Abstract
Aims
Obesity doubles the lifetime risk of developing heart failure. Current knowledge on the role of obesity in causing cardiac dysfunction is insufficient for optimal risk stratification. The aim of this study was first to estimate the prevalence of subclinical cardiac dysfunction in obesity patients and second to investigate the underlying pathophysiology.
Methods and results
The CARDIOBESE study is a cross‐sectional multicentre study of 100 obesity patients [body mass index (BMI) ≥ 35 kg/m2] without known cardiovascular disease and 50 age‐matched and gender‐matched non‐obese controls (BMI ≤ 30 kg/m2). Echocardiography was performed, blood samples were collected, and a Holter monitor was affixed. Fifty‐nine obesity patients [48 (42–50) years, 70% female] showed subclinical cardiac dysfunction: 57 patients had decreased global longitudinal strain (GLS), and two patients with normal GLS had either diastolic dysfunction or increased brain natriuretic peptide (BNP). Only one non‐obese control had diastolic dysfunction, and none had another sign of cardiac dysfunction. Multivariable logistic analysis identified male gender and standard deviation of all NN intervals (SDNN) index, which is a measure of autonomic dysfunction, as independent significant risk factors for subclinical cardiac dysfunction in obesity patients.
Conclusions
There was a high prevalence (61%) of subclinical cardiac dysfunction in obesity patients without known cardiovascular disease, which appeared to be best identified by GLS. Subclinical cardiac dysfunction in obesity was linked to autonomic dysfunction and male gender, and not to the presence of traditional cardiac risk factors, increased C‐reactive protein, increased BNP, increased high‐sensitivity troponin I, or increased left ventricular mass.
Keywords: Obesity/obese, Cardiac dysfunction, Global longitudinal strain, Speckle‐tracking echocardiography, Heart rate variability
Introduction
Obesity doubles the lifetime risk of developing heart failure 1 and is becoming a global epidemic. 2 In 2015, a total of 107.7 million children and 603.7 million adults were obese [body mass index (BMI) ≥ 30 kg/m2] worldwide. Since 1980, the prevalence of obesity has doubled in >70 countries. 3 Both overweight and obesity are associated with an increased risk of cardiovascular disease. 4 Despite the relatively consistent finding of increased prevalence of heart failure in obesity, the reason for this association remains unclear, and it seems to be a heterogeneous disorder. 5 , 6 Many factors have been suggested, such as insulin resistance, hypertension, and reduced high‐density lipoprotein cholesterol (HDL‐C). 7 However, the onset of heart failure in obesity patients cannot be fully explained by the presence of traditional cardiovascular risk factors. 8 The enormous and growing prevalence of obesity warrants efficient screening of obesity patients with the highest risk of cardiac dysfunction who may need further risk assessment, follow‐up, or even treatment. 9 Current knowledge on the role of obesity in causing cardiac dysfunction is insufficient to optimally develop such strategies for obesity patients. 10 Previous studies regarding the detection of the early stages of cardiac dysfunction have shown the benefits of newer diagnostic techniques such as speckle‐tracking echocardiography over left ventricular (LV) ejection fraction assessment, 11 , 12 also, for example, in patients with obstructive sleep apnoea syndrome without overt LV dysfunction. 13 The CARdiac Dysfunction In OBesity—Early Signs Evaluation (CARDIOBESE) study is the first study in which conventional and speckle‐tracking echocardiography, blood biomarkers, and Holter monitoring have been combined to study subclinical cardiac dysfunction in a cohort of obesity patients without known cardiovascular disease and non‐obese controls. The aim of the study was first to identify the prevalence of subclinical cardiac dysfunction in both groups and second to investigate the underlying pathophysiology by comparing obesity patients with and without cardiac dysfunction.
Methods
Study design
The protocol of the CARDIOBESE study has been described before. 14 In short, the CARDIOBESE study is a multicentre cross‐sectional study in which we prospectively enrolled 100 consecutive obesity patients who were referred for bariatric surgery in the Franciscus Gasthuis & Vlietland (75 patients) and Maasstad Ziekenhuis (25 patients), both in Rotterdam, the Netherlands. Patients were enrolled if they were between 35 and 65 years old, had a BMI of ≥35 kg/m2, and gave written informed consent. Patients with a suspicion of or known cardiovascular disease on the basis of the patients' history (as determined by questioning the patients and reviewing available medical files) were excluded. Fifty age‐matched and gender‐matched non‐obese (BMI < 30 kg/m2) controls, also without suspicion of cardiovascular disease, were enrolled. Controls were recruited using advertisements in a local newspaper or were personnel from the participating hospitals or family members or friends of personnel.
Conventional and advanced echocardiography was performed, blood samples were collected, and a Holter monitor was affixed for 24 h for heart rhythm registration in both the obesity patients and the non‐obese controls. This was done both to quantify the proportion of early signs of cardiac dysfunction and to determine if the prevalence of cardiac dysfunction in obesity patients is increased compared with the non‐obese controls. Also, a broad variety of parameters known to be related to obesity were collected to investigate the relation between cardiac dysfunction and obesity. The study complies with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Committee Toetsingscommissie Wetenschappelijk Onderzoek Rotterdam e.o. (TWOR). 14
Sample size calculation
The combination of parameters used to identify subclinical cardiac dysfunction has not been investigated in obesity patients before. A conservative estimate would be that cardiac dysfunction based on conventional echocardiography is present in 20% of obesity patients and 2.5% of age‐matched and gender‐matched non‐obese controls. 15 Given these estimates, to be able to reject the null hypothesis that cardiac dysfunction rates are equal between patients and controls, at least 97 obesity patients and 49 non‐obese controls have to be included in the analysis [alpha: 0.05 (two‐sided), power: 0.80, 2:1 ratio of patients:controls]. The use of more sensitive techniques may increase the proportion of non‐obese controls with an early sign of cardiac dysfunction. Nevertheless, the proportion of obesity patients with an early sign of cardiac dysfunction is expected to increase even more, assuring that the previous sample size calculation will still suffice.
