Tertiary lymphoid structures, adjacent to tumor nests, are sites where antitumor adaptive T and B cell immune responses are generated. Fridman et al. highlight the impact of B cells and the antibodies they produce in tumor immunity and a patient’s response to immunotherapy.
Abstract
Whereas T cells have been considered the major immune cells of the tumor microenvironment able to induce tumor regression and control cancer clinical outcome, a burst of recent publications pointed to the fact that B cells may also play a prominent role. Activated in germinal centers of tertiary lymphoid structures, B cells can directly present tumor-associated antigens to T cells or produce antibodies that increase antigen presentation to T cells or kill tumor cells, resulting in a beneficial clinical impact. Immune complexes can also increase inflammation, angiogenesis, and immunosuppression via macrophage and complement activation, resulting in deleterious impact.
Introduction
At the beginning of the millennium, it was demonstrated that the immune tumor microenvironment (TME), particularly the density, localization, and functional orientation of T cells, is of paramount importance for controlling tumor growth and spread (Galon et al., 2006; Fridman et al., 2012, 2017). It established the rationale for paradigm-changing immunotherapies using antibodies against immune checkpoint inhibitors (ICIs), such as CTLA-4, PD-1, and PD-L1, that have revolutionized the treatment of cancers (Sharma and Allison, 2015). Since these therapies aim to reinvigorate exhausted T cells, it is not surprising that they are mostly effective in highly mutated tumors, which are more likely to express more tumor-specific neoantigens, resulting in a strong T cell infiltrate, such as non–small cell lung cancer (NSCLC), melanoma, and microsatellite instable tumors (Rizvi et al., 2015). However, despite being very efficient and inducing long-term responses in many cancer types, most patients are resistant to ICI therapies (Hirsch et al., 2019), urging for the identification of other components of the immune TME that are involved in tumor control and may provide novel therapeutic approaches. In this respect, the role of B cells has, until recently, been underestimated. In fact, it had been reported in some murine models that B cells, and the antibodies they produce, may favor cancer occurrence and spread. In recent years, however, studies in human cancers showed that the density of B cells, particularly in tertiary lymphoid structures (TLSs) in the tumor-adjacent TME, correlates with favorable prognosis and predicts therapeutic response to ICIs even in tumors with low tumor mutational burden. We will put in perspective the different roles of B cells in cancer immunity, with emphasis on TLSs, and discuss the mechanisms underlying their effects and their use as prognostic biomarkers and therapeutic targets.
B cells in tumor immunology: Lessons from murine models
The question of the impact of B lymphocytes in the immune control of tumors has been addressed in several murine models, unraveling potential mechanisms of action of B cells in cancer immunity. In a transgenic mouse model of multistage epithelial carcinogenesis in genetically invalidated RAG1 mice, the group of L.M. Coussens (de Visser et al., 2005) observed a high reduction of innate cells infiltrate in premalignant skin and carcinoma incidence. Both were restored upon transfer of B cells or serum from tumor-bearing immunocompetent mice. The mechanism underlying these effects is that B lymphocytes are activated in tumor-developing mice and produce antibodies that deposit in the precancerous lesions, fueling chronic inflammation through Fcγ receptor (FcγR) activation of innate cells migrating into the preneoplastic and neoplastic TME. Whereas immune complexes did not activate complement in this model (Medler et al., 2018), complement was found to contribute to protumoral effects through antibody-driven chronic inflammation in CMT and TC1 lung cancer models (Roumenina et al., 2019a; Kwak et al., 2018). Immune complexes may also increase angiogenesis via the induction of vascular endothelial growth factor production by activated macrophages (Tan and Coussens, 2007). In addition, B cells may inhibit T cell responses in particular via the production of immunosuppressive cytokines (DeNardo et al., 2010). In models of fibrosarcoma and breast cancer (BC), B cell depletion with anti-IgM antibodies highly reduced the incidence of metastases as compared with control mice (Brodt and Gordon, 1978; Barbera-Guillem et al., 2000). In genetically invalidated deficient B cell mice, the growth of several types of tumors, including B16 melanoma, EL4 thymoma, and MC38 colon carcinoma, was also reduced (Qin et al., 1998; Shah et al., 2005). This effect was attributed to the lack of inhibition of the antitumor T cell response by B cell–produced cytokines such as IL-10 (Inoue et al., 2006). Finally, in a model of inflammation-driven hepatocellular carcinoma (HCC), B cell–rich TLSs were found to serve as a niche protecting tumor progenitor cells and favoring the growth of malignant cells via the production of lymphotoxin β (Finkin et al., 2015).
Altogether, these observations led to a “bad reputation” for B cells in cancer and, following several decades of unsuccessful searching for protective spontaneously produced antitumor antibodies, reinforced the paradigm that T cells, particularly CD8+/cytotoxic T cells, were the major, if not the only, component of beneficial antitumor immunity. A few reports challenged this statement, such as the observation that treatment of tumor-bearing mice with a B cell–depleting anti-CD20 antibody resulted in a large (more than twofold) increase in B16 melanoma volume and number of lung metastases. In this model, B cell depletion impaired induction of IFN-γ–producing T helper type 1 (Th1) cells (DiLillo et al., 2010), supporting the concept that B cells may be crucial for generating efficient T cell immunity. In conclusion, as in humans, where each patient is unique, in mice, each model is different and speaks for itself.
B cells in human cancers
The impact of B cells in cancer has been largely unraveled in studies performed in human tumors. The observation that CXCL13, a chemoattractant for B lymphocytes, is a major determinant of favorable prognosis suggests that the presence of B lymphocytes may be crucial. Thus, in colorectal cancer (CRC), an unsupervised screening for the impact of chemokine gene expression on cancer outcome revealed that tumors lacking CXCL13 had fewer intratumoral B cells and a worse prognosis than tumors expressing it (Bindea et al., 2013). The CXCL13 signature was also associated with favorable prognosis in melanoma (Helmink et al., 2020). In soft tissue sarcoma (STS), a high B cell signature significantly correlated with longer overall survival (OS), independently of the histology of the tumors, whereas there was no correlation between T cell signatures and OS. The B cell signature was the best predictor of OS, even when combined with CD8, PD-1, or CTLA-4 signatures (Petitprez et al., 2020). The fact that T follicular helper cells, which play a major role in B cell activation, correlate with favorable prognosis in breast (Gu-Trantien et al., 2017) and head and neck (Cillo et al., 2020) carcinoma also militates for a role of B cells in tumor immunity.
