Abstract
Aims
The mechanisms underlying the beneficial effect of ferric carboxymaltose (FCM) in patients with heart failure (HF) and iron deficiency (ID) have not been completely characterized. The Myocardial‐IRON trial was a double‐blind, randomized trial that evaluated myocardial iron repletion following FCM vs. placebo in 53 patients with HF and ID. In this post hoc analysis, we evaluated whether treatment with FCM was associated with cardiac magnetic resonance changes in left and right ventricular function (LVEF and RVEF, respectively) at different points of systolic dysfunction.
Methods and results
We included patients from the Myocardial‐IRON trial with left and right ventricular systolic dysfunction (LVSD and RVSD, respectively) at enrolment. Linear mixed regression models were used to evaluate changes at 7 and 30 days on LVEF and RVEF at cardiac magnetic resonance. At enrolment, 27 (50.9%) and 38 (71.7%) patients had LVEF < 40% (LVSD1) or <45% (LVSD2), respectively, and 10 (18.9%) and 17 (32.1%) patients had RVEF < 45% (RVSD1) or <51% in women and <52% in men (RVSD2), respectively. Treatment with FCM was associated with a significant improvement in LVEF at 30 days (LVSD1: Δ2.3%, P < 0.001; LVSD2: Δ4.1, P = 0.014). FCM was also associated with a significant and early improvement in RVEF at 7 days (RVSD1: Δ6.9%, P = 0.003; RVSD2: Δ3.2%, P = 0.003) that persisted at 30 days (RVSD1: Δ8.1%, P < 0.001; RVSD2: Δ4.7%, P < 0.001).
Conclusions
In patients with HF and systolic dysfunction with ID, FCM was associated with short‐term improvement in LVEF and, especially, in RVEF.
Keywords: Iron deficiency, Heart failure, Ventricular systolic function, Ferric carboxymaltose
Introduction
Iron deficiency (ID) is a common condition in patients with heart failure (HF), and it is associated with reduced functional capacity and worse prognosis. 1 , 2 , 3 Treatment with ferric carboxymaltose (FCM) has consistently been shown to improve surrogates of functional capacity and quality of life in patients with HF and reduced ejection fraction. 4 , 5 , 6 However, the mechanisms underlying such benefits remain controversial. 7 , 8 Different in vitro studies showed detrimental effects of ID on mitochondrial function among myoblasts and cardiomyocytes. 9 , 10 Iron treatment has been associated with improved muscular energetics in patients with HF. 11 However, evidence on the benefits of iron treatment on myocardial function is scarce. The Myocardial‐IRON trial of patients with chronic HF and ID showed that treatment with FCM causes short‐term changes in cardiac magnetic resonance (CMR) sequences suggestive of myocardial iron repletion. 12 Promising data from small clinical studies suggest that iron therapy may improve left ventricular ejection fraction (LVEF) on echocardiography, 13 , 14 but conflicting results have also been published. 15 Also, little is known about changes in the right ventricular ejection fraction (RVEF), especially on CMR, as a gold‐standard cardiac imaging technique.
In this subanalysis of the Myocardial‐IRON trial, we evaluated the association of treatment with FCM with short‐term changes in left and right ventricular systolic function in patients with established left and right ventricular systolic dysfunction (LVSD and RVSD, respectively) at enrolment.
Methods
Study sample and trial intervention
This is a post hoc analysis of an investigator‐initiated, multicentre, randomized, double‐blind, placebo‐controlled clinical trial designed to evaluate the effect of intravenous FCM vs. placebo on myocardial iron repletion estimated by T2* and T1 mapping CMR sequences, in patients with stable chronic HF (New York Heart Association Classes II and III), with systolic dysfunction (LVEF < 50%), and with ID (serum ferritin <100 or 100–299 μg/L with transferrin saturation <20%). Inclusion and exclusion criteria are listed in Supporting Information, Table S1 . The trial (NCT03398681) was conducted in five academic centres in Spain. Patients were randomized 1:1 to receive either FCM or placebo. FCM (Ferinject®, Vifor Pharma, Glattbrugg, Switzerland) was given intravenously as perfusion of 20 mL solution (equivalent to 1000 mg of iron) diluted in a sterile saline solution (0.9% NaCl) administered over at least 15 min. In the placebo group, an intravenous saline solution (0.9% weight/volume NaCl) was administered over the same time. The materials used in drug administration were entirely covered with aluminium foil, and the injection site shielded from the patient's view to ensure that patients were unaware of the treatment arm. Study personnel responsible for the study drug preparation and administration were not involved in any study assessment. At 30 days, patients assigned to placebo received intravenous FCM if ID persisted. The study design, protocol, and main results have been published and are available elsewhere. 12 , 16
Cardiac magnetic resonance
Cardiac magnetic resonance studies were performed by two experienced operators on a 1.5 T MR scanner (Essenza and Avanto, Siemens, Erlangen, Germany) using the spine and phased array six‐channel surface coils. All images were obtained with electrocardiographic gating and breath holding. Contiguous short‐axis cines from the atrioventricular ring to the apex were acquired at rest every 1 cm with steady‐state free precession imaging sequences (time resolution: 37 ms; voxel size: 1.7 × 1.7 × 7 mm).
