7:e7037 10.7717/peerj.7037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang Y., Wu S., Zhou Z., Ding X., Shi R., Thorne R.F., Zhang X.D., Hu W., and Wu M.. 2019b CircACC1 Regulates Assembly and Activation of AMPK Complex under Metabolic Stress. Cell Metab. 30:157–173.e7. 10.1016/j.cmet.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Lim S.H., Kim T.W., Hong Y.S., Han S.W., Lee K.H., Kang H.J., Hwang I.G., Lee J.Y., Kim H.S., Kim S.T., et al. 2015. A randomised, double-blind, placebo-controlled multi-centre phase III trial of XELIRI/FOLFIRI plus simvastatin for patients with metastatic colorectal cancer. Br. J. Cancer. 113:1421–1426. 10.1038/bjc.2015.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.Y., and Gustafsson J.A.. 2015. Targeting liver X receptors in cancer therapeutics. Nat. Rev. Cancer. 15:216–224. 10.1038/nrc3912 [DOI] [PubMed] [Google Scholar]
- Lin J., Yang R., Tarr P.T., Wu P.H., Handschin C., Li S., Yang W., Pei L., Uldry M., Tontonoz P., et al. 2005. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 120:261–273. 10.1016/j.cell.2004.11.043 [DOI] [PubMed] [Google Scholar]
- Lin R., Tao R., Gao X., Li T., Zhou X., Guan K.L., Xiong Y., and Lei Q.Y.. 2013. Acetylation stabilizes ATP-citrate lyase to promote lipid biosynthesis and tumor growth. Mol. Cell. 51:506–518. 10.1016/j.molcel.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.P., Cheng Z.L., He R.Y., Song L., Tian M.X., Zhou L.S., Groh B.S., Liu W.R., Ji M.B., Ding C., et al. 2016. Destabilization of Fatty Acid Synthase by Acetylation Inhibits De Novo Lipogenesis and Tumor Cell Growth. Cancer Res. 76:6924–6936. 10.1158/0008-5472.CAN-16-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L., Finer-Moore J., Stroud R.M., Luskey K.L., Brown M.S., and Goldstein J.L.. 1985. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J. Biol. Chem. 260:522–530. [PubMed] [Google Scholar]
- Liu L., Zhao X., Zhao L., Li J., Yang H., Zhu Z., Liu J., and Huang G.. 2016. Arginine Methylation of SREBP1a via PRMT5 Promotes De Novo Lipogenesis and Tumor Growth. Cancer Res. 76:1260–1272. 10.1158/0008-5472.CAN-15-1766 [DOI] [PubMed] [Google Scholar]
- Liu D., Wong C.C., Fu L., Chen H., Zhao L., Li C., Zhou Y., Zhang Y., Xu W., Yang Y., et al. 2018. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci. Transl. Med. 10:eaap9840 10.1126/scitranslmed.aap9840 [DOI] [PubMed] [Google Scholar]
- Luo D.X., Tong D.J., Rajput S., Wang C., Liao D.F., Cao D., and Maser E.. 2012. Targeting acetyl-CoA carboxylases: small molecular inhibitors and their therapeutic potential. Recent Patents Anticancer Drug Discov. 7:168–184. 10.2174/157489212799972918 [DOI] [PubMed] [Google Scholar]
- Luo J., Yang H., and Song B.L.. 2020. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21:225–245. 10.1038/s41580-019-0190-7 [DOI] [PubMed] [Google Scholar]
- Luong A., Hannah V.C., Brown M.S., and Goldstein J.L.. 2000. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 275:26458–26466. 10.1074/jbc.M004160200 [DOI] [PubMed] [Google Scholar]
- Ma J., Yan R., Zu X., Cheng J.M., Rao K., Liao D.F., and Cao D.. 2008. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J. Biol. Chem. 283:3418–3423. 10.1074/jbc.M707650200 [DOI] [PubMed] [Google Scholar]
- Ma M.K.F., Lau E.Y.T., Leung D.H.W., Lo J., Ho N.P.Y., Cheng L.K.W., Ma S., Lin C.H., Copland J.A., Ding J., et al. 2017. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J. Hepatol. 67:979–990. 10.1016/j.jhep.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Ma Y., Temkin S.M., Hawkridge A.M., Guo C., Wang W., Wang X.Y., and Fang X.. 2018. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 435:92–100. 10.1016/j.canlet.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.E., Pirman D., Chubukov V., Sleger T., Hayes S., Fan Z.P., Allen E.L., Chen Y., Huang L., Liu M., et al. 2019. A chemical biology screen identifies a vulnerability of neuroendocrine cancer cells to SQLE inhibition. Nat. Commun. 10:96 10.1038/s41467-018-07959-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Pichumani K., Vemireddy V., Hatanpaa K.J., Singh D.K., Sirasanagandla S., Nannepaga S., Piccirillo S.G., Kovacs Z., Foong C., et al. 2014. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 159:1603–1614. 10.1016/j.cell.2014.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez J.A., and Lupu R.. 2007. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 7:763–777. 10.1038/nrc2222 [DOI] [PubMed] [Google Scholar]
