Abstract
Aims
Cardiomyopathies are a heterogeneous group of disorders that increase the risk for atrial fibrillation (AF). The aim of the study is to assess the prevalence of AF, anticoagulation management, and risk of stroke/transient ischaemic attack (TIA) in patients with cardiomyopathy.
Methods and results
Three thousand two hundred eight consecutive adult patients with cardiomyopathy (34.9% female; median age: 55.0 years) were prospectively enrolled as part of the EURObservational Research Programme Cardiomyopathy/Myocarditis Registry. At baseline, 903 (28.2%) patients had AF (29.4% dilated, 27.5% hypertrophic, 51.5% restrictive, and 14.7% arrhythmogenic right ventricular cardiomyopathy, P < 0.001). AF was associated with more advanced New York Heart Association class (P < 0.001), increased prevalence of cardiovascular risk factors and co‐morbidities, and a history of stroke/TIA (P < 0.001). Oral anticoagulation was administered in 71.7% of patients with AF (vitamin K antagonist: 51.6%; direct oral anticoagulant: 20.1%). At 1 year follow‐up, the incidence of cardiovascular endpoints was as follows: stroke/TIA 1.85% (AF vs. non‐AF: 3.17% vs. 1.19%, P < 0.001), death from any cause 3.43% (AF vs. non‐AF: 5.39% vs. 2.50%, P < 0.001), and death from heart failure 1.67% (AF vs. non‐AF: 2.44% vs. 1.31%, P = 0.033). The independent predictors for stroke/TIA were as follows: AF [odds ratio (OR) 2.812, P = 0.005], history of stroke (OR 7.311, P = 0.010), and anaemia (OR 3.119, P = 0.006).
Conclusions
The study reveals a high prevalence and diverse distribution of AF in patients with cardiomyopathies, inadequate anticoagulation regimen, and high risk of stroke/TIA in this population.
Keywords: Hypertrophic cardiomyopathy, Dilated cardiomyopathy, Restrictive cardiomyopathy, Arrhythmogenic right ventricular cardiomyopathy, Atrial fibrillation, Anticoagulation
Introduction
Cardiomyopathies are heterogeneous disorders characterized by structural and functional abnormalities of the myocardium. All subtypes of cardiomyopathy are associated with an increased risk of atrial fibrillation (AF), 1 , 2 , 3 , 4 , 5 , 6 , 7 but current guidelines for the management of AF 8 lack detailed recommendations on its management.
The EURObservational Research Programme (EORP) Cardiomyopathy/Myocarditis Registry collects prospective data on patients with cardiomyopathy and myocarditis. Its general aim is to provide insight into clinical presentation and management of contemporary patients with heart muscle disease across a large range of centres in Europe so as to improve clinical service provision and therapy. 9
We hypothesized that a focused analysis of the data contained in the registry might give insight into the prevalence of AF and AF risk factors, reveal differences between different subtypes of cardiomyopathies, allow to compare the recommendations with real‐life data on anticoagulation regimens, and assess the risk of stroke in these populations.
The aim of this study was to determine the AF prevalence, anticoagulation management [vitamin K antagonist (VKA)/direct oral anticoagulant (DOAC)], and 1 year risk of stroke/transient ischaemic attack (TIA) in adult patients with cardiomyopathy enrolled into the EORP Cardiomyopathy/Myocarditis Registry. 9
Methods
General design
The EORP Cardiomyopathy/Myocarditis Registry conceived by the European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Disease is a prospective observational multinational registry of consecutive patients presenting to centres in European countries. The protocol of the registry and data on the participating centres are presented elsewhere. 9 The registry protocol was approved by each local ethics committee according to the local rules and regulations. Written informed consent was obtained from all participants before collection of any data. All diagnostic or therapeutic procedures and decisions were left to the discretion of an attending physician.
Baseline and 1 year follow‐up data (including demographic, clinical, cardiac, genetic, and therapeutic data) were collected using a web‐based system with an electronic case report form. The EORP department of the ESC was responsible for study management, data quality control, and statistical analyses.
Patients and cardiomyopathy subtypes
Four subtypes of cardiomyopathies were eligible for inclusion in the study: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), and arrhythmogenic right ventricular cardiomyopathy (ARVC). Patients were recruited between 1 December 2012 and 30 December 2016 in 68 centres located in 18 countries; obligatory number of the enrolled patient was 40 per centre. Patients met the following inclusion criteria: age above 18 years, willingness and ability to give informed consent, and a cardiomyopathy fulfilling standard diagnostic criteria for probands or relatives. General clinical characteristic of cardiomyopathy subtypes as well as a few important definitions used for analyses were reported in the first publication of the registry data. 9
Atrial fibrillation
Atrial fibrillation was defined as any form of AF (paroxysmal, persistent, and permanent). The whole cardiomyopathy population was divided into subjects with AF (AF population) and without AF (non‐AF population).
Anticoagulation and antiplatelet therapy
In the AF population, data on oral anticoagulation (OAC; VKA and DOAC) and antiplatelet therapy (aspirin and P2Y12 inhibitors) were obtained.
Clinical endpoints
The following adverse cardiovascular endpoints were reported at the 1 year follow‐up: stroke/TIA (fatal ischaemic stroke and non‐fatal stroke/TIA), death from any cause, death from heart failure, death from ischaemic stroke, death from haemorrhagic stroke, and death from systemic haemorrhage.