Transthoracic echocardiography
Two‐dimensional greyscale harmonic images were obtained in the left lateral decubitus position using a commercially available ultrasound system (EPIQ 7, Philips, the Netherlands), equipped with a broadband (1–5 MHz) X5‐1 transducer. All acquisitions and measurements were performed according to the current guidelines. 16 , 17
Speckle‐tracking echocardiography is a new echocardiographic imaging modality that is able to relatively angle‐independently quantify myocardial wall motion. Greyscale echocardiographic images consist of a speckled pattern. This pattern is not the actual image of the scatterers in the tissue itself but the interference pattern generated by the reflected ultrasound. Each region of the myocardium has its own unique speckle pattern that remains stable enough to allow spatial and temporal image processing with selection and recognition of speckles on the ultrasound image by dedicated software packages. The geometric position of the speckles changes from frame to frame with the surrounding tissue motion. Therefore, the geometric shift of each speckle represents local tissue motion, and by tracking these speckles, myocardial deformation parameters, such as strain, can be calculated. 18 To optimize speckle‐tracking echocardiography, apical images were obtained at a frame rate of 60 to 80 frames/s. Three consecutive cardiac cycles were acquired from all apical views (four‐chamber, two‐chamber, and three‐chamber). Subsequently, these cycles were transferred to a QLAB workstation (Version 10.2, Philips, the Netherlands) for offline speckle‐tracking analysis. Offline analyses were performed by two independent observers. In end‐diastole, automated border tracking was enabled, before manual adjustment using a ‘point and click approach’ to ensure that the endocardial and epicardial borders were included in the region of interest. When tracking was suboptimal, fine‐tuning was performed manually. Peak regional longitudinal strain was measured in 17 myocardial regions, and a weighted mean was used to derive global longitudinal strain (GLS) (Figure 1 ). 16
Figure 1.
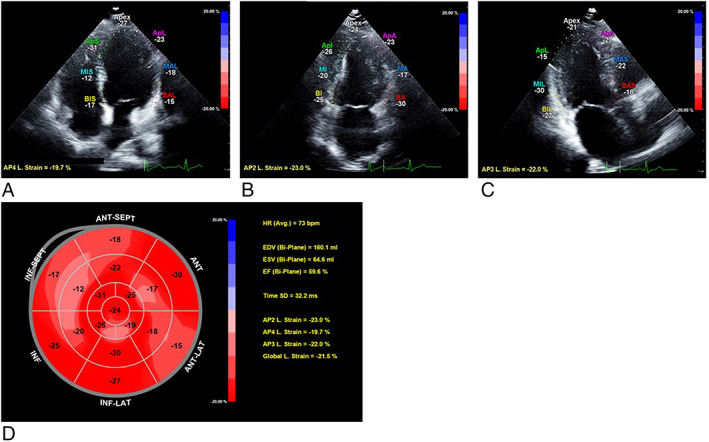
Measurement of global longitudinal strain (GLS) by speckle‐tracking analysis in an obesity patient [45‐year‐old woman, body mass index (BMI) 38.4 kg/m2]. (A) Apical four‐chamber view with measurement of longitudinal strain. (B) Apical two‐chamber view with measurement of longitudinal strain. (C) Apical three‐chamber view with measurement of longitudinal strain. (D) Bull's eye graph showing longitudinal strain for all myocardial segments, of which a weighted mean was used to derive GLS.
Blood tests
Non‐fasting blood samples were taken both for the study and as part of regular care, which included sodium, potassium, calcium, glucose, glycated haemoglobin (HbA1c), creatinine, estimated glomerular filtration rate (eGFR), alanine aminotransferase (ALAT), Apo‐lipoprotein B100, Lipoprotein a (Lp(a)), total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), HDL‐C, triglycerides, ferritin, active vitamin B12, folic acid, vitamin B1, vitamin B6, albumin, magnesium, vitamin D, haemoglobin, erythrocytes, thrombocytes, leucocytes, and thyroid‐stimulating hormone (TSH). In addition to the regular care path, high‐sensitivity cardiac troponin I (hs‐cTnI), C‐reactive protein, and brain natriuretic peptide (BNP) were determined. Hs‐cTnI was considered positive when ≥34 ng/L for male and >16 ng/L for female subjects.
Holter monitoring
Heart rhythm was recorded for 24 consecutive hours using a portable digital recorder (GE HEER Light, USA). The digital recorder was connected using stickers that were placed on the chest. Average heart rate, minimal heart rate, maximum heart rate, total premature atrial contractions (PACs), total premature ventricular contractions, the standard deviation of all NN (often also referred to as RR) intervals (SDNN), and SDNN index were measured. A 24 h recording of the SDNN reveals the sympathetic nervous system contribution to heart rate variability (HRV). 19 The SDNN index estimates the variability due to the factors affecting HRV within a 5 min period. It is calculated by first dividing the 24 h record into 288 five‐minute segments and then calculating the standard deviation of all NN intervals contained within each segment. 20
Cardiac dysfunction
With the use of echocardiography, Holter monitoring, and blood tests, cardiac dysfunction was in the current study defined as reduced LV ejection fraction (<52%), 16 decreased GLS (<95th percentile of the non‐obese controls, see Statistical analysis), diastolic dysfunction, 17 sustained supraventricular or (non)sustained ventricular arrhythmia, or an increased BNP (>30 pmol/L) or hs‐cTnI (≥34 ng/L for male and >16 ng/L for female subjects).
Statistical analysis
Normally distributed data are presented as means and standard deviation, skewed data as medians and inter‐quartile range, and categorical variables as percentages and frequencies. The normality of the data was checked by the Shapiro–Wilk test. Differences in both the clinical characteristics and parameters of cardiac function between obesity patients and the non‐obese controls were estimated by using generalized linear mixed models with obesity as the independent variable, and parameters were entered consecutively as the dependent variable, and the matched pairs were used as random intercepts. Missing variables were omitted. Differences in both clinical characteristics and parameters of cardiac function in obesity patients were tested by univariable logistic regression with cardiac dysfunction as the dependent variable. The Benjamini–Hochberg procedure, with a 5% false discovery rate, was used to correct for the multiple testing. 21
Patient characteristics statistically significant different between obesity patients with and without cardiac dysfunction in the univariable analyses were added to multivariable logistic regression analysis (method: backward stepwise analysis). Predicted probabilities of cardiac dysfunction in obesity patients obtained from the model were used to construct a receiver operating characteristic (ROC) curve, and the area under the ROC curve was calculated as an overall measure of discriminative ability. Sensitivity, specificity, positive predictive value, and negative predictive value and their 95% confidence intervals were calculated. A two‐tailed P‐value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS Version 25.0 and R Version 3.6.0.
Results
Clinical characteristics of obesity patients and non‐obese controls
Table 1 and Figure 2A show the characteristics of the obesity patients (n = 100) and the non‐obese controls (n = 50). Obesity patients had significantly increased weight, BMI, systolic blood pressure, waist circumference, and heart rate. Obesity patients also had significantly more frequent co‐morbidities such as diabetes mellitus and hypertension and more often used medication (angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, diuretics, and oral anti‐diabetics). Blood tests showed that obesity patients had significantly increased C‐reactive protein, leucocytes, glucose, HbA1c, Apo‐lipoprotein B100, triglycerides, and active vitamin B12. HDL‐C, albumin, and vitamin D were decreased in obesity patients. Echocardiography showed an increased LV mass in obesity patients, but when corrected for the body surface area (LV mass index), there was no significant difference between groups. Holter monitoring showed a significantly increased average and minimal heart rate in obesity patients. Obesity patients also had a significantly decreased SDNN and SDNN index.
