Abstract
Aims
Congestive splenomegaly is a classic sign of organ congestion in acute decompensated heart failure (ADHF). Shear wave elastography (SWE) allows the measurement of spleen stiffness (SS). We hypothesized that SS could quantify the severity of splenic congestion and predict adverse events in ADHF.
Methods and Results
This study included two cohorts: a haemodynamic cohort (62 HF patients) and an outcome cohort (115 ADHF patients). SS was measured by two‐dimensional SWE on the same day of right heart catheterization in the haemodynamic cohort. Right atrial pressure (RAP) independently correlated with SS (β = 0.32, P = 0.002). SS was measured in the outcome cohort before discharge. The 115 patients were divided into three groups on the basis of the tertile value of SS. The third tertile SS group had a higher prevalence of severe tricuspid regurgitation, higher N‐terminal B‐type natriuretic peptide (NT pro‐BNP), and larger right ventricular diastolic diameter, than had the first tertile group and the second tertile group. During a median follow‐up period of 105 (77–135) days, adverse events occurred in 25 patients (one death and 24 rehospitalizations for HF). The third tertile SS group had a significantly higher rate of adverse events (P < 0.001). A higher SS was independently associated with adverse events after adjusting for conventional validated risk score, liver function test, liver stiffness, and estimated RAP.
Conclusions
The degree of SS at discharge can be used as a marker of residual splenic congestion, which is predictive of adverse events in patients with ADHF.
Keywords: Congestive splenomegaly, Shear wave elastography, Acute decompensated heart failure, Cardio‐splenic axis
Introduction
Acute decompensated heart failure (ADHF) is often accompanied by organ congestion, such as congestive hepatopathy and splenomegaly, from increased right atrial pressure (RAP). 1 , 2 Organ congestion is strongly associated with poor outcome in heart failure (HF). 3 Although detection and monitoring of congestion are essential to HF management, the assessment of congestion is often challenging, especially when symptoms or physical signs are mild. 4 A simple and objective method of organ congestion assessment is crucial to improve HF management.
In severe congestive HF, elevated RAPs lead to liver congestion, and the high hepatic vein pressure can cause post‐sinusoidal portal hypertension, changing of portal venous flow patterns. 5 , 6 The flow pulsatility of the portal vein observed by ultrasound (US) correlates with the severity of HF. 7 However, there have been no methods to evaluate the congestion of the spleen in HF.
Recently, two‐dimensional elastography techniques have been used to measure the stiffness of organs as the liver and spleen. In the clinical field of gastroenterology, liver stiffness (LS) and spleen stiffness (SS) are two most widely used non‐invasive parameters for detecting oesophageal varices (EVs). Studies have reported that SS is significantly superior to LS for identifying the presence of EV and portal hypertension in patients with chronic liver disease. 8 , 9
In patients with HF, the clinical significance of SS has not been established. We hypothesized that SS may be predictive of the severity of congestion of the spleen and adverse events in HF. We sought to verify the relationship between SS and haemodynamic parameters and the outcome of HF through two studies: a haemodynamic study (Study 1) and an outcome study (Study 2).
Methods
Study design
This study was a single‐centre, observational, prospective study, which was approved by the ethics committee of Nihon University Itabashi Hospital (RK‐200218‐04). This study included two cohorts: a haemodynamic study (Study 1) cohort and an outcome study (Study 2) cohort. In the haemodynamic study, the relationship between SS and haemodynamic parameters was evaluated. Patients admitted with HF and scheduled for right heart catheterization (RHC) between September 2019 and February 2020 were prospectively enrolled. RHC was indicated in patients who required accurate haemodynamic monitoring for management of HF. The exclusion criteria included obesity [body mass index (BMI) > 30 kg/m2], organic hepatic disorder (viral hepatitis, cirrhosis, and hepatic tumours), haematologic malignancies (lymphomas, leukaemia, and myeloproliferative disorders), and low‐quality US images for SS measurement. Measurement of SS was performed on the same day as RHC.
In the outcome study, the association of SS with clinical and echocardiographic parameters and outcomes in ADHF was investigated. Patients admitted to the same hospital owing to ADHF between September 2019 and February 2020 were prospectively enrolled. ADHF was diagnosed according to the Framingham criteria. 10 The exclusion criteria were the same as those in Study 1. Measurement of SS was performed before discharge. All enrolees provided written informed consent for inclusion in these studies.
Study population
In the haemodynamic study, a total of 65 patients were screened. Patients with obesity (n = 1) and low‐quality US images for SS (n = 2) were excluded, leaving a total of 62 patients. In the outcome study, a total of 123 patients were screened. Patients with obesity (n = 2), organic hepatic disorder (n = 2), haematologic malignancies (n = 1), and low‐quality US images for SS (n = 3) were excluded; a total of 115 patients were included. Of these 115 patients, 28 patients were also included in the haemodynamic study, because the haemodynamic study included both haemodynamically stable HF patients who underwent RHC and ADHF patients who underwent RHC.
Data management
Patients' characteristics at discharge (baseline) and follow‐up data were obtained through a review of our hospital charts. The anonymized patient data were collected in Excel format by physicians or the clinical research coordinator at our institution.