LVEF and RVEF were calculated by semi‐automatic planimetry of endocardial and epicardial borders in the short‐axis view cine images. Further specifications of the CMR sequences are available elsewhere. 16 All measurements were made on the Syngo MR C15 (Siemens) platform by the same operator. CMR studies were performed at three time points: baseline and 7 and 30 days following treatment. CMR operators were blinded to treatment allocation.
Endpoints
The endpoints of this subanalysis were changes in LVEF and RVEF at 7 and 30 days after treatment in patients with established LVSD and RVSD at baseline. LVSD and RVSD were both defined at two cut‐offs: LVEF < 40% (LVSD1) or LVEF < 45% (LVSD2), and RVEF < 45% (RVSD1) or RVEF < 51% in women and <52% in men (RVSD2), based on recent recommendations from the European Association of Cardiovascular Imaging. 17
Secondary endpoints included the correlation between proxies of systemic iron repletion (ΔFerritin and ΔTSAT) and myocardial iron repletion (ΔT2* and ΔT1 mapping) and changes in LVEF and RVEF.
Statistical analysis
All statistical comparisons were performed under the intention‐to‐treat principle using Stata 15.1 (Stata Statistical Software, College Station, TX, USA). Observed mean ΔLVEF and ΔRVEF values across treatment allocation were reported and compared using the t‐test. Spearman correlation coefficient was used to explore the association among maximum absolute changes in parameters indicative of iron repletion (ΔFerritin, ΔTSAT, ΔT2*, and ΔT1 mapping) and absolute changes in LVEF and RVEF. Linear mixed regression models were used to evaluate the primary endpoints. All analyses were adjusted for age, gender, hospital (as a cluster variable), the interaction term treatment × visit (7 and 30 days), and the baseline value of the regressed outcome. As a prespecified analysis, no adjustment was made for multiple comparisons. Results from the linear mixed regression models are presented as least square means with their respective P‐values. A two‐sided P‐value of 0.05 was considered significant for all analyses.
Results
From May 2017 to June 2018, 53 patients were randomized to receive FCM (n = 27) or placebo (n = 26). All patients had ID at baseline. The mean age was 71.0 ± 9.8, and 13 (24.5%) patients were women. Ischaemic aetiology was present in 26 (49%) patients, and 50 (94.3%) were in NYHA Class II. Mean ± standard deviation LVEF and RVEF were 40.3 ± 10.4% and 56.3 ± 11.4%, and median (inter‐quartile range) were 39% (33–47) and 58% (49–63), respectively. Detailed baseline characteristics of the patients in the two study groups have been reported elsewhere. 12 , 16 At enrolment, LVSD1 and LVSD2 were found in 27 (50.9%) and 38 (71.7%) patients, respectively. The number of patients with RVSD1 and RVSD2 was 10 (18.9%) and 17 (32.1%), respectively. Baseline characteristics across treatment interventions at different cut‐offs of LVEF and RVEF are shown in Table 1 .
TABLE 1.
Baseline characteristics by treatment arm according to different cut points of left and right ventricular ejection fraction at enrolment
| Variables | LVEF < 40% | P‐value | LVEF < 45% | P‐value | RVEF < 45% | P‐value | RVEF < 51% in women or <52% in men | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | 27 (50.9) | 38 (71.7) | 10 (18.9%) | 17 (32.1%) | ||||||||
| Treatment arm | Placebo | Iron | Placebo | Iron | Placebo | Iron | Placebo | Iron | ||||
| n (%) | 17 (63) | 10 (37) | 20 (53) | 18 (47) | 4 (40) | 6 (60) | 7 (41) | 10 (59) | ||||
| Demographics and medical history | ||||||||||||
| Age (years) | 71 (67–73) | 70 (64–75) | 0.651 | 71 (65–78) | 73 (64–77) | 0.861 | 73 (68–82) | 67 (61–73) | 0.861 | 73 (67–78) | 65 (62–73) | 0.051 |
| Male, n (%) | 14 (82) | 10 (100) | 0.232 | 17 (85) | 14 (78) | 0.437 | 3 (75) | 4 (67) | 0.667 | 6 (86) | 7 (70) | 0.441 |
| Hypertension, n (%) | 12 (71) | 8 (80) | 0.475 | 13 (65) | 14 (78) | 0.307 | 4 (100) | 4 (67) | 0.333 | 5 (71) | 8 (80) | 0.559 |
| Diabetes mellitus, n (%) | 9 (53) | 9 (90) | 0.057 | 10 (50) | 12 (67) | 0.239 | 3 (75) | 3 (50) | 0.452 | 6 (86) | 6 (60) | 0.278 |
