Abstract
Aims
Functional mitral regurgitation (MR) (FMR) is common in heart failure with reduced ejection fraction and worsens morbidity and mortality, even when mild. The CARILLON® mitral contour system (Cardiac Dimensions, Kirkland, WA, USA), a mitral annuloplasty device delivered percutaneously to the coronary sinus, is designed to reduce the mitral annular dimension by virtue of the close anatomic relationship between the coronary sinus and the posterior mitral annulus. We performed a comprehensive individual patient data meta‐analysis of all studies that used CARILLON® device vs. control that have measured mitral regurgitation severity, left ventricular (LV) remodelling, functional status, and heart failure‐related outcomes in heart failure with reduced ejection fraction patients.
Methods and results
The Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE were searched in July 2020. Primary outcomes of interest were measures of MR severity, LV remodelling, New York Heart Association functional class and heart failure‐related outcomes [mortality and heart failure hospitalization (HFH) during follow up]. All data were received as individual patient and individual time point data‐points. Mean differences and 95% confidence intervals (CIs) were calculated for continuous data using a fixed‐effects model. Three studies (REDUCE FMR, TITAN and TITAN II) enrolling 209 participants were identified and included. Pooled analysis showed that, compared with control, CARILLON® device significantly improved both MR volume (mean difference MD ‐9.20, 95% C.I. −16.11 to −2.29 mL, P = 0.009) and MR grade (MD ‐1.12, 95% CI −1.36 to −0.88, P < 0.00001) and this was associated with a significant reduction in LA volume, MD −7.54 mL, 95% CI −14.90 to − 0.18, P = 0.04. Significant LV reverse remodelling was also seen in terms of EDV (MD −16.53, 95% CI −28.61 to −44.4 mL, P = 0.007), and a trend in ESV (MD −8.68, 95% CI −18.69 to −1.34 mL, P = 0.09) but no significant effect on LVEF (MD 0.88, 95% CI −1.52% to 2.38%, P = 0.47), due presumably to the greater residual MR in the control patients falsely elevating the LVEF. In addition, the CARILLON® device significantly improved New York Heart Association functional Class (MD −0.22, 95% CI −0.24 to −0.16, P < 0.00001), associated with a lower rate of HFH compared with controls (45.3% vs. 64%, respectively, P = 0.04). As a sensitivity analysis we also restricted the analyses to those patients with Class 3+/4+ MR at baseline. In this cohort, the echocardiographic results were similar, and the reduction in HFH rates was even more marked (43.9% vs. 82.9%, respectively, P = 0.04).
Conclusions
This comprehensive meta‐analysis of individual patient data has shown that CARILLON® device provides statistically significant and clinically meaningful benefits on MR severity, LA and LV volumes, and remodelling and rates of subsequent heart failure hospitalization
Keywords: Carillon device; Heart Failure; Meta‐analysis; Mitral Regurgitation
Introduction
Primary mitral regurgitation (MR) is a valve disorder that can cause heart failure, and its management in severe cases can involve valve surgery or percutaneous repair and even replacement. Functional (or secondary) MR (FMR) is a separate condition where valve leakage of a structurally normal valve occurs secondary to ventricular enlargement. FMR is common feature in patients with heart failure with reduced ejection fraction (HFrEF) 1 , 2 Patients with FMR and HFrEF have worse morbidity and mortality than patients with HFrEF without FMR, even when the MR is mild. 3 Medical therapy still represents the standard of care for HF patients with FMR, but there has been a growing interest in non‐surgical percutaneous devices for the management of this condition. Previous studies have shown that patients with FMR receiving optimal medical therapy alone have a poor prognosis with mortality rates as high as 50% at 5 years. 4 Despite satisfactory results in primary MR, surgical approaches used for the correction of FMR have not been successful in terms of mortality and HF re‐hospitalizations and are often considered contra‐indicated due to the increased surgical risk conferred by features such as impaired ejection fraction, advanced age, frailty, high operative risk, and other comorbidities. 5 , 6 Percutaneous mitral valve repair using the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) has been studied in high‐risk FMR patients. When deployed it creates a double orifice by bringing together the free edges of the anterior and posterior leaflets. 7 In the randomized Endovascular Valve Edge‐to‐Edge Repair Study (EVEREST) II trial, 8 trans‐catheter mitral‐leaflet approximation with the MitraClip® device was found to be less effective at reducing the severity of MR compared with surgery but with similar clinical outcomes, and fewer adverse events control compared with surgical mitral‐valve repair in patients with primary MR. Subsequently, two trials were performed comparing trans‐catheter mitral‐leaflet approximation with the MitraClip® device to control in patients with heart failure and secondary (functional) MR. These two studies presented conflicting results, with the Mitra‐FR trial 9 reporting no advantage of MitraClip® and the COAPT trial 10 showing significant clinical as well as mortality benefits.