Measurement of spleen stiffness and diameter
SS was measured by two‐dimensional shear wave elastography (SWE) using the Aplio 500 US machine (Canon Medical System, Otawara, Japan) with a 1 to 7 MHz broadband curved array transducer by experienced abdominal radiologists. SS measurements were acquired in the supine position with the left arm in maximum abduction and through a left intercostal space. 11 A rectangular box‐shaped area was placed at the middle portion of the spleen for scanning, and SS was calculated by placing a round region of interest (ROI) into the rectangular box (Figure 1). Thick and obvious vessels were not included in ROI. Five measurements were conducted, and the median of the five measurements was used for the SS value, expressed as kilopascal (kPa), according to the published European Federation of Societies for Ultrasound in Medicine and Biology Guidelines. 12 The longitudinal maximum spleen diameter was measured in the coronal plane and was expressed in millimetres.
Figure 1.
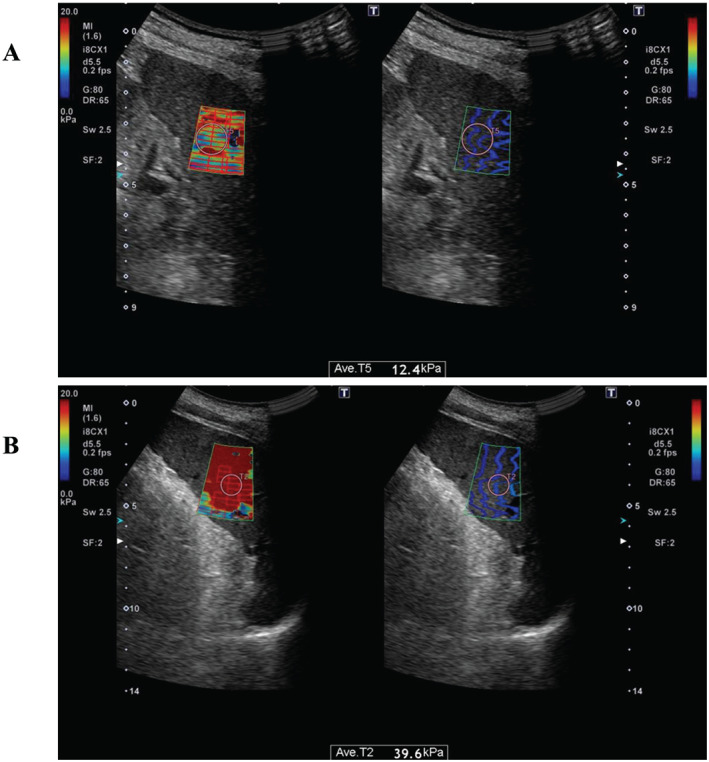
Images of two‐dimensional shear wave elastography for measurement of spleen stiffness (SS). The examiner places a region of interest into the rectangular box at middle portion of spleen in the elastogram map (left). The propagation map on the right was used as a reference to confirm the quality of the elastogram map. (A) Image of a patient in the low SS group. The median SS was 12.4 kPa. (B) Image of a patient in the high SS group. The median SS was 39.6 kPa.
Cardiac catheterization
RHC was performed with a Swan–Ganz catheter in the cardiac catheterization laboratory. All pressure data were measured at end expiration. The following haemodynamic pressures were measured: aortic systolic pressure, pulmonary artery wedge pressure (PAWP), pulmonary artery systolic pressure (PASP), mean pulmonary artery pressure (mPAP), RAP, and cardiac output (CO). The CO was measured using thermodilution or the Fick method, if tricuspid regurgitation (TR) was worse than moderate.
Echocardiography
In the outcome study, comprehensive transthoracic echocardiography was performed before discharge by experienced sonographers who were blinded to other data. Routine echocardiographic measurements were obtained according to published guidelines. 13 Left atrial diameter, left ventricular (LV) diastolic diameter, and LV systolic diameter were measured in the parasternal long‐axis view; and LV ejection fraction (LVEF) was measured using the modified Simpson method in the apical view or the Teichholz method. Right ventricular end‐diastolic diameter (RVDd) was measured at the basal ventricular level of the RV in end‐diastole. Valve regurgitations were graded on 4‐point scale (trivial, mild, moderate, or severe), using colour flow Doppler images. Estimated RAP was calculated based on inferior vena cava size and respiratory collapse rate, according to the published guidelines. 13
Evaluation of liver stiffness
Several non‐invasive markers have been reported for the assessment of LS in HF, such as transient elastography (TE), Fibrosis‐4 index, and non‐alcoholic fatty liver disease fibrosis score (NFS). 14 We measured Fibrosis‐4 index and NFS for LS evaluation. The Fibrosis‐4 index was calculated using the following formula: age (years) × AST (IU/L)/[platelet count (109/L) × square root of ALT (IU/L)]. 15 NFS was calculated as follows: S [−1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × diabetes mellitus (if present, given 1) + 0.95 × AST (U/L) to ALT (U/L) ratio − 0.013 × platelet count (109/L) − 0.66 × albumin (mg/L)]. 16
Follow‐up and clinical outcomes
Follow‐up began on the day of SS measurement. All patients underwent routine follow‐up at 2 weeks, 1 month, and every 2 months after the day of SS measurement at an outpatient clinic in our hospital. Patients were followed up until April 2020. Primary outcomes in the outcome study were all‐cause mortality and rehospitalization for HF.
