Abstract
Aims
High myocardial stiffness in heart failure with preserved ejection fraction (HFpEF) is attributed to comorbidity‐induced structural and functional remodelling through inflammation and oxidative stress affecting coronary microvascular endothelial cells and cardiomyocytes, which augments interstitial fibrosis and cardiomyocyte stiffness. In murine and human HFpEF myocardium, sodium glucose co‐transporter 2 (SGLT2) inhibition ameliorates cardiac microvascular endothelial cell and cardiomyocyte oxidative stress, while enhancing myocardial protein kinase G activity and lowering titin‐based cardiomyocyte stiffness. Failure of previous HFpEF outcome trials refocuses attention to improving pathophysiological insight and trial design with better phenotyping of patients and matching of therapeutic targets to prevailing pathogenetic mechanisms. SGLT2 inhibition could represent a viable therapeutic option especially in HFpEF patients in whom high diastolic left ventricular (LV) stiffness is predominantly caused by elevated cardiomyocyte stiffness and associated endothelial dysfunction, whereas HFpEF patients with extensive myocardial fibrosis might be less responsive. This study aims to investigate a stratified treatment approach, using dapagliflozin in heart failure patients with preserved ejection fraction without evidence of significant myocardial fibrosis.
Methods and results
The Stratified Treatment to Ameliorate DIAstolic left ventricular stiffness in early Heart Failure with preserved Ejection Fraction (STADIA‐HFpEF) is a Phase II, randomized, 2 × 2 crossover trial, evaluating the efficacy of 13 weeks of treatment with dapagliflozin 10 mg od in 26 patients with HFpEF, with normal cardiac magnetic resonance imaging‐derived extracellular volume. The co‐primary endpoint is echocardiographically derived change in E/e'/LV end‐diastolic volume index and change in mean LV e'.
Conclusions
The STADIA‐HFpEF trial will be the first study to evaluate the direct effects of dapagliflozin on amelioration of LV stiffness, using histological phenotyping to discern early HFpEF.
Keywords: HFpEF, Heart failure, Cardiomyocyte, Dapagliflozin
Introduction
Heart failure (HF) with preserved ejection fraction (EF; HFpEF) currently accounts for >50% of all HF cases, and its prevalence relative to HF with reduced EF (HFrEF) continues to rise at a rate of 1% per year. 1 Outcomes in patients with HFpEF and HFrEF are equally poor with 5‐year mortality rates up to 75% in both phenotypes. 2 In contrast to HFrEF, modern HF pharmacotherapy did not improve outcomes in HFpEF, which was attributed to incomplete understanding of HFpEF pathophysiology, patient heterogeneity, and suboptimal trial designs. 3 In the conceptual framework of HFpEF treatment, emphasis may need to shift from a ‘one size fits all’ strategy to an individualized approach based on phenotypic patient characterization and diagnostic/pathophysiological stratification of myocardial disease processes. 4 In the Stratified Treatment to Ameliorate DIAstolic left ventricular stiffness in early Heart Failure with preserved Ejection Fraction (STADIA‐HFpEF) trial, we propose to investigate a stratified and individualized treatment approach, based on matching of potential therapeutic efficacy to prevailing pathophysiological mechanisms of diastolic left ventricular (LV) stiffness in patients with HFpEF.
High diastolic left ventricular stiffness in heart failure with preserved ejection fraction
Heart failure with preserved EF is characterized by increased diastolic LV stiffness. 5 , 6 In the absence of endocardial or pericardial disease, high diastolic LV stiffness results from increased myocardial stiffness, which is regulated by the extracellular matrix (ECM) and the cardiomyocytes. 7 , 8 Collagen importantly determines ECM‐based stiffness through regulation by its total amount, the expression of collagen type I, and the degree of collagen crosslinking and is linked to diastolic LV dysfunction 9 and clinical outcomes 10 in patients with HFpEF. Additionally, increased cardiomyocyte stiffness also importantly contributes to high diastolic LV stiffness in HFpEF. 7
Cardiomyocyte stiffness is mainly determined by the elastic sarcomeric protein titin, which functions as a bidirectional spring, responsible for early diastolic recoil and late diastolic distensibility. 11 Titin‐based cardiomyocyte stiffness results from dynamic changes in expression of stiff (N2B) and compliant (N2BA) isoforms and from post‐translational modifications, including titin isoform phosphorylation and oxidative changes of the N2B segment. 11 , 12 Conditions associated with oxidative or physical stress can induce the formation of disulfide bridges in the human titin N2B sequence, leading to increased titin‐based cardiomyocyte stiffness. 13 In contrast, phosphorylation of titin at its N2B segment by protein kinase G (PKG) acutely increases its compliance. 11 , 12 , 14 Myocardial PKG activity is stimulated by natriuretic peptides (NPs) and nitric oxide (NO) mediated activation of cyclic guanosine monophosphate (cGMP). 14 In HFpEF, myocardial cGMP‐PKG signalling is down‐regulated because of impaired upstream NP and NO bioavailability, which hampers PKG‐mediated titin phosphorylation resulting in subsequent increased titin‐based cardiomyocyte stiffness and cardiomyocyte hypertrophy. 14 Impaired upstream NP‐cGMP and NO‐cGMP signalling in HFpEF could result from adverse effects inflicted by highly prevalent, especially metabolic, comorbidities in HFpEF, which induce NP down‐regulation, systemic inflammation, oxidative stress, and coronary microvascular endothelial dysfunction. 4
Improving cardiomyocyte cGMP‐PKG‐mediated titin phosphorylation was recently proposed as a viable option to improve LV compliance in HFpEF. 15 Therefore, pharmacological agents targeting systemic inflammation, oxidative stress, and microvascular endothelial dysfunction with subsequent stimulation of myocardial cGMP‐PKG activity could have high therapeutic potential in patients with HFpEF.