TABLE 1.
Clinical characteristics of the study population and parameters of cardiac function
| Non‐obese (n = 50) | Obese (n = 100) | P‐value | |
|---|---|---|---|
| Clinical characteristics | |||
| General characteristics | |||
| Age (years) | 50 (40–59) | 48 (42–50) | 0.02 * |
| Female (%) | 35 (70%) | 70 (70%) | >0.99 |
| Physical examination | |||
| Length (m) | 1.74 ± 0.1 | 1.71 ± 0.1 | 0.08 |
| Weight (kg) | 76 (64–82) | 123 (115–135) | <0.001 * |
| BMI (kg/m2) | 25 (22–28) | 42 (40–46) | <0.001 * |
| Systolic BP (mmHg) | 127 (118–136) | 140 (127–157) | <0.001 * |
| Diastolic BP (mmHg) | 78 (71–82) | 79 (72–88) | 0.11 |
| Waist circumference (cm) | 79 (74–89) | 131 (125–140) | <0.001 * |
| Heart rate (b.p.m.) | 64 ± 9 | 80 ± 13 | <0.001 * |
| Co‐morbidity | |||
| Diabetes mellitus | 0 | 22 (22%) | 0.007 * |
| Hypertension | 4 (8%) | 32 (32%) | 0.003 * |
| Hypercholesterolaemia | 5 (10%) | 18 (18%) | 0.21 |
| Current smoking | 7 (14%) | 17 (17%) | 0.63 |
| COPD | 1 (2%) | 5 (5%) | 0.39 |
| OSAS | 1 (2%) | 12 (12%) | 0.07 |
| Medication | |||
| Beta‐blockers | 0 | 8 (8%) | 0.03 |
| ACE inhibitors/ARBs | 2 (4%) | 24 (24%) | 0.008 * |
| Calcium channel blockers | 0 | 12 (12%) | 0.04 |
| Statins | 3 (6%) | 20 (20%) | 0.03 |
| Diuretics | 1 (2%) | 18 (18%) | 0.02 * |
| Insulin | 0 | 7 (7%) | 0.04 |
| Oral anti‐diabetics | 0 | 15 (15%) | 0.02 * |
| Blood tests | |||
| C‐reactive protein (mg/L) | 1 (0–2) | 6 (4–10) | <0.001 * |
| Glucose (mmol/L) | 5.1 (4.7–5.5) | 5.4 (4.8–6.2) | 0.006 * |
| HbA1c (mmol/mol) | 35 (33–37) | 39 (35–47) | <0.001 * |
| Creatinine (μmol/L) | 69 (65–75) | 72 (65–78) | 0.35 |
| eGFR (mL/min/1.73 m2) | 74 (69–79) | 90 (79–90) | <0.001 * |
| ALAT (U/L) | 22 (15–32) | 28 (20–37) | 0.64 |
| Apo‐lipoprotein B100 (g/L) | 0.90 (0.75–1.07) | 1.05 (0.91–1.30) | 0.007 * |
| Lipoprotein (a) (mg/L) | 172 (52–367) | 167 (71–522) | 0.80 |
| Total cholesterol (mmol/L) | 5.2 ± 1 | 5.3 ± 1 | 0.55 |
| LDL cholesterol (mmol/L) | 3.0 ± 1.0 | 3.2 ± 0.9 | 0.24 |
| HDL cholesterol (mmol/L) | 1.4 (0.94–1.80) | 1.1 (1.0–1.4) | <0.001 * |
| Triglycerides (mmol/L) | 1.25 (0.9–1.8) | 1.74 (1.3–2.6) | 0.01 * |
| Ferritin (μg/L) | 79 (34–149) | 90 (49–176) | 0.82 |
| Active vitamin B12 (pmol/L) | 96 (71–127) | 101 (70–130) | 0.002 * |
| Folic acid (nmol/L) | 17 (12–22) | 12 (8–16) | 0.002 * |
| Vitamin B1 (nmol/L) | 130 (98–144) | 140 (118–157) | 0.03 |
| Vitamin B6 (nmol/L) | 84 (74–114) | 69 (52–83) | 0.43 |
| Albumin (g/L) | 43 ± 2 | 41 ± 4 | <0.001 * |
| Magnesium (mmol/L) | 0.85 ± 0.05 | 0.81 ± 0.07 | <0.001 * |
| Vitamin D (nmol/L) | 60 (42–75) | 39 (27–61) | <0.001 * |
| Haemoglobin (mmol/L) | 8.6 (8.3–9.1) | 8.8 (8.2–9.2) | 0.21 |
| Erythrocytes (×1012/L) | 4.6 ± 0.4 | 4.9 ± 0.4 | <0.001 * |
| Thrombocytes (×109/L) | 231 ± 48 | 261 ± 70 | 0.009 * |
| Leucocytes (×109/L) | 5.9 (5.0–7.4) | 8.5 (6.9–9.6) | <0.001 * |
| TSH (mU/L) | 1.60 (1.1–1.9) | 1.63 (1.2–2.4) | 0.03 |
| Echocardiography parameters | |||
| LVM (g) | 148 (117–175) | 194 (149–231) | <0.001 * |
| LVM index (g/m2) | 79 (62–88) | 76 (64–92) | 0.16 |
| Holter monitoring | |||
| Average heart rate (b.p.m.) | 73 ± 10 | 83 ± 10 | <0.001 * |
| Minimal heart rate (b.p.m.) | 48 (41–50) | 52 (47–56) | <0.001 * |
| Maximum heart rate (b.p.m.) | 138 (125–155) | 136 (126–150) | 0.62 |
| SDNN (ms) | 160 (130–194) | 101 (71–141) | <0.001 * |
| SDNN index (ms) | 63 (49–79) | 47 (38–58) | <0.001 * |
| Parameters of cardiac function | |||
| Echocardiographic parameters | |||
| Mitral inflow E‐wave (cm/s) | 73 ± 13 | 69 ± 14 | 0.06 |
| Mitral inflow A‐wave (cm/s) | 64 ± 14 | 70 ± 14 | 0.003 * |
| E/A ratio | 1.20 (0.97–1.4) | 0.98 (0.87–1.1) | <0.001 * |
| Septal e′ velocity (cm/s) | 9 (7–10) | 8 (7–9) | 0.03 |
| Lateral e′ velocity (cm/s) | 13 (10–15) | 10 (8–13) | <0.001 * |
| E/e ratio | 8.0 (7.3–10) | 8.7 (7.2–10) | 0.25 |
| Deceleration time (s) | 0.18 (0.16–0.20) | 0.19 (0.17–0.22) | 0.25 |
| LA volume index (mL/m2) | 26 ± 6 | 26 ± 8 | 0.88 |
| TR velocity (cm/s) | 106 (89–199) | 99 (90–132) | 0.16 |
| Diastolic dysfunction (%) | 1 (2%) | 11 (11%) | 0.09 |
| LV ejection fraction (%) | 65 ± 5 | 57 ± 7 | <0.001 * |
| GLS (%) | −20 (−21 to −19) | −16 (−18 to −14) | <0.001 * |
| Blood tests | |||
| BNP (pmol/L) | 6 (3–9) | 5 (3–8) | 0.59 |
| Hs troponin I positive (%) | 0 | 1 (1%) | 0.37 |
| Holter monitoring | |||
| Total PAC per 24 h (n) | 9 (3–23) | 10 (2–34) | 0.11 |
| Total PVC per 24 h (n) | 4 (1–17) | 3 (0–22) | 0.69 |
| Supraventricular arrhythmia (%) | 0 | 1 (1%) | 0.37 |
| Ventricular arrhythmia (%) | 1 (2%) | 0 | 0.16 |
ACE, angiotensin‐converting enzyme; ALAT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; A‐wave, late diastolic transmitral flow velocity; BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; E‐wave, early diastolic transmitral flow velocity; GLS, global longitudinal strain; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; LA volume index, left atrial volume index; LDL, low‐density lipoprotein; LV, left ventricular; LVM index, left ventricular mass index; LVM, left ventricular mass; OSAS, obstructive sleep apnoea syndrome; PAC, premature atrial contraction; PVC, premature ventricular contraction SDNN index, mean of the standard deviations of all the NN intervals for each 5 min segment of a 24 h heart rate variability recording; SDNN, standard deviation of NN intervals; TR velocity, tricuspid regurgitation; TSH, thyroid‐stimulating hormone.