Statistical analysis
Continuous variables are presented as the median (inter‐quartile range) and categorical variables as number (percentage). The statistical significance of differences among the continuous variables of the three groups was calculated using a one‐way analysis of variance or the Kruskal–Wallis test, followed by the post‐hoc Tukey–Kramer honestly significant difference test or Steel–Dwass test. Categorical variables were compared by χ 2 test, followed by the post‐hoc Bonferroni correction. In the haemodynamic study, correlations of haemodynamic parameters with SS were tested by Pearson's correlation coefficient. Multivariate analysis was performed only on variables with a probability value < 0.1 in univariate analysis. The determinants of SS were identified using multiple linear regression analysis. A receiver operating characteristic (ROC) curve was used to detect the optimal cut‐off point of SS to predict elevated RAP (>10 mmHg, which was considered clinically significant in a previous study). 17 Intraobserver and interobserver agreements were analysed by calculating Lin's concordance correlation coefficient in 12 healthy volunteers. Intraobserver agreements were calculated as the agreement between the first and second SS of the single observer (Y. S.), whereas interobserver agreement was calculated as the agreement between the first SS of two observers (Y. S. and Y. A.).
In the outcome study cohort, patients were divided into three groups according to the tertile value of SS. Baseline characteristics at discharge were compared among these three groups. We used the Kaplan–Meier method to estimate event‐free survival, and the log‐rank test to compare group differences. The association of the SS (continuous variable) with cardiac events (all‐cause mortality and rehospitalization for HF) was evaluated using the Cox proportional‐hazards model, and hazard ratios with 95% confidence intervals (CIs) were calculated. To satisfy the model assumptions, a natural log transformation (ln) was applied to N‐terminal pro‐brain natriuretic peptide (NT pro‐BNP) data. Because of the small number of endpoints in this study, the model was adjusted using a conventional validated HF risk score, the MAGGIC risk score. This risk score was calculated on the basis of 13 clinically relevant risk factors [age, sex, BMI, systolic blood pressure, LVEF, creatinine level, current smoking, diabetes mellitus, chronic obstructive pulmonary disease, New York Heart Association (NYHA) class, HF duration > 18 months, beta‐blocker use, and angiotensin‐converting enzyme inhibitor use], as previously reported. 18 To avoid overfitting, we constructed multivariate models to adjust for the effect of variables associated with liver congestion [total bilirubin and γ‐glutamyl transferase (GGT)], LS (Fibrosis‐4 index and NFS), and central venous pressure (estimated RAP).
All statistical analyses were performed using JMP 13.0 (SAS Institute, Cary, NC, USA) and the R Statistics Version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses, P < 0.05 was considered statistically significant.
Results
Haemodynamic study
Patient cohort characteristics
The median (inter‐quartile range) SS was 14.5 (12.7–19.5) kPa. The clinical characteristics of the patients in the haemodynamic study are shown in Table 1. Almost 60% of patients had hypertension, 30% patients had ischaemic heart disease, and 16% patients had dilated cardiomyopathy. Median NT pro‐BNP was 921 pg/mL, 24% patients had moderate or severe TR, and median PAWP was 11 mmHg.
Table 1.
Patient characteristics of haemodynamic study
| Baseline clinical data | |
| Age, years | 69 (62–77) |
| Male, n (%) | 43 (69.3) |
| Body mass index, kg/m2 | 22.5 (20.2–25.5) |
| NYHA III or IV, n (%) | 5 (8.0%) |
| Diabetes mellitus, n (%) | 23 (37.0%) |
| Hypertension, n (%) | 38 (61.2%) |
| Aetiology | |
| IHD, n (%) | 19 (30.6) |
| DCM, n (%) | 10 (16.1) |
| HCM, n (%) | 1 (1.6) |
| HHD, n (%) | 6 (9.6) |
| VHD, n (%) | 15 (24.1) |
| Others, n (%) | 11 (17.7) |
| Laboratory data | |
| NT pro‐BNP, pg/mL | 921 (329–3279) |
| Echocardiographic data | |
| LVDd, mm | 51 (44–58) |
| LVEF, % | 50 (39–64) |
| LAD, mm | 43 (37–50) |
| RVDd, mm | 36 (30–42) |
| Moderate or severe MR, n (%) | 15 (24.1) |
| Moderate or severe TR, n (%) | 12 (19.3) |
| TRPG, mmHg | 16 (19.3) |
| Catheterization data | |
| Aortic systolic pressure, mmHg | 113 (103–131) |
| PAWP, mmHg | 11 (9–13) |
| PASP, mmHg | 25 (17–30) |
| mPAP, mmHg | 17 (12–20) |
| RAP, mmHg | 5 (3–7) |
| CO, L/min | 3.9 (3.1–4.9) |
| CI, L/min/m2 | 2.3 (1.9–4.9) |
CI, cardiac index; CO, cardiac output; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HHD, hypertensive heart disease; IHD, ischaemic heart disease; LAD, left atrial dimension; LVDd, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; mPAP, pulmonary arterial mean pressure; MR, mitral regurgitation, NT pro‐BNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary arterial systolic pressure; PAWP, pulmonary arterial wedge pressure; RAP, right atrial pressure; RVDd, right ventricle diastolic dimension; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation pressure gradient; VHD, valvular heart disease.
Values are expressed as median (inter‐quartile range) or % (n).
Correlation of spleen stiffness and haemodynamic parameters
Among haemodynamic parameters, PAWP (r = 0.37, P = 0.003), PASP (r = 0.48, P < 0.001), mPAP (r = 0.45, P < 0.001), and RAP (r = 0.47, P < 0.001) were positively correlated with SS (Table 2). Conversely, aortic systolic pressure, CO, and CI did not correlate with the SS. In multiple linear regression analysis, RAP independently correlated with SS (β = 0.32, P = 0.002) (Table 2). Figure 2 demonstrates the correlation of SS and RAP. ROC analysis revealed that the cut‐off value of SS to predict patients with RAP ≥ 10 mmHg was 28.0 kPa (area under ROC curve: 0.76, sensitivity: 0.72, specificity: 0.94, P < 0.001).