Cardiac effects of sodium glucose co‐transporter 2 inhibitors
In the DAPA‐HF trial, the sodium glucose co‐transporter 2 (SGLT2) inhibitor dapagliflozin improved outcomes in both HFrEF patients with and without type 2 diabetes mellitus (T2DM). 16 In addition to their glycaemia, volume load, and blood pressure‐lowering effects, SGLT2 inhibitors have also been shown to directly exert beneficial myocardial pleiotropic effects. These beneficial pleiotropic effects include improvement of inflammation, oxidative stress, 17 remodelling, 17 , 18 and mitochondrial dysfunction. 19 In rodent and human HFpEF experimental models, the SGLT2 inhibitor empagliflozin inhibited cardiac microvascular endothelial cell (CMEC) and cardiomyocyte oxidative stress and improved cardiomyocyte distensibility through stimulation of PKG‐mediated phosphorylation of titin. 20 , 21 Hence, SGLT2 inhibitors could be especially advantageous in HFpEF patients in whom impaired LV distensibility is predominantly caused by high cardiomyocyte stiffness and associated endothelial dysfunction. In contrast, HFpEF patients in whom impaired LV distensibility is predominantly caused by extensive myocardial fibrosis could be less likely to respond favourably to treatment.
Natriuretic peptides in heart failure with preserved ejection fraction
Non‐invasive evaluation of myocardial interstitial fibrosis is feasible with cardiac magnetic resonance T1 mapping‐based techniques. T1 mapping measures myocardial extracellular volume (ECV), which is associated with myocardial interstitial fibrosis in human myocardial biopsies, 22 invasively determined LV stiffness, 23 and plasma N‐terminal pro brain natriuretic peptide (NT‐proBNP) levels in individuals from the Multiethnic Atherosclerosis Study 24 and HFpEF patients. 25
Plasma NT‐proBNP levels are frequently low in HFpEF patients, 26 with a substantial proportion of patients with invasively confirmed HFpEF presenting with even normal values. 26 Low NT‐proBNP levels in HFpEF can be attributed to comorbidities inducing a relative state of NP deficiency, 27 which is specifically associated with the obesity HFpEF phenotype, 28 , 29 low LV diastolic wall stress due to concentric LV remodelling, 30 and a cushioning effect of epicardial fat, dampening LV diastolic distension. 28 Despite their suboptimal accuracy for diagnosing HFpEF, NP levels are associated with prognosis 26 , 31 , 32 and profibrotic biomarkers and cardiac remodelling. 33 In the PARAMOUNT trial, sacubitril/valsartan improved left atrial remodelling exclusively in HFpEF patients with below median levels of profibrotic biomarkers, including NT‐proBNP. 33 NP levels also predicted therapeutic efficiency and reverse cardiac remodelling in several, 31 , 32 , 33 but not all, 34 clinical trials.
In the I‐PRESERVE 31 and TOPCAT 32 trials, HFpEF patients with below median NT‐proBNP or lower tercile NPs demonstrated improved outcomes, which, however, was not replicated in the PARAGON HF trial investigating sacubitril/valsartan in HFpEF. 34
Diagnostic and pathophysiological stratification for patient‐tailored therapy
Importantly, markedly elevated NT‐proBNP levels were used as inclusion criterion in large Phase III HFpEF trials. 35 , 36 , 37 A relatively high NP cut‐off level increases the likelihood that recruited trial patients indeed have HF and enriches the event rate in the studied population. However, setting high the NP entry criteria will also skew the recruited HFpEF study population towards a more advanced myocardial fibrotic and hence potentially therapeutically less responsive phenotype. Skewing towards a more fibrotic HFpEF phenotype may potentially explain the neutral outcomes in previous Phase III HFpEF trials. In contrast, HFpEF patients in whom high diastolic LV stiffness is predominantly caused by elevated cardiomyocyte stiffness and associated endothelial dysfunction could potentially be more responsive to therapy.