More recently, the CARILLON® mitral contour system device (Kirkland, WA, USA) was developed to treat FMR in HF patients. The CARILLON® mitral contour system consists of a mitral annuloplasty device delivered percutaneously to the coronary sinus and is designed to reduce the mitral annular dimension by virtue of the close anatomic relationship between the coronary sinus and the posterior mitral annulus. In 2009, the CARILLON Mitral Annuloplasty Device European Union Study (AMADEUS), 11 a single‐arm study including 30 patients, showed significant improvements in functional capacity and quality of life with reduced MR over a 6 month follow‐up period. This was followed by three subsequent clinical trials. 12 , 13 , 14 We aimed to conduct a comprehensive individual patient data meta‐analysis of all studies that have used the CARILLON® mitral contour system® vs. control.
Methods
Search strategy
The Cochrane Database, MEDLINE, and EMBASE were searched in July 2020 to identify eligible human studies using the keyword: (‘CARILLON® mitral contour system®’). No language restrictions were applied. Reference lists of retrieved records were screened for further relevant studies. All review articles with a subject of ‘CARILLON® mitral contour system®’ and their reference lists were also searched. Clinical trials registers (http://www.clinicaltrials.gov, http://www.controlled‐trials.com) were searched for ongoing studies. Two authors selected studies independently (F.G. and A.J.S.C.), and disagreements were resolved by consensus. The results of study selection are presented in a flow diagram as depicted by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (Figure 1 ). All data (as individual patient data and individual time point data‐points) were received from the trial leaders or the sole sponsor of CARILLON® mitral contour system®.
Figure 1.
Study selection presented in a flow diagram as depicted by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement.
Study selection
A total of 44 potentially relevant records were screened, and three studies were identified.
The TITAN study 12 was a prospective, non‐randomized, non‐blinded, multi‐centre trial. Inclusion criteria were dilated ischaemic or non‐ischaemic cardiomyopathy; at least moderate (2+) FMR; LV ejection fraction (LVEF) <40%; New York Heart Association (NYHA) Class II–IV; 6 min walk distance (6MWD) 150–450 m; and stable HF medication regimen for at least 3 months. Key exclusion criteria were: hospitalization in the past 30 days for intravenous inotrope infusion; severe tricuspid regurgitation; serum creatinine >2.2 mg/dL; significant organic mitral valve pathology; and the presence of a pacing lead already in the coronary sinus. Patients in whom the device was implanted but which were then acutely recaptured for clinical indications (i.e. transient coronary compromise or <1 grade FMR reduction) underwent follow‐up assessments identical to the implanted cohort (baseline, 1, 6, and 12 months) and served as a non‐randomized comparison group. The implanted cohort underwent further safety and functional assessments up to 24 months. Quantitative measures of FMR, left ventricular (LV) dimensions and NYHA class were assessed in both groups up to 12 months. Safety and key functional data were assessed in the implanted cohort up to 24 months. Fifty‐three patients (36 in the permanent implant population and 17 in the device recaptured population) from seven European centres were included in the trial (Table 1). In the TITAN trial, the implanted cohort (n = 36) demonstrated significant reductions in FMR as represented by regurgitant volume [from 34.5 ± 11.5 mL to 17.4 ± 12.4 mL at 12 months (P < 0.001)] together with a significant reduction in LV diastolic volume [from 208.5 ± 62.0 mL to 178.9 ± 48.0 mL at 12 months (P = 0.015)] and systolic volume [from 151.8 ± 57.1 mL to 120.7 ± 43.2 mL at 12 months (P = 0.015)], whereas a progressive LV dilation was observed among the comparator group. Interestingly, the 6MWD markedly improved for the implanted patients (102.5 ± 164 m at 12 months (P = 0.014) and 131.9 ± 80 m at 24 months (P < 0.001).
Table 1.