Table 2.
Relationship between spleen stiffness and haemodynamic parameters
| Univariate | Multivariate | |||
|---|---|---|---|---|
| r | P | β | P | |
| Aortic systolic pressure | −0.16 | 0.16 | — | — |
| PAWP | 0.37 | 0.003 | 0.086 | 0.70 |
| PASP | 0.48 | <0.001 | 0.92 | 0.056 |
| mPAP | 0.45 | <0.001 | −0.70 | 0.22 |
| RAP | 0.47 | <0.001 | 0.32 | 0.002 |
| CO | −0.20 | 0.11 | — | — |
| CI | −0.12 | 0.34 | — | — |
Other abbreviations as in Table 1.
Figure 2.
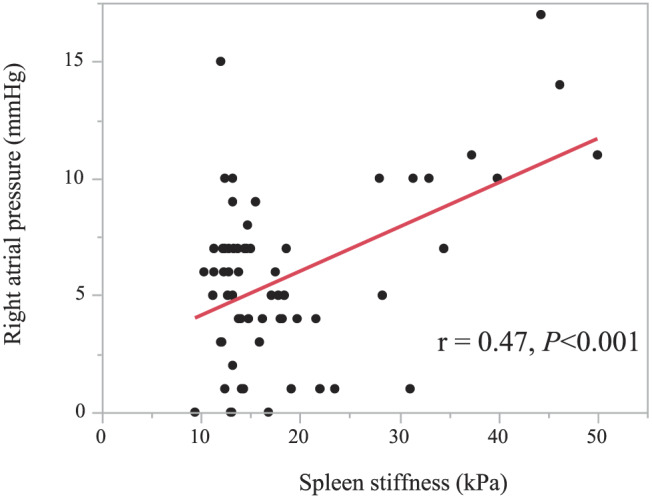
Correlation between spleen stiffness and right atrial pressure.
Reproducibility
Intraobserver and interobserver reproducibility was confirmed in 12 healthy volunteers. The median (inter‐quartile range) SS was 12.0 (12.7–19.5) kPa. All subjects had SS < 28.0 kPa. The intraclass correlation coefficients for intraobserver and interobserver agreement were 0.93 (0.80–0.98) and 0.91 (0.74–0.97).
Outcome study
Patient characteristics
In the outcome cohort, 115 patients with ADHF were divided into three groups on the basis of the tertile value of SS: first tertile group (SS ≤ 13.8 kPa, n = 38), second tertile group (13.8 < SS < 18.9 kPa, n = 38), and third tertile group (SS ≥ 18.9 kPa, n = 39). The median (inter‐quartile range) SS of 115 patients was 16.3 (13.2–23.5) kPa. Among 115 patients, 28 patients (24.3%) had SS ≥ 28.0 kPa, which derived from the cut‐off value for RAP ≥ 10 mmHg. The clinical characteristics of these groups are shown in Table 3. Age, systolic blood pressure, prevalence of hypertension, and aetiology of HF did not differ significantly among these groups. Spleen diameter also did not differ. The third tertile SS group had more advanced NYHA functional class and lower BMI and were treated with higher doses of diuretics. The third tertile SS group also had higher aspartate aminotransferase (AST), GGT, Fibrosis‐4 index, and NT pro‐BNP levels. In echocardiographic data, the third tertile SS group had a larger RVDd, higher TR pressure gradient (TRPG), higher estimated RAP, and a higher prevalence of moderate or severe TR, while left ventricular end‐diastolic dimension and LVEF did not differ significantly among the groups. SS was significantly correlated with estimated RAP (r = 0.57, P < 0.001).
Table 3.
