Abstract
Introduction
Diuretic resistance is a common complication impairing decongestion during hospitalization for acute decompensated heart failure (ADHF). The current understanding of diuretic resistance mechanisms in ADHF is based upon extrapolations from other disease states and healthy volunteers. However, accumulating evidence suggests that the dominant mechanisms in other populations have limited influence on diuretic response in ADHF. Additionally, the ability to rapidly and reliably diagnose diuretic resistance is inadequate using currently available tools.
Aims
The Mechanisms of Diuretic Resistance (MDR) Study is designed to rigorously investigate the mechanisms of diuretic resistance and develop tools to rapidly predict diuretic response in a prospective cohort hospitalized with ADHF.
Methods
Study assessments occur serially during the ADHF hospitalization and after discharge. Each assessment includes a supervised 6‐hour urine collection with baseline blood and timed spot urine collections following loop diuretic administration. Patient characteristics, medications, physical exam findings, and both in‐hospital and post‐discharge HF outcomes are collected. Patients with diuretic resistance are eligible for a randomized sub‐study comparing an increased loop diuretic dose with combination diuretic therapy of loop diuretic plus chlorothiazide.
Conclusions
The Mechanisms of Diuretic Resistance Study will establish a prospective patient cohort and biorepository to investigate the mechanisms of diuretic resistance and urine biomarkers to rapidly predict loop diuretic resistance.
Keywords: Heart failure, Diuretic, Diuretic resistance, Acute heart failure
Introduction
Acute decompensated heart failure (ADHF) is the most common hospital discharge diagnosis among Medicare beneficiaries, directly resulting in approximately 1 million hospitalizations annually and contributing to an additional 2 million hospitalizations as a secondary diagnosis. 1 , 2 Readmission rates remain remarkably high with nearly 50% of patients rehospitalized within 6 months. 2 Hypervolaemia is the chief problem in the majority of patients admitted with ADHF, and intravenous (IV) loop diuretic therapy is utilized in 80–90% of ADHF hospitalizations. 3 , 4 , 5 , 6 Contrary to significant advances in medical therapies for the treatment of chronic heart failure (HF), recent clinical trials of new ADHF therapeutics and treatment strategies have been negative. 7 , 8 , 9 , 10 , 11 , 12 , 13 Thus, loop diuretics remain the predominant therapy to treat fluid and sodium retention in ADHF and will remain so for the foreseeable future.
However, diuretic resistance (DR), defined qualitatively as a significantly reduced rate of diuresis/natriuresis to a diuretic, is common and makes the treatment of hypervolaemia in ADHF challenging. 14 , 15 Notably, an IV furosemide dose of 40 mg increases fractional excretion of sodium (FENa) to 20–25% and produces 3–4 L of urine in healthy volunteers. 16 , 17 The average patient with ADHF has a log reduction in response, producing only 300–400 mL of urine output per 40 mg of IV furosemide equivalents. 18 , 19 Even with high‐dose IV loop diuretics, the average peak FENa achieved is only ~5% in ADHF. 20
The majority of our current understanding of DR mechanisms in ADHF is extrapolated from studies of healthy volunteers and patients with hypertension and/or chronic kidney disease. 16 , 21 , 22 , 23 , 24 Mechanisms of DR that are dominant in other populations, such as reduced glomerular filtration rate, hypoalbuminaemia, and albuminuria, appear to have a minor influence on diuretic response in patients with HF compared with aberrations in tubular sodium handling. 18 , 25 , 26 , 27 In normal subjects, the negative sodium balance from a loop diuretic can be completely eliminated by a high‐sodium intake. 16 , 28 , 29 In contrast, several lines of evidence have suggested an equivalent or even superior diuretic response in patients with HF receiving higher sodium chloride intake. 30 , 31 , 32 , 33 Similarly, a loop diuretic infusion should unequivocally provide a profound advantage over bolus dosing based on traditional DR paradigms. 34 , 35 However, multiple clinical trials have failed to demonstrate a meaningful difference between bolus and continuous infusion in ADHF. 36 , 37
In addition to our limited understanding of the mechanisms of DR in human HF, our ability to diagnose DR is limited. In clinical practice and ADHF trials, changes in weight and fluid balance are the primary strategies to assess diuretic response. 13 , 36 , 38 , 39 , 40 Weight and net fluid loss should correlate perfectly. Yet even in clinical trials, weight and net fluid loss display poor correlation with each other and are notoriously difficult to accurately obtain even in these settings of rigorous monitoring. 41 It is reasonable to assume that the measurement of weight and fluid loss is worse in routine clinical practice, demonstrating the need for better tools to assess diuretic response.
