Abstract
Aims
Hypertrophic cardiomyopathy (HCM) is generally associated with mild disability and normal life expectancy. On the other hand, once the end‐stage phase of HCM characterized by left ventricular (LV) ejection fraction < 50% is established, patients with this subtype have a poor prognosis. This study clarifies the clinical parameters associated with progression to end‐stage HCM.
Methods and results
We retrospectively studied 157 HCM patients (age 59.9 ± 14.2 years, 104 men) with preserved LV systolic function in whom subsequent echocardiographic data were obtained for a period of >1 year. HCM progressed to end‐stage HCM in 13 patients (8.3%) of the 157 patients during a mean follow‐up period of 6.3 ± 2.8 years. Compared with patients who did not reach end‐stage HCM at the last evaluation, patients with progression to the end‐stage phase had lower ejection fraction, larger LV size, more enlarged left atrial diameter, longer follow‐up period, and higher frequency of an elevated concentration of high‐sensitivity cardiac troponin T (hs‐cTnT; >0.014 ng/mL) at registration. Multivariate analysis revealed that elevated hs‐cTnT was a significant predictor independent of lower LV ejection fraction for progression to end‐stage HCM. Furthermore, in patients with elevated hs‐cTnT levels, LV ejection fraction became significantly lower, LV end‐diastolic diameter increased, and LV wall thickness decreased during the follow‐up period, whereas those parameters did not change in the normal hs‐cTnT group.
Conclusions
In patients with HCM, an elevated hs‐cTnT was associated with progression of LV remodelling, and this biomarker can be useful for predicting progression to the end‐stage phase.
Keywords: Hypertrophic cardiomyopathy, High‐sensitivity cardiac troponin T, End‐stage phase, Left ventricular remodelling
Introduction
Hypertrophic cardiomyopathy (HCM) is a primary myocardial disorder that is generally associated with mild disability and normal life expectancy if sudden death can be prevented. 1 , 2 , 3 , 4 , 5 On the other hand, it is well known that HCM in a subset of patients progresses to ‘end‐stage’ or ‘dilated’ phase characterized by left ventricular (LV) systolic dysfunction, and patients with the end‐stage phase of HCM (end‐stage HCM) have a poor prognosis. 6 , 7 , 8 , 9 , 10 It is important to predict the clinical course in order to provide adequate interventions for those patients. However, there has been little information on clinical determinants associated with progression to end‐stage HCM from HCM with preserved LV systolic function. 6 Recently, cardiac troponins including high‐sensitivity cardiac troponin T (hs‐cTnT), as sensitive and specific markers of myocardial injury, have been reported to be elevated in patients with heart failure even in the absence of coronary artery stenosis. 11 , 12 , 13 , 14 Indeed, hs‐cTnT is a reliable indicator of subclinical and ongoing myocardial damage, and this biomarker can be associated with progression of LV remodelling in patients with heart failure.
The aim of this study was to identify the clinical parameters associated with progression to end‐stage HCM.
Methods
Subjects
We retrospectively studied 157 consecutive HCM patients who showed preserved LV systolic function (global LV ejection fraction ≥ 50%) at registration of this study and in whom serial echocardiography was performed during a follow‐up period of >1 year. We excluded patients with evidence of coronary artery disease and patients with renal failure (serum creatinine ≥ 3 mg/dL).
The diagnosis of HCM was based on echocardiographic demonstration of LV hypertrophy (LVH), that is, maximum LV wall thickness ≥ 15 mm, in the absence of another cardiac or systemic disease that could cause LVH. Patients with known metabolic disease or syndromic causes of LVH were excluded from the study. End‐stage HCM was defined as LV systolic dysfunction of global ejection fraction < 50%. This investigation was performed according to the Declaration of Helsinki. The study was approved by the Ethics Committee on Medical Research of Kochi Medical School, and informed consent was obtained from all patients or their parents in accordance with the guidelines of the Ethics Committee.