Clinical characteristics of patients stratified into three groups according to tertiles of spleen stiffness
| Item | First tertile SS ≤ 13.8 (n = 38) | Second tertile 13.8 < SS < 18.9 (n = 38) | Third tertile SS ≥ 18.9 (n = 39) | P value |
|---|---|---|---|---|
| Baseline clinical data | ||||
| Age, years | 73 (66–81) | 69 (56–77) | 76 (67–83) | 0.16 |
| Male, n (%) | 30 (78.9) | 23 (73.6) | 26 (66.6) | 0.47 |
| Body mass index, kg/m2 | 23.2 (20.7–25.3) | 23.3 (21.2–26.4) | 20.0 (18.6–23.7) * , † | 0.005 |
| NYHA III or IV, n (%) | 3 (7.8) | 1 (2.6) | 13 (33.3) * , † | <0.001 |
| Diabetes mellitus, n (%) | 12 (31.5) | 15 (39.4) | 20 (51.2) | 0.20 |
| Hypertension, n (%) | 26 (68.4) | 23 (60.5) | 27 (69.2) | 0.67 |
| COPD, n (%) | 8 (21.0) | 3 (7.8) | 3 (7.6) | 0.14 |
| Systolic blood pressure, mmHg | 111 (101–133) | 113 (103–133) | 113 (106–130) | 0.93 |
| MAGGIC risk score | 24 (20–28) | 23 (18–26) | 28 (23–32) * , † | <0.001 |
| Spleen diameter | 86 (79–98) | 86 (81–96) | 85 (74–96) | 0.68 |
| Aetiology | ||||
| IHD, n (%) | 12 (31.5) | 10 (26.3) | 9 (23.0) | 0.69 |
| DCM, n (%) | 4 (10.5) | 9 (23.6) | 6 (15.3) | 0.29 |
| HCM, n (%) | 3 (7.8) | 0 (0) | 3 (7.6) | 0.08 |
| HHD, n (%) | 7 (18.4) | 9 (23.6) | 14 (35.9) | 0.20 |
| VHD, n (%) | 5 (13.1) | 4 (10.5) | 2 (5.1) | 0.44 |
| Others, n (%) | 7 (18.4) | 6 (15.7) | 9 (23.0) | 0.71 |
| Medications | ||||
| ACE‐I or ARB, n (%) | 34 (89.4) | 33 (86.8) | 30 (76.9) | 0.28 |
| Beta‐blocker, n (%) | 31 (81.5) | 36 (94.7) | 33 (84.6) | 0.16 |
| Diuretics, n (%) | 30 (78.9) | 34 (89.4) | 35 (89.7) | 0.31 |
| Dose of loop diuretics (furosemide‐equivalent dose), mg | 20 (10–20) | 20 (17.5–20) | 20 (10–40) * | 0.021 |
| Laboratory data | ||||
| Haemoglobin, g/dL | 12.2 (10.9–13.6) | 12.9 (10.7–14.0) | 11.2 (10.1–12.6) | 0.14 |
| Platelet, ×103/μL | 219 (175–251) | 199 (173–228) | 202 (143–274) | 0.50 |
| Total bilirubin, mg/dL | 0.5 (0.3–0.6) | 0.5 (0.3–0.7) | 0.6 (0.3–0.8) | 0.35 |
| AST, U/L | 23 (19–28) | 22 (17–34) | 30 (20–39) * , † | 0.016 |
| ALT, U/L | 18 (12–26) | 20 (12–37) | 20 (13–34) | 0.44 |
| GGT, U/L | 32 (18–60) | 36 (21–47) | 61 (34–126) * , † | <0.001 |
| Albumin, g/dL | 3.4 (3.1–3.8) | 3.6 (3.3–4.0) | 3.3 (3.1–3.8) | 0.26 |
| Sodium, mEq/L | 141 (139–142) | 141 (138–143) | 140 (138–141) | 0.20 |
| eGFR, mL/min/1.73 m2 | 51 (35–72) | 53 (36–65) | 37 (23–57) | 0.14 |
| NT pro‐BNP, pg/mL | 1572 (402–2653) | 1535 (748–3645) | 3702 (1399–7399) * , † | 0.002 |
| Fibrosis‐4 index | 1.91 (1.34–2.36) | 1.72 (1.20–2.34) | 2.35 (1.56–4.32) † | <0.001 |
| NFS | −0.15 (−1.08 to 0.46) | −0.50 (−1.68 to 0.66) | 0.20 (−0.94 to 1.45) | 0.19 |
| Echocardiographic data | ||||
| LVDd, mm | 52 (45–59) | 53 (48–61) | 52 (43–58) | 0.22 |
| LVEF, % | 51 (36–63) | 42 (31–56) | 51 (35–62) | 0.15 |
| LAD, mm | 43 (38–50) | 46 (39–52) | 45 (40–50) | 0.79 |
| E, m/s | 71 (50–102) | 91 (52–114) | 86 (65–113) | 0.25 |
| e′ velocity, cm/s | 5.4 (3.7–7.4) | 5.8 (4.8–8.1) | 6.1 (4.2–8.2) | 0.30 |
| E/e′ ratio | 14.5 (11.2–17.9) | 11.6 (8.9–15.8) | 14.3 (10.1–19.6) | 0.39 |
| RVDd, mm | 33 (31–39) | 33 (30–38) | 42 (36–50) * , † | <0.001 |
| Moderate or severe MR, n (%) | 5 (13.1) | 12 (31.5) | 13 (33.3) | 0.068 |
| Moderate or severe TR, n (%) | 1 (2.6) | 6 (15.7) | 16 (41.0) * , † | <0.001 |
| TRPG, mmHg | 16 (5–25) | 14 (5–24) | 22 (15–35) * , † | 0.001 |
| Estimated RAP, mmHg | 3 (3–3) | 3 (3–3) | 8 (3–15) * , † | <0.001 |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; E, peak early diastolic transmitral flow velocity; e′, peak early diastolic septal mitral annular velocity; eGFR, estimated glomerular filtration rate; GGT, γ‐glutamyl transferase; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; NFS, non‐alcoholic fatty liver disease fibrosis score.
Values are the median (inter‐quartile range) or number (%). For multiple comparison, the ANOVA test was used for symmetrical continuous variables; the Kruskal–Wallis test for nonsymmetrical continuous variables; and the χ2 test for categorical variables. All‐pair comparisons were performed based on the Tukey–Kramer test for symmetrical continuous variables; the Steel–Dwass test for nonsymmetrical continuous variables; and the χ2 test with Bonferroni correction for categorical variables.
P < 0.05 vs. first tertile.
P < 0.05 vs. second tertile.