Statistical analysis
Univariate analysis was applied to both continuous and categorical variables. Continuous variables were reported as mean ± standard deviation. Among‐group comparisons were made using the non‐parametric test (Kruskal–Wallis test). Categorical variables were reported as percentages. Among‐group comparisons were made using the χ 2 test or Fisher's exact test if any expected cell count was less than five. Stepwise multivariable logistic regression analyses were performed to establish the relationship between the patient characteristics and (i) the presence of AF in each subtype of cardiomyopathies and (ii) the overall presence of stroke/TIA on follow‐up in whole cardiomyopathy population including into the model all the candidate variables (variables with P < 0.10 in univariate). A significance level of 0.05 was required for a variable to stay in the model. No interaction was tested. To verify that the models were optimal, Hosmer and Lemeshow goodness‐of‐fit test and per cent concordant were calculated. A two‐sided P‐value of <0.05 was considered as statistically significant. All analyses were performed using SAS statistical software, Version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographic data
Three thousand two hundred eight adult patients with cardiomyopathy (34.9% female; median age: 53.0) were recruited: 1260 (39.3%) with DCM, 1739 (54.2%) with HCM, 66 (2.1%) with RCM, and 143 (4.5%) with ARVC. Follow‐up data at 1 year were obtained in 2713 patients (84.6%), including 1105 (40.7%) with DCM, 1420 (52.3%) with HCM, 60 (2.2%) with RCM, and 128 (4.7%) with ARVC.
Rate of atrial fibrillation in cardiomyopathy population
Atrial fibrillation was present at baseline in 903 (28.2%) individuals: 370 (29.4%) with DCM, 478 (27.5%) with HCM, 34 (51.5%) with RCM, and 21 (14.7%) with ARVC (P < 0.001). Paroxysmal AF occurred mainly in AF patients with HCM (54.7%) and ARVC (83.3%). Permanent AF was the most common type in RCM (71.9%). Newly diagnosed AF was registered during follow‐up in 95 (3.0%) individuals: 41 (3.8%) with DCM, 48 (2.8%) with HCM, 3 (4.5%) with RCM, and 3 (2.1%) with ARVC. The proportion of patients with AF at baseline and at follow‐up in different cardiomyopathies is presented in Table 1 and Figure 1 .
TABLE 1.
Prevalence of AF in different types of cardiomyopathies
| Variable | Type of cardiomyopathy | P‐value | ||||
|---|---|---|---|---|---|---|
| All (N = 3208) | DCM (N = 1260) | HCM (N = 1739) | RCM (N = 66) | ARVC (N = 143) | ||
| AF at baseline | 903/3208 (28.15%) | 370/1260 (29.37%) | 478/1739 (27.49%) | 34/66 (51.52%) | 21/143 (14.69%) | <0.001 |
| Baseline—type of AF | ||||||
| Paroxysmal | 394/836 (47.13%) | 129/338 (38.17%) | 245/448 (54.69%) | 5/32 (15.63%) | 15/18 (83.33%) | <0.001 |
| Persistent | 149/836 (17.82%) | 64/338 (18.93%) | 79/448 (17.63%) | 4/32 (12.50%) | 2/18 (11.11%) | |
| Permanent | 293/836 (35.05%) | 145/338 (42.90%) | 124/448 (27.68%) | 23/32 (71.88%) | 1/18 (5.56%) | |
| AF at 1 year follow‐up | 998/3208 (31.11%) | 411/1260 (32.62%) | 526/1739 (30.25%) | 37/66 (56.06%) | 24/143 (16.78%) | <0.001 |
AF, atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy.
FIGURE 1.
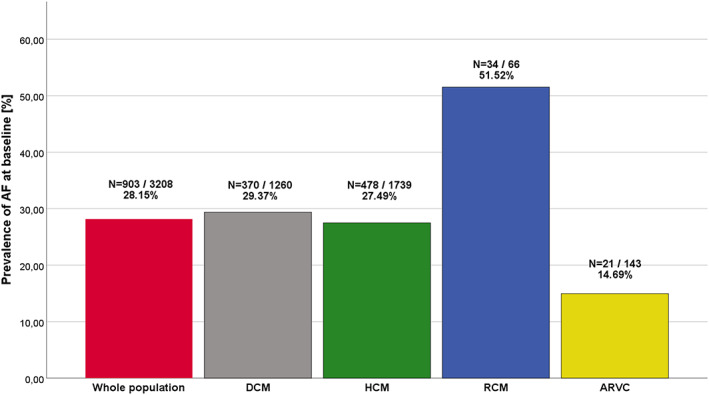
Prevalence of atrial fibrillation (AF) in cardiomyopathy populations at baseline. ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy.