Therefore, a rigorous investigation of DR in a real‐world ADHF population is required to better inform diagnostic strategies and understand which mechanisms predominate in ADHF, thus informing treatment strategy. The Mechanisms of Diuretic Resistance (MDR) Study was designed to rigorously investigate the mechanisms of DR and develop tools to rapidly predict diuretic response in a real‐world cohort of patients hospitalized with ADHF assessed serially across the hospital episode and after discharge.
Study design
The MDR Study seeks to establish a prospective patient cohort and biorepository, creating a premier data source for the study of DR in ADHF. The primary objective is to study DR mechanisms by combining the methodological rigour of a General Clinical Research Center (GCRC) in multiple study assessments with the generalizability of a heterogeneous, real‐world hospitalized ADHF population. The MDR Study is composed of serial assessments following loop diuretic administration, occurring at milestones during and after the ADHF hospital episode. Each timed urine collection is intensely supervised by research personnel with protocolized biospecimen collections to replicate the rigour of a research centre observation during a hospital stay for ADHF. However, the inclusion and exclusion criteria are designed to allow generalizability to a real‐world ADHF population. Additional objectives include studying the changes in the mechanisms of DR over time in the ADHF treatment episode, identifying urine variables to rapidly identify DR, and investigating the association between DR parameters and outcomes during and post‐hospital discharge (Table 1 ).
Table 1.
Example research questions to be investigated with the Mechanisms of Diuretic Resistance cohort
| What variables predict loop diuretic response? |
| Can widely available urine tests (i.e. urine electrolytes) allow rapid diagnosis of diuretic resistance? |
| Are specific mechanisms of diuretic resistance more responsive to escalating loop diuretic doses or various combinations of diuretic therapies? |
| Does tailoring diuretic therapy to the specific resistance mechanism improve natriuresis? |
| Can the mechanism of resistance be predicted using commonly available laboratory tests? |
| Which nephron location is primarily responsible for diuretic resistance? |
| Which specific ion transporters contribute significantly to diuretic resistance? |
| How does the loop diuretic natriuretic response and specific ion transporter activity change over an acute heart failure hospitalization episode? |
| How often is the initial empiric oral loop diuretic dose chosen an effective one? |
| What parameters can be used to detect diuretic resistance in outpatients where cumulative metrics, e.g. urine output, are unavailable? |
| What is the correlation between post‐discharge outcomes and variables such as diuretic resistance mechanisms or natriuretic response to combination diuretic therapies? |
The MDR Study is a prospective, observational cohort of patients hospitalized with ADHF at the two main hospitals in the Yale New Haven Hospital System (USA). After enrolment, patients are followed longitudinally with study assessments, termed ‘visits’, occurring at several milestones within the ADHF hospital episode, an outpatient study visit after hospital discharge, and outcomes assessment after hospital discharge. Within this study, patients with DR at the first study visit are randomized in an open‐label, parallel sub‐study comparing an increased dose of IV loop diuretic therapy with the addition of IV chlorothiazide to the same dose of IV loop diuretic therapy (NCT02546583).
The philosophy of the MDR Study is to investigate DR mechanisms in a heterogeneous ADHF population with real‐world generalizability. Via a pharmacy‐generated list, all patients prescribed an IV loop diuretic are screened daily. Full inclusion and exclusion criteria are listed in Table 2 . Key inclusion criteria include a clinical diagnosis of ADHF with at least one objective sign of hypervolaemia and projected need, based on the treating clinician's assessment, of IV loop diuretic therapy for at least 3 days. To maximize external validity, patients were not excluded based upon estimated glomerular filtration rate.
Table 2.
Mechanisms of Diuretic Resistance study criteria
| Inclusion criteria |
|---|
| Age ≥18 years |
| Clinical diagnosis of acute decompensated heart failure with at least one of the following objective signs of hypervolaemia: |
| • Oedema |
| • Rales |
| • Elevated jugular venous pressure |
| • Pre‐admission weight gain |
| Current use of bolus IV loop diuretic therapy and projected need by the treating clinician for continued treatment with IV diuretics for at least 3 days with the goal of significant fluid removal (>1 L net fluid loss per day) |
| Exclusion criteria |
|---|
| Inability to perform informed consent or comply with the serial urine collection procedures |
| Significant known bladder dysfunction or urinary incontinence |
| Haematocrit %3C21% or active bleeding |
IV, intravenous.