The STADIA‐HFpEF trial, investigating the capability of SGLT2 inhibitor dapagliflozin to improve diastolic LV distensibility in ‘early HFpEF’, is designed exactly for this purpose. In this regard, ‘early HFpEF’ refers to HFpEF patients with predominant cardiomyocyte and endothelial dysfunction without significant structural myocardial ECM remodelling. As dapagliflozin was shown to lower cardiomyocyte titin‐based stiffness in human HFpEF, dapagliflozin could especially be of therapeutic benefit in HFpEF patients with metabolic risk factors in whom high diastolic LV stiffness is primarily caused by increased cardiomyocyte derived stiffness. The STADIA‐HFpEF trial aims to select a more homogeneous HFpEF patient population in an earlier stage of disease progression (defined by lesser degrees of myocardial fibrosis), with a higher likelihood of responsiveness to therapeutic modulation of LV diastolic distensibility by dapagliflozin.
Methods
Trial design
STADIA‐HFpEF is a Phase II, single‐centre, prospective, double‐blind, randomized, 2 × 2 crossover, interventional study. Patients will be recruited from the outpatient clinic of the OLVG, Amsterdam, the Netherlands. After randomization, the duration of the study comprised two study periods of 13 weeks, separated by a washout period of 8 weeks. Patients will be randomized to either group A or group B (Figure 1 ). Group A will start with dapagliflozin 10 mg for 13 weeks, followed by 8 weeks of washout, followed by 13 weeks with placebo. Group B will start with 13 weeks with placebo, followed by 8 weeks of washout, followed by 13 weeks dapagliflozin 10 mg. Study visits will be planned at Weeks 1, 3, 6, 13, 22, 23, 25, 28, and 35. The trial is registered as ClinicalTrials.gov identifier NCT04475042.
Figure 1.
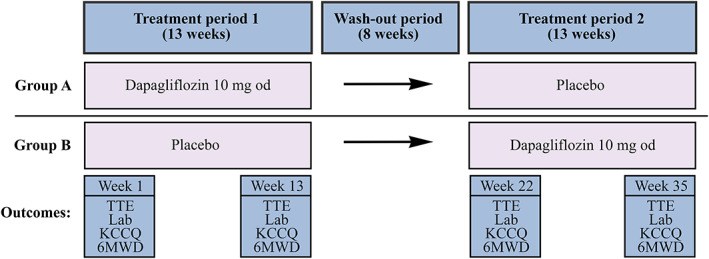
STADIA‐HFpEF study schedule with timing of outcome assessment. 6MWD, 6‐min walking distance; KCCQ, Kansas City Cardiomyopathy Questionnaire; Lab, laboratory testing; od, once daily; TTE, transthoracic echocardiogram.
Objectives
Our primary goal is to assess whether dapagliflozin, in comparison with placebo, improves LV distensibility in patients with early HFpEF. Co‐primary outcome measures will be change in echocardiographically derived E/e'/LV end‐diastolic volume (LVEDV) index and change in mean LV e' after 13 weeks of treatment with dapagliflozin 10 mg. Reliable quantification of LVEDV will be established by the use of an LV contrast agent. Secondary endpoints will be improvement of exercise tolerance (measured by 6‐min walking distance), quality of life (measured by Kansas City Cardiomyopathy Questionnaire), echocardiographically determined left atrial volume and function, diastolic and systolic LV and right ventricular function and systolic pulmonary artery pressure (Table 1 ), and multipanel biomarker profiles (Table 2 ).
Table 1.
Echocardiographic imaging techniques and parameters
| Echocardiography | |
|---|---|
| 2D imaging | |
| LV contrast imaging | |
| Doppler imaging (colour, spectral, and tissue) | |
| Myocardial deformation imaging—speckle tracking |
| Imaging parameters |
|---|
| Left ventricular volume, mass, and systolic and diastolic function |
| Right ventricular volume, systolic and diastolic function, and systolic pulmonary artery pressure |
| Left atrial volumes and function (including strain analysis and reservoir, conduit, and booster pump function) |
Table 2.