Spleen stiffness and clinical outcome
During a median (inter‐quartile range) follow‐up period of 105 (77–135) days, adverse events occurred in 25 (21.7%) patients, including death in one and rehospitalization for HF in 24. Kaplan–Meier analysis showed that the high SS group had a significantly higher rate of adverse events (P < 0.001, Figure 3). Univariate Cox regression analysis showed that a higher SS [along with older age, lower BMI, lower spleen diameter, higher NYHA functional class, higher ln (NT pro‐BNP), and higher MAGGIC risk score], LVEF, RVDd, and TRPG were significantly associated with a higher incidence of adverse events (Table 4). In multivariate Cox regression analysis, a higher SS remained associated with adverse events after adjusting for conventional validated risk score (MAGGIC risk score), liver function test, LS, and estimated RAP (Table 5).
Figure 3.
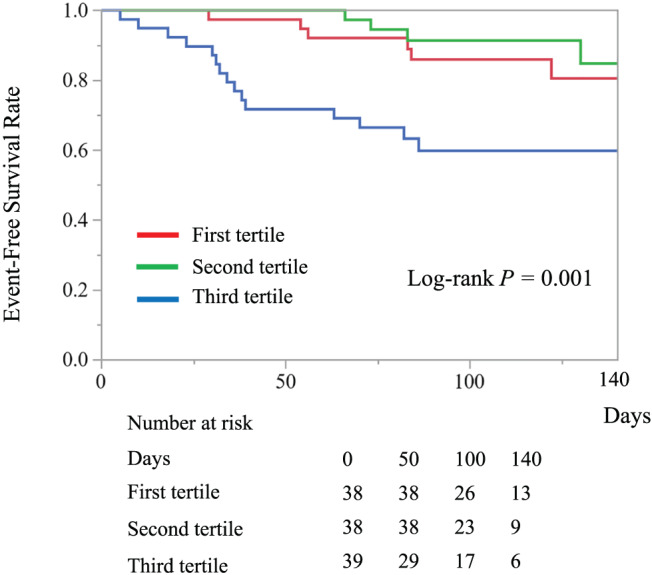
Kaplan–Meier curves of event‐free survival (all‐cause mortality and readmission for heart failure) for the three patient groups that comprise the first, second, and third tertiles of spleen stiffness.
Table 4.
Univariate Cox proportional‐hazards analysis for risk of a cardiac event
| Item | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Age (per 1 year increase) | 1.03 | 1.00–1.07 | 0.032 |
| Gender male | 0.52 | 0.23–1.20 | 0.12 |
| Body mass index (per 1 kg/m2 increase) | 0.83 | 0.72–0.94 | 0.004 |
| NYHA III or IV (vs. NYHA I or II) | 6.68 | 2.94–14.77 | <0.001 |
| Diabetes mellitus | 1.70 | 0.77–3.78 | 0.18 |
| Hypertension | 1.68 | 0.71–4.62 | 0.24 |
| COPD | 0.91 | 0.21 | 0.88 |
| Systolic blood pressure (per 1 mmHg increase) | 1.00 | 0.98–1.02 | 0.72 |
| Haemoglobin (per 1 g/dL increase) | 0.89 | 0.74–1.07 | 0.24 |
| Platelet count (per 1 × 103/μL increase) | 0.99 | 0.98–1.00 | 0.094 |
| Total bilirubin (per 1 mg/dL increase) | 2.60 | 0.52–10.93 | 0.22 |
| AST (per 1 U/L increase) | 1.01 | 0.98–1.03 | 0.28 |
| ALT (per 1 U/L increase) | 1.00 | 0.98–1.01 | 0.70 |
| GGT (per 1 U/L increase) | 1.00 | 0.99–1.00 | 0.21 |
| Albumin (per 1 g/dL increase) | 0.61 | 0.28–1.30 | 0.20 |
| Sodium (per 1 mmol/L increase) | 0.87 | 0.76–1.00 | 0.060 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.86 | 0.72–1.01 | 0.073 |
| ln (NT pro‐BNP) (per 1.0 increase) | 1.34 | 1.02–1.77 | 0.032 |
| Fibrosis‐4 index (per 1.0 increase) | 1.11 | 0.97–1.21 | 0.091 |
| NFS (per 1.0 increase) | 1.12 | 0.92–1.31 | 0.23 |
| LVDd (per 1 mm increase) | 0.97 | 0.93–1.02 | 0.34 |
| LVEF < 40% | 0.43 | 0.14–1.07 | 0.07 |
| LAD (per 1 mm increase) | 0.98 | 0.92–1.02 | 0.41 |
| RVDd (per 1 mm increase) | 1.07 | 1.01–1.12 | 0.047 |
| TRPG (per 1 mmHg increase) | 1.03 | 1.00–1.06 | 0.023 |
| Estimated RAP (per 1 mmHg increase) | 1.09 | 1.01–1.17 | 0.027 |
| MAGGIC risk score (per 1 increase) | 1.09 | 1.02–1.16 | 0.007 |
| Spleen diameter (per 1 mm increase) | 0.96 | 0.93–0.98 | 0.047 |
| Spleen stiffness (per 1 kPa increase) | 1.05 | 1.03–1.07 | <0.001 |
Table 5.
Multivariate Cox proportional‐hazards analysis for cardiac events
| Model | Spleen stiffness (per 1 kPa increase) | ||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| 1 (MAGGIC risk score) | 1.04 | 1.02–1.07 | <0.001 |
| 2 (Age, Gender, Total bilirubin, GGT) | 1.04 | 1.01–1.07 | 0.001 |
| 3 (Age, Gender, Fibrosis‐4 index) | 1.05 | 1.02–1.07 | <0.001 |
| 4 (Age, Gender, NFS) | 1.05 | 1.02–1.07 | <0.001 |
| 5 (Age, Gender, Estimated RAP) | 1.05 | 1.02–1.09 | <0.001 |
Other abbreviations are the same as in Table 1.