Differences between obesity patients and non‐obese controls. Values represent mean ± SD, median (Q1–Q3) or n (%). P‐values displayed were analysed by using generalized linear mixed models. Global longitudinal strain was available in 49 non‐obese controls and in 94 obesity patients. Left ventricular ejection fraction was available in 49 non‐obese controls and in 95 obesity patients.
Bold values are statistically significant at p<0.05; the bold values marked by * remain statistically significant after Benjamini – Hochberg correction.
Figure 2.
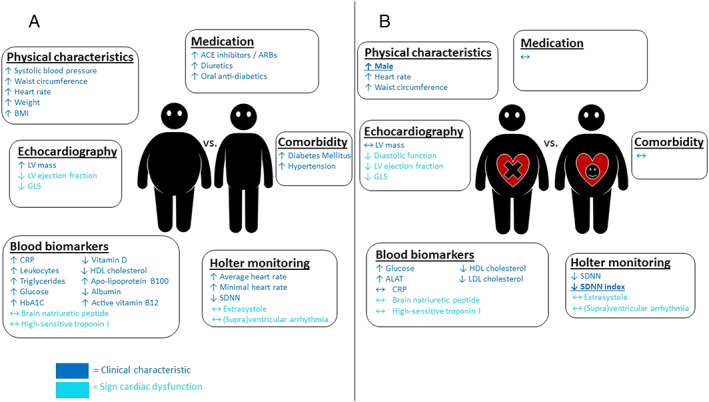
Difference in clinical characteristics and cardiac dysfunction parameters in (A) obesity patients vs. non‐obese controls. (B) Obesity patients with vs. obesity patients without cardiac dysfunction. Arrows indicate whether parameters were increased or decreased in obesity patients (A) or in obesity patients with cardiac dysfunction (B). Bold and underlined parameters are identified as significant risk factors for cardiac dysfunction in obesity patients by multivariate analysis. ACE, angiotensin‐converting enzyme; ALAT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; BMI, body mass index; CRP, C‐reactive protein; e′, early diastolic mitral annular velocity; E‐wave, early diastolic transmitral flow velocity; GLS, global longitudinal strain; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; LV, left ventricular; OSAS, obstructive sleep apnoea syndrome; SDNN, standard deviation of NN intervals.
Parameters of cardiac function in obesity patients and non‐obese controls
GLS was available in 49 non‐obese controls and 94 obesity patients (unavailable in the other subjects owing to insufficient echocardiographic image quality). Obesity patients had a significantly decreased GLS. With the use of a cut‐off value of 16.9% (95th percentile of the non‐obese controls), 57 (61%) obesity patients showed decreased GLS than did none of the controls (P < 0.001). The LV ejection fraction (57 ± 7 vs. 65 ± 5%, P < 0.001) was decreased as well, although only 24 (25% of 95 patients with available LV ejection fraction) of the obesity patients had an LV ejection fraction <52% (all with GLS < 16.9%). Also, obesity patients tended to have diastolic dysfunction more frequently (11% vs. 2%, P = 0.09). The septal e′ velocity and lateral e′ velocity were decreased. Levels of BNP and hs‐cTnI were comparable. One obesity patient had an episode of asymptomatic atrial flutter during 5 h recorded during Holter monitoring.
In total, 60 obesity patients showed at least one subclinical sign of cardiac dysfunction: 57 had a decreased GLS, one had diastolic dysfunction without an available GLS, one had a normal GLS (−17.3%) but an increased BNP (49 pmol/L, normal value < 30 pmol/L), and one had both a positive hs‐cTnI and a paroxysmal atrial flutter (GLS −18.6% in this patient). The latter patient was diagnosed with acromegaly after inclusion. We, therefore, decided to exclude this patient from the following sub‐analysis, focusing specifically on obesity patients with cardiac dysfunction.
Characteristics of obesity patients with subclinical signs of cardiac dysfunction
Table 2 and Figure 2B display the characteristics of obesity patients with (n = 59) and without (n = 40) cardiac dysfunction. Obesity patients with cardiac dysfunction were more often male and had an increased heart rate. There were no significant differences regarding the prevalence of co‐morbidities and medication use.
TABLE 2.
