Abstract
A 9-week feeding trial was conducted with juvenile red drum, Sciaenops ocellatus, to evaluate the use of soy oil as a fish oil replacement. Three primary protein sources (fishmeal - FM, soybean meal - SBM, and soy protein concentrate - SPC) were utilized with 100% fish oil (FM, SBM, SPC), 75% fish oil (SBM, SPC), or 50% fish oil (FM, SBM, SPC) as the lipid source. Traditional growth and performance metrics (specific growth rate, feed consumption, feed conversion ratio) were tracked and tissue samples (liver, muscle, plasma, adipose, and brain) were collected for gas chromatography-based fatty acid profiling. Ten lipid metabolism related genes were analyzed for potential expression differences between dietary treatments in liver and muscle tissues and whole body and fillet tissues were sampled for proximate composition analyses. Forty- four fatty acids were measured by gas chromatography-flame ionization detector (GC-FID) and evaluated with principle component analysis and ANOVA to understand the dietary influence on lipid metabolism and health. Significant differences in growth rate were observed with the SBM 50% fish oil diet outperforming the FM 100% fish oil reference diet. All other soy protein-based diets performed statistically equivalent to both FM reference diets (100% and 50% fish oil) in regard to growth, however all soy protein-based formulations had significantly lower feed conversion ratios than the fishmeal-based references (p < .001). Gene expression differences were not significant in most cases, however often trended similarly as the observed performance. Fatty acid profiles differed as a function of oil source, with no apparent influence by protein source, with C18:2n-6 (linoleic acid) being-the primary differentiator. Overall, the six soy protein, fishmeal-free formulations performed equivalently or better than the fishmeal references with up to 50% of fish oil replaced by soybean oil.
Keywords: Red drum, Fishmeal replacement, Fish oil replacement, Soy utilization, Fatty acid profiles
1. Introduction
As the world population increasingly relies on aquaculture for sources of healthy protein, the industry as a whole is transitioning away from feed ingredients with finite production and increasing costs, such as fishmeal and fish oil, to those that are more sustainably produced, environmentally friendly, and most importantly less expensive for the industry. Replacing fishmeal and fish oil as the primary protein and fat sources in feeds has been a high priority for research and production efforts for over a decade (FAO, 2018, 2016; Olsen and Hasan, 2012; Turchini et al., 2011). The utilization of soybeans as protein sources in various forms (hulled, de-hulled, meal, protein concentrates) and as a fat source (soy oil) has drastically increased due to soy’s reliable production levels, affordability, amino and fatty acid profiles, and palatability for most species (Casu et al., 2019; Davis and Arnold, 2004.; Krogdahl et al., 2003; Salze et al., 2010; Watson et al., 2014). However, every species reacts differently to soy and impacts to digestibility, palatability, and tolerance levels have been reported that can range from not detectable, to levels that prevent the use of high levels of soy-based ingredients (Bansemer et al., 2015; Sahlmann et al., 2013; Urán et al., 2008).
Red drum, Sciaenops ocellatus, is a euryhaline, eurythermal, relatively hardy, marine species that is an optimal candidate for aquaculture. The species is currently produced in several countries and is a prime candidate for the expansion of off-shore culture in the Gulf of Mexico. Several studies have examined the potential of soy protein products to be utilized by this species as a fishmeal replacement (Casu et al., 2017; Davis et al., 1995; McGoogan and Gatlin, 1997; Moxley et al., 2014; Watson et al., 2019) but few have examined the use of soybean oil as a fish oil replacement (Tucker et al., 1997).
The objective of this study was to evaluate the use of soybean oil as a fish oil replacement in fishmeal-free dietary formulations that rely on high levels of soy protein. Soy oil has been used to successfully replace fish oil as a lipid sources in feeds at varying inclusion levels (~1–33%) for serval species in aquaculture (Chou et al., 2004; González-félix et al., 2015; Sissener et al., 2009; Trushenski et al., 2011). Three basal diets were examined, a fishmeal-based reference feed with either 100% or 50% of the lipid being supplied by fish oil, a soybean meal formulation with three graded levels of soy oil inclusion (0, 25, and 50%), and a soy protein concentrate formulation with three graded levels of soy oil inclusion (0, 25, and 50%). Evaluation was based on the utilization of standard aquaculture metrics (growth rate, feed conversion ratios, proximate compositions); gas chromatography fatty acid profiling of plasma, liver, adipose, brain, and muscle tissues; and quantitative polymerase chain reaction (qPCR) analysis of lipid metabolism related genes. Combining traditional metrics with fatty acid profiling and gene expression assays provided a more in-depth analysis of the physiological response this species undergoes when fed alternative ingredients and may provide insight into how to further supplement and formulate feeds to optimize performance in this species.
2. Materials and methods
2.1. Diets
A nine-week feeding trial was conducted on juvenile red drum utilizing the highest performing SBM and SPC products from our previous work (Casu et al., 2019; Watson et al., 2019) incorporated into high inclusion level, practical formulations (36 g soy ingredient 100 g−1 diet, 47.0% crude protein (CP) and 10.6% crude lipid (CL)). In addition to soy protein, graded soybean oil inclusion at three levels (0%, 25%, and 50% of total added lipid) were utilized for each soy protein source to examine the effects of soy oil on key growth and production characteristics (growth rate, feed conversion ratio) as well as the fatty acid profile. A total of eight experimental diets were evaluated including two reference diets: a traditional fishmeal-based formulation with 100% fish oil and the same fishmeal formulation with a 50% inclusion level of soy oil. Alterations in squid meal, poultry meal, and wheat flour, to account for the reduction of fishmeal, were made to ensure diets were iso-nitrogenous and isolipidic (Table 1). The diets were produced with commercial manufacturing methods using a twin-screw cooking extruder (DNDL-44, Buhler AG, Uzwil, Switzerland) at the Bozeman Fish Technology Center, Bozeman, MT. The extruded mash was exposed to an average of 116 °C for 18-s in five barrel sections, and the last section was water cooled to an average temperature of 17 °C. Screws were rotating at 509 rpm. Steam was vented off in the last barrel section before the die head increasing pressure at the die head to approximately 29.8 bar (432 psi). The pellets were then dried in a pulse bed drier (Buhler AG) for 25 min at 102 °C and cooled at ambient air temperatures to reach final moisture levels of < 10%. Fish oil was top-dressed using vacuum coating (A.J Flauer Mixing, Ontario Canada) after the pellets were cooled. Diets were stored in plastic lined paper bags at room temperature until used. All diets were fed within four months of manufacture.
Table 1.
Composition of experimental diets for juvenile red drum.
| Grams 100 g−1 | FM 100%FO | FM 50% FO | SBM 100% FO | SBM 75% FO | SBM 50% FO | SPC 100% FO | SPC 75% FO | SPC 50% FO |
|---|---|---|---|---|---|---|---|---|
| Fish meal SeaPro 75 a | 36.50 | 36.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soybean meal (SBM) b | 0.00 | 0.00 | 36.00 | 36.00 | 36.00 | 0.00 | 0.00 | 0.00 |
| Soy protein concentrate c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 36.00 | 36.00 | 36.00 |
| Corn gluten meal 60% d | 9.20 | 9.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Poultry meal e | 0.00 | 0.00 | 9.139 | 9.139 | 9.139 | 3.147 | 3.147 | 3.147 |
| Wheat flour f | 37.45 | 37.45 | 20.602 | 20.602 | 20.602 | 24.5 | 24.5 | 24.5 |
| Fish Oil g | 7.50 | 3.75 | 8.60 | 6.45 | 4.30 | 10.50 | 7.875 | 5.25 |
| Soybean Oil h | 0.00 | 3.75 | 0.00 | 2.15 | 4.30 | 0.00 | 2.625 | 5.25 |
| Mixed nut meal i | 1.80 | 1.80 | 0.00 | 0.00 | 0.00 | 3.133 | 3.133 | 3.133 |
| Squid meal j | 1.00 | 1.00 | 15.414 | 15.414 | 15.414 | 12.00 | 12.00 | 12.00 |
| Taurine | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 | 1.70 |
| Lysine HCl | 0.85 | 0.85 | 2.20 | 2.20 | 2.20 | 2.30 | 2.30 | 2.30 |
| DL-Methionine | 0.10 | 0.10 | 0.81 | 0.81 | 0.81 | 0.82 | 0.82 | 0.82 |
| Threonine | 0.00 | 0.00 | 0.635 | 0.635 | 0.635 | 0.70 | 0.70 | 0.70 |
| Mono-Dical phosphate | 2.00 | 2.00 | 3.00 | 3.00 | 3.00 | 3.30 | 3.30 | 3.30 |
| Vitamin premix k | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline CL | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 |
| Vitamin C l | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Trace min premix m | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Analyzed composition, % as-is | ||||||||
| Dry matter | 93.6 | 92.3 | 93.3 | 91.9 | 91.6 | 92.7 | 93.6 | 92.9 |
| Crude protein | 48.4 | 49.3 | 46.1 | 45.5 | 45.6 | 47.1 | 46.7 | 47.5 |
| Crude lipid | 8.7 | 8.7 | 10.8 | 10.8 | 10.8 | 11.9 | 11.7 | 11.0 |
| Phosphorus | 0.85 | 0.88 | 1.41 | 1.45 | 1.41 | 1.37 | 1.34 | 1.31 |
BioOREGON Protein.
