Abstract
Aims
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune disorder that not only affects peripheral joints but also increases the risk for cardiovascular disease (CVD) and mortality. Heart failure (HF) appears to be one of the most important contributors to the excess mortality risk among patients with RA. We assessed the incidence of HF in patients with RA compared with age‐matched and sex‐matched non‐RA subjects, after accounting for traditional cardiovascular risk factors and clinical ischemic heart disease.
Methods and results
We performed an aggregate analysis on three studies of RA patients having listed manifestations of HF. We performed a meta‐regression analysis to evaluate the incidence of HF in RA patients with increased age and noted for any gender correlation. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using both fixed‐effects and random‐effects models. In the cumulative analysis of 5, 220, 883 patients, the incidence of HF was noted to be almost two‐fold higher in patients with RA compared with a matched control population (OR 1.78, 95% CI 1.22–2.60, P < 0.003), HTN (OR 1.66, 95% CI 1.24–2.23, P < 0.001), and diabetes (OR 1.57, 95% CI 1.36–1.81, P < 0.001). Women had three‐fold higher incidence of HF with RA (OR 3.38, 95% CI 2.59–4.40, P < 0.001). On meta‐regression, the incidence of HF increased further with older age (coefficient = 0.12, P = 0.0004).
Conclusions
Our systematic review that included over 5 million subjects confirms the suspected increased incidence of HF in RA patients. Women have the greatest risk for HF. Our analysis advocates the need for updating the current guidelines to incorporate screening and preventive methods for HF in RA patients.
Keywords: Rheumatoid arthritis, Congestive heart failure, Cardiovascular disease, Female gender, Inflammatory disorder
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic inflammatory autoimmune disorder most often characterized by peripheral and symmetrical joint inflammation. 1 , 2 , 3 In addition to chronic joint inflammation, patients with RA are at an increased risk for several extra‐articular manifestations. 3 RA has been shown to be an independent risk factor for cardiovascular disease (CVD) in a similar manner as older age, hypertension, diabetes mellitus, dyslipidemia, obesity, and tobacco use. 4 , 5 , 6 , 7 For example, there have been reports of an increased risk of myocardial infarction via worsening coronary atherosclerosis in women with RA, even in the absence of CVD risk factors. 8 It has further been suggested that CVD sequelae, such as myocardial infarction, arrhythmias, pericardial disease, and left ventricular failure, remain the most common cause of death in patients with RA. 9 , 10 , 11
Of the CVD manifestations of RA, heart failure (HF) appears to be one of the most important contributors to the excess mortality risk among patients. 5 , 12 , 13 , 14 Pro‐inflammatory cytokines involved in HF appear to be accentuated because of a chronic inflammatory state in RA patients. 15 Further animal models studying the inflammatory consequences that active RA has on contractile and molecular remodelling of the heart support the premise that there needs to be increased awareness of the incidence and prevalence of HF in RA patients. 16
To identify the percentage of the RA population that is at risk of HF, we present a novel systemic review and meta‐regression of literature that assesses the incidence of HF in patients with RA compared with age‐matched and sex‐matched non‐RA subjects, after accounting for traditional cardiovascular risk factors.
Methods
Study design/search strategy
A systematic review of the literature was performed. Two co‐authors (Y. K. and N. D.) independently searched published studies indexed in Ovid, Cochrane Central Register of Controlled Trials (CENTRAL) via the Wiley Interface, Web of Science Core Collection, MEDLINE, Embase, and Google Scholar from the beginning of the project on 1 October 2019 to 30 January 2020. We searched the terms ‘rheumatoid arthritis’ in all fields with each of the following keywords: heart failure, congestive heart failure, heart failure preserved ejection fraction, acute heart failure, systolic heart failure, diastolic heart failure, heart failure preserved ejection fraction, heart failure reduced ejection fraction, ischemic cardiomyopathy, idiopathic cardiomyopathy, cardiomyopathy, chronic heart failure, end‐stage heart failure, and cardiogenic shock. All electronically published papers were screened by titles or abstracts. We only reviewed entire papers if they were pertinent for inclusion for our study. All detected references were saved electronically in the Zotero reference management program, and all duplicates were identified and removed. The systematic search was supplemented by manual review of all references in the retrieved eligible studies.