CI, confidence interval.
Discussion
This study is the first to evaluate the association between SS measured by two‐dimensional SWE and haemodynamic parameters and outcomes of HF. We present four major findings. First, SS positively correlated with PAWP and right‐sided cardiac pressure in patients with HF but not with CO. Especially, RAP independently correlated with SS. Second, in ADHF patients, those with higher values of SS had a positive correlation with clinical parameters related to HF severity (NYHA functional class and NT pro‐BNP), hepatic congestion (GGT), and echocardiographic indices related to RV failure (RVDd, TRPG, severity of TR, estimated RAP). Third, higher SS values were strongly associated with poor clinical outcomes, independent of conventional HF risk score. Fourth, higher SS values were strongly associated with poor clinical outcomes, independent of liver function test, LS, and estimated RAP. These findings suggest that the degree of SS at discharge can be used as a reliable marker of residual spleen congestion, which reflects the severity of HF and risk of adverse clinical outcome in patients with ADHF.
In patients with severe congestive HF, elevated central venous pressure causes hepatic congestion, and high hepatic vein pressure can cause post‐sinusoidal portal hypertension and portal congestion. Congestive splenomegaly is a one of the classical major signs of organ congestion of HF. 2 However, severity of splenic congestion has not been quantitatively evaluated in HF patients because the methods to evaluate it have not yet been established.
SWE is a method to measure tissue stiffness. The mechanism of SWE is as follows: a high‐intensity pulse is transmitted to an ROI within the tissue and the excited tissue emit shear waves, whose propagation velocity reflects the degree of stiffness. 19 High shear wave velocity reflects a stiffer tissue. SWE allow a fast and non‐invasive measurement of tissue stiffness and could be used as an objective, quantitative, first‐line measurement tool for tissue stiffness. In the last few years, SS measurement has been receiving increased attention in predicting EV and clinically significant portal hypertension in patients with chronic liver disease, because portal hypertension leads to splenic congestion and fibrosis, which increases organ stiffness. 20 However, the clinical significance of SS in congestive HF patients has not been investigated.
In the present study, we hypothesized that SS reflects the severity of splenic congestion and can be a negative prognostic indicator in HF. Our results showed that SS positively correlated with PAWP and right‐sided cardiac pressure in patients with chronic HF, but not CO and CI. These results confirm that SS is related to systemic congestion rather than poor forward flow. These results are consistent with the previously reported theory that abdominal organ dysfunction in HF is related to congestion resulting from right‐sided failure rather than low tissue perfusion. 21 In our results, RAP was independently related to SS, thus confirming that SS is a reflection of the severity of splenic congestion in HF.
Residual congestion at discharge following hospitalization for ADHF is the strongest predictor of subsequent readmission and cardiovascular mortality. As a result, alleviating congestion has traditionally been one of the primary goals of management of HF. However, objective and quantitative assessment of congestion is often difficult, especially when symptoms are mild, HF status is unclear, or echocardiogram images are insufficient. Indeed, 50% of patients with ADHF were reported to be discharged with residual congestion. 22 In the present study, higher SS values were associated with HF severity, hepatic congestion, and severity of RV failure. SS measured by SWE could be one of the useful markers to evaluate the degree of residual congestion in ADHF.
The present study investigated the prognostic value of SS in patients with ADHF and also investigated the prognostic value after adjustment for conventional HF risk models. The MAGGIC risk score is a well‐established comprehensive risk model and has been validated in patients with ADHF. 23 We showed that SS values were associated with worse clinical outcomes even after adjustment for this risk score. The measurement of SS by SWE is real time, objective, and non‐invasive. SS provides additional information on conventional HF risk scores for prediction of negative clinical outcomes. Elevated SS values at discharge indicate residual organ congestion and may further be a sign of persistent haemodynamic congestion due to severe HF or insufficient decongestion therapy. Therefore, SS measured by SWE may be a useful marker for improving risk stratification and clinical management of patients with ADHF.
Recent studies suggest that LS is a representative non‐invasive marker of liver congestion due to elevated central venous pressure, and it provides prognostic information in patients with ADHF. 24 , 25 Although the prognostic relevance of LS measured by two‐dimensional SWE has not been fully evaluated, the two markers of LS that we calculated in the present study (Fibrosis‐4 index and NFS) were reported to be strongly associated with poor outcomes in patients with HF. 26 , 27 Our result showed that, even after adjustment for LS and estimated RAP, SS was associated with cardiac events in ADHF. Therefore, we consider that SS has additional predictive value over and above LS and estimated RAP. The independent association of SS with cardiac events may be due to SS reflecting the degree of splenic damage along with elevated central venous pressure. Several recent basic and clinical studies have suggested the existence of biological interaction between the cardiovascular system and spleen, the so‐called cardio‐splenic axis. 28 , 29 For example, one basic report demonstrated that activation of inflammatory mononuclear cells in the spleen plays a key role in progression of HF. 29 Our findings suggest the potential of SS as a reflection of end‐organ splenic damage caused by HF and provides insights into the cardio‐splenic axis.