ADM, 468 g/kg crude protein.
Nutrivance.
Rangen Feeds.
Rangen Feeds
IDF Inc., 832 g/kg protein.
Manildra Milling, 120 g/kg crude protein.
Omega Proteins Inc., Virginia Prime menhaden oil.
Jedwards, Inc.
Adaptive Bio Resources.
ARS 702; contributed, per kg diet; vitamin A 9650 IU; vitamin D 6600 IU; vitamin E 132 IU; vitamin K3 1.1 g: thiamin mononitrate 9.1 mg; riboflavin 9.6 mg; pyridoxine hydrochloride 13.7 mg; pantothenate DL‑calcium 46.5 mg; cyancobalamin 0.03 mg; nicotinic acid 21.8 mg; biotin 0.34 mg; folic acid 2.5 mg; inositol 600 mg.
Stay-C, 35%, DSM Nutritional Products.
Contributed in mg/kg of diet; manganese 13; iodine 5; copper 9; zinc 40.
2.2. Fish and experimental design
Juvenile red drum were obtained from South Carolina Department of Natural Resources stock enhancement program. All fish were from captive, wild red drum broodstock volitionally spawned at the Marine Resources Research Institute (MRRI) in Charleston, South Carolina. Larval fish from a single spawning event were transported and stocked into earthen ponds at the Waddell Mariculture Center (WMC) in Bluffton, South Carolina, in late summer of 2016. Fish were then transported back to the MRRI in the fall of 2016 at approximately 35-mm average length and cultured in a recirculating aquaculture system consisting of twenty-four 1600-l tanks utilizing drum filters, fluidized bed filters, protein fractionation for mechanical and biological filtration, and UV sterilizers. Fish were fed to apparent satiation twice daily with a standard commercial feed (40% CP and 10% CL) with excess feed removed from tanks after ten minutes of no visible feeding. Fish were acclimated from natural pond conditions to 25 °C over a period of two weeks at 0.5 °C day−1 and held at a salinity of 28–30 parts per thousand (‰) for the remainder of the experiment. Tank density was reduced to 27 fish per tank when a mean weight of ≈80 g per fish was obtained. Fish were fed Diet #1 as a conditioning diet for approximately two months prior to the initiation of the trial.
Ten fish were euthanized using tricaine methanesulfonate (MS-222, Argent Labs) at a concentration of 500 mg L−1 buffered with sodium bicarbonate for analysis of whole-body and fillet composition (dry matter (DM), CP, lipids, ash, gross energy, minerals) preceding the beginning of the experimental feeding trial. The total number of fish per tank was reduced to twenty-five with a mean individual weight of 204.9 g ± 5.8 g (SD). The eight diets were randomly assigned to three tanks per treatment. Total tank biomass was recorded on Day 0 and every three weeks until the conclusion of the trial.
On day 1, fish were transitioned to experimental diets and fed to apparent satiation twice daily or once daily on weekends, and total feed weight consumed was recorded. Any excess feed was removed from the system after 10 min by siphon. Water temperature, dissolved oxygen, pH, and salinity were recorded twice weekly on a subset of tanks (n = 6 tanks/sampling) and ammonia, nitrite and nitrate measured weekly (n = 6 tanks/week) using Hach reagents on a Hach DR3900 spectrophotometer (Hach Inc., Loveland, CO, USA). Water quality parameters were monitored and recorded throughout the trial (average ± SD): temperature, 24.80 ± 0.05 °C; dissolved oxygen, 5.14 ± 0.33 mg L−1; salinity, 28.31 ± 0.77‰; pH, 7.17 ± 0.10; ammonia, 0.19 ± 0.12 mg L−1; nitrite, 0.062 ± 0.016 mg L−1; nitrate, 5.9 ± 1.3 mg L−1. Additional fish (n = 20 fish/treatment) were sacrificed at the conclusion of the nine-week growth trial for whole-body and fillet composition. Proximate analysis of individual diet, fillet, and whole-body samples was performed by Clemson University Feed and Forage Laboratory, Clemson, South Carolina.
At the conclusion of the feeding trial, three fish per tank were randomly selected and anesthetized with MS-222 for individual weights and lengths. Blood samples were collected using a heparinized vacutainer drawn from the caudal vein midline just posterior of the anal fin. Fish were then euthanized with a lethal dose of MS-222 for 3 min prior to further dissection. The liver, brain, and a portion of the adipose were excised, rinsed clean with cold 3% saline solution, placed into labeled 5 mL cryovials, flash frozen in liquid nitrogen, and stored at −80 °C. The fish were then fully eviscerated and carcass weight recorded.
2.3. Fatty acid profiling
Seventy-two (nine replicates of eight diets) samples of muscle, plasma, and liver, and twenty-four (three replicates of eight diets) samples of adipose, brain, and feed pellets were cryohomogenized using a Retsch Cryomill (RETSCH GmbH, Haan Germany). Excess material for each tissue type was collected for a “pooled” quality control (QC) material. Lipids were extracted using a modified Bligh-Dyer extraction from the sample matrices and quality control materials with specified weights and internal standard volumes (Table S1) (Bligh and Dyer, 1959; Ostermann et al., 2014). Lipids were derivatized to fatty acid methyl esters (FAMEs) via acid-catalyzed hydrolysis and reaction with methyl acetate at 95 °C for 1 h (Lepage and Roy, 1986; Ostermannet al., 2014).
A gas chromatograph with flame ionization detector (GC-FID) (5890 N, Agilent, Santa Clara, CA, USA), an Rt-2560 GC column (100 m × 0.25 mm, 0.20-μm film thickness, Restek, Bellefonte, PA, USA) with a deactivated guard column (Siltek 10,026, 5 m × 0.25 mm, Restek), and wool-packed, focusing split liner (210–4004–5, Agilent, Santa Clara, CA, USA) was used for all experiments. Oven gradient was: initial 100 °C, hold 4 min; ramp 3.0 °C min−1; final 240 °C, hold 15 min. Other parameters included injector and detector temperatures at 225 °C and 285 °C, respectively; 2 μL injection volume; split ratio, 24:1; with helium as carrier gas at constant flow, 1.0 mL min−1 and velocity, 18 cm s−1.
FAME standards mix (GLC-463 Nu-Chek Prep, Elysian, MN, USA) supplemented with FAMEs of C18:2n-9 (1256, Matreya, State College, PA, USA); C21:0 (N-21-M, Nu-Chek Prep); C22:1n-9 (U-80-M, Nu-Chek Prep); C22:5n-6 (U-102-M, Nu-Chek Prep) were used for retention time alignment. Internal standard of C13:0 triglyceride (Nu-Chek Prep, T-135) was added pre-extraction (Table S1). Matrix-similar materials (to experimental biofluid/tissue) for both validation and quality controls consisted of NIST (Gaithersburg, MD, USA) SRM 1950 Metabolites in Frozen Human Plasma (plasma), SRM 1947 Lake Michigan Fish Tissue (muscle, liver, brain, adipose), and SRM 3290 Dry Cat Food (feed). Data processing for response factors and percentages for fatty acids and total fat followed previously reported methods (AOAC, 2002). Briefly, detector response factors were calculated by multiplication of the weight-to-peak area ratio of C13:0 to that of each FAME, which were then used to normalize calculations for experimental peak area ratios of FAME-to-internal standard. The calculated weight of each FAME was then converted to free fatty acid form by a conversion factor based upon respective molecular weights.
2.4. Gene expression
Homogenized sub-samples of liver and muscle from the conclusion of the feeding trial were utilized for gene expression assays. RNA was extracted with the Aurum total RNA fatty and fibrous tissue kit (Bio-Rad, Hercules, CA, USA), reconstituted into 50 μL water, and quantified using a Quantus fluorometer (Promega, Madison, WI, USA). RNA (1000 ng) from each sample was used in a reverse transcription reaction (iScript RT Supermix, Bio-Rad, Hercules, CA, USA) and diluted to 10 ng μL−1.