- Menendez J.A., Vellon L., Mehmi I., Oza B.P., Ropero S., Colomer R., and Lupu R.. 2004. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc. Natl. Acad. Sci. USA. 101:10715–10720. 10.1073/pnas.0403390101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L., et al. 2011. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 481:380–384. 10.1038/nature10602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H.K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., Kellum J., Warnick R., Contos M.J., and Sanyal A.J.. 2012. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15:665–674. 10.1016/j.cmet.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa K., Horiba T., Arimura N., Hirano Y., Inoue J., Emoto N., Shimano H., Shimizu M., and Sato R.. 2003. Sterol regulatory element-binding protein-2 interacts with hepatocyte nuclear factor-4 to enhance sterol isomerase gene expression in hepatocytes. J. Biol. Chem. 278:36176–36182. 10.1074/jbc.M302387200 [DOI] [PubMed] [Google Scholar]
- Moolenaar W.H., and Perrakis A.. 2011. Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12:674–679. 10.1038/nrm3188 [DOI] [PubMed] [Google Scholar]
- Moon S.H., Huang C.H., Houlihan S.L., Regunath K., Freed-Pastor W.A., Morris J.P. IV, Tschaharganeh D.F., Kastenhuber E.R., Barsotti A.M., Culp-Hill R., et al. 2019. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 176:564–580.e19. 10.1016/j.cell.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau K., Dizin E., Ray H., Luquain C., Lefai E., Foufelle F., Billaud M., Lenoir G.M., and Venezia N.D.. 2006. BRCA1 affects lipid synthesis through its interaction with acetyl-CoA carboxylase. J. Biol. Chem. 281:3172–3181. 10.1074/jbc.M504652200 [DOI] [PubMed] [Google Scholar]
- Morioka S., Sai K., Omori E., Ikeda Y., Matsumoto K., and Ninomiya-Tsuji J.. 2016. TAK1 regulates hepatic lipid homeostasis through SREBP. Oncogene. 35:3829–3838. 10.1038/onc.2015.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen P.J., Yu R., Longo J., Archer M.C., and Penn L.Z.. 2016. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer. 16:718–731. 10.1038/nrc.2016.76 [DOI] [PubMed] [Google Scholar]
- Nakamura M.T., and Nara T.Y.. 2004. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 24:345–376. 10.1146/annurev.nutr.24.121803.063211 [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Goldstein J.L., and Brown M.S.. 1988. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J. Biol. Chem. 263:8929–8937. [PubMed] [Google Scholar]
- Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S., et al. 2011. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17:1498–1503. 10.1038/nm.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohturfft A., and Zhang S.C.. 2009. Coordination of lipid metabolism in membrane biogenesis. Annu. Rev. Cell Dev. Biol. 25:539–566. 10.1146/annurev.cellbio.24.110707.175344 [DOI] [PubMed] [Google Scholar]
- Padanad M.S., Konstantinidou G., Venkateswaran N., Melegari M., Rindhe S., Mitsche M., Yang C., Batten K., Huffman K.E., Liu J., et al. 2016. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 16:1614–1628. 10.1016/j.celrep.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padyana A.K., Gross S., Jin L., Cianchetta G., Narayanaswamy R., Wang F., Wang R., Fang C., Lv X., Biller S.A., et al. 2019. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun. 10:97 10.1038/s41467-018-07928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Fan Z., Wang Z., Dai Q., Xiang Z., Yuan F., Yan M., Zhu Z., Liu B., and Li C.. 2019. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. 38:52 10.1186/s13046-019-1049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.B., Lee C.S., Jang J.H., Ghim J., Kim Y.J., You S., Hwang D., Suh P.G., and Ryu S.H.. 2012. Phospholipase signalling networks in cancer. Nat. Rev. Cancer. 12:782–792. 10.1038/nrc3379 [DOI] [PubMed] [Google Scholar]
- Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C.S., Berenguer A., Prats N., Toll A., Hueto J.A., et al. 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 541:41–45. 10.1038/nature20791 [DOI] [PubMed] [Google Scholar]
- Peck B., and Schulze A.. 2016. Lipid desaturation - the next step in targeting lipogenesis in cancer? FEBS J. 283:2767–2778. 10.1111/febs.13681 [DOI] [PubMed] [Google Scholar]
- Penfold L., Woods A., Muckett P., Nikitin A.Y., Kent T.R., Zhang S., Graham R., Pollard A., and Carling D.. 2018. CAMKK2 Promotes Prostate Cancer Independently of AMPK via Increased Lipogenesis. Cancer Res. 78:6747–6761. 10.1158/0008-5472.CAN-18-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., and Sabatini D.M.. 2011. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 146:408–420. 10.1016/j.cell.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L.S., Smift A.L., Croteau N.J., Ferrick D.A., and Wu M.. 2011. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta. 1807:726–734. 10.1016/j.bbabio.2010.10.022 [DOI] [PubMed] [Google Scholar]
- Porter J.A., Young K.E., and Beachy P.A.. 1996. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 274:255–259. 10.1126/science.274.5285.255 [DOI] [PubMed] [Google Scholar]
- Potapova I.A., El-Maghrabi M.R., Doronin S.V., and Benjamin W.B.. 2000. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 39:1169–1179. 10.1021/bi992159y [DOI] [PubMed] [Google Scholar]
- Potze L., Di Franco S., Grandela C., Pras-Raves M.L., Picavet D.I., van Veen H.A., van Lenthe H., Mullauer F.B., van der Wel N.N., Luyf A., et al. 2016. Betulinic acid induces a novel cell death pathway that depends on cardiolipin modification. Oncogene. 35:427–437. 10.1038/onc.2015.102 [DOI] [PubMed] [Google Scholar]
- Poulsen Ll., Siersbæk M., and Mandrup S.. 2012. PPARs: fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 23:631–639. 10.1016/j.semcdb.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Rawson R.B. 2003. The SREBP pathway--insights from Insigs and insects. Nat. Rev. Mol. Cell Biol. 4:631–640. 10.1038/nrm1174 [DOI] [PubMed] [Google Scholar]
- Rengan R., Mick R., Pryma D., Rosen M.A., Lin L.L., Maity A.M., Evans T.L., Stevenson J.P., Langer C.J., Kucharczuk J., et al. 2012. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J. Thorac. Oncol. 7:709–715. 10.1097/JTO.0b013e3182435aa6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa J.J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.M., Shimomura I., Shan B., Brown M.S., Goldstein J.L., and Mangelsdorf D.J.. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14:2819–2830. 10.1101/gad.844900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios Garcia M., Steinbauer B., Srivastava K., Singhal M., Mattijssen F., Maida A., Christian S., Hess-Stumpp H., Augustin H.G., Müller-Decker K., et al. 2017. Acetyl-CoA Carboxylase 1-Dependent Protein Acetylation Controls Breast Cancer Metastasis and Recurrence. Cell Metab. 26:842–855.e5. 10.1016/j.cmet.2017.09.018 [DOI] [PubMed] [Google Scholar]
- Röhrig F., and Schulze A.. 2016. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 16:732–749. 10.1038/nrc.2016.89 [DOI] [PubMed] [Google Scholar]
- Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F., and Ido Y.. 2010. AMPK and SIRT1: a long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 298:E751–E760. 10.1152/ajpendo.00745.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I., Harmancey R., Fiegl M., Kantarjian H., Konopleva M., Korchin B., Kaluarachchi K., Bornmann W., Duvvuri S., Taegtmeyer H., and Andreeff M.. 2010. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Invest. 120:142–156. 10.1172/JCI38942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R., Goldstein J.L., and Brown M.S.. 1993. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc. Natl. Acad. Sci. USA. 90:9261–9265. 10.1073/pnas.90.20.9261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug Z.T., Peck B., Jones D.T., Zhang Q., Grosskurth S., Alam I.S., Goodwin L.M., Smethurst E., Mason S., Blyth K., et al. 2015. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 27:57–71. 10.1016/j.ccell.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl M.J., Ottensmeier C.H., Cullen M., Schmid P., Ngai Y., Muthukumar D., Thompson J., Harden S., Middleton G., Fife K.M., et al. 2017. Multicenter, Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Pravastatin Added to First-Line Standard Chemotherapy in Small-Cell Lung Cancer (LUNGSTAR). J. Clin. Oncol. 