Study eligibility
Randomized, prospective, retrospective, cross‐sectional, and non‐randomized controlled studies were considered for inclusion if they reported the incidence of HF in patients with RA compared with control patients without RA. The inclusion criteria were as follows 1 : observational studies (case–control, cross‐sectional, or cohort studies) published as original studies to evaluate the association between RA and HF 2 ; odds ratios (ORs), relative risks, hazard ratios, or standardized incidence ratios with 95% confidence intervals (CIs) were provided 3 ; and non‐rheumatoid participants were used as the reference group in cross‐sectional and cohort studies, and participants without HF were used as the reference group in case–control study. When the needed data were not directly found in the published articles, we obtained the necessary data from the authors through electronic communication or via reviewing their supplemental reports.
We excluded all studies that did not meet these criteria or that reported insufficient data. We used the checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modeling Studies for study quality assessment. The search methods and study selection/eligibility criteria are outlined in Figure 1 . 17
Figure 1.
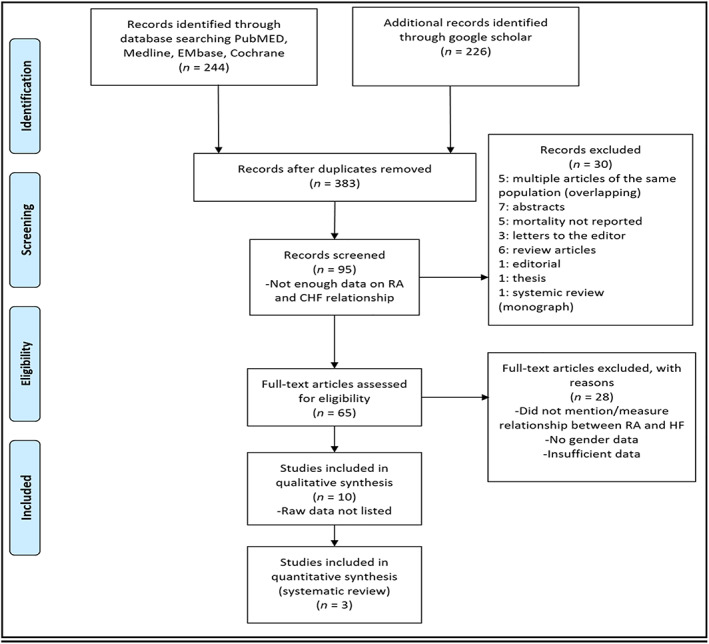
Flow chart of studies screened and included in our systematic review. CHF, congestive heart failure; HF, heart failure; RA, rheumatoid arthritis.
Patients were selected per RA classification criteria. For the Khalid et al. study, the diagnosis of RA was established by rheumatologists. 18 For the Logstrup et al. study, the diagnosis of RA was determined by using the Danish National Patient Register to identify all patients with a first time primary or secondary diagnosis of RA in Denmark since 1995 until July 2016 using ICD‐10 codes (M05.0–M05.9 and M06.0–M06.9), index date was defined as the time of first primary or secondary diagnosis of RA, and both hospital inpatient and outpatient contacts were included. 18 Finally, for the Mantel et al. study, patients with RA were identified as patients 18 years of age or older with at least two visits at inpatient or outpatient specialist clinics with a listed diagnosis of RA in the National Patient Registry, including at least one visit at an internal medicine or rheumatology clinic between 2006 and 2012. 18 All diagnoses of RA were based on the American College of Rheumatology (ACR) criteria or the European League Against Rheumatism (EULAR) criteria. 2 , 18
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, title of the article, study design, year of study, country of origin, year of publication, sample size, characteristics of included participants, definition of HF, method used to diagnose RA and HF, mean duration of follow‐up, and adjusted effect estimates with 95% CI. The two investigators mentioned earlier independently performed this data extraction.
Statistical analysis
We performed an aggregate systematic review on three studies of 5, 220, 883 individuals, 89, 145 patients with RA compared with a matched control population of 4, 651, 419, which compared the incidence of HF over varying time periods to a large number of matched controls from the same registries. Summary ORs and 95% CIs were estimated using a random‐effects model.