A total of ten target genes were analyzed (Table 2) with elongation factor 1-alpha (EF1A) used as a common reference gene across all qPCR panels, multiplex and singleplex. Delta 4-desaturase sphingolipid 1 (DEGS1), fatty acid synthase (FASN), and peroxisome proliferator activated receptor alpha (PPARA) were analyzed individually using Sso Advanced SYBR Green (Bio-Rad, Hercules, CA, USA) with 800-μM primer concentrations in 10-μL reaction volumes. Acyl-coenzyme A thioesterase 1 (ACOT1), Acyl-coenzyme A oxidase 1 (ACOX1), apolipoprotein A-IV (APOA4), Carnitine O-palmitoyltransferase 1 (CPT1), elongation of very long chain fatty acids 1 (ELOVL1), glucose-6-phosphate isomerase (G6PI), and glycerol kinase 5 (GK5) were analyzed in two multiplexed reaction sets using iQ Multiplex Powermix (Bio-Rad, Hercules, CA, USA) with 800 μM primer and 400 μM probe concentrations in 10 μL reaction volumes. All samples were run with 10 ng total RNA in triplicate on a Bio-Rad CFX96 Touch Real Time PCR Detection system (Bio-Rad, Hercules, CA, USA).
Table 2.
Primer sequences and accession numbers for genes utilized in singleplex and multiplex expression assays of liver and muscle tissue of juvenile red drum.
| Gene name | Primer sequence | Tm (°C) | Accession number |
|---|---|---|---|
| Acyl-coenzyme A Thioesterase 1 (ACOT1) Forward | 5′-GGGACGTTTCCTCTTCCTGG-3′ | 62.5 | MH660389 |
| Acyl-coenzyme A Thioesterase 1 (ACOT1) Reverse | 5′-TAATGTCCTGCTCCGGGGTA-3′ | 60.5 | |
| Acyl-coenzyme A Thioesterase 1 (ACOT1) Probe | 5′-Cy55-ACGAGATGGTGGAGCGGCTG-IAbRQSp-3′ | 64.6 | |
| Acyl-coenzyme A Oxidase 1 (ACOX1) Forward | 5′-GCTGTTCGTCACCAGTCTGA-3′ | 60.5 | MH660390 |
| Acyl-coenzyme A Oxidase 1 (ACOX1) Reverse | 5′-GCGGTGGTAAGTTTCCCTCA-3′ | 60.5 | |
| Acyl-coenzyme A Oxidase 1 (ACOX1) Probe | 5′-TYE665-TCCAGGAGAGCCAGAGCCCC-IAbRQSp-3′ | 66.6 | |
| Apolipoprotein A-IV (APOA4) Forward | 5′-TGAGGCAAGGTGACAACAAG-3′ | 59.9 | KX171009 |
| Apolipoprotein A-IV (APOA4) Reverse | 5′-CAGCAAGGACCTTCATGGTT-3′ | 60.1 | |
| Apolipoprotein A-IV (APOA4) Probe | 5′−6-FAM-CCAAGTGCTCCCAGGTGGAC-IABkFQ-3′ | 65.3 | |
| Carnitine O-palmitoyltransferase 1 (CPT1) Forward | 5′-CAGCTTGGCTATCAACCCCA-3′ | 60.5 | MH660394 |
| Carnitine O-palmitoyltransferase 1 (CPT1) Reverse | 5′-CTACCTCTCTGGCCTCCACT-3′ | 62.5 | |
| Carnitine O-palmitoyltransferase 1 (CPT1) Probe | 5′-HEX-AGGGCTCCCTGGGTACACTCC-IAbRQSp-3′ | 67.3 | |
| Elongation of Very Long Chain Fatty Acids 1 (ELOVL1) Forward | 5′-CTTCGTGCTGAGGAAAAAGC-3′ | 60.1 | KX171014 |
| Elongation of Very Long Chain Fatty Acids 1 (ELOVL1) Reverse | 5′-CATGATCACGTGGACGGTAG-3′ | 60.0 | |
| Elongation of Very Long Chain Fatty Acids 1 (ELOVL1) Probe | 5′-HEX-TTCCTATGCCCCTGGTGGAA-IABkFQ-3′ | 64.8 | |
| Glucose-6-Phosphate Isomerase (G6PI) Forward | 5′-GGAACGTTCTGATCCAGAGG-3′ | 59.7 | KX171015 |
| Glucose-6-Phosphate Isomerase (G6PI) Reverse | 5′-GACTGGGTTGGAGGTCGTTA-3′ | 60.0 | |
| Glucose-6-Phosphate Isomerase (G6PI) Probe | 5′-HEX-ACGTGCAGAGCGATGGACAG-IABkFQ-3′ | 64.9 | |
| Glycerol Kinase 5 (GK5) Forward | 5′-TCATGAAGGTGCCTGTTCCC-3′ | 60.5 | MH592587 |
| Glycerol Kinase 5 (GK5) Reverse | 5′-ACCCGTCCATCTTTGGCATT-3′ | 58.4 | |
| Glycerol Kinase 5 (GK5) Probe | 5′−6-FAM-ACATGGCTGCCTGCTGGTCC-IAbRQSp-3′ | 64.6 | |
| Delta 4-desaturase, Sphingolipid 1 (DEGS1) Forward | 5′-TCACCTTCAACGTGGGCTAC-3′ | 60.5 | MH660393 |
| Delta 4-desaturase, Sphingolipid 1 (DEGS1) Reverse | 5′-TACAGGACCCTCAGCCATGA-3′ | 60.5 | |
| Fatty Acid Synthase (FASN) Forward | 5′-ACCAGATCCCCCTCCAGTAC-3′ | 62.5 | MH660391 |
| Fatty Acid Synthase (FASN) Reverse | 5′-CTCTCAGGCTCATTGTGGCA-3′ | 60.5 | |
| Peroxisome Proliferator Activated Receptor Alpha (PPARA) Forward | 5′-GGGGACACAGTGTGGAAGAG-3′ | 62.5 | MH660392 |
| Peroxisome Proliferator Activated Receptor Alpha (PPARA) Reverse | 5′-TGCCCACTCCCTACTTAGCT-3′ | 60.5 | |
| Translation Elongation Factor 1-alpha (EF1A) Forward | 5′-GCTCGTTTCGAGGAAATCAC-3′ | 59.8 | KJ958539 |
| Translation Elongation Factor 1-alpha (EF1A) Reverse | 5′-CATCCCTTGAACCAGCTCAT-3′ | 60.1 | |
| Translation Elongation Factor 1-alpha (EF1A) Probe | 5′-TEX615-ATGGCACGGAGACAACATGC-IAbRQSp-3′ | 64.8 |
2.5. Calculations and statistical analyses
Standard performance parameters utilized in this feeding trial to compare treatments were:
Weight gain, % = (final weight − initial weight) / initial weight × 100
Specific growth rate, SGR = ln (final weight − initial weight) / (days × 100)
Feed Intake, FI = average total feed consumed per fish throughout trial (g)
Feed conversion ratio, FCR = grams fed / grams weight gained
Condition factor, K = (weight (g) × 100) / (length (cm))3
Hepatosomatic index, HSI = (liver weight / body weight) × 100
The effects of experimental treatments were compared using ANOVA with Tukey’s post-hoc analyses, as needed, within R statistical software (v3.0.2, Core, 2013) to examine the effects of the protein source and lipid ratios on performance with significance set to p < .05.
Multivariate statistical analyses were performed for fatty acid profiles using principal component analysis (PCA) with MetaboAnalyst 4.0 (Chong et al., 2018) by comparing the eight experimental diets among each matrix and then further subgrouping of diets by protein source (i.e., FM, SBM, SPC) or percentage of fish oil (i.e., 0%, 25%, 50%). Following common practices detailed by van den Berg et al. (2006), features with greater than 50% of values missing were omitted. Missing values were replaced by small-value imputation, which is equivalent to half of the minimum positive value in the entire data array, and all data were scaled using the pareto method. Three outliers were removed due to unrecoverable errors during either sample collection or handling: one from muscle and two from plasma. For PCA scores plots with visually ambiguous separation, Microsoft Excel was used to perform a two-sample Student’s t-test assuming unequal variance using the scores for the compared groups. Precision (coefficient of variance) was calculated for each technical replicate (pooled QCs SRMs) among the different matrices (Table S2), to ensure that technical variance (due to either method or instrumental factors) and measurement performance was sound (Parsons et al., 2009). A one-way ANOVA followed by a Tukey HSD post-hoc test were performed to test for significant differences in fatty acids among diets using R statistical software (v3.0.2, Core, 2013).
Statistics to evaluate significant differences in gene expression, as compared to the reference diet, were run utilizing the Bio-Rad CFXMaestro software.