Characteristics of the trials included
| REDUCE FMR trial | TITAN trial | TITAN II trial | |||
| Treatment (n = 73) | Control (n = 47) |
Treatment (n = 36) |
Control (n = 17) |
Treatment (n = 36) |
|
| Gender (Male) (%) | 72.4 | 72.7 | 75 | 82 | 67 |
| Mean age (years) | 70.1 ± 9.7 | 69.1 ± 8.9 | 62.4 ± 12.7 | 62.6 ± 13.1 | 70.6 ± 8.5 |
| Ischemic aetiology (n, %) | 59 (67.8) | 21 (63.6) | 24 (66.7) | 10 (58.8) | 21 (58) |
| Diabetes (n, %) | 24 (27.6) | 12 (36.4) | 6 (16.7) | 5 (29.4) | 11 (31) |
| NYHA functional class (%) | |||||
| II | 44.8 | 48.5 | — | 6 | 5.6 |
| III | 52.9 | 51.5 | 63.8 | 94 | 88.8 |
| IV | 2.3 | — | 5.5 | — | 5.6 |
| Atrial fibrillation (n, %) | 57 (58.6) | 20 (60.6) | 12 (33.3) | 2 (11.8) | 17 (47) |
| Device (ICD or PPM) (n, %) | 43 (49.4) | 12 (36.4) | 6 (16.7) | 2 (11.8) | — |
| 6MWT distance (m) | 306.4 ± 90.5 | 292.6 ± 91.5 | 302 ± 73.6 | 338 ± 83.4 | 294.1 ± 83 |
| LVEF (%) | 34 ± 9 | 37 ± 9 | 29 ± 7 | 27 ± 8 | 34 ± 10 |
| LVEDV (mL) | 187 ± 65.6 | 188.6 ± 75.7 | 208.5 ± 62.0 | 237.4 ± 96.7 | 174.4 ± 51.2 |
| LVESV (mL) | 127.4 ± 56.1 | 122.0 ± 59.8 | 151.7 ± 57.1 | 177.7 ± 91.9 | 119.8 ± 39.6 |
| Mitral regurgitant volume (mL/beat) | 40.4 ± 23.9 | 38.1 ± 24.0 | 34.5 ± 11.5 | 39.9 ± 13.2 | 34.4 ± 13.5 |
| Mitral regurgitant grade (%) | |||||
| 1+ | 28.7 | 32.3 | |||
| 2+ | 39.1 | 25.8 | 19.4 | 11.8 | 28 |
| 3+ | 26.4 | 35.5 | 55.6 | 58.8 | 61 |
| 4+ | 5.7 | 6.5 | 25.0 | 29.4 | 11 |
6MWT, 6 min walking test; ICD, implantable cardioverter defibrillator; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; PPM, pace‐maker.
The TITAN II study 13 was a prospective, single‐arm, multi‐centre safety study using a modified version of the Carillon device and undertaken at five centres in Germany, Poland, and France. The primary endpoint was the rate of major adverse events at 30 days, defined as: death, myocardial infarction, cardiac perforation requiring intervention, device embolization, or the occurrence of surgery or percutaneous intervention related to the device. Secondary safety endpoints included mortality and major adverse event rates up to 12 months. Secondary efficacy endpoints included evaluation of mitral valve competence based on quantitative echocardiographic parameters and MR grade changes over 12 months. Data from 36 patients undergoing the Carillon procedure were analysed. A significant reduction in FMR and improvements in functional class (83.3% improved at 6 months and 77.3% at 12 months follow‐up compared with baseline) and 6MWTD (from 294.1 ± 83 m at baseline to 381 ± 130 m at 12 months follow up) similar to previous studies were documented. In addition, the issues observed in previous studies (i.e. device fractures in the high strain region of the proximal anchor) were not reported.
Individual patient data from the TITAN and TITAN II studies were pooled together. As TITAN II do not have a control group, we added this as a treatment only arm, compared for exploratory purposes against the control (recaptured devices) group of TITAN I, given the similarity of patients recruited into TITAN and TITAN II. All patients were on optimal medical therapy.
The REDUCE FMR trial 14 was a double‐blind, multi‐centre, randomized, proof‐of‐concept, sham‐controlled trial of the Carillon® mitral contour system. The pre‐specified primary endpoint was a comparison of changes in mitral regurgitant volumes at 1 year compared with baseline, between the treatment group and the sham control group, as assessed by the independent, blinded echocardiographic core laboratory using quantitative echocardiography. Secondary safety endpoints were major adverse events, defined as death, myocardial infarction, device embolization, vessel erosion, cardiac perforation, need for cardiac surgery or percutaneous coronary intervention associated with device failure, and heart failure hospitalizations. Eligibility screening included an age of at least 18 years, symptoms of NYHA functional Class II, III, or IV, an LV ejection fraction (LVEF) of <50%, an LV end‐diastolic diameter more than 55 mm, and an FMR grade of 2+, 3+, or 4+, despite the use of stable (≥3 months) guideline‐directed medical therapy. In addition, patients had to have the ability to complete a 6MWD of between 150 and 450 m to confirm exercise limitation, and establishing a capacity for serial 6 min walk testing. Key exclusion criteria included percutaneous coronary intervention in the previous 30 days, prior mitral valve surgery, significant organic mitral valve pathology, severe mitral annular calcification, and existing or indication for cardiac resynchronization therapy. In REDUCE FMR trial, after screening, 120 patients were randomized, 87 to treatment and 33 to sham control (Table 1). In the REDUCE FMR trial, the investigators reported a statistically significant reduction in mitral regurgitant volume in the treatment group compared with the control group (a decrease of 7.1 mL/beat vs. an increase of 3.3 mL/beat, respectively; P = 0.049). Additionally, there was a significant reduction in LV volumes in patients receiving the device compared with controls [LV end‐diastolic volume (LVEDV) decrease of 10.4 mL (95% confidence interval [CI] −18.5 to −2.4) vs. an increase of 6.5 mL (95% CI −5.1 to 18.2); P = 0.03, and LV end‐systolic volume (LVESV) decrease of 6.2 mL (95% CI −12.8 to 0.4) vs. an increase of 6.1 mL (95% CI −1.42 to 13.6); P = 0.04].
Additionally, two ongoing trials were identified, but as the results of these are not available, they have not been considered further is this meta‐analysis.