| Dyslipidaemia, n (%) | 11 (65) | 9 (90) | 0.161 | 12 (60) | 15 (83) | 0.110 | 4 (100) | 6 (100) | 1.000 | 5 (71) | 8 (80) | 0.559 |
| History of IHD, n (%) | 7 (41) | 6 (60) | 0.293 | 8 (40) | 8 (44) | 0.520 | 0 (0) | 1 (17) | 0.600 | 1 (14) | 3 (30) | 0.441 |
| Admission for AHF in last year, n (%) | 11 (65) | 5 (50) | 0.363 | 13 (65) | 10 (55) | 0.396 | 1 (25) | 4 (67) | 0.262 | 4 (57) | 5 (50) | 0.581 |
| History of atrial fibrillation, n (%) | 9 (53) | 3 (30) | 0.226 | 11 (55) | 5 (28) | 0.085 | 3 (75) | 3 (50) | 0.452 | 5 (71) | 5 (50) | 0.354 |
| Vital signs | ||||||||||||
| HR at baseline (b.p.m.) | 68 (64–84) | 73 (71–80) | 0.763 | 68 (64–81) | 73 (67–80) | 0.753 | 92 (71–104) | 77 (73–80) | 0.588 | 84 (59–100) | 79 (73–86) | 0.845 |
| Systolic blood pressure (mmHg) | 123 (113–131) | 111 (97–120) | 0.138 | 124 (113–139) | 118 (109–132) | 0.306 | 122 (120–125) | 105 (90–117) | 0.306 | 122 (113–129) | 111 (97–120) | 0.187 |
| Diastolic blood pressure (mmHg) | 66 (63–73) | 62 (58–68) | 0.151 | 66 (62–74) | 63 (58–70) | 0.232 | 68 (59–75) | 65 (59–70) | 0.213 | 65 (61–75) | 65 (59–70) | 0.353 |
| Laboratory | ||||||||||||
| Haemoglobin (g/dL) | 13.7 (13.1–14.6) | 13.2 (13–13.3) | 0.152 | 13.6 (12.9–14.6) | 12.8 (11.9–13.3) | 0.018 | 14.5 (13.6–14.7) | 12.7 (11.9–13.3) | 0.018 | 14.4 (12.8–14.8) | 12.9 (11.9–13.3) | 0.117 |
| Haematocrit (%) | 44.0 (40.6–45) | 41.5 (38.1–43) | 0.258 | 43.7 (40.5–45) | 39.6 (38–43) | 0.025 | 46.1 (43.1–47.5) | 40.0 (38–43) | 0.025 | 45.3 (41–47.2) | 41.5 (38–43) | 0.129 |
| Transferrin saturation (%) | 14.9 (9.0–20.5) | 15.3 (12.0–19.2) | 0.821 | 14.5 (9.3–20.2) | 14.1 (11.0–19.2) | 0.815 | 12.2 (9.6–18.4) | 14.1 (12–16) | 0.815 | 9.6 (9–14.9) | 14.1 (12–16) | 0.241 |
| Ferritin (ng/mL) | 48 (30–85) | 61 (56–81) | 0.353 | 48 (26–88) | 61 (48–99) | 0.273 | 36 (23–45) | 72 (56–271) | 0.273 | 42.0 (25–48) | 74 (59–101) | 0.004 |
| Creatinine (mg/dL) | 1.11 (0.95–1.46) | 1.11 (0.94–1.24) | 0.812 | 1.18 (0.95–1.51) | 1.12 (0.97–1.39) | 0.826 | 1.29 (1.06–1.44) | 1.05 (0.94–1.52) | 0.826 | 1.42 (1.11–1.48) | 1.12 (0.94–1.61) | 0.696 |
| eGFR (mL/min/1.73 m2) | 67.6 (46.8–79.3) | 69.6 (59.4–86.1) | 0.379 | 58.4 (45.5–79.2) | 59.7 (51.8–71.3) | 0.826 | 49.5 (48–64.5) | 62.0 (45–86.1) | 0.803 | 49.3 (46.8–70.6) | 58.4 (41.9.71.3) | 0.922 |
| Serum sodium (mEq/L) | 141 (140–142) | 140 (138–142) | 0.761 | 140 (139–142) | 140 (138–142) | 0.614 | 139 (136–141) | 140 (138–141) | 0.614 | 141 (137–144) | 140 (138–142) | 0.806 |
| NT‐proBNP (pg/mL) | 1760 (1026–2667) | 2628 (1728–2836) | 0.303 | 1503 (976–2898) | 2269 (1220–2836) | 0.511 | 2083 (1508–2352) | 3190 (2110–4998) | 0.502 | 2177 (1760–3999) | 2738 (2110–3952) | 0.588 |
| CMR parameters | ||||||||||||
| LVEDVI (mL/m2) | 119 (105–137) | 128 (104–148) | 0.900 | 122 (101–141) | 111 (98–141) | 0.334 | 115 (98–127) | 110 (73–141) | 0.334 | 105 (91–126) | 110 (78–141) | 0.922 |
| LVESVI (mL/m2) | 85 (68–99) | 86 (72–101) | 0.920 | 83 (67–95) | 73 (54–87) | 0.156 | 78 (62–94) | 73 (46–87) | 0.152 | 68 (55–89) | 64 (46–87) | 0.588 |
| LVEF (%) a | 31.7 ± 5.6 | 32.0 ± 5.3 | 0.919 | 33.6 ± 6.8 | 37.1 ± 7.2 | 0.912 | 31.7 ± 7.1 | 35.2 ± 7.3 | 0.139 | 34.4 ± 7.4 | 39 ± 6 | 0.238 |
| LVEF (%) | 33 (28–36) | 32 (27–38) | 0.919 | 34 (29–38) | 38 (31–44) | 0.139 | 33 (27–37) | 38 (27–41) | 0.280 | 36 (30–39) | 39 (38–44) | 0.238 |
| RVEF (%) a | 53.5 ± 10.5 | 51.3 ± 13.4 | 0.725 | 54.1 ± 10.1 | 52.9 ± 12.9 | 0.725 | 37.7 ± 2.6 | 38.3 ± 7.5 | 0.965 | 42.3 ± 6.4 | 42.8 ± 8.1 | 0.578 |
| RVEF (%) | 57 (48–59) | 53 (43–53) | 0.725 | 57 (47–61) | 53 (43–63) | 0.965 | 37 (35–39) | 40 (37–43) | 0.286 | 41 (36–48) | 44 (39–49) | 0.558 |
| T2* (ms) | 36 (32–42) | 41 (34–46) | 0.279 | 37 (32–42) | 39 (34–46) | 0.292 | 52 (40–68) | 39 (34–45) | 0.292 | 42.3 (33–59) | 42.8 (37–46) | 0.695 |
| T1 mapping (ms) | 1082 (1064–1132) | 1078 (1067–1096) | 0.651 | 1073 (1031–1130) | 1082 (1067–1122) | 0.293 | 1116 (1087–1142) | 1079 (1050–1096) | 0.292 | 1101 (1064–1138) | 1081 (1050–1096) | 0.435 |
| Medical treatment | ||||||||||||