Biomarker assessment STADIA‐HFpEF
| Pathophysiological domain | Biomarker |
|---|---|
| Inflammation | hs‐CRP, TNF‐α, TNFR‐1, IL‐1, IL‐6, IL‐18 sICAM‐3, TGF‐β, MPO, MCP‐1 |
| Oxidative stress | Ox‐LDL, 8‐iso‐PGF2α, 8‐OHdG, H2O2, LPO, GSH |
| Endothelial function | E‐selectin, endothelin‐1, ICAM‐1, VCAM‐1, Von Willebrand Factor, NOx |
| Collagen/extracellular matrix turnover | MMP‐1, MMP‐2, MMP‐3, MMP‐7, MMP‐8, MMP‐9, TIMP‐1, TIMP‐4, CITP, PICP, PIIINP, FGF23, GDF‐15, ST2, galectin‐3, Tenascin‐C |
| Adipokines | Adiponectin, Leptin |
| Renal | Cystatin‐C, NGAL, urea |
| Cardiomyocyte stress | NTproBNP |
| Intracellular interactions | cGMP |
cGMP, cyclic guanosine monophosphate; CITP, C‐terminal telopeptide of collagen type I; FGF‐23, fibroblast growth factor‐23; GDF‐15, growth differentiation factor‐15; GSH, reduced gluthatione; hs‐CRP, highly sensitive C‐reactive protein; H2O2 , hydrogen peroxide; ICAM‐1, intercellular adhesion molecule‐1; IL‐1, interleukin‐1; IL‐6, interleukin‐6; IL‐18, interleukin‐18; LPO, lipid peroxide; MCP‐1, monocyte chemoattractant protein‐1; MMP, matrix metalloproteinase; MPO, myeloperoxidase; NGAL, neutrophil gelatinase‐associated lipocalin; NOx, nitrite/nitrate; NTproBNP, N‐terminal pro B‐type natriuretic peptide; Ox‐LDL, oxidized low‐density lipoprotein; PICP, procollagen type I carboxy‐terminal propeptide; PIIINP, procollagen III amino‐terminal peptide; sICAM‐3, soluble intercellular adhesion molecule 3; ST2, suppression of tumorigencity‐2; TGF‐β, transforming growth factor‐β; TIMP‐1, tissue inhibitor of metalloproteinase‐1; TIMP‐4, tissue inhibitor of metalloproteinase‐4; TNF‐α, tumour necrosis factor receptor‐α; TNFR‐1, tumour necrosis factor receptor‐1; VCAM‐1, vascular adhesion cell protein‐1; 8‐iso‐PGF2α, 8‐iso‐prostaglandin F2α; 8‐OhdG, 8‐hydroxy‐2′‐deoxyguanosine.
Patient population
The STADIA‐HFpEF trial will recruit symptomatic (New York Heart Association classes II–IV) HFpEF patients (Figure 2 ). Patients visiting the outpatient clinic will be screened for eligibility. A more homogeneous HFpEF trial population will be recruited as all patients are referred for extensive imaging (echocardiography and magnetic resonance imaging and, if applicable, bone scintigraphy) and, if applicable, invasive (coronary angiography and/or bicycle exercise right heart catheterization) studies before assessment of trial eligibility. The STADIA‐HFpEF trial will exclude HFpEF patients with concomitant obstructive coronary disease, significant valvular disease, and infiltrative or genetic cardiomyopathies. As a consequence of our aim to enrol patients with ‘early HFpEF’, in whom NP values are generally low to normal, NP levels will not be part of the inclusion criteria. To ascertain that recruited patients indeed have HFpEF, additional selection criteria are incorporated in the eligibility strategy (Table 3 and Figure 2 ). Patients need to have met at least one out of the following criteria: (i) H2FpEF score ≥ 6 38 ; (ii) HFA‐PEFF score ≥ 5 39 ; and (iii) pulmonary capillary wedge pressure > 15 mmHg at rest or >25 mmHg with exercise assessed with right heart catheterization. 30
Figure 2.

STADIA‐HFpEF inclusion criteria. A cardiac MRI‐derived extracellular volume (ECV) cut‐off level of ≤29% will select HFpEF patients in whom impaired diastolic left ventricular distensibility is predominantly caused by high cardiomyocyte stiffness. The putative mechanisms of action of dapagliflozin are (i) inhibition of endothelial reactive oxygen species (ROS); (ii) inhibition of ROS in the cardiomyocyte; and (iii) enhancing protein kinase G (PKG) activity. Inhibition of endothelial ROS increases nitric oxide (NO) bioavailability, which stimulates soluble guanylate cyclase (sGC) activity and subsequent cyclic guanosine monophosphate (cGMP) generation and PKG activity. PKG phosphorylates titin at its N2B segment, improving cardiomyocyte compliance. Inhibition of cardiomyocyte ROS directly results in down‐regulation of disulfide bridges (S–S) on the N2B isoform of titin, improving its compliance. CMEC, cardiac microvascular endothelial cell.