The AFIRE trial—a prospective, multi‐centre clinical trial assessing the effectiveness of the CARILLON® Mitral Contour System in treating patients with moderate‐to‐severe atrial functional MR (aFMR) that is not yet recruiting; and
The CARILLON trial—assessment of the Carillon® Mitral Contour System® in treating FMR associated with heart failure, a prospective, multi‐centre, randomized, double‐blind trial to assess the safety and efficacy of the CARILLON® Mitral Contour System® in treating subjects with FMR associated with heart failure, compared with a randomized control group, which is medically managed according to heart failure guidelines (final data collection date for primary outcome measure expected on 1 October 2022).
Outcome measures
Primary outcomes of interest were the echocardiographic parameters mitral regurgitant volume and MR grade to assess the severity of MR, and LV end‐diastolic (EDV) and end‐systolic volumes (ESV) to assess LV remodelling. LVEF was also estimated, even though it is misleading in the presence of MR, as it gives a false measure of effective LV output, as blood ejected back into the left atrium is considered as useful as blood ejected into the ascending aorta which of course it is not. NYHA class and the rates of mortality and heart failure hospitalizations (HFH) during follow‐up were also assessed. NYHA class was also assessed as it provides a simple way of classifying the severity of HF on patient functional capacity. It classifies patients in one of four categories based on their limitation during physical activity. 15
Statistical analysis
Revman 5.3 (Nordic Cochrane Centre, Copenhagen, Denmarrk) was used to conduct meta‐analyses for outcome measures. Data used were continuous and were reported as mean and standard deviation. Results were presented as weighted mean differences for continuous data, along with the 95% CIs. Comparisons between groups for continuous variables were made using t tests. A Mantel–Haenzel random‐effects model was adopted taking into account potential heterogeneity across studies. The I 2 statistic was used to explore statistical heterogeneity. An Egger plot was produced to identify sources of publication bias. P values ≤0.05 for two‐sided tests were considered to be statistically significant.
Results
Description of studies
The three studies included in this review included an aggregate of 209 subjects (Table 1). The baseline characteristics of all patients were similar: the most common aetiology of HF was ischaemic (about 2/3 of study population), and the majority of the participants were of males with NYHA Classifications III. All studies were multi‐centre studies. Withdrawals and associated reasons were described in each trial and there was no evidence of selective outcome reporting.
Pooled analysis showed that, compared with control, the CARILLON® mitral contour system significantly improved mitral regurgitant volume (MD −9.20, 95% C.I. −16.11 to −2.29 mL, P = 0.009), MR grade (MD −1.12, 95% CI −1.36 to −0.88, P < 0.00001), LA volume (MD −7.54 mL, 95% CI −14.90 to − 0.18, P = 0.04) and NYHA functional class (MD −0.22, 95% CI −0.24 to −0.16, P < 0.00001), (Figure 2 C–E ). In addition, EDV [mean difference (MD) −16.53, 95% CI −28.61 to −44.4 mL, P = 0.007] (Figure 2 A ) was reduced, showing beneficial LV remodelling with a trend to a reduction in ESV (MD −8.68, 95% CI −18.69 to −1.34 mL, P = 0.09) (Figure 2 B ). This smaller effect on ESV compared with EDV probably reflects the fact that treated patients had smaller degrees of MR, and therefore, the LV was no longer emptying as much into the left atrium. This was consistent with the fact that there was no significant effect on LVEF (MD 0.88, 95% CI −1.52% to 2.38%, P = 0.47). In addition implanted HF patients showed lower rates of HF‐related hospitalization compared with controls (45.3% vs. 64%, respectively, P = 0.04) (Figure 3 ), but there was no difference in mortality. The prevalence of NYHA class at baseline in each of the trials is presented in Table 1. Post‐procedure NYHA class improved by at least 1 point score in 58/105 patients (55.2%) whereas deterioration was observed in 8/105 patients (7.6%). In patients with baseline NYHA III/IV nearly 70% improved by at least one class (69.9%; 51/73) change text in figure below from red to black
Figure 2.