Patients with DR at Study Visit 1, defined as a cumulative 6 h sodium output %3C100 mmol following the Visit 1 IV loop diuretic dose, are eligible for a randomized sub‐study occurring as Study Visit 2. While additional inclusion and exclusion criteria exist to qualify for the randomized sub‐study (Table 3 ), these criteria are limited to ensure that a diverse, representative cohort is included while ensuring patient safety. A cumulative 6 h sodium output %3C100 mmol was chosen as this would rarely result in a significant net negative daily sodium balance (maximum possible sodium balance of only negative 70 mmol/day assuming twice daily diuretic dosing and a 130 mmol dietary sodium intake per day). 42 , 43 , 44 Similar to prior studies, patients with an IV loop diuretic dose >160 mg IV furosemide equivalents at Visit 1 are not included, even if they met the criteria for DR, because their loop diuretic bolus dose would exceed 400 mg IV furosemide equivalents if randomized to the 2.5 times the Visit 1 IV diuretic treatment arm. 36 Patients not meeting the full inclusion and exclusion criteria listed in Table 3 are not included in the randomized sub‐study but are eligible for subsequent study visits.
Table 3.
Mechanisms of Diuretic Resistance additional criteria for randomized sub‐study
| Inclusion criteria |
|---|
| Cumulative 6 h sodium output %3C100 mmol following Visit 1 IV loop diuretic dose |
| Visit 1 IV loop diuretic dose ≤160 mg IV furosemide equivalents a |
| Serum sodium >125 mEq/L |
| At least 6 h since last dose of diuretic |
| Inclusion criteria |
|---|
| Current use or projected future requirement by the treating physician for thiazide diuretics |
| Use of high‐dose mineralocorticoid receptor antagonist therapy (≥50 mg of spironolactone or ≥100 mg of eplerenone) or amiloride |
IV, intravenous.
40 mg IV furosemide = 1 mg IV bumetanide.
Study assessments
A flow diagram of the study assessment timeline is provided in Figure 1 A . After consent, patients undergo serial study ‘visits’ during the ADHF hospital episode. Each visit includes a supervised 6 h urine collection timed to loop diuretic administration with protocolized biospecimen collections. Visit 1 occurs early in the ADHF treatment course during open‐label IV loop diuretic therapy. Patients with DR at Visit 1 can be included in the randomized sub‐study, termed Visit 2. At Visit 2, patients are randomized to open label increase in loop diuretic therapy (2.5 times the Visit 1 IV loop diuretic dose) or combination of loop dose and thiazide diuretic therapy (1 times the IV loop diuretic at Visit 1 plus 500 mg of IV chlorothiazide, unless the estimated glomerular filtration rate is %3C30 mL/min/1.73 m2 in which case they receive a 1000 mg dose) the following day. Visit 2 is not conducted in patients who do not meet the sub‐study inclusion and exclusion criteria in Table 3 . Additional reasons for not having Visit 2 include both medical (e.g. haemodynamic instability, diuretic discontinuation, use of non‐study diuretic therapies, altered mental status, and new bladder incontinence or retention) and logistical (patient refusal, new loose stools preventing urine collection, transfer to hospital ward where study procedures are not possible, etc.). With the exception of Visit 2, the diuretic therapy is at the discretion of the treating medical provider. Visit 3 occurs during IV diuretic therapy later in the hospital stay, ideally on the last day of IV diuretic therapy when able to be predicted. Visit 4 occurs upon transition to oral diuretic therapy prior to hospital discharge. Visit 5 occurs in a clinical research centre after hospital discharge on oral diuretic therapy. After discharge, patients are prospectively monitored for rehospitalization and death via electronic medical records and the Social Security Death Index.
Figure 1.