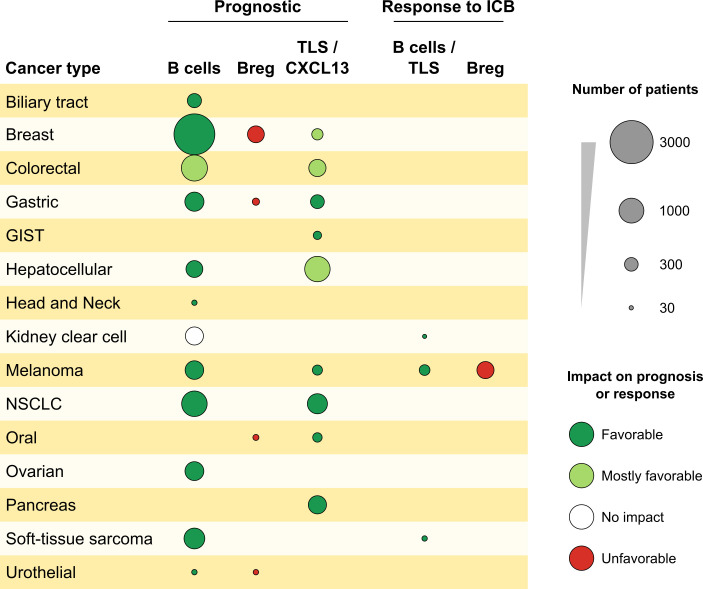
Direct evidence for the impact of B cells came from studies showing that their intratumoral density is associated with good prognosis in BC (Mahmoud et al., 2012), CRC (Edin et al., 2019; Berntsson et al., 2018), NSCLC (Germain et al., 2014), head and neck cancer (van Herpen et al., 2008), ovarian cancer (Nielsen et al., 2012; Milne et al., 2009; Santoiemma et al., 2016), biliary tract cancer (Goeppert et al., 2013), primary cutaneous melanoma (Garg et al., 2016), metastatic melanoma (Cabrita et al., 2020), and HCC (Shi et al., 2013; Garnelo et al., 2017). In CRC, B cells were found associated with good outcome (Edin et al., 2019; Berntsson et al., 2018). Analysis of clonal diversity of B cell infiltrates showed that decreased diversity was associated with improved survival in primary cutaneous melanoma but with diminished OS in renal cell carcinoma (Iglesia et al., 2014, 2016; Selitsky et al., 2019). Such clonal expansion is suggestive, but not demonstrative, of the presence of anticancer B cells and does not inform about their specificity. The presence of plasma cells in BC (Yeong et al., 2018), at the vicinity of CD8T cells in ovarian cancer, and the expression of their signature in breast (Seow et al., 2020) and ovarian cancer (Nielsen et al., 2012) were associated with favorable outcome (Table 1 and Fig. 1). However, an analysis of 54 cohorts of encompassing 25 cancer types revealed that although the prognostic impact of tumor-infiltrating B cells was positive in 50% of the studies, it was deleterious or neutral in 9% and 41%, respectively (Wouters and Nelson, 2018). A few studies addressed the question of the role of B regulatory cells (B reg cells) in human cancers. The frequencies of CD19+-IL-10+ B reg cells correlated with shorter OS in bladder cancer (Zirakzadeh et al., 2020) and BC (Murakami et al., 2019), and the coexistence of B reg cells with regulatory T cells (T reg cells) correlates with shorter metastasis-free survival in BC (Ishigami et al., 2019).
Table 1. Prognostic impact of B cells and TLS in human cancers.
| Cancer type | Number of cases | Result | Reference |
|---|---|---|---|
| Biliary tract cancer | 323 | CD20 and CD8 infiltration correlate with improved OS | Goeppert et al., 2013 |
| BC | 60 | The B cell–attracting chemokine CXCL13 correlates with PFS long-term survival | Gu-Trantien et al., 2017 |
| BC | 1,200 | CD20+ infiltration correlates with DSS and PFS | Mahmoud et al., 2012 |
| BC | 269 | CD38+ plasma cells infiltration correlates with better disease-free survival, but not OS | Yeong et al., 2018 |
| BC | 489 | Coexistence of B reg and T reg cells in TLS correlates with shorter metastasis-free survival | Ishigami et al., 2019 |
| BC | 728 | B cell signatures correlates with improved PFS | Iglesia et al, 2016 |
| ccRCC | 526 (TCGA); 28 | B cell lineage signature demonstrates no impact on survival; B cell signature correlates with response to ICIs | Helmink et al., 2020 |
| CRC | 107 | B (CD20) cell and T (CD8) cell infiltration and B cell–attracting CXCL13 chemokine correlate with longer PFS | Bindea et al., 2013 |
| CRC | 351 stage II and III | TLS density correlates with longer PFS in stage II, but not stage III, patients | Di Caro et al., 2014 |
| CRC | 557 | CD20, CD138, and IGKC infiltration correlate with improved OS | Berntsson et al., 2018 |
| CRC | 316 | High infiltration of CD20+ B cells correlates with improved DSS and slightly increases the prognostic effect of CD8+ T lymphocytes while having no prognostic effect on patients with tumors poorly infiltrated by CD8+ cytotoxic T lymphocytes | Edin et al., 2019 |
| Gastric cancer | 59 | IL-10+CD19+ cells are associated with shorter survival | Murakami et al., 2019 |
| Gastric cancer | 226 | Majority of B cells in gastric cancer are located in TLSs and associated with better OS | Yamakoshi et al., 2020 |
| Gastrointestinal stromal tumors | 111 | TLS density (H&E) correlates with longer OS and PFS | Lin et al., 2020 |
| HCC | 462 | TLS density (H&E) correlates with longer OS and PFS | Li et al., 2020a |
| HCC | 273 and 226 | Density of mature TLS (H&E) and TLS signature associated with lower risk of early recurrence | Calderaro et al., 2019 |
| HCC | 120 and 200 | CD20+ B cells located in the invasive margin colocalize and correlate with CD8, and a positive correlation is found between the density of B cells and OS or RFS; simultaneously, high densities of CD20+ and CD8+ cells are significantly associated with both prolonged OS and recurrence-free survival | Shi et al., 2013 |
| HCC | 112 | Density of tumor-infiltrating CD19+ B cells correlates with superior survival; CD20 and CD3 correlates with high intratumoral densities of both CD20+ B cells, and CD3+ T cells had longer survival compared with those with low densities of both subsets | Garnelo et al., 2017 |
| Head and neck cancer | 30 | Peritumoral B cell infiltration correlates with prolonged OS irrespective of IL-12 treatment | van Herpen et al., 2008 |
| Lung | 118 | Follicular CD20+ B cells and TLS density correlate with longer DSS | Germain et al., 2014 |
| Lung | 138 | TLSs are associated with good prognosis in untreated lung squamous cell carcinoma; corticosteroids may be detrimental to TLS formation | Siliņa et al., 2018 |
| Melanoma, primary cutaneous | 82 | Mature DC/T cell aggregate density correlates with favorable prognosis | Ladányi et al., 2007 |
| Melanoma | 25 | Frequency of plasmablast-like cells and naive B cells in pretherapy melanomas is higher in patients responding to ICB | Griss et al., 2019 |
| Melanoma | 164 | CD20 and CD8 infiltration correlates with improved survival; a CD20+ and CD8+ cell combination is the best prognosticator of survival; TLS gene signature predicts prognosis and response to ICI | Cabrita et al., 2020 |
| Melanoma | 136, 28, and 21 | B cell lineage signature demonstrates prolonged OS; CD20+, B cell signature, and TLS densities predict response to ICI | Helmink et al., 2020 |
| Melanoma, primary cutaneous | 98 | CD20 infiltration associated with longer OS | Garg et al., 2016 |
| Melanoma, primary cutaneous | 473 | Memory B cell score and increased clonality of the BCR repertoire are associated with longer OS; lack of an assembled BCR in pretreatment tumor tissues is associated with a lack of antitumor response to a CTLA-4 inhibitor; B reg cell score is associated with shorter OS and lack of response to CTLA4 | Selitsky et al., 2019 |
| Lung | 118 | Follicular CD20+ B cell and TLS density correlates with longer DSS | Germain et al., 2014 |
| Oral cancer | 65 | TLS density correlates with longer PFS and OS | Li et al., 2020b |
| Ovarian cancer | 172 | Tumor-infiltrating plasma cells are associated with TLSs, cytolytic response, and favorable prognosis | Kroeger et al., 2016 |
| Ovarian cancer | 194 | CD20+ infiltration improves the favorable impact of CD8+ T cells on DSS; tumors positive for both CD8 and CD20 TIL showed markedly greater DSS compared with those positive for CD8 TIL alone | Nielsen et al., 2012 |
| Ovarian cancer | 199 | CD20+ and CD8+ immune infiltrates and the combination correlate with improved DSS | Milne et al., 2009 |
| Ovarian cancer | 135 | CD20 infiltrate correlates with improved 5-yr and 10-yr survival | Santoiemma et al., 2016 |
| Ovarian cancer, omental metastases | 92 | B cells support the development of antitumor immune response | Montfort et al., 2017 |
| Pancreas | 534 | TLS density (H&E) correlate with longer DSS | Hiraoka et al., 2015 |
| Several tumor types | 3,485 | B and T cell signatures correlate with improved OS in BC (821), lung cancer (267), and melanoma (329); BCR diversity associated with OS in melanoma (329) and RCC (461) | Iglesia et al, 2016 |
| STS | 496 and 47 | B cell lineage signature associated with longer OS independently of T cell signature and PD1, PD-L1, and Foxp3 gene expression; TLS-rich group predicts PFS and response to ICIs independently of STS histology | Petitprez et al., 2020 |
| Urothelial urinary bladder cancer | 31 | CD20+ B cells in follicle-like structures correlate with longer survival; B reg cells may negatively affect prognosis | Zirakzadeh et al., 2020 |
ccRCC, clear cell renal cell cancer; DSS, disease-specific survival; ICB, immune check-point blocker; IGKC, immunoglobulin K constant domain; RFS, relapse-free survival; TIL, tumor-infiltrating lymphocytes.