Demographic and clinical characteristics of atrial fibrillation and non‐atrial fibrillation populations at baseline
There were some differences in baseline characteristics between patients with and without AF (Table 2 ). Age at enrolment (58.7 ± 13.6 vs. 50.9 ± 15.2 years, P < 0.001) and age at the first evaluation in the centre (54.0 ± 14.8 vs. 47.3 ± 15.9 years, P < 0.001) were greater in AF subjects. Patients with AF had larger body mass index (27.5 ± 5.0 vs. 26.8 ± 4.9 kg/m2, P < 0.001). New York Heart Association (NYHA) class was more advanced in AF (NYHA I/II/III/IV: 18.2/48.4/28.3/5.1%) than in non‐AF subjects (NYHA I/II/III/IV: 32.7/45.3/18.5/3.5%, P < 0.001). History of arrhythmias: sustained ventricular tachycardia (14.4% vs. 9.9%, P < 0.001), resuscitated ventricular fibrillation/cardiac arrest (5.1% vs. 3.6%, P = 0.048), and atrioventricular block (11.8% vs. 8.4%, P = 0.015) were more frequent in AF than in non‐AF population. History of stroke/TIA was positive in 10.3% of AF population and in 4.6% of non‐AF population (P < 0.001).
TABLE 2.
Demographic and clinical characteristics in AF and non‐AF populations of cardiomyopathy patients
| Variable | AF (N = 998) | Non‐AF (N = 2210) | P‐value | OR [95% CI] | OR P‐value |
|---|---|---|---|---|---|
| Age at enrolment (years), mean ± SD | 58.7 (±13.6) | 50.9 (±15.2) | <0.001 | 1.038 [1.032–1.044] | <0.001 |
| Age at first evaluation in the centre (years), mean ± SD | 54.0 (±14.8) | 47.3 (±15.9) | <0.001 | 1.029 [1.023–1.034] | <0.001 |
| Gender—female | 327/998 (32.77%) | 792/2210 (35.84%) | 0.091 | 0.873 [0.745–1.022] | 0.091 |
| Body mass index (kg/m2), mean ± SD | 27.5 (±5.0) | 26.8 (±4.9) | <0.001 | 1.029 [1.013–1.045] | <0.001 |
| Inherited metabolic disorder | 2/638 (0.31%) | 19/1436 (1.32%) | 0.054 | 0.238 [0.055–1.024] | 0.054 |
| Mitochondrial disorder | 2/996 (0.20%) | 8/2202 (0.36%) | 0.734 | 0.554 [0.117–2.610] | 0.455 |
| Neuromuscular diseases | 10/638 (1.57%) | 20/1455 (1.37%) | 0.733 | 1.143 [0.532–2.455] | 0.733 |
| NYHA class | |||||
| NYHA I | 160/877 (18.24%) | 573/1755 (32.65%) | <0.001 | Reference | Reference |
| NYHA II | 424/877 (48.35%) | 795/1755 (45.30%) | 1.910 [1.546–2.359] | <0.001 | |
| NYHA III | 248/877 (28.28%) | 325/1755 (18.52%) | 2.733 [2.148–3.477] | <0.001 | |
| NYHA IV | 45/877 (5.13%) | 62/1755 (3.53%) | 2.599 [1.705–3.964] | <0.001 | |
| History of arrhythmias | |||||
| History of sustained VT | 144/998 (14.43%) | 218/2210 (9.86%) | <0.001 | 1.541 [1.230–1.930] | <0.001 |
| History of resuscitated VF/cardiac arrest | 51/998 (5.11%) | 80/2210 (3.62%) | 0.048 | 1.434 [1.001–2.054] | 0.049 |
| History of AV block | 75/638 (11.76%) | 122/1455 (8.38%) | 0.015 | 1.456 [1.074–1.973] | 0.015 |
| History of BBB | 161/638 (25.24%) | 295/1455 (20.27%) | 0.011 | 1.327 [1.066–1.653] | 0.012 |
| Family history of sudden death | 168/897 (18.73%) | 356/2072 (17.18%) | 0.310 | 1.111 [0.907–1.361] | 0.310 |
| History of stroke: TIA or stroke | 102/991 (10.29%) | 101/2200 (4.59%) | <0.001 | 2.384 [1.791–3.174] | <0.001 |
| Co‐morbidities | |||||
| Arterial hypertension | 409/998 (40.98%) | 755/2210 (34.16%) | <0.001 | 1.338 [1.147–1.561] | <0.001 |
| Diabetes mellitus type I or II | 157/998 (15.73%) | 246/2210 (11.13%) | <0.001 | 1.490 [1.201–1.850] | <0.001 |
| Hyperlipidaemia/dyslipidaemia | 396/998 (39.68%) | 757/2210 (34.25%) | 0.003 | 1.263 [1.082–1.473] | 0.003 |
| Renal impairment | 177/998 (17.74%) | 168/2210 (7.60%) | <0.001 | 2.620 [2.090–3.284] | <0.001 |
| Chronic obstructive pulmonary disease | 68/998 (6.81%) | 90/2210 (4.07%) | <0.001 | 1.723 [1.246–2.382] | 0.001 |
| Anaemia | 80/990 (8.08%) | 113/2187 (5.17%) | 0.001 | 1.614 [1.199–2.171] | 0.002 |
| Lifestyle | |||||
| Physical activity | 346/776 (44.59%) | 918/1719 (53.40%) | <0.001 | 0.702 [0.592–0.832] | <0.001 |
| None | 430/776 (55.41%) | 801/1719 (46.60%) | <0.001 | Reference | Reference |
| Occasionally | 213/776 (27.45%) | 497/1719 (28.91%) | 0.798 [0.654–0.974] | 0.026 | |
| Regularly | 120/776 (15.46%) | 352/1719 (20.48%) | 0.635 [0.501–0.806] | <0.001 | |
| Intensely | 13/776 (1.68%) | 69/1719 (4.01%) | 0.351 [0.192–0.642] | <0.001 | |
| Alcohol use (any amount) | 278/820 (33.90%) | 569/1825 (31.18%) | 0.165 | 1.132 [0.950–1.349] | 0.165 |
| Smoking (current or former) | 310/927 (33.44%) | 725/2051 (35.35%) | 0.311 | 0.919 [0.780–1.082] | 0.312 |
AF, atrial fibrillation; AV, atrioventricular; BBB, bundle branch block; CI, confidence interval; NYHA, New York Heart Association; OR, odds ratio; SD, standard deviation; TIA, transient ischaemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
The following co‐morbidities were more prevalent in cardiomyopathy patients with AF: arterial hypertension (41.0% vs. 34.2%, P < 0.001), diabetes mellitus type II (15.0% vs. 10.4%, P < 0.001), hyperlipidaemia (39.7% vs. 34.3%, P = 0.003), renal impairment (17.7% vs. 7.6%, P < 0.001), chronic obstructive pulmonary disease (6.8% vs. 4.1%, P < 0.001), and anaemia (8.1% vs. 5.2%, P < 0.001). Patients with AF reported less physical activity than those without AF (44.6% vs. 53.4%, P < 0.001).