| Diuretics, n (%) | 16 (94) | 9 (90) | 0.631 | 18 (90) | 16 (89) | 0.656 | 4 (100) | 5 (83) | 0.600 | 7 (100) | 9 (90) | 0.588 |
| MRA, n (%) | 11 (65) | 4 (40) | 0.402 | 12 (60) | 7 (39) | 0.135 | 4 (100) | 1 (17) | 0.024 | 4 (57) | 3 (30) | 0.268 |
| Beta‐blockers, n (%) | 13 (76) | 10 (100) | 0.136 | 15 (75) | 17 (94) | 0.115 | 2 (50) | 5 (83) | 0.333 | 4 (70) | 9 (90) | 0.162 |
| ACEI or ARB, n (%) | 7 (41) | 5 (50) | 0.481 | 7 (35) | 9 (50) | 0.272 | 1 (25) | 4 (67) | 0.262 | 3 (43) | 7 (70) | 0.268 |
| Sacubitril/valsartan, n (%) | 4 (23) | 4 (40) | 0.316 | 5 (20) | 7 (39) | 0.284 | 2 (50) | 1 (17) | 0.333 | 2 (28) | 1 (10) | 0.360 |
ACEI, angiotensin‐converting enzyme inhibitor; AHF, acute heart failure; ARB, angiotensin II receptor blocker; CMR, cardiac magnetic resonance; eGFR, estimated glomerular filtration rate; HR, heart rate; IHD, ischaemic heart disease; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; RVEF, right ventricular ejection fraction.
Values are expressed as median (inter‐quartile range); categorical variables are presented as percentages.
Values are expressed as mean ± standard deviation.
Iron treatment and left ventricular systolic function
In the whole sample, baseline LVEF was not different according to treatment allocation (P = 0.128). LVEF also did not significantly differ between FCM and placebo at 7 days (Figure 1 A ). Among patients with LVSD1 and LVSD2, we did not find significant differences in baseline characteristics across treatment interventions (Table 1 ). In the prespecified subgroups, observed means of LVEF across treatment allocation are presented in Supporting Information, Figure S1 . Overall, LVEF was higher in the active group at 30 days.
FIGURE 1.
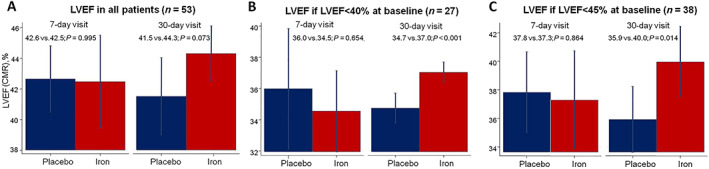
Differences in left ventricular ejection fraction (LVEF) on cardiac magnetic resonance (CMR) at 7 and 30 days following the administration of ferric carboxymaltose in patients included in the Myocardial‐IRON trial. Values are presented as the least square means from each mixed linear regression model. All models were adjusted by hospital (as a cluster variable), the interaction term treatment × visit (7 and 30 days), age, gender, and the baseline (pretreatment) value of the regressed outcome. (A) LVEF differences in all patients. (B) LVEF differences in patients with baseline LVEF < 40%. (C) LVEF differences in patients with baseline LVEF < 45%.
The inferential analysis showed that treatment with FCM was associated with a significant improvement in LVEF at 30 days (LVSD1: Δ2.3%, P < 0.001; LVSD2: Δ4.1, P = 0.014), but not at 7 days (Figure 1 B and 1 C ). Individual predicted values are shown in Supporting Information, Figure S2 . The improvement in LVEF was mainly at the expense of a reduction of the left ventricular end‐systolic volumes (Supporting Information, Figure S3 ). In patients with systolic dysfunction at baseline, we could not find any significant correlations among changes in proxies of iron repletion and changes in ventricular systolic function (Supporting Information, Table S2 ).
Iron treatment and right ventricular systolic function
In the entire sample, RVEF was not different across treatment arms (P = 0.908), and we did not find significant differences in RVEF changes across treatment arms at Day 7 or Day 30 (Figure 2 A ). Among patients with RVSD1 or RVSD2, we did not identify significant differences in RVEF or other important baseline characteristics across both treatment arms (Table 1 ). In both RVEF subgroups, the observed means of RVEF along the visits after the intervention were mainly higher in the active arm (Supporting Information, Figure S1 ). In multivariate setting, FCM was associated with a significant improvement in RVEF at 7 days (RVSD1: Δ6.9%, P = 0.003; RVSD2: Δ3.2%, P = 0.003) and 30 days (RVSD1: Δ8.1%, P < 0.001; RVSD2, Δ4.7%, P < 0.001; Figure 2 B and 2 C ). Individual predicted values are shown in Supporting Information, Figure S2 . We could not find a significant statistical association between proxies of iron repletion and changes in RVEF (Supporting Information, Table S2 ).