The Hartung–Knapp–Sidik–Jonkman method was employed to complete the statistical data using MedCalc software with (i) a summary of data from individual studies; (ii) an investigation of the studies' heterogeneity graphically and statistically; (iii) calculation of clustered indexes; (iv) exploration of heterogeneity; and (v) graphical illustration via forest plots. Our assumption of heterogeneity was tested for each planned analysis using the Cochrane Q heterogeneity and I 2 statistics with each of the following values: low (0–25%), moderate (25–75%), or high (>75%). Random effects models using the Mantel–Haenszel method and fixed‐effects models were used. Meta‐regression was analysed using a generalization of Littenberg and Moses linear model weighted by inverse of the variance or study size unweighted.
A secondary analysis was performed using the DerSimonian and Laird method and fixed‐effects models through Comprehensive Meta‐Analysis Software. Heterogeneity was assessed using the χ 2 test and I 2 statistic.
Study quality and publication bias
Study quality was assessed using the Newcastle–Ottawa scale (0–9 points) using the methodology described by Downs and Black.
All statistical tests were two tailed, and the type I error rate was set at 5%. Only two‐sided tests with a significance level of 0.05 were used. CIs of individual studies were calculated from the available data. A sensitivity analysis was also conducted. Publication bias was assessed using funnel plots and the Egger and Begg test using I 2 and Q statistics for incidence of HF.
Results
Study selection and patient characteristics
The process of study selection is presented in Figure 1 . From the initial 244 papers identified from the search strategy, 226 additional studies were considered to be potentially eligible, following the exclusion of duplicates. After full review, three studies were included in the final analysis (Table 1 ). The characteristics of the three studies included in the systematic review are presented in Table 1 . All three studies were based on analysis of prospective national registries in Europe and, therefore, multicentre in nature, including inpatients, outpatients, and non‐patient residents. The number of RA patients in each registry varied between the range of 12, 943–62, 732 RA patients and a matched control sample range from 113, 684 to 4, 280, 882 individuals. The total for all three studies reported on 89, 145 patients and a matched control population of 4, 651, 419 individuals, with a weighted mean age of 59 years and weighted 70% women noted. The matched controls were age and sexed matched in a ratio that varied.
TABLE 1.
List of selected studies in our systematic review with their demographics, duration, sample size, mean age, and primary outcomes assessed
| Author and publication year | Country/registry | Duration | RA patient size | Control patient size | Mean age (years) | Primary outcomes assessed | ||
|---|---|---|---|---|---|---|---|---|
| Female (n, %) | Male (n, %) | Female (n, %) | Male (n, %) | |||||
| Khalid 2017 retrospective study | Denmark/Danish | 4 years (2008–2012) | 17, 941; 73.7% | 6,402 26.3% | 2, 172, 815; 50.8% | 2, 108, 067; 49.2% | 60.7 | Incidence of HF |
| Logstrup 2017 prospective study | Denmark/Danish | 21 years (1995–2016) | 36, 176; 69.8% | 15, 683 31.2% | 179 559, 70.0% | 77, 094; 30.0% | 57.0 | Incidence of HF |
| Mantel 2017 retrospective study | Sweden/Swedish | 6 years (2006–2012) | 8,972; 69.3% | 3,971 30.7% | 77 478, 68.2% | 36, 206; 31.8% | 57.0 | Incidence of HF |
Defining outcomes
Our key outcome we studied was HF in RA patients. HF was defined using primary and secondary International Classification of Diseases codes (ICD‐10). All studies used the following ICD‐10 codes to define HF: DI50, DI110, DI130, and DI132.
Two independent Danish registry studies were reported, one by Logstrup et al. and the other by Khalid et al. The latter included patients diagnosed with RA by a rheumatologist since 1978, excluded all HF at the cohort sampling 2008 to 2012, and reported on incident HF over this 5‐ year period of time. The control group was not matched for age, but it included all those without HF diagnosis prior to 2008 and then looked at the HF diagnosis. The researchers changed the diagnosis of HF to include not only an ICD‐10 HF diagnosis but also diagnosed based on loop diuretic treatment. For data on women vs. men, we had to make assumptions of proportion because these data were not explicitly listed in the Khalid et al. report.