35:1506–1514. 10.1200/JCO.2016.69.7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S., Kim Y.C., Byun S., Choi S., Xiao Z., Iwamori N., Zhang Y., Wang C., Ma J., Ge K., et al. 2018. Fasting-induced JMJD3 histone demethylase epigenetically activates mitochondrial fatty acid β-oxidation. J. Clin. Invest. 128:3144–3159. 10.1172/JCI97736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W., and Espenshade P.J.. 2012. Expanding roles for SREBP in metabolism. Cell Metab. 16:414–419. 10.1016/j.cmet.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L.J., and Brown A.J.. 2013. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 288:18707–18715. 10.1074/jbc.R113.479808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L.J., Howe V., Scott N.A., Luu W., Phan L., Berk J.M., Hochstrasser M., and Brown A.J.. 2019. Cholesterol increases protein levels of the E3 ligase MARCH6 and thereby stimulates protein degradation. J. Biol. Chem. 294:2436–2448. 10.1074/jbc.RA118.005069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., and Sato R.. 2017. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nat. Rev. Endocrinol. 13:710–730. 10.1038/nrendo.2017.91 [DOI] [PubMed] [Google Scholar]
- Sivanand S., Rhoades S., Jiang Q., Lee J.V., Benci J., Zhang J., Yuan S., Viney I., Zhao S., Carrer A., et al. 2017. Nuclear Acetyl-CoA Production by ACLY Promotes Homologous Recombination. Mol. Cell. 67:252–265.e6. 10.1016/j.molcel.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaebjornsson M.T., Janaki-Raman S., and Schulze A.. 2020. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 31:62–76. 10.1016/j.cmet.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Stevenson J., Luu W., Kristiana I., and Brown A.J.. 2014. Squalene mono-oxygenase, a key enzyme in cholesterol synthesis, is stabilized by unsaturated fatty acids. Biochem. J. 461:435–442. 10.1042/BJ20131404 [DOI] [PubMed] [Google Scholar]
- Su X., and Abumrad N.A.. 2009. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol. Metab. 20:72–77. 10.1016/j.tem.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist A., Bengoechea-Alonso M.T., Ye X., Lukiyanchuk V., Jin J., Harper J.W., and Ericsson J.. 2005. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 1:379–391. 10.1016/j.cmet.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Svensson R.U., Parker S.J., Eichner L.J., Kolar M.J., Wallace M., Brun S.N., Lombardo P.S., Van Nostrand J.L., Hutchins A., Vera L., et al. 2016. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22:1108–1119. 10.1038/nm.4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Xiong X., DePinho R.A., Deng C.X., and Dong X.C.. 2013. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J. Lipid Res. 54:2745–2753. 10.1194/jlr.M039339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfay L., Paul B.T., Konstorum A., Deng Z., Cox A.O., Lee J., Furdui C.M., Hegde P., Torti F.M., and Torti S.V.. 2019. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 79:5355–5366. 10.1158/0008-5472.CAN-19-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Nakatsu Y., Yamamotoya T., Ono H., Inoue Y., Inoue M.K., Mizuno Y., Matsunaga Y., Kushiyama A., Sakoda H., et al. 2019. Prolyl isomerase Pin1 binds to and stabilizes acetyl CoA carboxylase 1 protein, thereby supporting cancer cell proliferation. Oncotarget. 10:1637–1648. 10.18632/oncotarget.26691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.K., Yang F., Jiang K., Ji J.Y., Watts J.L., Purushotham A., Boss O., Hirsch M.L., Ribich S., Smith J.J., et al. 2010. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 24:1403–1417. 10.1101/gad.1901210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.D., Wu H., Fu G.B., Zhang H.L., Zhou X., Tang L., Dong L.W., Qin C.J., Huang S., Zhao L.H., et al. 2016. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology. 63:1272–1286. 10.1002/hep.28415 [DOI] [PubMed] [Google Scholar]