Figure 1.
Impact of B cells and TLSs on prognosis and response to ICIs. Analysis of 15 different tumor types. The surface of the spot is proportional to the number of patients for each cancer, with a favorable impact (green), mostly favorable impact (light green), unfavorable impact (red), or no impact (white) on PFS or OS and therapeutic response to ICIs.
Intratumoral B cells undergo isotypic switch and produce IgG or IgA antibodies directed against tumor antigens upon in vitro culture, as shown in NSCLC (Germain et al., 2014) and ovarian cancer (Montfort et al., 2017). The impact of the isotypes of antibodies produced by intratumoral B cells is critical for their Fc-dependent activities, which can be mediated through Fc receptors or complement activation. Indeed, by activating natural killer (NK) cells or macrophages via antibody-dependent cellular cytotoxicity (ADCC; Clynes and Ravetch, 1995) and macrophages via antibody-dependent cellular phagocytosis (ADCP; Gül and van Egmond, 2015), IgG antibodies may participate in antitumor activity, locally and systematically, as illustrated by the favorable prognostic impact of circulating IgG anti-MUC1 antibodies in breast, pancreatic, and gastric cancers (Hamanaka et al., 2003; Kurtenkov et al., 2007; Fremd et al., 2015). Analysis of RNA-sequencing data from cutaneous melanoma tumors from The Cancer Genome Atlas also showed an association between high levels of IgG1/IGH transcripts and favorable prognosis (Bolotin et al., 2017). In contrast, in clear cell renal cell cancer, tumor-bound IgG participates in the activation of the classical complement pathway in situ, fueling chronic inflammation and yielding poor prognosis. This occurs through the binding of complement component C1q, originating from macrophages, on tumor-associated IgG antibodies. Recruitment of tumor cell–produced C1r, C1s, C4, C2, C3, and C5 allows complement activation and chronic inflammation (Roumenina et al., 2019a). A protumoral role of B cells has been also been found in squamous cell carcinoma (Affara et al., 2014) occurring by deposition of IgG-containing immune complexes that foster FcγR-dependent activation of myeloid cells and fuel inflammation. In NSCLC, the classical pathway is activated in part in an IgM-dependent manner (Kwak et al., 2018), conferring poor prognosis (Ajona et al., 2013). The impact of antitumor IgA antibodies on patient prognosis seems deleterious, as suggested by analysis of IgA/IgGH transcripts in melanoma and in situ analyses in melanoma and bladder cancer (Bosisio et al., 2016; Welinder et al., 2016). In HCC, IgA+ B cells inhibit cytotoxic T cell responses that prevent hepatocarcinogenesis in the inflamed liver (Shalapour et al., 2017). The impact of B cells, although undoubtful, may therefore be a tumor contexture–dependent double-edged sword.
B cells and TLSs
Comprehensive analyses of the TME in different human cancers led to the observation that in human tumors, B cells were mostly located in TLSs. TLSs are ectopic lymphoid organs that develop in inflamed tissues in the context of chronic antigen stimulation. In cancers, proimmunogenic inflammation can also be induced or increased by treatments such as chemotherapy (Lu et al., 2020; Kuwabara et al., 2019) or radiotherapy (Boivin et al., 2018). TLSs are formed in inflamed sites, starting upon contact of IL-7–secreting stromal cells with tissue-resident monocytic cells, Th17 cells, or B cells in a CXCL13-rich milieu (Buckley et al., 2015; Nayar et al., 2016; Barone et al., 2016; Jones et al., 2016). CCL21 and CXCL12 participate in lymphocytes recruitment, while CXCL13 and CCL19, together with adhesion molecules, govern the structural organization of the forming TLSs (Pitzalis et al., 2014). TLSs are sites of generation of immune responses to locally produced antigens, developing even in the absence of lymph nodes in lymphotoxin α invalidated mice (Moyron-Quiroz et al., 2006). Mature TLSs contain a T cell zone, where mature dendritic cells (DCs) present antigen to T cells, and a prominent B cell zone organized in a germinal center (GC), with follicular DCs (FDCs) and proliferating B cells, expression of activation-induced deaminase, and BCL6 allowing class switch and maturation toward plasma cells (Sautès-Fridman et al., 2019). They are surrounded by high endothelial venules (Ager, 2017).
In 2008, it was reported that TLSs were present in NSCLC tumors and that their density correlated with favorable prognosis (Dieu-Nosjean et al., 2008). Subsequently, it was shown that T cells in TLS+ tumors presented a Th1/cytotoxic functional orientation (Goc et al., 2014) and that B cells were also activated in TLS GCs to proliferate and differentiate into plasma cells producing antibodies to tumor-associated antigens. A high density of B cell follicles correlated with longer progression-free survival (PFS) and OS in NSCLC (Germain et al., 2014). The presence of TLSs was reported in many cancer types, including melanoma (Ladányi et al., 2007), bladder (Zirakzadeh et al., 2020), colorectal (Di Caro et al., 2014; Posch et al., 2017), gastric (Yamakoshi et al., 2020), gastrointestinal stromal tumor (Lin et al., 2020), HCC (Li et al., 2020a; Calderaro et al., 2019) ovarian (Kroeger et al., 2016), oral (Li et al., 2020b), squamous lung (Siliņa et al., 2018), and pancreatic carcinomas (Hiraoka et al., 2015; Table 1 and Fig. 1; reviewed in Sautès-Fridman et al., 2019). In general, the presence of TLSs inside or adjacent to tumor nests correlated with favorable prognosis (Sautès-Fridman et al., 2019). The degree of TLS maturation seems to be important, since GC-containing TLSs were the best predictors of lack of cancer recurrence in stage II/III CRC (Posch et al., 2017), squamous cell carcinoma (Siliņa et al., 2018), and HCC (Calderaro et al., 2019), whereas precancerous lesions with immature TLSs progressed toward malignancy (Meylan et al., 2020). Indeed, in premalignant early stages of HCC, TLSs found in high-grade dysplastic and early HCC nodules were immature, in the form of lymphoid aggregates without a fully matured GC. Their presence correlated with markers of inflammation, immunosuppression, and immune exhaustion, which may favor transition to HCC (Meylan et al., 2020). This finding is reminiscent of the work of Finkin et al. (2015) showing that TLSs may serve as niches protecting tumor cells in a murine model of HCC. Together with the reports in murine models of premalignant stages (de Visser et al., 2005), they support a different impact of TLSs and B cells according to the stage of cancer. Altogether, these studies indicate that TLSs may shape cancer-controlling immunity and that B cells inside GCs are important in this process and influence clinical outcome.
B cells predict therapeutic response to immunotherapy by ICIs
Whereas immunotherapy using antibodies to ICIs aims to reinvigorate effector T lymphocytes, several publications pointed to the fact that B cells may be major players of therapeutic efficacy.