FIGURE 2.
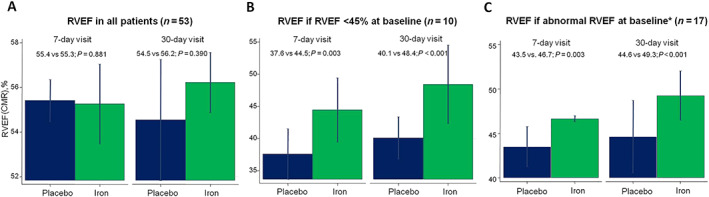
Differences in right ventricular ejection fraction (RVEF) on cardiac magnetic resonance (CMR) at 7 and 30 days following the administration of ferric carboxymaltose in patients included in the Myocardial‐IRON trial. Values are presented as the least square means from each mixed linear regression model. All models were adjusted by hospital (as a cluster variable), the interaction term treatment × visit (7 and 30 days), age, gender, and the baseline (pretreatment) value of the regressed outcome. (A) RVEF differences in all patients. (B) RVEF differences in patients with baseline RVEF < 45%. (C) RVEF differences in patients with RVEF < 51% in women and <52% in men*.
In a sensitivity analysis including only patients with RVSD at baseline, the improvement in LVEF was highly significant at 30 days, and changes in LVEF were, at least, similar in magnitude than those found for RVEF (ΔLVEF when RVSD1:10.5%, P = 0.003; ΔLVEF when RVSD2: 6.4%, P = 0.003) as it is shown in Figure 3 .
FIGURE 3.
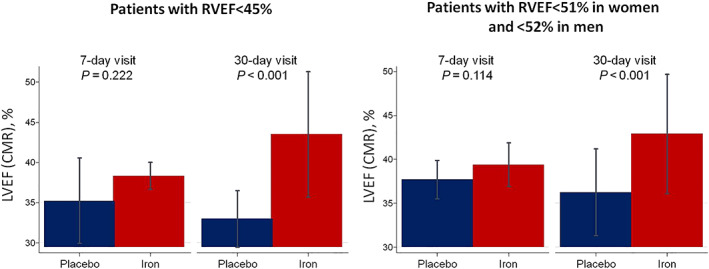
Differences in left ventricular ejection fraction (LVEF) on cardiac magnetic resonance (CMR) following the administration of ferric carboxymaltose in patients with right ventricular systolic dysfunction at enrolment. All models were adjusted by hospital (as a cluster variable), the interaction term treatment × visit (7 and 30 days), age, gender, and the baseline (pretreatment) value of the regressed outcome. RVEF, right ventricular ejection fraction.
Discussion
In this post hoc analysis of the Myocardial‐IRON trial conducted in patients with ID and systolic dysfunction at baseline, FCM was associated with a significant short‐term improvement in both LVEF and RVEF vs. placebo. The increase in RVEF was larger in magnitude and already present at 7 days following iron therapy. To the best of our knowledge, this is the first report of iron repletion resulting in a significant short‐term improvement of RVEF evaluated by CMR in patients with baseline RVSD.
Ferric carboxymaltose treatment and changes in left ventricular ejection fraction
Iron is an essential micronutrient in the mitochondrial function and energy production. Accumulating data from in vitro and basic research indicate that ID has deleterious effects on the contractile function of cardiomyocytes that can be reversed following iron repletion. 9 , 18 In a recent study on human embryonic cardiomyocytes derived from stem cells, ID resulted in impaired energy production with a profound decrease in the amount of phosphocreatine and ATP. 10 This mitochondrial dysfunction severely hampered mechanical function. The restitution of intracellular iron reversed the negative effects of ID on the cardiomyocytes. 10 In an animal model of ID, iron sucrose treatment prevented myocardial fibrosis and improved cardiac function. 13 Surprisingly, despite the growing evidence and research on the benefits of intravenous iron treatment in HF, 2 , 5 , 6 , 19 , 20 there are limited data on the effect of iron repletion on LVEF, especially in clinical studies on real‐world patients with HF. Some observational and interventional studies have suggested that iron therapy may be related to reverse cardiac remodelling and LVEF improvement on echocardiography. 13 , 15 , 21 , 22 Gaber et al. evaluated the effects of ID correction with iron dextran in 40 patients with LVEF < 40% and ID without anaemia. In this study, LVEF did not change significantly following the correction of ID (32 ± 8% vs. 34 ± 9%). However, there was an increase in the S′‐wave velocity and an improvement in peak systolic strain. 15 Usmanov et al. evaluated the effects of 26 weeks' treatment with intravenous iron sucrose (no control group) in 32 advanced HF who had anaemia and renal dysfunction, showing an improvement in LVEF and reduction in left ventricular volumes. However, this study does not allow us to separate the effect of correcting anaemia vs. iron treatment per se. 14 In the Myocardial‐IRON trial, albeit changes consistent with myocardial iron repletion on CMR sequences, we did not find an improvement in LVEF with FCM treatment. However, there was a trend towards increasing in LVEF at 30 days (44.8% vs. 40.9%, respectively; P = 0.056). Of note, the mean LVEF value on patients included in the trial was 40.3%. In the present subanalysis, when we restricted the analysis on patients with LVSD at enrolment (mean LVEF in LVSD1 and LVSD2 were 31.8% and 35.3%, respectively), differences were more evident and became significant. Our results are hypothesis generating but are in line with prior data, showing an improvement in LVEF at 30 days in patients with baseline LVSD after treatment with FCM.