Study results
The incidence of HF was noted to be approximately two‐fold higher in patients with RA compared with a matched control population (OR 1.78, 95% CI 1.22–2.60, P < 0.003) as shown in Figure 2 . Female gender had approximately three‐fold higher incidence of HF with RA (OR 3.38, 95% CI 2.59–4.40, P < 0.001). The incidence of HF was noted to be approximately 1.7‐fold higher in patients with RA and HTN compared with a matched control population (OR 1.66, 95% CI 1.24–2.23, P < 0.001) as shown in Figure 4 . The incidence of HF was noted to be approximately 1.7‐fold higher in patients with RA and diabetes, both type 1 and type 2, compared with a matched control population (OR 1.66, 95% CI 1.24–2.23, P < 0.001) as shown in Figure 5 . Fixed and random models led to mostly similar outcomes in all of the noted analyses; the Q and I 2 values indicated significant heterogeneity (Figures 2 , 3 , 4 , 5 ), so forest plots are shown.
Figure 2.
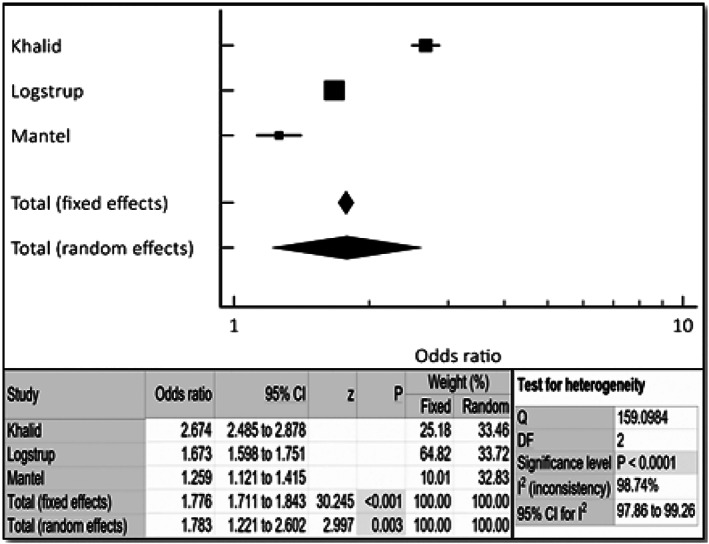
Cumulative incidence of heart failure in rheumatoid arthritis patients via combined fixed‐effects and random‐effects forest plot. Odds ratio, confidence interval (CI), and weight of studies for incidence of heart failure in rheumatoid arthritis patients. Test for heterogeneity of studies for heart failure in rheumatoid arthritis patients. DF, degrees of freedom; I 2, heterogeneity; Q, Q‐value; z, z‐score.
Figure 4.
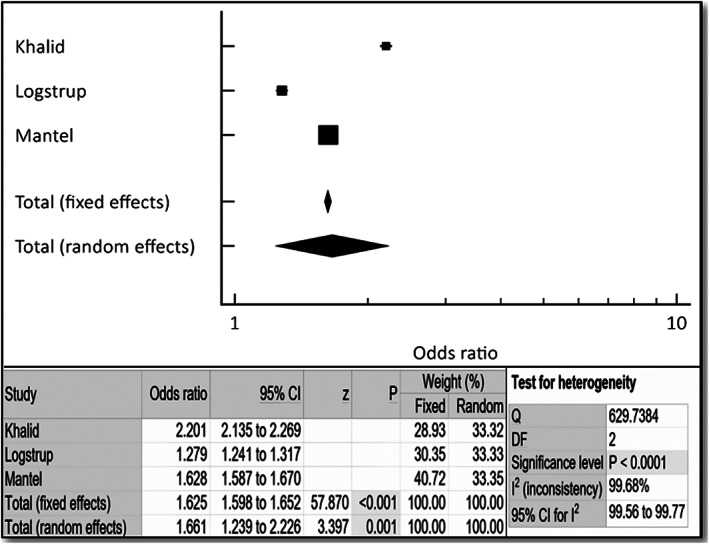
Forest plot depicting odds ratio for hypertension in patients with rheumatoid arthritis (RA) vs. control. Odds ratio, confidence interval (CI), and weight of studies for incidence of heart failure in RA patients with hypertension. Test for heterogeneity of studies for heart failure in female RA patients with hypertension. DF, degrees of freedom; I 2, heterogeneity; Q, Q‐value; z, z‐score.
Figure 5.