Multivariate logistic regression analysis revealed that the independent predictors of AF in DCM population were as follows: age at enrolment [odds ratio (OR) 1.042, P < 0.001] and left atrium diameter (OR 1.069, P < 0.001); in the HCM population: age at enrolment (OR 1.068, P < 0.001), left ventricle ejection fraction (OR 0.978, P < 0.001), and left atrium diameter (OR 1.094, P < 0.001).
Oral anticoagulation (vitamin K antagonist/direct oral anticoagulant) and antiplatelet therapy in atrial fibrillation population at baseline
Oral anticoagulation was administered in 71.7% of patients with AF: 51.6% patients were treated with VKA and 20.1% with DOAC. Frequency of OAC administration in cardiomyopathy subtypes was as follows: DCM (75.9%), RCM (75.7%), HCM (69.5%), and ARVC (43.5%). Antiplatelet therapy was administered in 17.5% of AF patients, of whom 88.0% received aspirin. Detailed data on anticoagulant and antiplatelet therapy in different cardiomyopathies are presented in Table 3 .
TABLE 3.
Current anticoagulation regimen (VKA/DOAC) in patients with AF and various subtypes of cardiomyopathies
| Variable | Patients with AF (N = 998) | DCM with AF (N = 411) | HCM with AF (N = 526) | RCM with AF (N = 37) | ARVC with AF (N = 24) | P‐value |
|---|---|---|---|---|---|---|
| Oral anticoagulant treatment | 713/994 (71.73%) | 311/410 (75.85%) | 364/524 (69.47%) | 28/37 (75.68%) | 10/23 (43.48%) | 0.003 |
| Oral anticoagulant treatment | ||||||
| No treatment | 281/994 (28.27%) | 99/410 (24.15%) | 160/524 (30.53%) | 9/37 (24.32%) | 13/23 (56.52%) | 0.014 |
| DOAC | 200/994 (20.12%) | 80/410 (19.51%) | 110/524 (20.99%) | 7/37 (18.92%) | 3/23 (13.04%) | |
| VKA | 513/994 (51.61%) | 231/410 (56.34%) | 254/524 (48.47%) | 21/37 (56.76%) | 7/23 (30.43%) | |
| Antiplatelet therapy | 175/998 (17.54%) | 57/411 (13.87%) | 107/526 (20.34%) | 8/37 (21.62%) | 3/24 (12.50%) | 0.057 |
| Aspirin | 154/973 (15.83%) | 47/400 (11.75%) | 97/514 (18.87%) | 8/37 (21.62%) | 2/22 (9.09%) | 0.017 |
AF, atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; DOAC, direct oral anticoagulant; HCM, hypertrophic cardiomyopathy; RCM, restrictive cardiomyopathy; VKA, vitamin K antagonist.
Clinical endpoints in atrial fibrillation and non‐atrial fibrillation populations
At 1 year follow‐up, the incidence of adverse cardiovascular endpoints differed among AF and non‐AF populations. Annual incidence of stroke/TIA was higher in AF as compared with non‐AF population (3.17% vs. 1.19%, P < 0.001). Death from any cause (5.39% vs. 2.50%, P < 0.001) and death from heart failure (2.44% vs. 1.31%, P = 0.033) were higher in the AF population. Comparison of other endpoints—death from ischaemic stroke, death from haemorrhagic stroke, and death from systemic haemorrhage did not differ between the AF and non‐AF populations (Table 4 ).
TABLE 4.