Table 3.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| 1. Age ≥ 18 years at time of screening |
| 2. Symptomatic chronic heart failure patients with diagnosis of heart failure and |
| •NYHA classes II–IV |
| •Preserved systolic LV function, defined by LVEF ≥ 50% and LV end‐diastolic volume index < 97 mL/m2 |
| •Evidence of diastolic LV dysfunction and at least 1 out of the 5 following additional criteria: |
| ∘H2FPEF score ≥ 6 |
| ∘HFA‐PEFF score ≥ 5 |
| ∘Pulmonary capillary wedge pressure > 15 mmHg at rest or >25 mmHg with exercise assessed with right heart catheterization |
| 3. Cardiac MRI T1‐derived extracellular volume < 29% at screening |
| 4. Oral diuretics, if prescribed to the patient according to local guidelines and at the discretion of the investigator, should be stable for at least 1 week prior to baseline visit |
| 5. Signed and dated written informed consent in accordance with GCP and local legislation prior to admission to the trial |
| Exclusion criteria |
|---|
| 1. Reduced systolic LV function (LVEF < 50%), measured at any time point in the history of the patient |
| 2. Obstructive coronary artery disease with evidence of ischaemia |
| 3. Myocardial infarction, coronary artery bypass graft surgery, or other major cardiovascular surgery, stroke, or TIA in past 90 days prior to screening visit |
| 4. More than mild valve stenosis |
| 5. More than moderate aortic and/or mitral valve regurgitation |
| 6. Cardiomyopathy based on infiltrative diseases (e.g. amyloidosis), accumulation diseases (e.g. haemochromatosis, Fabry disease), muscular dystrophies, cardiomyopathy with reversible causes (stress cardiomyopathy), hypertrophic (obstructive) cardiomyopathy, or known pericardial constriction |
| 7. History of mitral valve repair or replacement |
| 8. Atrial fibrillation or atrial flutter with a resting heart rate > 110 bpm at screening |
| 9. Acute decompensation that requires intravenous loop diuretics |
| 10. Systolic blood pressure ≥ 180 mmHg. If SBP > 150 mmHg and <180 mmHg, the patient should be receiving at least three antihypertensive drugs at screening or baseline visit |
| 11. Symptomatic hypotension and/or a SBP < 100 mmHg at screening or baseline visit |
| 12. Impaired renal function, defined as eGFR < 30 mL/min/1.73 m2 |
| 13. Indication of liver disease, defined by serum levels of either ALT (SGPT), AST (SGOT), or alkaline phosphatase above 3× upper limit of normal or history of cirrhosis with evidence of portal hypertension |
| 14. Haemoglobin < 9 g/dL at screening |
| 15. Chronic obstructive pulmonary disease, more than GOLD class 2 |
| 16. Pulmonary function test with FEV1/FVC < 80% |
| 17. Primary pulmonary arterial hypertension |
| 18. Type 1 diabetes mellitus |
| 19. History of ketoacidosis |
| 20. Any documented active or suspected malignancy or history of malignancy within 2 years prior to screening, except appropriately treated basal cell carcinoma of the skin or in situ carcinoma of uterine cervix or low‐risk prostate cancer (biopsy Gleason score of ≤6 and clinical stage T1c or T2a) |
| 21. Current use or prior use of a SGLT‐2 inhibitor or combined SGLT‐1 and 2 inhibitor within 3 months prior to screening visit. Discontinuation of a SGLT‐2 inhibitor or combined SGLT‐1 and 2 inhibitor for the purposes of study enrolment is not permitted |
| 22. Pregnancy or lactation |
| 23. Any (clinical) condition that, in the investigator's opinion, would jeopardize patients safety while participating in this trial, or may prevent the patient from adhering to the trial protocol |
To select HFpEF patients with predominant cardiomyocyte and endothelial dysfunction, without significant structural myocardial ECM remodelling, only patients with a normal (<29% 40 ) MRI‐derived ECV will be included. All inclusion and exclusion criteria are listed in Table 3 .
Investigational medicinal product
Dapagliflozin is administrated orally via encapsulated tablets. Previously, it was demonstrated that the use of SGLT2 inhibitors is safe in patients with HF, regardless of presence of T2DM. 16 , 41 The most important but rare side effect of SGLT2 inhibitors in patients with T2DM is diabetic ketoacidosis. The most common side effect is urinary tract infection. There will be close monitoring of adverse events during the follow‐up visits. Dapagliflozin is contraindicated in severe renal impairment (estimated glomerular filtration rate < 30 mL/min/1.73 m2). Several studies demonstrated that in patients with estimated glomerular filtration rate between 30 and 60 mL/min/1.73 m2, dapagliflozin was not only safe, but even potentially advantageous. 41 Compliance during the study will be checked by pill counting.
Outcome measurements
Echocardiographic measurements will be performed on an EPIQ 7C ultrasound system (Philips Health Systems Benelux, Andover, USA). All echocardiographic examinations will be performed by one designated experienced cardiac echocardiographer (blinded to treatment allocation), to prevent interobserver variability. One other echocardiographer will be appointed to perform examinations if abovementioned is not present on study site. Measurements will be performed following a standardized protocol involving two‐dimensional, M‐mode, Doppler, tissue Doppler, and 2D speckle tracking echocardiography in accordance with current recommendations. 42 , 43
Quality of life will be measured by the Kansas City Cardiomyopathy Questionnaire. The questionnaire will be completed by the subject under supervision of a study team member. Exercise capacity will be assessed by 6‐min walk distance. Biomarker measurements will be performed according to standard recommended analysis techniques, 21 including ELISA, also using real‐time PCR. Transcriptomic profile will be evaluated using single‐cell RNA sequencing.