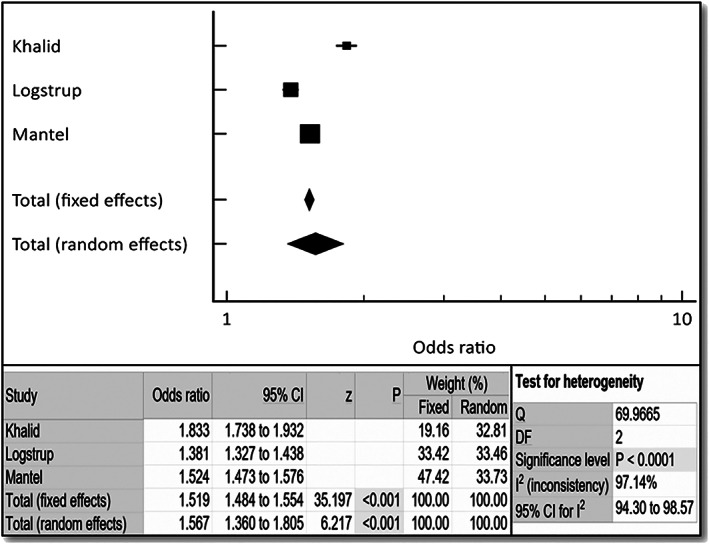
Forest plot depicting odds ratio for diabetes mellitus in patients with rheumatoid arthritis (RA) vs. control. Odds ratio, confidence interval (CI), and weight of studies for incidence of heart failure in RA patients with diabetes mellitus. Test for heterogeneity of studies for heart failure in female RA patients with diabetes mellitus. DF, degrees of freedom; I 2, heterogeneity; Q, Q‐value; z, z‐score.
Figure 3.
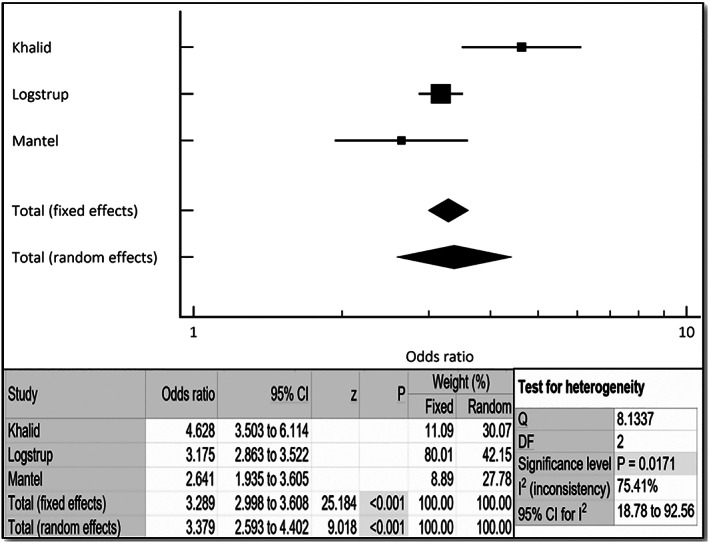
Cumulative incidence of heart failure in female rheumatoid arthritis patients via combined fixed and random effects. Odds ratio, confidence interval (CI), and weight of studies for incidence of heart failure in female rheumatoid arthritis patients. Test for heterogeneity of studies for heart failure in female rheumatoid arthritis patients. DF, degrees of freedom; I 2, heterogeneity; Q, Q‐value; z, z‐score.
On meta‐regression analysis (Figure 6 ), when plotting log OR of incidence of HF in RA patients (y‐axis) against age (x‐axis), incidence of HF was noted to be increased further with older age (coefficient = 0.1047, P = 0.0004).
Figure 6.
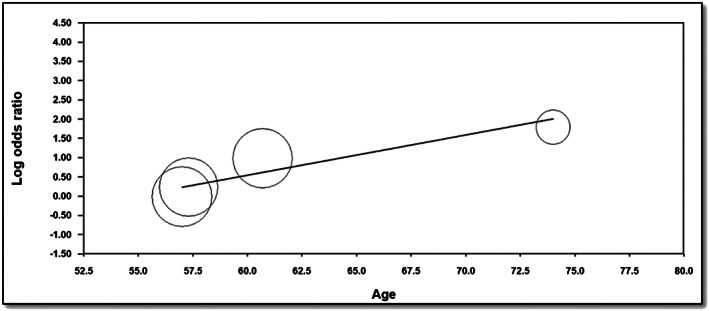
Meta‐regression to assess for incidence of heart failure in rheumatoid arthritis patients with ageing.