Clinical evaluation
Evaluation of patients included medical history, clinical examination, 12‐lead electrocardiography, and M‐mode, two‐dimensional (2‐D), and Doppler echocardiography. Maximum LV wall thickness was defined as the greatest thickness in any single segment. LV end‐diastolic diameter (LVEDD) and end‐systolic diameter were measured from M‐mode and 2‐D images obtained from parasternal long‐axis views. Global ejection fraction was determined by the modified Simpson method from apical two‐chamber and four‐chamber views. LV outflow tract gradient was calculated from continuous‐wave Doppler using the simplified Bernoulli equation. Based on morphologic and haemodynamic assessments by echocardiography, we divided the patients into the following five groups: (i) end‐stage HCM; (ii) hypertrophic obstructive cardiomyopathy, defined as the presence of basal LV outflow tract obstruction (gradient ≥ 30 mmHg at rest); (iii) midventricular obstruction, defined as the presence of systolic LV cavity obliteration at the midventricle creating midventricular obstruction with a peak systolic gradient ≥ 30 mmHg at rest; (iv) apical HCM, defined as hypertrophy confined to the LV apex, and (v) others, HCM without obstruction other than end‐stage HCM and apical HCM.
Measurements of high‐sensitivity cardiac troponin T
Peripheral venous blood samples were collected for measurements of biomarkers at the time of clinical evaluation. Aliquots were stored at −80°. Serum hs‐cTnT was measured by Elecsys Troponin T‐High Sensitive immunoassay (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The normal range is ≤0.014 ng/mL (99th percentile).
Statistical analysis
All data are expressed as mean ± SD or frequency (percentage). Comparisons of clinical characteristics between normal and elevated hs‐cTnT groups were assessed using Student's t‐test for normally distributed variables. Pearson's χ 2 test was used for comparisons between categorical variables, and Fisher's exact test was used when expected frequency was lower than 5. Clinical characteristics were all first tested, and all variables with a P value < 0.05 were then taken forward to be considered for inclusion in the multivariate model. Optimal cut‐off values for progression to end‐stage HCM was determined by using receiver operating characteristic (ROC) curves. Multivariate logistic regression analysis was performed to estimate the odds ratios for predictors of progression to end‐stage HCM. Changes of echocardiographic findings in normal or elevated hs‐cTnT groups were assessed using the paired t‐test. A probability value of <0.05 was considered significant. All statistical analyses were performed using SPSS version 21 (IBM Corporation, Armonk, NY).
Results
Baseline characteristics
Clinical characteristics of the patients in the present study are summarized in Table 1 . The patients were aged from 18 to 87 years (mean age, 59.9 ± 14.2 years), and 104 (66%) of the patients were male. Of the 157 patients, 101 (64%) were New York Heart Association (NYHA) functional class I, 53 (34%) were NYHA class II, and only three (2%) were NYHA class III. LV ejection fraction was 70.0 ± 7.8%, and all of the patients showed LV ejection fraction ≥ 50%. Maximum LV wall thickness was 20.2 ± 3.8 mm, and 23 (15%) of the patients showed LV outflow tract obstruction at rest (pressure gradient ≥ 30 mmHg). Twenty‐eight (18%) of the patients had documentation of atrial fibrillation. Serum hs‐cTnT ranged from 0.003 to 0.130 ng/mL (mean, 0.019 ± 0.020 ng/mL; median, 0.014 ng/mL), and 78 (50%) of the patients showed elevated hs‐cTnT values (>0.014 ng/mL).
Table 1.
Baseline clinical characteristics at registration of patients with and without progression to end‐stage hypertrophic cardiomyopathy
| Overall cohort n = 157 | Progression to end‐stage HCM (+) n = 13 | Progression to end‐stage HCM (−) n = 144 | P | |