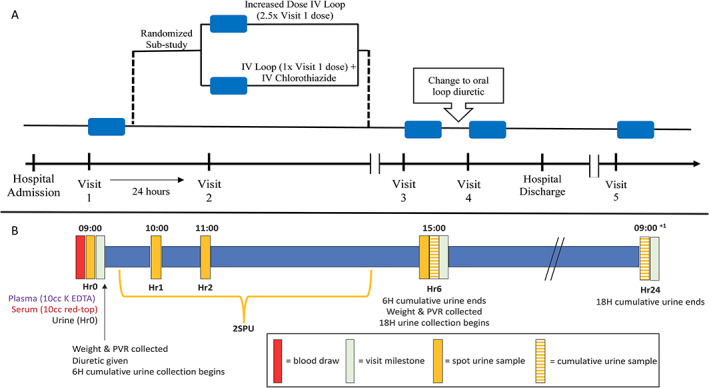
Study assessment diagram. (A) Patients hospitalized with acute decompensated heart failure enrolled in the Mechanisms of Diuretic Resistance Study undergo serial ‘visits’, indicated by a blue box. Visits 1, 3, and 4 occur during open‐label intravenous (IV) loop diuretic therapy. Patients who are diuretic resistant at Visit 1 and meet sub‐study criteria are randomized to increased loop diuretic therapy or combination of loop and thiazide diuretic therapy at Visit 2 the following day. Visit 3 occurs during IV diuretic therapy later in the hospital stay. Visit 4 occurs on the day of oral diuretic therapy prior to hospital discharge. Visit 5 occurs in a clinical research centre after hospital discharge on oral diuretic therapy. (B) Each visit includes a supervised 6 h urine collection with urine and blood sample collection and standing weight measurements timed to a dose of loop diuretic. Spot urine is collected at Hours 0, 1, 2, and 6. A spot sample from the second spontaneous urination (2SPU) is also collected if it occurred outside of these timed spot urine samples. A sample from the 6 and 18 h cumulative urine collections is also collected. H, cumulative hours; Hr, hour of biospecimen collection; PVR, post‐void residual.
Each study visit includes a 6 h supervised urine collection with timed spot urine collections (Figure 1 B ). In the 3 h prior to loop diuretic administration, a urine sample is collected any time the patient urinates, and the time and full volume of the urination are recorded as contingency urine. Prior to diuretic administration, patients are instructed to empty their bladder, which is confirmed with post‐void bladder ultrasound. A standing weight with serum, plasma, and urine collections is performed at Hour 0 (Hr0). If the patient is unable to urinate at Hr0, the contingency urine from the most recent void in the previous 3 h is saved as Hr0. After diuretic administration, spot urine is collected at Hours 1 (Hr1), 2 (Hr2), and 6 (Hr6). A spot sample from the second spontaneously produced urination is also collected if it occurred outside of these timed spot urine samples. The time and volume of each spot urination are recorded. The 6 h cumulative urine collection is measured via graduated cylinder to the nearest 5 mL. To account for urine sampled throughout the visit, sample volumes are added back to calculate the total urine output. After the 6 h supervised urine collection, an additional 18 h urine collection is initiated at Hr6, to quantify 24 h urine output. The 18 h urine collection is measured for volume, and a sample is collected.
A full list of all study assessments is provided in Table 4 . After consent and enrolment in the MDR Study, admission and prior‐to‐admission variables are collected from the electronic medical record. Some visits have specific additional assessments unique to the study visit, which are also displayed in Table 4 . A validated method of loop diuretic dose logarithmic transformation will be utilized when expressing diuretic response in relation to increasing diuretic dose. 26 Sodium and urine output will be the predominant descriptors utilized for diuretic response. Sodium‐based diuretic response will be described using cumulative sodium output, representing the absolute sodium excretion rate of the kidney, and FENa, representing natriuresis ‘per nephron’. Post‐discharge outcomes are prospectively assessed for mortality and rehospitalization back to the Yale New Haven Hospital System.
Table 4.
Study assessments
| Admission assessments a |
| Demographics and co‐morbidities |
| Concomitant medications |
| Baseline clinically obtained laboratory values |
| Physical exam |
| Vital signs and weight |
| Assessments repeated at each study visit |
| Physical exam assessment a |
| All diuretic medications a |
| Clinically obtained laboratory values a |
| Standing weight at baseline and Hr6 |
| Post‐void residual at baseline and Hr6 |
| 6 h cumulative urine sodium (mmol) |
| Assessments unique to specific study visit |
| Visit 1 |
| Clinical Assessment Survey |
| Visit 4 |
| Clinical Assessment Survey |
| Visit 5 |
| Clinical Assessment Survey |
| Concomitant medication list |
| Hospital discharge a |
| Weight |
| Discharge medications |
| Clinically obtained laboratory values (CBC, CMP, and LFTs) at discharge, peak, and nadir during hospitalization |
| Assessments of heart failure outcomes |
| All‐cause mortality |
| First rehospitalization |
CBC, complete blood count; CMP, complete metabolic panel; Hr6, Hour 6; LFTs, liver function tests.