Assessment and publication bias analysis
All three studies had high scores on the Newcastle–Ottawa scale, and only one study had a score of 6 (Supporting Information, Table 1 in Appendix S1 ). All studies defined the study objective, described the outcomes and confounders, and outlined the main findings (Supporting Information, Table 2 in Appendix S1 ). We performed Q and I 2 analyses to assess for heterogeneity for meta‐regression, which can be found in Supporting Information, Appendix S2 .
The funnel plot analysis was performed (Supporting Information, Appendices S3 – S6 ) and did not reveal asymmetry around the axis for the treatment effect in the assessed outcomes (P < 0.05), suggesting very low likelihood of publication bias.
Discussion
Patients with or without extra‐articular manifestations of RA (such as lymphadenopathy, skin nodules, osteoporosis, pericarditis, pleuritis, splenomegaly, and anemia) are at increased risk for coronary artery disease (CAD) and HF. 4 , 5 , 7 , 18 Bhatia et al. in their small study of asymptomatic RA patients have shown that there was a 3.5% incidence of HF with ejection fraction <50% (8/226 RA patients), when screened with an echocardiogram. 18 Currently, no professional organizations' RA management guidelines address screening for HF for asymptomatic patients with RA. 19 Published RA studies suggested addressing modifiable risk factors for patients with RA and CAD, such as hypertension, diabetes, high cholesterol, and smoking. 5 , 14 , 20 , 21 Our aggregate analysis of three large reports found that there was a two‐fold increase in the incidence of HF in the RA population compared with the non‐RA matched control population (Figure 2 ). There was an increase in the incidence of HF in RA patients with older age (Figure 4 ), a non‐modifiable risk factor. We further found the incidence of HF to be approximately 1.7‐fold higher in patients with RA and diabetes, both type 1 and type 2, compared with a matched control population (OR 2.092, 95% CI 1.46–3.00, P < 0.001). Also, the incidence of HF was noted to be approximately 1.7‐fold higher in patients with RA and HTN compared with a matched control population (OR 2.092, 95% CI 1.46–3.00, P < 0.001). Not enough data were available to perform subgroup analyses for incidence of HF in patients with RA/tobacco abuse and RA/hyperlipidaemia.
Blum and Adawi 8 in their review had reported that women with RA are at an increased risk of cardiovascular events and HF compared with matched controls. In our aggregate analysis, women with RA were found to have a three‐fold increased risk of HF (Figure 3 ). Traditionally, the Pooled Cohort risk equation developed by the American College of Cardiology/American Heart Association is used to estimate the atherosclerotic disease risk utilizing traditional risk factors, such as gender, age, race, cholesterol levels, systolic blood pressure, diabetes, and smoking status, to determine the risk of CVD. 22 As it is recognized now that RA patients have a higher risk of CAD and myocardial infarction, in addition to the traditional risk factors, it is recommended that the traditional 10 year risk index for estimating myocardial infarction should be multiplied by 1.5 when estimating risk in RA patients. This implies that additional ischemic and/or non‐ischemic mechanisms play a role and may make the case for registries or longitudinal studies to incorporate disease activity measures as has been performed in a single small German prospective study. 14
Finally, HF is a major growing public health problem due to rising healthcare costs and hospitalizations. Specific patient subgroups with HF that have increased inflammation at baseline, such as patients with RA, must be identified early and monitored much more vigilantly for screening and prevention to minimize the morbidity and mortality from HF. Even though hypertension and ischemic heart disease are well‐established risk factors for HF, there must be an emphasis on non‐traditional risk factors for HF, such as RA. Currently, important risk factors such as RA are understudied and overlooked, but they nonetheless very significantly increase morbidity and mortality. The increased risk in morbidity and mortality for RA patients with HF, which has been proven in multiple studies to exist even in the absence of any other risk factors, progresses extremely rapidly after initial RA onset. It is associated with high inflammatory and disease activity. It is essential for these patients to have routine follow‐up with cardiologists to help mitigate their risk of developing HF and managing HF once it is diagnosed. Very few studies in the current literature have examined the relationship of inflammatory and rheumatological diseases on HF, and almost none have reviewed multiple studies in a systematic review.