Sample size
We aim to recruit 26 subjects that complete treatment period and all study visits. The sample size calculation was based on previous studies of e' improvement after SGLT2 inhibitor administration. Verma et al. demonstrated that, in T2DM patients with established cardiovascular disease, empagliflozin 10 mg for 3 months induced a 14% increase in mean lateral e' (8.5 ± 1.6 to 9.7 ± 1.2 cm/s; P = 0.002). 44 Empagliflozin administration to skinned cardiomyocytes isolated from LV biopsies from HFpEF patients resulted in a 24% improvement in cardiomyocyte passive stiffness. 20 Hence, based on e' improvement with empagliflozin from 8.5 to 9.7 ± 1.6 SD cm/s to reach 90% power with alpha 0.05, n = 11 per group is needed, thus 22 in total. With a dropout of 15% taken into account, the total group should consist of 26 patients, with 13 patients per group.
Statistical analysis
The primary analysis set for efficacy will be analysed according to the intention‐to‐treat approach. Per‐protocol analysis will be conducted as secondary analysis set. The aim is to reject the null hypothesis that dapagliflozin does not change the LV stiffness in patients with early stage HFpEF. The co‐primary outcomes, the E/e'/LVEDV index quotient and e', will be quantified as mean differences between intervention conditions. A linear mixed effects regression model with E/e'/LVEDV index as dependent variable will be used to analyse the treatment effect on the primary outcome. The linear mixed effects model will include a random intercept to account for correlation of repeated measures within patients arising from the crossover design. The main effect of the treatment will be estimated by including a fixed effect for treatment allocation (dapagliflozin or placebo) in the model. Another fixed effect for the sequence of the period (first or second) will be included in the model to control for a period effect. An interaction term for treatment × period will be included to test for presence of a carryover effect. A similar model will be used to estimate effects for e' and for secondary endpoints. Results will be presented as mean difference with 95% confidence interval. P values will be two‐sided and considered statistically significant with P < 0.05. Statistical analysis will be performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
Study management and committees
STADIA‐HFpEF is an investigator‐initiated study, conducted by the research team under the guidance of primary investigator LvH. The trial is funded by AstraZeneca BV, The Hague, the Netherlands. AstraZeneca will have no involvement in the conduct of this study; the analysis of data; or the drafting, editing, and publication of the paper. The study will be conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The protocol and informed consent form are approved by the Institutional Review Board of Amsterdam University Medical Centre and the local Institutional Review Board.
Discussion
Sodium glucose co‐transporter 2 inhibitors
Several studies have recently demonstrated beneficial myocardial pleiotropic effects of SGLT2 inhibitors. Dapagliflozin improved echocardiographically determined LV filling pressures in T2DM patients with stable HF after 6 months of treatment. 45 In cardiomyocytes isolated from LV biopsies from HFpEF patients, empagliflozin directly improved cardiomyocyte stiffness through enhanced phosphorylation of myofilamentary proteins including titin. 20 In addition, in a co‐culture model of human CMECs and rat adult cardiomyocytes, it was demonstrated that CMECs exert a direct positive effect on cardiomyocyte contractility and relaxation. This effect, which was mediated by CMEC‐derived NO, was diminished after CMEC stimulation with tumour necrosis factor‐α and restored by adding empagliflozin. 41 Furthermore, empagliflozin restored NO bioavailability and reduced mitochondrial and cytoplasmic reactive oxygen species production after tumour necrosis factor‐α stimulation in CMECs. 41
In order to elucidate the exact cardiac molecular target of SGLT2 inhibitors, Kolijn et al. investigated the acute mechanisms of empagliflozin in vitro in human myocardium from patients with HFpEF and in vivo in murine zucker diabetic fatty (ZDF) obese rats. 21 Empagliflozin‐induced improvement of LV stiffness was attributed to enhanced phosphorylation of titin and troponin‐I and to reduction in oxidative stress/eNOS‐dependent PKGIα oxidation, leading to increased levels of NO, soluble guanylate cyclase activity, and cGMP and PKGIα concentration. These results confirm the direct effect of SGLT2 inhibitors on the NO‐cGMP‐PKG pathway in HFpEF patients.
Targeting early heart failure with preserved ejection fraction
Pathophysiological stratification based on myocardial disease processes in diastolic LV stiffness, including the role of metabolic comorbidities, is now increasingly gaining attention. Recently, the importance of stratification of HFpEF patients with matching of therapeutic targets to prevailing pathophysiological mechanisms was recognized, leading to small‐scale clinical initiatives to investigate this approach. 46 The PIROUETTE trial will investigate the efficacy of pirfenidone to improve myocardial ECV in selected HFpEF patients with cardiac MRI T1 mapping ECV values ≥ 27% cut‐off levels at baseline. 46
Several other studies have recently been initiated to evaluate the effects of SGLT2 inhibitors on HFpEF, 47 , 48 although these studies do not differentiate between different phenotypes of HFpEF. Moreover, in each of these studies, patient selection is based on elevated NT‐proBNP, risking inclusion of HFpEF patients with mostly fibrosis‐based diastolic LV stiffness. The STADIA‐HFpEF trial will be the first study to evaluate the direct effects of dapagliflozin on amelioration of LV stiffness, using histological phenotyping and pathophysiological stratification targeting early HFpEF.
Conflict of interest
None declared.