Generated during standard of care and collected from electronic medical record.
Urine and blood samples
Each urine sample is divided into two conicals for analysis. A 15 mL urine conical is analysed via urine dipstick for pH and standard urine analyses, centrifuged for 10 min at 4°C and 1500 g, and then divided into up to ten 1 mL aliquots for storage in the biorepository. In addition, up to 42.5 mL of urine is treated with protease inhibitor and stored to isolate microvesicles at a later time point.
Serum is allowed to clot at room temperature for no %3C60 min and then put on ice for 10 min prior to centrifugation. Plasma is refrigerated until separation is visible and qualitatively analysed for haemolysis prior to centrifugation. Serum and plasma are then centrifuged for 10 min at 4°C and 1500 g. Up to ten 0.5 mL aliquots from each serum and plasma collection, along with the plasma sample's buffy coat for DNA, are saved. After aliquoting, all blood and urine samples are stored at −80°C in continuously monitored freezers and registered in the biospecimen collection console of OnCore, a biospecimen management system integrated with the electronic medical record.
Study population
Beginning in 2015, the MDR Study aimed to enroll 200 participants. The overall cohort size was calculated to provide adequate power to validate our previously published loop diuretic natriuretic response prediction equation, a primary study question in Table 1 . 42 Detecting a statistically significant difference (P %3C 0.05) with an area under the curve (AUC) of ≥0.8 with a power of 90% only requires 34 patients. As such, we focused rather on the size of the confidence interval of the AUC. With a population of 200 participants, there was 90% power to detect a 95% confidence interval error margin of 0.06 and 0.04 for an AUC of 0.8 and 0.9, respectively (α = 0.05). All other study question power calculations were performed as a derivative of this primary study population size.
We projected that ~50–66% of patients in the total cohort would qualify for the randomized sub‐study (Visit 2). At 2 years of enrollment, only 26% (n = 60) of 228 enrolled participants were randomized in the sub‐study. The Visit 2 enrollment target was modified to a goal of 100 patients with expansion of the total study population size to reach that goal. To date, the MDR Study has enrolled 460 total participants, with enrollment into the Visit 2 sub‐study nearly complete. Enrollment is estimated to be completed by July 2020.
Discussion
The MDR Study will facilitate investigation into the multitude of clinical questions surrounding DR in ADHF. The central study goals are rapid identification of patients with DR and identification of DR mechanisms in ADHF. By developing the knowledge base and tools to rapidly identify DR, days of ‘wasted’ hospitalization with ineffective diuresis can potentially be avoided. Understanding the mechanism(s) of DR in patients with ADHF is a critical step in improving care in this population. As multiple medications with antagonistic activity at renal sodium transporters/channels are already available, knowledge of the nephron segment location and specific transporters responsible for DR can be immediately translated to patient care at low cost.
Limitations of the MDR Study are extensions of intentionally enrolling a heterogeneous, real‐world cohort. In an effort to capture this population, patients hospitalized with ADHF are enrolled even if completion of all longitudinal assessment visits is not expected. Reasons for non‐completion of all visits could be medical (e.g. worsening creatinine with cessation of IV diuretic therapy) or at the participant level (e.g. unlikely to return for an outpatient follow‐up visit). Despite an intensely supervised 6 h urine collection, missing or spilled urine voids still infrequently occur. Missing voids are documented for inclusion in analyses. Study data that are generated during standard‐of‐care medical orders may be missing in some patients based upon unavailable data.
The MDR Study will provide a methodologically rigorous dataset and biorepository to study the mechanisms of DR longitudinally in a diverse real‐world population of patients hospitalized with ADHF. The planned investigations will provide unique insight on the specific mechanisms of DR and impact of different diuretic strategies to restore diuretic response. The findings will provide critical information towards our understanding of DR in patients with ADHF on contemporary medical management.
Conflict of interest
J.M.T. reports grants and personal fees from Sequana Medical, BMS, 3ive Labs, Boehringer Ingelheim, Sanofi, and FIRE1; personal fees from AstraZeneca, Novartis, Cardionomic, Bayer, MagentaMed, Reprieve Medical, W.L. Gore, and Windtree Therapeutics; and grants from Otsuka and Abbott outside the submitted work. Ms.M. reports personal fees from Sequana Medical.