In our study, we observed a reduction of left ventricular volumes following FCM treatment, but they were more marked and evident on left ventricular end‐systolic volume. This might reflect that FCM, at least at the short term, had a predominant effect on cardiac contractility instead of true remodelling (left ventricular end‐diastolic being less dependent on the contractility). However, further research will be necessary to provide definite answers. For instance, the IRON‐CRT trial is currently evaluating whether FCM is capable of improving LVEF and cardiac remodelling in patients with HF and reduced ejection fraction with an incomplete response to cardiac resynchronization therapy. 23 A planned echocardiographic substudy of the EFFECT‐HF study will also provide valuable information on this topic. 24 Future studies focusing on advance cardiac imaging techniques able to detect subtle changes in contractility, such as myocardial deformation analyses, and larger randomized controlled trials will help to better elucidate the potential benefits of iron treatment in the heart.
Ferric carboxymaltose treatment and changes in right ventricular ejection fraction
There are very limited data on the effect of iron therapy on the right heart. The myocardial iron load is reduced in the right ventricle in advanced HF. 25 Alioglu et al. showed that ID anaemia was negatively associated with right ventricular myocardial function indexes in a small study performed in children. 26 There is evidence from basic research that ID may be involved in the pathogenesis of pulmonary hypertension. In an animal model, ID rapidly promoted pulmonary vascular remodelling, pulmonary hypertension, and right ventricular hypertrophy. 27 Pulmonary vascular remodelling and haemodynamic changes induced by ID in rats were reversed by iron replacement. 27 The potential benefit of iron replacement therapy in the right ventricle and pulmonary circulation is an area of utmost interest. However, the potential benefit of iron on the right heart in HF has not been described yet. Darbepoetin α improved left and right ventricular function by echocardiography in a small randomized study on patients with anaemia and chronic HF, but this was not a study of iron repletion treatment. 28 Our research shows for the first time that treatment with FCM is associated with an early improvement in RVEF, which is maintained at 30 days. In addition, the magnitude of the changes appears to be higher than those found for the left ventricle (7–8% absolute increase in patients with RVEF < 45% at enrolment). Interestingly, changes in LVEF were bigger in magnitude (6–10%) when the analyses were limited to patients with baseline RVSD. The latter suggests that the improvement in RVEF may be, to a large extent, secondary to an increase in LVEF as a result of interventricular dependence in patients with biventricular dysfunction. However, the mechanisms underlying the improvement in RVEF following FCM treatment remain elusive. Other possibilities deserve to be mentioned. FCM may also directly exert positive effects on right myocardial contractility, induce positive changes in the pulmonary circulation, and therefore improve right ventricular to pulmonary artery coupling. In this work, we did not find a significant correlation among parameters of iron repletion and changes in ventricular systolic function. However, it may be due to a type II error given the limited sample size.
The improvement of RVEF following FCM treatment should be considered hypothesis generating. Still, it may help disentangle part of the mechanisms underlying the clinical benefits related to iron repletion therapy in HF. For instance, FCM can improve decongestion in the short term, as it has been reported in a recent substudy from the FAIR‐HF trial. 29 The early improvement in right ventricular performance in our study may partly help to explain this symptomatic benefit. The potential association of iron status and right HF should be confirmed and explored in upcoming studies.
Limitations
The present study has several limitations. First, this is a small post hoc analysis in which residual confounding may play an unmeasurable critical role. To confirm the beneficial effect of FCM on left and right ventricular function, a further randomized clinical trial, assuming a power of 0.9 and difference of 2 ± 4% in LVEF and 4 ± 5% in RVEF at 30 days favouring the active treatment, should at least include 80 and 52 patients, respectively. Second, more than half of the patients were non‐ischaemic, and the proportion of those with very severely reduced LVEF was low. Left gadolinium enhancement imaging was not performed systematically per study protocol. Thus, extrapolation of these findings to less selected samples, patients with advanced ischaemic heart disease with extensive scars, and more severe forms of systolic dysfunction remains to be confirmed. Third, due to the small sample size and study design, we could not evaluate the effect of FMC on pulmonary artery pressures or other pulmonary circulation parameters, which could help to explain the benefit of FCM on the right heart. Fourth, the lack of advanced imaging methods for evaluating systolic dysfunction should be also be remarked. Albeit the sample size is small, the placebo‐controlled nature of the trial, with investigators and operators blinded to treatment intervention, and the use of CMR as a standard gold technique in LVEF and RVEF assessment are the major strengths of the study. 30 , 31
Conclusions
In this post hoc analysis from the Myocardial‐IRON trial on patients with stable HF and ID with systolic dysfunction at baseline, treatment with FCM was associated with short‐term improvements in LVEF on CRM but especially in RVEF. Further trials should confirm these findings and explore the potential benefit of FCM on the right heart.