Clinical characteristics and parameters of cardiac function in obesity patients with and without cardiac dysfunction
| Obese with normal cardiac function (n = 40) | Obese with cardiac dysfunction (n = 59) | P‐value | |
|---|---|---|---|
| Clinical characteristics | |||
| General characteristics | |||
| Age (years) | 47 (42–52) | 49 (42–56) | 0.53 |
| Female (%) | 35 (88%) | 35 (59%) | 0.004 * |
| Physical examination | |||
| Length (m) | 1.69 ± 0.1 | 1.73 ± 0.1 | 0.045 |
| Weight (kg) | 123 (115–132) | 124 (114–138) | 0.28 |
| BMI (kg/m2) | 43 (40–46) | 42 (40–45) | 0.56 |
| Systolic blood pressure (mmHg) | 139 ± 21 | 144 ± 20 | 0.08 |
| Diastolic blood pressure (mmHg) | 75 (70–84) | 80 (73–89) | 0.06 |
| Waist circumference (cm) | 128 (122–134) | 137 (127–141) | 0.048 |
| Heart rate (b.p.m.) | 76 ± 11 | 83 ± 14 | 0.019 * |
| Co‐morbidity | |||
| Diabetes mellitus | 8 (20%) | 13 (22%) | 0.81 |
| Hypertension | 11 (28%) | 20 (34%) | 0.27 |
| Hypercholesterolaemia | 8 (20%) | 10 (17%) | 0.89 |
| Current smoking | 7 (18%) | 9 (15%) | 0.77 |
| COPD | 3 (8%) | 2 (3%) | 0.37 |
| OSAS | 3 (8%) | 8 (14%) | 0.35 |
| Medication | |||
| Beta‐blockers | 3 (8%) | 5 (9%) | 0.36 |
| ACE inhibitors/ARBs | 10 (27%) | 13 (23%) | 0.53 |
| Calcium channel blockers | 3 (8%) | 7 (12%) | 0.13 |
| Statins | 5 (13%) | 14 (25%) | 0.12 |
| Diuretics | 6 (16%) | 11 (19%) | 0.13 |
| Insulin | 2 (5%) | 5 (9%) | 0.51 |
| Oral anti‐diabetics | 5 (13%) | 9 (16%) | 0.70 |
| Blood tests | |||
| C‐reactive protein (mg/L) | 7 (3–10) | 6 (4–11) | 0.70 |
| Glucose (mmol/L) | 5.0 (4.6–5.6) | 5.6 (5.1–6.7) | 0.01 * |
| HbA1c (mmol/mol) | 37 (35–43) | 40 (36–51) | 0.05 |
| Creatinine (μmol/L) | 68 (64–78) | 73 (66–77) | 0.32 |
| eGFR (mL/min/1.73 m2) | 87 (79–90) | 90 (80–90) | 0.43 |
| ALAT (U/L) | 23 (16–33) | 31 (22–45) | 0.003 * |
| Apo‐lipoprotein B100 (g/L) | 1.12 ± 0.3 | 1.04 ± 0.3 | 0.67 |
| Lipoprotein (a) (mg/L) | 190 (65–599) | 149 (71–386) | 0.10 |
| Total cholesterol (mmol/L) | 5.5 ± 0.8 | 5.1 ± 1.1 | 0.025 |
| LDL cholesterol (mmol/L) | 3.5 ± 0.8 | 3.0 ± 0.9 | 0.003 * |
| HDL cholesterol (mmol/L) | 1.3 (1.0–1.5) | 1.1 (1.0–1.3) | 0.009 * |
| Triglycerides (mmol/L) | 1.4 (1.0–2.0) | 2.1 (1.5–3.0) | 0.09 |
| Ferritin (μg/L) | 87 (49–136) | 92 (58–189) | 0.14 |
| Active vitamin B12 (pmol/L) | 93 (61–128) | 108 (71–197) | 0.22 |
| Folic acid (nmol/L) | 11 (8–16) | 12 (9–16) | 0.45 |
| Vitamin B1 (nmol/L) | 13 ± 29 | 145 ± 22 | 0.08 |
| Vitamin B6 (nmol/L) | 65 (54–91) | 70 (52–80) | 0.39 |
| Albumin (g/L) | 42 (40–44) | 42 (39–44) | 0.83 |
| Magnesium (mmol/L) | 0.81 ± 0.06 | 0.81 ± 0.07 | 0.45 |
| Vitamin D (nmol/L) | 39 (28–60) | 39 (27–61) | 0.95 |
| Haemoglobin (mmol/L) | 8.6 (8.0–9.0) | 8.9 (8.4–9.3) | 0.11 |
| Erythrocytes (×1012/L) | 4.8 ± 0.4 | 5.0 ± 0.3 | 0.03 |
| Thrombocytes (×109/L) | 270 ± 54 | 255 ± 79 | 0.22 |
| Leucocytes (×109/L) | 8.4 (6.8–9.4) | 8.5 (7.0–10.2) | 0.48 |
| TSH (mU/L) | 1.5 (1.2–2.5) | 1.7 (1.2–2.4) | 0.94 |
| Echocardiographic parameters | |||
| LVM (g) | 169 (140–215) | 203 (156–241) | 0.10 |
| LVM index (g/m2) | 72 (59–88) | 81 (67–94) | 0.16 |
| Holter monitoring | |||
| Average heart rate (b.p.m.) | 81 ± 9 | 83 ± 10 | 0.35 |
| Minimal heart rate (b.p.m.) | 50 (46–55) | 53 (47–56) | 0.66 |
| Maximum heart rate (b.p.m.) | 142 (129–152) | 132 (125–146) | 0.06 |
| SDNN (ms) | 121 ± 48 | 99 ± 42 | 0.026 |
| SDNN index (ms) | 54 ± 16 | 45 ± 14 | 0.015 * |
| Parameters of cardiac function | |||
| Echocardiographic parameters | |||
| Mitral inflow E‐wave (cm/s) | 75 ± 15 | 65 ± 12 | 0.007 * |
| Mitral inflow A‐wave (cm/s) | 70 ± 11 | 70 ± 15 | 0.68 |
| E/A ratio | 1.1 (0.92–1.20) | 0.96 (0.80–1.10) | 0.029 |
| Septal e′ velocity (cm/s) | 8 ± 2 | 8 ± 2 | 0.22 |
| Lateral e′ velocity (cm/s) | 12 ± 3 | 10 ± 3 | 0.002 * |
| E/e′‐ratio | 9.1 (7.5–10.3) | 8.4 (6.9–9.7) | 0.27 |
| Deceleration time (s) | 0.19 ± 0.04 | 0.19 ± 0.04 | 0.93 |
| LA volume index (mL/m2) | 25 ± 7 | 26 ± 8 | 0.52 |
| TR velocity (cm/s) | 113 (91–140) | 93 (86–125) | 0.55 |
| Diastolic dysfunction (%) | 0 | 10 (17%) | 0.001 * |
| LV ejection fraction (%) | 62 ± 6 | 54 ± 7 | <0.001 * |
| GLS | −19.2 ± 1.3 | −14.4 ± 2.1 | <0.001 * |
| Blood tests | |||
| BNP (pmol/L) | 6 (4–11) | 4 (3–6) | 0.29 |
| hs‐troponin I positive (%) | 0 | 0 | |
| Holter monitoring | |||
| Total PAC per 24 h | 12 (3–38) | 8 (2–27) | 0.42 |
| Total PVC per 24 h | 2 (0–16) | 3 (0–28) | 0.98 |
| Supraventricular arrhythmia (%) | 0 | 0 | |
| Ventricular arrhythmia (%) | 0 | 0 | |
ACE, angiotensin‐converting enzyme; ALAT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; A‐wave, late diastolic transmitral flow velocity; BMI, body mass index; BNP, brain natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; E‐wave, early diastolic transmitral flow velocity; GLS, global longitudinal strain; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; LA volume index, left atrial volume index; LDL, low‐density lipoprotein; LV, left ventricular; LVM index, left ventricular mass index; LVM, left ventricular mass; OSAS, obstructive sleep apnoea syndrome; PAC, premature atrial contraction; PVC, premature ventricular contraction SDNN index, mean of the standard deviations of all the NN intervals for each 5 min segment of a 24 h heart rate variability recording; SDNN, standard deviation of NN intervals; TR velocity, tricuspid regurgitation; TSH, thyroid‐stimulating hormone.