- Wang T., Fahrmann J.F., Lee H., Li Y.J., Tripathi S.C., Yue C., Zhang C., Lifshitz V., Song J., Yuan Y., et al. 2018. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 27:136–150.e5. 10.1016/j.cmet.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Tao B., Fan Q., Wang S., Chen L., Zhang J., Hao Y., Dong S., Wang Z., Wang W., et al. 2019. Fatty-acid receptor CD36 functions as a hydrogen sulfide-targeted receptor with its Cys333-Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine. 45:108–123. 10.1016/j.ebiom.2019.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M.J., Clark A.K., Selth L.A., Haynes V.R., Lister N., Rebello R., Porter L.H., Niranjan B., Whitby S.T., Lo J., et al. 2019. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 11:eaau5758 10.1126/scitranslmed.aau5758 [DOI] [PubMed] [Google Scholar]
- Wei J., and Tong L.. 2015. Crystal structure of the 500-kDa yeast acetyl-CoA carboxylase holoenzyme dimer. Nature. 526:723–727. 10.1038/nature15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E., Hatzivassiliou G., Sachdeva U.M., Bui T.V., Cross J.R., and Thompson C.B.. 2009. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 324:1076–1080. 10.1126/science.1164097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.M., Fokas E., Dutton S.J., Patel N., Hawkins M.A., Eccles C., Chu K.Y., Durrant L., Abraham A.G., Partridge M., et al. 2016. ARCII: A phase II trial of the HIV protease inhibitor Nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother. Oncol. 119:306–311. 10.1016/j.radonc.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Xie Y., Yu Z., Xiao H., Jiang G., Zhou X., Yang Y., Li X., Zhao M., Li L., et al. 2018. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell. 175:1059–1073.e21. 10.1016/j.cell.2018.08.070 [DOI] [PubMed] [Google Scholar]
- Xiao X., Tang J.J., Peng C., Wang Y., Fu L., Qiu Z.P., Xiong Y., Yang L.F., Cui H.W., He X.L., et al. 2017. Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol. Cell. 66:154–162.e10. 10.1016/j.molcel.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Xie H., Tang C.H., Song J.H., Mancuso A., Del Valle J.R., Cao J., Xiang Y., Dang C.V., Lan R., Sanchez D.J., et al. 2018. IRE1α RNase-dependent lipid homeostasis promotes survival in Myc-transformed cancers. J. Clin. Invest. 128:1300–1316. 10.1172/JCI95864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X., Wang S.C., Xia W., Zou Y., Shao R., Kwong K.Y., Yu Z., Zhang S., Miller S., Huang L., and Hung M.C.. 2000. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat. Med. 6:189–195. 10.1038/72294 [DOI] [PubMed] [Google Scholar]
- Xu D., Wang Z., Zhang Y., Jiang W., Pan Y., Song B.L., and Chen Y.. 2015. PAQR3 modulates cholesterol homeostasis by anchoring Scap/SREBP complex to the Golgi apparatus. Nat. Commun. 6:8100 10.1038/ncomms9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Wang Z., Xia Y., Shao F., Xia W., Wei Y., Li X., Qian X., Lee J.H., Du L., et al. 2020. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 580:530–535. 10.1038/s41586-020-2183-2 [DOI] [PubMed] [Google Scholar]
- Yabe D., Brown M.S., and Goldstein J.L.. 2002. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl. Acad. Sci. USA. 99:12753–12758. 10.1073/pnas.162488899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Zhang G., Bie F., Lv Y., Ma Y., Ma M., Wang Y., Hao X., Yuan N., and Jiang X.. 2017. Eugenol inhibits oxidative phosphorylation and fatty acid oxidation via downregulation of c-Myc/PGC-1β/ERRα signaling pathway in MCF10A-ras cells. Sci. Rep. 7:12920 10.1038/s41598-017-13505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Espenshade P.J., Wright M.E., Yabe D., Gong Y., Aebersold R., Goldstein J.L., and Brown M.S.. 2002a Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 110:489–500. 10.1016/S0092-8674(02)00872-3 [DOI] [PubMed] [Google Scholar]
- Yang Y.A., Han W.F., Morin P.J., Chrest F.J., and Pizer E.S.. 2002b Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res. 279:80–90. 10.1006/excr.2002.5600 [DOI] [PubMed] [Google Scholar]