|---|---|---|---|---|
| Age at registration, years | 59.9 ± 14.2 | 61.2 ± 15.8 | 59.5 ± 14.1 | 0.734 |
| Gender: male, n (%) | 104 (66%) | 11 (85%) | 93 (65%) | 0.221 |
| Age at diagnosis, years | 53.5 ± 15.8 | 46.2 ± 16.9 | 54.1 ± 15.6 | 0.086 |
| Follow‐up period, years | 6.3 ± 2.8 | 7.5 ± 2.0 | 6.1 ± 2.8 | 0.039 |
| Family history of HCM, n (%) | 51 (32%) | 7 (54%) | 44 (31%) | 0.120 |
| Presence of AF, n (%) | 28 (18%) | 5 (38%) | 23 (16%) | 0.057 |
| NYHA functional class: ≥II, n (%) | 56 (36%) | 7 (54%) | 49 (34%) | 0.225 |
| Echocardiographic data at registration | ||||
| Subtype, n (%) | 0.065 | |||
| HOCM | 23 (15%) | 0 (0%) | 23 (16%) | |
| MVO | 6 (4%) | 0 (0%) | 6 (4%) | |
| Apical HCM | 24 (15%) | 0 (0%) | 24 (17%) | |
| Others | 104 (66%) | 13 (100%) | 91 (63%) | |
| Presence of LV outflow obstruction, n (%) | 23 (15%) | 0 (0%) | 23 (16%) | 0.218 |
| LV ejection fraction, % | 70.0 ± 7.8 | 59.6 ± 6.0 | 71.0 ± 7.3 | <0.001 |
| LV end‐diastolic diameter, mm | 45.4 ± 6.0 | 49.8 ± 5.8 | 45.0 ± 5.8 | 0.005 |
| Maximum LV wall thickness, mm | 20.2 ± 3.8 | 19.5 ± 4.4 | 20.3 ± 3.8 | 0.508 |
| Left atrial diameter, mm | 43.9 ± 7.0 | 49.8 ± 7.4 | 43.4 ± 6.7 | 0.001 |
| E/e′ (septal) | 12.6 ± 8.6 | 12.8 ± 4.2 | 12.6 ± 8.9 | 0.954 |
| E/e′ (lateral) | 8.7 ± 4.8 | 8.3 ± 2.5 | 8.8 ± 4.9 | 0.735 |
| Hs‐cTnT value, ng/mL | 0.019 ± 0.020 | 0.033 ± 0.034 | 0.018 ± 0.019 | 0.012 |
| Elevated hs‐cTnT value: >0.014 ng/mL, n (%) | 78 (50%) | 11 (85%) | 67 (47%) | 0.009 |
AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; hs‐cTnT, high‐sensitivity cardiac troponin T; LV, left ventricular; MVO, midventricular obstruction; NYHA, New York Heart Association.
Values are mean ± SD and n (%).
Progression to end‐stage hypertrophic cardiomyopathy
Figure 1 shows the changes of LV ejection fraction in each patient during the mean follow‐up period of 6.3 ± 2.8 years. HCM progressed to end‐stage HCM in 13 (8%) of the patients, and the rate of progression to end‐stage HCM was 1.3%/year. Table 1 shows baseline clinical characteristics at registration of the study patients with and without progression to end‐stage HCM. Patients with progression to end‐stage HCM had a tendency to have earlier diagnosis of HCM, although age at registration of this study did not differ between the two groups. The follow‐up period was significantly longer in patients with progression to end‐stage HCM. Both the percentage of patients with a family history of HCM and the percentage of patients with atrial fibrillation were higher in the progression group than in the non‐progression group. Results of echocardiography showed that LV ejection fraction was lower and that LVEDD and left atrial diameter were larger in patients with progression to end‐stage HCM than in patients without progression to end‐stage HCM. The percentage of patients with elevated hs‐cTnT was higher in the progression group. Significant differences were found between the two groups in the follow‐up period, LV ejection fraction, LVEDD, let atrial diameter, and the percentage of patients with elevated hs‐cTnT value.
Figure 1.
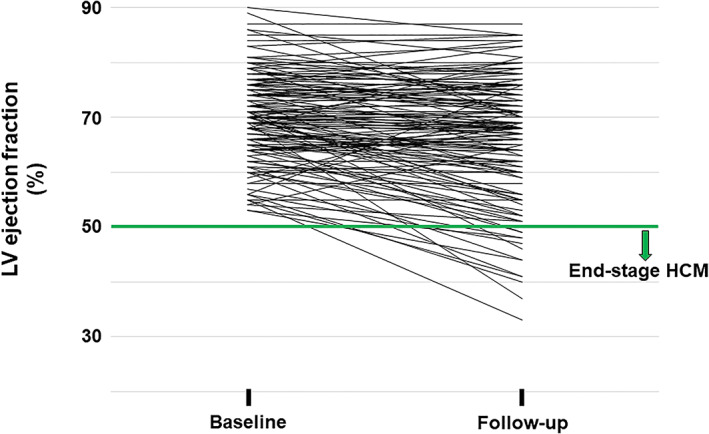
Longitudinal changes of LV ejection fraction in 157 patients with hypertrophic cardiomyopathy. LV, left ventricular; HCM, hypertrophic cardiomyopathy.
Multivariate logistic regression analysis was performed to clarify the determinants of progression to end‐stage HCM after we divided the patients into two groups according to each cut‐off value of the five parameters (follow‐up period, LV ejection fraction, LVEDD, left atrial diameter, and hs‐cTnT value) determined using ROC curves. Table 2 shows that elevated hs‐cTnT value (>0.014 ng/mL) was a significant predictor independent of LV ejection fraction among the five determinants. On the other hand, follow‐up period, LVEDD, and let atrial diameter were not significant.