Values represent mean ± SD, median (Q1–Q3) or n (%). P‐values displayed were analysed by using univariable logistic regression.
Bold values are statistically significant at p<0.05; the bold values marked by * remain statistically significant after Benjamini – Hochberg correction.
Blood tests showed increased glucose and ALAT in obesity patients with cardiac dysfunction. Also, these patients had decreased levels of LDL‐C and HDL‐C. Hs‐cTnI and C‐reactive protein were not significantly different.
Obesity patients with cardiac dysfunction tended to have an increased LV mass and LV mass index. They also had more often diastolic dysfunction. Holter monitoring showed a decreased SDNN index in obesity patients with cardiac dysfunction.
Odds ratios and predictive model of cardiac dysfunction in obesity patients
Univariable logistic regression analysis showed that cardiac dysfunction was associated with male gender, SDNN index, SDNN, length, waist circumference, heart rate, glucose, ALAT, total cholesterol, LDL‐C, HDL‐C, and erythrocytes. The multivariable logistic analysis identified male gender and SDNN index as independent significant risk factors for subclinical cardiac dysfunction in obesity patients (Table 3 ). The ROC curve is shown in Figure 3 . The area under the ROC curve was 0.81 (95% CI: 0.71–0.91, P < 0.001). Sensitivity was 74% (95% CI: 57–86%), specificity 62% (95% CI: 45–77%), positive predictive value 67% (95% CI: 50–80%), and negative predictive value 70% (95% CI: 51–84%).
TABLE 3.
Univariable and multivariable logistic regression analysis in obesity patients, with presence of cardiac dysfunction as the dependent variable
| Variable | Univariate P‐value | Multivariate P‐value |
|---|---|---|
| Male gender | 0.004 | 0.001 |
| SDNN index | 0.015 | 0.015 |
| SDNN | 0.026 | |
| Waist circumference | 0.048 | |
| Heart rate | 0.019 | |
| Glucose | 0.01 | |
| ALAT | 0.003 | |
| Total cholesterol | 0.025 | |
| LDL cholesterol | 0.003 | |
| HDL cholesterol | 0.009 | |
| Erythrocytes | 0.03 | |
| Length | 0.045 |
ALAT, alanine aminotransferase; SDNN index, mean of the standard deviations of all the NN intervals for each 5 min segment of a 24 h heart rate variability recording; SDNN, standard deviation of NN intervals.
Variables displayed were statistically significant different between obesity patients with and without cardiac dysfunction. Multivariate logistic regression analysis; method: backward stepwise analysis.
Figure 3.
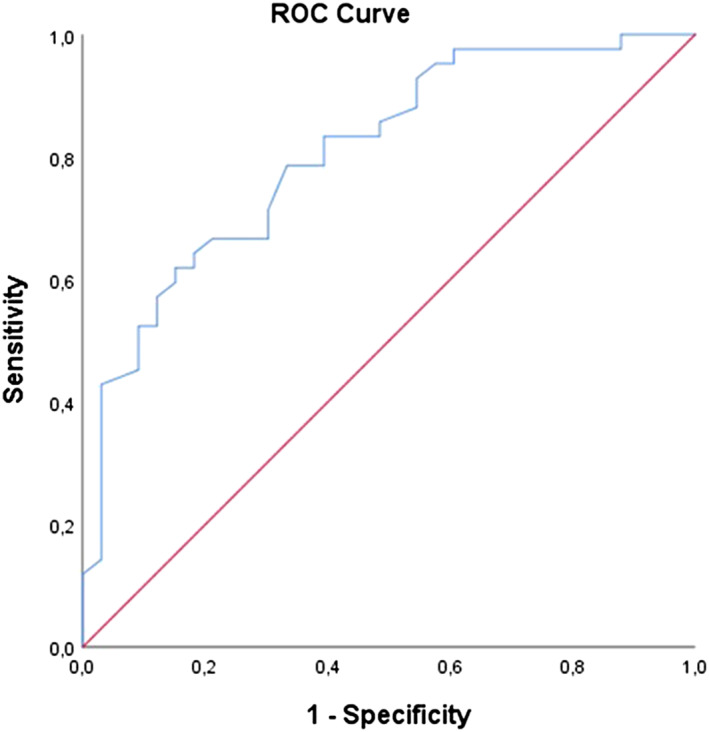
ROC curve for the prediction model for cardiac dysfunction in obesity patients. Model; combination of SDNN, SDNN index, gender, ALAT, glucose, and triglycerides. Area under the curve = 0.72 (95% CI: 0.61–0.82, P < 0.001). ALAT, alanine aminotransferase; ROC, receiver operating characteristic; SDNN, standard deviation of all NN intervals.
Discussion
The main findings of the current study are (i) that there is a high prevalence (61%) of subclinical cardiac dysfunction in obesity patients (BMI ≥ 35 kg/m2) without suspicion of or known cardiovascular disease; (ii) in the vast majority of patients with subclinical cardiac dysfunction, this was identified by abnormal GLS, clearly more than by other echocardiographic parameters, arrhythmias, increased BNP or high‐sensitivity troponin I (hs‐TnI); and (iii) decreased HRV as measured by SDNN index and male gender are predictors of subclinical cardiac dysfunction in obesity patients.
The association between abnormalities in cardiac structure and function is known in obesity patients. 1 Because of the ongoing obesity epidemic, efficient screening for (subclinical) cardiac dysfunction in these patients is needed. 22 The current knowledge of the early signs of cardiac dysfunction in obesity patients is not optimal to develop such screening tools.