In STS, B cell and plasma cell signatures were pathognomonic of an immune-rich sarcoma immune class also characterized by the presence of TLSs. Patients with this sarcoma immune class highly responded (50%) to pembrolizumab, an anti–PD-1 antibody, whereas none of the patients from immune desert classes responded (Petitprez et al., 2020). This finding may change the medical care of STS patients, who are considered poor responders to ICIs. It has generated a prospective clinical trial in which patients to be treated are selected on the basis of B cell rich TLS (ClinicalTrials.gov identifier NCT02406781). In melanoma, TLS and B cell signatures, and not T cell signatures, predicted therapeutic responses to pembrolizumab and ipilimumab, an anti–CTLA-4 antibody. B cells in tumors of responding patients exhibited oligoclonal repertoires of the Ig genes as compared with the polyclonal B cell repertoires of nonresponding patients. Moreover, B cells and TLS densities increased during treatment in responding, but not nonresponding, patients (Helmink et al., 2020), and a TLS gene signature synergized with a T effector signature to predict responses to ICIs with anti–PD-1 and anti–CTLA-4 antibodies (Cabrita et al., 2020). In contrast, a B reg cell signature was associated with lack of response to anti–CTLA-4 in cutaneous melanoma (Selitsky et al., 2019).
Not all B cell subtypes are likely to participate in response to ICIs, and recent data suggest that plasmablasts are in particular more frequent in responder patients (Griss et al., 2019). Indeed, multiomics predictions are not absolute, but they provide significant predictive correlations that open a new field of investigations integrating B cells as major players and potential targets of novel immunotherapeutic approaches. Single-cell transcriptomics analysis of tumor-infiltrating B cells allowed for the identification of transcriptomic programs that are specifically expressed in B cell–infiltrating ICI-responsive tumors (Helmink et al., 2020). Such analyses may pave the way to better understand how each B cell subtype contributes to patient survival and response to ICIs.
B cells and cancer: Mechanisms of action
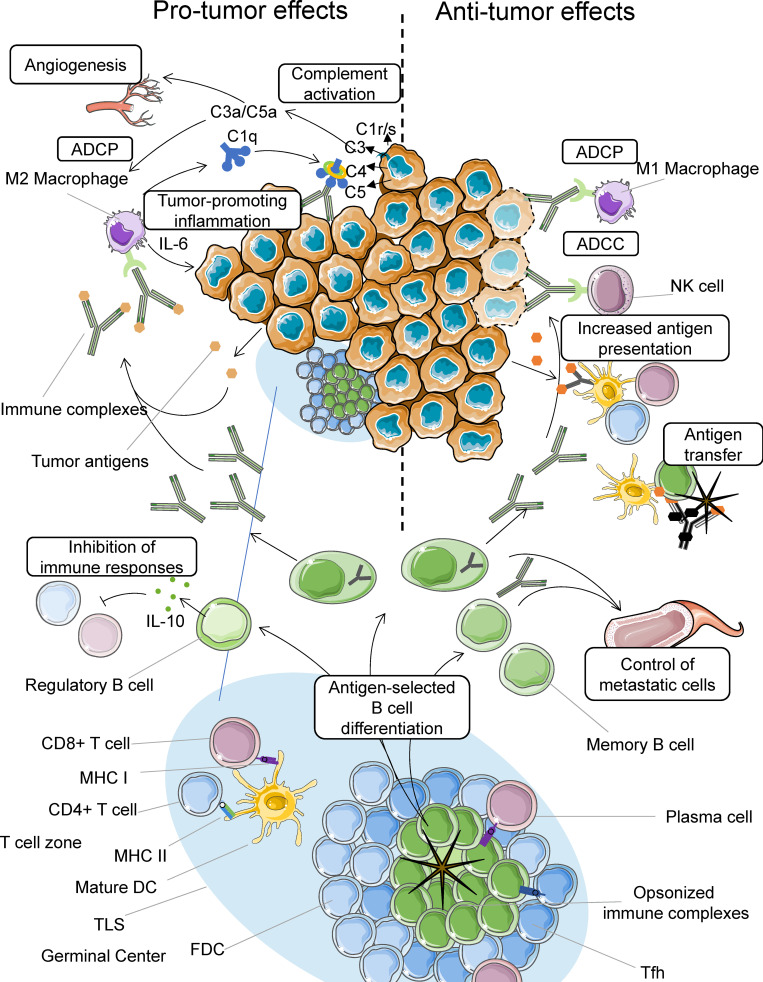
The functions of B cells are multiple. Although a truly mechanistic aspect of their impact is still missing, Fig. 2 illustrates potential mechanisms that may operate in the participation of B cell to tumor immunity.
Figure 2.
Mechanisms of action of B cells in cancer. In GCs of TLSs, B cells are selected by antigen presented by FDC-associated immune complexes. They are activated with the help of T follicular helper (Tfh) cells, proliferate, and differentiate into memory B cells and plasma cells. B cells present antigens to CD4 and CD8 T cells. Plasma cells produce IgG antibodies, which may increase antigen presentation to T cells after uptake of immune complexes by DCs and kill or engulf tumor cells via ADCC and ADCP, respectively (right). B cells can also transfer antigen directly to DCs or via complement receptor 2 to FDCs in the form of opsonized immune complexes. Memory B cells and antibodies circulate in the blood, where they help control potential metastatic cells (right). In contrast, binding of immune complexes to macrophages results in their activation and the production of proinflammatory mediators that exert protumor activities. Tumor cell–bound antibodies can also activate complement, which fuels inflammation and activates endothelial cells, promoting tumor growth and spread (left). B reg cells may also inhibit immune responses via the production of IL-10.
Antigen presentation
B cells recognize and internalize native proteins and glycoproteins via the B cell receptor (BCR). They internalize the proteins thanks to the immunotyrosine activation motif of the CD79α and β signaling chains associated with the antigen-recognizing Ig within the BCR. Subsequent cytoplasmic processing of the proteins allows antigenic peptide association with MHC class II molecules and presentation of the complex to CD4 T helper cells and with MHC class I–associated peptides to CD8T cells. This mechanism of T cell activation occurs not only in lymph nodes but also in TLSs (Bruno et al., 2017; Garaud et al., 2019; Wouters and Nelson, 2018). Close contact between BCR-bound antigen and DCs favors antigen transfer to the latter and supports efficient antigen presentation to T cells (Harvey et al., 2014). In addition, B cells can capture immune complexes via complement receptors and transfer these antigens to FDCs (Phan et al., 2007), increasing the GC response. In tumors, B cells may therefore enhance tumor-associated antigen presentation to proximal T cells, resulting in a stronger T cell response.
In TLS GCs, plasma cells are generated and produce IgG antibodies to tumor-associated antigens, and the immune complexes formed are internalized by DCs, which process the antigens and present them in a very efficient way to CD4 and CD8 T cells. It is known that the quantity of antigen necessary to induce a T cell response is much lower (1,000–10,000 times) when internalized via an immune complex than its native counterpart (Kalergis and Ravetch, 2002). This mechanism may explain why B cells are crucial to obtain efficient T cell responses in poorly mutated tumors in which the antigenic load is low and therefore not sufficient to directly activate T cells. It may be the case in STS, ovarian cancer, or renal cell carcinoma. It may also amplify T cell responses in tumors with high tumor mutational burden such as melanoma or NSCLC.
Another important modulator of B cell activation is complement, which can be activated on dying tumor cells, as recently shown in BC tumors in response to chemotherapy. Through binding to CR2 expressed by B cells, complement cleavage product C3b induces the generation of an ICOS-L+ B cell subset that boosts T cell immunity by enhancing tumor-specific CD8T cells and the Th1/T reg cell ratio. Emergence of this B cell subset is associated with improved therapeutic efficacy of neoadjuvant chemotherapy in BC, particularly in triple-negative tumors, and prolonged survival of patients (Lu et al., 2020; Sautès-Fridman and Roumenina, 2020).