Incidence of adverse cardiovascular endpoints in 12 month follow‐up among AF and non‐AF populations of cardiomyopathy patients
| Variable | AF | Non‐AF | P‐value | OR [95% CI] | OR P‐value |
|---|---|---|---|---|---|
| Stroke/TIA | 26/820 (3.17%) | 21/1763 (1.19%) | <0.001 | 2.714 [1.518–4.853] | <0.001 |
| Death from any cause | 47/872 (5.39%) | 46/1840 (2.50%) | <0.001 | 2.222 [1.467–3.364] | <0.001 |
| Death from heart failure | 21/862 (2.44%) | 24/1832 (1.31%) | 0.033 | 1.881 [1.041–3.398] | 0.036 |
| Death from arrhythmia | 2/862 (0.23%) | 5/1832 (0.27%) | 1.000 | 0.850 [0.165–4.389] | 0.846 |
| Death from ischaemic stroke | 2/862 (0.23%) | 1/1832 (0.05%) | 0.242 | 4.241 [0.385–46.755] | 0.238 |
| Death from haemorrhagic stroke | 1/862 (0.12%) | 1/1832 (0.05%) | 0.538 | 2.126 [0.133–34.037] | 0.594 |
| Death from systemic haemorrhage | 1/862 (0.12%) | 0/1832 (0.00%) | 0.320 | 212910.950 [0.000–1] | 0.969 |
| Death from peripheral embolism | 0/862 (0.00%) | 0/1832 (0.00%) | NC | NC | NC |
AF, atrial fibrillation; CI, confidence interval; NC, not calculable; OR, odds ratio; TIA, transient ischaemic attack.
The following differences between incidence of clinical endpoints were observed in AF vs. non‐AF patients of DCM population (annual incidence of stroke/TIA: 3.97% vs. 1.88%, P = 0.045; death from any cause: 6.68% vs. 2.87%, P = 0.003; and death from heart failure: 3.25% vs. 1.10%, P = 0.012) and in AF vs. non‐AF patients of HCM population (annual incidence of stroke/TIA: 2.64% vs. 0.85%, P = 0.009).
Incidence and risk factors for stroke/transient ischaemic attack in cardiomyopathy population
At 1 year follow‐up, stroke/TIA incidences in the whole cardiomyopathy population occurred in 47 (1.82%) subjects. Univariate logistic regression analysis revealed that the following factors were associated with the incidence of stroke/TIA: age at enrolment (OR 1.024, P = 0.032), age at first evaluation in the centre (OR 1.025, P = 0.021), mitochondrial disorder (OR 13.945, P = 0.020), history of stroke/TIA (OR 6.795, P < 0.001), diabetes mellitus type I or II (OR 2.838, P = 0.001), renal impairment (OR 2.193, P = 0.028), chronic obstructive pulmonary disease (OR 2.528, P = 0.039), and anaemia (OR 3.252, P = 0.003) (OAC and AF considered as fixed covariates). Multivariate analysis identified the following independent predictors for stroke/TIA on follow‐up: AF (OR 2.812, P = 0.005), history of stroke (OR 7.311, P = 0.010), and anaemia (OR 3.119, P = 0.006). Results of multivariate analysis are presented in Table 5 .
TABLE 5.
Multivariate logistic regression analysis of different baseline demographic and clinical variables associated with the overall presence of stroke/TIA on follow‐up
| Variable | Global P‐value | OR [95% CI] | OR P‐value |
|---|---|---|---|
| Oral anticoagulant treatment | |||
| DOAC vs. no | 0.365 | 0.635 [0.213–1.894] | 0.742 |
| VKA vs. no | 0.565 [0.253–1.262] | 0.380 | |
| AF at baseline or at 1 year | 0.005 | 2.812 [1.361–5.810] | 0.005 |
| History of stroke/TIA | |||
| Stroke vs. no | <0.001 | 7.311 [3.363–15.894] | 0.010 |
| TIA vs. no | 5.749 [2.109–15.674] | 0.143 | |
| Anaemia | 0.006 | 3.119 [1.387–7.014] | 0.006 |
| Sample size: 2394/2583 | |||
| Hosmer–Lemeshow goodness of fit: Stat = 3.55 with 4 d.f. and six groups. P‐value = 0.470 | |||
AF, atrial fibrillation; CI, confidence interval; DOAC, direct oral anticoagulant; OR, odds ratio; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
Discussion
We present data from the EORP registry on the prevalence of AF and AF risk factors in a contemporary European population of cardiomyopathy patients. The prevalence of AF was relatively high in the whole population with the highest prevalence of AF in patients with RCM.
We found significant differences between AF and non‐AF populations in terms of demographic and clinical characteristics. In particular, the presence of AF was associated with more severe symptoms, increased prevalence of cardiovascular risk factors and co‐morbidities, and an increased incidence of stroke and death. Anticoagulation was possibly inadequate in patients with cardiomyopathy and concomitant AF, because it was administered in less than three quarters of this population.