In our study, we were unable to identify subclinical cardiac dysfunction in obesity patients by Holter monitoring or assessment of hs‐cTnI and BNP. The frequency of extrasystole was not increased, and although obesity is a known risk factor for atrial fibrillation, 23 none of the obesity patients showed atrial fibrillation during the 24 h Holter monitoring. There was one patient with an atrial flutter and a positive hs‐cTnI, but he was diagnosed with acromegaly after inclusion and therefore does not represent the typical obesity patients without known cardiac disease.
However, we were able to identify a high prevalence of subclinical cardiac dysfunction on the basis of a decreased GLS by advanced echocardiography in 57 (61% of the 94 obesity patients with available GLS) of the obesity patients. GLS performed much better as compared with conventional echocardiography parameters such as LV ejection fraction (decreased in 29% of the obesity patients) and diastolic function (abnormal in only 11% of the obesity patients). Currently, GLS assessment by speckle‐tracking echocardiography is broadly available, as echo machines from all well‐known vendors are generally equipped with speckle‐tracking software. Strain can be assessed in three directions (longitudinal, circumferential, and radial), with longitudinal strain known to be the most reproducible. It is therefore recommended to use GLS as a parameter of LV systolic function. 16 Recently, we demonstrated that the assessment of GLS is feasible and reproducible in obesity patients as well. 24 As said, in the current study, we identified GLS as the best parameter to diagnose cardiac dysfunction in obesity patients. Therefore, when looking for subclinical cardiac abnormalities in these patients, GLS assessment by speckle‐tracking echocardiography seems to be the best diagnostic technique.
So far, the pathophysiology of obesity leading to cardiac dysfunction is incompletely understood, and it was hypothesized that it is most likely multifactorial. 3 , 25 While previous studies examined the association between heart failure and obesity, 1 , 26 the CARDIOBESE study is the first to investigate the relation between subclinical cardiac dysfunction and obesity using a combination of techniques to simultaneously investigate different aspects that may all play a role. The transthoracic echocardiogram may identify direct local effects of obesity such as increased LV mass, systemic influences caused by secretion of adipokines by the fat tissue may be revealed by the blood tests, and the Holter monitor may identify autonomic dysfunction by assessment of HRV.
In our study, there were no differences between obesity patients with and without subclinical cardiac dysfunction regarding the presence of traditional cardiac risk factors, such as diabetes mellitus, hypertension, and hypercholesterolaemia. These results are consistent with previous studies, which showed that the onset of heart failure in obesity cannot be fully explained by these risk factors. 27 Also, obesity patients are known to have a state of chronic low‐grade inflammation. 28 Although increased circulating levels of C‐reactive protein were observed in obesity patients compared with non‐obese controls, there was no difference between obesity patients with and without subclinical cardiac dysfunction. Therefore, at least as far as C‐reactive protein reflects systemic inflammation, this was not likely to be an explanation of subclinical cardiac dysfunction in our patients. Increased cardiac filling pressures or cardiomyocyte damage have been suggested to play a role 29 ; however, BNP and hs‐cTnI were comparable between obesity patients and non‐obese controls and between obesity patients with and without cardiac dysfunction in our study. Finally, the LV mass and LV mass index were comparable between obesity patients with and without subclinical cardiac dysfunction. Therefore, the local effect of increased LV mass does not seem to play a major role in obesity leading to subclinical cardiac dysfunction.
Nevertheless, the SDNN index as a measure of HRV and thereby of autonomic dysfunction was strikingly different between obesity patients with and without subclinical cardiac dysfunction and was identified as an independent risk factor for cardiac dysfunction by multivariable analysis. The SDNN index estimates the variability due to the factors affecting HRV within a 5 min period. 30 Even a slight variation in the autonomic regulation of the heart changes the heart rate and rhythm. 31 The analysis of HRV thereby provides a non‐invasive tool to characterize autonomic function. Depressed HRV has been confirmed to be a prognostic marker and is correlated with morbidity and mortality. 32 , 33 , 34 Furthermore, sympathetic nervous system dysfunction is crucial in the development of heart failure. 35 Previous studies already described a decreased HRV in obesity patients, 31 , 36 linking this to inflammatory processes. 37 , 38 However, our study not only confirmed the presence of a decreased HRV in obesity patients, but it is also the first to show that decreased HRV may play a crucial role in the development of cardiac dysfunction in these patients. Therefore, the analysis of HRV may be a useful and simple non‐invasive method to further investigate the effect of obesity on cardiac function.
The multivariable analysis identified not only SDNN index but also male gender as a significant risk factor for cardiac dysfunction in obesity patients. Previous studies already described an association between obesity and more severe heart failure symptoms in male patients compared with female patients. 39 Also, overweight and obese men have higher adjusted mortality than normal‐weight men, whereas a BMI in the overweight range was associated with a survival benefit in women. 40 However, the reason for these findings is not clear. It cannot be excluded that there are unidentified confounders related to gender that may explain the suggested relationship between male gender and cardiac dysfunction in obesity in our study. Further studies are needed to clarify this issue.
Limitations
GLS was missing in seven obesity patients because of insufficient image quality. This may have affected the identified prevalence of cardiac dysfunction. In addition, cardiac magnetic resonance could be of added value, when investigating myocardial characteristics. However, this was not available in the CARDIOBESE study. Also, the study contained a relatively large number of women (70%), which may have influenced the observed relationship between gender and subclinical cardiac dysfunction.
When studying HRV, one can select several parameters. 41 In our study, we only used SDNN and SDNN index as markers of HRV to limit the total number of parameters studied. They were chosen because no currently recognized HRV measure provides better prognostic information than the time domain HRV measures assessing overall HRV. 41 In parallel, we only used C‐reactive protein and leucocytes as inflammatory markers, also to limit the total number of parameters studied and because of limited testing that can be done in our clinical laboratory. To further investigate the role of inflammation, further studies are needed. Also, blood samples were obtained in the non‐fasting state, which could have influenced our results. Finally, although obesity is usually defined as a BMI ≥ 30 kg/m2, all patients in our study had a BMI ≥ 35 kg/m2, because they were included at the outpatient clinic for screening for bariatric surgery (BMI ≥ 35 kg/m2 is a condition to qualify for bariatric surgery). Therefore, the conclusions may only be applied to morbidly obese patients and not to obesity patients in general.
Conclusions
There was a high prevalence (61%) of subclinical cardiac dysfunction in obesity patients without known cardiovascular disease, which appeared to be best identified by GLS. Subclinical cardiac dysfunction in obesity was linked to autonomic dysfunction and male gender and not to the presence of traditional cardiac risk factors, increased C‐reactive protein, increased BNP, increased hs‐TnI, or increased LV mass.
Conflict of interest
None declared.
Funding
This work was supported by a grant from Stichting BeterKeten.