Funding
This publication was made possible by the National Institutes of Health(NIH)/National Heart, Lung, and Blood Institute Grant R01HL128973, Award 5T32HL007950 (to MG), P30DK079310 from the National Institute of Diabetes and Digestive and Kidney Diseases, and by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Sciences, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Cox, Z. L. , Fleming, J. , Ivey‐Miranda, J. , Griffin, M. , Mahoney, D. , Jackson, K. , Hodson, D. Z. , Thomas, D. Jr , Gomez, N. , Rao, V. S. , and Testani, J. M. (2020) Mechanisms of Diuretic Resistance Study: design and rationale. ESC Heart Failure, 7: 4458–4464. 10.1002/ehf2.12949.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, van Wagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Giamouzis G, Kalogeropoulos A, Georgiopoulou V, Laskar S, Smith AL, Dunbar S, Triposkiadis F, Butler J. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail 2011; 17: 54–75. [DOI] [PubMed] [Google Scholar]
- 3. Thibodeau JT, Drazner MH. The role of the clinical examination in patients with heart failure. JACC Heart Fail 2018; 6: 543–551. [DOI] [PubMed] [Google Scholar]
- 4. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2007; 153: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 6. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 7. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J, Investigators T‐A. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 9. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M, Investigators REiAHF . Serelaxin, recombinant human relaxin‐2, for treatment of acute heart failure (RELAX‐AHF): a randomised, placebo‐controlled trial. Lancet 2013; 381: 29–39. [DOI] [PubMed] [Google Scholar]
- 10. Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, Patel CB, Echols M, Khouri MG, Tauras JM, Gupta D, Monds P, Roberts R, O'Connor CM. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017; 69: 1399–1406. [DOI] [PubMed] [Google Scholar]
- 11. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne‐Nickens P, Hernandez AF, Braunwald E, National Heart Lung and Blood Institute Heart Failure Clinical Research Network . Efficacy and safety of spironolactone in acute heart failure: the ATHENA‐HF randomized clinical trial. JAMA Cardiol 2017; 2: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, Gualandro DM, de Oliveira Junior MT, Sabti Z, Muller B, Noveanu M, Socrates T, Ziller R, Bayes‐Genis A, Sionis A, Simon P, Michou E, Gujer S, Gori T, Wenzel P, Pfister O, Conen D, Kapos I, Kobza R, Rickli H, Breidthardt T, Munzel T, Erne P, Mueller C, GALACTIC Investigators . Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA 2019; 322: 2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367: 2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox ZL, Testani JM. Loop diuretic resistance complicating acute heart failure. Heart Fail Rev 2020; 25: 133–145. [DOI] [PubMed] [Google Scholar]
- 15. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 137–155. [DOI] [PubMed] [Google Scholar]
- 16. Brater DC. Diuretic therapy. N Engl J Med 1998; 339: 387–395. [DOI] [PubMed] [Google Scholar]
- 17. Brenner BM, Rector FC. Brenner & Rector's the Kidney, 8th ed. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 18. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WH. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014; 7: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ter Maaten JM, Dunning AM, Valente MA, Damman K, Ezekowitz JA, Califf RM, Starling RC, van der Meer P, O'Connor CM, Schulte PJ, Testani JM, Hernandez AF, Tang WH, Voors AA. Diuretic response in acute heart failure—an analysis from ASCEND‐HF. Am Heart J 2015; 170: 313–321. [DOI] [PubMed] [Google Scholar]
- 20. Hanberg JS, Rao V, Ter Maaten JM, Laur O, Brisco MA, Perry Wilson F, Grodin JL, Assefa M, Samuel Broughton J, Planavsky NJ, Ahmad T, Bellumkonda L, Tang WH, Parikh CR, Testani JM. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail 2016; 9: e003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol 2002; 13: 798–805. [DOI] [PubMed] [Google Scholar]
- 22. Loon NR, Wilcox CS, Unwin RJ. Mechanism of impaired natriuretic response to furosemide during prolonged therapy. Kidney Int 1989; 36: 682–689. [DOI] [PubMed] [Google Scholar]
- 23. Nakahama H, Orita Y, Yamazaki M, Itoh S, Okuda T, Yamaji A, Miwa Y, Yanase M, Fukuhara Y, Kamada T. Pharmacokinetic and pharmacodynamic interactions between furosemide and hydrochlorothiazide in nephrotic patients. Nephron 1988; 49: 223–227. [DOI] [PubMed] [Google Scholar]