Atrial fibrillation in different cardiomyopathy populations
Data on AF prevalence in patients with cardiomyopathies are scarce. According to the EORP registry, 29.4% of cardiomyopathy patients were affected by AF. These findings are similar to those of a previous analysis, 10 which showed a prevalence of AF in patients with inherited cardiomyopathies ranging from 11% to 33%, with the highest values in patients with HCM and familial DCM. Another study reported AF in a third of patients with familial DCM. 11
On the other hand, data presented in ESC guidelines on AF indicate 5–15% AF prevalence in HCM 12 , 13 and even >40% in patients with ARVC. 14
In the EORP registry population, the highest prevalence of AF was observed in patients with RCM followed by DCM and HCM. This difference almost certainly reflects disease‐specific characteristics that contribute to the pathogenesis of AF; for example, increased left ventricular filling pressure or secondary dilatation of left atrium underlies the high prevalence of AF in RCM, DCM, and HCM, 3 , 4 , 5 whereas AF in ARVC with dominant right ventricular involvement is not typical. 6
Our analysis also revealed that patients with AF were older and more symptomatic and had more cardiovascular risk factors and co‐morbidities. Moreover, the median age varied by cardiomyopathy subtype, being the lowest in ARVC and the highest in patients with RCM. This distribution of age and disease severity at diagnosis may also partially explain differences in the prevalence of AF. Many cardiovascular diseases as well as unhealthy lifestyle are associated with the risk of AF and its complications. 8 , 15 Our observations in AF cardiomyopathy patients are in accordance with these data. Thus, identification of co‐morbidities as well as their prevention and treatment are important to prevent AF also in patients with cardiomyopathy.
Oral anticoagulation (vitamin K antagonist/direct oral anticoagulant) and antiplatelet therapy in atrial fibrillation population
Almost a third of patients with AF did not receive anticoagulants during the observation period. This is consistent with data from the EORP‐AF pilot registry, 16 in which OAC was used in 80.1% of patients with AF. Other contemporary registries presenting data on OAC in the general AF population report rates of anticoagulation that vary from 46% to 97%. 17 , 18
Anticoagulation is associated with a lower incidence of thromboembolic events, 19 and the CHA2DS2–VASc score is used as a method of stratifying patients with AF for therapy. 8 However, CHA2DS2–VASc has not been specifically tested in patients with cardiomyopathies, 8 and retrospective evidence in HCM suggests that it performs suboptimally with respect to stroke prediction. 20 , 21 Conversely, genotype, age, and, most importantly, left atrial dimension are more predictive for AF and thromboembolism in patients with HCM and DCM. 4 , 10 , 15 , 22 , 23 Given that AF increases the risk of thromboembolic events in patients with HCM to a greater extent than in the general population, the first occurrence of AF should be an indication for lifelong OAC. 7 , 24 While there is more experience with VKA, there are no data suggesting that DOAC cannot be used in these patients. 7 Currently, there is no consensus on the indications and the choice of anticoagulation in other types of cardiomyopathies. Although antiplatelet agents are not indicated for prevention of thromboembolic events, 8 17.5% of our AF patients received this therapy. This registry highlights the need for further work in this area.
Clinical endpoints and risk factors for stroke/transient ischaemic attack
The EORP registry confirmed worse prognosis for the population with cardiomyopathy and concurrent AF. The annual incidence of stroke/TIA was approximately three times higher in AF population, and the annual incidence of stroke/TIA is comparable with the adjusted stroke rate (3.2% per year) in non‐valvular AF population with a CHA2DS2–VASc score of 3 (population not receiving anticoagulation). 8
In a meta‐analysis by Guttmann et al., 20 the annual incidence of thromboembolic events was 3.75% in patients with HCM and AF. According to a Korean database, 21 the risk of stroke in HCM population with AF and without any CHA2DS2–VASc risk factors was similar to that of AF general population with CHA2DS2–VASc score of 3. These data are comparable with our results; the annual incidence of stroke/TIA was more than twice higher in AF patients with HCM (2.65% vs. 0.85%).
In a cohort of European patients with AF from the EORP‐AF pilot registry, the composite of stroke/TIA/peripheral embolism/all‐cause death at 3 years occurred in 18.2%. 16
The incidence of death from any cause (5.59% vs. 2.50%) and death from heart failure (2.44% vs. 1.41%) was twice higher in AF population than in non‐AF population. Similar observations regarded our AF and non‐AF subjects with DCM. It corresponds with recently published studies 25 , 26 , 27 and meta‐analyses 28 that demonstrated the increased risk of sudden cardiac death with AF in the general population. Sudden cardiac death has been found to be the most common cause of death in AF populations (22.3–31.7%). 26
Comparison of other clinical endpoints, that is, death from ischaemic stroke, death from haemorrhagic stroke, and death from systemic haemorrhage that may be related to AF or anticoagulation, did not reveal any differences, which may be linked to limited number of events.
It is well documented that AF coexists and interacts with other cardiovascular risk factors both in general 9 , 29 and in cardiomyopathy population. 15 , 30 Thus, AF itself and AF as an element of complex interactions may worsen the prognosis for AF population. We found that classic cardiovascular risk factors, that is, age, diabetes mellitus, and renal impairment, were associated with the incidence of stroke/TIA in the cardiomyopathy population. The AF at baseline, previous incidence of stroke and anaemia were independent risk factors for the stroke/TIA on follow‐up. It suggests that both monitoring for AF diagnosis and complex ‘upstream therapy’ including modification of lifestyle risk factors and treatment of co‐morbidities are necessary to prevent cerebral events in patients with cardiomyopathies.