3. Results
3.1. Feeding trial performance results
Traditional performance metrics (SGR, weight gain, FCR, K and HSI) are presented in Table 3 (Proximate compositions are presented in Tables S3 and S4). The most significant result is that the fishmeal-based reference formulation with 100% FO was the lowest performing diet overall with significantly lower SGR (0.93 ± 0.05), weight gain (79.41 ± 5.79%), and FCR (1.63 ± 0.07) (mean ± SD) than at least one soy protein-based formulation. Noticeably, both fishmeal formulations had significantly higher FCR’s than all tested soy-based formulations (R, ANOVA, p < .001). There were no significant differences in K (R, ANOVA, p = .052) or HSI (R, ANOVA, p = .173) between diets.
Table 3.
Performance characteristics from 9-week feeding trial with juvenile red drum. Values with different superscripts Parsons et al., 2009 are significantly different from one another (mean ± SD, R, ANOVA, p < .05).
| Diet | Initial Weight (g) | SGR1 | Weight Gain (%)2 | FI3 | FCR4 | K5 | HSI6 |
|---|---|---|---|---|---|---|---|
| FM 100%FO | 205.27 ± 9.5 | 0.93 ± 0.05a | 79.41 ± 5.79a | 314.89 ± 21.09a | 1.63 ± 0.07a | 1.24 ± 0.15 | 2.35 ± 0.60 |
| FM 50%FO | 207.46 ± 2.7 | 0.94 ± 0.01a,b | 81.28 ± 1.54a | 380.24 ± 12.15b | 1.89 ± 0.04b | 1.26 ± 0.09 | 2.73 ± 0.56 |
| SBM 100%FO | 210.49 ± 3.7 | 1.11 ± 0.07a,b | 101.57 ± 8.52a,b | 327.03 ± 7.43a | 1.30 ± 0.06c | 1.37 ± 0.11 | 2.50 ± 1.05 |
| SBM 75%FO | 203.05 ± 5.5 | 1.03 ± 0.01a,b | 91.01 ± 1.25a,b | 306.32 ± 6.97a | 1.39 ± 0.04c | 1.32 ± 0.13 | 2.09 ± 0.54 |
| SBM 50%FO | 204.36 ± 3.1 | 1.14 ± 0.04b | 104.56 ± 4.56b | 315.00 ± 5.61a | 1.25 ± 0.00c | 1.34 ± 0.11 | 2.36 ± 0.52 |
| SPC 100%FO | 203.97 ± 2.0 | 1.05 ± 0.13a,b | 94.42 ± 15.59a,b | 290.89 ± 25.78a | 1.29 ± 0.12c | 1.39 ± 0.14 | 2.01 ± 0.44 |
| SPC 75%FO | 202.64 ± 12.2 | 1.11 ± 0.07a,b | 101.46 ± 8.82a,b | 298.83 ± 17.85a | 1.22 ± 0.03c | 1.38 ± 0.12 | 2.27 ± 0.42 |
| SPC 50%FO | 202.13 ± 1.0 | 1.01 ± 0.08a,b | 88.77 ± 9.47a,b | 289.92 ± 36.12a | 1.39 ± 0.09c | 1.28 ± 0.11 | 1.97 ± 0.63 |
| P | 0.732 | 0.009 | 0.01 | < 0.001 | < 0.001 | 0.052 | 0.173 |
Specific growth rate, SGR = ln (final weight − initial weight) / (days × 100).
Weight gain, percent of initial weight, WG% = (final weight − initial weight) / initial weight × 100.
Feed Intake, FI = total average feed (g) consumed per fish throughout trial.
Feed conversion ratio, FCR = grams fed / grams weight gained.
Condition factor, K = (weight (g) × 100) / (length (cm))3.
Hepatosomatic index, HSI = (liver weight / body weight) × 100.
3.2. Fatty acid profile results
Muscle, plasma, and feed fatty acid profiles were similar as a function of dietary soy oil percentage (Fig. 1). Muscle (PC1, explained variance (EV) 49%) and plasma (PC1, EV 60.7%) displayed the same FA profile trends with clustering observed among 50% FO diets (SBM and SPC); 50% FO (FM) and 75% FO (SBM and SPC) diets; and 100% FO (FM, SBM, and SPC) diets. Feeds similarly differed by soy oil percentage as observed in the muscle and plasma (PC1, EV 85.2%, Fig. 1) and protein sources (FM diets vs. soy formulations were distinctly different in FA profile (PC2, EV 10%)). For each matrix, the top-three loadings from the component with greatest EV (PC1) are provided in Table 4, which show C18:2n-6 being the fatty acid primarily driving separation in the PCA scores plots. The %FA for linoleic acid (C18:2n-6) are provided in Table 5, where groupings based on percent soy oil is similar to the diet groupings observed in PCA, following normalization by the lowest percentage amount within each matrix (i.e., 10.6% for feed, 10.2% for muscle, 5.66% for plasma). Liver, brain, and adipose tissue had indistinguishable free fatty acid profiles across all diet formulations (data not shown).
Fig. 1.
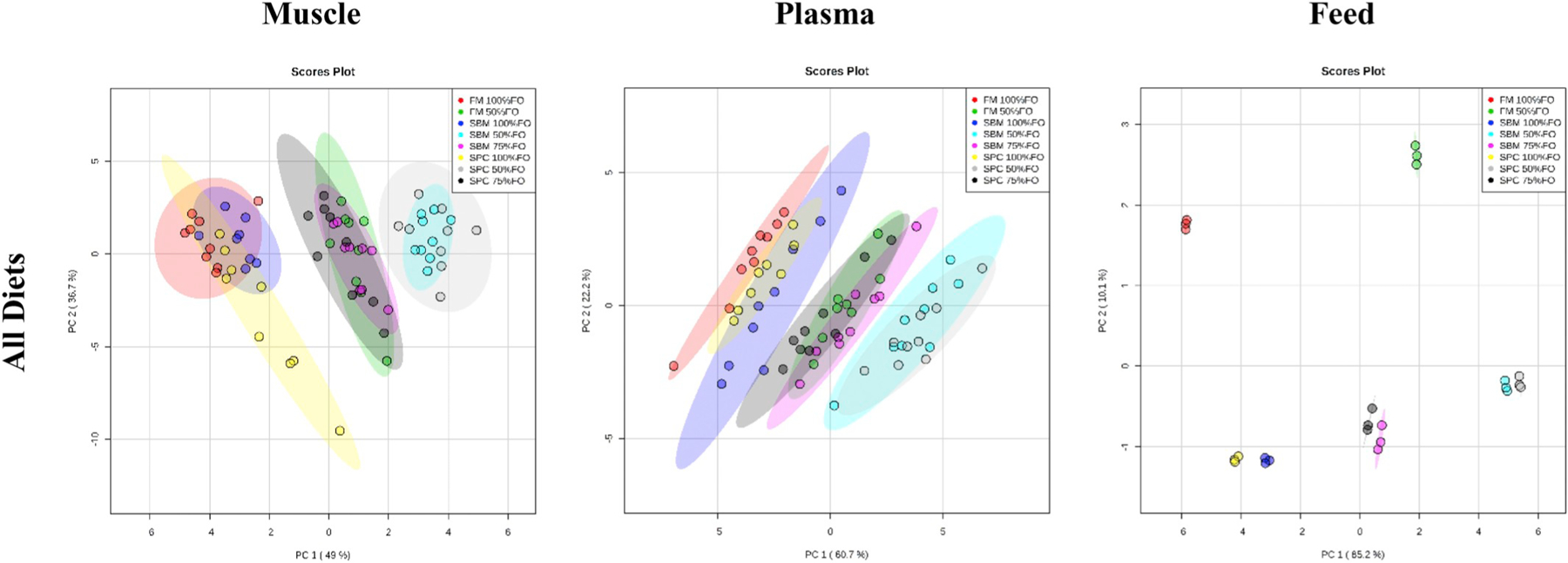
Fish muscle, plasma, and feed fatty acid profile analysis (PCA scores plots).
Table 4.
The fatty acids with the top three loadings from component 1 in PCA.
| Feed |
Muscle |
Plasma |
|||
|---|---|---|---|---|---|
| Component 1 | Loadings | Component 1 | Loadings | Component 1 | Loadings |
| C18: 2n-6 (linoleic acid) | 0.677 | C18: 2n-6 (linoleic acid) | 0.719 | C18: 2n-6 (linoleic acid) | 0.612 |
| 20: 5n-3 (EPA) | 0.325 | 22: 6n-3 (DHA) | 0.405 | 20: 5n-3 (EPA) | 0.573 |
| 22: 6n-3 (DHA) | 0.308 | 20: 5n-3 (EPA) | 0.385 | 22: 6n-3 (DHA) | 0.314 |
Table 5.