- Yang P., Su C., Luo X., Zeng H., Zhao L., Wei L., Zhang X., Varghese Z., Moorhead J.F., Chen Y., and Ruan X.Z.. 2018. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 438:76–85. 10.1016/j.canlet.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Yang X., Shao F., Shi S., Feng X., Wang W., Wang Y., Guo W., Wang J., Gao S., Gao Y., et al. 2019. Prognostic Impact of Metabolism Reprogramming Markers Acetyl-CoA Synthetase 2 Phosphorylation and Ketohexokinase-A Expression in Non-Small-Cell Lung Carcinoma. Front. Oncol. 9:1123 10.3389/fonc.2019.01123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wang L., and Jia R.. 2020. Role of de novo cholesterol synthesis enzymes in cancer. J. Cancer. 11:1761–1767. 10.7150/jca.38598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies J.L., Zhang H.H., Menon S., Liu S., Yecies D., Lipovsky A.I., Gorgun C., Kwiatkowski D.J., Hotamisligil G.S., Lee C.H., and Manning B.D.. 2011. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14:21–32. 10.1016/j.cmet.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Coates H.W., Chua N.K., Hashimoto Y., Brown A.J., and Ohgane K.. 2020. A key mammalian cholesterol synthesis enzyme, squalene monooxygenase, is allosterically stabilized by its substrate. Proc. Natl. Acad. Sci. USA. 117:7150–7158. 10.1073/pnas.1915923117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.Y., Theusch E., Lo K., Mangravite L.M., Naidoo D., Kutilova M., and Medina M.W.. 2014. HNRNPA1 regulates HMGCR alternative splicing and modulates cellular cholesterol metabolism. Hum. Mol. Genet. 23:319–332. 10.1093/hmg/ddt422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S., Li J., Lee S.Y., Lee H.J., Shao T., Song B., Cheng L., Masterson T.A., Liu X., Ratliff T.L., and Cheng J.X.. 2014. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 19:393–406. 10.1016/j.cmet.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg K., Yao Y., Reilly P.T., Kannan K., Kiarash R., Mason J., Huang P., Sawyer S.K., Fuerth B., Faubert B., et al. 2011. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25:1041–1051. 10.1101/gad.1987211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N., Hong C., Boyadjian R., and Tontonoz P.. 2009. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 325:100–104. 10.1126/science.1168974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N., Sharpe L.J., Loregger A., Kristiana I., Cook E.C., Phan L., Stevenson J., and Brown A.J.. 2014. The E3 ubiquitin ligase MARCH6 degrades squalene monooxygenase and affects 3-hydroxy-3-methyl-glutaryl coenzyme A reductase and the cholesterol synthesis pathway. Mol. Cell. Biol. 34:1262–1270. 10.1128/MCB.01140-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Lu M., Mori K., Luo S., Lee A.S., Zhu Y., and Shyy J.Y.. 2004. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J. 23:950–958. 10.1038/sj.emboj.7600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Liu J., Huang G., Zhao Y., Yue X., Wu H., Li J., Zhu J., Shen Z., Haffty B.G., et al. 2016. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Genes Dev. 30:1956–1970. 10.1101/gad.283283.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Song F., Zhao X., Jiang H., Wu X., Wang B., Zhou M., Tian M., Shi B., Wang H., et al. 2017a EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol. Cancer. 16:127 10.1186/s12943-017-0704-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Rajbhandari P., Priest C., Sandhu J., Wu X., Temel R., Castrillo A., de Aguiar Vallim T.Q., Sallam T., and Tontonoz P.. 2017b Inhibition of cholesterol biosynthesis through RNF145-dependent ubiquitination of SCAP. eLife. 6:e28766 10.7554/eLife.28766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.F., Iwasaki Y., Nishiyama M., Taguchi T., Tsugita M., Okazaki M., Nakayama S., Kambayashi M., Fujimoto S., Hashimoto K., et al. 2012. Liver X receptor α is involved in the transcriptional regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene. Diabetes. 61:1062–1071. 10.2337/db11-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Jang C., Liu J., Uehara K., Gilbert M., Izzo L., Zeng X., Trefely S., Fernandez S., Carrer A., et al. 2020. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 579:586–591. 10.1038/s41586-020-2101-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Zhan J., Fang W., Zhao Y., Yang Y., Hou X., Zhang Z., He X., Zhang Y., Huang Y., and Zhang L.. 2017. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer. 17:269 10.1186/s12885-017-3239-z [DOI] [PMC free article] [PubMed] [Google Scholar]