Funding
This work was supported by AstraZeneca BV, The Hague, the Netherlands (ESR‐19‐20378).
Scheffer, M. , Driessen‐Waaijer, A. , Hamdani, N. , Landzaat, J. W. D. , Jonkman, N. H. , Paulus, W. J. , and van Heerebeek, L. (2020) Stratified Treatment of Heart Failure with preserved Ejection Fraction: rationale and design of the STADIA‐HFpEF trial. ESC Heart Failure, 7: 4478–4487. 10.1002/ehf2.13055.
Clinical trial registration: ClinicalTrials.gov identifier NCT04475042.
References
- 1. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 2. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol 2017; 70: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 3. Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne‐Nickens P, Nkulikiyinka R, Lewis GD, Gomberg‐Maitland M, O'Connor CM, Stockbridge N, Califf RM, Konstam MA, Januzzi JL, Solomon SD, Borlaug BA, Shah SJ, Redfield MM, Felker GM. Heart failure with preserved ejection fraction expert panel report: Current controversies and implications for clinical trials. J Am Coll Cardiol HF. 2018; 6: 619–632. [DOI] [PubMed] [Google Scholar]
- 4. Shah S, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134: 165–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004; 350: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 6. Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschöpe C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008; 117: 2051–2060. [DOI] [PubMed] [Google Scholar]
- 7. Van Heerebeek L, Borbély A, Niessen HWM, Bronzwaer JGF, Van Der Velden J, Stienen GJM, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 2006; 113: 1966–1973. [DOI] [PubMed] [Google Scholar]
- 8. Zile MR, Baicu CF, Ikonomidis J, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, van Buren P, Meyer M, Redfield MA, Bull DL, Granzier H, le Winter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction. Circulation 2015; 131: 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss HP, Tschpe C. Diastolic tissue doppler indexes correlate with the degree of collagen expression and cross‐linking in heart failure and normal ejection fraction. J Am Coll Cardiol 2011; 57: 977–985. [DOI] [PubMed] [Google Scholar]
- 10. López B, Ravassa S, González A, Zubillaga E, Bonavila C, Bergés M, Echegaray K, Beaumont J, Moreno MU, San José G, Larman M, Querejeta R, Díez J. Myocardial collagen cross‐linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol 2016; 67: 251–260. [DOI] [PubMed] [Google Scholar]
- 11. Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res 2014; 114: 1052–1068. [DOI] [PubMed] [Google Scholar]
- 12. Borbély A, Falcao‐Pires I, van Heerebeek L, Hamdani N, Édes I, Gavina C, Leite‐Moreira AF, Bronzwaer JGF, Papp Z, van der Velden J, Stienen GJM, Paulus WJ. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 2009; 104: 780–786. [DOI] [PubMed] [Google Scholar]
- 13. Grützner A, Garcia‐Manyes S, Kötter S, Badilla CL, Fernandez JM, Linke WA. Modulation of titin‐based stiffness by disulfide bonding in the cardiac titin N2‐B unique sequence. Biophys J 2009; 97: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Heerebeek L, Hamdani N, Falcão‐Pires I, Leite‐Moreira AF, Begieneman MPV, Bronzwaer JGF, Van Der Velden J, Stienen GJM, Laarman GJ, Somsen A, Verheugt FWA, Niessen HWM, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012; 126: 830–839. [DOI] [PubMed] [Google Scholar]
- 15. Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC, Roessig L, Stasch JP, Solomon SD, Paulus WJ, Butler J. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc 2013; 2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Lohlavek JB, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 17. Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019; 18: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium‐glucose co‐transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol 2019; 18: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee TI, Chen YC, Lin YK, Chung CC, Lu YY, Kao YH, Chen YJ. Empagliflozin attenuates myocardial sodium and calcium dysregulation and reverses cardiac remodeling in streptozotocin‐induced diabetic rats. Int J Mol Sci 2019; 20: 1680 10.3390/ijms20071680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C, Tirilomis P, Mustroph J, Renner A, Gummert J, Fischer T, Van Linthout S, Tschöpe C, Streckfuss‐Bömeke K, Hasenfuss G, Maier LS, Hamdani N, Sossalla S. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 2018; 20: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 21. Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovács Á, Fülöp GÁ, Falcão‐Pires I, Reusch PH, van Linthout S, Papp Z, van Heerebeek L, Vecchione C, Maier LS, Ciccarelli M, Tschöpe C, Mügge A, Bagi Z, Sossalla S, Hamdani N. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro‐inflammatory‐oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res 2020; 12: cvaa123 10.1093/cvr/cvaa123 [DOI] [PubMed] [Google Scholar]
- 22. Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast‐enhanced T1 mapping. J Am Coll Cardiol 2008; 52: 1574–1580. [DOI] [PubMed] [Google Scholar]
- 23. Rommel KP, Von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lücke C, Gutberlet M, Linke A, Schuler G, Lurz P. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2016; 67: 1815–1825. [DOI] [PubMed] [Google Scholar]