Table 2.
Predictors of progression to end‐stage hypertrophic cardiomyopathy (multivariate logistic regression analysis)
| Odds ratio (95% CI) | P | |
|---|---|---|
| Follow‐up period: >7.2 years | 2.025 (0.503–8.162) | 0.321 |
| LV ejection fraction: <65% | 13.818 (3.137–60.862) | 0.001 |
| LV end‐diastolic diameter: >47 mm | 4.242 (0.879–20.462) | 0.072 |
| Left atrial diameter: >47 mm | 2.065 (0.467–9.123) | 0.339 |
| Hs‐cTnT value: >0.014 ng/mL | 6.467 (1.114–37.554) | 0.038 |
hs‐cTnT, high‐sensitivity cardiac troponin T; LV, left ventricular.
95% CI, 95% confidence interval.
Measurements of high‐sensitivity cardiac troponin T and echocardiographic changes
The patients were divided into two groups by hs‐cTnT values: a normal hs‐cTnT group (hs‐cTnT ≤ 0.014 ng/mL) and an elevated hs‐cTnT group. Figure 2 shows changes of LV ejection fraction in each patient with normal hs‐cTnT value or elevated hs‐cTnT value (left figure, LV ejection fraction < 65% at baseline; right figure, LV ejection fraction ≥ 65% at baseline). Among patients with baseline LV ejection fraction < 65%, patients with elevated hs‐cTnT value had significantly more frequent progression to end‐stage HCM than had those with normal hs‐cTnT value (nine of 21 patients in elevated hs‐cTnT group vs. one of 16 patients in the normal hs‐cTnT group, P = 0.023), although the follow‐up period was not different in those two groups. In patients with baseline LV ejection fraction ≥ 65%, there was no significant difference in progression to end‐stage HCM between the two groups (two of 57 patients in the elevated hs‐cTnT group vs. one of 63 patients in the normal hs‐cTnT group, P = 0.604). However, with regard to reaching LV ejection fraction < 65% at follow‐up, patients with elevated hs‐cTnT value had significantly more frequent progression to LV ejection fraction < 65% than had those with normal hs‐cTnT value (19 of 57 patients in the elevated hs‐cTnT group vs. five of 63 patients in the normal hs‐cTnT group, P = 0.001).
Figure 2.
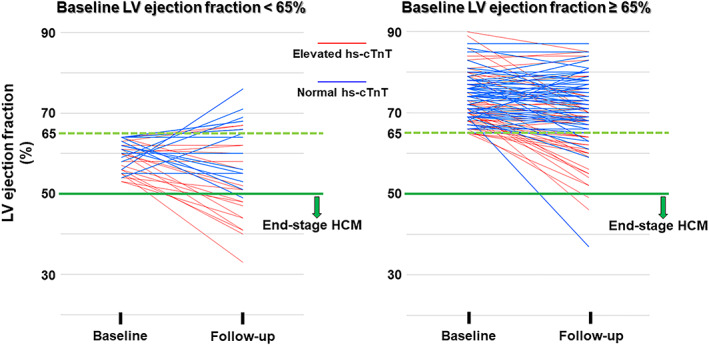
Changes of LV ejection fraction in each patient with normal hs‐cTnT value or elevated hs‐cTnT value (left, LV ejection fraction < 65% at baseline; right, LV ejection fraction ≥65% at baseline). LV, left ventricular; Hs‐cTnT, high‐sensitivity cardiac troponin T; HCM, hypertrophic cardiomyopathy.
Table 3 shows echocardiographic changes according to hs‐cTnT values. In the initial echocardiographic findings, maximum LV wall thicknesses was greater and left atrial diameter was larger in patients with elevated hs‐cTnT levels than in patients with normal hs‐cTnT levels, although LV ejection fraction and LVEDD were not significantly different between the two groups. In follow‐up echocardiographic findings, LV ejection fraction became significantly lower and LVEDD became larger in the elevated hs‐cTnT group. On the other hand, maximum LV wall thickness was still significantly different in the two groups, but the difference was less prominent.
Table 3.