There are several limitations to this study. First, the study was performed at a single centre and included a small number of patients with a short follow‐up period. Second, although we visualized the morphology of the spleen by B‐mode US imaging and excluded patients with diseases affecting splenic stiffness (organic hepatic disorder and haematologic malignancies), an underlying histology of spleen fibrosis might have been present. Third, we measured SS by SWE only at discharge, so the changes of SS values during HF treatment were not evaluated. Therefore, the relationship between decreasing SS value with treatment and the clinical outcome of HF has been unclear. Fourth, although TE is one of the widely used methods to assess LS, in this study, we did not use this method to measure LS. Finally, this study included relatively haemodynamically stable HF patients in the haemodynamic study and a higher prevalence of HF with preserved ejection fraction patients in the outcome study. Therefore, further large‐scale multicentre studies are needed to confirm our findings in general ADHF patients.
Conclusions
Increased SS reflects elevated RAP, severity of HF, and RV failure. SS on discharge may be a useful prognostic marker in patients with ADHF. This non‐invasive technique of US imaging is useful to assess the degree of organ congestion and risk stratification of patients with ADHF.
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
Funding
This study was funded by Nihon University School of Medicine 50th Anniversary Fund Research Grant (2020) (Y.S.) and Grant‐in‐Aid for Early‐Career Scientists (Y.S.).
Saito, Y. , Matsumoto, N. , Aizawa, Y. , Fukamachi, D. , Kitano, D. , Kazuto, T. , Tamaki, T. , Fujito, H. , Sezai, A. , and Okumura, Y. (2020) Clinical significance of spleen stiffness in patients with acute decompensated heart failure. ESC Heart Failure, 7: 4005–4014. 10.1002/ehf2.13001.
References
- 1. Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009; 11: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ibrahim M, Sorour A, Elsherif A. Splenic enlargement in congestive heart failure and active rheumatic infection. Brit Heart J 1951; 13: 212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure‐related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol 2008; 52: 428–434. [DOI] [PubMed] [Google Scholar]
- 4. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, Fillieux L, Abraham WT, Januzzi J Jr, Sebbag L, Zannad F, Mebazaa A, Rossignol P. Integrative assessment of congestion in heart failure throughout the patient journey. JACC: Heart Fail 2018; 6: 273–285. [DOI] [PubMed] [Google Scholar]
- 5. Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, Uchino H. “Congestion index” of the portal vein. Am J Roentgenol 1986; 146: 735–739. [DOI] [PubMed] [Google Scholar]
- 6. Merkel C, Sacerdoti D, Bolognesi M, Bombonato G, Gatta A. Doppler sonography and hepatic vein catheterization in portal hypertension: assessment of agreement in evaluating severity and response to treatment. J Hepatol 1998; 28: 622–630. [DOI] [PubMed] [Google Scholar]
- 7. Denault AY, Azzam MA, Beaubien‐Souligny W. Imaging portal venous flow to aid assessment of right ventricular dysfunction. Can J Anaesth 2018; 65: 1260–1261. [DOI] [PubMed] [Google Scholar]
- 8. Ma X, Wang L, Wu H, Feng Y, Han X, Bu H, Zhu Q. Spleen stiffness is superior to liver stiffness for predicting esophageal varices in chronic liver disease: a meta‐analysis. PloS One 2016; 11: e0165786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tseng Y, Li F, Wang J, Chen S, Jiang W, Shen X, Wu S. Spleen and liver stiffness for noninvasive assessment of portal hypertension in cirrhotic patients with large esophageal varices. Journal of clinical ultrasound: JCU 2018; 46: 442–449. [DOI] [PubMed] [Google Scholar]
- 10. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 11. Albayrak E, Server S. The relationship of spleen stiffness value measured by shear wave elastography with age, gender, and spleen size in healthy volunteers. J Med Ultrason 2019; 46: 195–199. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich‐Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall der Medizin 2017; 38: e48. [DOI] [PubMed] [Google Scholar]
- 13. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 14. Khan MS, Siddiqi TJ, Khan SU, Shah SJ, VanWagner LB, Khan SS. Association of liver stiffness and cardiovascular outcomes in patients with heart failure: a systematic review and meta‐analysis. Eur J Prev Cardiol 2020; 27: 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45: 846–854. [DOI] [PubMed] [Google Scholar]
- 17. Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007; 20: 857–861. [DOI] [PubMed] [Google Scholar]
- 18. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 19. Muller M, Gennisson JL, Deffieux T, Tanter M, Fink M. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med Biol 2009; 35: 219–229. [DOI] [PubMed] [Google Scholar]
- 20. Abraldes JG, Reverter E, Berzigotti A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology 2013; 57: 1278–1280. [DOI] [PubMed] [Google Scholar]
- 21. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 23. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K, Yoshikawa T. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail 2018; 5: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taniguchi T, Ohtani T, Kioka H, Tsukamoto Y, Onishi T, Nakamoto K, Katsimichas T, Sengoku K, Chimura M, Hashimoto H, Yamaguchi O, Sawa Y, Sakata Y. Liver stiffness reflecting right‐sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging 2019; 12: 955–964. [DOI] [PubMed] [Google Scholar]
- 25. Saito Y, Kato M, Nagashima K, Monno K, Aizawa Y, Okumura Y, Matsumoto N, Moriyama M, Hirayama A. Prognostic relevance of liver stiffness assessed by transient elastography in patients with acute decompensated heart failure. Circ J 2018; 82: 1822–1829. [DOI] [PubMed] [Google Scholar]
- 26. Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver stiffness assessed by Fibrosis‐4 index predicts mortality in patients with heart failure. Open Heart 2017; 4: e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer‐Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015; 8: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 2014; 114: 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]