Antibody production
In addition to amplifying T cell immunity via antigen presentation, antibodies produced by plasma cells in TLS GCs exert effector functions. Antibodies directed against tumor-associated antigens such as LAGE-1, MAGE antigens, and NY-ESO-1 were detected in supernatants of tumor-infiltrating B cells from half of the NSCLC patients (Germain et al., 2014). Antibodies against the tumor-associated antigen MUC1 overexpressed in tumors under an unglycosylated form and against ganglioside GD3, CEA, MUC1, and FN1 have been detected in BC (Montfort et al., 2017; Coronella et al., 2002; Garaud et al., 2019; Pavoni et al., 2007). These are tumor-associated rather than cancer-specific antibodies (such as mutated RAS), but they may be efficient in antitumor responses, since they recognize and bind to membrane antigens expressed by tumor cells. One of the interests of B cells for responses to immunotherapy is that although effector T cells almost always recognize patient’s selective private neoantigens (Tran et al., 2017), B cells and the antibodies they produce may recognize shared tumor-associated antigens (Heesters et al., 2016). IgG antibodies bind to FcγRs on NK cells and macrophages, activating them to destroy tumor cells via ADCC.
ADCC killing of target cells is very efficient, even when these cells have a low antigen load. It may, however, not be very efficient in solid tumors, as NK cells are scarce and often anergic (Platonova et al., 2011). Macrophages, which are often the most prominent tumor-infiltrating hematopoietic cells, may act as effector cells, inducing the killing of tumor cells via ADCC (Clynes and Ravetch, 1995) or their phagocytosis via ADCP (Gül and van Egmond, 2015). Following ADCP, macrophages may also up-regulate PD-L1 and indoleamine 2,3-dioxygenase and support local immunosuppression (Su et al., 2018). In addition, macrophages may be immunosuppressive cells through the production of proangiogenic (vascular endothelial growth factor) or immunosuppressive (TGF-β) cytokines (Campa et al., 2015; Amornsiripanitch et al., 2010). By activating macrophages, IgG immune complexes may also support chronic inflammation (Clynes and Ravetch, 1995; Sylvestre et al., 1996), angiogenesis, and immunosuppression, thus favoring tumor growth. A similar mechanism may operate when tumor cell–bound IgG activated locally produced complement with the production of the anaphylatoxins proangiogenic and proinflammatory complement components C3a and C5a (Roumenina et al., 2019b). Complement activation could also lead to tumor cell killing, but it seems unlikely, since malignant tumor cells in solid tumors are generally equipped with complement-inhibiting molecules (CD46, CD55, and CD59; Roumenina et al., 2019b). Interestingly, antibodies against the complement regulator factor H (FH) were found in patients with NSCLC and were shown to protect against tumor progression (Campa et al., 2015; Amornsiripanitch et al., 2010). These antibodies recognize a FH neoantigen produced by the tumor cells. B cells from anti-FH–positive patients were sorted and used to select an antibody with therapeutic properties in mouse models (Bushey et al., 2016). Although it was suggested that the anti-FH antibodies stimulate complement-mediated cancer cell killing, the classical pathway is deleterious in NSCLC (Ajona et al., 2013; Kwak et al., 2018), indicating that further studies are needed to unravel the complex mechanisms by which the B cells and produced Ig affect tumor progression.
In general, the IgA isotype is often characteristic of the B reg cell/T reg cell circuit that regulates mucosal inflammation and T reg cells producing TGF-β, which mediates isotype class switching to IgA (Stavnezer and Kang, 2009). In HCC, IgA+ B cells inhibit cytotoxic T cell responses that prevent hepatocarcinogenesis in the inflamed liver (Shalapour et al., 2017).
In addition, two studies suggest that activated B cells may directly induce tumor cell apoptosis (Garnelo et al., 2017; Jahrsdörfer et al., 2006).
Regulatory role
Tumor B cells produce antitumor antibodies of IgA isotype, as shown in NSCLC (Germain et al., 2014). These IgA-producing B cells were shown to modulate CD8T cells cytotoxicity in an HCC mouse model (Shalapour et al., 2017). Through production of immunosuppressive cytokines such as IL-10 (Shen and Fillatreau, 2015), B reg cells may locally inhibit T cell activation toward tumor-associated antigens.
Concluding remarks
In conclusion, B cells may be a double-edged sword, resulting either in tumor cell destruction by increasing T cell responses and via ADCC or in tumor growth by fueling chronic inflammation, angiogenesis, or immunosuppression via immune complex formation or complement activation. However, we still have a poor understanding of the heterogeneity and diversity of B cell subsets in tumors, which poses a major obstacle and dilemma when targeting B cells as oncological treatments. In addition, as for T cells, most intratumoral B cells may be bystanders and not antitumoral. The respective importance of bystander and cancer-specific B cells needs to be further evaluated in this emerging field.
The exploding investigations in this new field will not only provide a better understanding of antitumor immunity but also propose novel prognostic and predictive markers as well as novel therapeutic targets. Thus, the analysis of intratumoral B cells by single-cell technology, the identification of their Ig repertoire, and the characterization of the antibodies they produce in long-survivor immunotherapy-responder patients will permit the design of new therapeutic monoclonal antibodies. We are at the very beginning of a newly open and very promising avenue.
Acknowledgments
This work was supported by the Institut national de la santé et de la recherche médicale; Sorbonne Université, Université de Paris; the Site Intégré de Recherche sur le Cancer (Cancer Research for Personalized Medicine); and Laboratory of Excellence LabeX Immunooncology.
Author contributions: W.H. Fridman, F. Petitprez, L.T. Roumenina, and C. Sautès-Fridman wrote the manuscript. M. Meylan, C.-M. Sun, and T.W.-W. Chen edited the manuscript.
References
- Affara N.I., Ruffell B., Medler T.R., Gunderson A.J., Johansson M., Bornstein S., Bergsland E., Steinhoff M., Li Y., Gong Q., et al. 2014. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 25:809–821. 10.1016/j.ccr.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ager A. 2017. High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Front. Immunol. 8:45 10.3389/fimmu.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajona D., Pajares M.J., Corrales L., Perez-Gracia J.L., Agorreta J., Lozano M.D., Torre W., Massion P.P., de-Torres J.P., Jantus-Lewintre E., et al. 2013. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J. Natl. Cancer Inst. 105:1385–1393. 10.1093/jnci/djt205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amornsiripanitch N., Hong S., Campa M.J., Frank M.M., Gottlin E.B., and Patz E.F. Jr. 2010. Complement factor H autoantibodies are associated with early stage NSCLC. Clin. Cancer Res. 16:3226–3231. 10.1158/1078-0432.CCR-10-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera-Guillem E., Nelson M.B., Barr B., Nyhus J.K., May K.F. Jr., Feng L., and Sampsel J.W.. 2000. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol. Immunother. 48:541–549. 10.1007/PL00006672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F., Gardner D.H., Nayar S., Steinthal N., Buckley C.D., and Luther S.A.. 2016. Stromal Fibroblasts in Tertiary Lymphoid Structures: A Novel Target in Chronic Inflammation. Front. Immunol. 7:477 10.3389/fimmu.2016.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsson J., Eberhard J., Nodin B., Leandersson K., Larsson A.H., and Jirström K.. 2018. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: Relationship with sidedness and prognosis. OncoImmunology. 7:e1465165 10.1080/2162402X.2018.1465165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G., Mlecnik B., Tosolini M., Kirilovsky A., Waldner M., Obenauf A.C., Angell H., Fredriksen T., Lafontaine L., Berger A., et al. 2013. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 39:782–795. 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Boivin G., Kalambaden P., Faget J., Rusakiewicz S., Montay-Gruel P., Meylan E., Bourhis J., Lesec G., and Vozenin M.-C.. 2018. Cellular Composition and Contribution of Tertiary Lymphoid Structures to Tumor Immune Infiltration and Modulation by Radiation Therapy. Front. Oncol. 8:256 10.3389/fonc.2018.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin D.A., Poslavsky S., Davydov A.N., Frenkel F.E., Fanchi L., Zolotareva O.I., Hemmers S., Putintseva E.V., Obraztsova A.S., Shugay M., et al. 2017. Antigen receptor repertoire profiling from RNA-seq data. Nat. Biotechnol. 35:908–911. 10.1038/nbt.3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosisio F.M., Wilmott J.S., Volders N., Mercier M., Wouters J., Stas M., Blokx W.A., Massi D., Thompson J.F., Scolyer R.A., et al. 2016. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod. Pathol. 29:347–358. 10.1038/modpathol.2016.28 [DOI] [PubMed] [Google Scholar]