Study limitations
Several limitations of our study warrant consideration. Although the quality assessment methods and bias assessments methods used in our studies were of high quality and of minimal bias, the Q and I 2 determined a high degree of heterogeneity between the studies. We therefore thought that it is most prudent to present these data as a forest plot and that an meta‐analysis would not be appropriate and potentially misleading. Then, as with any systematic review of observational studies, variations in the inclusion criteria and endpoint definitions, measurements, capture, and confirmation are all potential sources of heterogeneity among studies that analyse registries. We could not access patient‐level data to allow adjustment for other covariates that might influence the incidence of HF, including medications, laboratory data, and other imaging parameters. Although reproducibility analyses have been provided, prospective multicentre studies are needed to confirm the relationship between HF and RA. Additionally, an overestimation of the pooled effect sizes is possible, as the small number of studies limits the assessment of publication bias. Furthermore, only one of our studies, Mantel et al., differentiated between HF with reduced or preserved ejection fraction in the association between HF and RA. Our analyses also consisted of only European RA patients; as a result, other factors could impact risk of HF, such as environmental, geographical, dietary, cultural, genetic, race/ethnicity, and socio‐economic status. Because of the nature of RA, this patient population often has more frequent healthcare surveillance, which inadvertently could lead to having more diagnostic tests and subsequent diagnosis. With this realization, surveillance bias would be an unavoidable entity because patients with RA are more likely to be diagnosed with HF and reported earlier than non‐RA patients. Finally, registries may have limited availability of treatment data and underreporting of outcomes if a patient leaves the registry or is not adequately followed up.
Conclusions
Our aggregate analysis of three large studies demonstrates an increased incidence of HF in patients with RA, especially in women. This suggests that current RA management guidelines not only address the CVD risk factors that may lead to ischaemic HF but also consider incorporating screening for either symptomatic or asymptomatic HF in RA patients. Additional investigation into the factors that lead to an increase of non‐ischemic HF in RA patients appears to be warranted.
Conflict of interest
None of the authors report any financial or other conflicts of interests. We state that our study complies with the Declaration of Helsinki; given that this manuscript is a systematic review, we did not require approval from a local ethics committee.
Author contributions
Y.K. is the primary author of the manuscript. He is responsible for the conception and design, analysis and interpretation of data, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. N.D., A.S., K.D., K.B., A.K., A.R., and A.L. participated in drafting of the manuscript and revising it critically for important intellectual content and helped with the final approval of the manuscript submitted.
Supporting information
Appendix S1. Newcastle‐Ottawa scale and quality data.
Appendix S2. Q and I2 for Meta‐Regression.
Appendix S3. Funnel plot analysis to assess for potential publication bias ‐ CHF.
Appendix S4. Funnel plot analysis to assess for potential publication bias ‐ Women.
Appendix S5. Funnel plot analysis to assess for potential publication bias ‐ Hypertension.
Appendix S6. Funnel plot analysis to assess for potential publication bias ‐ Diabetes Mellitus.
Khalid, Y. , Dasu, N. , Shah, A. , Brown, K. , Kaell, A. , Levine, A. , Dasu, K. , and Raminfard, A. (2020) Incidence of congestive heart failure in rheumatoid arthritis: a review of literature and meta‐regression analysis. ESC Heart Failure, 7: 3745–3753. 10.1002/ehf2.12947.