- 24. Liu C‐Y, Heckbert SR, Lai S, Ambale‐Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC, Bluemke DA. Association of elevated NT‐proBNP with myocardial fibrosis in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2017; 70: 3102–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duca F, Kammerlander AA, Zotter‐Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: Insights from a prospective cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging 2016; 9: 1–11. [DOI] [PubMed] [Google Scholar]
- 26. Van Veldhuisen DJ, Linssen GCM, Jaarsma T, Van Gilst WH, Hoes AW, Tijssen JGP, Paulus WJ, Voors AA, Hillege HL. B‐type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013; 61: 1498–1506. [DOI] [PubMed] [Google Scholar]
- 27. Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care 2014; 37: 2899–2908. [DOI] [PubMed] [Google Scholar]
- 28. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Packer M, Kitzman DW. Obesity‐related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium‐glucose cotransporter‐2. J Am Coll Cardiol HF. 2018; 6: 633–639. [DOI] [PubMed] [Google Scholar]
- 30. Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction. Circulation 2017; 135: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, McMurray JJ, Zile MR, Komajda M, Massie BM, Carson PE. Prognostic value of baseline plasma amino‐terminal pro‐brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction findings from the I‐PRESERVE trial. Circ Heart Fail 2011; 4: 569–577. [DOI] [PubMed] [Google Scholar]
- 32. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, Desai AS, O'Meara E, Fleg JL, Pfeffer MA, Pitt B, Solomon SD. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. J Am Coll Cardiol HF 2017; 5: 241–252. [DOI] [PubMed] [Google Scholar]
- 33. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, Prescott MF, Shi V, Lefkowitz M, McMurray JJV, Solomon SD. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction. Circ Heart Fail 2016; 9: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cunningham JW, Vaduganathan M, Claggett BL, Zile MR, Anand IS, Packer M, Zannad F, Lam CSP, Janssens S, Jhund PS, Kober L, Rouleau J, Shah SJ, Chopra VK, Shi VC, Lefkowitz MP, Prescott MF, Pfeffer MA, McMurray JJV, Solomon SD. Effects of sacubitril/valsartan on N‐terminal pro‐B‐type natriuretic peptide in heart failure with preserved ejection fraction. J Am Coll Cardiol HF. 2020; 8: 372–381. [DOI] [PubMed] [Google Scholar]
- 35. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 36. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 37. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen H‐D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1–11. [DOI] [PubMed] [Google Scholar]
- 38. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pieske B, Tschöpe C, De Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske‐Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: The HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 40. Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart 2012; 98: 1436–1441. [DOI] [PubMed] [Google Scholar]
- 41. Juni RP, Kuster DWD, Goebel M, Helmes M, Musters RJP, van der Velden J, Koolwijk P, Paulus WJ, van Hinsbergh VWM. Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. JACC Basic Transl Sci 2019; 4: 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Retzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 43. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 1961–1977. [DOI] [PubMed] [Google Scholar]
- 44. Verma S, Garg A, Yan AT, Gupta AK, Al‐Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial. Diabetes Care 2016; 39: e212–e213. [DOI] [PubMed] [Google Scholar]
- 45. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol 2018; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewis GA, Schelbert EB, Naish JH, Bedson E, Dodd S, Eccleson H, Clayton D, Jimenez BD, McDonagh T, Williams SG, Cooper A, Cunnington C, Ahmed FZ, Viswesvaraiah R, Russell S, Neubauer S, Williamson PR, Miller CA. Pirfenidone in heart failure with preserved ejection fraction—rationale and design of the PIROUETTE trial. Cardiovasc Drugs Ther 2019; 33: 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, Packer M, Anker SD, Butler J, Filippatos GS, Zannad F, George J, Brueckmann M, Perrone S, Nicholls S, Janssens S, Bocchi E, Giannetti N, Verma S, Jian Z, Gomez Mesa JE, Spinar J, Böhm M, Merkely B, Chopra V, Senni M, Taddi S, Tsutsui H, Chuquiure E, la Rocca HPB, Ponikowski P, Vinereanu D, Sim D, Choi DJ, Juanatey JRG, Squire I, Butler J, Januzzi J, Pina I, Pocock SJ, Carson P, Doehner W, Miller A, Haas M, Pehrson S, Komajda M, Anand I, Teerlink J, Rabinstein A, Steiner T, Kamel H, Tsivgoulis G, Lewis J, Freston J, Kaplowitz N, Mann J, Petrie M, Bernstein R, Cheung A, Green J, Januzzi J, Kaul S, Ping CLS, Lip G, Marx N, McCullough P, Mehta C, Ponikowski P, Rosenstock J, Sattar N, Scirica B, Tsutsui H, Verma S, Wanner C, Welty FK, Parhofer KG, Clayton T, Pedersen TR, Lees KR, Konstam MA, Greenberg B, Palmer M. Evaluation of the effects of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 48. Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (DELIVER) [Internet]. 2018. https://clinicaltrials.gov/ct2/show/NCT03619213 (22 September 2020).