Echocardiographic changes according to high‐sensitivity cardiac troponin T values
|
Normal hs‐cTnT n = 79 |
Elevated hs‐cTnT n = 78 |
P | |
|---|---|---|---|
| Follow‐up period, years | 6.3 ± 2.8 | 6.2 ± 2.8 | 0.754 |
| Initial echocardiographic findings | |||
| LV ejection fraction, % | 71.2 ± 7.1 | 68.9 ± 8.3 | 0.061 |
| LV end‐diastolic diameter, mm | 45.0 ± 5.2 | 45.7 ± 6.7 | 0.462 |
| Maximum LV wall thickness, mm | 19.0 ± 2.8 | 21.4 ± 4.3 | <0.001 |
| Left atrial diameter, mm | 41.7 ± 5.8 | 46.1 ± 7.4 | <0.001 |
| Follow‐up echocardiographic findings | |||
| LV ejection fraction, % | 70.3 ± 8.7 | 63.6 ± 11.4 | <0.001 |
| LV end‐diastolic diameter, mm | 45.3 ± 5.7 | 47.7 ± 7.4 | 0.020 |
| Maximum LV wall thickness, mm | 18.5 ± 3.2 | 19.6 ± 3.6 | 0.035 |
| Left atrial diameter, mm | 43.3 ± 6.4 | 47.6 ± 9.5 | 0.001 |
hs‐cTnT, high‐sensitivity cardiac troponin T; LV, left ventricular.
Values are mean ± SD and n (%).
Figure 3 shows changes of echocardiographic indices in each group. In the normal hs‐cTnT group, LV ejection fraction, LVEDD, and maximum LV wall thickness did not change in the follow‐up period. On the other hand, in patients with elevated hs‐cTnT levels, LV ejection fraction became significantly lower, LVEDD increased, and maximum LV wall thickness decreased during the follow‐up period. Left atrial diameter became larger in both the normal hs‐cTnT group and the elevated hs‐cTnT group.
Figure 3.
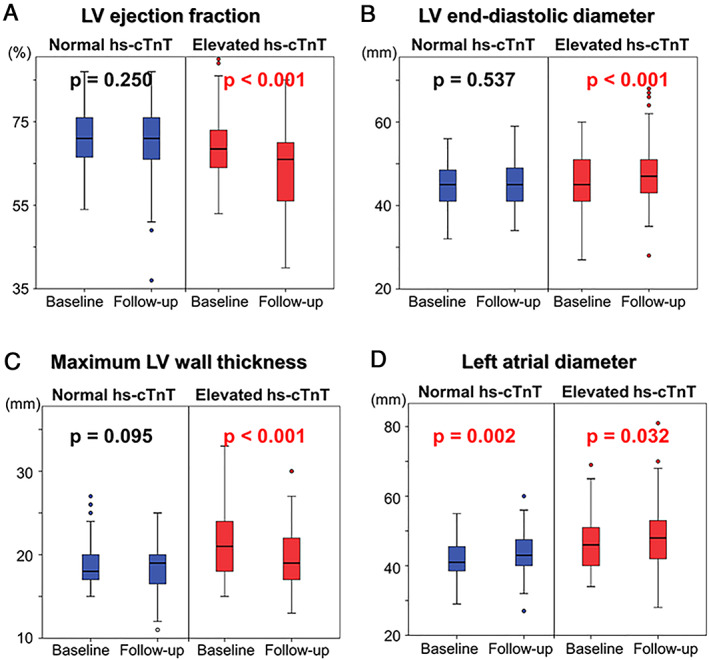
(A) Changes of LV ejection fraction in the normal hs‐cTnT group and elevated hs‐cTnT group. (B) Changes of LV end‐diastolic diameter in the normal hs‐cTnT group and elevated hs‐cTnT group. (C) Changes of maximum LV wall thickness in the normal hs‐cTnT group and elevated hs‐cTnT group. (D) Changes of left atrial diameter in the normal hs‐cTnT group and elevated hs‐cTnT group. LV, left ventricular; Hs‐cTnT, high‐sensitivity cardiac troponin T.
Furthermore, in the elevated hs‐cTnT group, we divided the patients into two groups by the median value of hs‐cTnT in the abnormal range: a mildly elevated hs‐cTnT group (0.014 ng/mL < hs‐cTnT < 0.023 ng/mL) and a highly elevated hs‐cTnT group (≥0.023 ng/mL). Although the follow‐up period was not different in those two groups, patients with a highly elevated hs‐cTnT value tended to show more reduced LV ejection fraction than patients with a mildly elevated hs‐cTnT value (delta LV ejection fraction from baseline to final evaluation: −6.6 ± 7.6% vs. −4.0 ± 6.5%, P = 0.105).