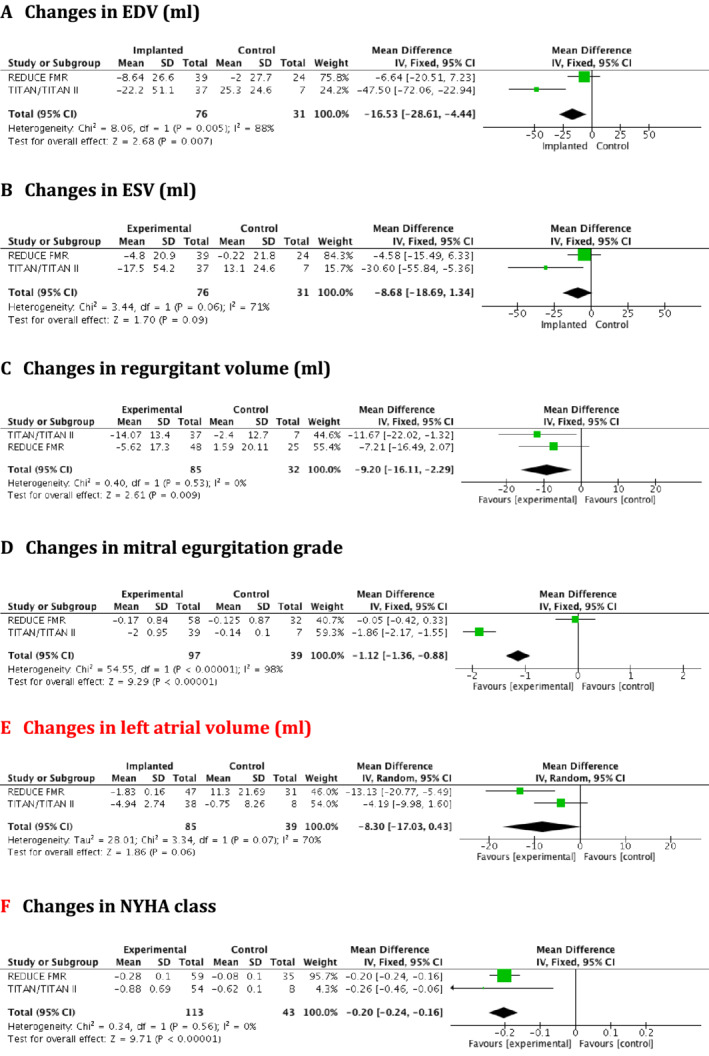
(A–F) Forest plots for changes in left ventricular remodelling, mitral regurgitation severity, left atrial volume, and New York Heart Association class at 12 months follow up. CI, confidence interval.
Figure 3.
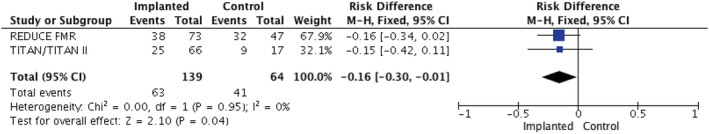
Forest plot for total heart failure related hospitalizations. CI, confidence interval.
Sensitivity analysis
Because of an imbalance in the severity of MR in patients randomized to Carillon vs. control, seen only in REDUCE FMR, we excluded all patients with Class 1 or 2 (mild) MR who were over‐represented in the intervention group in REDUCE FMR (see Table 1) and repeated the analyses with subjects only with moderate to severe MR (Class 3+/4+) at baseline, a group likely to be the target population for this device.
Table 2 shows data from the 66 implanted and the 35 non‐implanted control patients with baseline 3+/4 + MR. Compared with control, there was a significant early improvement in the severity of the MR at 1 month (−0.84 compared with −0.41, P = 0.024) which grew with time, to a 12 month difference of −1.05 vs. −0.20, P = 0.002. This was also reflected in a reduced regurgitant volume both at 6 months (−13.86 mL vs. +0.47 mL, P < 0.001) and at 12 months (−13.86 mL vs. +0.47 mL, P = 0.002). LVEF showed significant early improvement at 1 month (from 30.29% to 32.82%, Δ + 3.41, P = 0.029), an effect that was no longer significant at 6 or 12 months, presumably because of the greater regurgitant volumes seen in the control patients at these time‐points generating falsely high LVEF values. Compared with control, there was also a significant improvement in both LVEDV (−23.50 mL vs. +12.41 mL, P = 0.002) and LVESV (−21.25 mL vs. +8.89 mL, P = 0.004) at 12 months. These favourable effects on MR severity and LV remodelling may help explain the lower rate of HF‐related hospitalizations seen in treated patients compared with controls (43.9% vs. 82.9%, P = 0.04).
Table 2.
Changes in echo‐Doppler ventricular indices, and regurgitant volume in MR class 3+/4 + patients
| Baseline | 1 month | Δ* | P | 6 month | Δ* | P | 12 month | Δ* | P | |
| LVEDV (mL) | ||||||||||
| Implant (n = 66) | 209.62 | 207.23 | −1.95 | 0.908 | 199.18 | −10.11 | 0.013 | 193.77 | −23.50 | 0.002 |
| Non‐implant (n = 35) | 212.24 | 208.12 | −1.32 | 227.54 | 6.63 | 211.20 | 12.41 | |||
| LVESV (mL) | ||||||||||
| Implant (n = 66) | 147.76 | 142.10 | −6.41 | 0.503 | 138.52 | −9.96 | 0.173 | 132.29 | −21.25 | 0.004 |
| Non‐implant (n = 35) | 147.18 | 143.22 | −2.97 | 148.69 | −1.11 | 138.38 | 8.89 | |||
| LVEF (%) | ||||||||||
| Implant (n = 66) | 30.29 | 32.82 | 3.41 | 0.029 | 32.45 | 2.01 | 0.930 | 32.60 | 2.59 | 0.177 |
| Non‐implant (n = 35) | 32.73 | 34.26 | 0.67 | 36.68 | 2.15 | 35.73 | −0.34 | |||
| Mitral regurgitation grade | ||||||||||
| Implant (n = 66) | 3.30 | 2.55 | −0.84 | 0.024 | 2.37 | −0.91 | 0.001 | 2.26 | −1.05 | 0.002 |
| Non‐implant (n = 35) | 3.23 | 2.90 | −0.41 | 3.00 | −0.17 | 2.95 | −0.20 | |||
| RV (mL/beat) | ||||||||||
| Implant (n = 66) | 45.41 | 36.65 | −7.98 | 0.731 | 29.70 | −13.86 | 0.001 | 30.68 | −13.85 | 0.009 |
| Non‐implant (n = 35) | 50.91 | 48.20 | −6.77 | 52.80 | 0.47 | 50.89 | −1.27 | |||
Δ* represents paired changes from baseline; LVEDV, left ventricular end‐diastolic volume; LFEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume, RV, regurgitant volume.