Conflict of interest
J.N. received board speaker fees and travel expenses from Novartis, Roche Diagnostics, Abbott, Rovi, Vifor Pharma, Daiichi Sankyo, Novonordisk, Boehringer Ingelheim, and AstraZeneca (modest). L.F. received speaker fees and travel expenses from Novartis (modest). J.S. received speaker fees from AstraZeneca, Abbott, and Edwards Lifesciences (modest). A.B.‐G. received board membership fees and travel expenses from Novartis, Roche Diagnostics, Vifor Pharma, and Critical Diagnostics (modest). All other authors declare no competing interests.
Funding
This work was supported in part by an unrestricted grant from Vifor Pharma and Proyectos de Investigación de la Sección de Insuficiencia Cardiaca 2017 from Sociedad Española de Cardiología.
Supporting information
Table S1. Inclusion and exclusion criteria in the Myocardial‐IRON trial.
Table S2. Spearman correlations among changes in parameters of iron repletion and changes in left and right ventricular systolic function
Figure S1. Observed means of left and right ventricular ejection fraction on cardiac magnetic resonance at baseline, 7 and 30 days across treatment allocation in patients included in the Myocardial‐IRON trial. CMR: cardiac magnetic resonance; LVEF: left ventricular ejection fraction; NS: non‐significant; RVEF: right ventricular ejection fraction.
Figure S2. Individual predicted values of left and right ventricular ejection fraction at 7 and 30 days across treatment allocation in patients included in the Myocardial‐IRON trial. All models were adjusted by hospital (as a cluster variable), the interaction term treatment*visit (7 and 30‐d), age, gender, and the baseline (pre‐treatment) value of the regressed outcome CMR: cardiac magnetic resonance; LVEF: left ventricular ejection fraction; RVEF: right ventricular ejection fraction.
Figure S3. Absolute changes in indexed left ventricular volumes at 7 and 30 days following the administration of ferric carboxymaltose in patients included in the Myocardial‐IRON trial. LVEF: left ventricular ejection fraction; LVEDV: left ventricular end‐diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end‐systolic volume; MRI: magnetic resonance imaging.
Santas, E. , Miñana, G. , Cardells, I. , Palau, P. , Llàcer, P. , Fácila, L. , Almenar, L. , López‐Lereu, M. P. , Monmeneu, J. V. , Sanchis, J. , Maceira, A. M. , Bayés‐Genís, A. , Núñez, J. , and Myocardial‐IRON Investigators (2020) Short‐term changes in left and right systolic function following ferric carboxymaltose: a substudy of the Myocardial‐IRON trial. ESC Heart Failure, 7: 4222–4230. 10.1002/ehf2.13053.
References
- 1. Rocha BML, Cunha GJL, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol 2018; 71: 782–793. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Phjantahti‐Maroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Núñez J, Comín‐Colet J, Miñana G, Núñez E, Santas E, Mollar A, Valero E, García‐Blas S, Cardells I, Bodí V, Chorro FJ, Sanchis J. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail 2016; 18: 798–802. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P, FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghafourian K, Chang HC, Ardehali H. Intravenous iron therapy in heart failure: a different perspective. Eur J Heart Fail 2019; 21: 703–714. [DOI] [PubMed] [Google Scholar]
- 8. Ghafourian K, Shapiro JS, Goodman L, Ardehali H. Iron and heart failure. J Am Coll Cardiol Basic Trans Science 2020; 5: 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobak KA, Radwańska M, Dzięgała M, Kasztura M, Josiak K, Banasiak W, Ponikowski P, Jankowska EA. Structural and functional abnormalities in iron‐depleted heart. Heart Fail Rev 2019; 24: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, Rodenburg RJ, van Veldhuisen DJ, de Boer RA, van der Meer P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail 2018; 20: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charles‐Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, Monaghan M, Amin‐Youssef G, Kemp GJ, Shah AM, Okonko DO. Effect of iron isomaltoside on skeletal muscle energetic in patients with chronic heart failure and iron deficiency: FERRIC II randomized mechanistic trial. Circulation 2019; 139: 2386–2398. [DOI] [PubMed] [Google Scholar]
- 12. Nuñez J, Minana G, Cardells I, Palau P, Llacer P, Facila L, Almenar L, Lopez‐Lereu MP, Monmeneu JV, Amiguet M, Gonzalez J, Serrano A, Montagud V, Lopez‐Vilella R, Valero E, Garcia‐Blas S, Bodi V, de la Espriella‐Juan R, Lupon J, Navarro J, Gorriz JL, Sanchis J, Chorro FJ, Comin‐Colet J, Bayes‐Genis A, Myocardial IId . Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the Myocardial‐IRON trial. J Am Heart Assoc 2020. 18; 9: e014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toblli JE, Cao G, Rivas C, Giani JF, Dominici FP. Intravenous iron sucrose reverses anemia‐induced cardiac remodeling, prevents myocardial fibrosis, and improves cardiac function by attenuating oxidative/nitrosative stress and inflammation. Int J Cardiol 2016; 212: 84–91. [DOI] [PubMed] [Google Scholar]
- 14. Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol 2008; 21: 236–242. [PubMed] [Google Scholar]
- 15. Gaber R, Kotb NA, Ghazy M, Nagy HM, Salama M, Elhendy A. Tissue Doppler and strain rate imaging detect improvement of myocardial function in iron deficient patients with congestive heart failure after iron replacement therapy. Echocardiography 2012; 29: 13–18. [DOI] [PubMed] [Google Scholar]