Discussion
HCM phenotype itself is a slowly progressive disorder that manifests remarkable evolution of clinical features throughout life. 15 , 16 End‐stage HCM, in which there is usually a hypokinetic LV wall with ventricular dilatation, is now recognized as an important clinical entity in part of the HCM disease spectrum. This type of HCM is generally associated with unfavourable events including heart failure events and sudden cardiac deaths. 6 , 7 , 8 , 9 , 10 However, there is little information on risk factors of progression to end‐stage HCM. The main finding of this study is that an elevated hs‐cTnT value is associated with a greater risk of dilated‐hypokinetic evolution of HCM. Serum hs‐cTnT value > 0.014 ng/mL and LV ejection fraction < 65%, with these cut‐off values determined by ROC curves in our patient's cohort, were significant predictors for progression to end‐stage HCM. Although end‐stage HCM is generally defined as LV systolic dysfunction of global ejection fraction < 50%, true systolic function might start to decline in HCM patients with LV ejection fraction of around 65%. Recently, Stokke et al. reported that increased wall thickness and/or reduced end‐diastolic volume augmented ejection fraction and could maintain a normal ejection fraction despite reduced shortening. 17 Olivotto et al. mentioned patterns of disease progression in HCM in their review article. 16 In the paper, an LV ejection fraction in the low–normal range of 50% to 65% was defined as ‘adverse remodelling’ translating into increasing LV fibrosis and worsening function with relatively preserved clinical and haemodynamic balance. In our study, even in HCM patients with baseline LV ejection fraction < 65%, almost all patients with normal hs‐cTnT value remained in preserved ejection fraction > 50% at follow‐up. On the other hand, patients with elevated hs‐cTnT value had significantly more frequent progression to end‐stage HCM.
Serum hs‐cTnT has been used to detect myocardial tissue damage. In our previous study regarding the utility of hs‐cTnT as a prognostic marker in HCM, assessment of the relationships of serum hs‐cTnT levels with baseline clinical characteristics showed that abnormal hs‐cTnT value (>0.014 ng/mL) was associated with findings supporting clinical deterioration in HCM, including presence of atrial fibrillation, severity of heart failure symptoms as judged by NYHA functional class, prevalence of syncope, greater wall thickness, and larger left atrial diameter. 14 We also found that an abnormal hs‐cTnT value itself and the degree of abnormality in hs‐cTnT value were related to a greater risk of adverse cardiovascular events, particularly heart failure events. 14 With regard to disease severity in HCM, cardiac magnetic resonance imaging (MRI) examination with late gadolinium enhancement (LGE) is now the diagnostic gold standard. The presence of LGE in MRI has been reported to be associated with arrhythmic events and heart failure symptoms in HCM patients, and the presence of this fibrosis indicated by MRI is an important marker for identifying patients at risk for progressive disease. 18 , 19 , 20 , 21 , 22 , 23 A lower LV ejection fraction is related to a higher percentage of LGE of the LV mass in patients with HCM. Although we did not evaluate the presence and extent of fibrosis using late gadolinium‐enhanced MRI, Moreno et al. showed in their cross‐sectional HCM study that hs‐cTnT levels were increased in patients with gadolinium enhancement in cardiac MRI. 24
The mechanisms of myocyte injury and release of hs‐cTnT in HCM remain unresolved. We speculate that they may be caused by relative myocardial ischaemia. Petersen et al. reported that patients with HCM showed a reduced myocardial perfusion reserve, as assessed by MRI, which was in proportion to the magnitude of hypertrophy. 25 Furthermore, they found that the decreased prevalence of myocardial fibrosis assessed by delayed contrast enhancement of MRI was accompanied by increasing hyperaemic myocardial blood flow. This observation suggests a pathophysiological link between repetitive hypoperfusion during stress and development of myocardial fibrosis. Similarly, Olivotto et al. reported a relationship between microvascular dysfunction assessed by positron emission tomography using dipyridamole myocardial blood flow and LV systolic dysfunction in HCM. 26 They found that severe microvascular dysfunction was a potent long‐term predictor of adverse LV remodelling and progression to the end‐stage phase. From these findings, serum hs‐cTnT level may reflect microvessel ischaemia resulting in myocardial replacement fibrosis in HCM and may be able to predict an increase in the extent of fibrosis.