Limitations
There are limitations intrinsic to all registries including selection bias and lack of adjudication. The total number of enrolled patients was not very high; however, it resulted from the design of the EORP Cardiomyopathy/Myocarditis Registry. As most patients were enrolled in tertiary referral centres, the results may not be generalizable. There were some incomplete data at baseline, and a number of patients were lost to follow‐up (15%). Some cases of AF might have been undetected because the study was not focused on AF specifically, and the diagnosis of AF was not verified by objective methods such as prolonged electrocardiographic monitoring. The percentage of patients with a rare underlying disease, that is, inherited metabolic disorders, mitochondrial disorder, and neuromuscular diseases, was low. The small size of the subgroup with these disorders does not allow for constructive remarks regarding their potent role in a stroke aetiology. The data in the EORP registry have been collected between 2012 and 2016, 9 and the anticoagulation was administered according to the current recommendations. However, we should be aware of the fact that the specific recommendations have been set only for patients with HCM and AF. 7 The indications for OAC in AF patients with DCM, RCM, and ARVC are not yet well established; however, probably all cardiomyopathy patients with structural abnormalities and AF should be anticoagulated. In patients with AF and DCM, a decreased left ventricular ejection fraction/heart failure increases a priori thromboembolic risk and is used as a recommendation for OAC.
We did not analyse CHA2DS2–VASc score, because it is not a verified tool for the thromboembolic risk stratification in AF cardiomyopathy patients, especially in HCM subjects. However, we have analysed separately different risk factors for AF prevalence and for stroke/TIA occurrence with all factors included in the CHA2DS2–VASc scoring.
Relatively short follow‐up constitutes another limitation of the study. Because there are no many subjects who experienced a stroke/TIA during follow‐up, we had to limit the analysis of risk factors for stroke/TIA in the whole cardiomyopathy population.
Summary
The EORP Cardiomyopathy/Myocarditis Registry reveals the high prevalence of AF in patients with cardiomyopathy. It shows that AF is associated with an increased risk of stroke/TIA and an increased rate of death from any cause and death from heart failure. A substantial proportion of patients with AF did not receive OAC, and the importance of modifiable factors associated with AF occurrence was highlighted. More efficient AF prevention and treatment, especially through a better anticoagulation and ‘upstream therapy’, represent major goals for improvement of prognosis in patients with cardiomyopathies and AF.
Conflict of interest
None declared.
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular International (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer AG (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific Corporation (2009–2012), the Bristol‐Myers Squibb–Pfizer Alliance (2011–2019), Daiichi Sankyo Europe GmbH (2011–2020), the Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014–2017), Edwards Lifesciences (2016–2019), Gedeon Richter Plc. (2014–2016), Menarini International Operations (2009–2012), MSD–Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed Foundation (2014–2016), Sanofi (2009–2011), Servier (2009–2021), and Vifor Pharma (2019–2022).
Supporting information
Appendix S1. List of registry committees and Investigators.
Acknowledgements
We thank the EORP Oversight Committee and the Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP department from the ESC by Rachid Mir Hassaine as Clinical Project Manager, Emanuela Fiorucci, Myriam Glemot, and Patti‐Ann McNeill as Project Officers, and Marème Konté and Sebastien Authier as Data Managers. Statistical analyses were performed by Cécile Laroche. Overall activities were coordinated and supervised by Dr Aldo P. Maggioni (EORP Scientific Coordinator). All investigators are listed in Supporting Information, Appendix S1 .
Mizia‐Stec, K. , Caforio, A. L. P. , Charron, P. , Gimeno, J. R. , Elliott, P. , Kaski, J. P. , Maggioni, A. P. , Tavazzi, L. , Rigopoulos, A. G. , Laroche, C. , Frigy, A. , Zachara, E. , Pena‐Pena, M. L. , Olusegun‐Joseph, A. , Pinto, Y. , Sala, S. , Drago, F. , Blagova, O. , Reznik, E. , and Tendera, M. (2020) Atrial fibrillation, anticoagulation management and risk of stroke in the Cardiomyopathy/Myocarditis registry of the EURObservational Research Programme of the European Society of Cardiology. ESC Heart Failure, 7: 3601–3609. 10.1002/ehf2.12854.
These authors are members of the European Reference Network on Heart Diseases (ERN GUARD‐Heart).