Percent free fatty acid (%FA) for C18:2n-6 (linoleic acid). Errors are standard deviation, n = 9.
| C18:2n-6 | |||
|---|---|---|---|
| Diet | %FA feed | %FA muscle | %FA plasma |
| FM 100%FO | 10.6 ± 0.1 | 10.2 ± 1.0 | 5.66 ± 0.5 |
| FM 50%FO | 24.7 ± 0.2 | 17.3 ± 0.9 | 11.6 ± 2.0 |
| SBM 100%FO | 14.6 ± 0.1 | 12.3 ± 3.0 | 7.84 ± 0.9 |
| SBM 75%FO | 21.6 ± 0.2 | 16.7 ± 0.4 | 12.8 ± 0.7 |
| SBM 50%FO | 29.4 ± 0.1 | 20.9 ± 0.9 | 16.3 ± 1.0 |
| SPC 100%FO | 12.7 ± 0.3 | 10.7 ± 0.6 | 7.38 ± 2.0 |
| SPC 75%FO | 20.9 ± 0.3 | 16.2 ± 0.6 | 11.5 ± 0.7 |
| SPC 50%FO | 30.3 ± 0.1 | 21.5 ± 1.0 | 17.3 ± 1.0 |
To elaborate on the contribution of the dietary constituents, fatty acid profile analyses were conducted within the constraints of only one diet variable: soy oil percentage (Fig. 2) or primary protein source (Fig. 3). For Fig. 2, partial or complete separation of fishmeal diets was observed, which is largely due to residual fish oil. Across diets with the same primary protein source (Fig. 3), dietary oil has a definitive and obvious influence on FA profiles as clear separation was observed between all the groupings.
Fig. 2.
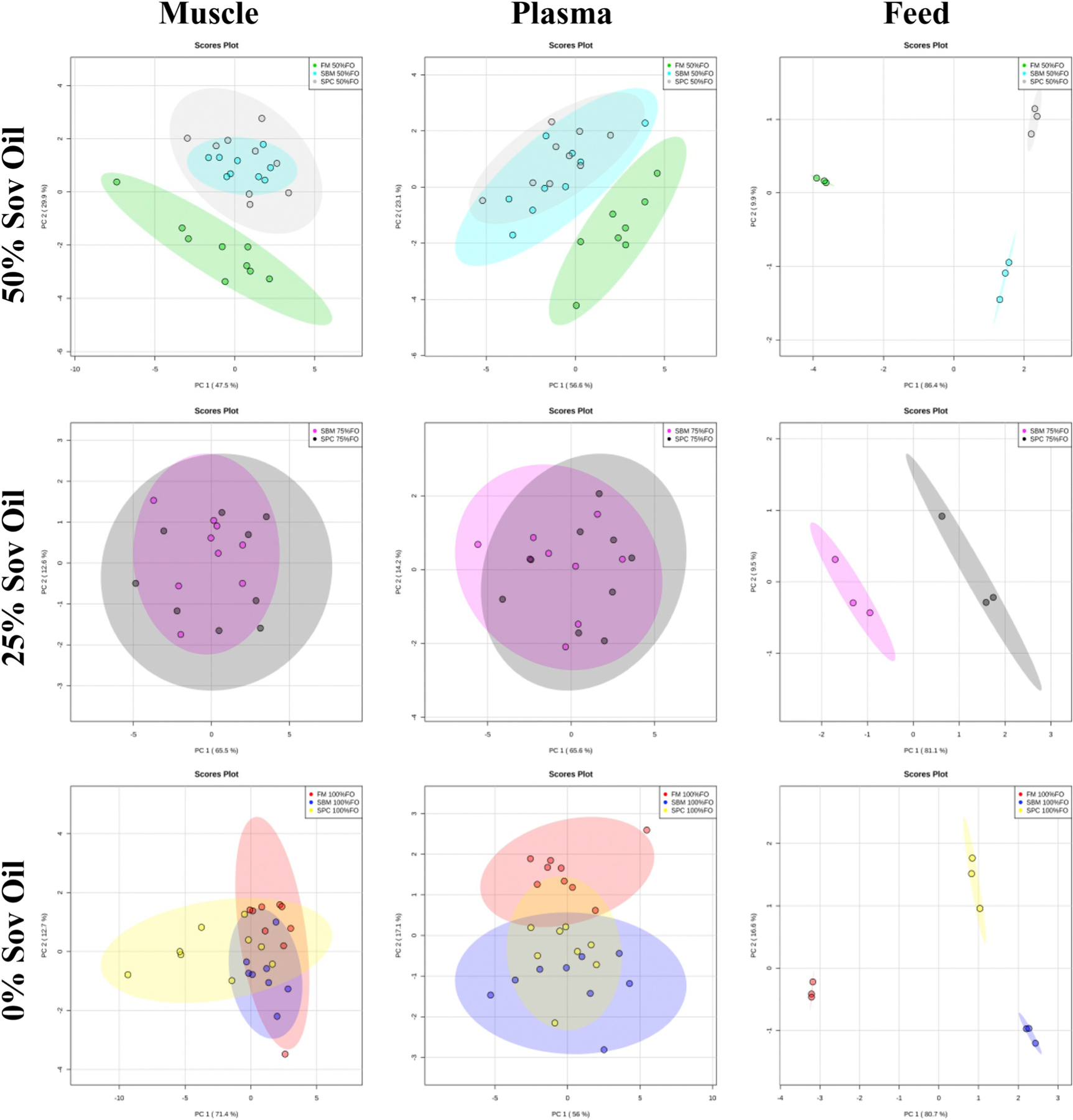
PCA scores plots from fatty acid profile analysis of muscle, plasma, and feed based on dietary oil formulation (100%FO, 75%FO, 50%FO). Plots display data by protein source: fishmeal (FM), soybean meal (SBM), or soy protein concentrate (SPC).
Fig. 3.
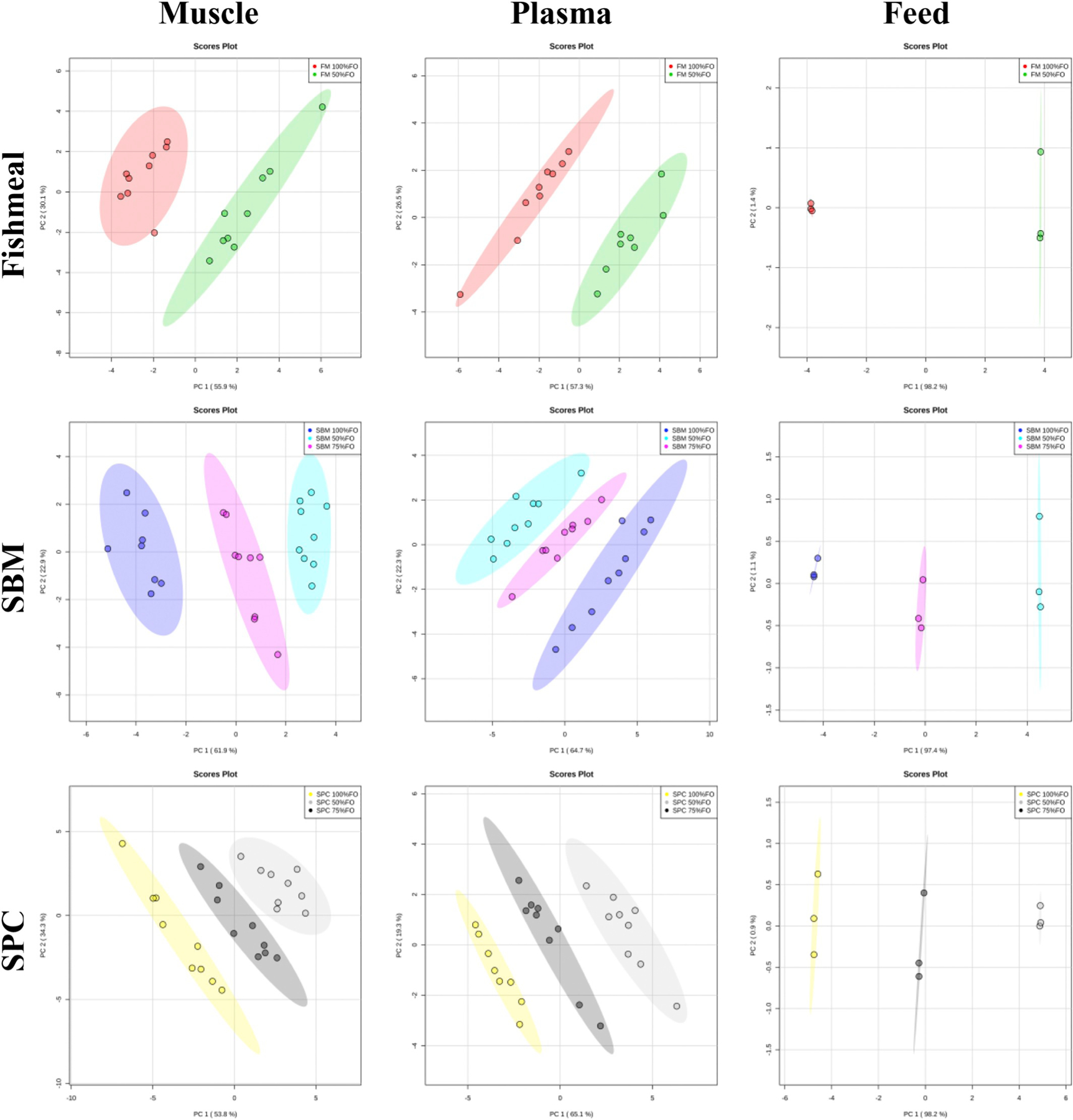
PCA scores plots from fatty acid profile analysis of muscle, plasma, and feed based on dietary protein source (fishmeal, soybean meal (SBM), or soy protein concentrate (SPC)). Plots display data by oil inclusion: 100%FO, 75%FO, 50%FO.