Biagini et al. reported that young age at diagnosis, family history of HCM, and greater wall thickness were incremental risk factors for dilated‐hypokinetic HCM. 6 In our study, we also found that there was a tendency for progression to the end‐stage phase in patients with a young age at diagnosis and a family history of HCM. However, maximum LV wall thickness was not associated with dilated‐hypokinetic evolution. In this matter, wall thickness itself is not a fixed value; it can be changeable in the patients' lifelong remodelling, like a dramatic change in patients with end‐stage HCM. Values of wall thickness differ even in the same patients depending on the disease stage, and hypokinetic wall motion generally progresses with wall thinning. A recent study showed that not LV wall thickness but LGE mass itself was associated with subsequent LGE progression. 27 Therefore, it is difficult to predict progression to the end‐stage phase by only using wall thickness at any time point. On the other hand, hs‐cTnT, which seems to reflect ongoing myocardial damage, may be a more reliable biomarker to predict lifelong LV remodelling at any stage.
Limitations
There are several limitations in the present study. This was a single‐centre study, and it was not prospective. In addition, our study cohort differs from tertiary centre cohorts in which referral patterns are skewed toward patients perceived to be at high risk. The cohort in the present study was a community‐based cohort and included less high‐risk patients than those in major referral centres. Additional prospective studies are needed to verify our results. Next, we did not analyse serial measurements of hs‐cTnT in this study, and we had no data on whether there were any interventions to reduce the concentrations of this biomarker, although hs‐cTnT values were relatively static in our experience (data not shown). Serum hs‐cTnT value is generally known to rise temporarily when the patient is in decompensated heart failure status, particularly acute deterioration phase. Therefore, the timing of the measurement is important to evaluate the clinical significance of this biomarker in HCM patients. In our current investigation, almost all patients were assessed in outpatient clinic, and only 2% of the patients were NYHA class III. Finally, in our retrospective study, we were not able to find in the abnormal hs‐cTnT group whether the risk of progression to the end‐stage phase of HCM increases with an increase in the hs‐cTnT value. However, it is likely that a prospective study would show that a higher degree of abnormality is associated with a greater risk and faster progression of dilated‐hypokinetic evolution of HCM.
Conclusions
In patients with HCM, an elevated serum concentration of hs‐cTnT was associated with progression of LV remodelling, and this biomarker can be useful for predicting progression to the end‐stage phase.
Conflict of interest
None declared.
Funding
This work was supported, in part, by Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science.
Kubo, T. , Ochi, Y. , Baba, Y. , Sugiura, K. , Takahashi, A. , Hirota, T. , Yamanaka, S. , Yamasaki, N. , Doi, Y. L. , and Kitaoka, H. (2020) Elevation of high‐sensitivity cardiac troponin T and left ventricular remodelling in hypertrophic cardiomyopathy. ESC Heart Failure, 7: 3593–3600. 10.1002/ehf2.12852.
References
- 1. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018; 379: 655–668. [DOI] [PubMed] [Google Scholar]
- 2. Cecchi F, Olivotto I, Montereggi A, Santoro G, Dolara A, Maron BJ. Hypertrophic cardiomyopathy in Tuscany: clinical course and outcome in an unselected regional population. J Am Coll Cardiol 1995; 26: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy‐related death: revisited in a large non‐referral‐based patient population. Circulation 2000; 102: 858–864. [DOI] [PubMed] [Google Scholar]
- 4. Kofflard MJM, Ten Cate FJ, van der Lee C, van Domberg RT. Hypertrophic cardiomyopathy in a large community‐based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol 2003; 41: 987–993. [DOI] [PubMed] [Google Scholar]
- 5. Kubo T, Hirota T, Baba Y, Ochi Y, Takahashi A, Yamasaki N, Hamashige N, Yamamoto K, Kondo F, Bando K, Yamada E, Furuno T, Yabe T, Doi YL, Kitaoka H. Patients' characteristics and clinical course of hypertrophic cardiomyopathy in a regional Japanese cohort—results from Kochi RYOMA Study. Circ J 2018; 82: 824–830. [DOI] [PubMed] [Google Scholar]