References
- 1. Harnden K, Pease C, Jackson A. Rheumatoid arthritis. BMJ 2016; 23: 352 https://www.bmj.com/content/352/bmj.i387 (16 April 2020). [DOI] [PubMed] [Google Scholar]
- 2. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018; 320: 1360. [DOI] [PubMed] [Google Scholar]
- 3. McInnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. N Engl J Med 2011; 365: 2205–2219. [DOI] [PubMed] [Google Scholar]
- 4. Gkaliagkousi E, Gavriilaki E, Doumas M, Petidis K, Aslanidis S, Stella D. Cardiovascular risk in rheumatoid arthritis. J Clin Rheumatol 2012; 18: 9. [DOI] [PubMed] [Google Scholar]
- 5. Nicola PJ, Maradit‐Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, Gabriel SE. The risk of congestive heart failure in rheumatoid arthritis: a population‐based study over 46 years. Arthritis Rheum 2005; 52: 412–420. [DOI] [PubMed] [Google Scholar]
- 6. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JAE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003; 107: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 7. Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel PLCM, Gabriel SE, Matteson EL, Kvien TK, Douglas K, Sandoo A, Arts E, Wållberg‐Jonsson S, Innala L, Karpouzas G, Dessein PH, Tsang L, el‐Gabalawy H, Hitchon C, Ramos VP, Yáñez IC, Sfikakis PP, Zampeli E, Gonzalez‐Gay MA, Corrales A, Laar MV, Vonkeman HE, Meek I, Semb AG, A Trans‐Atlantic Cardiovascular Consortium for Rheumatoid Arthritis (ATACC‐RA) . Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018; 77: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blum A, Adawi M. Rheumatoid arthritis (RA) and cardiovascular disease. Autoimmun Rev 2019; 18: 679–690. [DOI] [PubMed] [Google Scholar]
- 9. Tournadre A, Mathieu S, Soubrier M. Managing cardiovascular risk in patients with inflammatory arthritis: practical considerations. Ther Adv Musculoskelet Dis 2016; 8: 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Generali E, Carrara G, Kallikourdis M, Condorelli G, Bortoluzzi A, Scirè CA, Selmi C. Risk of hospitalization for heart failure in rheumatoid arthritis patients treated with etanercept and abatacept. Rheumatol Int 2019; 39: 239–243. [DOI] [PubMed] [Google Scholar]
- 11. Lee TH, Song GG, Choi SJ, Seok H, Jung JH. Relationship of rheumatoid arthritis and coronary artery disease in the Korean population: a nationwide cross‐sectional study. Adv Rheumatol 2019; 59: 40. [DOI] [PubMed] [Google Scholar]
- 12. Gabriel SE. Heart disease and rheumatoid arthritis: understanding the risks. Ann Rheum Dis. 2010; 69: i61–i64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology 2006; 45: iv4–iv7. [DOI] [PubMed] [Google Scholar]
- 14. Schau T, Gottwald M, Arbach O, Seifert M, Schöpp M, Neuß M, Butter C, Zänker M. Increased prevalence of diastolic heart failure in patients with rheumatoid arthritis correlates with active disease, but not with treatment type. J Rheumatol 2015; 42: 2029–2037. [DOI] [PubMed] [Google Scholar]
- 15. Khalid U, Egeberg A, Ahlehoff O, Lane D, Gislason GH, Lip GYH, Hansen PR. Incident heart failure in patients with rheumatoid arthritis: a nationwide cohort study. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2018; 7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5850154/ (1 March 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pironti G, Bersellini‐Farinotti A, Agalave NM, Sandor K, Fernandez‐Zafra T, Jurczak A, Lund LH, Svensson CI, Andersson DC. Cardiomyopathy, oxidative stress and impaired contractility in a rheumatoid arthritis mouse model. Heart 2018; 104: 2026–2034. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 19. Bhatia GS, Sosin MD, Patel JV, Grindulis KA, Khattak FH, Hughes EA, Lip GYH, Davis RC. Left ventricular systolic dysfunction in rheumatoid disease: an unrecognized burden? J Am Coll Cardiol 2006; 47: 1169–1174. [DOI] [PubMed] [Google Scholar]
- 20. Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med 2017; 27: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Løgstrup BB, Ellingsen T, Pedersen AB, Kjærsgaard A, Bøtker H‐E, Maeng M. Development of heart failure in patients with rheumatoid arthritis: a Danish population‐based study. Eur J Clin Invest 2018; 48: e12915. [DOI] [PubMed] [Google Scholar]
- 22. Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol 2017; 69: 1275–1285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Newcastle‐Ottawa scale and quality data.
Appendix S2. Q and I2 for Meta‐Regression.
Appendix S3. Funnel plot analysis to assess for potential publication bias ‐ CHF.
Appendix S4. Funnel plot analysis to assess for potential publication bias ‐ Women.
Appendix S5. Funnel plot analysis to assess for potential publication bias ‐ Hypertension.
Appendix S6. Funnel plot analysis to assess for potential publication bias ‐ Diabetes Mellitus.