Discussion
FMR, defined as mitral insufficiency secondary to inadequate leaflet movement due to either LV wall motion abnormalities or to ventricular and mitral annulus dilatation, is common in patients with advanced systolic heart failure 1 , 2 contributing to morbidity and mortality. 3
This comprehensive meta‐analysis of individual patient data has shown that CARILLON® mitral contour system device provides statistically significant and clinically meaningful benefits in LV volumes, NYHA functional status, and indexes of mitral valve performance (MR grade and regurgitation volume) in HF patients with FMR. In addition, more compromised (3+ and 4+ MR grade) HF patients undergoing CARILLON® mitral contour system device implant showed a significant early improvement in LA and LV remodelling, MR volume and MR grade and in NYHA class, that are all sustained long‐term (see also Figure 4 ). There was also a significant effect on the rate of hospitalization for heart failure, seen in both the total cohort and a sensitivity analysis restricted to those with more severe (Class 3+/4+) MR. In these trials, treatment changes were at the discretion of the treating physician, so it was possible that treatment differed between the device and control groups. We cannot entirely exclude a small effect, but neither baseline nor follow‐up medical therapies significantly differed between the device and control groups overall.
Figure 4.
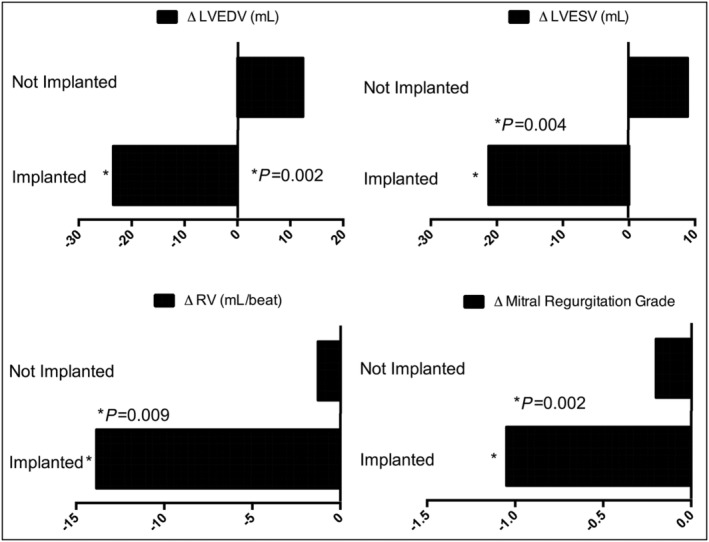
Changes in echo‐Doppler ventricular indices, MR grade and regurgitant volume in MR class 3+/4+ patients at 12 month follow‐up.LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MR, mitral regurgitation; RV, regurgitant volume.
The substantial reductions in LVEDVs seen with the CARILLON® device in the meta‐analysis (−23.5 mL compared with baseline in those starting with 3+ or 4+ MR) stands in contrast to the lack of a reduction in LVEDV in similarly severe FMR patients in either trial of MitraClip®, Mitra‐FR (−2 mL median change from baseline) or COAPT (−1.1 mL mean change from baseline), perhaps because the CARILLON® device actually reduces mitral annular size as opposed to merely clipping the valve leaflets together. The two largest trials of MitraClip® have recruited patients much later in the natural history of FMR when the remodelling and MR has progressed further. The CARILLON® device is designed to be used in a wider range of FMR patients and because it has no direct effect on the valve leaflets it can be deployed earlier (when reversal of LV remodelling is still possible) and actually reduce end‐diastolic volumes and still leave the patient eligible for later leaflet clipping if needed.