- 6. Biagini E, Coccolo F, Ferlito M, Perugini E, Rocchi G, Bacchi‐Reggiani L, Lofiego C, Boriani G, Prandstraller D, Picchio FM, Branzi A, Rapezzi C. Dilated‐hypokinesis evolution of hypertrophic cardiomyopathy. Prevalence, incidence, risk factors, and prognostic implications in pediatrics and adult patients. J Am Coll Cardiol 2005; 46: 1543–1550. [DOI] [PubMed] [Google Scholar]
- 7. Thaman R, Gimeno JR, Mutphy RT, Kubo T, Sachdev B, Mogensen J, Elliott PM, McKenna WJ. Prevalence and clinical significance of systolic impairment in hypertrophic cardiomyopathy. Heart 2005; 91: 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey‐Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end‐stage phase of hypertrophic cardiomyopathy. Circulation 2006; 114: 216–225. [DOI] [PubMed] [Google Scholar]
- 9. Hamada T, Kubo T, Kitaoka H, Hirota T, Hoshikawa E, Hayato K, Shimizu Y, Okawa M, Yamasaki N, Matsumura Y, Yabe T, Takata J, Doi YL. Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin Cardiol 2010; 33: E24–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goto D, Kinugawa S, Hamaguchi S, Sakakibara M, Tsuchihashi‐Makaya M, Yokota T, Yamada S, Yokoshiki H, Tsutsui H, JCARE‐CARD Investigators . Clinical characteristics and outcomes of dilated phase of hypertrophic cardiomyopathy: report from the registry data in Japan. J Cardiol 2013; 61: 65–70. [DOI] [PubMed] [Google Scholar]
- 11. Sato Y, Yamada T, Taniguchi R, Nagai K, Makiyama T, Okada H, Kataoka K, Ito H, Matsumori A, Sasayama S, Takatsu Y. Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 2001; 103: 369–374. [DOI] [PubMed] [Google Scholar]
- 12. Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108: 833–838. [DOI] [PubMed] [Google Scholar]
- 13. Kawahara C, Tsutamoto T, Nishiyama K, Yamaji M, Sakai H, Fujii M, Yamamoto T, Horie M. Prognostic role of high‐sensitivity cardiac troponin T in patients with nonischemic dilated cardiomyopathy. Circ J 2011; 75: 656–661. [DOI] [PubMed] [Google Scholar]
- 14. Kubo T, Kitaoka H, Yamanaka S, Hirota T, Baba Y, Hayashi K, Iiyama T, Kumagai N, Tanioka K, Yamasaki N, Matsumura Y, Furuno T, Sugiura T, Doi YL. Significance of high‐sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol 2013; 62: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 15. Kubo T, Kitaoka H, Okawa M, Nishinaga M, Doi YL. Hypertrophic cardiomyopathy in the elderly. Geriatr Gerontol Int 2010; 10: 9–16. [DOI] [PubMed] [Google Scholar]
- 16. Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail 2012; 5: 535–546. [DOI] [PubMed] [Google Scholar]
- 17. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 2017; 70: 942–954. [DOI] [PubMed] [Google Scholar]
- 18. Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004; 43: 2260–2264. [DOI] [PubMed] [Google Scholar]
- 19. Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol 2003; 41: 1561–1567. [DOI] [PubMed] [Google Scholar]
- 20. Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol 2008; 51: 1369–1374. [DOI] [PubMed] [Google Scholar]
- 21. Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, Lesser JR, Udelson JE, Manning WJ, Maron BJ. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail 2008; 1: 184–191. [DOI] [PubMed] [Google Scholar]
- 22. Leonardi S, Raineri C, De Ferrari GM, Ghio S, Scelsi L, Pasotti M, Tagliani M, Valentin A, Dore R, Raisaro A, Arbustini E. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009; 30: 2003–2010. [DOI] [PubMed] [Google Scholar]
- 23. O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli‐Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010; 56: 867–874. [DOI] [PubMed] [Google Scholar]
- 24. Moreno V, Hernandez‐Romero D, Vilchez JA, Garcia‐Honrubia A, Campronero F, Casas T, Gonzalez J, Martinez P, Climent V, de la Morena G, Valdes M, Marin F. Serum levels of high‐sensitivity troponin T: a novel marker for cardiac remodeling in patients with hypertrophic cardiomyopathy. J Card Fail 2010; 16: 950–956. [DOI] [PubMed] [Google Scholar]
- 25. Petersen SE, Jerosch‐Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 2007; 115: 2418–2425. [DOI] [PubMed] [Google Scholar]
- 26. Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG. Relevance of coronary microvascular flow impairment to long‐term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006; 47: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 27. Raman B, Ariga R, Spartera M, Spartera M, Sivalokanathan S, Chan K, Dass S, Petersen SE, Daniels MJ, Francis J, Smillie R, Lewandowski AJ, Ohuma EO, Rodgers C, Kramer CM, Mahmod M, Watkins H, Neubauer S. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging 2019; 20: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]