Snelder, S. M. , de Groot‐de Laat, L. E. , Biter, L. U. , Castro Cabezas, M. , Pouw, N. , Birnie, E. , Boxma‐de Klerk, B. M. , Klaassen, R. A. , Zijlstra, F. , and van Dalen, B. M. (2020) Subclinical cardiac dysfunction in obesity patients is linked to autonomic dysfunction: findings from the CARDIOBESE study. ESC Heart Failure, 7: 3726–3737. 10.1002/ehf2.12942.
Contributor Information
Bianca M. Boxma‐de Klerk, Email: b.boxma@franciscus.nl.
Bas M. van Dalen, Email: b.vandalen@franciscus.nl.
References
- 1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collaboration NCDRF . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afshin A, Reitsma MB, Murray CJL. Health effects of overweight and obesity in 195 countries. N Engl J Med 2017; 377: 1496–1497. [DOI] [PubMed] [Google Scholar]
- 4. Global BMIMC , Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G, Lewington S. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet 2016; 388: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Packer M. The conundrum of patients with obesity, exercise intolerance, elevated ventricular filling pressures and a measured ejection fraction in the normal range. Eur J Heart Fail 2019; 21: 156–162. [DOI] [PubMed] [Google Scholar]
- 6. Cescau A, Van Aelst LNL, Baudet M, Cohen Solal A, Logeart D. High body mass index is a predictor of left ventricular reverse remodelling in heart failure with reduced ejection fraction. ESC Heart Fail 2017; 4: 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: pathophysiology and management. J Am Coll Cardiol 2018; 71: 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang YC, Liang CS, Gopal DM, Ayalon N, Donohue C, Santhanakrishnan R, Sandhu H, Perez AJ, Downing J, Gokce N, Colucci WS, Ho JE. Preclinical systolic and diastolic dysfunctions in metabolically healthy and unhealthy obese individuals. Circ Heart Fail 2015; 8: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner L, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 10. Finer N. Weight loss for patients with obesity and heart failure. Eur Heart J 2019; 40: 2139–2141. [DOI] [PubMed] [Google Scholar]
- 11. Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all‐cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013; 61: 2365–2373. [DOI] [PubMed] [Google Scholar]
- 12. Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr 2011; 24: 322–332 e6. [DOI] [PubMed] [Google Scholar]
- 13. Manov EI, Runev NM, Shabani RA, Vasileva DG, Cherneva RV, Donova TI, Georgiev OB, Petrova DS. Early left ventricular function abnormalities in obstructive sleep apnea. J Cardiovasc Dis Diagn 2014;2: 1–4. [Google Scholar]
- 14. Snelder SM, de Groot‐de Laat LE, Biter LU, Cabezas MC, van de Geijn GJ, Birnie E, Boxma‐de Klerk B, Klaassen RA, Zijlstra F, van Dalen BM. Cross‐sectional and prospective follow‐up study to detect early signs of cardiac dysfunction in obesity: protocol of the CARDIOBESE study. BMJ Open 2018; 8: e025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, Tébar FJ, Valdés M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 2003; 89: 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 18. van Dalen BM, Tzikas A, Soliman OI, Heuvelman HJ, Vletter WB, ten Cate FJ, Geleijnse ML. Assessment of subendocardial contractile function in aortic stenosis: a study using speckle tracking echocardiography. Echocardiography 2013; 30: 293–300. [DOI] [PubMed] [Google Scholar]
- 19. Grant CC, van Rensburg DC, Strydom N, Viljoen M. Importance of tachogram length and period of recording during noninvasive investigation of the autonomic nervous system. Ann Noninvasive Electrocardiol 2011; 16: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 2017; 5: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 1995; 57: 289–300. [Google Scholar]
- 22. Weismann D, Wiedmann S, Bala M, Frantz S, Fassnacht M. Obesity and heart failure. Internist (Berl) 2015; 56: 121–126. [DOI] [PubMed] [Google Scholar]
- 23. Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 2005; 118: 489–495. [DOI] [PubMed] [Google Scholar]
- 24. Snelder SM, Younge JO, Dereci A, van Velzen JE, Akkerhuis JM, de Groot‐de Laat LE, Zijlstra F, van Dalen BM. Feasibility and reproducibility of transthoracic echocardiography in obese patients. J Am Soc Echocardiogr 2019; 32: 1491–1493.e5. [DOI] [PubMed] [Google Scholar]
- 25. Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016; 68: 200–203. [DOI] [PubMed] [Google Scholar]
- 26. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose‐response meta‐analysis of prospective studies. Circulation 2016; 133: 639–649. [DOI] [PubMed] [Google Scholar]
- 27. Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc 2016; 5, e003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542: 177–185. [DOI] [PubMed] [Google Scholar]
- 29. Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Dávila‐Román VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 2004; 43: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 30. Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration—a risk of CVD. Diabetes Metab Syndr Obes 2017; 10: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis MJ. Heart rate variability analysis: a tool to assess cardiac autonomic function. Comput Inform Nurs 2005; 23: 335–341. [DOI] [PubMed] [Google Scholar]
- 32. Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, Hnatkova K, Schömig A, Huikuri H, Bunde A, Malik M, Schmidt G. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 2006; 367: 1674–1681. [DOI] [PubMed] [Google Scholar]
- 33. Ali A, Holm H, Molvin J, Bachus E, Tasevska‐Dinevska G, Fedorowski A, Jujic A, Magnusson M. Autonomic dysfunction is associated with cardiac remodelling in heart failure patients. ESC Heart Fail 2018; 5: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Forslund L, Bjorkander I, Ericson M, Held C, Kahan T, Rehnqvist N, Hjemdahl P. Prognostic implications of autonomic function assessed by analyses of catecholamines and heart rate variability in stable angina pectoris. Heart 2002; 87: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014; 114: 1815–1826. [DOI] [PubMed] [Google Scholar]
- 36. Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol 1999; 83: 1242–1247. [DOI] [PubMed] [Google Scholar]
- 37. Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, Thayer JF. Heart rate variability and inflammation: a meta‐analysis of human studies. Brain Behav Immun 2019; 80: 219–226. [DOI] [PubMed] [Google Scholar]
- 38. Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V. Decreased heart rate variability is associated with higher levels of inflammation in middle‐aged men. Am Heart J 2008; 156: 759 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heo S, Moser DK, Pressler SJ, Dunbar SB, Lee KS, Kim J, Lennie TA. Association between obesity and heart failure symptoms in male and female patients. Clin Obes 2017; 7: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC Heart Fail 2015; 3: 917–926. [DOI] [PubMed] [Google Scholar]
- 41. Heart rate variability: standards of measurement, physiological interpretation and clinical use . Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93: 1043–1065. [PubMed] [Google Scholar]