- Brodt P., and Gordon J.. 1978. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J. Immunol. 121:359–362. [PubMed] [Google Scholar]
- Bruno T.C., Ebner P.J., Moore B.L., Squalls O.G., Waugh K.A., Eruslanov E.B., Singhal S., Mitchell J.D., Franklin W.A., Merrick D.T., et al. 2017. Antigen-Presenting Intratumoral B Cells Affect CD4+ TIL Phenotypes in Non-Small Cell Lung Cancer Patients. Cancer Immunol. Res. 5:898–907. 10.1158/2326-6066.CIR-17-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C.D., Barone F., Nayar S., Bénézech C., and Caamaño J.. 2015. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu. Rev. Immunol. 33:715–745. 10.1146/annurev-immunol-032713-120252 [DOI] [PubMed] [Google Scholar]
- Bushey R.T., Moody M.A., Nicely N.L., Haynes B.F., Alam S.M., Keir S.T., Bentley R.C., Roy Choudhury K., Gottlin E.B., Campa M.J., et al. 2016. A Therapeutic Antibody for Cancer, Derived from Single Human B Cells. Cell Rep. 15:1505–1513. 10.1016/j.celrep.2016.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita R., Lauss M., Sanna A., Donia M., Skaarup Larsen M., Mitra S., Johansson I., Phung B., Harbst K., Vallon-Christersson J., et al. 2020. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 577:561–565. 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- Calderaro J., Petitprez F., Becht E., Laurent A., Hirsch T.Z., Rousseau B., Luciani A., Amaddeo G., Derman J., Charpy C., et al. 2019. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 70:58–65. 10.1016/j.jhep.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Campa M.J., Gottlin E.B., Bushey R.T., and Patz E.F. Jr. 2015. Complement Factor H Antibodies from Lung Cancer Patients Induce Complement-Dependent Lysis of Tumor Cells, Suggesting a Novel Immunotherapeutic Strategy. Cancer Immunol. Res. 3:1325–1332. 10.1158/2326-6066.CIR-15-0122 [DOI] [PubMed] [Google Scholar]
- Cillo A.R., Kürten C.H.L., Tabib T., Qi Z., Onkar S., Wang T., Liu A., Duvvuri U., Kim S., Soose R.J., et al. 2020. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity. 52:183–199.e9. 10.1016/j.immuni.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R., and Ravetch J.V.. 1995. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 3:21–26. 10.1016/1074-7613(95)90155-8 [DOI] [PubMed] [Google Scholar]
- Coronella J.A., Spier C., Welch M., Trevor K.T., Stopeck A.T., Villar H., and Hersh E.M.. 2002. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J. Immunol. 169:1829–1836. 10.4049/jimmunol.169.4.1829 [DOI] [PubMed] [Google Scholar]
- de Visser K.E., Korets L.V., and Coussens L.M.. 2005. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 7:411–423. 10.1016/j.ccr.2005.04.014 [DOI] [PubMed] [Google Scholar]
- DeNardo D.G., Andreu P., and Coussens L.M.. 2010. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 29:309–316. 10.1007/s10555-010-9223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caro G., Bergomas F., Grizzi F., Doni A., Bianchi P., Malesci A., Laghi L., Allavena P., Mantovani A., and Marchesi F.. 2014. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 20:2147–2158. 10.1158/1078-0432.CCR-13-2590 [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean M.-C., Antoine M., Danel C., Heudes D., Wislez M., Poulot V., Rabbe N., Laurans L., Tartour E., de Chaisemartin L., et al. 2008. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 26:4410–4417. 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- DiLillo D.J., Yanaba K., and Tedder T.F.. 2010. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. 184:4006–4016. 10.4049/jimmunol.0903009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin S., Kaprio T., Hagström J., Larsson P., Mustonen H., Böckelman C., Strigård K., Gunnarsson U., Haglund C., and Palmqvist R.. 2019. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci. Rep. 9:19997 10.1038/s41598-019-56441-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkin S., Yuan D., Stein I., Taniguchi K., Weber A., Unger K., Browning J.L., Goossens N., Nakagawa S., Gunasekaran G., et al. 2015. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 16:1235–1244. 10.1038/ni.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremd C., Stefanovic S., Beckhove P., Pritsch M., Lim H., Wallwiener M., Heil J., Golatta M., Rom J., Sohn C., et al. 2015. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. OncoImmunology. 5:e1057387 10.1080/2162402X.2015.1057387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman W.H., Pagès F., Sautès-Fridman C., and Galon J.. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 12:298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- Fridman W.H., Zitvogel L., Sautès-Fridman C., and Kroemer G.. 2017. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14:717–734. 10.1038/nrclinonc.2017.101 [DOI] [PubMed] [Google Scholar]
- Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- Garaud S., Buisseret L., Solinas C., Gu-Trantien C., de Wind A., Van den Eynden G., Naveaux C., Lodewyckx J.-N., Boisson A., Duvillier H., et al. 2019. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 5:129641 10.1172/jci.insight.129641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg K., Maurer M., Griss J., Brüggen M.-C., Wolf I.H., Wagner C., Willi N., Mertz K.D., and Wagner S.N.. 2016. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum. Pathol. 54:157–164. 10.1016/j.humpath.2016.03.022 [DOI] [PubMed] [Google Scholar]
- Garnelo M., Tan A., Her Z., Yeong J., Lim C.J., Chen J., Lim K.H., Weber A., Chow P., Chung A., et al. 2017. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 66:342–351. 10.1136/gutjnl-2015-310814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain C., Gnjatic S., Tamzalit F., Knockaert S., Remark R., Goc J., Lepelley A., Becht E., Katsahian S., Bizouard G., et al. 2014. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 189:832–844. 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- Goc J., Germain C., Vo-Bourgais T.K.D., Lupo A., Klein C., Knockaert S., de Chaisemartin L., Ouakrim H., Becht E., Alifano M., et al. 2014. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 74:705–715. 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- Goeppert B., Frauenschuh L., Zucknick M., Stenzinger A., Andrulis M., Klauschen F., Joehrens K., Warth A., Renner M., Mehrabi A., et al. 2013. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br. J. Cancer. 109:2665–2674. 10.1038/bjc.2013.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griss J., Bauer W., Wagner C., Simon M., Chen M., Grabmeier-Pfistershammer K., Maurer-Granofszky M., Roka F., Penz T., Bock C., et al. 2019. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10:4186 10.1038/s41467-019-12160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu-Trantien C., Migliori E., Buisseret L., de Wind A., Brohée S., Garaud S., Noël G., Dang Chi V.L., Lodewyckx J.N., Naveaux C., et al. 2017. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2:91487 10.1172/jci.insight.91487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül N., and van Egmond M.. 2015. Antibody-Dependent Phagocytosis of Tumor Cells by Macrophages: A Potent Effector Mechanism of Monoclonal Antibody Therapy of Cancer. Cancer Res. 75:5008–5013. 10.1158/0008-5472.CAN-15-1330 [DOI] [PubMed] [Google Scholar]