3.3. Gene expression results
Quantitative PCR gene expression results for liver (Table 6) and muscle (Table 7) show several genes with significant differences in expression. All expression levels were normalized to the fishmeal-based diet with 100% fish oil (FM 100%FO). No consistent trends were observed when significant differences did arise based on increasing soy oil inclusion within each protein source. In the liver, ACOX1, APOA4, CPT1, DEGS1, and ELOVL1 all resulted in at least one diet treatment showing significant expression differences. In muscle, significant differences were observed in ACOT1, CPT1A, FASN, GK5, and PPARA, and were almost exclusively driven by lower expression of each of those genes in fish fed the SPC 50%FO diet.
Table 6.
Gene expression in liver of juvenile red drum fed varying protein sources and soy oil levels. Fold expression difference ( ± SEM) compared to fishmeal and fish oil reference diet (FM 100%FO). Values with different superscripts are significantly different from one another (p < .05, ANOVA).
| Diet | ACOT1 | ACOX1 | APOA4 | CPT1A | DEGS1 | ELOVL1 | FASN | G6P1 | GK5 | PPARA |
|---|---|---|---|---|---|---|---|---|---|---|
| FM 100%FO | 1.00 ± 0.41 | 1.00 ± 0.85a,b | 1.00 ± 0.49a | 1.00 ± 0.58a | 1.00 ± 0.79a | 1.00 ± 0.31a,b | 1.00 ± 0.51 | 1.00 ± 0.44 | 1.00 ± 0.37 | 1.00 ± 0.38 |
| FM 50%FO | 1.80 ± 0.20 | 0.39 ± 0.69b | 1.97 ± 0.26a,b | 2.93 ± 0.22b | 0.08 ± 0.63b | 2.00 ± 0.21a,b | 2.54 ± 0.25 | 1.93 ± 0.17 | 1.41 ± 0.28 | 1.28 ± 0.17 |
| SBM 100%FO | 1.71 ± 0.22 | 5.77 ± 0.59a | 1.98 ± 0.18a,b | 1.12 ± 0.23a | 0.23 ± 0.69a,b | 1.20 ± 0.16a,b | 1.97 ± 0.29 | 1.77 ± 0.37 | 2.18 ± 0.35 | 1.08 ± 0.12 |
| SBM 75%FO | 1.13 ± 0.36 | 2.45 ± 0.67a,b | 1.14 ± 0.34a | 2.40 ± 0.37b | 0.54 ± 0.47a,b | 1.44 ± 0.35a,b | 0.65 ± 0.92 | 2.21 ± 0.41 | 2.00 ± 0.24 | 0.77 ± 0.015 |
| SBM 50%FO | 1.41 ± 0.26 | 1.86 ± 0.94a,b | 1.58 ± 0.15a,b | 0.99 ± 0.56a | 0.33 ± 0.60a,b | 0.82 ± 0.49a | 2.52 ± 0.36 | 1.62 ± 0.33 | 1.17 ± 0.51 | 1.15 ± 0.25 |
| SPC 100%FO | 1.64 ± 0.24 | 1.99 ± 0.71a,b | 1.69 ± 0.27a,b | 1.36 ± 0.25a | 0.10 ± 0.67a,b | 1.45 ± 0.18a,b | 1.35 ± 0.72 | 3.18 ± 0.34 | 1.57 ± 0.31 | 1.14 ± 0.17 |
| SPC 75%FO | 1.06 ± 0.31 | 3.90 ± 0.71a,b | 1.41 ± 0.25a,b | 3.03 ± 0.25b | 0.63 ± 0.66a | 2.02 ± 0.15b | 2.09 ± 0.35 | 2.45 ± 0.42 | 1.86 ± 0.26 | 1.12 ± 0.19 |
| SPC 50%FO | 2.26 ± 0.19 | 4.87 ± 0.41a | 2.85 ± 0.19b | 1.14 ± 0.30a | 0.36 ± 0.55a,b | 1.28 ± 0.30a,b | 2.56 ± 0.36 | 3.03 ± 0.45 | 2.16 ± 0.35 | 1.29 ± 0.015 |
| p value (ANOVA) | 0.067 | 0.019 | 0.012 | 0.009 | 0.002 | 0.031 | 0.081 | 0.062 | 0.191 | 0.312 |
Table 7.
Gene expression in muscle of juvenile red drum fed varying protein sources and soy oil levels. Fold expression difference ( ± SEM) compared to fishmeal and fish oil reference diet (FM 100%FO). Values with different superscripts are significantly different from one another (p < .05, ANOVA).
| Diet | ACOT1 | ACOX1 | APOA4 | CPT1A | DEGS1 | ELOVL1 | FASN | G6P1 | GK5 | PPARA |
|---|---|---|---|---|---|---|---|---|---|---|
| FM 100%FO | 1.00 ± 0.26a,b | 1.00 ± 0.26 | 1.00 ± 0.25 | 1.00 ± 0.20a,b | 1.00 ± 0.32 | 1.00 ± 0.21 | 1.00 ± 0.21a,b | 1.00 ± 0.27 | 1.00 ± 0.28a,b | 1.00 ± 0.18a,b |
| FM 50%FO | 0.96 ± 0.17a,b | 0.79 ± 0.22 | 1.16 ± 0.15 | 0.93 ± 0.19a,b | 1.44 ± 0.17 | 0.99 ± 0.14 | 1.00 ± .018a,b | 0.98 ± 0.17 | 1.15 ± 0.16a | 1.26 ± 0.12a |
| SBM 100%FO | 0.85 ± 0.19a,b | 0.68 ± 0.19 | 0.82 ± 0.26 | 0.62 ± 0.13a,b | 1.50 ± 0.24 | 0.79 ± 0.16 | 0.96 ± 0.19a,b | 0.79 ± 0.21 | 0.65 ± 0.23a,b | 1.00 ± 0.16a,b |
| SBM 75%FO | 1.11 ± 0.23a,b | 0.76 ± 0.22 | 1.05 ± 0.16 | 1.00 ± 0.15a,b | 0.98 ± 0.24 | 1.05 ± 0.20 | 1.42 ± 0.16a | 1.09 ± 0.16 | 1.07 ± 0.26a | 1.46 ± 0.13a |
| SBM 50%FO | 0.87 ± 0.15a,b | 1.02 ± 0.06 | 0.81 ± 0.23 | 0.75 ± 0.23a,b | 1.88 ± 0.14 | 0.86 ± 0.18 | 0.85 ± 0.30a,b | 0.80 ± 0.20 | 0.63 ± 0.36a,b | 0.90 ± 0.24a,b |
| SPC 100%FO | 1.19 ± 0.22a,b | 0.63 ± 0.30 | 1.34 ± 0.22 | 1.08 ± 0.23a | 0.90 ± 0.33 | 1.07 ± 0.19 | 1.26 ± 0.25a,b | 1.15 ± 0.21 | 1.28 ± 0.23a | 1.45 ± 0.17a |
| SPC 75%FO | 1.32 ± 0.16a | 0.70 ± 0.14 | 1.28 ± 0.22 | 1.08 ± 0.18a | 1.49 ± 0.20 | 0.92 ± 0.16 | 1.11 ± 0.24a,b | 0.94 ± 0.18 | 1.26 ± 0.27a | 1.41 ± 0.18a |
| SPC 50%FO | 0.67 ± 0.24b | 0.72 ± 0.17 | 0.63 ± 0.46 | 0.56 ± 0.24b | 1.10 ± 0.37 | 0.62 ± 0.25 | 0.60 ± 0.40b | 0.62 ± 0.38 | 0.43 ± 0.41b | 0.61 ± 0.39b |
| p value (ANOVA) | 0.05 | 0.179 | 0.062 | 0.003 | 0.059 | 0.084 | 0.042 | 0.164 | 0.001 | < 0.001 |
4. Discussion
Replacing up to 50% of fish oil with 50% soy oil did not have a negative effect on performance in juvenile red drum in this study in diets relying primarily on soybean meal or soy protein concentrate. These results are similar to those observed by other researchers in regards to high levels of fishmeal or fish oil being successfully replaced by alternative ingredients for juvenile red drum (Casu et al., 2017; Moxley et al., 2014; Perez-Velazquez et al., 2018; Rossi et al., 2013; Watson et al., 2019). The performance results are in contract to Tucker et al. (1997) who observed reduced performance in small juvenile red drum (0.3–9.4 g) when diets exceeded 1.5% soy oil, although these differences may be attributable to the different life stage evaluated or other significant differences in feed formulations tested. In fact, in the current study, the soybean meal-based diet with 50% fish oil had a significantly higher SGR than the fishmeal-based reference with 100% fish oil. The absence of a drop-off in performance at the highest inclusion level of soy oil tested (50%) confirms this species is a high-quality candidate for intensive aquaculture as it is amenable to high levels of fishmeal and fish oil replacements, given that other ingredients are available to meet the known nutritional requirements. Further increases in soy oil inclusion level, with and without individual fatty acid supplementations (i.e., docosahexaenoic acid (DHA), arachidonic acid (ARA), eicosapentaenoic acid (EPA)) to meet known requirements should continue to be evaluated.