Current European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines recommend for mitral valve surgery (mitral annuloplasty or chordal‐sparing MV replacement) for severe FMR with LVEF >30% in patients already planned for CABG, but only weak ‘may be considered’ advice if surgery is not otherwise planned, because the evidence for surgical intervention for FMR is so limited. 16 The MitraClip® device is the current standard for the treatment of severe FMR, but patient selection is crucial. Patients with symptomatic HFrEF and moderate or severe SMR should be assessed by a multi‐disciplinary heart team, to first optimize guideline‐directed medical therapy and then consider the merits of mitral intervention either by device or surgery. Patients should be assessed in euvolaemia and with as near their normal heart rate as possible. It has been estimated that the COAPT trial entry criteria should be used to consider patients for transcatheter mitral leaflet clipping, but that these may be satisfied in fewer than 5% of otherwise eligible FMR patients, indicating the need for other treatment approaches. The CARILLON® device, being easier to deploy, requiring as few as five cases to become proficient, compared with more than 50 for the MitraClip® device, and being non‐destructive to the mitral valve leaflets, may be an excellent option in earlier FMR, where the reduction in severity of FMR and left atrial and ventricular volumes documented in this meta‐analysis may prove to be an excellent option to delay the need for MitraClip®. It is also a much easier technique for non‐specialist structural heart interventional cardiologists to master.
The treatment of FMR is a rapidly changing field of medical practice. Transcatheter mitral valve interventions are also the subject of considerable innovation. Other percutaneous techniques now approved for commercial use in Europe include the CARILLON® device (Carillon Mitral Contour System, Cardiac Dimensions, Kirkland), the direct annuloplasty using the Cardioband Mitral System (Edwards Lifesciences, Irvine, CA, USA), and edge‐to‐edge repair using the PASCAL Mitral Valve Repair System (Edwards Lifesciences). Adequately powered clinical trials will be essential to inform the optimal use of these devices. Recent registries have shown the safety of percutaneous mitral valve interventions are improving and not showing worse outcomes in reduced LVEF patients, 17 but that further education in how best to apply recent advances may be needed. 18 An update advice statement has recently been published by the Heart Failure Association of the ESC. 19 New evidence continues to accumulate showing the value of percutaneous mitral valve procedures. 20 Other recent reviews and advice documents have covered this field. 21 , 22
Study limitations and conclusions
In conclusion, this comprehensive meta‐analysis of individual patient data has shown that CARILLON® device provides statistically significant and clinically meaningful benefits in LV volumes, NYHA functional class and indexes of mitral valve performance, along with a reduction in the rate of subsequent HFH. Significant improvements were seen in LVEDV but not in LVEF. This is possibly due to the removal of a low resistance outlet for LV emptying which had prior to correction lead to a falsely reassuring pre‐treatment LVEF. The number of patients and trials was modest, but the clear evidence of LV remodelling suggests this may translate into improved outcomes.23 Future studies are awaited in order to define the impact on this intervention on major clinical outcomes and also to determine whether earlier intervention with the CARILLON® device could delay the need for the more destructive (to the mitral valve leaflets) MitraClip® device.
Conflict of interest
Professor Coats declares having received honoraria and/or lecture fees from Astra Zeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia. Francesco Giallauria, Anna Di Lorenzo, Alessandro Parlato, Crescenzo Testa, Emanuele Bobbio, and Carlo Vigorito declare that they have no conflict of interest.
Giallauria, F. , Di Lorenzo, A. , Parlato, A. , Testa, C. , Bobbio, E. , Vigorito, C. , and Coats, A. J. S. (2020) Individual patient data meta‐analysis of the effects of the CARILLON® mitral contour system. ESC Heart Failure, 7: 3383–3391. 10.1002/ehf2.13125.
References
- 1. De Marchena E, Badiye A, Robalino G, Junttila J, Atapattu S, Nakamura M, De Canniere D, Salerno T. Respective prevalence of the different Carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg 2011; 26: 385–392. [DOI] [PubMed] [Google Scholar]
- 2. Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail 2004; 10: 285–2,911. [DOI] [PubMed] [Google Scholar]
- 3. Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003; 91: 538–543. [DOI] [PubMed] [Google Scholar]
- 4. Goel S, Bajaj N, Aggarwal B, Gupta S, Poddar KL, Ige M, Bdair H, Anabtawi A, Rahim S, Whitlow PL, Tuzcu EM. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure. J Am Coll Cardiol 2014; 63: 185–186. [DOI] [PubMed] [Google Scholar]
- 5. DiSalvo TG, Acker MA, Dec GW, Byrne JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol 2010; 55: 272–282. [DOI] [PubMed] [Google Scholar]
- 6. Mirabel M, Lung B, Baron G, Messika‐Zeitoun D, Détaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 7. Mack MJ. New techniques for percutaneous repair of the mitral valve. Heart Fail Rev 2006; 11: 259–268. [DOI] [PubMed] [Google Scholar]