- Hamanaka Y., Suehiro Y., Fukui M., Shikichi K., Imai K., and Hinoda Y.. 2003. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int. J. Cancer. 103:97–100. 10.1002/ijc.10801 [DOI] [PubMed] [Google Scholar]
- Harvey B.P., Raycroft M.T., Quan T.E., Rudenga B.J., Roman R.M., Craft J., and Mamula M.J.. 2014. Transfer of antigen from human B cells to dendritic cells. Mol. Immunol. 58:56–65. 10.1016/j.molimm.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesters B.A., van der Poel C.E., Das A., and Carroll M.C.. 2016. Antigen Presentation to B Cells. Trends Immunol. 37:844–854. 10.1016/j.it.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Helmink B.A., Reddy S.M., Gao J., Zhang S., Basar R., Thakur R., Yizhak K., Sade-Feldman M., Blando J., Han G., et al. 2020. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 577:549–555. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N., Ino Y., Yamazaki-Itoh R., Kanai Y., Kosuge T., and Shimada K.. 2015. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer. 112:1782–1790. 10.1038/bjc.2015.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch L., Zitvogel L., Eggermont A., and Marabelle A.. 2019. PD-Loma: a cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer. 120:3–5. 10.1038/s41416-018-0294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesia M.D., Vincent B.G., Parker J.S., Hoadley K.A., Carey L.A., Perou C.M., and Serody J.S.. 2014. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin. Cancer Res. 20:3818–3829. 10.1158/1078-0432.CCR-13-3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesia M.D., Parker J.S., Hoadley K.A., Serody J.S., Perou C.M., and Vincent B.G.. 2016. Genomic Analysis of Immune Cell Infiltrates Across 11 Tumor Types. J. Natl. Cancer Inst. 108:djw144 10.1093/jnci/djw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Leitner W.W., Golding B., and Scott D.. 2006. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 66:7741–7747. 10.1158/0008-5472.CAN-05-3766 [DOI] [PubMed] [Google Scholar]
- Ishigami E., Sakakibara M., Sakakibara J., Masuda T., Fujimoto H., Hayama S., Nagashima T., Sangai T., Nakagawa A., Nakatani Y., and Otsuka M.. 2019. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer. 26:180–189. 10.1007/s12282-018-0910-4 [DOI] [PubMed] [Google Scholar]
- Jahrsdörfer B., Blackwell S.E., Wooldridge J.E., Huang J., Andreski M.W., Jacobus L.S., Taylor C.M., and Weiner G.J.. 2006. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 108:2712–2719. 10.1182/blood-2006-03-014001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.W., Hill D.G., and Jones S.A.. 2016. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 7:401 10.3389/fimmu.2016.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalergis A.M., and Ravetch J.V.. 2002. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. J. Exp. Med. 195:1653–1659. 10.1084/jem.20020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D.R., Milne K., and Nelson B.H.. 2016. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 22:3005–3015. 10.1158/1078-0432.CCR-15-2762 [DOI] [PubMed] [Google Scholar]
- Kurtenkov O., Klaamas K., Mensdorff-Pouilly S., Miljukhina L., Shljapnikova L., and Chuzmarov V.. 2007. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 46:316–323. 10.1080/02841860601055441 [DOI] [PubMed] [Google Scholar]
- Kuwabara S., Tsuchikawa T., Nakamura T., Hatanaka Y., Hatanaka K.C., Sasaki K., Ono M., Umemoto K., Suzuki T., Sato O., et al. 2019. Prognostic relevance of tertiary lymphoid organs following neoadjuvant chemoradiotherapy in pancreatic ductal adenocarcinoma. Cancer Sci. 110:1853–1862. 10.1111/cas.14023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.W., Laskowski J., Li H.Y., McSharry M.V., Sippel T.R., Bullock B.L., Johnson A.M., Poczobutt J.M., Neuwelt A.J., Malkoski S.P., et al. 2018. Complement Activation via a C3a Receptor Pathway Alters CD4+ T Lymphocytes and Mediates Lung Cancer Progression. Cancer Res. 78:143–156. 10.1158/0008-5472.CAN-17-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladányi A., Kiss J., Somlai B., Gilde K., Fejős Z., Mohos A., Gaudi I., and Tímár J.. 2007. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol. Immunother. 56:1459–1469. 10.1007/s00262-007-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang J., Liu H., Lan T., Xu L., Wang G., Yuan K., and Wu H.. 2020a Existence of intratumoral tertiary lymphoid structures is associated with immune cells infiltration and predicts better prognosis in early-stage hepatocellular carcinoma. Aging (Albany NY). 12:3451–3472. 10.18632/aging.102821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Guo Q., Zhang X., Dong X., Liu W., Zhang A., Li Y., Yan J., Jia G., Zheng Z., et al. 2020b Oral cancer-associated tertiary lymphoid structures: gene expression profile and prognostic value. Clin. Exp. Immunol. 199:172–181. 10.1111/cei.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Tao P., Wang J., Ma L., Jiang Q., Li J., Zhang G., Liu J., Zhang Y., Hou Y., et al. 2020. Tumor-associated tertiary lymphoid structure predicts postoperative outcomes in patients with primary gastrointestinal stromal tumors. OncoImmunology. 9:1747339 10.1080/2162402X.2020.1747339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Zhao Q., Liao J.-Y., Song E., Xia Q., Pan J., Li Y., Li J., Zhou B., Ye Y., et al. 2020. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell. 180:1081–1097.e24. 10.1016/j.cell.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Mahmoud S.M.A., Lee A.H.S., Paish E.C., Macmillan R.D., Ellis I.O., and Green A.R.. 2012. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 132:545–553. 10.1007/s10549-011-1620-1 [DOI] [PubMed] [Google Scholar]
- Medler T.R., Murugan D., Horton W., Kumar S., Cotechini T., Forsyth A.M., Leyshock P., Leitenberger J.J., Kulesz-Martin M., Margolin A.A., et al. 2018. Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell. 34:561–578.e6. 10.1016/j.ccell.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan M., Petitprez F., Lacroix L., Di Tommaso L., Roncalli M., Bougoüin A., Laurent A., Amaddeo G., Sommacale D., Regnault H., et al. 2020. Early hepatic lesions display immature tertiary lymphoid structures and show elevated expression of immune inhibitory and immunosuppressive molecules. Clin. Cancer Res. 26:4381–4389. 10.1158/1078-0432.CCR-19-2929 [DOI] [PubMed] [Google Scholar]
- Milne K., Köbel M., Kalloger S.E., Barnes R.O., Gao D., Gilks C.B., Watson P.H., and Nelson B.H.. 2009. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 4:e6412 10.1371/journal.pone.0006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montfort A., Pearce O., Maniati E., Vincent B.G., Bixby L., Böhm S., Dowe T., Wilkes E.H., Chakravarty P., Thompson R., et al. 2017. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 23:250–262. 10.1158/1078-0432.CCR-16-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyron-Quiroz J.E., Rangel-Moreno J., Hartson L., Kusser K., Tighe M.P., Klonowski K.D., Lefrançois L., Cauley L.S., Harmsen A.G., Lund F.E., and Randall T.D.. 2006. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 25:643–654. 10.1016/j.immuni.2006.08.022 [DOI] [PubMed] [Google Scholar]
- Murakami Y., Saito H., Shimizu S., Kono Y., Shishido Y., Miyatani K., Matsunaga T., Fukumoto Y., Ashida K., Sakabe T., et al. 2019. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci. Rep. 9:13083 10.1038/s41598-019-49581-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar S., Campos J., Chung M.M., Navarro-Núñez L., Chachlani M., Steinthal N., Gardner D.H., Rankin P., Cloake T., Caamaño J.H., et al. 2016. Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin α1β2 in Newly Formed Tertiary Lymphoid Structures. J. Immunol. 197:1957–1967. 10.4049/jimmunol.1500686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.S., Sahota R.A., Milne K., Kost S.E., Nesslinger N.J., Watson P.H., and Nelson B.H.. 2012. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 18:3281–3292. 10.1158/1078-0432.CCR-12-0234 [DOI] [PubMed] [Google Scholar]