References
- 1. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention . Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006; 113: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 3. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non‐dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2016; 37: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 4. Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet 2017; 389: 1253–1267. [DOI] [PubMed] [Google Scholar]
- 5. Mogensen J, Arbustini E. Restrictive cardiomyopathy. Curr Opin Cardiol 2009; 24: 214–220. [DOI] [PubMed] [Google Scholar]
- 6. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017; 376: 61–72. [DOI] [PubMed] [Google Scholar]
- 7. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2733–2779. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, ESC Scientific Document Group . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962. [DOI] [PubMed] [Google Scholar]
- 9. Charron P, Elliott PM, Gimeno JR, Caforio ALP, Kaski JP, Tavazzi L, Tendera M, Maupain C, Laroche C, Rubis P, Jurcut R. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: baseline data and contemporary management of adult patients with cardiomyopathies. Eur Heart J 2018; 39: 1784–1793. [DOI] [PubMed] [Google Scholar]
- 10. Yeung C, Enriquez A, Suarez‐Fuster L, Baranchuk A. Atrial fibrillation in patients with inherited cardiomyopathies. Europace 2019; 21: 22–32. [DOI] [PubMed] [Google Scholar]
- 11. Grunig E, Tasman JA, Kucherer H, Franz W, Kubler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol 1998; 31: 186–194. [DOI] [PubMed] [Google Scholar]
- 12. Losi MA, Betocchi S, Aversa M, Lombardi R, Miranda M, D'Alessandro G, Cacace A, Tocchetti CG, Barbati G, Chiariello M. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol 2004; 94: 895–900. [DOI] [PubMed] [Google Scholar]
- 13. Maron BJ, Olivotto I, Bellone P, Conte MR, Cecchi F, Flygenring BP, Casey SA, Gohman TE, Bongioanni S, Spirito P. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002; 39: 301–307. [DOI] [PubMed] [Google Scholar]
- 14. Chu AF, Zado E, Marchlinski FE. Atrial arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and ventricular tachycardia. Am J Cardiol 2010; 106: 720–722. [DOI] [PubMed] [Google Scholar]
- 15. Guttmann OP, Pavlou M, O'Mahony C, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, Garcia‐Pavia P, McKenna WJ, Omar RZ, Elliott PM, Hypertrophic Cardiomyopathy Outcomes Investigators . Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart 2017; 103: 672–678. [DOI] [PubMed] [Google Scholar]
- 16. Boriani G, Proietti M, Laroche C, Diemberger I, Popescu MI, Riahi S, Shantsila A, Dan GA, Tavazzi L, Maggioni AP, Lip GYH, EORP‐AF Pilot General Registry Investigators . Changes to oral anticoagulant therapy and risk of death over a 3‐year follow‐up of a contemporary cohort of European patients with atrial fibrillation final report of the EURObservational Research Programme on Atrial Fibrillation (EORP‐AF) pilot general registry. Int J Cardiol 2018; 271: 68–74. [DOI] [PubMed] [Google Scholar]
- 17. Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, Ezekowitz MD, Fonarow GC, Gersh BJ, Goldhaber S, Haas S, Hacke W, Kowey PR, Ansell J, Mahaffey KW, Naccarelli G, Reiffel JA, Turpie A, Verheugt F, Piccini JP, Kakkar A, Peterson ED, Fox KAA, GARFIELD‐AF; ORBIT‐AF Investigators . International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD‐AF, ORBIT‐AF I, and ORBIT‐AF II registries. Am Heart J 2017; 194: 132–140. [DOI] [PubMed] [Google Scholar]
- 18. Verheugt FWA, Gao H, Al Lahmeed W, Ambrosio G, Angchaisuksiri P, Atar D, Bassand JP, Camm AJ, Cools F, Eikelboom J, Kayani G, Lim TW, Misselwitz F, Pieper KS, van Eickels M, Kakkar AK, Investigators GARFIELD‐AF. Characteristics of patients with atrial fibrillation prescribed antiplatelet monotherapy compared with those on anticoagulants: insights from the GARFIELD‐AF registry. Eur Heart J 2018; 39: 464–473. [DOI] [PubMed] [Google Scholar]
- 19. Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, Goldhaber SZ, Goto S, Haas S, Hacke W, Kayani G, Mantovani LG, Misselwitz F, Ten Cate H, Turpie AG, Verheugt FW, Kakkar AK, Investigators GARFIELD‐AF. Two‐year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD‐AF. Eur Heart J 2016; 37: 2882–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart 2014; 100: 465–472. [DOI] [PubMed] [Google Scholar]
- 21. Jung H, Yang PS, Sung JH, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Lip GYH, Joung B. Hypertrophic cardiomyopathy in patients with atrial fibrillation: prevalence and associated stroke risks in a nationwide cohort study. Thromb Haemost 2019; 119: 285–293. [DOI] [PubMed] [Google Scholar]
- 22. Spirito P. Atrial fibrillation in hypertrophic cardiomyopathy: new light on an old problem. Circulation 2017; 136: 2437–2439. [DOI] [PubMed] [Google Scholar]
- 23. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, Rastegar H, Estes NAM, Maron MS, Maron BJ. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017; 136: 2420–2436. [DOI] [PubMed] [Google Scholar]
- 24. Camm CF, Camm AJ. Atrial fibrillation and anticoagulation in hypertrophic cardiomyopathy. Arrhythm Electrophysiol Rev 2017; 6: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. JAMA Intern Med 2013; 173: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eisen A, Ruff CT, Braunwald E, Nordio F, Corbalán R, Dalby A, Dorobantu M, Mercuri M, Lanz H, Rutman H, Wiviott SD, Antman EM, Giugliano RP. Sudden cardiac death in patients with atrial fibrillation: insights from the ENGAGE AF‐TIMI 48 trial. J Am Heart Assoc 2016; 5: e003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pujades‐Rodriguez M, Guttmann OP, Gonzalez‐Izquierdo A, Duyx B, O'Mahony C, Elliott P, Hemingway H. Identifying unmet clinical need in hypertrophic cardiomyopathy using national electronic health records. PLoS One 2018; 13: e0191214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rattanawong P, Upala S, Riangwiwat T, Jaruvongvanich V, Sanguankeo A, Vutthikraivit W, Chung EH. Atrial fibrillation is associated with sudden cardiac death: a systematic review and meta‐analysis. J Interv Card Electrophysiol 2018; 51: 91–104. [DOI] [PubMed] [Google Scholar]
- 29. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014; 129: 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyazawa K, Lip GYH. Atrial fibrillation and hypertrophic cardiomyopathy: co‐existing conditions with additive risks. Hellenic J Cardiol 2017; 58: 340–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of registry committees and Investigators.