- 24. Phakdeekitcharoen B, Boonyawat K. The added‐up albumin enhances the diuretic effect of furosemide in patients with hypoalbuminemic chronic kidney disease: a randomized controlled study. BMC Nephrol 2012; 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco‐Bacik MA, Wilson FP, Jacoby D, Chen M, Tang WHW, Cherney DZI, Ellison DH, Testani JM. Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol JASN 2017; 28: 3414–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ter Maaten JM, Rao VS, Hanberg JS, Perry Wilson F, Bellumkonda L, Assefa M, Sam Broughton J, D'Ambrosi J, Wilson Tang WH, Damman K, Voors AA, Ellison DH, Testani JM. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail 2017; 19: 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charokopos A, Griffin M, Rao VS, Inker L, Sury K, Asher J, Turner J, Mahoney D, Cox ZL, Wilson FP, Testani JM. Serum and urine albumin and response to loop diuretics in heart failure. Clin J Am Soc Nephrol 2019; 14: 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001; 96: 132–143. [DOI] [PubMed] [Google Scholar]
- 29. Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Dunbar SB, Reilly CM, Sands JM, Fonarow GC, Jessup M, Gheorghiade M, Yancy C, Butler J. Dietary sodium intake in heart failure. Circulation 2012; 126: 479–485. [DOI] [PubMed] [Google Scholar]
- 30. Paterna S, Di Pasquale P, Parrinello G, Amato P, Cardinale A, Follone G, Giubilato A, Licata G. Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail 2000; 2: 305–313. [DOI] [PubMed] [Google Scholar]
- 31. Paterna S, Di Pasquale P, Parrinello G, Fornaciari E, Di Gaudio F, Fasullo S, Giammanco M, Sarullo FM, Licata G. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high‐dose furosemide and hypertonic saline solution versus high‐dose furosemide alone in refractory congestive heart failure: a double‐blind study. J Am Coll Cardiol 2005; 45: 1997–2003. [DOI] [PubMed] [Google Scholar]
- 32. Licata G, Di Pasquale P, Parrinello G, Cardinale A, Scandurra A, Follone G, Argano C, Tuttolomondo A, Paterna S. Effects of high‐dose furosemide and small‐volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long‐term effects. Am Heart J 2003; 145: 459–466. [DOI] [PubMed] [Google Scholar]
- 33. Griffin M, Soufer A, Goljo E, Colna M, Rao VS, Jeon S, Raghavendra P, D'Ambrosi J, Riello R, Coca SG, Mahoney D, Jacoby D, Ahmad T, Chen M, Tang WHW, Turner J, Mullens W, Wilson FP, Testani JM. Real world use of hypertonic saline in refractory acute decompensated heart failure: a U.S. center's experience. JACC Heart Fail 2020; 8: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellison DH, Felker GM. Diuretic treatment in heart failure. N Engl J Med 2017; 377: 1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Felker GM, O'Connor CM, Braunwald E. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail 2009; 2: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alqahtani F, Koulouridis I, Susantitaphong P, Dahal K, Jaber BL. A meta‐analysis of continuous vs intermittent infusion of loop diuretics in hospitalized patients. J Crit Care 2014; 29: 10–17. [DOI] [PubMed] [Google Scholar]
- 38. Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE Jr, McMurray JJ, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62: 1495–1539. [DOI] [PubMed] [Google Scholar]
- 39. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, le Winter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila‐Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM. Low‐dose dopamine or low‐dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310: 2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cox ZL, Hung R, Lenihan DJ, Testani JM. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, Coca SG, Tang WH. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med 2015; 128: 776–831.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Rapid and highly accurate prediction of poor loop diuretic natriuretic response in patients with heart failure. Circ Heart Fail 2016; 9: e002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furosemide [package insert]. Sanofi‐Aventis L, Bridgewater, NJ; November 2012.
- 44. Hodson DZ, Griffin M, Mahoney D, Raghavendra P, Ahmad T, Turner J, Wilson FP, Tang WHW, Rao VS, Collins SP, Mullens W, Testani JM. Natriuretic response is highly variable and associated with 6‐month survival: insights from the ROSE‐AHF trial. JACC Heart Fail 2019; 7: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]