According to Lochmann and Gatlin’s (1993) definitive study on the essential fatty acid requirements for red drum, the optimal values are 0.5–1.0% for highly unsaturated fatty acids (HUFA, n-3), with a performance decline observed at 1.5% and pronounced decline at 2.5%, and 0.5% for the sum of both EPA and DHA. For our eight diets, the percentage of HUFA n-3 fatty acids respective to the dry weight of the diets are 2.03% for FM(0% SO), 1.64% for FM(50% SO), 1.93% for SBM (0% SO), 1.70% for SBM(25% SO), 1.46% for SBM(50% SO), 2.25% for SPC(0% SO), 1.93% for SPC(25% SO), and 1.52% for SPC(50% SO). Therefore, although ours do not surpass the upper limit of 2.5% where declining performance was observed. For our eight diets, the percentage of EPA and DHA (combined) respective to the dry weight of diets are 1.77% for FM(0% SO), 1.25% for FM(50% SO), 1.56% for SBM(0% SO), 1.24% for SBM(25% SO), 0.92% for SBM(50% SO), 1.85% for SPC(0% SO), 1.43% for SPC(25% SO), and 0.94% for SPC(50% SO). There are no known upper limits for EPA and DHA stipulated for red drum.
The limited differences in gene expression of lipid related genes in both the liver and muscle are also indicators that significant energy is not being shifted into various lipid anabolic or catabolic pathways in order to meet nutritional needs for growth. This result could be due to a lack of ability of the animals to up or down regulate expression levels to respond to changing needs based on dietary input, post-translational modification of enzyme activity levels, or more likely, based on the growth results on the formulations evaluated here, the animals were not nutritionally stressed enough to require a molecular level response to individual lipid or fatty acid levels.
As expected however, the overall fatty acid profiles of multiple tissues show differences based directly on feed level inputs, a common finding in fish, especially in regards to essential fatty acids (Fountoulaki et al., 2009; Trushenski et al., 2012; Trushenski and Boesenberg, 2009; Watson et al., 2013). Lipid source and inclusion percentage are the primary factors affecting lipid metabolism in red drum, while the protein source is impactful at a high level of soy oil (50%). Liver, adipose, and brain did not exhibit statistically significant grouping and considering that these tissues have a much higher mass percent of fat than muscle and plasma, it may be inferred that metabolic inclusion of feed-derived fatty acids did not occur to any appreciable extent in this nine-week feeding trial of juvenile red drum. Across diets with the same soy oil percentage, soy protein sources had little effect on the FA profile of muscle and plasma (Fig. 2). Yet, in the cases of 0% soy oil for muscle and plasma a Student’s t-test of PCA scores showed of diet SPC 100% FO from diets SBM 100% FO and also FM 100% FO in PC1% for muscle (Casu et al., 2017). In plasma analysis of 0% soy oil incorporation, the same soy protein source effects were observed (p < .05; SPC 100% FO from diets SBM 100% FO and also FM 100% FO), however in PC2; and clearly SBM 100% FO and FM 100% FO FA profiles were distinct. The FA primarily driving overall separation was C18:2n-6 (linoleic acid), which is understandable considering is the most abundant fatty acid in soy oil, comprising 47.5% (mass fraction) (Ivanov et al., 2010).
To explore the extent of incorporation of fatty acids from feed to tissue as a function of increasing soy oil amount, the %FA values (Tables S5, S6, S7) of each FA measured in plasma and muscle were normalized by the %FA value at each of the eight diets and expressed as fold change, which were then arranged in order of increasing soy oil (i.e., 0% FM; 0% SPC; 0% SBM; 25% SPC; 25% SBM; 50% FM; 50% SPC; 50% SBM). Only those fatty acids with an average fold change of either greater than +1 or less than −1 across all eight diets were plotted (Fig. S1), which resulted in five fatty acids meeting the criteria and listed here with common name or abbreviation and (fold change): 18:3n-6, γ-linolenic acid (+2.96); 22:6n-3, DHA (+2.14); 22:5n-3, DPA (+1.75); 22:5n-6 (+1.29); 20:4n-6, arachidonic acid (+1.11). There were no fatty acids with an average fold change of tissue incorporation less than −1 as a function of increasing soy oil percentage. It is notable that all the fatty acids listed are PUFAs and the identity of the latter four are not unexpected considering the increasing levels of fish oil. However, the high and increasing inclusion of 18:3n-6 is not immediately clear, although it may be hypothesized that the fatty acid is not being incorporated into tissue at an appreciable extent and being transported and/or stored, which may be supported by the much higher fold change values in plasma over muscle.
5. Conclusions
Overall, juvenile red drum fed all six of the soy protein, fishmeal free feeds outperformed the fish fed the fishmeal-based reference feeds. The incorporation of limited animal protein sources (poultry and squid meals) allowed for the total elimination of fishmeal without loss of performance. FAMEs were measured using a robust method developed using GC-FID across several fish tissue types. Muscle and plasma were acutely impacted by the %FO during this nine-week feeding trial, and protein source affects lipid metabolism at high soy incorporation (50%). The significant fatty acid driving the separation among the diets in multivariate analysis was C18:2n-6 (linoleic acid) and correlated with the percentage of soy oil inclusion, which is composed mostly of that fatty acid. The similarity of fatty acid profiles between each diet and subsequent tissues of fish fed that diet coupled with limited differences in differential gene expression of multiple genes indicates a limited ability of this species to alter consumed fatty acids, which makes identifying specific dietary requirements for individual lipid species and fatty acids critical as feed formulations rapidly change between significantly different sources. The equivalent and in one case (SBM 50%SO) significantly improved performance from soy-based feeds with soy oil inclusion up to 50% as a fish oil replacement indicates that increased levels of alternative oils still meet the nutritional requirements for this species, a question that should be evaluated further. The results herein may provide new insight toward the optimization of essential and other fatty acids for the proper growth and development of farm-raised fish for human consumption and underscore the ability of soy protein and oil sources to deliver balanced nutrition.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the Soy Aquaculture Alliance and the United Soybean Board for funding this study and Alina Hall, Kaylie Anne Costa, and Wiley Sinkus for laboratory assistance during extraction. This is contribution #827 from the South Carolina Department of Natural Resources’ Marine Resources Research Institute.
Footnotes
Publisher's Disclaimer: Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aquaculture.2020.735627.