- 16. Miñana G, Cardells I, Palau P, Llacer P, Fácila L, Almenar L, Lopez‐Lereu MP, Monmeneu JV, Amiguet M, González J, Serrano A, Montagud V, López‐Vilella R, Valero E, García‐Blas S, Bodí V, de la Espriella‐Juan R, Sanchis J, Chorro FJ, Bayés‐Genís A, Núñez J, Myocardial‐IRON Investigators . Changes in myocardial iron content following administration of intravenous iron (Myocardial‐IRON): study design. Clin Cardiol 2018; 41: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen SE, Khanji MY, Plein S, Lancelloti P, Bucciarelli‐Ducci C. European Association of Cardiovascular Imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging 2019; 20: 1321–1331. [DOI] [PubMed] [Google Scholar]
- 18. Bakogiannis C, Briasoulis A, Mouselimis D, Tsarouchas A, Papageorgiu N, Papadopoulus C, Fragakis N, Vassilikos V. Iron deficiency as therapeutic target in heart failure: a translational approach. Heart Fail Rev 2019; 25: 173–182. [DOI] [PubMed] [Google Scholar]
- 19. Jankowka EA, Tkcazyszyn M, Suchoki T, Drodz M, von Haeling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 20. Zhou X, Xu W, Xu Y, Quian Z. Iron supplementation improves cardiovascular outcomes in patients with heart failure. Am J Med 2019; 132: 955–963. [DOI] [PubMed] [Google Scholar]
- 21. Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ 2015; 24: 686–695. [DOI] [PubMed] [Google Scholar]
- 22. Núñez J, Monmeneu JV, Mollar A, Núñez E, Bodí V, Miñana G, García‐Blas S, Santas E, Aguero J, Chorro FJ, Sanchis J, López‐Lereu MP. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail 2016; 3: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martens P, Dupont M, Dauw J, Somers F, Herbots L, Timmermans P, Verweft J, Mullens W. Rationale and design of the IRON‐CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy. ESC Heart Fail 2019; 6: 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, Cohen‐Solal A, EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lescek P, Sochanowicz B, Szperl M, Kolsut P, Brzoska K, Piotrowski W, Rywik TM, Danko B, Polkowska‐Montero H, Rozanski JM, Kruszewski M. Myocardial iron homeostasis in advanced chronic heart failure patients. Int J Cardiol 2012; 159: 47–52. [DOI] [PubMed] [Google Scholar]
- 26. Alioglu B, Cetin II, Emeksiz ZS, Dindar N, Tapci E, Dallar Y. Iron deficiency anemia in infants: does it really affect the myocardial functions? Pediatr Hematol Oncol 2013; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 27. Cotroneo E, Ashek A, Wang L, Wharton J, Dubois O, Bozorgi S, Busbridge M, Alavian KN, Wilkins MR, Zhao L. Iron homeostasis and pulmonary hypertension: iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res 2015; 116: 1680–1690. [DOI] [PubMed] [Google Scholar]
- 28. Parissis JT, Kourea K, Panou F, Farmakis D, Paraskevaidis I, Ikonomidis I, Filippatos G, Kremastinos DT. Effects of darbepoetin α on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J 2008; 155:751: e751–e757. [DOI] [PubMed] [Google Scholar]
- 29. Okonko DO, Jouhra F, Abu‐Own H, Filippatos G, Colet JC, Suki C, Mori C, Ponikowski P, Anker SD, FAIR‐HF Investigators . Effect of ferric carboxymaltose on calculated plasma volumen status and clinical congestion: a FAIR‐HF substudy. ESC Heart Fail 2019; 6: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellenger NG, Burgess MI, Ray SG, Lahiti A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are the interchangeable? Eur Heart J 2000; 21: 1387–1396. [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Prakasa K, Bomma C, Tandri H, Dalal D, James C, Tichnell C, Corretti M, Bluemke D, Calkins H, Abraham TP. Comparison of novel echocardiographic parameters of right ventricular function with ejection fraction by cardiac magnetic resonance. J Am Soc Echocardiogr 2007; 20: 1058–1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria in the Myocardial‐IRON trial.
Table S2. Spearman correlations among changes in parameters of iron repletion and changes in left and right ventricular systolic function
Figure S1. Observed means of left and right ventricular ejection fraction on cardiac magnetic resonance at baseline, 7 and 30 days across treatment allocation in patients included in the Myocardial‐IRON trial. CMR: cardiac magnetic resonance; LVEF: left ventricular ejection fraction; NS: non‐significant; RVEF: right ventricular ejection fraction.
Figure S2. Individual predicted values of left and right ventricular ejection fraction at 7 and 30 days across treatment allocation in patients included in the Myocardial‐IRON trial. All models were adjusted by hospital (as a cluster variable), the interaction term treatment*visit (7 and 30‐d), age, gender, and the baseline (pre‐treatment) value of the regressed outcome CMR: cardiac magnetic resonance; LVEF: left ventricular ejection fraction; RVEF: right ventricular ejection fraction.
Figure S3. Absolute changes in indexed left ventricular volumes at 7 and 30 days following the administration of ferric carboxymaltose in patients included in the Myocardial‐IRON trial. LVEF: left ventricular ejection fraction; LVEDV: left ventricular end‐diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end‐systolic volume; MRI: magnetic resonance imaging.