- 8. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L, EVEREST II Investigators . Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011; 364: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 9. Obadia JF, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N, Investigators M‐F . Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 10. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, COAPT Investigators . Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 11. Schofer J, Siminiak T, Haude M, Herrman JP, Vainer J, Wu JC, Levy WC, Mauri L, Feldman T, Kwong RY, Kaye DM, Duffy SJ, Tübler T, Degen H, Brandt MC, Van Bibber R, Goldberg S, Reuter DG, Hoppe UC. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009; 120: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siminiak T, Wu JC, Haude M, Hoppe UC, Sadowski J, Lipiecki J, Fajadet J, Shah AM, Feldman T, Kaye DM, Goldberg SL, Levy WC, Solomon SD, Reuter DG. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN trial. Eur J Heart Fail 2012; 14: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipiecki J, Siminiak T, Sievert H, Müller‐Ehmsen J, Degen H, Wu JC, Schandrin C, Kalmucki P, Hofmann I, Reuter D, Goldberg SL, Haude M. Coronary sinus‐based percutaneous annuloplasty as treatment for functional mitral regurgitation: the TITAN II trial. Open Heart 2016; 3: e000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Witte KK, Lipiecki J, Siminiak T, Meredith IT, Malkin CJ, Goldberg SL, Stark MA, von Bardeleben RS, Cremer PC, Jaber WA, Celermajer DS, Kaye DM, Sievert H. The REDUCE FMR trial: a randomized sham‐controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail 2019; 7: 945–955. [DOI] [PubMed] [Google Scholar]
- 15. The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed. Boston, Mass: Little, Brown & Co; 1994. p 253–256. [Google Scholar]
- 16. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Lung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL, Roffi M, Alfieri O, Agewall S, Ahlsson A, Barbato E, Bueno H, Collet J‐P, Coman IM, Czerny M, Delgado V, Fitzsimons D, Folliguet T, Gaemperli O, Habib G, Harringer W, Haude M, Hindricks G, Katus HA, Knuuti J, Kolh P, Leclercq C, McDonagh TA, Piepoli MF, Pierard LA, Ponikowski P, Rosano GMC, Ruschitzka F, Shlyakhto E, Simpson IA, Sousa‐Uva M, Stepinska J, Tarantini G, Tchetche D, Aboyans V, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet J‐P, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Juni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh T, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Zamorano JL, Kzhdryan HK, Mascherbauer J, Samadov F, Shumavets V, Camp GV, Loncar D, Lovric D, Georgiou GM, Linhartova K, Ihlemann N, Abdelhamid M, Pern T, Turpeinen A, Srbinovska‐Kostovska E, Cohen A, Bakhutashvili Z, Ince H, Vavuranakis M, Temesvari A, Gudnason T, Mylotte D, Kuperstein R, Indolfi C, Pya Y, Bajraktari G, Kerimkulova A, Rudzitis A, Mizariene V, Lebrun F, Demarco DC, Oukerraj L, Bouma BJ, Steigen TK, Komar M, De Moura Branco LM, Popescu BA, Uspenskiy V, Foscoli M, Jovovic L, Simkova I, Bunc M, de Prada JAV, Stagmo M, Kaufmann BA, Mahdhaoui A, Bozkurt E, Nesukay E, Brecker SJD, ESC Scientific Document Group . 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 17. Geis NA, Puls M, Lubos E, Zuern CS, Franke J, Schueler R, von Bardeleben RS, Boekstegers P, Ouarrak T, Zahn R, Ince H, Senges J, Katus HA, Bekeredjian R. Safety and efficacy of MitraClip therapy in patients with severely impaired left ventricular ejection fraction: results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2018; 20: 598–608. [DOI] [PubMed] [Google Scholar]
- 18. Iung B, Delgado V, Lazure P, Murray S, Sirnes PA, Rosenhek R, Price S, Metra M, Carrera C, De Bonis M, Haude M, Hindricks G, Bax J, Vahanian A. Educational needs and application of guidelines in the management of patients with mitral regurgitation. A European mixed‐methods study. Eur Heart J 2018; 39: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 19. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 20. Gyoten T, Schenk S, Rochor K, Herwig V, Harnath A, Grimmig O, Just S, Fritzsche D, Messroghli D. Outcome comparison of mitral valve surgery and MitraClip therapy in patients with severely reduced left ventricular dysfunction. ESC Heart Failure 2020; 7: 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keßler M, Seeger J, Muche R, Wohrle J, Rottbauer W, Markovic S. Predictors of rehospitalization after percutaneous edge‐to‐edge mitral valve repair by MitraClip implantation. Eur J Heart Fail 2019; 21: 182–192. [DOI] [PubMed] [Google Scholar]
- 22. Praz F, Grasso C, Taramasso M, Baumbach A, Piazza N, Tamburino C, Windecker S, Maisano F, Prendergast B. Mitral regurgitation in heart failure: time for a rethink. Eur Heart J 2019; 40: 2189–2193. [DOI] [PubMed] [Google Scholar]
- 23. Adamo M, Godino C, Giannini C, Scotti A, Liga R, Curello S, Fiorina C, Chiari E, Chizzola G, Abbenante A, Visco E, Branca L, Fiorelli F, Agricola E, Stella S, Lombardi C, Colombo A, Petronio AS, Metra M, Ettori F. Left ventricular reverse remodelling predicts long‐term outcomes in patients with functional mitral regurgitation undergoing MitraClip therapy: results from a multicentre registry. Eur J Heart Fail 2018; 21: 196–204. [DOI] [PubMed] [Google Scholar]