References
- AOAC, 2002. AOAC Official method 996.06: Fat (total, saturated, and unsaturated) in foods - Hydrolytic Extraction Gas Chromatographic method. AOAC Int
- Bansemer MS, Forder REA, Howarth GS, Suitor GM, Bowyer J, Stone DAJ, 2015. The effect of dietary soybean meal and soy protein concentrate on the intestinal mucus layer and development of subacute enteritis in yellowtail kingfish (Seriola lalandi) at suboptimal water temperature. Aquac. Nutr 300–310. 10.1111/anu.12160. [DOI]
- Bligh EG, Dyer WJ, 1959. A rapid methid of total lipid extraction and purification. Can. J. Biochem. Physiol 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Casu F, Watson AM, Yost J, Leffler JW, Gaylord TG, Barrows FT, Sandifer PA, Denson MR, Bearden DW, 2017. Metabolomics analysis of effects of commercial soy-based protein products in red drum (Sciaenops ocellatus). J. Proteome Res 16, 2481–2494. 10.1021/acs.jproteome.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu F, Watson AM, Yost J, Leffler JW, Gaylord TG, Barrows FT, Sandifer PA, Denson MR, Bearden DW, 2019. Investigation of graded-level soybean meal diets in red drum (Sciaenops ocellatus) using NMR-based metabolomics analysis. Comp. Biochem. Physiol. D Genomics Proteomics 29, 173–184. 10.1016/j.cbd.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J, 2018. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46, W486–W494. 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RL, Her BY, Su MS, Hwang G, Wu YH, Chen HY, 2004. Substituting fish meal with soybean meal in diets of juvenile cobia Rachycentron canadum. Aquaculture 229, 325–333. 10.1016/S0044-8486(03)00395-8. [DOI] [Google Scholar]
- Core TR, 2013. R: A Language and Environment for Statistical Computing
- Davis DA, Arnold CR, 2004. Production diets : replacement of fish meal with soybean meal. J. Appl. Aquac 15, 173–181. [Google Scholar]
- Davis DA, Jirsa D, Arnold CR, 1995. Evaluation of soybean proteins as replacements for menhaden fish meal in practical diets for the red drum Sciaenops ocellatus. J. World Aquacult. Soc 26, 48–58. [Google Scholar]
- FAO, 2016. The state of world fisheries and aquaculture. In: The State of World Fisheries and Aquaculture, https://doi.org/92-5-105177-1.
- FAO, 2018. State of Fisheries and Aquaculture in the world https://doi.org/issn 10. [Google Scholar]
- Fountoulaki E, Vasilaki A, Hurtado R, Grigorakis K, Karacostas I, Nengas I, Rigos G, Kotzamanis Y, Venou B, Alexis MN, 2009. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile. Aquaculture 289, 317–326. 10.1016/j.aquaculture.2009.01.023. [DOI] [Google Scholar]
- González-félix ML, Minjarez-osorio C, Perez-velazquez M, Urquidez-bejarano P, 2015. Influence of dietary lipid on growth performance and body composition of the Gulf corvina, Cynoscion othonopterus. Aquaculture 448, 401–409. 10.1016/j.aquaculture.2015.06.031. [DOI] [Google Scholar]
- Ivanov DS, Lević JD, Sredanović SA, 2010. Fatty acid composition of various soybean products. Food Feed Res 37, 65–70. [Google Scholar]
- Krogdahl A, Bakke-McKellep AM, Baeverfjord G, 2003. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr 9, 361–371. 10.1046/j.1365-2095.2003.00264.x. [DOI] [Google Scholar]
- Lepage G, Roy CC, 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res 27, 114–120. [PubMed] [Google Scholar]
- Lochmann RT, Gatlin DM, 1993. Essential fatty acid requirement of juvenile red drum (Sciaenops ocellatus). Fish Physiol. Biochem 12, 221–235. 10.1007/BF00004370. [DOI] [PubMed] [Google Scholar]
- McGoogan BB, Gatlin DM, 1997. Effects of replacing fish meal with soybean meal in diets for red drum Sciaenops ocellatus and potential for palatability enhancement. J. World Aquacult. Soc 28, 374–385. [Google Scholar]
- Moxley JD, Rossi W, Buentello A, Pohlenz C, Gatlin DM, Tomasso JR, 2014. Replacement of fish meal with plant feedstuffs in the diet of red drum, Sciaenops ocellatus : Effects on Production Characteristics and Tolerance to Aquaculture-Related Stressors. J. World Aquacult. Soc 45, 192–198. 10.1111/jwas.12106. [DOI] [Google Scholar]
- Olsen RL, Hasan MR, 2012. A limited supply of fishmeal: impact on future increases in global aquaculture production. Trends Food Sci. Technol 27, 120–128. 10.1016/j.tifs.2012.06.003. [DOI] [Google Scholar]
- Ostermann AI, Müller M, Willenberg I, Schebb NH, 2014. Determining the fatty acid composition in plasma and tissues as fatty acid methyl esters using gas chromatography - a comparison of different derivatization and extraction procedures. Prostaglandins Leukot. Essent. Fat. Acids 91, 235–241. 10.1016/j.plefa.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Parsons HM, Ekman DR, Collette TW, Viant MR, 2009. Spectral relative standard deviation: a practical benchmark in metabolomics. Analyst 134, 478–485. 10.1039/b808986h. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez M, Gatlin DM, González-Félix ML, García-Ortega A, 2018. Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture 487, 41–50. 10.1016/j.aquaculture.2018.01.001. [DOI] [Google Scholar]
- Rossi W, Moxely D, Buentello A, Pohlenz C, Gatlin DM, 2013. Replacement of fishmeal with novel plant feedstuffs in the diet of red drum Sciaenops ocellatus : an assessment of nutritional value. Aquac. Nutr 19, 72–81. 10.1111/anu.12073. [DOI] [Google Scholar]
- Sahlmann C, Sutherland BJG, Kortner TM, Koop BF, Krogdahl A, Bakke AM, 2013. Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish Immunol 34, 599–609. 10.1016/j.fsi.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Salze G, McLean E, Battle PR, Schwarz MH, Craig SR, 2010. Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia, Rachycentron canadum. Aquaculture 298, 294–299. 10.1016/j.aquaculture.2009.11.003. [DOI] [Google Scholar]
- Sissener NH, Sanden M, Bakke AM, Krogdahl Å, Hemre G-I, 2009. A long term trial with Atlantic salmon (Salmo salar L.) fed genetically modified soy; focusing general health and performance before, during and after the parr-smolt transformation. Aquaculture 294, 108–117. 10.1016/j.aquaculture.2009.05.002. [DOI] [Google Scholar]
- Trushenski JT, Boesenberg J, 2009. Influence of dietary fish oil concentration and finishing duration on beneficial fatty acid profile restoration in sunshine bass Morone chrysops ♀ x M. saxatilis ♂. Aquaculture 296, 277–283. 10.1016/j.aquaculture.2009.08.019. [DOI] [Google Scholar]
- Trushenski J, Schwarz M, Lewis H, Laporte J, Delbos B, Takeuchi R, Sampaio LA, 2011. Effect of replacing dietary fish oil with soybean oil on production performance and fillet lipid and fatty acid composition of juvenile cobia Rachycentron canadum. Aquac. Nutr 17, 437–447. 10.1111/j.1365-2095.2010.00779.x. [DOI] [Google Scholar]
- Trushenski J, Schwarz M, Bergman A, Rombenso A, Delbos B, 2012. DHA is essential, EPA appears largely expendable, in meeting the n−3 long-chain poly-unsaturated fatty acid requirements of juvenile cobia Rachycentron canadum. Aquaculture 326–329, 81–89 10.1016/j.aquaculture.2011.11.033. [DOI]
- Tucker JW, Lellis WA, Vermeer GK, Roberts DE, Woodward PN, 1997. The effects of experimental starter diets with different levels of soybean or menhaden oil on red drum (Sciaenops ocellatus). Aquaculture 149, 323–339. [Google Scholar]
- Turchini G, Ng W-K, Tocher DR, 2011. Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds CRC Press, Boca Raton FL. [Google Scholar]
- Urán PA, Gonçalves AA, Taverne-Thiele JJ, Schrama JW, Verreth JAJ, Rombout JHWM, 2008. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immunol 25, 751–760. 10.1016/j.fsi.2008.02.013. [DOI] [PubMed] [Google Scholar]
- van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ, 2006. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 7, 1–15. 10.1186/1471-2164-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AM, Barrows FT, Place AR, 2013. Taurine supplementation of plant derived protein and n-3 fatty acids are critical for optimal growth and development of cobia, Rachycentron canadum. Lipids 48, 899–913. 10.1007/s11745-013-3814-2. [DOI] [PubMed] [Google Scholar]
- Watson AM, Buentello A, Place AR, 2014. Partial replacement of fishmeal, poultry by-product meal and soy protein concentrate with two non-genetically modified soybean cultivars in diets for juvenile cobia, Rachycentron canadum. Aquaculture 434, 129–136. 10.1016/j.aquaculture.2014.08.003. [DOI] [Google Scholar]
- Watson AM, Casu F, Bearden DW, Yost J, Denson MR, Gaylord TG, Anderson P, Sandifer PA, Leffler JW, Barrows FT, 2019. Investigation of graded levels of soybean meal diets for red drum, Sciaenops ocellatus, using quantitative PCR derived biomarkers. Comp. Biochem. Physiol. D Genomics Proteomics 29, 274–285. 10.1016/j.cbd.2019.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