- Pavoni E., Monteriù G., Santapaola D., Petronzelli F., Anastasi A.M., Pelliccia A., D’Alessio V., De Santis R., and Minenkova O.. 2007. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 7:70 10.1186/1472-6750-7-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitprez F., de Reyniès A., Keung E.Z., Chen T.W.-W., Sun C.-M., Calderaro J., Jeng Y.-M., Hsiao L.-P., Lacroix L., Bougoüin A., et al. 2020. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 577:556–560. 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- Phan T.G., Grigorova I., Okada T., and Cyster J.G.. 2007. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8:992–1000. 10.1038/ni1494 [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Jones G.W., Bombardieri M., and Jones S.A.. 2014. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14:447–462. 10.1038/nri3700 [DOI] [PubMed] [Google Scholar]
- Platonova S., Cherfils-Vicini J., Damotte D., Crozet L., Vieillard V., Validire P., André P., Dieu-Nosjean M.-C., Alifano M., Régnard J.-F., et al. 2011. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 71:5412–5422. 10.1158/0008-5472.CAN-10-4179 [DOI] [PubMed] [Google Scholar]
- Posch F., Silina K., Leibl S., Mündlein A., Moch H., Siebenhüner A., Samaras P., Riedl J., Stotz M., Szkandera J., et al. 2017. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. OncoImmunology. 7:e1378844 10.1080/2162402X.2017.1378844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z., Richter G., Schüler T., Ibe S., Cao X., and Blankenstein T.. 1998. B cells inhibit induction of T cell-dependent tumor immunity. Nat. Med. 4:627–630. 10.1038/nm0598-627 [DOI] [PubMed] [Google Scholar]
- Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 348:124–128. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumenina L.T., Daugan M.V., Noé R., Petitprez F., Vano Y.A., Sanchez-Salas R., Becht E., Meilleroux J., Clec’h B.L., Giraldo N.A., et al. 2019a Tumor Cells Hijack Macrophage-Produced Complement C1q to Promote Tumor Growth. Cancer Immunol. Res. 7:1091–1105. 10.1158/2326-6066.CIR-18-0891 [DOI] [PubMed] [Google Scholar]
- Roumenina L.T., Daugan M.V., Petitprez F., Sautès-Fridman C., and Fridman W.H.. 2019b Context-dependent roles of complement in cancer. Nat. Rev. Cancer. 19:698–715. 10.1038/s41568-019-0210-0 [DOI] [PubMed] [Google Scholar]
- Santoiemma P.P., Reyes C., Wang L.-P., McLane M.W., Feldman M.D., Tanyi J.L., and Powell D.J. Jr. 2016. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 143:120–127. 10.1016/j.ygyno.2016.07.105 [DOI] [PubMed] [Google Scholar]
- Sautès-Fridman C., and Roumenina L.T.. 2020. B cells and complement at the forefront of chemotherapy. Nat. Rev. Clin. Oncol. 17:393–394. 10.1038/s41571-020-0376-0 [DOI] [PubMed] [Google Scholar]
- Sautès-Fridman C., Petitprez F., Calderaro J., and Fridman W.H.. 2019. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 19:307–325. 10.1038/s41568-019-0144-6 [DOI] [PubMed] [Google Scholar]
- Selitsky S.R., Mose L.E., Smith C.C., Chai S., Hoadley K.A., Dittmer D.P., Moschos S.J., Parker J.S., and Vincent B.G.. 2019. Prognostic value of B cells in cutaneous melanoma. Genome Med. 11:36 10.1186/s13073-019-0647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow D.Y.B., Yeong J.P.S., Lim J.X., Chia N., Lim J.C.T., Ong C.C.H., Tan P.H., and Iqbal J.. 2020. Tertiary lymphoid structures and associated plasma cells play an important role in the biology of triple-negative breast cancers. Breast Cancer Res. Treat. 180:369–377. 10.1007/s10549-020-05548-y [DOI] [PubMed] [Google Scholar]
- Shah S., Divekar A.A., Hilchey S.P., Cho H.-M., Newman C.L., Shin S.-U., Nechustan H., Challita-Eid P.M., Segal B.M., Yi K.H., and Rosenblatt J.D.. 2005. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int. J. Cancer. 117:574–586. 10.1002/ijc.21177 [DOI] [PubMed] [Google Scholar]
- Shalapour S., Lin X.-J., Bastian I.N., Brain J., Burt A.D., Aksenov A.A., Vrbanac A.F., Li W., Perkins A., Matsutani T., et al. 2017. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 551:340–345. 10.1038/nature24302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., and Allison J.P.. 2015. The future of immune checkpoint therapy. Science. 348:56–61. 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- Shen P., and Fillatreau S.. 2015. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15:441–451. 10.1038/nri3857 [DOI] [PubMed] [Google Scholar]
- Shi J.-Y., Gao Q., Wang Z.-C., Zhou J., Wang X.-Y., Min Z.-H., Shi Y.-H., Shi G.-M., Ding Z.-B., Ke A.-W., et al. 2013. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 19:5994–6005. 10.1158/1078-0432.CCR-12-3497 [DOI] [PubMed] [Google Scholar]
- Siliņa K., Soltermann A., Attar F.M., Casanova R., Uckeley Z.M., Thut H., Wandres M., Isajevs S., Cheng P., Curioni-Fontecedro A., et al. 2018. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 78:1308–1320. 10.1158/0008-5472.CAN-17-1987 [DOI] [PubMed] [Google Scholar]
- Stavnezer J., and Kang J.. 2009. The surprising discovery that TGF beta specifically induces the IgA class switch. J. Immunol. 182:5–7. 10.4049/jimmunol.182.1.5 [DOI] [PubMed] [Google Scholar]
- Su S., Zhao J., Xing Y., Zhang X., Liu J., Ouyang Q., Chen J., Su F., Liu Q., and Song E.. 2018. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell. 175:442–457.e23. 10.1016/j.cell.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Sylvestre D., Clynes R., Ma M., Warren H., Carroll M.C., and Ravetch J.V.. 1996. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J. Exp. Med. 184:2385–2392. 10.1084/jem.184.6.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.-T., and Coussens L.M.. 2007. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 19:209–216. 10.1016/j.coi.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Tran E., Robbins P.F., and Rosenberg S.A.. 2017. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat. Immunol. 18:255–262. 10.1038/ni.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen C.M.L., van der Voort R., van der Laak J.A.W.M., Klasen I.S., de Graaf A.O., van Kempen L.C.L., de Vries I.J.M., Boer T.D., Dolstra H., Torensma R., et al. 2008. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int. J. Cancer. 123:2354–2361. 10.1002/ijc.23756 [DOI] [PubMed] [Google Scholar]
- Welinder C., Jirström K., Lehn S., Nodin B., Marko-Varga G., Blixt O., Danielsson L., and Jansson B.. 2016. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon. 2:e00143 10.1016/j.heliyon.2016.e00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters M.C.A., and Nelson B.H.. 2018. Prognostic Significance of Tumor-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 24:6125–6135. 10.1158/1078-0432.CCR-18-1481 [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y., Tanaka H., Sakimura C., Deguchi S., Mori T., Tamura T., Toyokawa T., Muguruma K., Hirakawa K., and Ohira M.. 2020. Immunological potential of tertiary lymphoid structures surrounding the primary tumor in gastric cancer. Int. J. Oncol. 57:171–182. 10.3892/ijo.2020.5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong J., Lim J.C.T., Lee B., Li H., Chia N., Ong C.C.H., Lye W.K., Putti T.C., Dent R., Lim E., et al. 2018. High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer. Front. Immunol. 9:1209 10.3389/fimmu.2018.01209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirakzadeh A.A., Sherif A., Rosenblatt R., Ahlén Bergman E., Winerdal M., Yang D., Cederwall J., Jakobsson V., Hyllienmark M., Winqvist O., and Marits P.. 2020. Tumour-associated B cells in urothelial urinary bladder cancer. Scand. J. Immunol. 91:e12830 10.1111/sji.12830 [DOI] [PubMed] [Google Scholar]